Abstract
Endotoxin is often used to activate NF-κB in vitro when assessing NLRP3 inflammasome activation by various exogenous particles including nanoparticles. However, the endogenous source of this signal 1 is unknown. High-mobility group box 1 (HMGB1) is known to play a critical role in acute lung injury, however the potential contribution of the alarmin HMGB1 to NLRP3 Inflammasome activation has not been determined in response to nanoparticles in vivo. In this study, the ability of multi-walled carbon nanotubes (MWCNT) to cause release of HMGB1 in vitro and in vivo, as well as the potential of HMGB1 to function as signal 1 in vitro and in vivo, was determined. HMGB1 activity in vivo was assessed by administration of HMGB1 neutralization antibodies following MWCNT exposure. Caspase-1−/− mice were utilized to elucidate the dependence of HMGB1 secretion on NLRP3 inflammasome activity. MWCNT exposure increased extracellular HMGB1 levels in primary alveolar macrophages from C57Bl/6 mice and C10 mouse epithelial cell culture supernatants, and in C57Bl/6 mouse lung lavage fluid. MWCNT-induced HMGB1 secretion was dependent upon caspase-1. HMGB1 increased MWCNT-induced IL-1β release from macrophages in vitro, and neutralization of extracellular HMGB1 reduced MWCNT-induced IL-1β secretion in vivo. HMGB1 neutralization was accompanied with overall decreased inflammation. In summary, this study suggests extracellular HMGB1 participates in NLRP3 inflammasome activity and regulates IL-1β associated sterile inflammation induced by MWCNT.
Keywords: Caspase-1, DAMP, IL-1β, MWCNT, sterile inflammation
Introduction
Multi-walled carbon nanotubes have remarkable physiochemical properties that will advance the fields of medicine, electronics and engineering. However, the high demand for these materials, in concert with their toxicity potential, may pose a future health risk for humans. Pulmonary exposure studies in murine models have revealed that MWCNT exposure has negative consequences, including chronic inflammation leading to granuloma formation and fibrosis, along with asthma-like pathology (Glista-Baker et al., 2014; Hamilton et al., 2007, 2012, 2013; Mercer et al., 2011, 2013). The mechanisms that promote chronic inflammation are not clear, but activation of the NLRP3 inflammasome in macrophages and IL-1β signaling has been shown to be necessary for MWCNT-induced inflammation (Girtsman et al., 2012; Hamilton et al., 2012; Palomaki et al., 2011).
The NLRP3 Inflammasome is a multi-protein scaffolding complex that is assembled in macrophages following MWCNT exposure. Assembly of the NLRP3 Inflammasome complex results in activation of caspase-1, which in turn, processes pro-IL-1β and pro-IL-18 to their active, pyrogenic forms (Martinon et al., 2002). However, assembly of the NLRP3 complex alone does not result in IL-1β secretion in vitro (Ghonime et al., 2014), for a signal (often described as signal 1) is required for production of pro-IL-1β, usually accomplished by adding low levels of endotoxin. This two hit method is widely used to assess the inflammatory potential of particles in vitro. However, in mice kept in SPF facilities treated with MWCNT or in conditions of sterile injury in humans, endotoxin likely plays a minimal role, suggesting some other endogenous signal is involved.
High-mobility group box 1 (HMGB1) is a nuclear architectural protein that is secreted from injured/dead cells (Pisetsky, 2014; Scaffidi et al., 2002; Stros, 2010; Yang et al., 2010b), as well as actively secreted from cells of monocytic origin (Gardella et al., 2002). Secreted HMGB1 belongs to a large family of Danger Associated Molecular Patterns (DAMPs) ligands, and has been implicated as an activator of NF-κB in sterile inflammation. It has been reported that HMGB1 is secreted via a non-conventional NLRP3 Inflammasome-mediated mechanism (Lamkanfi et al., 2010; Willingham et al., 2009). Once outside the cell, HMGB1 has been shown to bind TLR4 and/or the Receptor for Advanced Glycation End-products (RAGE) (Park et al., 2006; Ullah et al., 2014), and through this mechanism is suggested to mediate sepsis/ LPS induced acute lung injury (ALI), hemorrhagic shock and ventilator-induced ALI (Kim et al., 2005; Ueno et al., 2004; van Zoelen et al., 2008; Wang et al., 1999, 2004). HMGB1 has also been identified as a mediator of bleomycin-induced fibrosis, likely through regulating the severity of the acute inflammatory response (Hamada et al., 2008). Although HMGB1 has been shown to play specific roles in these injury models, the secretory pathway of HMGB1 and extracellular activity in MWCNT-induced inflammation, which has characteristics distinct from other exposure models, is unknown. Additionally, whether HMGB1 contributes to NLRP3 Inflammasome-mediated inflammation following MWCNT exposure, or whether other DAMPs are more critical to this activity, is not known.
The objective of this study was to elucidate the contribution of HMGB1 to MWCNT-induced acute inflammation. Specifically, we hypothesized that HMGB1 has the ability to regulate NLRP3 Inflammasome activity, and therefore, targeting extracellular HMGB1 signaling or pathways that regulate HMGB1 secretion will be mechanisms to decrease NLRP3 Inflammasome activity. In this study, we assessed for the presence and activity of HMGB1 following MWCNT exposure in vivo and in vitro. The MWCNT used in this study has previously been shown to induce a robust inflammatory and pathological response (Girtsman et al., 2012; Hamilton et al., 2012). The potential of extracellular HMGB1 to act on primary AM is of particular focus in this study since previous findings report strong correlations between AM production of IL-1β and pathology score following MWCNT exposure (Hamilton et al., 2012). Furthermore, caspase-1−/− mice were used to assess the contribution of NLRP3 Inflammasome activation to HMGB1 secretion. Together, this study describes a critical role for HMGB1 signaling in MWCNT-induced NLRP3 Inflammasome activity and associated inflammation.
Methods
MWCNT preparation
The MWCNT selected for these studies, designated FA-21 (Sun Innovations, Inc., Fremont, CA; www.nanomaterialstore.com), was selected due to its potent NLRP3 Inflammasome activity and has been characterized elsewhere (Hamilton et al., 2012). This specific MWCNT is 1 of 24 samples provided by Dr. Nigel Walker and Brad Collins at the National Toxicology Program (NTP) at the National Institute of Environmental Health Sciences (NIEHS). Purity and metal content of the MWCNT were determined using thermal gravimetric analysis (TGA) and X-ray fluorescence spectrometry, respectively. Diameter and agglomeration state of the MWCNT were determined by transmission electron microscopy (TEM) and dynamic light scattering (DLS), respectively. This pro-inflammatory MWCNT has high nickel contamination (~5.54%), and forms agglomerates that range from 122 to 469 nm depending on the dispersion media. Additionally, the MWCNT is free from endotoxin contamination, a critical quality for investigating the sterile inflammatory response. Prior to in vivo exposure by instillation or dispersion in vitro, MWCNT were suspended in dispersion medium [DM, 0.6 mg/ml mouse serum albumin (Sigma–Aldrich, Saint Louis, MO) and 0.01 mg/ ml 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (Sigma–Aldrich) in PBS] and suspended using sonication for 1 min (Porter et al., 2008).
Animals
C57Bl/6 and caspase-1−/− mice (2 months old) were housed in specific pathogen free and controlled environmental conditions (22 ± 2 °C; 30–40% humidity, 12 h light, 12 h dark cycle) and provided food and water ad libitum. All procedures were performed under protocols approved by the IACUC of the University of Montana.
Alveolar macrophage isolation and culture
Mice were euthanized by sodium pentobarbital (Euthasol™ Shering-Plough, Lot # 1JRR11V), and the lungs with the heart were removed. After lavage, AM were isolated by centrifugation (400 μg, 5 min). Retrieved cells were counted using a Coulter Z2 particle counter (Beckman Coulter, Miami, FL). AM were suspended in RPMI media supplemented with 10% fetal bovine serum (FBS), sodium pyruvate, and supplemented with an antimycotic/antibiotic cocktail (Mediatech, Manassas, VA). Cells were suspended at 1 × 106 cells/ml and exposed to MWCNT (25 μg/ml, 2.5 μg/105 cells) for 24 h at 37 °C in a water-jacketed CO2 incubator (ThermoForma, Houston, TX) in a 96-well culture plate. Recombinant HMGB1 (LPS free) was purchased from HMGBiotech. Cells were exposed to LPS (10 ng/ ml) or rHMGB1 (dose response: 0, 0.5, 1 and 2 μg/ml) at the same time as the MWCNT. rHMGB1 was formulated by the manufacture to be the disulfide isoform, or cytokine active form (Yang et al., 2010a, 2012). After 24 h of culture with the particle and treatment groups, supernatants were collected and assessed for extracellular IL-1β and/or HMGB1.
C10 cell culture and exposure
C10 epithelial cells, an immortalized non-transformed type II pneumocytic cell line cultured from BALB/c mice, were generously provided by Dr. Galya Orr (Pacific Northwest National Laboratories, Richland, WA). C10 cells were maintained in media identical to that used for AM. Cells were removed by trypsinization for 5–10 min at 37 °C, and plated at 80% density for 3 h prior to exposure to allow for adherence and acclimation. Cells were treated with MWCNT (25 μg/ml) for 24 h, after which the cell supernatants were collected, and debri removed by centrifugation in order to assess extracellular HMGB1 levels by Western blot.
In vivo experiments
Mice were exposed to MWCNT (2 mg/kg or 50 μg/25 g mouse) by oropharyngeal aspiration (Lacher et al., 2010). Briefly, mice were anaesthetized using isoflurane inhalation and the MWCNT prepared in DM were delivered into the back of the throat while holding the tongue to the side, allowing for aspiration into the lungs. For HMGB1 neutralization studies, mice were instilled with chicken anti-HMGB1 IgY or Control IgY (Shinotest, Japan), or vehicle (PBS) only via oropharyngeal aspiration 1 h following MWCNT instillations. After 1 day, the lungs were lavaged as described with ice cold PBS (pH 7.4). AM were removed by centrifugation (400 μg, 5 min, 4 °C) and cell counts obtained using the Coulter Z2 particle counter. Portions of the cells were stained for differential analysis with Wright’s Giemsa stain using a Hematek 2000 autostainer (Miles-Bayer-Siemens Diagnostics, Deerfield, IL). The acellular lavage was retained for assessment of IL-1β and HMGB1.
Assessment of endogenous HMGB1 in vitro
Multi-walled carbon nanotubes or DM were instilled into C57Bl/6 or caspase-1−/− mice as previously described and after 24 h mice were euthanized and lungs lavaged by instilling and retrieving 1 ml cold PBS repeatedly (3×) to maximize concentration of extracellular HMGB1 in the lavage fluid. HMGB1 was either retained or removed from the lavage fluid by immunoprecipitation. Naïve AM were isolated as described and exposed to MWCNT (25 μg/ml) in vitro and the lung lavage fluid from MWCNT- or DM-treated mice with or without HMGB1. Prior to treatment, lavage fluid was supplemented with 1% FCS to prevent AM starvation. After 24 h, supernatants were collected and assessed for IL-1β production.
HMGB1 immunoprecipitation and Western-blot analysis
Protein A coated magnetic Dynabeads® (Life Technologies) were prepared according to manufacturer’s instructions, and coated with 5 μg of anti-HMGB1 antibody (C-terminal epitope, Sigma–Aldrich). About 1.5 mg of the bead/antibody conjugates were added to 1 ml of the lavage fluid and incubated overnight at 4 °C with gentle tumbling. HMGB1 was then immunoprecipitated by magnetic separation, and the remaining lavage fluid and immunoprecipitated product were assessed for the presence of HMGB1 by traditional Western-blot analysis to confirm that HMGB1 had been successfully removed. Briefly, 30 μl of sample including: cell supernatant, lavage fluid or immunoprecipitated HMGB1 was loaded on a 12–4% Bis–Tris polyacrylamide gel and run for 1 h at 150 V. Protein was electrophoresed onto a PVDF membrane, and blocked with 5% non-fat dry milk in Tris-buffered saline. After blocking, the membrane was incubated overnight at 4 °C with anti-HMGB1 antibody (1:1000), washed 3 times, and then detected using a donkey anti-rabbit horseradish peroxidase-coupled secondary antibody (1:10 000). After washing three more times, the blot was developed using Fempto™ chemo-luminescence detection reagents (Pierce, Thermo Scientific, Rockford, IL).
HMGB1 assay
High-mobility group box 1 was measured by ELISA using commercially available antibodies (R&D Systems, Minneapolis MN; EMD Millipore, Billerica, MA, Santa-Cruz Biotech, Dallas, TX) and previously validated protocols (Dave et al., 2009; Liou et al., 2012). Slight adjustments were made to these protocols, including decreased blocking time (2 h) in 4% BSA in PBS, 2 h of sample incubation with the primary antibody, followed by a 2-h detection antibody incubation. ELISA specificity was confirmed by Western blot. Lavage samples were run immediately on the ELISA in order to remove variability and potential degradation caused by freeze-thaw.
Cytokine assays
IL-1β and TNF-α were measured using mouse Duo-Set ELISA (R&D Systems, Minneapolis, MN) following the manufacture’s protocol. Total Protein was measured using the BCA assay (Pierce, Thermo Scientific, Rockford, IL).
Statistical analysis
Statistical analyses involved comparison of means using a one- or two-way ANOVA followed by Dunnett’s test or Bonferroni’s test to compensate for increased type I error. All probabilities were two-tailed unless otherwise stated. Statistical power was >0.8. Statistical significance was defined as a probability of type I error occurring at <5% (p<0.05). The minimum number of experimental replications was 3. Graphics and analyses were performed on PRISM 5.0.
Results
MWCNT exposure increases extracellular HMGB1 levels
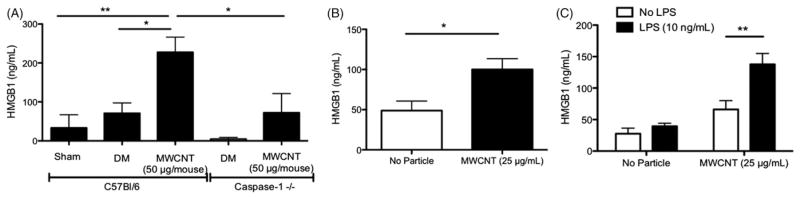
The ability of MWCNT to induce HMGB1 secretion was assessed by instilling C57Bl/6 (WT) mice with 50 μg/mouse (2 mg/kg) of particle or DM, and after 24 h, mice lungs were lavaged and HMGB1 concentrations were measured in the lavage fluid by ELISA. The MWCNT dose used for the in vivo studies was selected based on prior results showing it was the lowest amount required for reproducible measurements of IL-1β dependent inflammation and pathology (Girtsman et al., 2012). The physiochemical characteristics and NLRP3 Inflammasome activating potential of the MWCNT used in these studies has been previously reported (Hamilton et al., 2012). MWCNT exposure increased HMGB1 in the lung lavage fluid (Figure 1A). In order to assess potential sources of HMGB1 following MWCNT exposure in the lung, isolated primary AM and C10 cells were treated with 25 μg/ml MWCNT and extracellular HMGB1 concentrations were assessed after 24 h. The dosage chosen for in vitro studies was selected to eliminate interference of the particle in colorimetric/fluorescent assays that occurs at higher doses, and yet this lower dose retains the ability to induce a significant IL-1β response. MWCNT exposure resulted in increased extracellular HMGB1 in C10 cell supernatant (Figure 1B). Likewise, MWCNT exposure induced a non-significant increase of extracellular HMGB1 in AM supernatants, which was significantly enhanced by stimulation with low levels of LPS (Figure 1C). C10 cells are susceptible to MWCNT cytotoxicity, as measured by the LDH assay (Beamer et al., 2013). Similarly, MWCNT are cytotoxic to AM (Hamilton et al., 2012), suggesting that passive release is a likely source of part of the extracellular HMGB1 pool. Endotoxin stimulated enhancement of HMGB1 secretion in AM also suggests that there is active secretion as well. Together, the data in Figure 1 demonstrate MWCNT induce HMGB1 secretion in the lung, and both epithelial and AM are plausible sources of HMGB1 through passive and active secretion.
Figure 1.
HMGB1 levels following MWCNT exposure in vitro and in vivo. (A) HMGB1 levels in whole lung lavage fluid 24 h following MWCNT (50 μg/mouse) or DM instillation in C57Bl/6 or caspase-1−/− null mice. HMGB1 levels were assessed in sham (non-instilled mice) to assess injury caused by instillation of the vehicle. (B) Extracellular HMGB1 detected in C10 cell supernatants following 24 h of exposure to MWCNT (25 μg/ml). (C) HMGB1 levels in primary AM cell supernatant 24 h following MWCNT (25 μg/ml) exposure with (black bar) or without (white bar) LPS (10 ng/ml). *p<0.05, **p<0.01.
HMGB1 secretion is dependent on caspase-1
The NLRP3 Inflammasome activates caspase-1, which in turn facilitates pro-IL-1β processing and secretion. Reports suggest that HMGB1 secretion is dependent upon NLRP3 Inflammasome activation of caspase-1 (Lamkanfi et al., 2010; Willingham et al., 2009). However, the dependence of HMGB1 secretion on caspase-1 has not been evaluated with nanoparticles such as MWCNT. Therefore, we instilled caspase-1−/− mice with MWCNT or DM, and after 24 h, assessed HMGB1 concentrations in whole lung lavage fluid. Caspase-1−/− mice had significantly less HMGB1 than WT (Figure 1A), confirming that HMGB1 secretion is dependent upon caspase-1 in MWCNT exposure models.
Native HMGB1, but not rHMGB1, contributes to signal 1 of NLRP3 inflammasome activation
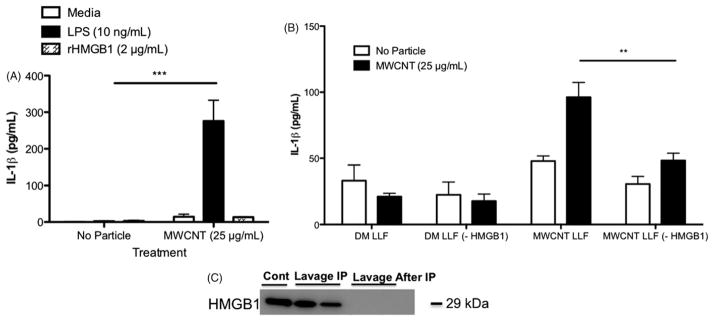
The contribution of HMGB1 to particle-induced inflammatory response, specifically the ability of HMGB1 to act as signal 1, has not been investigated. To elucidate whether HMGB1 can act as the NF-κB activating signal in vitro, primary AM were exposed to MWCNT and a dose response of commercially available disulfide form rHMGB1, reported to have NF-κB activating function (Yang et al., 2010a, 2012). rHMGB1 failed to induce IL-1β secretion at any dose in primary AM (high dose shown, Figure 2A), as well as in two other macrophage-like cell models, including: THP-1 and Bone Marrow derived Macrophages (Supplementary Materials S1A and B). Likewise, TNF-α was not increased in any of these models (data not shown). Specific HMGB1 isoforms have been identified to contribute more to different disease models, and the rHMGB1 used in this proposal, which has been reported to signal through TLR4 (Yang et al., 2010a), may not be comparable to endogenous HMGB1 in the lung following MWCNT exposure. To elucidate the ability of endogenous, native HMGB1 (nHMGB1) to act as signal 1, primary AM were isolated from C57Bl/6 and exposed to MWCNT in vitro, then treated with cell fee lung lavage fluid obtained from DM or MWCNT-exposed mice (24-h exposure period in vivo before isolation of cell free lung lavage fluid). In parallel, a second group of MWCNT-exposed C57Bl/6 AM was treated with lung lavage fluid from DM or MWCNT-exposed mice, where HMGB1 had been removed by immunoprecipitation (Figure 2C). Treatment with lung lavage fluid from MWCNT-treated mice enhanced IL-1β secretion in MWCNT-exposed AM, demonstrating that an endogenous signal 1 is present and soluble in the lung lavage fluid of MWCNT-treated mice (Figure 2B). Removal of HMGB1 by immunoprecipiation from the lung lavage fluid prior to treatment resulted in significantly less IL-1β production from MWCNT-exposed AM, indicating that HMGB1 is that endogenous signal 1 following MWCNT exposure. Immunoblotting and Coomassie blue staining revealed that the majority of immunoprecipitated protein was HMGB1 (Supplementary Material S2). Similar experiments were performed in which C57Bl/6 AM were exposed to MWCNT with lavage fluid from caspase-1−/− mice. Lavage fluid isolated from caspase-1−/− mice exposed to MWCNT failed to induce IL-1β secretion from C57Bl/6 AM exposed to MWCNT in vitro (data not shown), supporting the reported role of caspase-1 in HMGB1 secretion and signaling.
Figure 2.
nHMGB1 not rHMGB1 participates in inflammasome activity in vitro. (A) IL-1β in primary AM cell supernatants 24 h following MWCNT exposure and treatment with either LPS (10 ng/ml) or rHMGB1 (2 μg/ml). (B) IL-1β in primary AM cell supernatants after 24-h exposure to MWCNT (black bar, 25 μg/ml) in vitro, and exposure to cell free lung lavage fluid from DM-exposed mice (DM LLF) or MWCNT-exposed mice (MWCNT LLF). nHMGB1 was immunoprecipitated out of the lavage fluid to form treatment groups DM LLF (-HMGB1) and MWCNT LLF (-HMGB1). (C) Representative Western blot confirming that nHMGB1 was removed from the lung lavage fluid prior to addition to primary AM in vitro. **p<0.01, ***p<0.001.
HMGB1 neutralization decreases IL-1β release in vivo
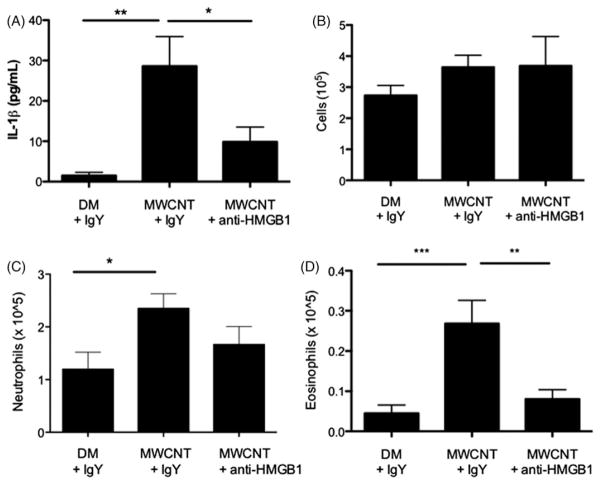
To translate the contribution of HMGB1 to NLRP3 Inflammasome activity in vivo, mice were administered HMGB1 neutralizing antibodies or control IgY antibodies by oropharyngeal aspiration 1 hr following MWCNT instillation. HMGB1 neutralization significantly decreased IL-1β levels in the lung lavage fluid at 1 day (Figure 3A), along with decreased inflammatory markers including neutrophil (trending but non-significant), and eosinophil counts (Figure 3C and D). Additionally, there was a non-significant, but trended decrease in total protein and TNF-α, (Supplementary Material S3A and B). However, HMGB1 neutralization did not decrease total cell counts (Figure 3B). Treatment with the isotype control and anti-HMGB1 antibodies induced an increase in neutrophils versus vehicle only (data not shown), however, this had no effect on IL-1β levels, and appeared to be non-inflammatory. Despite the increase in neutrophil numbers with antibody instillation, the anti-inflammatory trends of HMGB1 neutralization remained consistent. Together, these data support a specific role for extracellular HMGB1 in mediating NLRP3 inflammasome activity, specifically the secretion of IL-1β, in vivo. Furthermore, these data suggest an important role for HMGB1/IL-1β dependent recruitment of eosinophils in C57Bl/6 mice exposed to MWCNT.
Figure 3.
Neutralization of HMGB1 decreases IL-1β secretion and associated inflammation. (A) IL-1β concentration in lung lavage fluid 1 day following MWCNT instillation and treatment with HMGB1 neutralizing antibodies or control antibodies, (B) total cell counts, (C) neutrophil counts and (D) eosinophil counts in the lung lavage fluid 1 day following treatment MWCNT treatment and administration of antibodies. *p<0.05, **p<0.01, ***p<0.001.
Caspase-1−/− mice have decreased NLRP3 inflammasome activity
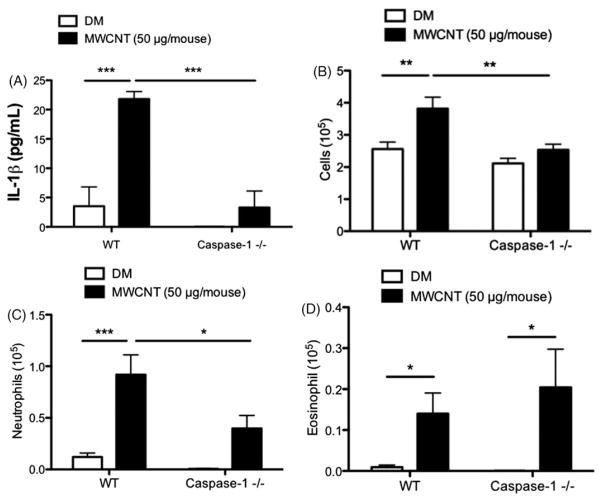
Multi-walled carbon nanotubes exposure in caspase-1−/− mice resulted in decreased IL-1β release, along with other standard inflammatory parameters including: total cell count and neutrophil counts (Figure 4A–C). These data support the critical role of caspase-1 processing of IL-1β and HMGB1 signaling in MWCNT-induced inflammation. However, MWCNT-exposed caspase-1−/− mice retained similar levels of eosinophil recruitment compared to WT control (Figure 4D). This suggests that despite decreased HMGB1 levels in the lavage fluid, caspase-1−/− mice have a compensating, HMGB1 independent pathway for recruitment of eosinophils.
Figure 4.
Caspase-1−/− mice have decreased IL-1β and associated inflammation. (A) IL-1β in lung lavage fluid 1 day following MWCNT instillation, (B) total cell counts, (C) neutrophil counts and (D) eosinophil counts in the lung lavage fluid after 1 day. *p<0.05, **p<0.01, ***p<0.001.
Discussion
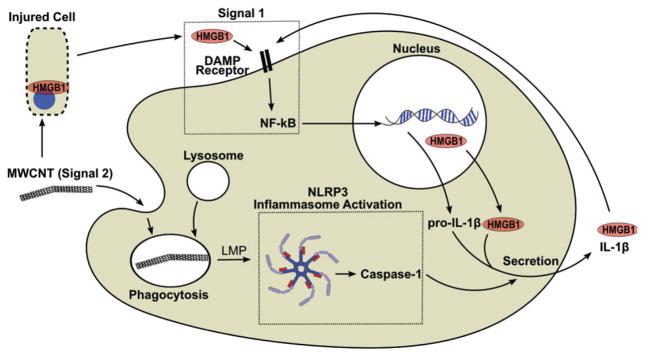
Due to the diversity of physiochemical properties and potential modifications of MWCNT, in vitro screening of inflammatory potential will be critical to identifying their potential to cause lung disease. Past studies by our laboratory have demonstrated that MWCNT physiochemical properties that promote more lL-1β secretion also result in greater pathology (Hamilton et al., 2012, 2013). This assessment currently requires stimulation of macrophages with endotoxin, a practice widespread when assessing inflammasome activation by inhaled particles. Pre-sensitization to LPS has been shown to enhance MWCNT-induced inflammation and fibrosis, and LPS has been shown to be a major contributor to environmental particle induced lung disease by integrating with the hazardous material in the environment (Cesta et al., 2010; Schwartz, 2001). However, it is important to consider the sterile nature of MWCNT-induced inflammation in vivo. The MWCNT used in these studies were free from LPS contamination, primarily due to the manufacturing process that requires high temperatures and controlled conditions. The mice used in these studies were kept in SPF conditions, where the contribution of LPS to NLRP3 Inflammasome activity is not evident. The ‘‘danger theory’’ of the inflammatory response indicates that sterile injury is sufficient for NLRP3 inflammasome activation and associated disease through the release of endogenous DAMPs, including HMGB1 (Chen & Nunez, 2010). The current two hit hypothesis for MWCNT in vitro activation includes MWCNT activation of the NLRP3 Inflammasome through lysosomal membrane permeablization (Hamilton et al., 2012), and the activation of NF-kB by an endogenous DAMP (Figure 5).
Figure 5.
Model depicts the contribution of HMGB1 to MWCNT-induced inflammation. Macrophages phagocytose MWCNT (signal 2), which induce lysosomal membrane permeablization (LMP), leading to activation of the NLRP3 Inflammasome and caspase-1. Signal 1 is required for activation of NF-κB, which is accomplished by activation of a DAMP recognition receptor such as RAGE or TLR4. HMGB1 is released passively by injured cells or by active secretion from macrophages, and participates in Signal 1.
Results from this study implicate that HMGB1 plays an integral role in sterile activation of the NLRP3 Inflammasome. The contribution of HMGB1 to sterile inflammation has been of recent interest due to its ability to bind TLR4/RAGE leading to NF-κB activation (Park et al., 2006; Ullah et al., 2014). While most studies on HMGB1-mediated inflammasome activity have been done following exposure to high levels of endotoxin, bleomycin or mechanical stress (Hamada et al., 2008; van Zoelen et al., 2008; Wang et al., 1999), the contribution of HMGB1 to MWCNT-induced inflammation and disease has not hitherto been elucidated. Not only did MWCNT exposure elevate levels of HMGB1, but also we confirmed native HMGB1 participated in NLRP3 inflammasome activation.
High-mobility group box 1 secretion has been observed to be through both active (from monocytic cells) and passive mechanisms (secondary to necrosis or apoptosis) (Gardella et al., 2002; Pisetsky, 2014; Scaffidi et al., 2002; Willingham et al., 2009). Increased cytotoxicity has been observed with MWCNT exposure in epithelial cells and AM (Beamer et al., 2013; Hamilton et al., 2012), however the pre-dominant form of MWCNT-induced cell death is conflicting, and likely depends upon particle physiochemical and surface properties. However, increased HMGB1 secretion from primary AM exposed to LPS and MWCNT, along with decreased secretion from Caspase-1−/− mice, suggests that a portion of the extracellular HMGB1 present following MWCNT exposure is processed through an active caspase-1 mediated secretory mechanism. Higher levels of LPS than those used in this proposal have been shown to induce HMGB1 translocation from the nucleus to the cytosol, where it is then secreted, like IL-1β, through an unconventional pathway (Gardella et al., 2002). The observation that HMGB1 secretion was dependent on caspase-1 activation suggests that the NLRP3 Inflammasome is integral to the secretory pathway, and is not limited to processing of cytokine precursors.
Others have reported that specific HMGB1 isoforms are required for pro-inflammatory, cytokine-like function or chemokine activity. Specifically, a disulfide bridge between cysteine residues 23 and 45, along with a reduced cysteine residue at 106, constitutes HMGB1 with cytokine-like activity (Venereau et al., 2012; Yang et al., 2010a, 2012). Fully reduced HMGB1 is considered to be primarily chemotactic. The failure of the disulfide rHMGB1 isoform to act as signal 1 in our model suggests that the endogenous form in the lung, following MWCNT exposure, is distinct from the commercial product. Though most of the HMGB1 immunoprecipiated in these studies was soluble (Figure 2C), it is possible that there was co-immunoprecipiation of immune complexes present at low levels with greater specificity to activate NF-κB, that were not detected do to the insensitive nature of coomassie blue. Others have demonstrated HMGB1 binds to IL-1β, LPS and extracellular DNA in situ, and that these complexes have increased immune-stimulatory activity (Hreggvidsdottir et al., 2009; Sha et al., 2008). Alternatively, since HMGB1 secretion is dependent upon caspase-1, it is possible that HMGB1 is targeted by caspase-1 prior to secretion, and that this cleaved isoform is important in extracellular signaling. However, HMGB1 targeting by caspase-1 has had conflicting reports and was not assessed in these studies (Lamkanfi et al., 2010; Leblanc et al., 2014). Furthermore, the integration of the NLRP3 Inflammasome into the unconventional secretory pathway may be a pathway around direct caspase-1 cleavage of HMGB1 (Dupont et al., 2011).
IL-1β signaling has been shown to be a significant contributor to neutrophil and eosinophil recruitment after MWCNT exposure (Girtsman et al., 2012). IL-1R null mice have decreased eosinophil and neutrophil recruitment 24 h after MWCNT exposure. Likewise, HMGB1 neutralization in this study, resulting in decreased IL-1β, resulted in decreased eosinophil counts and a decreasing trend in neutrophil recruitment. Similarly, HMGB1 neutralization has been reported to decrease eosinophil recruitment in a murine asthma model using Ova/Albumin sensitization (Shim et al., 2012). The mechanism for HMGB1 recruitment of eosinophil and neutrophils remains to be defined, though general cell migration towards HMGB1 has been reported to be dependent on CXCL12 and sustained NF-κB activation (Kew et al., 2012).
We present a conceptual model for the role of extracellular HMGB1 activity in MWCNT-induced inflammation in Figure 5. This study demonstrates extracellular HMGB1 in MWCNT exposure regulates NLRP3 inflammasome activity by participating in the NF-κB activating step. Though HMGB1 neutralization results in reduced acute NLRP3 Inflammasome activity, the dependence of MWCNT-induced long-term pathology on HMGB1 remains to be determined. It is plausible that HMGB1 functions as part of the NF-κB activating signal in many other models of particle exposure, which require NLRP3 Inflammasome activity. Therefore, assessment of MWCNT bioactivity (including NLRP3 inflammasome activity) should include measuring the ability of MWCNT and other particles to induce HMGB1 secretion.
Conclusions
These studies establish extracellular HMGB1 as a regulator of NLRP3 inflammasome activity in vivo following MWCNT exposure. HMGB1 secretion is dependent upon caspase-1, and this study provides evidence that targeting HMGB1 signaling through extracellular neutralization or secretion pathways (caspase-1 dependent) may have therapeutic implications, however, further studies are needed. Finally, the potency of MWCNT to induce HMGB1 secretion should be considered in future studies delineating inflammatory potential of MWCNT and conceivably other inhaled particulates that act through a similar mechanism.
Acknowledgments
The authors thank Center for Environmental Health Sciences core scientists, Mary Buford (Inhalation and Pulmonary Physiology Core), Brittan Postma (Animal Core), Lou Herritt (Molecular Histology and Fluorescence Imaging Core) and Dr Chris Migliaccio (Inhalation and Pulmonary Physiology Core Director) and Ray Hamilton for contributions of expertise and advice needed to conduct the experiments discussed in this manuscript. The content is solely the responsibility of the authors, and does not necessarily represent the official views of the NIH or PhRMA Foundation.
Footnotes
Supplementary material available online
Supplementary materials S1–S3
Declaration of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of this manuscript. Research reported in this publication was supported grants from the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under award number P30GM103338, National Institute of Environmental Health Sciences under award number RC2-ES018742 and a Pre-doctoral fellowship from PhRMA Foundation (Forrest Jessop).
References
- Beamer CA, Girtsman TA, Seaver BP, Finsaas KJ, Migliaccio CT, Perry VK, et al. IL-33 mediates multi-walled carbon nanotube (MWCNT)-induced airway hyper-reactivity via the mobilization of innate helper cells in the lung. Nanotoxicology. 2013;7:1070–81. doi: 10.3109/17435390.2012.702230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesta MF, Ryman-Rasmussen JP, Wallace DG, Masinde T, Hurlburt G, Taylor AJ, Bonner JC. Bacterial lipopolysaccharide enhances PDGF signaling and pulmonary fibrosis in rats exposed to carbon nanotubes. Am J Respir Cell Mol Biol. 2010;43:142–51. doi: 10.1165/rcmb.2009-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–37. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave SH, Tilstra JS, Matsuoka K, Li F, Demarco RA, Beer-Stolz D, Sepulveda AR, et al. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J Leukoc Biol. 2009;86:633–43. doi: 10.1189/jlb.1008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont N, Jiang S, Pilli M, Ornatowski W, Bhattacharya D, Deretic V. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1beta. EMBO J. 2011;30:4701–11. doi: 10.1038/emboj.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghonime MG, Shamaa OR, Das S, Eldomany RA, Fernandes-Alnemri T, Alnemri ES, et al. Inflammasome priming by lipopolysaccharide is dependent upon ERK signaling and proteasome function. J Immunol. 2014;192:3881–8. doi: 10.4049/jimmunol.1301974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girtsman TA, Beamer CA, Wu N, Buford M, Holian A. IL-1R signalling is critical for regulation of multi-walled carbon nanotubes-induced acute lung inflammation in C57Bl/6 mice. Nanotoxicology. 2012;8:17–27. doi: 10.3109/17435390.2012.744110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glista-Baker EE, Taylor AJ, Sayers BC, Thompson EA, Bonner JC. Nickel nanoparticles cause exaggerated lung and airway remodeling in mice lacking the T-box transcription factor, TBX21 (T-bet) Particle Fibre Toxicol. 2014;11:7. doi: 10.1186/1743-8977-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada N, Maeyama T, Kawaguchi T, Yoshimi M, Fukumoto J, Yamada M, et al. The role of high mobility group box1 in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39:440–7. doi: 10.1165/rcmb.2007-0330OC. [DOI] [PubMed] [Google Scholar]
- Hamilton RF, Buford MC, Wood MB, Arnone B, Morandi M, Holian A. Engineered carbon nanoparticles alter macrophage immune function and initiate airway hyper-responsiveness in the BALB/c mouse model. Nanotoxicology. 2007;1:104–17. [Google Scholar]
- Hamilton RF, Jr, Buford M, Xiang C, Wu N, Holian A. NLRP3 inflammasome activation in murine alveolar macrophages and related lung pathology is associated with MWCNT nickel contamination. Inhal Toxicol. 2012;24:995–1008. doi: 10.3109/08958378.2012.745633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RFJ, Wu Z, Mitra S, Shaw PK, Holian A. Effect of MWCNT size, carboxylation, and purification on in vitro and in vivo toxicity, inflammation and lung pathology. Part Fibre Toxicol. 2013;10:57. doi: 10.1186/1743-8977-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hreggvidsdottir HS, Ostberg T, Wahamaa H, Schierbeck H, Aveberger AC, Klevenvall L, et al. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 2009;86:655–62. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- Kew RR, Penzo M, Habiel DM, Marcu KB. The IKKalpha-dependent NF-kappaB p52/RelB noncanonical pathway is essential to sustain a CXCL12 autocrine loop in cells migrating in response to HMGB1. J Immunol. 2012;188:2380–6. doi: 10.4049/jimmunol.1102454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288:L958–65. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- Lacher SE, Johnson C, Jessop F, Holian A, Migliaccio CT. Murine pulmonary inflammation model: a comparative study of anesthesia and instillation methods. Inhal Toxicol. 2010;22:77–83. doi: 10.3109/08958370902929969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Sarkar A, vande Walle L, Vitari AC, Amer AO, Wewers MD, et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol. 2010;185:4385–92. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc PM, Doggett TA, Choi J, Hancock MA, Durocher Y, Frank F, et al. An Immunogenic Peptide in the A-box of HMGB1 Protein Reverses Apoptosis-induced Tolerance through RAGE Receptor. J Biol Chem. 2014;289:7777–86. doi: 10.1074/jbc.M113.541474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou TG, Adler FR, Keogh RH, Li Y, Jensen JL, Walsh W, et al. Sputum biomarkers and the prediction of clinical outcomes in patients with cystic fibrosis. PLoS One. 2012;7:e42748. doi: 10.1371/journal.pone.0042748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Hubbs AF, Scabilloni JF, Wang L, Battelli LA, Friend S, et al. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Particle Fibre Toxicol. 2011;8:21. doi: 10.1186/1743-8977-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Hubbs AF, Battelli LA, McKinney W, Friend S, et al. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Particle Fibre Toxicol. 2013;10:33. doi: 10.1186/1743-8977-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomaki J, Valimaki E, Sund J, Vippola M, Clausen PA, Jensen KA, et al. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano. 2011;5:6861–70. doi: 10.1021/nn200595c. [DOI] [PubMed] [Google Scholar]
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–24. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- Pisetsky DS. The Expression of HMGB1 on microparticles released during cell activation and cell death in vitro and in vivo. Mol Med. 2014;20:158–63. doi: 10.2119/molmed.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D, Sriram K, Wolfarth M, Jefferson A, Schwegler-BERRY D, Andrew M, Castranova V. A biocompatible medium for nanoparticle dispersion. Nanotoxicology. 2008;2:144–154. [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Schwartz DA. Inhaled endotoxin, a risk for airway disease in some people. Respir Physiol. 2001;128:47–55. doi: 10.1016/s0034-5687(01)00264-x. [DOI] [PubMed] [Google Scholar]
- Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180:2531–7. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- Shim EJ, Chun E, Lee HS, Bang BR, Kim TW, Cho SH, et al. The role of high-mobility group box-1 (HMGB1) in the pathogenesis of asthma. Clin Exp Allergy. 2012;42:958–65. doi: 10.1111/j.1365-2222.2012.03998.x. [DOI] [PubMed] [Google Scholar]
- Stros M. HMGB proteins: interactions with DNA and chromatin. Biochim Biophys Acta. 2010;1799:101–13. doi: 10.1016/j.bbagrm.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170:1310–16. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- Ullah MA, Loh Z, Gan WJ, Zhang V, Yang H, Li JH, et al. Receptor for advanced glycation end products and its ligand high-mobility group box-1 mediate allergic airway sensitization and airway inflammation. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2013.12.1035. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- van Zoelen MA, Ishizaka A, Wolthuls EK, Choi G, van der Poll T, Schultz MJ. Pulmonary levels of high-mobility group box 1 during mechanical ventilation and ventilator-associated pneumonia. Shock. 2008;29:441–5. doi: 10.1097/SHK.0b013e318157eddd. [DOI] [PubMed] [Google Scholar]
- Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, de Marchis F, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–28. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–31. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, et al. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol. 2009;183:2008–15. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA. 2010a;107:11942–7. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Rivera Z, Jube S, Nasu M, Bertino P, Goparaju C, et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci USA. 2010b;107:12611–16. doi: 10.1073/pnas.1006542107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Lundback P, Ottosson L, Erlandsson-Harris H, Venereau E, Bianchi ME, et al. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1) Mol Med. 2012;18:250–9. doi: 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]