Abstract
Exposure to elevated levels of the toxic metals inorganic arsenic (iAs) and cadmium (Cd) represents a major global health problem. These metals often occur as mixtures in the environment, creating the potential for interactive or synergistic biological effects different from those observed in single exposure conditions. In the present study, environmental mixtures collected from two waste sites in China and comparable mixtures prepared in the laboratory were tested for toxicogenomic response in placental JEG-3 cells. These cells serve as a model for evaluating cellular responses to exposures during pregnancy. One of the mixtures was predominated by iAs and one by Cd. Six gene biomarkers were measured in order to evaluate the effects from the metals mixtures using dose and time-course experiments including: heme oxygenase 1 (HO-1) and metallothionein isoforms (MT1A, MT1F and MT1G) previously shown to be preferentially induced by exposure to either iAs or Cd, and metal transporter genes aquaporin-9 (AQP9) and ATPase, Cu2+ transporting, beta polypeptide (ATP7B). There was a significant increase in the mRNA expression levels of ATP7B, HO-1, MT1A, MT1F, and MT1G in mixture-treated cells compared to the iAs or Cd only-treated cells. Notably, the genomic responses were observed at concentrations significantly lower than levels found at the environmental collection sites. These data demonstrate that metal mixtures increase the expression of gene biomarkers in placental JEG-3 cells in a synergistic manner. Taken together, the data suggest that toxic metals that co-occur may induce detrimental health effects that are currently underestimated when analyzed as single metals.
Keywords: Toxicogenomics, biomarkers, real-world mixtures, gene expression, synergism, mRNA
INTRODUCTION
Exposure to elevated levels of the toxic metals inorganic arsenic (iAs) and cadmium (Cd) is a global health problem affecting millions of people (Ratnaike, 2003; Satarug et al., 2003; Waalkes, 2003; Jomova et al., 2011). Both iAs and Cd occur naturally in the environment, and their concentrations may be elevated to potentially toxic levels at certain waste sites (Fay & Mumtaz, 1996). There is evidence that metals predominantly occur as mixtures, also potentially in association with other non-metals toxic substances (Balistrieri & Mebane, 2014). Exposure to these metals is of concern as elevated levels have been associated with neurological, reproductive, cardiovascular and carcinogenic effects (Liaw et al., 2009; Moore et al., 2002; Rahman et al., 2007; Smith et al., 2006; Tsai et al., 2003; von Ehrenstein et al., 2006; Wasserman et al., 2007; Yuan et al., 2007).
In addition to the health effects associated with exposures during adulthood, exposures to susceptible populations such as pregnant women and the developing fetus are of concern. This is because iAs can readily cross the placenta (Concha et al, 1998) and Cd, while doing so less readily, also crosses the placenta (Iyengar and Rapp, 2001; Zhang et al. 2004). Epidemiologic studies support relationships between chronic iAs exposure and increased risk of spontaneous abortion, stillbirth, preterm birth, and neonatal death in pregnant women (Ahmad et al., 2001; von Ehrenstein et al., 2006; Rahman et al., 2007). Similar to iAs, exposure to elevated levels of Cd is also associated with reproductive and developmental effects. This stems from the limited capacity of the body to respond to Cd exposure, as the metal does not undergo metabolic degradation to less toxic species and is poorly excreted (Waalkes, 2003; Arita & Costa, 2009). In pregnant women, Cd accumulation in the placenta causes inhibition of trophoblastic invasion, decreased steroidogenesis, and adjusted handling of nutritive essential metals that are deleterious to fetal and maternal health (reviewed by Estaban-Vasallo et al., 2012). Consequently, Cd exposure has been associated with birth outcome effects such as lower birth weight and decreased birth height (Salpietro et al., 2002; Chisolm & Handorf, 1996; Zhang et al., 2004).
Changes in mRNA expression levels can be used as biomarkers that indicate disturbances in cellular metabolic pathways leading to cell death or disease and as such, are valuable predictors of exposure and/or xenobiotic toxicity. Since iAs and Cd activate the induction of specific metal-responsive and oxidative stress-inducible genes such as those that encode for metallothioneins and heme oxygenase (Choi & Alam, 1996; Menzel et al., 1998), it is possible to use these genes as biomarkers/indicators of exposure to iAs and/or Cd. Heme oxygenase 1 (HO1) has been shown to be induced by iAs and its importance in cellular stress response has been established (Applegate et al, 1991; Choi & Alam, 1996; Menzel et al., 1998). HO1 has also been implicated as a gene biomarker of iAs exposure (Menzel et al., 1998). Cd is also capable of inducing the HO1 gene (Menzel et al., 1998), but is a preferential potent inducer of metallothionein expression. Metallothioneins (MTs) are the primary gene biomarkers of Cd exposure because of their high capacity to bind Cd through the thiol group of their cysteine residues, thereby resulting in increased expression levels relative to Cd. (Chisolm & Handorf, 1996; Wang & Fowler, 2008). In addition to HO1 and MTs detailed above, other genes of interest related to metals response are the metal transporter genes aquaporin-9 (AQP9) and Cu2+ transporting ATP-ase ATP7B). AQP9 is a transmembrane, solute transporting protein that facilitates the passage of glycerol and other non-charged solutes (Tsukaguchi et al., 1999). It has been shown to control the transmembrane transport of iAs, thereby playing a critical role in sensitivity of cells towards iAs cytotoxicity (Leung et al., 2006). ATP7B belongs to the P-type adenosine triphosphatase family that includes a number of membrane proteins specialized in the transport of cations across cell membranes (Lutsenko and Kaplan, 1995). ATP7B plays a key role in the normal cellular distribution of copper in liver, kidney, placenta (Petrukhin et al., 1994) and brain (Lutsenko et al., 2003). Cd binding can also occur at the thiol group of cysteine residues of ATP7B.
Previous studies have shown that concurrent exposure to mixtures of iAs and Cd may result in additive or synergistic effects that are not seen in single component exposures (Liu et al., 2000; Madden et al., 2002; Wang & Fowler, 2008). In the present study the metal responsive stress biomarkers HO-1 and MT and metal transporter genes AQP9 and ATP7B were measured in placental JEG-3 cells exposed to iAs or Cd alone, and environmental and laboratory mixtures of both metals. The environmental mixtures were obtained as water samples collected from two highly contaminated sites in China in order to evaluate interactive effects from concomitant exposures. The first site represents one of the largest known Cd spills in history, on the Longjiang River in Guangxi Province, China that serves as a drinking water source for 3.7 million residents (Zhang et al. 2013). The second site was at an iAs herbicide operation in the Pearl River watershed in Guangdong Province, China. The iAs collected was present as arsenite. To assess uniformity to the environmental mixtures, comparable laboratory samples were prepared using the same concentrations of iAs and Cd present in the environmental samples. Given the interest in understanding potential environmental factors that could impact reproductive health, the JEG-3 human choriocarcinoma cell line was used in the present study as a model for investigating the effects of metals exposure in the placenta (Guiñazú et al., 2012; Huang & Leung, 2009; Letcher & Holsteijn, 1999; Ronco et al., 2010).
2. MATERIALS AND METHODS
Water Sample Collection and Analysis
Water samples were collected from two contaminated sites in China. Water samples were obtained using a diffusive gradient in thin film (DGT) passive sampler (DGT Research Ltd, Lancaster, UK) with Chelex gel for Cd and ferrihydrite gel for iAs. These samplers provide a surrogate measurement for the bioavailable or labile fraction of metals in water as described elsewhere (Huynh et al. 2012). The DGT devices were deployed in triplicate for a period of two days in the surface water near the sites of the putative sources of the metals and downstream from the sources providing a gradient of exposure. For each of the contaminated sites, the DGT samplers were deployed and collected at six points along a transect downstream from the putative source, representing a total of 12 collections. Upon retrieval, the DGTs were eluted with 1.5 mL of 3 M Ultrapure HNO3 for 24 h, prior to analysis by inductively coupled mass spectroscopy (ICP-MS, X Series 2 Thermo Fischer Scientific, Waltham, MA, USA). The resin gels were removed from the DGT samplers and metals eluted in 1M HNO3 (pH 5.0) for 24h. Procedural blanks were less than 1% of the lowest measured concentration, matrix spike samples resulted in mean recoveries of 98% (As) and 101% (Cd), and laboratory duplicate analyses were always less than 5% relative percent difference.
Six total treatments were used for the in vitro assays. Two represented dilutions of the environmentally collected samples with the highest levels of iAs or Cd selected from 12 total river samples. Two were laboratory-generated treatments with the same concentrations of iAs and Cd as the environmental samples. The final two treatments represented single metals. The first treatment, referred to as the iAs-environmental mixture (iAs-EM), was carried out at a final concentration of 0.08μM iAs and 0.0013μM Cd. This resulted from a 60-fold dilution of the original environmental sample with the highest iAs concentration from the Pearl River watershed (4.8μM iAs). The original sample also had measurable levels of Cd (0.08μM). The second treatment, referred to as the iAs laboratory-generated mixture (iAs-LM), was modeled after the environmental mixture with a final concentration of 0.08μM iAs and 0.0013μM Cd. The third treatment, referred to as the Cd environmental mixture (Cd-EM), had final concentrations of 0.1μM Cd and 0.002μM iAs. This resulted from a 60-fold dilution of the original environmental sample with the highest concentration of Cd from the Longjiang River (6.07 μM Cd). The original sample also had measurable levels of iAs (0.12μM). The fourth treatment, referred to as the Cd-laboratory-generated mixture (Cd-LM), was modeled after the environmental mixture with a final concentration of 0.1μM Cd and 0.002μM iAs. These were compared to two single metals treatments of iAs (iAs) 0.08μM or Cd (Cd) 0.1μM, both prepared in the laboratory. All the mixtures were stored under the same conditions as controls in the laboratory with the iAs samples made fresh prior to treatment.
Cell Culture and iAs and Cd Treatments
The JEG-3 choriocarcinoma cell line was purchased from the American Type Culture Collection (Manassas, VA). JEG-3 cells were grown in Dulbecco’s modified Eagle's Minimum Essential Medium, supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin and 1mM sodium pyruvate at 37C in 5% CO2. Cells were plated at 5 x 106 cells per 25cm3 flask and incubated under standard conditions until achieving 80–90% confluence. To study the effects of iAs and Cd mixtures in vitro, JEG-3 cells were cultured in a 6-well culture plate for 24h at 0.5 x 106 cells per well. Cells were serum starved in 2 ml of serum-free DMEM supplemented with 1% penicillin/streptomycin, and 1mM sodium pyruvate for 4hrs. Cells were exposed to identical concentrations of iAs and Cd present in the environmental mixtures by combining 0.08μM sodium arsenite (Na2AsO3) and 0.0013μM cadmium chloride (CdCl2) for the iAs-LM and 0.1μM cadmium chloride (CdCl2) and 0.002μM sodium arsenite (Na2AsO3) for the Cd-LM. To assess the effect of the mixtures, cells were either treated with the iAs-EM, iAs-LM, Cd-EM or the Cd-LM. For the metal only treatments, the concentrations were selected to be identical to the final concentrations of iAs and Cd in the mixtures. Therefore, cells were treated with 0.08μM sodium arsenite (Na2AsO3) or 0.1μM cadmium chloride (CdCl2). Control cells were treated with dilute nitric acid at (pH 5.0). All the cells in each treatment group were exposed for a total of 2 hrs, 4 hrs, 8 hrs and 24 hrs. Cells were then harvested for RNA isolation.
Cytotoxicity Assay
For the cytotoxicity analysis, JEG-3 cells were seeded at 1.5 x 104 cells per well in 96-well culture plates and incubated until achieving 80–90% confluence. Cells were serum starved in 200 μl of serum-free DMEM supplemented with 1% penicillin/streptomycin, and 1mM sodium pyruvate for 4hrs. To assess the cytotoxic effects of the mixtures and single metal exposures, cells were treated as detailed above and incubated for 24hrs. Resazurin dye (10μg/L) was added to the culture medium present in the wells containing both treated cells and controls followed by incubation for 3 hrs to allow viable cells to convert resazurin to the fluorescent resorufin product. After incubation, fluorescence was measured using Promega® spectrophotometer micro-plate reader at an excitation wavelength of 560 nm and an emission wavelength of 590 nm according to the manufacturer’s information. The conversion of resazurin to fluorescent resorufin is proportional to the number of metabolically active, viable JEG-3 cells present in the population.
mRNA expression assessment by Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
Treated cells and controls were homogenized in TRIzol (Life Technologies) and mRNA isolated according to the standard TRIzol protocol. Extracted RNA was quantified with a Nanodrop 1000 spectrophotometer (Thermo Scientific, Waltham, MA). RNA integrity was verified with a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). iAs and Cd-induced changes in mRNA expression were measured using real-time reverse transcriptase polymerase chain reaction (RT-PCR) by employing QuantiTect Primer Assays (Qiagen) in conjunction with QuantiTect SYBR® Green PCR kits (Qiagen) on the Stratagene Mx3005P QPCR System (Agilent Technologies). Specifically, AQP9, ATP7B, HO-1, MT1A, MT1F, and MT1G were evaluated for changes in mRNA transcript levels induced by exposure to iAs or Cd alone, or the iAs or Cd environmental or laboratory-generated mixtures. The resulting RT-PCR cycle times were normalized against the housekeeping gene GAPDH, and fold changes in expression were calculated based on the Δ ΔCT method (Livak & Schmittgen, 2001).
Statistical Analysis
Each experiment was performed in at least biological triplicate and the results are presented as mean ± standard error (SE). Statistical significance of the differences in mRNA expression levels between the exposed and control JEG-3 samples were calculated using a one-way analysis of variance (ANOVA) test followed by Tukey’s post-hoc comparison. Statistical significance was set as p<0.05.
3. RESULTS
Collected environmental samples contain mixtures of iAs and Cd at detectable levels
Metals analysis was carried out on the DGT samples. The samples collected at the iAs herbicide facility in the Pearl River watershed came from waters that ranged from 17.7 ± 2.5 μg/L (0.24 ± 0.013 μM) up to 352 ± 10.4 μg/L (4.7 ± 0.14 μM) of total dissolved iAs. The DGT samples collected in the Longjiang River came from waters with dissolved Cd ranging from 27 ± 2.5 μg/L (0.24 ± 0.02 μM) up to 623 ± 67 μg/L (5.54 ± 0.61 μM). Thus, these samples represent very high levels of real-world contamination to iAs and Cd.
The DGT samplers were deployed in these waters for two days and the resulting final elution concentrations from the sampling points ranged from 14.98–360 μg/L (0.2 to 4.8 μM) of iAs and 25–680 μg/L (0.22 to 6.07 μM) of Cd. Although other metals were present in these waters, their concentrations were several orders of magnitude below those of the iAs and Cd (data not shown). For the purposes of this study, two metal-mixtures were used from the DGT elution samples for the in-vitro assays and compared to iAs and Cd (Table 1).
Table 1.
Sample composition, pre-dilution and post-dilution concentrations. Post-dilution concentrations are the treatment/exposure concentrations used in in-vitro assays.
| Pre-dilution Concentration (μM) | Post-dilution Concentration (μM) | ||||
|---|---|---|---|---|---|
| Sample ID | Sample Source | iAs | Cd | iAs | Cd |
| iAs environmental mixture (iAs-EM) | Pearl River Watershed | 4.80 | 0.08 | 0.08 | 0.0013 |
| Cd environmental mixture (Cd-EM) | Longjiang River | 0.12 | 6.07 | 0.002 | 0.1 |
| iAs alone | Commercially obtained Na2AsO3 | 10 | n/a | 0.08 | — |
| Cd alone | Commercially obtained CdCl2 | n/a | 10 | — | 0.10 |
No significant cytotoxicity resulting from any of the treatments at 24hrs
The cellular viability of JEG-3 cells after treatment (24 hrs) with the iAs and Cd mixtures or to iAs or Cd alone is shown in Figure 1. There was no statistically significant cytotoxicity observed following any of the treatments.
Figure 1.
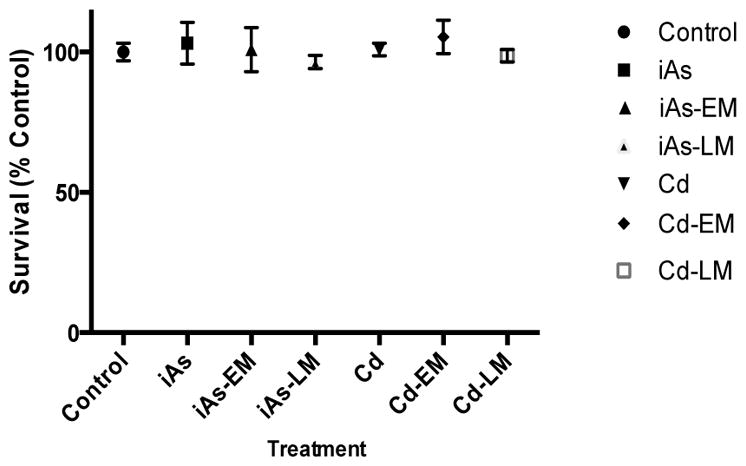
Cell survival of JEG3 cells exposed to iAs alone (iAs) (0.08 μM iAs), an iAs environmental mixture (iAs-EM) (0.08 μM iAs, 0.0013 μM Cd), an iAs laboratory-generated mixture (iAs-LM) (0.08 μM iAs, 0.0013 μM Cd), Cd alone (Cd) (0.1 μM Cd), a Cd environmental mixture (Cd-EM) (0.1 μM Cd, 0.002 μM iAs), or a Cd laboratory-generated mixture (iAs-LM)(0.1 μM Cd, 0.002 μM iAs).
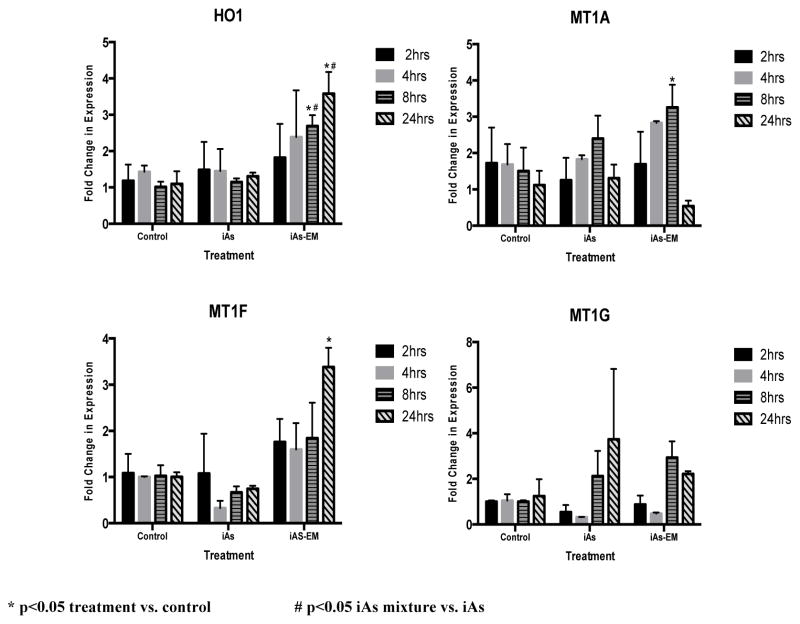
The iAs environmental mixture increases HO-1 mRNA expression to a greater extent than iAs alone
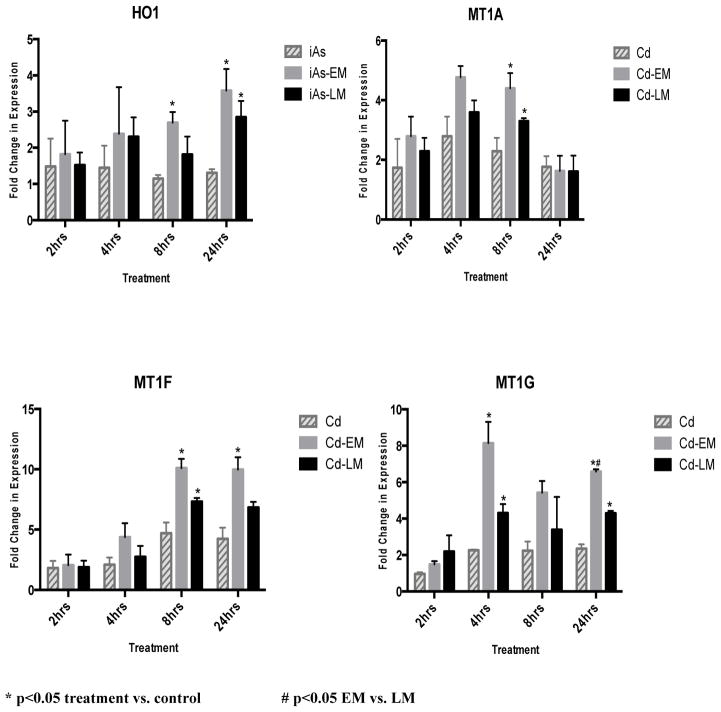
In order to compare the induction of the oxidative stress biomarker HO-1 between iAs and the environmental mixture, JEG-3 cells were treated and incubated for 2, 4, 8 and 24 hours with the iAs mixture or 0.08μM of Na2AsO3, which is comparable to the iAs content in the mixture. After the 2 hr and 4 hr incubation periods, there was no significant increase in the expression of HO-1 resulting from any of the iAs treatments (Figure 2). At the 8-hr and 24-hr time-periods, the iAs-EM treatment resulted in a significant (p<0.05) fold increase of 2.6 and 3.6, respectively, in HO-1 mRNA expression compared to controls (Figure 2). When the iAs-EM was compared to iAs, there was a significant (p<0.05) average increase in fold change of 2.6-fold and 2.7-fold in the expression level of HO-1 at the 8 and 24 hr time points, respectively (Figure 2).
Figure 2.
Fold changes of HO-1, MT1A, MT1F, and MT1G mRNA expression levels in cells treated with iAs alone (iAs) (0.08 uM iAs) or the iAs environmental mixture (iAs-EM) (0.08 uM As, 0.0013uM Cd) relative to untreated cells (control) at 2, 4, 8 or 24 hours of exposure.
The iAs environmental mixture induces MT1A, MT1F, and MT1G mRNA expression to a greater extent than iAs alone
In order to compare the induction of the biomarkers MT1A, MT1F, and MT1G between the iAs-EM and iAs, JEG-3 cells were treated for 2, 4, 8 and 24 hrs with the iAs-EM or iAs (0.08μM of Na2AsO3), which is comparable to the iAs concentration in the mixture. There was no significant increase in the levels of mRNA expression in cells treated with iAs compared to untreated controls at any of the time points. At 8 hrs, the cells treated with the iAs-EM induced a 3.3 fold (p<0.05) change increase in MIT1A mRNA expression. There was no difference in the levels of mRNA expression in MT1F between cells treated with iAs and untreated controls at any of the time points. The iAs-EM resulted in a statistically significant (p<0.05) increase of 3.4 fold in MT1F expression at the 24 hr time point (Figure 2). As with MT1F, there was no change in mRNA expression in MT1G between cells treated with iAs and untreated controls at any of the time points, nor did the iAs-EM alter MT1G expression.
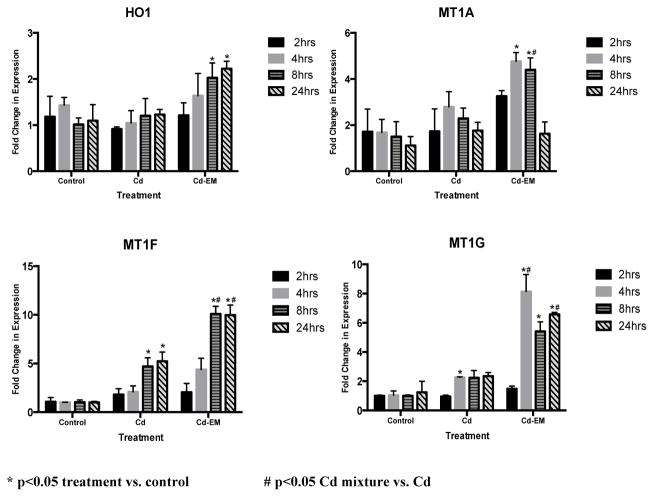
The Cd environmental mixture induces HO-1 mRNA expression to a greater extent than Cd alone
JEG-3 cells were treated for 2, 4, 8 and 24 hrs with the Cd-EM, or Cd (0.1μM of CdCl2), which is, comparable to the Cd concentration in the environmental mixture. There was no change in mRNA expression in HO-1 between cells treated with Cd and untreated controls at any of the time points. In contrast, there was a statistically significant (p<0.05) 2 and 2.2 fold increase in the expression level of HO-1 induced by the Cd-EM when compared to the untreated controls and Cd at the 8 and 24 hr time points, respectively (Figure 3).
Figure 3.
Fold changes of HO-1, MT1A, MT1F, and MT1G mRNA expression levels in cells treated with Cd alone (Cd) (0.1 uM Cd) or the Cd environmental mixture (Cd-EM) (0.1 uM Cd, 0.002 uM iAs) relative to untreated cells (control) at 2, 4, 8 or 24 hours of exposure.
The Cd environmental mixture induces greater expression of MT1A, MT1F, and MT1G mRNA than Cd alone
To compare the induction of metallothionein-1 isoforms MT1A, MT1F, and MT1G by the Cd-EM or Cd, JEG-3 cells were treated for 2, 4, 8 and 24 hrs with the Cd-EM or 0.1μM of CdCl2, which is comparable to the Cd concentration in the environmental mixture. At 4 and 8 hrs, there were statistically significant (p<0.05) fold change increases of 4.7 and 4.4 in the expression levels of MT1A respectively induced by the Cd-EM compared to the untreated controls. At 8 hrs, there was a statistically significant (p<0.05) 1.6 fold increase in the expression level of MT1A induced by the Cd-EM compared to Cd.
There were significant (p<0.05) increases in the expression levels of MT1F of 4.7 and 5.2 fold for Cd relative to the controls at the 8 and 24 hr time points, respectively. For the Cd-EM, MT1F displayed a 10.1 and 10 fold for Cd-EM relative to the controls at the 8 and 24 hr time points, respectively. The Cd-EM induced a greater increase in the expression levels of MT1F (2.1 and 1.9 fold, p<0.05) compared to Cd at the 8 and 24 hr time point, respectively (Figure 3).
There was a significant (p<0.05) increase in the expression level of MT1G of 2.3 fold for Cd relative to the controls at the 4 hr time point (Figure 3). At the 4, 8 and 24 hr time points, there was a significant (p<0.05) increase in the expression level of MT1G of 8.1, 5.4, and 6.6 fold induced by Cd-EM compared to controls. At the 4 and 24 hr time points, Cd-EM induced significant (p<0.05) increases of 3.6 and 2.8 fold changes in MT1G expression compared to Cd.
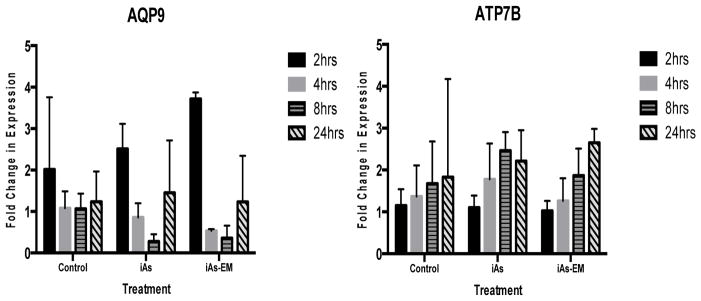
Neither AQP9 nor ATP7B display altered mRNA expression in response to iAs alone or the iAs environmental mixture
Neither iAs-EM nor iAs altered the expression of AQP9 in statistically significant manner (Figure 4). Similarly, neither iAs-EM nor iAs altered the expression of ATP7B in a significant manner (Figure 4).
Figure 4.
Fold changes of AQP9 and ATP7B mRNA expression levels in cells treated with iAs alone (iAs) (0.08 uM iAs) or the iAs environmental mixture (iAs-EM) (0.08 uM As, 0.0013uM Cd) relative to untreated cells (control) at 2, 4, 8 or 24 hours of exposure.
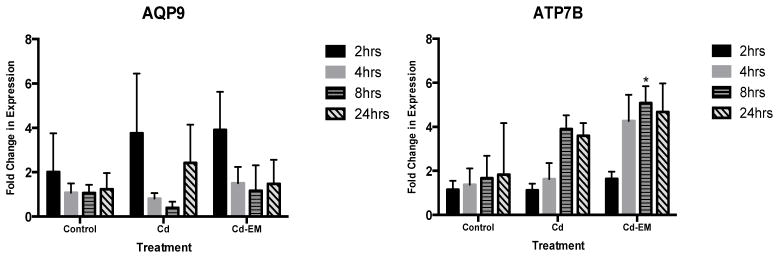
Induction of AQP9 and ATP7B mRNA expression in response to Cd alone versus the Cd environmental mixture
There was no statistically significant induction of AQP9 by Cd or Cd-EM at any time points (Figure 5). ATP7B showed a significant (p<0.05) increase with a 5.1 fold change in expression in response to the Cd-EM at the 8 hr time point (Figure 5).
Figure 5.
Fold changes of AQP9 and ATP7B mRNA expression in cells treated with Cd alone (Cd) (0.1 uM Cd) or the Cd environmental mixture (Cd-EM) (0.1 uM Cd, 0.002 uM iAs) versus untreated cells (control) at 2, 4, 8 or 24 hours of exposure.
Comparison of the environmental mixture and laboratory-generated mixtures
In order to compare the induction of the biomarkers HO1, MT1A, MT1F and MT1G between the iAs-EM and the iAs-LM, JEG-3 cells were treated independently for 2, 4, 8 and 24 hrs with each of the mixtures. Generally, the iAs or Cd environmental mixture induced a higher expression of these genes compared to the laboratory-generated mixtures at most time points. To note, the laboratory-generated mixtures induced expression to a greater extent than of the single metals treatments (Figure 6).
Figure 6.
Fold changes of HO1, MT1A, MT1F, and MT1G in cells treated with the environmental mixtures or laboratory-generated mixtures. For HO1, the iAs environmental mixture (iAs-EM) was compared to iAs laboratory mixture (iAs-LM). For MT1A, MT1F and MT1G the Cd environmental mixture (Cd-EM) was compared to the Cd laboratory mixture (Cd-LM). Both mixture types were also compared to either iAs alone (iAs) or Cd alone (Cd).
DISCUSSION
In the present study, we set out to establish whether real world environmental mixtures containing predominantly iAs or predominantly Cd collected from two contaminated water sites in China induce the expression of key gene biomarkers in placental JEG-3 cells. These cells serve as a model system for the assessment of placental toxicity and are relevant to health effects related to exposures during pregnancy (Tuan et al., 1991; Ikeda et al., 2010). We investigated iAs and Cd-induced changes in the expression levels of six targeted genes, namely AQP9, ATP7B, HO-1, MTIA, MT1F, and MT1G. The expression levels of these genes were compared between iAs and Cd treatments alone versus the two environmental mixtures in the JEG-3 cell line. We also compared the environmental mixtures to laboratory-generated mixtures with matched iAs and Cd concentrations. Both of the environmental mixtures induced the expression of the gene biomarkers to a greater extent than the individual metals. In addition, the environmental mixtures tended to have increased expression relative to the laboratory-generated mixtures. The effects of the metals mixtures on gene expression were greater than additive and thus indicate a synergistic mode of action.
Exposure to the iAs mixture resulted in a greater increase in HO-1 expression than iAs alone in placental JEG-3 cells in a time-dependent manner with the highest induction apparent at 24 hrs. The increased expression of HO-1 is of interest as it is an indicator of oxidative (O2) stress (Choi & Alam, 1996). O2 stress has been shown to be a sensitive endpoint for evaluating the effects of metal mixtures (Fowler & Mahaffey, 1978; Yanez et al., 1991; Fowler et al., 2004; Madden et al., 2001). Metals are known to target the mitochondria, the major intracellular source of reactive oxygen species (ROSs) that contribute to the oxidant-antioxidant imbalance in O2 stress (Fowler & Mahaffey, 1978; Wang & Fowler, 2008). O2 stress has been linked to many deleterious effects of iAs (reviewed by Jomova et al., 2011) and has also been linked to placental-related pregnancy disorders (Jauniaux et al., 2006). Increased O2 stress in the placenta of women with placental-related pregnancy disorders such as preeclampsia and miscarriages has been well-documented (Hubel et al., 1989; Wang et al., 1991; Uotila et al., 1993; Walsh, 1994; Walsh and Wang, 1995; Poranen et al., 1996; Wang and Walsh, 1998; Staff et al., 1999; Wang and Walsh, 2001; Zusterzeel et al., 2001; Vanderlie et al., 2005). The oxidant-antioxidant imbalance renders the placental tissue more prone to injury from free radicals, leading to lipid peroxidation that contributes to maternal endothelial dysfunction and release of vasoconstriction agents typically observed in these disorders (Jauniaux et al., 2006). Taken together our data suggest that exposure to metal mixtures may increase cellular oxidative stress that can potentially contribute to the pathogenesis of placental-related pregnancy disorders.
It is interesting that the Cd mixture induced a greater expression of MTs than Cd alone with MT1A, MT1F and MT1G showing increased expression levels as early as 4 hrs after treatment. While it is not surprising that MTs increase, as they are known response biomarkers of Cd exposure, it is interesting that in the presence of iAs their induction is greater. Metallothioneins are important metal-binding stress proteins with the capacity to bind to Cd through the thiol group of their cysteine residues (Chisolm & Handorf, 1996; Wang & Fowler, 2008). The most well characterized mechanism of Cd-induced expression of MTs is via the metal regulatory transcription factor (MTF1). Cd activates MTF1, which recognizes and binds the MRE (metal response element) sequence present in the MT promoter region causing transcription of these genes (LaRochelle et al., 2001; Waisberg et al., 2003). Interestingly, studies have shown that other metals such as iAs and Cu are also capable of inducing MTs, though to a lower extent than Cd, and this induction is related to MTF1 activity (Andrews, 2000; Falnoga et al., 2012; He & Ma, 2009; Murata & Gong, 1999). Hence, it is possible that the increased expression levels of MT1A, MT1F, and MT1G induced by the Cd mixture are due to a common transcriptional response induced by iAs and Cd. The HO-1 and MT family of genes were more responsive in expression than the two other genes tested, including AQP9. Thus while AQP9 is a known transmembrane transporter of iAs (Chau et al., 2015), the results of the present study suggest minimal transcriptional change in JEG3 cells to these metals.
The present study focuses on toxic metals in the environment, specifically iAs and Cd. We are mindful that environmental mixtures can include other inorganic as well as organic contaminants. Still the research focus here is dependent on the sampling methodology of the DGT samplers. As these do not accumulate organic chemicals, these measures are not present in the tested samples. Importantly, measurements of other metals and inorganic chemicals in these same waters indicates that concentrations of other metals, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, legacy chlorinated pesticides and currently used pesticides are all several orders of magnitude below the concentrations of iAs and Cd (data not shown). Strikingly, the induction of the gene expression resulting from the laboratory-generated mixtures examined in our study was not as high as in response to the environmental mixtures. These data suggest that other unmeasured factors indeed may impact the expression of these genes in the JEG3 cells. However, the laboratory-generated mixture-induced expression was statistically significantly greater than that of the single metal treatments for the following genes: HO1, MT1A, MT1F and MT1G. This confirms our hypothesis that the effects of metals mixtures on gene expression are greater than additive and thus indicate a synergistic mode of action. Using environmental mixtures simulates real-world exposures to a variety of toxicogenomic compounds and is therefore more relevant to understanding the effects of these compounds on human health and susceptible populations in particular.
In summary, the results of this study demonstrate that iAs and Cd together, are capable of inducing metal-responsive and oxidative stress biomarker gene expression in JEG-3 placental cells even at significantly lower concentrations than those found at highly polluted sites. Evaluating these biomarker responses in the placenta is the first step towards the ideal goal of predicting the effect of toxicants on maternal-fetal health. Of additional importance is the fact that mixtures induced a synergistic response related to gene induction. This synergism needs to be taken into consideration when conducting risk assessments involving toxic metals commonly found in highly contaminated sites, such as Superfund sites in the United States, so as not to underestimate risk.
HIGHLIGHTS.
Toxicogenomic responses of environmental metals mixtures assessed
Induction of ATP7B, HO-1, MT1A, MT1F and MT1G by metals mixtures observed in placental cells
Higher gene induction in response to metals mixtures versus single metal treatments
Acknowledgments
Sample collection and analysis was coordinated by Ying Li. This work was supported by grants from the National Institute of Environmental Health Sciences (NIEHS) (P42-ES005948, T32-ES007126 and R01-ES019315).
ABBREVIATIONS
- iAs
inorganic Arsenic
- Cd
Cadmium
- mRNA
messenger RNA
- HO-1
Heme Oxygenase 1
- MT
Metallothionein
- AQP9
Aquaporin-9
- ATP7B
Cu2+ transporting ATP-ase
- DGT
diffusive gradient in thin film
- O2 stress
oxidative stress
References
- 1.Ahmad Sa, Sayed MH, Barua S, Khan MH, Faruquee MH, Jalil a, … Talukder HK. Arsenic in drinking water and pregnancy outcomes. Envr Hlth Persp. 2001;109(6):629–31. doi: 10.1289/ehp.01109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Bio Pharmacol. 2000;59(1):95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 3.Arita A, Costa M. Epigenetics in metal carcinogenesis: nickel, arsenic, chromium and cadmium. Metallomics. 2009;1(3):222–8. doi: 10.1039/b903049b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balistrieri L, Mebane C. Predicting the toxicity of metal mixtures. Science of The Total Envr. 2014;466–467:788–799. doi: 10.1016/j.scitotenv.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 5.Chisolm JC, Handorf CR. Further observations on the etiology of preeclampsia: Mobilization of toxic cadmium-metallothionein into the serum during pregnancy. Med Hypotheses. 1996;47(2):123–128. doi: 10.1016/s0306-9877(96)90451-x. [DOI] [PubMed] [Google Scholar]
- 6.Choi AMK, Alam J. Heme Oxygenase-1: Function, Regulation, and Implication of a Novel Stress-inducible Protein in Oxidant-induced Lung Injury. Am J Resp Cell and Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- 7.Concha G, Vogler G, Lezcano D, Nermell B, Vahter M. Exposure to inorganic arsenic metabolites during early human development. Tox Scie. 1998;44(2):185–90. doi: 10.1006/toxs.1998.2486. [DOI] [PubMed] [Google Scholar]
- 8.Estaban-Vasallo Maria D, Aragones Nuria, Marina Pollan, L-AG, P-GB Mercury, Cadmium, and Lead Levels in Human Placenta: A Systematic Review. Envr Hlth Persp. 2012;120(10):1369–1377. doi: 10.1289/ehp.1204952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falnoga I, Zelenik Pevec A, Šlejkovec Z, Žnidarič MT, Zajc I, Mlakar SJ, Marc J. Arsenic trioxide (ATO) influences the gene expression of metallothioneins in human glioblastoma cells. Biol Trace Element Res. 2012;149(3):331–9. doi: 10.1007/s12011-012-9431-8. [DOI] [PubMed] [Google Scholar]
- 10.Fay RM, Mumtaz MM. Development of a priority list of chemical mixtures occurring at 1188 hazardous waste sites, using the hazdat database. Food Chem Tox. 1996;34(11–12):1163–1165. doi: 10.1016/s0278-6915(97)00090-2. [DOI] [PubMed] [Google Scholar]
- 11.Fowler BA, Mahaffey KR. Interactions among Lead, Cadmium and Arsenic in Relation to Porphyrin Excretion Patterns. Envr Hlth Persp. 1978;25:87–90. doi: 10.1289/ehp.782587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guiñazú N, Rena V, Genti-Raimondi S, Rivero V, Magnarelli G. Effects of the organophosphate insecticides phosmet and chlorpyrifos on trophoblast JEG-3 cell death, proliferation and inflammatory molecule production. Tox in Vitro. 2012;26(3):406–13. doi: 10.1016/j.tiv.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 13.He X, Ma Q. Induction of metallothionein I by arsenic via metal-activated transcription factor 1: critical role of C-terminal cysteine residues in arsenic sensing. J Biol Chem. 2009;284(19):12609–21. doi: 10.1074/jbc.M901204200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang H, Leung LK. Bisphenol A downregulates CYP19 transcription in JEG-3 cells. Tox Letters. 2009;189(3):248–52. doi: 10.1016/j.toxlet.2009.06.853. [DOI] [PubMed] [Google Scholar]
- 15.Hubel CA, Roberts JM, Taylor RN, Musci TJ, Rogers GM, McLaughlin MK. Lipid peroxidation in pregnancy: New perspectives on preeclampsia. Am J Obstet Gynecol. 1989;161:1025–1034. doi: 10.1016/0002-9378(89)90778-3. [DOI] [PubMed] [Google Scholar]
- 16.Huynh T, Zhang H, Noller B. Evaluation and Application of the Diffusive Gradients in Thin Films Technique Using a Mixed-Binding Gel Layer for Measuring Inorganic Arsenic and Metals in Mining Impacted Water and Soil. Analyt Chem. 2012;84(22):9988–9995. doi: 10.1021/ac302430b. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda K, Utoguchi N, Tsutsui H, Yamaue S, Homemoto M, Nakao E, Hukunaga Y, Yamasaki K, Myotoku M, Hirotani Y. In vitro approaches to evaluate placental drug transport by using differentiating JEG-3 human choriocarcinoma cells. Basic & Clin Pharmacol & Tox. 2010;108:138–145. doi: 10.1111/j.1742-7843.2010.00634.x. [DOI] [PubMed] [Google Scholar]
- 18.Iyengar GV, Rapp A. Human placenta as a “dual” biomarker for monitoring fetal and maternal environment with special reference to potentially toxic trace elements. Part 3: toxic trace elements in placenta and placenta as a biomarker for these elements. Sci Total Environ. 2001;280(1–3):221–238. doi: 10.1016/s0048-9697(01)00827-0. [DOI] [PubMed] [Google Scholar]
- 19.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Repro Update. 2006;12(6):747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Valko M. Arsenic: toxicity, oxidative stress and human disease. J App Tox. 2011;31(2):95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 21.LaRochelle O, Gagné V, Charron J, Soh JW, Séguin C. Phosphorylation Is Involved in the Activation of Metal-regulatory Transcription Factor I in Response to Metal Ions. J Biol Chem. 2001;276(45):41879–41888. doi: 10.1074/jbc.M108313200. [DOI] [PubMed] [Google Scholar]
- 22.Letcher R, van Holsteijn I. Cytotoxicity and aromatase (CYP19) activity modulation by organochlorines in human placental JEG-3 and JAR choriocarcinoma cells. Tox and App Pharmacol. 1999;160(1):10–20. doi: 10.1006/taap.1999.8746. [DOI] [PubMed] [Google Scholar]
- 23.Leung J, Pang A, Yuen W, Kwong Y, Tse EWC. Relationship of expression of aquaglycceporin 9 with arsenic uptake and sensitivity in leukemia cells. Blood. 2007;109:740–746. doi: 10.1182/blood-2006-04-019588. [DOI] [PubMed] [Google Scholar]
- 24.Liaw, Jan Increased childhood liver cancer mortality and arsenic in drinking water in Northern Chile. Cancer Epidemiol Biomarkers. 2009;27(8):417–428. doi: 10.1158/1055-9965.EPI-07-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Liu Y, Habeebu SM, Waalkes MP, Klaassen CD. Chronic combined exposure to cadmium and arsenic exacerbates nephrotoxicity, particularly in metallothionein-I/II null mice. Toxicology. 2000;147(3):157–66. doi: 10.1016/s0300-483x(00)00194-3. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Lutsenko S, Kaplan JH. Organization of P-type ATPases; Significance of structural diversity. Biochem. 1995;34(48):15607–15613. doi: 10.1021/bi00048a001. [DOI] [PubMed] [Google Scholar]
- 28.Lutsenko S, Tsivkosvii R, Walker JM. Functional properties of the human copper-transporting ATPase ATP7B (the Wilson's disease protein) and regulation by metallochaperone Atox1. Ann N Y Acad Sci. 2003;986:204–211. doi: 10.1111/j.1749-6632.2003.tb07161.x. [DOI] [PubMed] [Google Scholar]
- 29.Madden E. A Comparison of 60, 70, and 90 kDa stress protein expression in normal rat NRK-52 and human HK-2 kidney cell lines following in vitro exposure to arsenite and cadmium alone or in combination. J Biochem. 2002;16(1):24–32. doi: 10.1002/jbt.10015. [DOI] [PubMed] [Google Scholar]
- 30.Menzel DB, Rasmussen RE, Lee E, Meacher DM, Said B, Hamadeh H, … Roth RN. Human lymphocyte heme oxygenase 1 as a response biomarker to inorganic arsenic. Biochem Biophys Res Comm. 1998;250(3):653–6. doi: 10.1006/bbrc.1998.9363. [DOI] [PubMed] [Google Scholar]
- 31.Moore LE, Lu M, Smith AH. Childhood cancer incidence and arsenic exposure in drinking water in Nevada. Arch Env Health. 2002;57:201–206. doi: 10.1080/00039890209602937. [DOI] [PubMed] [Google Scholar]
- 32.Murata M, Gong P. Differential metal response and regulation of human heavy metal-inducible genes. J Cell Biol. 1999;113:105–113. doi: 10.1002/(SICI)1097-4652(199907)180:1<105::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 33.Petrukhin K, Lutsenko S, Chernov I, Ross BM, Kaplan JH, Gilliam TC. Characterization of the Wilson disease gene encoding a P-type copper transporting ATPase: genomic organization, alternative splicing, and structure/function predictions. Human Mol Genet. 1994;3(9):1647–56. doi: 10.1093/hmg/3.9.1647. [DOI] [PubMed] [Google Scholar]
- 34.Poranen AK, Ekblad U, Uotila P, Ahotuba M. Lipid peroxidation and antioxidants in normal and pre-eclamptic pregnancies. Placenta. 1996;17:401–405. doi: 10.1016/s0143-4004(96)90021-1. [DOI] [PubMed] [Google Scholar]
- 35.Rahman A, Vahter M, Ekström E-C, Rahman M, Golam Mustafa AHM, Wahed MA, Persson L-A. Association of arsenic exposure during pregnancy with fetal loss and infant death: a cohort study in Bangladesh. Am J Epi. 2007;165(12):1389–96. doi: 10.1093/aje/kwm025. [DOI] [PubMed] [Google Scholar]
- 36.Rahman A, Vahter M, Ekström E-C, Rahman M, Golam Mustafa AHM, Wahed MA, Persson L-A. Association of arsenic exposure during pregnancy with fetal loss and infant death: a cohort study in Bangladesh. Am J Epi. 2007;165(12):1389–96. doi: 10.1093/aje/kwm025. [DOI] [PubMed] [Google Scholar]
- 37.Ratnaike R. Acute and chronic arsenic toxicity. Postgrad Med J. 2003;79(933):391–396. doi: 10.1136/pmj.79.933.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ronco AM, Llaguno E, Epuñan MJ, Llanos MN. Effect of cadmium on cortisol production and 11beta-hydroxysteroid dehydrogenase 2 expression by cultured human choriocarcinoma cells (JEG-3) Tox in Vitro. 2010;24(6):1532–7. doi: 10.1016/j.tiv.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PEB, Williams DJ, Moore MR. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Tox Letters. 2003;137(1–2):65–83. doi: 10.1016/s0378-4274(02)00381-8. [DOI] [PubMed] [Google Scholar]
- 40.Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, Selvin S. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Envr Hlth Persp. 2006;114(8):1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staff AC, Ranheim T, Khoury J, Henriksen T. Increased contents of phospholipids, cholesterol, and lipid peroxides in decidua basalis in women with preeclampsia. Am J Obstet Gynecol. 1999;180:587–592. doi: 10.1016/s0002-9378(99)70259-0. [DOI] [PubMed] [Google Scholar]
- 42.Tsai SY, Chou HY, The HW, Chen CM, Chen CJ. The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. NeuroToxicology. 2003;24:747–753. doi: 10.1016/S0161-813X(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 43.Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Functional and molecular characterization of the human neutral solute channel aquaporin-9. Am J Physiol. 1999;277:F685–F696. doi: 10.1152/ajprenal.1999.277.5.F685. [DOI] [PubMed] [Google Scholar]
- 44.Tuan RS, Moore CJ, Brittingham JW, Kirwin JJ, Akins RE, Wong M. In vitro study of placental trophoblast calcium uptake using JEG-3 human choriocarcinoma cells. J Cell Sci. 1991;3:333–342. doi: 10.1242/jcs.98.3.333. [DOI] [PubMed] [Google Scholar]
- 45.Uotila JT, Tuimala RJ, Aarnio TM. Findings on lipid peroxidation and antioxidant function in hypertensive complications of pregnancy. Br J Obstet Gynaecol. 1993;100:270–276. doi: 10.1111/j.1471-0528.1993.tb15242.x. [DOI] [PubMed] [Google Scholar]
- 46.Vanderlie J, Venardos K, Clifton VL, Gude NM, Clarke FM, Perkins AV. Increased biological oxidation and reduced anti-oxidant enzyme activity in pre-eclamptic placentae. Placenta. 2005;26:53–58. doi: 10.1016/j.placenta.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Von Ehrenstein OS, Guha Mazumder DN, Hira-Smith M, Ghosh N, Yuan Y, Windham G, Smith AH. Pregnancy outcomes, infant mortality, and arsenic in drinking water in West Bengal, India. Am J Epi. 2006;163(7):662–9. doi: 10.1093/aje/kwj089. [DOI] [PubMed] [Google Scholar]
- 48.Waalkes M. Cadmium carcinogenesis. Fund Mol Mech Muta. 2003;533(1–2):107–120. doi: 10.1016/j.mrfmmm.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003;192:95–117. doi: 10.1016/s0300-483x(03)00305-6. [DOI] [PubMed] [Google Scholar]
- 50.Walsh SW. Lipid peroxidation in pregnancy. Hypertens Pregnancy. 1994;13:1–31. [Google Scholar]
- 51.Walsh SW, Wang Y. Trophoblast and placental villous core production of lipid peroxides, thromboxane, and prostacyclin in preeclampsia. J Clin Endocrinol Metab. 1995;80:1888–1893. doi: 10.1210/jcem.80.6.7775637. [DOI] [PubMed] [Google Scholar]
- 52.Wang G, Fowler BA. Roles of biomarkers in evaluating interactions among mixtures of lead, cadmium and arsenic. Tox and App Pharmacol. 2008;233(1):92–9. doi: 10.1016/j.taap.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Walsh SW. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta. 1998;19:581–586. doi: 10.1016/s0143-4004(98)90018-2. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Walsh SW. Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in preeclampsia. Placenta. 2001;22:206–212. doi: 10.1053/plac.2000.0608. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Walsh SW, Guo J, Zhang J. The imbalance between thromboxane and prostacyclin in preeclampsia is associated with an imbalance between lipid peroxides and vitamin E in maternal blood. Am J Obstet Gynecol. 1991;165:1695–1700. doi: 10.1016/0002-9378(91)90017-l. [DOI] [PubMed] [Google Scholar]
- 56.Wasserman Ga, Liu X, Parvez F, Ahsan H, Factor-Litvak P, Kline J, Graziano JH. Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh. Envr Hlth Persp. 2007;115(2):285–289. doi: 10.1289/ehp.9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan Y, Marshall G, Ferreccio C, Steinmaus C, Selvin S, Liaw J, Smith AH. Acute myocardial infarction mortality in comparison with lung and bladder cancer mortality in arsenic-exposed region II of Chile from 1950 to 2000. Am J Epi. 2007;166(12):1381–1391. doi: 10.1093/aje/kwm238. [DOI] [PubMed] [Google Scholar]
- 58.Zhang YL, Zhao YC, Wang JX, Zhu HD, Liu QF, Fan YG, Fan TQ. Effect of environmental exposure to cadmium on pregnancy outcome and fetal growth: A study on healthy pregnant women in China. J Env Sci Hlth, Part A. 2004;39(9):2507–2515. doi: 10.1081/ese-200026331. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Yang D, Lu Z. Introduction and reflection of cadmium pollution accident occurred in LongJiang River of GuangXi Province. Chi J Chem Edu. 2013;34(6):1–2. [Google Scholar]
- 60.Zusterzeel PLM, Rutten H, Roelofs HMJ, Peters WHM, Steegers EAP. Protein carbonyls in decidua and placenta of pre-eclamptic women as markers for oxidative stress. Placenta. 2001;22:213–219. doi: 10.1053/plac.2000.0606. [DOI] [PubMed] [Google Scholar]