Abstract
Background:
Antibiotic resistance is a worldwide problem in acne patients due to regional prescription practices, patient compliance, and genomic variability in Propionibacterium acnes, though the effect of treatment on the resistance has not been comprehensively analyzed.
Aims:
Our primary objective was to assess the level of antibiotic resistance in the Indian patients and to assess whether there was a difference in the resistance across common treatment groups.
Subjects and Methods:
A cross-sectional, institutional based study was undertaken and three groups of patients were analyzed, treatment naïve, those on antibiotics and patients on benzoyl peroxide (BPO) and/isotretinoin. The follicular content was sampled and the culture was verified with 16S rRNA polymerase chain reaction, genomic sequencing, and pulsed-field gel electrophoresis. Minimum inhibitory concentration (MIC) assessment was done for erythromycin (ERY), azithromycin (AZI), clindamycin (CL), tetracycline (TET), doxycycline (DOX), minocycline (MINO), and levofloxacin (LEVO). The four groups of patients were compared for any difference in the resistant strains.
Results:
Of the 52 P. acnes strains isolated (80 patients), high resistance was observed to AZI (100%), ERY (98%), CL (90.4%), DOX (44.2%), and TETs (30.8%). Low resistance was observed to MINO (1.9%) and LEVO (9.6%). Statistical difference was seen in the resistance between CL and TETs; DOX/LEVO and DOX/MINO (P < 0.001). High MIC90 (≥256 μg/ml) was seen with CL, macrolides, and TETs; moreover, low MIC90 was observed to DOX (16 μg/ml), MINO (8 μg/ml), and LEVO (4 μg/ml). Though the treatment group with isotretinoin/BPO had the least number of resistant strains there was no statistical difference in the antibiotic resistance among the various groups of patients.
Conclusions:
High resistance was seen among the P. acnes strains to macrolides-lincosamides (AZI and CL) while MINO and LEVO resistance was low.
Keywords: Acne, antibiotics, azithromycin, benzoyl peroxide, clindamycin, levofloxacin, minocycline, resistance
Introduction
What was known?
Antibiotic resistance in acne patients is a global phenomenon
Macrolides and clindamycin are found to be commonly resistant to in various studies across the world
The use of benzoyl peroxide and retinoids can help obviate resistance.
Antibiotic resistance in acne has been reported from across the globe[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21] and is dependent on multiple factors including variable prescription practices,[1,2,3,6,7,16] patient compliance,[1,2,3,6,7] use of concomitant therapy,[1,2] and also on the methods adopted for isolation of (surface or follicular isolation).[22]
A crucial determinant of resistance is the method of assessing the minimum inhibitory concentration (MIC) levels, which is not uniform across studies,[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21] with some using CLSI[23] or EUCAST,[24] while others using country specific or self-devised MIC values.[13,14,15,16,25] Conversely, some authors believe that the role of Propionibacterium acnes may be overestimated[26] and it may be useful to administer therapies that do not target P. acnes, like retinoids, benzoyl peroxide (BPO), and other nonantibiotics (Zn).[27]
The existing studies do not usually focus on the effect of the type of treatment on the resistance seen. It is believed that P. acnes strains isolated from patients on antibiotics have more resistance than those on retinoids (tretinoin, adapalene, and isotretinoin) or BPO.[2,4,8,28] Thus, it is recommended to avoid using antibiotics for long periods and to add retinoids or BPO along with them or as the sole agents depending on the severity of acne.[28] The premise that the resistance to P. acnes would be less in patients of BPO/oral isotretinoin has not been formally examined previously with other treatment groups. The primary objectives of our study was to: (1) Determine the prevalence of antibiotic resistance to P. acnes in the outpatient department attendees of a tertiary level hospital, and (2) to compare the resistance to P. acnes among commonly used treatment groups in India. It was our premise that patient on retinoids and/or BPO would have less resistance than patients on antibiotics (oral and/topical).
Subjects and Methods
A cross-sectional study was conducted in the Department of Dermatology and Sexually Transmitted Diseases of a tertiary referral hospital and the Institute of Genomics and Integrative Biology, Delhi from November 2012 to February 2014. Ethics Committee clearance was taken at the Institute, Delhi University, and Council of Scientific and Industrial Research (CSIR) independently as it was an inter-institutional program. A total of 80 random patients of acne vulgaris were selected with the exclusion criteria of pregnant/lactating females, patients with hormonal acne, patients without comedones, and patients unwilling for sampling from their lesions. A predesigned performa was used, and patients were divided into four subgroups on the basis of the treatment they were on. Group A were treatment naïve cases with no history of previous oral or topical treatment for acne, Group B included patients who received antibiotic treatment (topical and/or oral), Group C had patients who received retinoids and/or BPO while Group D were constituted by patients who received a combination of retinoid (topical/oral) or benzyl peroxide with antibiotics. Patients, who had taken treatment for at least 4 weeks, were considered as being on treatment in the study. The acne severity was graded using the global acne grading system.[29]
The identification and confirmation of genotypic mutation of P. acnes required a sequential step by step identification protocol based on the work of Ross et al.[2] and Oprica et al.[3]
Specimen collection and processing
After cleansing the sample area with a clean alcohol-imbibed cotton swab, open comedones (blackheads) were sampled, from the face, using a sterile comedone extractor. For a sampling of closed comedones (whiteheads), the lesion was ruptured using a 25 mm × 35 mm hypodermic needle followed by a collection of the contents using the comedone extractor.
The specimens were transported in sterile and anaerobic conditions using brain heart infusion (BHI) broth (HiMedia). The specimens were cultured on anaerobic BHI plate containing 5% defibrinated sheep blood and incubated in anaerobic condition using anaerobic jar (GasPak Anaerobic System, HiMedia) at 37°C for 7–10 days followed by sub-culturing to isolate the pure culture. Morphologically, colonies were differentiated from the basis of their color, shape, and size.[30] The isolated culture was divided into two parts; one part was frozen at −80°C and another part was used to perform antimicrobial resistance study at the same time.
Microbial identification
The initial microbial identification was performed by Gram-staining and biochemical tests, including catalase and indole tests.[30] Molecular methods were used to further confirm these isolated microbes. Sequencing of 16S rRNA gene was performed to confirm the isolated organism from the acne lesions to be P. acnes. Genomic DNA was extracted from the pure bacterial cultures using QIAGEN QIAamp® genomic DNA extraction kit and followed by 0.8% agarose (Sigma-Aldrich) gel electrophoresis to check their quality while the NanoDrop (NanoDrop spectrophotometer ND-1000) was used to estimate the concentration of genomic DNA. This step was followed by polymerase chain reaction (PCR) using two different sets of universal primers, forward primer (5’-AGAGTTTGATCCTGGCTCAG-3’), and reverse primer (5’-ACGGCTACCTTGTTACGACTT-3’).[31,32,33] QIAGEN QIAquick Gel extraction kit was used to purify the PCR product of agarose gel and followed by 16S rRNA gene sequencing using ABI-PRISM 377 automated DNA sequencer.
Determination of minimum inhibitory concentration
Quantitative evaluation of antibacterial activity was performed by estimating the MIC for commercially available drugs on the skin isolated microbial flora from acne patients, employing the micro-well serial dilution method. Antibiotic susceptibility and resistance breakpoints were defined as prescribed by the Clinical and Laboratory Standards Institute[23] as follows: Clindamycin (CL) ≥8 μg/ml, erythromycin (ERY) ≥2 μg/ml, tetracycline (TET) ≥16 μg/ml, minocycline (MINO) ≥16 μg/ml, doxycycline (DOX) ≥4 μg/ml, levofloxacin (LEVO) ≥8 μg/ml and azithromycin (AZI) ≥4 μg/ml. For the quality control of antibiotic tests the following strains were used: P. acne – microbial type culture collection (MTCC) 1951, S. aureus – MTCC 740, B. cereus – MTCC 430, B. subtilis – MTCC 121, E. coli – MTCC 1586 (the MTCC and gene bank, is a national facility funded jointly by the Department of Biotechnology and the CSIR, Government of India and is at the Institute of Microbial Technology, Chandigarh).
Statistical analysis
The data generated were compared by the Chi-square test. If the expected value in any of the cells of a contingency table was below 5, Fisher's exact test was used. SAS software package for windows version 9.2 was used for statistical analysis and P < 0.05 were considered statistically significant.
Results
Of the 80 patients studied, there were 42 (52.5%) males and 38 (47.5%) females. The majority of the patients (83.8%) were in the age group of <25 years with more males than females (47.5%). Using the global acne grading system,[29] the majority of the patients had moderate acne (48.8%), 30% patients had mild acne, and 21.2% had severe acne.
The patients were analyzed by dividing them into four groups according to the treatment they were on for ≥4 weeks [Table 1]. There were 22 patients each in the groups not on treatment and on antibiotics alone and 18 each in the group of retinoids and/or BPO.[34] There was no statistical difference among the groups with respect to age, sex, duration of disease, or severity (P ≥ 0.05).
Table 1.
An overview of the treatment groups and the therapy administered
A total of 52 strains of P. acnes were isolated (isolation rate of 65%) [Table 2] including some novel P. acnes strains KC874899.1, KF268368.1, KF268366.1 (http://www.ncbi.nlm.nih.gov/nuccore/). Of the rest, staphylococcal epidermis was the commonest organism isolated (n = 47).
Table 2.
Organisms isolated after culture of samples and genomic validation

Propionibacterium acnes antimicrobial susceptibility
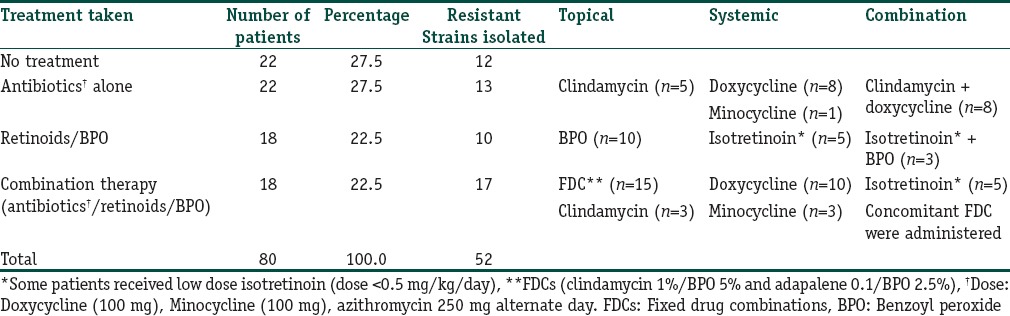
High resistance [Table 3] was observed to macrolides-lincosamides (ERY, CL, and AZI: 98%, 90.4%, and 100%, respectively) while a low level was noted with MINO and LEVO [Table 3]. Cross resistance to CL in ERY resistant P. acnes was found in 46 patients (88.4%) while cross resistance between TET and DOX was seen in eight patients (15.4%). All P. acnes strains were resistant to at least one antibiotic (100%), none (0%) were sensitive to all the antibiotics, and 1 P. acnes strain was resistant to all the antibiotics (1.9%). Resistance to the macrolides was statistically more [Figure 1] as compared to cyclines (TET, DOX, and MINO) and LEVO (P < 0.001). Similarly, the resistance to DOX was more than MINO and LEVO (P < 0.001) [Figure 1].
Table 3.
Susceptibility of Propionibacterium acnes to various antibiotics
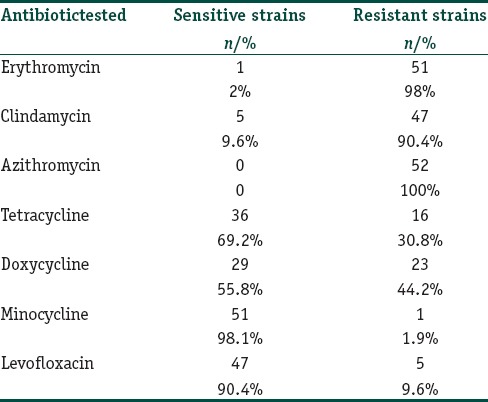
Figure 1.
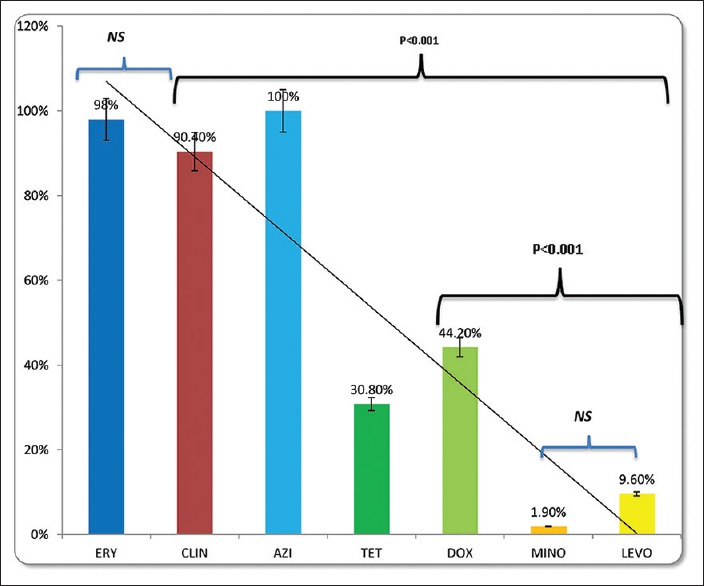
Propionibacterium acnes resistance of commonly to used antibiotics in acne. Statistically significant difference was observed between, CL/TETs, LEVO DOX, and MINO/LEVO. ERY: Erythromycin, CL: Clindamycin, AZI: Azithromycin, TET: Tetracycline, DOX: Doxycycline, MINO: Minocycline, LEVO: levofloxacin. NS: not significant
Minimum inhibitory concentration values
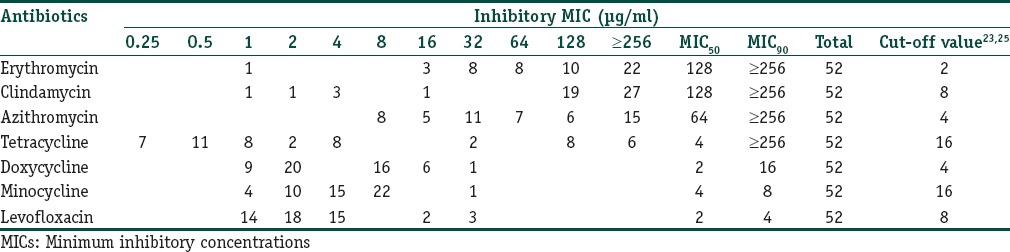
The MIC values of the strains are listed in Table 4. The macrolides, CL, and TET had the highest MIC90 values, followed by DOX, MINO, and LEVO.
Table 4.
MICs of antimicrobials against Propionibacterium acnes
Propionibacterium acnes antimicrobial resistance in the treatment groups
The treatment naïve group [Table 5] had high resistance to macrolides and CL (75–100%), followed by TET and DOX (33–41%), with a low resistance to MINO and LEVO (0–8.3%). A similar trend was noticed in the other treatment groups.
Table 5.
Resistance patterns to antibiotics in the treatment groups*

As the 4th group [Table 1] on combination therapy had heterogeneous treatment interventions this was not included in the intergroup analysis. On intergroup comparison, there was no statistical difference among the groups (P > 0.05). While the lowest number of resistant strains was seen in the group on retinoids and/or BPO, the difference with the other groups was not statistically significant (P > 0.05).
Comparison of the prevalence of antibiotic resistance to P. acnes in the subgroups according to age, sex, disease duration, and severity of acne did not reveal any significant difference except in CL resistance which was seen in patients having duration of disease of more than 2 years (P = 0.049).
Discussion
Our study revealed a high level of resistance to macrolides and CL (98% to ERY, 90.4% to CL, and 100 % to AZI) with a low resistance to MINO (1.9%) and LEVO (9.6%). The MIC values also largely correlated with the high resistant antibiotics with a high MIC90 seen to macrolides and CL (≥256 μg/ml), and low MIC90 values to MINO (8 μg/ml) and LEVO (4 μg/ml). On comparison of the treatment groups, the least number of resistant strains were seen in the retinoids and/or BPO group, but there was no statistically significant difference within the treatment groups with respect to the antibiotic resistant strains.
Though there are numerous studies on antibiotic resistance in acne,[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,21] there is no study that has compared various treatment groups, namely antibiotics and retinoids and/or BPO, with respect to the pattern of antibiotic resistance.
There are three issues that are relevant to a study of resistance in acne vulgaris. The first is the presence of resistant P. acnes to various antibiotics, which has been noticed with most macrolides and TETs and is due to the long treatment duration and concomitant use of oral and topical antibiotics.[1,2,3] The worldwide data on resistance [Table 6] should be interpreted with respect to regional prescription practices.[2,3] The low levels of resistance in Hungary[2] is due to the restricted acne prescriptions. The use of multiple adjunctive therapies accounts for the high levels of resistance in Spain [Table 6].[2] Oprica et al.[3] reported a variable resistance, ranging from 83% in Croatia, 60% in Italy to 0% in The Netherlands. There was more resistance in the Southern Europe as compared to the Western Europe. Notably in countries with no “nonhospital” use of antibiotics (Netherlands) there was no problem of resistance. Though it is has been proposed that resistance across certain areas may be explained by specific mutations and clones of P. acnes[36] this has not been consistently proved.[37]
Table 6.
An overview of seminal studies on antibiotic resistance in acne vulgaris
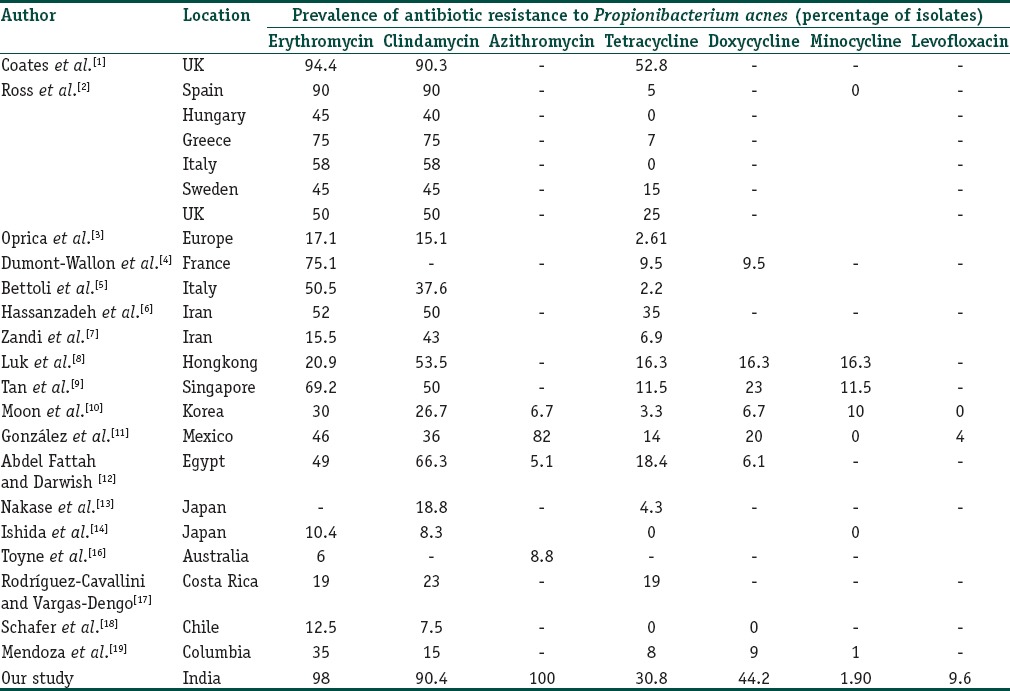
Our study largely mirrors the trend of high resistance to macrolides and CL and low level of resistance to MINO and LEVO has seen across the world [Table 6]. This is due to prescription practices that cause macrolides resistance which drives resistance to CL and often to TET. The high levels in our study are due to the widespread use of macrolides and CL, with a prolonged course of therapy, mostly due to the nonadherence by patients of the physicians advice to restrict antibiotic usage and duration. Furthermore, “over the counter” availability of drugs without prescription is a major contributing factor leading to misuse of the antibiotics. The universal (100%) resistance to AZI is akin to another study from Mexico[11] and highlights the misuse of this drug for acne. Its use in acne is surprising as it is essentially a macrolides, a class to which resistance has been known to occur.[1,2,3] As AZI is commonly used to treat a variety of systemic infections, its use should be restricted and discouraged in acne.[27,35] The resistance to TET and DOX was high in our study with low resistance to MINO [Figure 1]. The low resistance to MINO is in consonance with studies across the world[1,2,3,4,5,6,7,10,11,12,13,14,15,16,17,18] and is probably as the drug is highly lipophilic and thus has a better penetration leading to enhanced efficacy. LEVO is a reserve drug for acne[10,11,38,39] that is prescribed in Japan. Though low resistance levels have been seen in our study, the drug should be used sparingly, lest it is misused, such as AZI.
The second important issue that is not well-researched is whether various therapeutic interventions can impact on the level of resistance.[28] This is largely dependent on the level of background resistance, a high level of which was seen in the treatment naïve group [Table 5]. Coates et al.[1] had observed that it is difficult to correlate the use of antibiotics with the emergence of resistant commensal organisms. A study from Australia[15] found that patients who had not received any antibiotics for last 4 months had no resistance. We cannot rule out the use of antibiotics for nondermatological indications in the treatment naïve group, which is a possible reason for the high levels of resistance. Interestingly, another study[18] revealed that patients may have resistance to antibiotics which they have never been used before. This has been explained by the dissemination of resistance by person-to-person contact, which explained the prevalence of resistant P. acnes in household contacts of patients with acne.[2] Thus, the high resistance levels in the treatment naïve group can be explained by, the colonization with resistant clones, dissemination of antibiotic resistant genes and the possible treatment with antibiotics for other diseases.
It is believed that the antibiotic group, due to selection pressure, would have more resistance than the treatment naïve group, but this was not seen in our study [Table 5]. While studies have shown that resistance levels correlate with antibiotic usage,[2,3] other authors[7] have not found this correlation, similar to our work. Two studies[10,12] have found that prior antibiotic therapy tended to result in higher mean MICs of isolates, although the differences were not statistically significant. A study from Korea[10] found that while the MIC tended to be higher in the patient group with prior use of antimicrobial agents, this was statistically significant only for DOX. As resistant strains are mutation based and permanent, they will persist[37,41] and this explains the lack of correlation among the group on antibiotics vis-a-vis the treatment naïve group [Table 5].
The lack of significant difference between the retinoids and/or BPO group and the antibiotic group is an interesting finding as it is conventionally believed that being “nonantibiotics,” resistant strains are not seen with their use. The effect of BPO against sensitive and resistant strains of P. acnes[35] is time dependent.[41] A study[41] revealed that at the end of 4 weeks though the ERY/CL resistant strains decreased, they were not completely eliminated.[40] Ross et al. found that in Spain,[2] BPO did not reduce resistant strains, due to the high background resistance to macrolides/CL, similar to our study. Oprica et al.[41] elegantly demonstrated that patients on isotretinoin had resistant strains even post-therapy in spite of a favorable clinical response. Coates et al.[42] had also demonstrated that isotretinoin did not significantly reverse the resistant strains of P. acnes. Our study reiterates the finding that retinoids and/or BPO cannot always eliminate or markedly reduce the resistant strains in acne[41,42] especially if there is a high background resistance as seen in our study [Table 5]. However, it is possible that if a longer period of treatment was administered, this group of patients might have had a markedly reduced P. acnes resistance; though we may point out that in our work a high background resistance was noted and this resistance is mutational based and is not reversible.[2,3]
The method of isolation of P. acnes is an important aspect[22] as different sampling/study methodologies will likely target anatomically distinct P. acnes populations with, at least theoretically, distinct pathogenic potentials. P. acnes at the stratum corneum is, like S. epidermidis, a commensal. Recent studies on genomic analysis of P. acnes have led to the discovery of various phylotypes (IA 1, IA 2, IB, IC, II, and III).[43] Techniques such as swab and scrape are inappropriate to isolate P. acnes.[22] Sadly, there is a lack of uniformity in studies with a large number of studies using surface skin isolation methods,[1,2,3,4,15] which may not always reflect the pathogenic strains of P. acnes.[22] The confirmation of the organism requires the use 16S rRNA based PCR and sequencing or other techniques such as fluorescence in situ hybridization, which has been used in a few studies.[8,9,10,16,18] Our technique included a combination of follicular content sampling and genomic confirmation, which makes our isolation and identification of P. acnes accurate though it does add to the cost of the study [Table 6].
The inherent drawback in our study is that we did not study discrete groups of patients, on a single drug, which though is an ideal scenario, is not always practiced. Our groups represented a realistic prescription practice that has been observed by Coates et al.,[1] wherein topical and oral agents are used in succession and in combination. Another drawback, largely due to the cost constraints of our protocol, was the lack of sequential (weekly) isolation and confirmation of resistant P. acnes, which could have helped to map the sequential decrease in resistant strains on BPO. The lack of any statistically significant changes may also be partially contributed by the low sample size and power of the present study.
Antibiotic resistance in P. acnes presents a worldwide problem for the treatment of acne and geographical variations in resistance are bound to be present [Table 6]. Thus, each country has to enforce a specific antibiotic policy for acne. Though it is difficult to defend the often long-term use of antibiotics for acne, until effective new nonantibiotic treatments for acne with acceptable side effects are available, antibiotics will probably remain the preferred drug in moderate grades of acne.[28,44,45] To minimize the resistance problem certain measures are advocated, like avoiding antibiotics for milder forms of acne and using antibiotics in combination with topical retinoids or BPO if antibiotics must be administrated for longer than 2 months.[27,28,45,46,47] Probably adding other agents such as zinc and azealic acid may help to obviate the development of resistance.[27,47] However, the misuse of antibiotics in acne, if they exceed the MIC levels “in vitro,” will lead to an epidemiological problem and predispose to transfer of resistance to other organisms and patients. Most importantly higher MIC may drive the antibiotics out of the realms of clinical use as breakpoint levels will be reached soon with most antibiotics.
Financial support and sponsorship
We gratefully acknowledge the financial assistance from the CSIR– TOUCH (BSC0302) project. The author is also thankful to CSIR, New Delhi for the financial support (SRF).
Conflicts of interest
There are no conflicts of interest.
What is new?
The type of therapy may not affect the resistance levels to macrolideslincosamides if there is a high background resistance
Minocycline resistance is still low and this drug may be a valid treatment option
BPO/isotretinoin are effective drugs though they may not markedly affect the resistant strains, though a prolonged duration of therapy may help.
References
- 1.Coates P, Vyakrnam S, Eady EA, Jones CE, Cove JH, Cunliffe WJ. Prevalence of antibiotic-resistant propionibacteria on the skin of acne patients: 10-year surveillance data and snapshot distribution study. Br J Dermatol. 2002;146:840–8. doi: 10.1046/j.1365-2133.2002.04690.x. [DOI] [PubMed] [Google Scholar]
- 2.Ross JI, Snelling AM, Carnegie E, Coates P, Cunliffe WJ, Bettoli V, et al. Antibiotic-resistant acne: Lessons from Europe. Br J Dermatol. 2003;148:467–78. doi: 10.1046/j.1365-2133.2003.05067.x. [DOI] [PubMed] [Google Scholar]
- 3.Oprica C, Nord CE. ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. European surveillance study on the antibiotic susceptibility of Propionibacterium acnes. Clin Microbiol Infect. 2005;11:204–13. doi: 10.1111/j.1469-0691.2004.01055.x. [DOI] [PubMed] [Google Scholar]
- 4.Dumont-Wallon G, Moyse D, Blouin E, Dréno B. Bacterial resistance in French acne patients. Int J Dermatol. 2010;49:283–8. doi: 10.1111/j.1365-4632.2009.04270.x. [DOI] [PubMed] [Google Scholar]
- 5.Bettoli V, Borghi A, Rossi R, Ferroni M, Rigolin F, Virgili A. Antibiotic resistance of propionibacteria. Four years’ experience of a large number of cases in Italy. Dermatology. 2006;212:206–7. doi: 10.1159/000090665. [DOI] [PubMed] [Google Scholar]
- 6.Hassanzadeh P, Bahmani M, Mehrabani D. Bacterial resistance to antibiotics in acne vulgaris: An in vitro study. Indian J Dermatol. 2008;53:122–4. doi: 10.4103/0019-5154.43213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zandi S, Vares B, Abdollahi H. Determination of microbial agents of acne vulgaris and Propionibacterium acnes antibiotic resistance in patients referred to dermatology clinics in Kerman, Iran. Jundishapur J Microbiol. 2011;4:17–22. [Google Scholar]
- 8.Luk NM, Hui M, Lee HC, Fu LH, Liu ZH, Lam LY, et al. Antibiotic-resistant Propionibacterium acnes among acne patients in a regional skin centre in Hong Kong. J Eur Acad Dermatol Venereol. 2013;27:31–6. doi: 10.1111/j.1468-3083.2011.04351.x. [DOI] [PubMed] [Google Scholar]
- 9.Tan HH, Tan AW, Barkham T, Yan XY, Zhu M. Community-based study of acne vulgaris in adolescents in Singapore. Br J Dermatol. 2007;157:547–51. doi: 10.1111/j.1365-2133.2007.08087.x. [DOI] [PubMed] [Google Scholar]
- 10.Moon SH, Roh HS, Kim YH, Kim JE, Ko JY, Ro YS. Antibiotic resistance of microbial strains isolated from Korean acne patients. J Dermatol. 2012;39:833–7. doi: 10.1111/j.1346-8138.2012.01626.x. [DOI] [PubMed] [Google Scholar]
- 11.González R, Welsh O, Ocampo J, Hinojosa-Robles RM, Vera-Cabrera L, Delaney ML, et al. In vitro antimicrobial susceptibility of Propionibacterium acnes isolated from acne patients in Northern Mexico. Int J Dermatol. 2010;49:1003–7. doi: 10.1111/j.1365-4632.2010.04506.x. [DOI] [PubMed] [Google Scholar]
- 12.Abdel Fattah NS, Darwish YW. In vitro antibiotic susceptibility patterns of Propionibacterium acnes isolated from acne patients: An Egyptian university hospital-based study. J Eur Acad Dermatol Venereol. 2013;27:1546–51. doi: 10.1111/jdv.12057. [DOI] [PubMed] [Google Scholar]
- 13.Nakase K, Nakaminami H, Takenaka Y, Hayashi N, Kawashima M, Noguchi N. Relationship between the severity of acne vulgaris and antimicrobial resistance of bacteria isolated from acne lesions in a hospital in Japan. J Med Microbiol. 2014;63:721–8. doi: 10.1099/jmm.0.067611-0. [DOI] [PubMed] [Google Scholar]
- 14.Ishida N, Nakaminami H, Noguchi N, Kurokawa I, Nishijima S, Sasatsu M. Antimicrobial susceptibilities of Propionibacterium acnes isolated from patients with acne vulgaris. Microbiol Immunol. 2008;52:621–4. doi: 10.1111/j.1348-0421.2008.00081.x. [DOI] [PubMed] [Google Scholar]
- 15.Kurokawa I, Nishijima S, Kawabata S. Antimicrobial susceptibility of Propionibacterium acnes isolated from acne vulgaris. Eur J Dermatol. 1999;9:25–8. [PubMed] [Google Scholar]
- 16.Toyne H, Webber C, Collignon P, Dwan K, Kljakovic M. Propionibacterium acnes (P. acnes) resistance and antibiotic use in patients attending Australian general practice. Australas J Dermatol. 2012;53:106–11. doi: 10.1111/j.1440-0960.2011.00867.x. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Cavallini E, Vargas-Dengo P. Bacterial etiology and antibiotic susceptibility in patients with acne. Rev Biomed. 2004;15:101–6. [Google Scholar]
- 18.Schafer F, Fich F, Lam M, Gárate C, Wozniak A, Garcia P. Antimicrobial susceptibility and genetic characteristics of Propionibacterium acnes isolated from patients with acne. Int J Dermatol. 2013;52:418–25. doi: 10.1111/j.1365-4632.2011.05371.x. [DOI] [PubMed] [Google Scholar]
- 19.Mendoza N, Hernandez PO, Tyring SK, Haitz KA, Motta A. Antimicrobial susceptibility of Propionibacterium acnes isolates from acne patients in Colombia. Int J Dermatol. 2013;52:688–92. doi: 10.1111/j.1365-4632.2011.05403.x. [DOI] [PubMed] [Google Scholar]
- 20.Dreno B, Reynaud A, Moyse D, Habert H, Richet H. Erythromycin-resistance of cutaneous bacterial flora in acne. Eur J Dermatol. 2001;11:549–53. [PubMed] [Google Scholar]
- 21.Oprica C, Emtestam L, Lapins J, Borglund E, Nyberg F, Stenlund K, et al. Antibiotic-resistant Propionibacterium acnes on the skin of patients with moderate to severe acne in Stockholm. Anaerobe. 2004;10:155–64. doi: 10.1016/j.anaerobe.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Alexeyev OA, Jahns AC. Sampling and detection of skin Propionibacterium acnes: Current status. Anaerobe. 2012;18:479–83. doi: 10.1016/j.anaerobe.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Hecht DW, Citron DM, Cox M, Jacobus N, Jenkins SG, Onderdonk A, et al. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute (CLSI); 2007. [Last accessed on 2014 Jan 10]. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard-seventh edition. Available from: http://www.clsi.org/source/orders/free/m11a7.pdf . [Google Scholar]
- 24.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 4.0. 2014. [Last accessed on 2014 Jan]. Available from: http://www.eucast.org .
- 25.Merriam CV, Citron DM, Tyrrell KL, Warren YA, Goldstein EJ. In vitro activity of azithromycin and nine comparator agents against 296 strains of oral anaerobes and 31 strains of Eikenella corrodens. Int J Antimicrob Agents. 2006;28:244–8. doi: 10.1016/j.ijantimicag.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Shaheen B, Gonzalez M. A microbial aetiology of acne: What is the evidence? Br J Dermatol. 2011;165:474–85. doi: 10.1111/j.1365-2133.2011.10375.x. [DOI] [PubMed] [Google Scholar]
- 27.Sardana K, Garg VK. Antibiotic resistance in acne: Is it time to look beyond antibiotics and Propionobacterium acnes? Int J Dermatol. 2014;53:917–9. doi: 10.1111/ijd.12445. [DOI] [PubMed] [Google Scholar]
- 28.Thiboutot D, Gollnick H, Bettoli V, Dréno B, Kang S, Leyden JJ, et al. New insights into the management of acne: An update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 Suppl):S1–50. doi: 10.1016/j.jaad.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 29.Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36:416–8. doi: 10.1046/j.1365-4362.1997.00099.x. [DOI] [PubMed] [Google Scholar]
- 30.Rodloff AC, Hillier SL, Moncla BJ. Peptostreptococcus, Propionibacterium, Lactobacillus, Actinomycetes and other nonspore forming anaerobic Gram-positive bacteria. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of Clinical Microbiology. Washington, DC: American Society for Microbiology; 1999. pp. 672–89. [Google Scholar]
- 31.Sacchi CT, Whitney AM, Mayer LW, Morey R, Steigerwalt A, Boras A, et al. Sequencing of 16S rRNA gene: A rapid tool for identification of Bacillus anthracis. Emerg Infect Dis. 2002;8:1117–23. doi: 10.3201/eid0810.020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pathak R, Kumar R, Gautam HK. Cross-Species induction and enhancement of antimicrobial properties in response to gamma irradiation in Exiguobacterium sp. HKG 126. Indian J Microbiol. 2013;53:130–6. doi: 10.1007/s12088-013-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ting DT, Chern KC, Meister DM, Hallb GS, Knappb CC, Doyleb LJ, et al. Evaluation of Propionibacterium acnes isolates using contour-clamped homogenous electric field gel electrophoresis. Anaerobe. 1999;5:579–82. [Google Scholar]
- 34.Sardana K, Garg VK. Low-dose isotretinoin in acne vulgaris: A critical review. Br J Dermatol. 2011;165:698–700. doi: 10.1111/j.1365-2133.2011.10440.x. [DOI] [PubMed] [Google Scholar]
- 35.Layton AM. Disorders of the sebaceous glands. In: Burns DA, Breathnach SM, Cox NH, Griffiths CE, editors. Rook's Textbook of Dermatology. 8th ed. Vol. 4. West Sussex: Blackwell Publishing Ltd; 2010. pp. 42-47–42-60. [Google Scholar]
- 36.Ross JI, Snelling AM, Eady EA, Cove JH, Cunliffe WJ, Leyden JJ, et al. Phenotypic and genotypic characterization of antibiotic-resistant Propionibacterium acnes isolated from acne patients attending dermatology clinics in Europe, the USA, Japan and Australia. Br J Dermatol. 2001;144:339–46. doi: 10.1046/j.1365-2133.2001.03956.x. [DOI] [PubMed] [Google Scholar]
- 37.Oprica C, Löfmark S, Lund B, Edlund C, Emtestam L, Nord CE. Genetic basis of resistance in Propionibacterium acnes strains isolated from diverse types of infection in different European countries. Anaerobe. 2005;11:137–43. doi: 10.1016/j.anaerobe.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Kawada A, Wada T, Oiso N. Clinical effectiveness of once-daily levofloxacin for inflammatory acne with high concentrations in the lesions. J Dermatol. 2012;39:94–6. doi: 10.1111/j.1346-8138.2011.01216.x. [DOI] [PubMed] [Google Scholar]
- 39.Kawada A, Aragane Y, Tezuka T. Levofloxacin is effective for inflammatory acne and achieves high levels in the lesions: An open study. Dermatology. 2002;204:301–2. doi: 10.1159/000063365. [DOI] [PubMed] [Google Scholar]
- 40.Leyden JJ, Preston N, Osborn C, Gottschalk RW. In-vivo effectiveness of adapalene 0.1%/benzoyl peroxide 2.5% gel on antibiotic-sensitive and resistant Propionibacterium acnes. J Clin Aesthet Dermatol. 2011;4:22–6. [PMC free article] [PubMed] [Google Scholar]
- 41.Oprica C, Emtestam L, Hagströmer L, Nord CE. Clinical and microbiological comparisons of isotretinoin vs. tetracycline in acne vulgaris. Acta Derm Venereol. 2007;87:246–54. doi: 10.2340/00015555-0211. [DOI] [PubMed] [Google Scholar]
- 42.Coates P, Vyakrnam S, Ravenscroft JC, Stables GI, Cunliffe WJ, Leyden JJ, et al. Efficacy of oral isotretinoin in the control of skin and nasal colonization by antibiotic-resistant propionibacteria in patients with acne. Br J Dermatol. 2005;153:1126–36. doi: 10.1111/j.1365-2133.2005.06897.x. [DOI] [PubMed] [Google Scholar]
- 43.Fitz-Gibbon S, Tomida S, Chiu BH, Nguyen L, Du C, Liu M, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013;133:2152–60. doi: 10.1038/jid.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nast A, Dréno B, Bettoli V, Degitz K, Erdmann R, Finlay AY, et al. European evidence-based (S3) guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012;26(Suppl 1):1–29. doi: 10.1111/j.1468-3083.2011.04374.x. [DOI] [PubMed] [Google Scholar]
- 45.Thiboutot D. Dermatologists do not yet fully understand the clinical significance of antibiotic use and bacterial resistance in patients with acne: Comment on “Antibiotics, acne, and Staphylococcus aureus colonization. Arch Dermatol. 2011;147:921–2. doi: 10.1001/archdermatol.2011.201. [DOI] [PubMed] [Google Scholar]
- 46.Sardana K, Chugh S, Garg VK. The role of zinc in acne and prevention of resistance: Have we missed the “base” effect? Int J Dermatol. 2014;53:125–7. doi: 10.1111/ijd.12264. [DOI] [PubMed] [Google Scholar]
- 47.Sardana K, Gupta T, Garg VK, Ghunawat S. Antibiotic resistance to Propionobacterium acnes: Worldwide scenario, diagnosis and management. Expert Rev Anti Infect Ther. 2015;13:883–96. doi: 10.1586/14787210.2015.1040765. [DOI] [PubMed] [Google Scholar]


