Abstract
The bulk of the lens consists of tightly packed fiber cells. Because mature lens fibers lack mitochondria and other organelles, lens homeostasis relies on a monolayer of epithelial cells at the anterior surface. The detection of various signaling pathways in lens epithelial cells suggests they respond to stimuli that influence lens function. Focusing on Src Family Kinases (SFKs) and Transient Receptor Potential Vanilloid 4 (TRPV4), we tested whether the epithelium can sense and respond to an event that occurs in fiber mass. The pig lens was subjected to localized freeze-thaw (FT) damage to fibers at posterior pole then the lens was incubated for 1–10 min in Krebs solution at 37°C. Transient SFK activation in the epithelium was detectable at 1 min. Using a western blot approach, the ion channel TRPV4 was detected in the epithelium but was sparse or absent in fiber cells. Even though TRPV4 expression appears low at the actual site of FT damage to the fibers, SFK activation in the epithelium was suppressed in lenses subjected to FT damage then incubated with the TRPV4 antagonist HC067047 (10 μM). Na,K-ATPase activity was examined because previous studies report changes of Na,K-ATPase activity associated with SFK activation. Na,K-ATPase activity doubled in the epithelium removed from FT-damaged lenses and the response was prevented by HC067047 or the SFK inhibitor PP2 (10 μM). Similar changes were observed in response to fiber damage caused by injection of 5μl hyperosmotic NaCl or mannitol solution beneath the surface of the posterior pole. The findings point to a TRPV4-dependent mechanism that enables the epithelial cells to detect remote damage in the fiber mass and respond within minutes by activating SFK and increasing Na,K-ATPase activity. Because TRPV4 channels are mechanosensitive, we speculate they may be stimulated by swelling of the lens structure caused by damage to the fibers. Increased Na,K-ATPase activity gives the lens greater capacity to control ion concentrations in the fiber mass and the Na,K-ATPase response may reflect the critical contribution of the epithelium to lens ion homeostasis.
Keywords: Lens epithelium, Lens fiber, Na, K-ATPase activity, Remote sensing, Src family kinase, TRPV4 channel
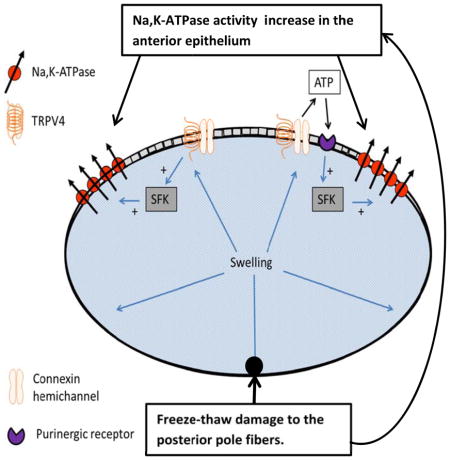
Graphical abstract
Localized damage to fibers at the lens posterior pole caused a rapid increase in Src family kinase (SFK) activation in the epithelium. Inhibition of a mechanosensitive ion channel TRPV4 channel prevented the SFK response. Localized fiber damage also caused a TRPV4- and SFK-dependent increase of Na,K-ATPase activity in the epithelium. Importantly, TRPV4 channel protein was determined to be sparse or absent in fibers although it is abundant in the epithelium. The findings point to a TRPV4-dependent mechanism that enables the epithelial cells to remotely detect damage in the fiber mass and respond within minutes by activating SFK and increasing Na,K-ATPase activity. Higher Na,K-ATPase activity would give the lens greater capacity to control ion concentrations in the fiber mass.
1. Introduction
The bulk of the mammalian lens consists of tightly packed fiber cells carefully arranged in a hexagonal array (Bassnett et al., 2011). The anterior surface of the fiber cell mass is covered by an epithelial monolayer and new fiber cells are formed by mitosis and elongation of cells at the periphery of the epithelial sheet. Fully differentiated mature lens fibers lack mitochondria, endoplasmic reticulum and nuclei. These unusual cellular specializations contribute to a low degree of light scattering but as a result of such differentiation, lens fibers are unable to function in isolation and their homeostasis relies on the anterior monolayer of epithelial cells. Metabolic substrates such as glucose and amino acids enter the lens and lactate is exported via transporters in the epithelium. The epithelium also plays a critical role in active sodium-potassium transport. Lens epithelial cells have a high Na,K-ATPase activity (Tamiya et al., 2003). In contrast, mature fiber cells that account for the bulk of the lens structure have negligible Na,K-ATPase activity (Delamere and Dean, 1993). Sodium and potassium homeostasis of the fiber mass, and thus water homeostasis, is supported to a large extent by Na,K-ATPase activity in epithelial cells at the anterior-equatorial lens surface (Gao et al., 2000).
The detection of ERK, Src, TGFβ, Wnt/β-catenin, Smad3 and other signaling pathways in lens epithelial cells points to the potential for these cells to respond to cues that influence lens function (Dawes et al., 2013; Leonard et al., 2013). External stimuli may take the form of growth factors, hormones, peptides such as endothelin-1 or muscarinic and purinergic agonists in the aqueous humor (Duncan and Collison, 2002; Sanderson et al., 2014). Here we consider the possibility that epithelial cells also are able to sense and respond to events that occur in lens fibers.
Src tyrosine kinase and related Src family kinases (SFKs) have been detected in the lens epithelium of several species (Tamiya and Delamere, 2005; Zhou et al., 2007). SFKs are expressed in most cells serve as points of control in a wide variety of signaling pathways (Ingley, 2008). In chick lens there is convincing evidence that c-Src promotes epithelial cell proliferation and the maintenance of an epithelial phenotype while Fyn, a different SFK, promotes lens fiber morphogenesis (Leonard et al., 2013). In porcine lens, SFK activation has been shown to increase Na,K-ATPase activity in the epithelium of lenses that are exposed to purinergic agonists (Tamiya et al., 2007) or hyposmotic solution (Shahidullah et al., 2012a). The present studies show evidence for rapid SFK activation and an increase of Na,K-ATPase activity in the epithelium in response to damage at a remote region of the lens fiber mass. Our data suggests that SFK response mechanism in the epithelium is dependent on activation of the TRPV4 ion channel. Studies by other investigators suggest TRPV4 activation is mechanosensitive (Luo et al., 2014). In a previous study TRPV4 was shown to be involved in the response of the lens to osmotic swelling (Shahidullah et al., 2012b).
2. Materials and Methods
2.1. Materials
Ouabain, HC067047, PP2, alamethicin and DMSO were purchased from Sigma, St. Louis, MO, USA. Other chemicals including those for preparing the Krebs solution were purchased from Sigma St. Louis, MO, USA. Rabbit anti TRPV4 polyclonal antibody was purchased from Abcam (Cambridge, MA). Rabbit polyclonal anti- phospho-Src-family (Tyr 416) kinase antibody and rabbit polyclonal anti-β-actin antibody were obtained from Cell Signaling Technology (Danvers, MA, USA). Mouse monoclonal anti-GAPDH monoclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz. CA, USA). Goat anti-rabbit secondary antibody conjugated with IRDye 680 and goat anti-mouse secondary antibody conjugated with IR Dye 800 were obtained from LICOR Biosciences (Lincoln, NE, USA).
2.2. Lenses
Porcine eyes were obtained from the University of Arizona meat Science Laboratory or from West Valley Processing Meat processors, Buckeye AZ or Hatfield Quality Meats (Philadelphia, PA. The use of porcine eye tissues was approved by the University of Arizona Institutional Animal Care and Use Committee and conformed to the ARVO Resolution for the Use of Animals in Ophthalmic and Vision Research. The posterior of the eye was dissected open and the zonules were cut, allowing the intact lens to be removed and transferred to Krebs solution that contained (in mM) 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 25 NaHCO3, 2.5 CaCl2, 1 MgCl2, and 5.5 glucose. Prior to use the Krebs solution was equilibrated with 5% CO2 for 30–40 min and adjusted to pH 7.4. Lenses were allowed to equilibrate in Krebs solution for 3h at 37°C and 5% CO2 in a humidified incubator prior to use.
2.3. Damage to the posterior surface of the lens
The freeze-thaw maneuver was carried out on lenses placed in a 24mm culture well and partially immersed in Krebs solution such that the posterior surface protruded above the solution surface. The posterior pole of the lens was touched for 10 sec with a 2.5 mm diameter stainless steel probe that had been cooled in liquid nitrogen, and then a Pasteur pipette was used to flush 37°C Krebs solution onto the probe to thaw, release and cover the lens with solution. In specified experiments, damage to a localized area of posterior fiber mass was achieved by injecting 5 μl of hyperosmotic (2400 mOsm) NaCl solution approximately 1.0 mm beneath the posterior capsule using a 30 gauge Hamilton syringe needle. Following the damage maneuver, the intact lens was incubated 1 – 10 min in Krebs solution. Then, the lens was removed from the Krebs solution and the epithelium was isolated from the fiber mass using fine forceps as described earlier (Shahidullah et al., 2012b). The epithelium samples were immediately snap frozen and stored at −80°C until further use.
2.4. Na,K-ATPase activity
The frozen epithelium-capsule from each lens was homogenized in 350 μl of an ice-cold double-strength ATPase assay buffer that contained (mM): L-Histidine, 80; NaCl 200; KCl, 10; MgCl2, 6.0; EGTA, 2.0 (pH 7.4) plus 10 μl/ml of a Protease Inhibitors Cocktail (Thermo Scientific, Rockford, IL). The homogenization protocol, which was carried out on ice, was 4 cycles of 15 sec at 5 sec intervals using a Misonix S3000 sonicator at a 6 Watt power setting (Misonix, New York, USA). The homogenate was centrifuged at 13,000 g for 30 min at 4°C to remove nuclei, larger mitochondria and unbroken debris. Then the supernatant was used to measure Na,K-ATPase activity. Protein in the supernatant was measured by the bicinchoninic acid (BCA) assay (Smith et al., 1985)(Pierce Biotechnology, Rockford, IL, USA), using bovine serum albumin as a standard.
Na,K-ATPase activity was measured using a modification of a method described earlier (Shahidullah and Delamere, 2006). In brief, 150 μl of the supernatant was placed in duplicate tubes containing 50 μl of double-strength ice-cold Na,K-ATPase buffer. To ensure access of ions and ATP to membrane vesicles, alamethicin (5 μl) was added to give a final approximate concentration of 0.1 mg alamethicin per mg of protein (Xie et al., 1989). Ouabain, a selective inhibitor of Na,K-ATPase (Wallick and Schwartz, 1988), was added to half the tubes (final concentration 300 μM). Previous studies (data not shown) indicate 300 μM ouabain causes maximal inhibition of porcine lens Na,K-ATPase. Remainder of the tubes received an equivalent volume of distilled water. An additional 150 μl of distilled water was added to each tube. The tubes were pre-incubated at 37°C for 5 min then concentrated ATP solution (40 μl) was added to each tube (final ATP concentration of 2.0 mM), bringing the total volume of the assay mixture to 400 μl and the concentration of the double-strength Na,K-ATPase buffer to single strength. The assay mixture was incubated in the dark at 37°C for 30 min with slow shaking. At the end of the incubation period, the ATP hydrolysis reaction was stopped by the addition of 150 μl of 15% ice-cold trichloroacetic acid and the tubes were placed on ice for 30 min with occasional mixing by shaking to encourage protein precipitation.
ATP hydrolysis was determined by measuring the amount of inorganic phosphate released in each reaction tube. The tubes were placed in a centrifuge at 3000 rpm for 15 min at 4°C then 400 μl of the supernatant was removed and mixed with 400 μl of 4.0% FeSO4 solution in ammonium molybdate (1.25 g of ammonium molybdate in 100 ml of 2.5N sulphuric acid). Standard solutions, which contained NaH2PO4 and were equivalent to 0, 62.5, 125, 250 and 500 nmoles of PO4−3, were treated similarly. The mixture was allowed to sit for 5 min at room temperature, and then 200 μl of each standard or sample was transferred to a 96-well plate and absorbance was measured at 750 nm using a Perkin Elmer plate reader (Victor V1420-040, Perkin Elmer, CT, USA). Na,K-ATPase activity was calculated as the difference between ATP hydrolysis in the presence and absence of ouabain and values are presented as nmoles ATP hydrolyzed per milligram protein per 30 min. Because Na,K-ATPase activity was variable between batches of eyes, data from different experiments were not pooled.
2.5. Western blot
The entire epithelium was isolated as described above or stainless steel trephines were used to harvest separate samples of central and equatorial epithelium and fibers from different regions. Using a modification of an approach described earlier(Delamere and Dean, 1993) lenses were frozen on a slurry of dry ice powder and 2-methyl-butane and cored using a 4 mm diameter trephine to isolate central epithelium, anterior surface fibers and posterior surface fibers. Samples of equatorial fibers and epithelium were collected from outside a 7 mm trephine core. Samples were placed in an Eppendorf tube containing ice-cold lysis buffer that contained (in mM) 50 HEPES, 150 NaCl, 1 EDTA, 10 sodium fluoride, 10 sodium pyrophosphate, 2 sodium orthovanadate, 10% glycerol, 1% Triton X-100, 1% sodium deoxycholate, protease inhibitor cocktail at manufacturer’s recommended concentration (Roche Applied Science, Mannheim, Germany) (pH of 7.5). Following homogenization for 1 min (4 strokes of 15 sec at 5 sec interval) using a Misonix S3000 sonicator at 6W power setting (Misonix, New York, USA) the mixture was centrifuged at 13,000g for 25 min at 4°C to remove nuclei, larger mitochondria and unbroken debris. The supernatant was subjected to western blot analysis and also protein measurement by the bicinchoninic acid assay (Smith et al., 1985). For western blot, proteins were separated by 7.5% SDS-PAGE electrophoresis and transferred to nitrocellulose membrane. The membrane was kept overnight at 4°C in blocking buffer (LI-COR, Lincoln, NE) then incubated overnight at 4°C with one or more of the following primary antibodies: rabbit anti TRPV4 polyclonal antibody (1 μg/ml), rabbit polyclonal anti- phospho-Src-family (Tyr 416) antibody (1:1,000); rabbit polyclonal anti-β-actin antibody (1:5,000) and mouse monoclonal anti- GAPDH monoclonal antibody (1:5,000). After three 5-min washes with a mixture of Tris-buffered saline and Tween 20, the nitrocellulose membrane was incubated at room temperature for 60 to 90 min with goat anti-rabbit secondary antibody conjugated with IRDye 680 or goat anti-mouse secondary antibody conjugated with IR Dye 800 (1:20,000) (LI-COR, Lincoln, NE). Then the membrane was washed three times with Tris-buffered saline + Tween 20 and three times with PBS. Bands were detected and quantified using an Odyssey infrared scanner (LI-COR, Lincoln, NE). By using two secondary antibodies that are detectable at different infra-red wavelengths, we were able to probe for β-actin simultaneously with either TRPV4 or pY416 SFK. This allowed TRPV4 or pY416 SFK band density to be normalized to β-actin band density in each lane. Different western blots commonly show some variability and in previous studies we have seen differences between batches of lenses. Slight differences between aliquots of primary or secondary antibody could also introduce variability. To minimize variability we designed experiments using control and treated lenses obtained from the same batch of eyes in experiments run together on the same day. Then, for western blot analysis, the samples from control and treated lenses were placed on adjacent lanes and processed with the same antibody and blocking solutions.
2.6. Statistical analysis
Results are expressed as the mean ± SEM of data from a specified number of independent experiments. Statistical comparisons were made by on- way analysis of variance followed by the Bonferroni post hoc multiple comparison test. To validate the homogeneity of variance assumption, we performed Levene’s test. A probability (p) value of <0.05 was considered significant.
3. Results
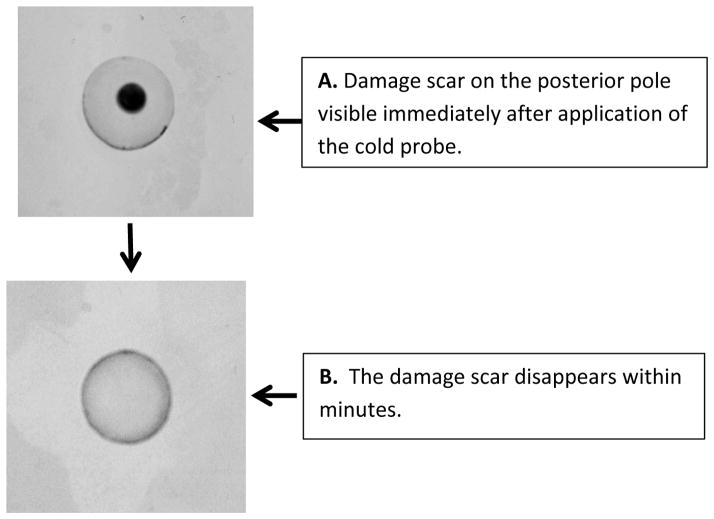
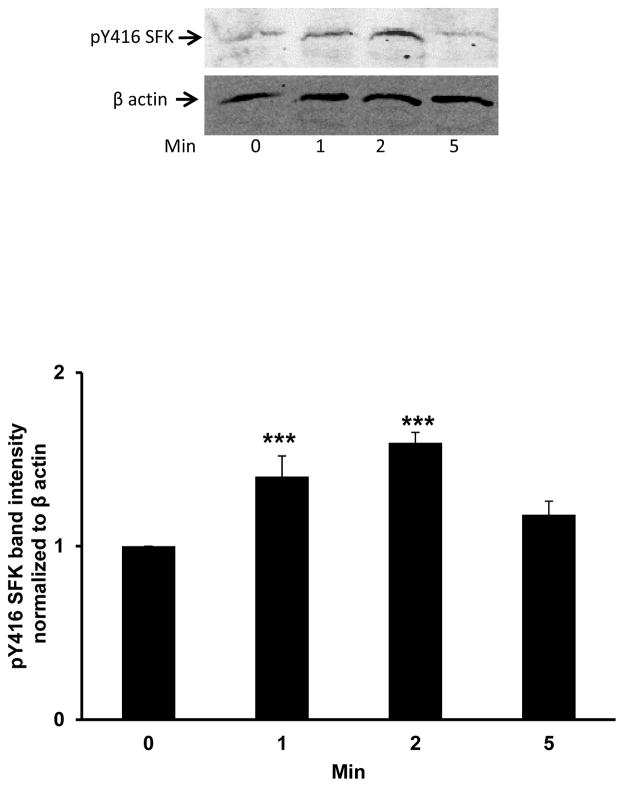
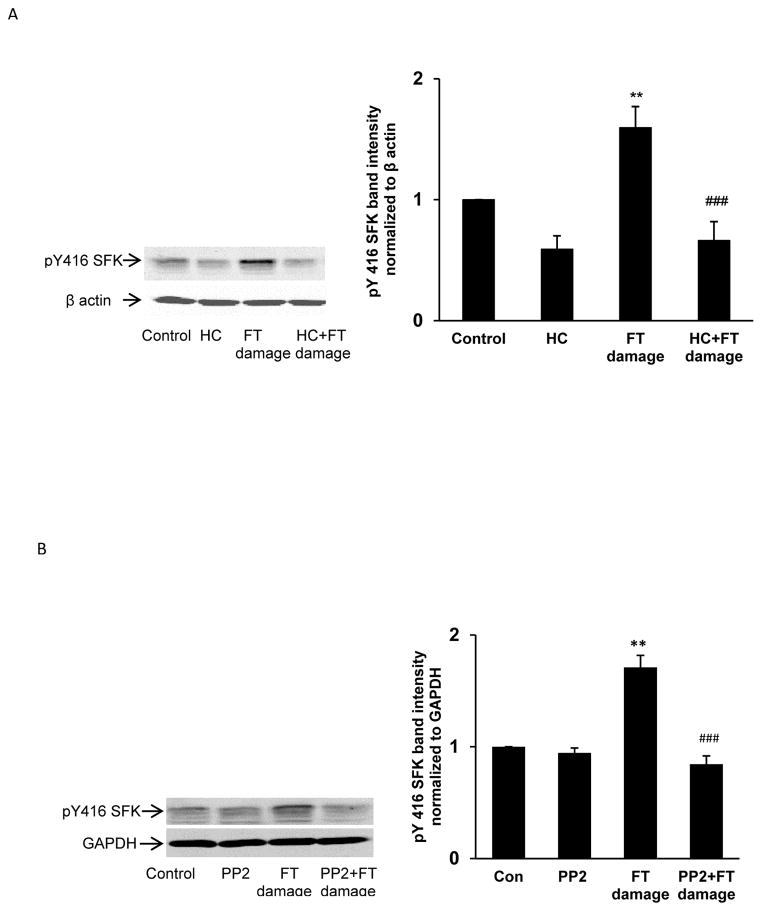
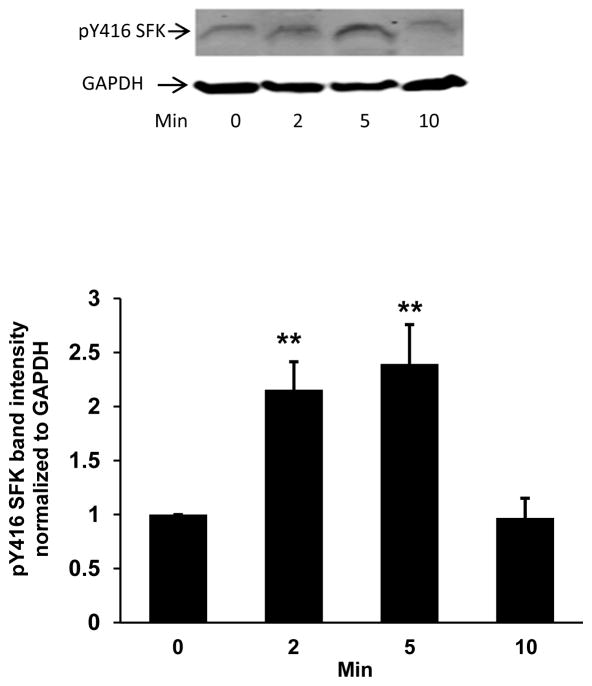
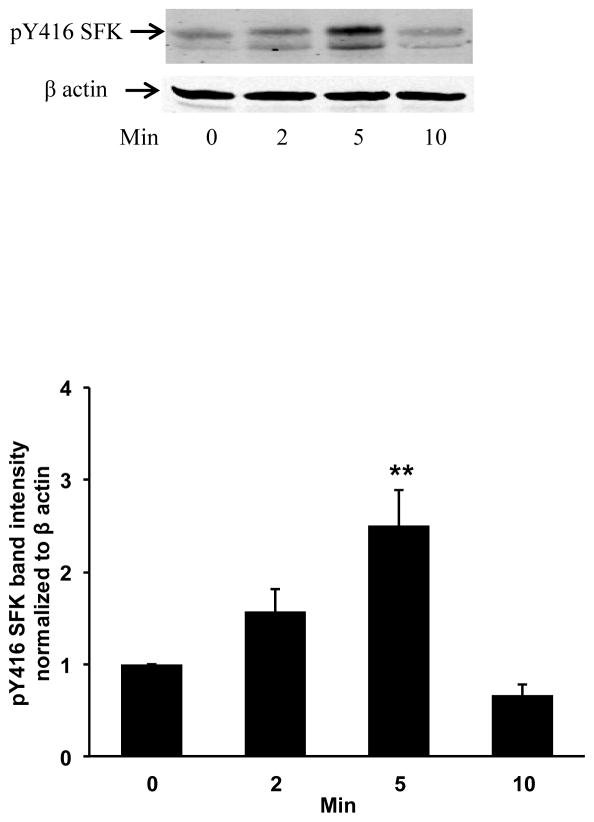
Porcine lenses were subjected to localized freeze-thaw damage to fibers at the posterior surface, and then incubated in Krebs solution at 37°C. Although the maneuver initially caused a localized opacity at the posterior surface, transparency was restored within a few minutes (Fig. 1). Src family tyrosine kinase (SFK) activation was examined in the epithelium removed from intact lenses that had been subjected to localized freeze-thaw damage then returned to control Krebs solution for 1 – 5 min. Western blot analysis showed a transient increase of activated SFK, which is phosphorylated at Y416. SFK activation peaked at 2 min (Fig. 2). SFK activation in the epithelium was suppressed when lenses were subjected to freeze-thaw damage and then incubated for 2 min in the presence of HC067047 (10 μM), a TRPV4 antagonist, or in the presence of an SFK inhibitor, PP2 (Fig. 3).
Fig. 1.
The freeze-thaw damage maneuver initially caused an area of opacity where the 2.5 mm diameter probe had touched the posterior surface of the lens (A). Transparency was restored within minutes (B).
Fig. 2.
Western blot showing the time course of Src family kinase (SFK) activation in the epithelium of lenses that had been subjected to freeze-thaw (FT) damage at the posterior pole then incubated in control Krebs solution for 1 – 5 min. The typical western blot shows pY416-SFK (upper) and β-actin (lower), which was used as a loading control. The bar graph shows densitometric analysis of pY416-SFK normalized to β-actin. The data are the mean ± SEM of results from 5 independent experiments. ***(p<0.001) indicates a significant difference compared to control.
Fig. 3.
The influence of TRPV4 antagonist HC067047 (panel A) and SFK inhibitor PP2 (Panel B) on SFK activation in the epithelium of lenses subjected to freeze-thaw (FT) damage. Lenses were preincubated with or without 10 μM HC067047 (20 min), or 10 μM PP2 (40 min), subjected to the FT maneuver, then incubated for a further 2 min in the continued presence or absence of HC067047 or PP2, respectively. Control lenses were not damaged. On the left of each panel the typical western blot shows pY416-SFK (upper) and β-actin or GAPDH (lower) which was used as loading control. The bar graphs (right on both panels) show densitometric analysis of pY416-SFK normalized to β-actin/GAPDH. The results are the mean ± SEM of data from 3 independent experiments. ** (p<0.01) indicates a significant difference compared to control and ### (p<0.001) indicates a significant difference compared to the FT-damage group.
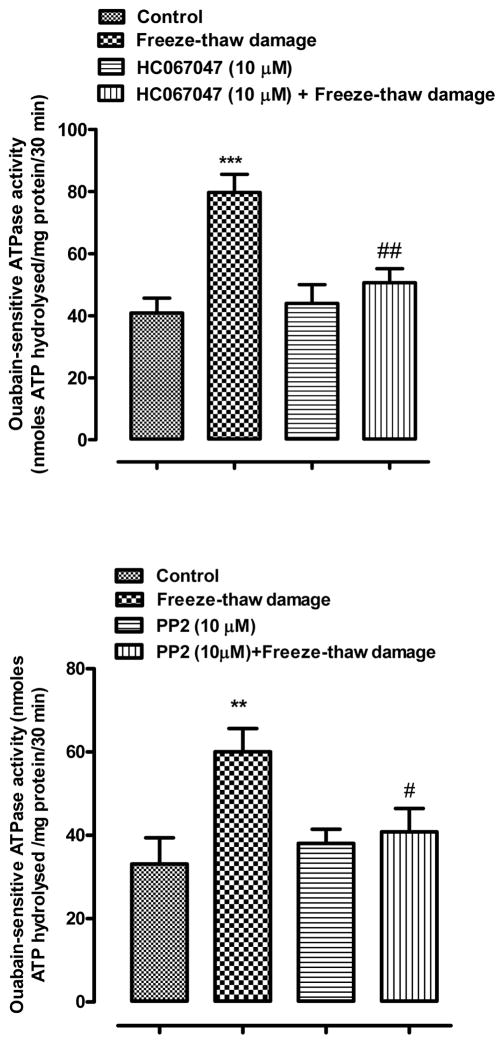
Na,K-ATPase activity was examined in the lens epithelium because previous studies have associated changes of Na,K-ATPase activity with SFK activation. Na,K-ATPase activity (ouabain sensitive ATP hydrolysis) was almost doubled in the epithelium removed from lenses subjected to freeze-thaw damage and then incubated for 2 min in control conditions (Fig. 4). In contrast, no significant increase in Na,K-ATPase activity was observed in damaged lenses that were exposed to the TRPV4 antagonist HC067047 (10 μM) (Fig. 4). Importantly, the damage-associated increase in Na,K-ATPase activity also did not occur in lenses that were incubated in the presence of the SFK inhibitor PP2 (10 μM) (Fig. 4).
Fig. 4.
The influence of PP2 (upper panel) and HC067047 (lower panel) on Na,K-ATPase activity in the epithelium of lenses subjected to freeze-thaw (FT) damage. Lenses were preincubated with or without 10 μM PP2 (40 min), or 10 μM HC067047 (20 min), subjected to the FT maneuver, then incubated for a further 2 min in the continued presence or absence of PP2 or HC067047. Control lenses were not damaged. The results are the mean ± SEM of data from 4–6 independent experiments. ** (p<0.01) and *** (p<0.001) indicate significant differences compared to the Control group, while # (p<0.05) and ## (p<0.01) indicate a significant difference compared to the FT group. Levene’s test show homoscedasticity of data for all groups in panel A (p=0.330) and panel B (p=0.558).
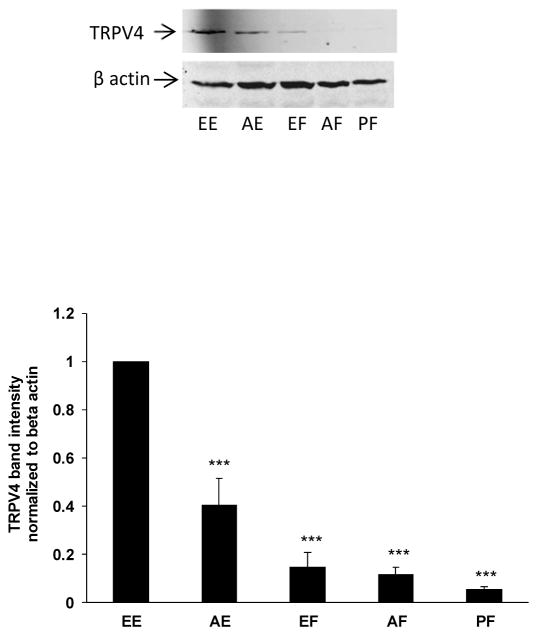
Western blot analysis was carried out to detect TRPV4 protein. TRPV4 band density was normalized to β-actin band density within each lane. The results indicated robust TRPV4 protein expression in the lens epithelium (Fig. 5). TRPV4 immunoreactive band density was higher at the equatorial region of the epithelium compared to the anterior pole. TRPV4 band density was low or undetectable in samples obtained from different regions of the lens fiber mass. A TRPV4 band was not observed in samples of lens fibers taken from the posterior pole region of the lens.
Fig. 5.
Western blot showing TRPV4 protein detected in different regions of the epithelium and cortical fiber mass. The different lens regions are: EE equatorial epithelium; AE anterior epithelium; EF equatorial fibers; AF anterior fibers; PF posterior fibers. The typical western blot shows TRPV4 (upper) and β-actin (lower), which was used as a loading control. The bar graph shows densitometric analysis of TRPV4 band density normalized to β-actin. The results are the mean ± SEM of data from 3 different lenses. ***(p<0.01) indicates a significant difference compared to band density observed in the equatorial epithelium.
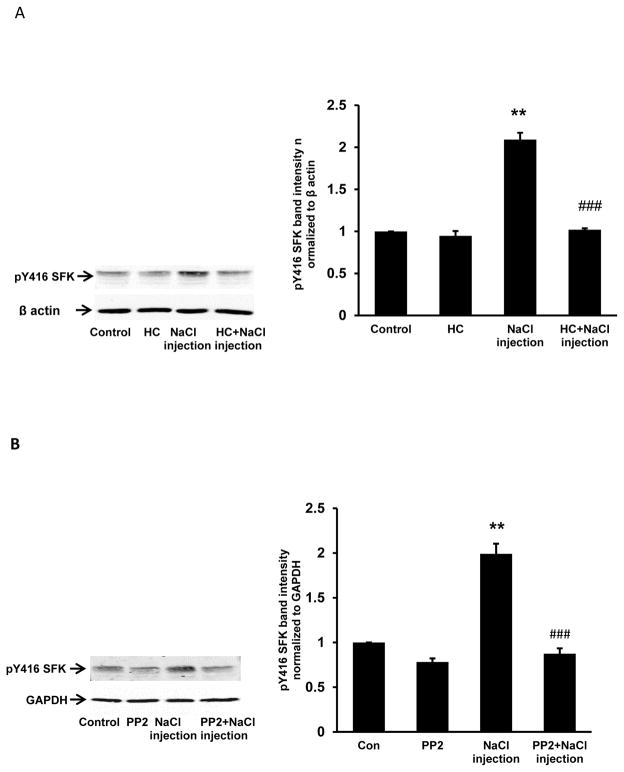
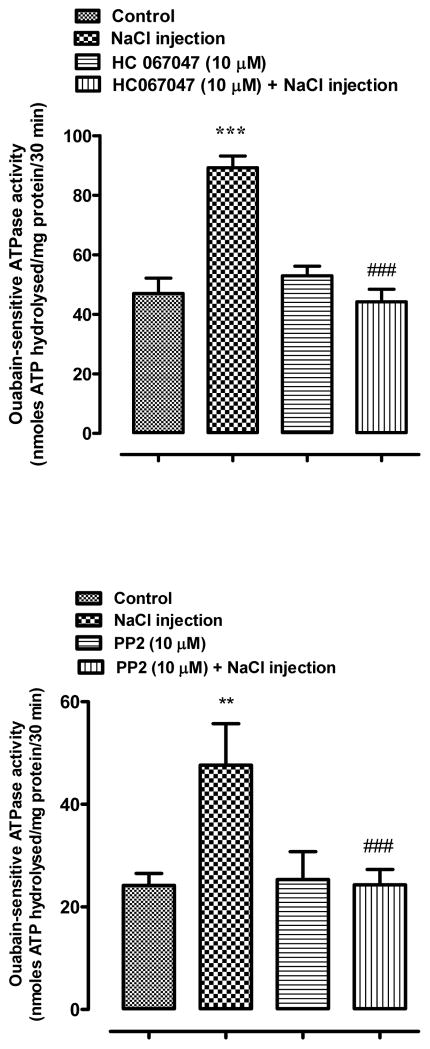
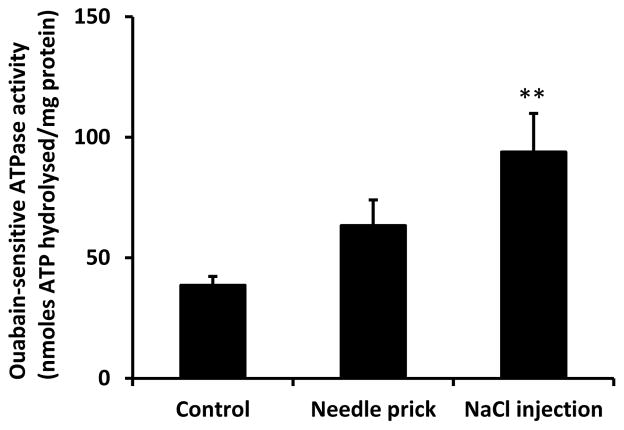
Studies were carried out to examine responses to fiber damage at the posterior pole caused by a different perturbation. Transient SFK activation was observed in the epithelium removed from lenses that had been incubated 1–10 min in control Krebs solution after being subjected to injection of 5 μl hyperosmotic NaCl solution into the fiber mass in order to cause osmotic swelling (Fig. 6). The NaCl solution was placed approximately 1 mm beneath the surface at the posterior pole. SFK activation was not observed when lenses subjected to this maneuver were incubated in the presence of the TRPV4 antagonist HC067047 or in the presence of an SFK inhibitor, PP2 (Fig. 7). When lenses subjected to NaCl injection were incubated in control Krebs solution for 5 min, a twofold increase of Na,K-ATPase activity was detected in the epithelium (Fig. 8). The Na,K-ATPase increase was not observed in lenses incubated in the presence of either HC067047 or SFK inhibitor PP2 (Fig. 8). Na,K-ATPase activity showed a tendency to increase but was not significantly altered in lenses subjected only to the needle puncture without NaCl injection (Fig. 9). If the damage response has a threshold, needle puncture alone may fall just below it. To consider whether the lens response was linked to an osmotic effect rather than a sodium ion concentration effect, some lenses were subjected to injection of 5 μl hyperosmotic mannitol solution into the fiber mass. Mannitol injection caused robust SFK activation (Fig. 10).
Fig. 6.
Western blot showing the time course of Src family kinase (SFK) activation in the epithelium of lenses incubated in control Krebs solution for 1 – 10 min after receiving 5 μl of hyperosmotic NaCl solution injected ~1 mm beneath the capsule of the posterior pole. The typical western blot shows pY416-SFK (upper) and GAPDH (lower), which was used as a loading control. The bar graph shows densitometric analysis of pY416-SFK normalized to GAPDH. The data are the mean ± SEM of results from 3 independent experiments. **(p<0.01) indicates a significant difference compared to band density at time zero (control).
Fig. 7.
The influence of HC067047 (panel A) or PP2 (panel B) on SFK activation in the epithelium of lenses that received 5 μl of hyperosmotic NaCl solution injected ~1 mm beneath the posterior pole. Lenses were preincubated with or without 10 μM HC067047 (20 min), or 10 μM PP2 (40 min), subjected to the injection maneuver, then incubated for a further 5 min in the continued presence or absence of HC067047 or PP2, respectively. Control lenses did not receive an injection. On the left of each panel the typical western blot shows pY416-SFK (upper) and β-actin (lower) which was used as loading control. The bar graphs show densitometric analysis of pY416-SFK normalized to β-actin. The results are the mean ± SEM of data from 3 independent experiments. ** (p<0.01) indicates a significant difference compared to control and ### (p<0.001) indicates a significant difference compared to the NaCl injection group.
Fig. 8.
The influence of HC067047 (upper panel) and PP2 (lower panel) on Na,K-ATPase activity in the epithelium of lenses that received 5 μl of hyperosmotic NaCl solution (2400 mOsm) injected ~1 mm beneath the capsule of the posterior pole. Lenses were preincubated with or without 10 μM HC067047 (20 min), or 10 μM PP2 (40 min), subjected to the 5 μl injection, then incubated for a further 5 min in the continued presence or absence of HC067047 or PP2. Control lenses did not receive an injection. The results are the mean ±} SEM of data from 3 independent experiments. ** (p<0.01) and *** (p<0.001) indicates significant differences compared to the Control group, while ### (p<0.01) indicates a significant difference compared to the NaCl injection group. Levene’s test show homoscedasticity of data for all groups in panel A (p=0.459) and panel B (p=0.218).
Fig. 9.
Na,K-ATPase activity in the epithelium of lenses incubated in Krebs solution for 5 min after receiving a 30 Ga needle prick alone or a needle prick and the injection 5 μl of hyperosmotic NaCl solution. The results are the mean ± SEM of date from 4–6 independent experiments. ** indicates a significant difference (p<0.01) compared to control. Levene’s test show homoscedasticity of data (p=0.468).
Fig. 10.
Western blot showing the time course of SFK activation in the epithelium of lenses incubated in Krebs solution for 5 min after receiving 5 μl of hyperosmotic mannitol solution (2400 mOsm) injected ~1 mm beneath the capsule of the posterior pole. The typical western blot shows pY416-SFK (upper) and β-actin (lower), which was used as a loading control. The bar graph shows densitometric analysis of pY416-SFK normalized to β-actin. The results are the mean ± SEM of data from 3 independent experiments. ** (p<0.01) indicates a significant difference compared to control (time zero).
4. Discussion
The lens epithelium was found capable of responding to damage that occurs in a distant region of the fiber cell mass. After freeze-thaw damage to the surface of the lens at the posterior pole, the epithelial cells displayed SFK activation that was detectable within 1 min. SFK activation in the epithelium also was observed in response to a different damage maneuver that involved needle puncture and deposit of 5 μl hyperosmotic solution, either NaCl or mannitol, beneath the surface of the posterior pole. SFKs play a role in a variety of cell signaling pathways and their activation occurs in response to diverse stimuli. SFKs are converted from an inactive to active state by phosphorylation and the transient nature of SFK activation observed in the lens epithelium was consistent with SFK responses observed in other tissues (Ingley, 2008). Importantly, the SFK response was associated with a major functional change in the epithelium since a doubling of Na,K-ATPase activity was observed following the damage maneuvers and was prevented by SFK inhibition with PP2. While the findings are consistent with a role for SFK, and PP2 indeed prevented Y416 phosphorylation of SFK, PP2 is understood to have non-specific effects (Brandvold et al., 2012) and we are unable to rule out off target effects on other protein kinases.
In the lens, as in all cells and tissues, active transport by Na,K-ATPase compensates for the passive inward leak of sodium ions and outward leak of potassium ions. Na,K-ATPase in the lens epithelium plays a particularly critical role in ion homeostasis of the entire lens structure (Gao et al., 2000) because fiber cells have little or no Na,K-ATPase activity (Delamere and Dean, 1993). On this basis, the observed doubling of Na,K-ATPase activity in the epithelium following the damage maneuver would increase the capacity of the lens to maintain ion homeostasis. Ion homeostasis has an osmotic impact on water homeostasis since failure to maintain appropriate cellular concentrations of sodium and potassium causes the fiber mass to accumulate water. The porcine lens is a large multicellular structure and the distance between fiber cells at the posterior pole and the epithelium monolayer at the anterior surface is considerable. Nevertheless, the SFK response points to the ability of the epithelium to detect and respond to a perturbation event that occurs at a remote region of the fiber mass. Even though the damage was restricted to a region of fiber mass close to the lens surface at the posterior pole, it is feasible that a signaling molecule originating from damaged cells, or damage-induced changes in calcium or ATP, could spread throughout the lens cortex and thus to the epithelium via diffusion from cell-to-cell between coupled fibers as well as along the length of individual fiber cells, each of which extends from the posterior to the anterior suture. However, the required diffusion time would be too long to explain a response in epithelium that is detectable at 1 min. We also considered the possibility that because cells throughout the fiber mass as well as the epithelium are functionally coupled (Bassnett et al., 1994; Beyer and Berthoud, 2014; Mathias et al., 2007), depolarization caused by damage at the posterior pole might have an immediate influence on the remainder of the lens. However, a depolarization-mediated response is not consistent with the ability of HC067047 to prevent SFK response. Instead, the findings point to a critical role for TRPV4 ion channels.
TRPV4 channels, which are observed in a variety of cells, respond to various mechanical stimuli and are recognized for their response to osmotic cell swelling (Liedtke, 2005a). Although TRPV4 ion channels are poorly selective between different cations, there is evidence that many TRPV4-dependent responses are linked to calcium entry that occurs when the channels open (Tamiya et al., 2003). Interestingly, in cultured lens epithelial cells TRPV4 activation causes a particularly robust and long-lasting pattern of calcium entry that appears to occur as the result of connexin hemichannel opening (Mandal et al., 2015) An apparent linkage between TRPV4 channels and SFK activation in the intact porcine lens has been reported previously in a study in which TRPV4 antagonists HC067047 and RN1734 both were found to prevent SFK activation that takes place in the epithelium of lenses that are exposed to hyposmotic conditions (Shahidullah et al., 2012b). In those experiments the lens was fully immersed in hyposmotic solution and as a consequence it was not possible to distinguish the direct swelling response of the epithelial cells to hyposmotic solution from a possible mechanosensitive response to osmotic swelling of the entire lens structure. In the same studies, SFK activation was detected in the epithelium of lenses exposed to the TRPV4 agonist GSK1016790A.
Even though the epithelial SFK activation response to fiber damage was suppressed by a TRPV4 antagonist, indicating TRPV4-dependence, Western blot analysis revealed scarcely detectable TRPV4 protein expression in the posterior pole region of the lens that was damaged. TRPV4 abundance in the epithelium was robust compared to lens fibers. This raises the interesting possibility that damage to fibers might cause TRPV4 activation in the epithelium. TRPV4 channel opening is reported to occur in response to mechanical stimuli such as shear stress, cell swelling and movement of the primary cilium (Köttgen et al., 2008; Liedtke, 2005b; Luo et al., 2014). By disrupting fiber cell integrity, the freeze-thaw damage maneuver used in these studies would be expected to lead to water entry and localized swelling, as would deposit of 5 μl hyperosmotic NaCl solution. Consistent with an osmotic swelling response, 5 μl hyperosmotic NaCl and mannitol solution injected at the posterior pole produced similar SFK activation. We speculate that swelling of the lens structure caused by damage to the fiber mass might activate mechanosensitive TRPV4 channels in the epithelium. This notion is difficult to test experimentally because TRPV4-associated SFK activation occurred within minutes and there is no way to measure small changes in lens volume that might occur over such a short time span. Lenses subjected to damage then incubated for 30 min showed no detectable weight increase (data not shown). While the present studies are based on experimental maneuvers to disrupt tissue integrity and increase ion permeability, it has long been understood that cortical cataract is associated with increased ion permeability and water accumulation (Lucas et al., 1986) and even normal human lenses become detectably more cation permeable with age (Duncan et al., 1989). Pathophysiological conditions like exposure to ionizing irradiation also increase lens ion permeability and cause swelling (Lambert and Kinoshita, 1967). In subjects with poorly controlled diabetes, the accumulation of sugar alcohol in the lens causes osmotic swelling and ion imbalance (Bron et al., 1993).
Most lens fiber cells have low Na,K-ATPase activity, the exception being fibers close to the surface at the lens equator (Delamere and Dean, 1993). In comparison, the epithelium has a high Na,K-ATPase activity (Tamiya et al., 2003) and the epithelial monolayer plays a critical role in maintenance of steady-state sodium-potassium concentrations of cells in the fiber mass that constitutes the bulk of the lens (Gao et al., 2011; Mathias et al., 2007). SFK expression in the lens epithelium has been described previously (Tamiya and Delamere, 2005) and there have been several reports of changes of Na,K-ATPase activity associated with SFK activation (Mandal et al., 2011; Tamiya and Delamere, 2005; Tamiya et al., 2007). Here, we report SFK-dependent doubling of Na,K-ATPase activity in the epithelium that occurs within minutes of damage to fibers at the posterior pole. TRPV4 channels are close to absent in the fibers and this makes it noteworthy that the SFK and Na,K-ATPase responses to fiber damage can be prevented by a TRPV4 antagonist HC067047. The findings point to a TRPV4 response in the epithelium where TRPV4 is abundant. Importantly, the increase of Na,K-ATPase activity is completely eliminated by HC067047, making it unlikely that an alternate mechanism of Na,K-ATPase activation operates to a significant degree. Although the epithelial cells were far removed from the point of damage, the Na,K-ATPase activity response may reflect the critical contribution of the epithelium to sodium-potassium homeostasis for the entire lens.
Highlights.
Damage to posterior pole fibers activates Src family kinase in the epithelium.
Src family kinase activation increases Na,K-ATPase activity in the epithelium.
The SFK and Na,K-ATPase responses are TRPV4-dependent.
TRPV4 is sparse or absent in the posterior fibers but abundant in the epithelium.
Using TRPV4, the epithelium remotely detects and responds to damage in the fibers.
Acknowledgments
Funding: This research was supported by a grant from the National Institute of Health, Grant number: NIH EY009532.
The authors thankful to the University of Arizona Meat Science Laboratory, West Valley Meat Processing, Buckeye, Phoenix and Hatfield Quality Meat, Pennsylvania for supplying porcine eyes. This research was supported by a grant from the National Institute of Health (Grant: NIH EY009532).
Footnotes
Conflict of interest
The authors do not have any conflict of interest to declare.
Authorship Contributions
Research concept and design: Mohammad Shahidullah and Nicholas A Delamere.
Conducted experiments: Amritlal Mandal and Mohammad Shahidullah.
Data analysis and interpretation: Mohammad Shahidullah and Amritlal Mandal.
Wrote manuscript: Mohammad Shahidullah and Nicholas A Delamere.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bassnett S, Kuszak JR, Reinisch L, Brown HG, Beebe DC. Intercellular communication between epithelial and fiber cells of the eye lens. Journal of Cell Science. 1994;107:799–811. doi: 10.1242/jcs.107.4.799. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Shi Y, Vrensen GF. Biological glass: structural determinants of eye lens transparency. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2011;366:1250–1264. doi: 10.1098/rstb.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer EC, Berthoud VM. Connexin hemichannels in the lens. Frontiers in Physiology. 2014;5:20. doi: 10.3389/fphys.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandvold KR, Steffey ME, Fox CC, Soellner MB. Development of a Highly Selective c-Src Kinase Inhibitor. ACS Chemical Biology. 2012;7:1393–1398. doi: 10.1021/cb300172e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron AJ, Sparrow J, Brown NA, Harding JJ, Blakytny R. The lens in diabetes. Eye. 1993;7:260–275. doi: 10.1038/eye.1993.60. [DOI] [PubMed] [Google Scholar]
- Dawes LJ, Sugiyama Y, Tanedo AS, Lovicu FJ, McAvoy JW. Wnt-frizzled signaling is part of an FGF-induced cascade that promotes lens fiber differentiation. Invest Ophthalmol Vis Sci. 2013;54:1582–1590. doi: 10.1167/iovs.12-11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamere NA, Dean WL. Distribution of lens sodium-potassium-adenosine triphosphatase. Invest Ophthalmol Vis Sci. 1993;34:2159–2163. [PubMed] [Google Scholar]
- Duncan G, Collison DJ. Calcium signalling in ocular tissues: functional activity of G-protein and tyrosine-kinase coupled receptors. Exp Eye Res. 2002;75:377–389. [PubMed] [Google Scholar]
- Duncan G, Hightower KR, Gandolfi SA, Tomlinson J, Maraini G. Human lens membrane cation permeability increases with age. Investigative Ophthalmology & Visual Science. 1989;30:1855–1859. [PubMed] [Google Scholar]
- Gao J, Sun X, Moore LC, White TW, Brink PR, Mathias RT. Lens intracellular hydrostatic pressure is generated by the circulation of sodium and modulated by gap junction coupling. The Journal of general physiology. 2011;137:507–520. doi: 10.1085/jgp.201010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Sun X, Yatsula V, Wymore RS, Mathias RT. Isoform-specific function and distribution of Na/K pumps in the frog lens epithelium. Journal of Membrane Biology. 2000;178:89–101. doi: 10.1007/s002320010017. [DOI] [PubMed] [Google Scholar]
- Ingley E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochimica et biophysica acta. 2008;1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Köttgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. The Journal of Cell Biology. 2008;182:437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert BW, Kinoshita JH. The effects of ionizing irradiation on lens cation permeability, transport, and hydration. Investigative Ophthalmology. 1967;6:624–634. [PubMed] [Google Scholar]
- Leonard M, Zhang L, Bleaken BM, Menko AS. Distinct roles for N-Cadherin linked c-Src and fyn kinases in lens development. Developmental dynamics : an official publication of the American Association of Anatomists. 2013;242:469–484. doi: 10.1002/dvdy.23935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W. TRPV4 as osmosensor: a transgenic approach. Pflugers Archiv : European journal of physiology. 2005a;451:176–180. doi: 10.1007/s00424-005-1449-8. [DOI] [PubMed] [Google Scholar]
- Liedtke W. TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. The Journal of physiology. 2005b;567:53–58. doi: 10.1113/jphysiol.2005.088963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas VA, Duncan G, Davies P. Membrane permeability characteristics of perfused human senile cataractous lenses. Experimental Eye Research. 1986;42:151–165. doi: 10.1016/0014-4835(86)90039-4. [DOI] [PubMed] [Google Scholar]
- Luo N, Conwell MD, Chen X, Kettenhofen CI, Westlake CJ, Cantor LB, Wells CD, Weinreb RN, Corson TW, Spandau DF, Joos KM, Iomini C, Obukhov AG, Sun Y. Primary cilia signaling mediates intraocular pressure sensation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:12871–12876. doi: 10.1073/pnas.1323292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal A, Shahidullah M, Beimgraben C, Delamere NA. The effect of endothelin-1 on Src-family tyrosine kinases and Na,K-ATPase activity in porcine lens epithelium. Journal of cellular physiology. 2011;226:2555–2561. doi: 10.1002/jcp.22602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal A, Shahidullah M, Delamere NA. Calcium entry via connexin hemichannels in lens epithelium. Exp Eye Res. 2015;132:52–58. doi: 10.1016/j.exer.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias RT, Kistler J, Donaldson P. The lens circulation. The Journal of membrane biology. 2007;216:1–16. doi: 10.1007/s00232-007-9019-y. [DOI] [PubMed] [Google Scholar]
- Sanderson J, Dartt DA, Trinkaus-Randall V, Pintor J, Civan MM, Delamere NA, Fletcher EL, Salt TE, Grosche A, Mitchell CH. Purines in the eye: recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Muller cells, lens, trabecular meshwork, cornea and lacrimal gland. Exp Eye Res. 2014;127:270–279. doi: 10.1016/j.exer.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidullah M, Delamere NA. NO donors inhibit Na,K-ATPase activity by a protein kinase G-dependent mechanism in the nonpigmented ciliary epithelium of the porcine eye. Br J Pharmacol. 2006;148:871–880. doi: 10.1038/sj.bjp.0706795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Beimgraben C, Delamere NA. Hyposmotic stress causes ATP release and stimulates Na,K-ATPase activity in porcine lens. Journal of cellular physiology. 2012a;227:1428–1437. doi: 10.1002/jcp.22858. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Delamere NA. TRPV4 in porcine lens epithelium regulates hemichannel-mediated ATP release and Na-K-ATPase activity. American Journal of Physiology - Cell Physiology. 2012b;302:C1751–1761. doi: 10.1152/ajpcell.00010.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Analytical Biochemistry. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tamiya S, Dean WL, Paterson CA, Delamere NA. Regional distribution of Na,K-ATPase activity in porcine lens epithelium. Invest Ophthalmol Vis Sci. 2003;44:4395–4399. doi: 10.1167/iovs.03-0287. [DOI] [PubMed] [Google Scholar]
- Tamiya S, Delamere NA. Studies of tyrosine phosphorylation and Src family tyrosine kinases in the lens epithelium. Invest Ophthalmol Vis Sci. 2005;46:2076–2081. doi: 10.1167/iovs.04-1199. [DOI] [PubMed] [Google Scholar]
- Tamiya S, Okafor MC, Delamere NA. Purinergic agonists stimulate lens Na-K-ATPase-mediated transport via a Src tyrosine kinase-dependent pathway. American journal of physiology Cell physiology. 2007;293:C790–796. doi: 10.1152/ajpcell.00579.2006. [DOI] [PubMed] [Google Scholar]
- Wallick ET, Schwartz A. Interaction of cardiac glycosides with Na+,K+-ATPase. Methods in Enzymology. 1988;156:201–213. doi: 10.1016/0076-6879(88)56022-6. [DOI] [PubMed] [Google Scholar]
- Xie ZJ, Wang YH, Ganjeizadeh M, McGee R, Jr, Askari A. Determination of total (Na+ + K+)-ATPase activity of isolated or cultured cells. Analytical Biochemistry. 1989;183:215–219. doi: 10.1016/0003-2697(89)90470-3. [DOI] [PubMed] [Google Scholar]
- Zhou J, Leonard M, Van Bockstaele E, Menko AS. Mechanism of Src kinase induction of cortical cataract following exposure to stress: destabilization of cell-cell junctions. Mol Vis. 2007;13:1298–1310. [PubMed] [Google Scholar]