Significance
Drosophila is a popular model system for the study of olfaction. However, the physiological properties of its olfactory sensory neurons, both intrinsic and responsive, remain unclear. We have succeeded, for the first time, in patch-clamp recording from targeted Drosophila OSNs, revealing the distinct signaling of odorant receptors (Ors) and ionotropic receptors (Irs). We found that Ir-driven receptor currents did not adapt, whereas Or responses strongly adapted. Surprisingly, although Or adaptation increased odor sensitivity at high odor backgrounds, it reduced odor sensitivity at low backgrounds. Adaptation permeates all senses, and the finding of dynamic gain control by adaptation in Drosophila Or-expressing OSNs sheds light on the understanding of adaptation in other sensory systems.
Keywords: Drosophila, olfaction, chemoreceptor, sensory adaptation, OSN
Abstract
In Drosophila, olfactory sensory neurons (OSNs) rely primarily on two types of chemoreceptors, odorant receptors (Ors) and ionotropic receptors (Irs), to convert odor stimuli into neural activity. The cellular signaling of these receptors in their native OSNs remains unclear because of the difficulty of obtaining intracellular recordings from Drosophila OSNs. Here, we developed an antennal preparation that enabled the first recordings (to our knowledge) from targeted Drosophila OSNs through a patch-clamp technique. We found that brief odor pulses triggered graded inward receptor currents with distinct response kinetics and current–voltage relationships between Or- and Ir-driven responses. When stimulated with long-step odors, the receptor current of Ir-expressing OSNs did not adapt. In contrast, Or-expressing OSNs showed a strong Ca2+-dependent adaptation. The adaptation-induced changes in odor sensitivity obeyed the Weber–Fechner relation; however, surprisingly, the incremental sensitivity was reduced at low odor backgrounds but increased at high odor backgrounds. Our model for odor adaptation revealed two opposing effects of adaptation, desensitization and prevention of saturation, in dynamically adjusting odor sensitivity and extending the sensory operating range.
From insects to mammals, the sense of smell begins with odor detection by olfactory sensory neurons (OSNs) (1–6). Recently, rapid advances have been made in understanding chemoreceptors in Drosophila OSNs (7–9). To date, Drosophila is the only model organism for which odor selectivity is known for most of its odorant receptors (Ors) (10, 11), and an Or expression pattern has been mapped to OSNs (12, 13). In addition, another family of chemoreceptors called ionotropic receptors (Irs) has been identified and characterized (14–16). These two types of chemoreceptors respond to different odors, thus endowing Drosophila OSNs with unique and complementary properties for odor detection (17). In contrast to the advanced molecular understanding of these two types of chemoreceptors, the mechanisms of their cellular signaling in native OSNs remain unclear, particularly hampered by the technical difficulty of carrying out patch-clamp recordings of Drosophila OSNs.
Drosophila OSNs are encased in hair-like sensilla in the antennae and maxillary palps, with each sensillum containing the dendrites of one to four OSNs that are wrapped by sheath cells (18). The responses of native Drosophila OSNs to odors have traditionally been measured by electroantennogram (EAG) (19), which extracellularly measures the potentials across the entire antenna. In addition, single-sensillum recording (SSR) was developed to provide a higher spatial resolution by measuring the local field potentials (LFPs) from a single sensillum (20–24). These methods, especially SSR, have greatly advanced understanding of the odor selectivity of both Ors and Irs (10, 11, 14). However, because sheath cells and other OSNs also contribute to EAG and SSR signals (25), the response characteristics obtained by such measurements are often contaminated. Patch-clamp recordings of single OSNs could ideally overcome this issue while facilitating the experimental manipulations of a cell’s membrane potential; however, this standard method has unfortunately not yet been routinely applied to Drosophila OSNs.
Here, we developed a Drosophila antennal preparation and succeeded in performing patch-clamp recordings of single identified OSNs. By using a fast solution change system to deliver liquid-phase odor stimuli, we investigated the response properties of odor-induced receptor currents of Drosophila OSNs. We found that OSNs expressing Ors exhibited slow response kinetics, outward receptor current rectification, and strong adaptation to odors. We further demonstrated that this adaptation was produced by a Ca2+ influx into OSNs because it could be eliminated by voltage clamping at positive holding potentials, by removing extracellular Ca2+, or by removing internal free Ca2+ with a Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA). Importantly, in contrast to the long-held view that adaptation simply increases sensitivity, we found that Or-mediated adaptation selectively reduced odor-signaling gain at low odor backgrounds but increased the gain at high odor backgrounds, thereby extending the dynamic odor-operating range. In contrast, odor-induced receptor currents in Ir-expressing OSNs showed fast response kinetics and, surprisingly, did not adapt.
Results
Patch-Clamp Recordings of Odor Responses in Or-Expressing OSNs.
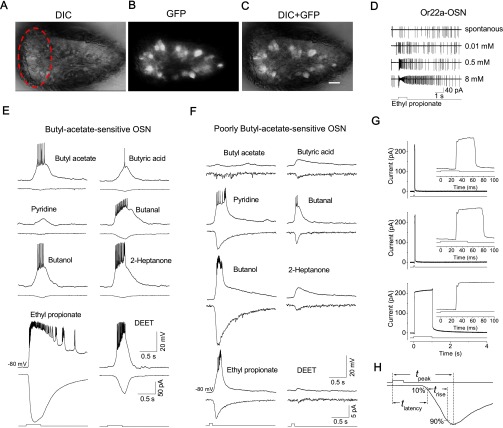
Drosophila OSNs are bipolar neurons, each with a single chemosensitive dendrite protruding into a small, hair-like sensillum covered by a cuticle. In the antenna, ∼80% of the total 1,200 OSNs are Or-expressing OSNs that coexpress Orco and odorant-binding Ors (12, 13, 26), the former of which is a coreceptor present in all Or-expressing OSNs (26). We have succeeded in developing an antennal-slice preparation (Fig. 1A and Materials and Methods) that allows perforated-patch recording of a genetically labeled OSN. OSNs were identified by expressing mCD8-GFP via a binary Gal4/UAS expression system in Orco-Gal4 flies (Fig. S1 A–C). With a stepper-driven, rapid solution-switching system to translate the interface between two solution streams across the recorded cell (27), we were able to apply brief and precise odor pulses to the recorded OSNs (Fig. 1B). The OSNs appeared healthy and remained healthy with a stable membrane potential for up to 2 h. Under cell-attached recordings, the OSNs showed a strong firing ability similar to that observed for the in vivo SSR preparation (Fig. S1D). For the odor stimulus, we typically used butyl acetate, a common fruit odor that activates many Ors (11). We found that most Or-expressing OSNs in the dorsomedial part of the antenna were butyl acetate responsive (Fig. S1E), whereas others were poorly responsive to butyl acetate (Fig. S1F). In the absence of the Orco protein (26), we found that these OSNs lost their odor responses completely.
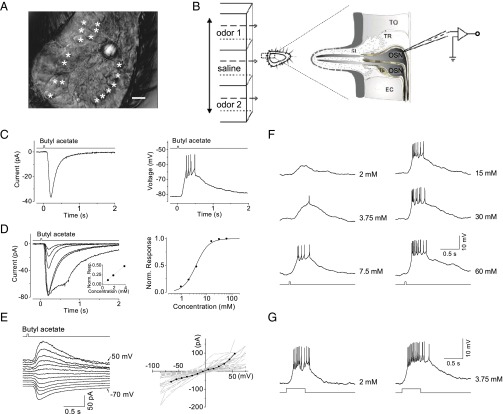
Fig. 1.
Odor responses of Or-expressing OSNs. (A) Infrared–differential interference contrast (IR-DIC) image of an antennal slice. The third segment of an antenna was transversely cut into three slices (Materials and Methods). The antennal slice was stabilized on the recording chamber, with one cutting end facing up and all sensilla pointing horizontally. The OSN cell bodies, marked by asterisks, are exposed. (Scale bar: 5 µm.) (B) Odor stimulation and recording arrangement. A three-barrel array, moved horizontally by a stepper, is placed ∼50 µm away from the antennal slice, with the middle barrel containing Drosophila saline and the flanking barrels containing odor solutions. The expanded diagram on the right illustrates a patch-clamp recording of an OSN. EC, epithelium cell; SL, sensillum lymph; TE, thecogen cell; TO, tomorgen cell; TR, trichogen cell. (C, Left) Inward receptor current recorded under voltage clamping (−82 mV) and a 35-ms pulse of 4 mM butyl acetate. (Right) The same response as that on the Left but under current clamping, showing action potential firing. (D, Left) A family of superimposed responses to a 35-ms pulse of butyl acetate at 1, 2, 4, 15, 30, and 60 mM. This is the same cell as that shown in C. Each trace is the average of three to five trials. (Right) The normalized dose–response relationship of the response family on the Left. The curve is the Hill equation, R/Rmax = Cm/(Cm + K1/2m), where R is the response amplitude at the transient peak, Rmax is the maximum (i.e., saturated) response, C is the odorant concentration, K1/2 is the odor concentration that half-saturates the response, and m is the Hill coefficient. In this experiment, K1/2 = 3.7 mM, m = 1.7. The Inset shows that the relationship is actually linear at its foot. (E, Left) The current–voltage relationship of the butyl acetate-elicited response. The cell was voltage clamped at −70 mV and then stepped to the indicated voltages in 10-mV increments. Odor stimulation: 35-ms pulses of 15 mM butyl acetate. (Right) Collective relationships of 34 Or-expressing OSNs indicate moderate outward rectification, with a reversal potential of −9 ± 13 mV (mean ± SD). Black dots (interconnected by straight lines) correspond to data from the OSN on the Left. (F) Dose dependence of spike firing. The traces show the membrane depolarization (current clamped) induced by brief (35-ms) pulses of butyl acetate at concentrations of 2, 3.75, 7.5, 15, 30, and 60 mM. The resting membrane potential was −73 mV. (G) Temporal integration of the odor response. The same cell produces no spike firing in response to the 35-ms, 2 mM butyl acetate stimulus in F but fires robustly in response to the 500-ms, 2 mM butyl acetate and fires even more robustly to 500-ms, 3.75 mM butyl acetate. In each panel, the top trace (C–E) and bottom trace (F and G) indicate the timing of the voltage trigger controlling the solution change (see Fig. S1 legend for details). C and D from the same OSN; E from another OSN; and F and G from yet another OSN.
Fig. S1.
Identification of Or-expressing OSNs and the measurement of response kinetics. (A) DIC image of an antennal slice from a fly with GFP expression in Or-expressing OSNs. The dorsomedial part of the antenna is marked with a dashed ellipse. (B) Fluorescent image of GFP-labeled OSNs. (C) Overlay of the DIC and fluorescent images. (Scale bar: 5 µm.) (D) Spike responses of an Or22a-expressing OSN. Under cell-attached recordings, the recorded Or22a-OSN showed spontaneous firing at a frequency of 4 Hz and responded to ethyl propionate in a dose-dependent manner. The maximal firing rate was about 160 Hz, which was close to that reported for the in vivo SSR preparation (10, 11). (E) A butyl acetate-sensitive OSN. In each pair of traces, the top trace shows the voltage response (current clamped) and the bottom trace shows the receptor current (voltage clamped at −80 mV), elicited by the indicated odors. The bottom trace indicates the timing of the voltage trigger controlling the solution change. Each odor was applied at 3 mM for 400 ms. (F) A poorly butyl acetate-sensitive OSN. Each odor was applied at 3 mM for 100 ms. (G) Measurement of odor arrival and departure. A patch-clamp electrode filled with intracellular solution was positioned with its tip ∼5 µm above the sensillum. Upon switching the perfusion saline to fourfold diluted (i.e., 25%) saline, a large outward liquid-junction current was detected by the patch-clamp electrode. This junction current indicates the true time course of solution change at the location of the sensillum and, thus, the time course of odor delivery to a recorded OSN in a real experiment—versus the timing of the voltage trigger controlling the solution change indicated by the trace below. As shown, application of a 35-ms (Top), 50-ms (Middle), or 1-s pulse (Bottom) of diluted saline elicits a junction current of corresponding duration. The Inset in each panel shows the same recording on an expanded timescale, low-pass filtered at 2 kHz, and sampled at 5 kHz. The time delay between the junction current and voltage trigger is 28 ms. The rise time of the junction current (10–90%) is 1 ms. For voltage trigger pulses of 35 ms, 50 ms, and 1 s, the time width of the junction-current profile at half-height is 36, 51, and 1,002 ms, respectively, thus indicating the timing of odor arrival and departure. (H) Measurement of response kinetics parameters. The top trace shows the symbolic timings of odor arrival and departure at the OSN dendrite based on the junction-current measurements in G together with an additional 5-ms delay for odor penetration into and diffusion in a sensillum. tlatency, response latency, i.e., the time duration from odor arrival to 10% of the response peak; tpeak, time to peak, i.e., time duration from odor arrival to the transient peak of the response; trise, rise time from 10% to 90% of the response. In all of the text/supporting figures, only the timing of the voltage trigger controlling the solution change is shown; however, all tpeak and tlatency values provided in the text and Table 1 have already taken into account the above corrections.
In Fig. 1C, Left, a brief (35-ms) pulse of 4 mM butyl acetate elicited an inward current of approximately −40 pA from a representative, voltage-clamped Or-expressing OSN. Across cells, the saturated response ranged from −20 to −160 pA (−51 ± 31 pA, mean ± SD; n = 25). The response amplitude increased with increasing concentration (Fig. 1D, Left), with a dynamic range covering 1–2 log units of butyl acetate concentration (Fig. 1D, Right). The dose–response relationship was approximately linear at its foot (Fig. 1D, Left, Inset) but was supralinear overall (Hill coefficient, 1.7 for this cell), broadly similar to the behavior of vertebrate OSNs (27, 28). As expected from OSNs expressing different Ors, the sensitivity to butyl acetate varied widely across randomly recorded Or-expressing OSNs, with a half-saturating concentration of 0.6–17 mM (10 cells; 3.7 mM in Fig. 1D) for 35-ms pulses, but the Hill coefficient was fairly constant (1.5 ± 0.3; n = 10). The receptor current elicited by butyl acetate showed a moderate outward rectification in the physiological voltage range with a reversal potential of −9 ± 13 mV (n = 34) (Fig. 1E), suggesting the involvement of a nonselective transduction cation channel.
Under current clamping, the responsive OSNs depolarized to generate action potentials, yielding higher firing frequencies at higher odor concentrations (Fig. 1F). When the duration of odor simulation was prolonged to 500 ms, the same recorded OSN responded strongly with a high firing frequency (Fig. 1G, Left) to butyl acetate at 2 mM, a concentration that did not trigger any firing with a 35-ms pulse (Fig. 1F). The response increased further for butyl acetate at 3.75 mM and 500 ms (Fig. 1G, Right). Thus, both the concentration and duration of odor stimulation contribute to the response strength, explaining why a brief pulse (e.g., 35 ms) requires a relatively high concentration to trigger a substantial response, as shown above. The concentrations used here do not correspond directly to those used in the air-phase odor stimulation in SSR (see Supporting Information for details).
To examine the response kinetics, we followed the strategy adopted for vertebrate photoreceptors by focusing on small responses at the foot of the dose–response curve, where linearity holds and intrinsic kinetics is revealed (29). The profile of the small receptor current showed a sigmoidal rise, with a latency (tlatency) of 66 ± 25 ms (n = 25) (i.e., time from odor arrival to 10% of response peak amplitude; Fig. S1 G and H) and a time-to-peak (tpeak) of 138 ± 42 ms (n = 25) (Table 1). The rise time (trise, from 10% to 90% of response) of the receptor current was 62 ± 23 ms (n = 25). The integration time of the response (tint) (30), which provides a representation of the overall response duration independent of its specific waveform (Table 1 legend) and is useful for performing comparisons across OSNs and even comparisons with sensory neurons of other modalities, was 250 ± 103 ms (n = 25). We also found that the responses of a given OSN to different odors typically exhibited comparable rising phases but sometimes widely variable falling phases (10). The reason for this variability remains unclear.
Table 1.
Parameters of odor-induced receptor currents
| OSN types | Rmax, pA | tlatency, ms | tpeak, ms | trise, ms | tint, ms | Cell no. |
| Orco-expressing OSNs (in situ) | −51 ± 31 | 66 ± 25 | 138 ± 42 | 62 ± 23 | 250 ± 103 | 25 |
| Ir-expressing OSNs (in situ) | −49 ± 35 | 35 ± 16 | 104 ± 25 | 52 ± 14 | 225 ± 106 | 10 |
| Or47a-expressing OSNs (in situ) | −94 ± 53 | 67 ± 35 | 132 ± 46 | 55 ± 24 | 275 ± 86 | 10 |
| Or47a-expressing OSNs (dendrite out) | −103 ± 65 | 64 ± 36 | 128 ± 45 | 53 ± 18 | 251 ± 79 | 5 |
| Or22a-expressing OSNs (in situ) | −63 ± 36 | 65 ± 17 | 135 ± 23 | 58 ± 21 | 246 ± 65 | 15 |
Note: Rmax is the transient-peak amplitude of the saturated odor response. Integration time of odor response, tint, is defined as ∫f(t)dt/fp, where f(t) is the response waveform and fp is the waveform’s transient-peak amplitude. Other parameters are defined in Fig. S1H. All data are given as mean ± SD, derived from responses filtered at DC-2 kHz.
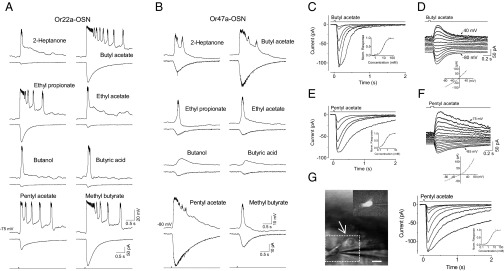
In addition, we examined two additional Drosophila lines in which OSNs expressing Or22a and Or47a were GFP labeled. The odors that we found effective for these OSNs (Fig. 2 A and B) were broadly similar to those previously identified for Or22a and Or47a expressed in “empty neurons” lacking endogenous Ors (11). By using butyl acetate and pentyl acetate as the stimulus for Or22a- and Or47a-expressing OSNs, respectively, we found that these OSNs produced odor responses with similar properties to those described above (Fig. 2 C–F), including the maximum amplitude (Table 1), response kinetics (Table 1), and current–voltage relationship.
Fig. 2.
Odor responses of Or22a- and Or47a-expressing OSNs. (A) The odor spectrum of an Or22a-expressing OSN. In each pair of traces, the top trace shows the voltage response (current clamped) and the bottom trace shows the inward receptor current (voltage clamped at −75 mV) elicited by the indicated odors (10 mM, applied for 35 ms). Each of the multiple peaks in the odor responses obtained via current-clamped recordings represents a burst of action potential firing, which also occurs in some OSNs when injected with depolarizing currents. Note the voltage trace is faster than the current trace, possibly due to the contribution of voltage-gated conductance to voltage changes. (B) The odor spectrum of an Or47a-expressing OSN. (C) The odor response family of an Or22a-expressing OSN. Odor stimulations with 35-ms pulses of butyl acetate at concentrations of 1, 2, 8, 12, 40, and 60 mM. The Inset shows the corresponding normalized dose–response relationship. The curve is the Hill equation (K1/2 = 9 mM, m = 1.9). (D) The current–voltage relationship for the response in an Or22a-expressing OSN. The voltage is clamped at −80 mV and stepped to +40 mV in 10-mV increments. Odor stimulation: 35-ms pulses of 10 mM butyl acetate. (E) The odor response family of an Or47a-expressing OSN. Odor stimulations via 35-ms pulses of pentyl acetate at concentration of 0.25, 0.5, 1, 2, and 7.5 mM. The Inset shows the corresponding normalized dose–response relationship. The curve is the Hill equation (K1/2 = 0.6 mM, m = 1.8). (F) The current–voltage relationship for the response of an Or47a-expressing OSN. The voltage is clamped at −85 mV and stepped to +75 mV in 10-mV increments. Odor stimulation: 75-ms pulses of 1 mM pentyl acetate. (G) Odor responses of an Or47a-expressing OSN with its dendrite exposed. (Left) Image of a dendrite-out OSN. The arrow indicates the exposed dendrite. The Inset illustrates GFP fluorescence in the dashed box region. (Scale bar: 5 µm.) (Right) Odor response family for the OSN on the Left. The Inset is the normalized dose–response relationship.
Next, we compared the responses of an in situ Or47a-expressing OSN (i.e., with its chemosensory dendrite inside the sensillum, corresponding to the experiments described so far) with those of an Or47a-expressing OSN with its dendrite directly exposed to the bath perfusion solution (Fig. 2G and Materials and Methods). We found that the responses under the two experimental conditions were remarkably similar (Fig. 2 C and G, and Table 1), except for the slightly higher half-saturating pentyl acetate concentration associated with the dendrite-out OSNs (in situ: 1.7 ± 1.1 mM, n = 10; versus dendrite-out: 2.4 ± 1.5 mM, n = 5). These data suggest that when pentyl acetate is applied in the liquid phase, the intrasensillar lymph is not important for odor response kinetics and sensitivity. Alternatively, a partial lymph washout or dilution may have occurred for in situ ORNs due to the continuous bath perfusion.
Odor Responses in Ir-Expressing OSNs.
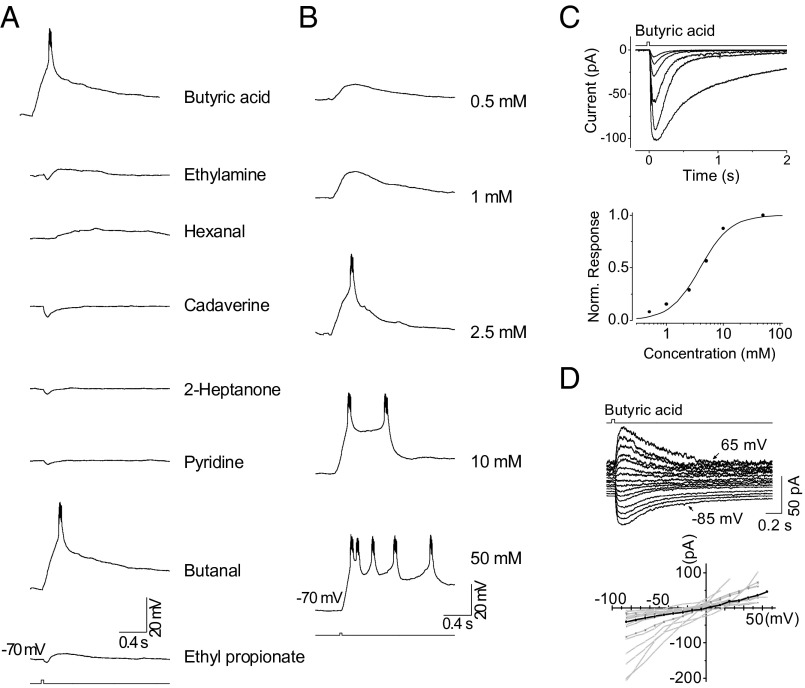
For comparison, we recorded Ir-expressing OSNs, which compose 20% of the total 1,200 OSNs in the antenna. To avoid any confounding Or-mediated signals, we focused on GFP-negative OSNs in Orco-Gal4;;Orco2,UAS-mCD8-GFP flies, which express either Irs or the gustatory receptors Gr21a/Gr63a (16). These two subpopulations of chemoreceptive cells can be distinguished from each other by their distinct responsivities to a panel of odorants (Gr21a/Gr63a respond mainly to CO2). In the Orco−/− background, any potential influence of a neighboring Or-expressing OSN would also be eliminated (31). Thus, we were able to record a subpopulation of Ir-expressing OSNs with a distinct odor spectrum (Fig. 3A) similar to that of ac3A neurons (16, 32). When stimulated with butyric acid, the OSN fired bursts of action potentials, with increasing firing frequency at higher odor concentrations (Fig. 3B). Under voltage clamping, an inward receptor current was elicited from these cells by butyric acid, with a peak amplitude that was graded with concentration (Fig. 3C). The Hill coefficient of the overall dose–response relationship across these OSNs was 1.2 ± 0.4 (n = 10). The response to butyric acid showed an approximately linear current–voltage relationship, with a reversal potential of −11 ± 15 mV (Fig. 3D; n = 17). In data from 10 cells, the average tlatency, trise, tpeak, and tint were 35 ± 16, 52 ± 14, 104 ± 25, and 225 ± 106 ms, respectively (Table 1). The key difference between Or- and Ir-expressing OSNs is the shorter tlatency and tpeak of the latter, suggesting that a difference may exist in the odor signaling mechanisms of Drosophila Ors and Irs.
Fig. 3.
The odor responses of Ir-expressing OSNs. (A) Odor spectrum. Traces show the voltage responses (current clamped) elicited by the indicated odors. Each odor was delivered at 25 mM and applied for 35 ms. (B) The dose dependence of spike firing. The same Ir-OSN as that in A is shown. Traces show the membrane depolarization elicited by 35-ms pulses of butyric acid at the indicated concentrations. (C) The odor response family (Top) and dose–response relationship (Bottom). A 35-ms pulse of butyric acid at concentrations of 0.5, 1, 2.5, 5, 10, and 50 mM. The curve fit to the dose–response relationship is the Hill equation (K1/2 = 4.0 mM, m = 1.6). (D, Top) Current–voltage relationship of the response of an OSN to butyric acid (35 ms, 25 mM). The cell is stepped from −85 to 65 mV in 10-mV increments. (Bottom) Collected current–voltage relationships from 17 Ir-expressing OSNs, with responses elicited by butyric acid. Black dots (interconnected by straight lines) correspond to data from the OSN at the Top.
Adaptation of the Receptor Current in OSNs.
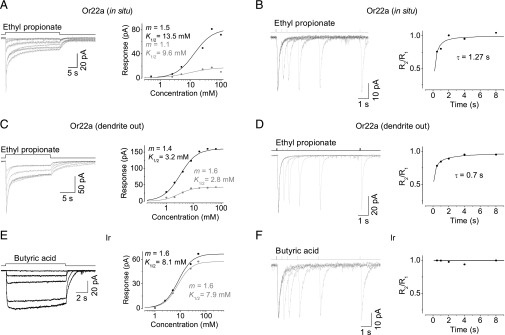
To determine the adaptation of odor responses, we first recorded the responses of Or22a-expressing OSNs to a 30-s odor step. We chose ethyl propionate, a potent excitatory odor recognized by Or22a (Fig. 2A) with relatively high water solubility, as the stimulus. The receptor current rose to a transient peak, and then substantially decreased and was maintained a steady response in the presence of the odor step, an indicator of adaptation (Fig. 4A; n = 22). At higher concentrations, the amplitude of the transient peak became larger and eventually saturated. In contrast, the steady current first increased with concentrations and then decreased at the highest concentration tested, possibly due to nonspecific inhibition (33). A broadly similar adaptation was also observed with butyl acetate and pentyl acetate. To investigate the time course of recovery from adaptation, we examined responses to paired-pulse stimulations at varied interpulse intervals. At an interval of 500 ms, the second pulse induced a smaller receptor current relative to the first, indicating the existence of adaptation produced by the first pulse; at longer intervals, the reduction of the receptor current gradually recovered (Fig. 4B; n = 12). The presence of residual adaptation even when the response to the first pulse had already decayed to near zero suggests that the adaptation was produced by changes associated with receptor current generation.
Fig. 4.
Adaptation of Drosophila OSNs. (A) Adaptation in Or22a-expressing OSNs. (Left) Receptor current responses to 30-s steps of ethyl propionate at concentrations of 0.5, 2.5, 5, 25, 50, and 100 mM. (Right) The dose–response relationship from the response family on the Left, with black points representing the receptor current amplitude at transient peaks and the gray points representing the steady state. The curves are derived from the Hill equation, with K1/2 = 13.5 and 9.6 mM, m = 1.5 and 1.1, for a transient peak and the steady-state response, respectively. (B) Adaptation recovery in Or22a-expressing OSNs. (Left) The same OSN as that recorded in A showed recovery from adaptation by paired-pulse stimulations at intervals of 0.5, 1, 2, 4, and 8 s. (Right) The normalized responses between the two pulses plotted against the intervals. The curve is fit with an exponential function with a time constant of 1.27 s. R1 and R2 are the amplitudes of transient-peak responses to the first and second odor pulses, respectively. (C) Odor adaptation in Or22a-expressing OSNs with dendrites exposed. (D) Adaptation recovery in Or22a-expressing OSNs with dendrites exposed. (E) No adaptation was observed in Ir-expressing OSNs. Odor stimulation: 15-s steps of butyric acid at concentrations of 1, 2.5, 5, 30, and 50 mM. (F) Responses to paired-pulse stimulation in Ir-expressing OSNs.
This adaptation may be caused by perineuronal effects, such as the depletion of odorant-binding proteins or ionic concentration changes in sensillar lymph (34), or by desensitized cellular signaling intrinsic to the OSNs. To distinguish between these possibilities, we examined the adaptation of OSNs with their sensory dendrites pulled out of the sensillar cavities to preclude any perineuronal effects. Nonetheless, we observed a similar adaptation to odor steps and similar recovery kinetics associated with paired-pulse adaptation (Fig. 4 C and D; n = 6). These results demonstrated that the adaptation is produced by the desensitization of intrinsic signaling in OSNs.
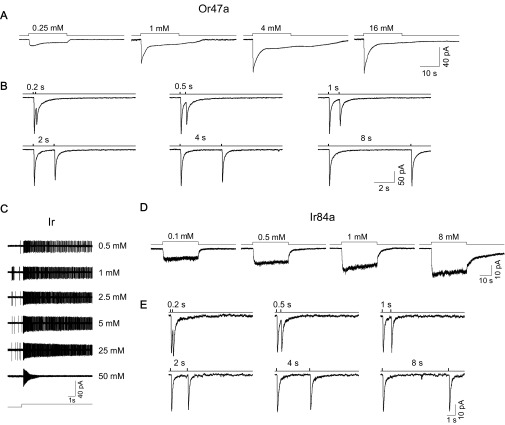
Or47a-expressing OSNs also showed strong adaptation to a long step of pentyl acetate and exhibited a similar recovery from the adaptation induced by paired-pulse stimulation (Fig. S2 A and B). In addition, randomly recorded Orco-expressing OSNs also exhibited adaptation, further supporting the idea that adaptation is a general feature of Or-expressing OSNs. Interestingly, Or-expressing OSNs with a slow falling phase in their short-pulse responses showed a relatively weaker adaptation.
Fig. S2.
Adaptation in Or47a- and Ir-expressing OSNs. (A) Adaptation to odor steps in Or47a-expressing OSNs. Odor stimulation: 20-s steps of pentyl acetate at concentrations of 0.25, 1, 4, and 16 mM. (B) Adaptation recovery in Or47a-expressing OSNs. Odor stimulation: 35-ms pulses of 4 mM pentyl acetate at intervals of 0.2, 0.5, 1, 2, 4, and 8 s. (C) Spike adaptation in ac3A OSNs. Under cell-attached recordings, spike firings in response to long steps of butyric acid at concentrations of 0.5, 1, 2.5, 5, 25, and 50 mM were recorded. Note the strong spike adaptation at a concentration of 50 mM. (D) Receptor current responses to odor steps in Ir84a-expressing OSNs. Under voltage-clamp recording, the receptor current showed no adaptation to 30-s steps of phenylacetaldehyde at concentrations of 0.1, 0.5, 1, and 8 mM. (E) Receptor current responses to paired pulses in Ir84a-expressing OSNs. Odor stimulation: 35-ms pulses of 16 mM phenylacetaldehyde at intervals of 0.2, 0.5, 1, 2, 4, and 8 s. A and B from the same Or47a-expressing OSN; D and E from two different Ir84a-expressing OSNs.
Next, we investigated adaptation in Ir-expressing OSNs. We recorded receptor currents of ac3A OSNs and found, in contrast, that they did not show adaptation. The receptor current rose and then remained at a steady level during the step stimulation (Fig. 4E; n = 10). When examined with the paired-pulse protocol, the two pulses triggered identical receptor currents (Fig. 4F) at all interpulse intervals tested, further indicating that Ir-expressing OSNs did not adapt. Interestingly, spike firing in these Ir-expressing OSNs did show adaptation. In cell-attached recordings, the recorded OSN fired action potentials at the onset of an odor step, which was followed by a gradual reduction in spike amplitude and even a complete loss of spikes in the presence of a 30-s odor step at a high concentration (Fig. S2C). This spike adaptation probably resulted from a change in the spike-generating mechanism, such as the inactivation of voltage-gated sodium channels (34). Similar spike adaptation in Ir-expressing OSNs has been shown in one (15), but not in another (35), study via SSR. In addition, we obtained similar results from OSNs expressing Ir84a (Fig. S2D); receptor currents recorded from Ir84a-expressing OSNs did not exhibit adaptation to long steps of phenylacetaldehyde or when probed with paired-pulse stimulations, but spike firing adapted strongly at high odor concentrations.
Changes in Sensitivity and Kinetics During Adaptation.
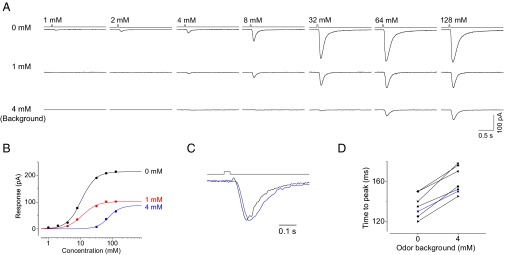
In vertebrate OSNs, the hallmarks of adaptation are a reduction of sensitivity and a prolongation of response kinetics (36–39). We next investigated changes in sensitivity and response kinetics during adaptation in Or22a-expressing OSNs by measuring incremental responses to 35-ms pulses on odor backgrounds. We found that higher concentrations were required to elicit the same criterion response on odor backgrounds, indicating reduced sensitivity in the presence of background odors (Fig. 5 A and B). Additionally, the amplitude of the saturated response became smaller in the presence of background odors. Notably, a prolongation of the 128 mM ethyl-propionate pulse from 35 to 150 ms was needed to saturate incremental responses in a 4 mM background. In addition to a sensitivity change, background odors also prolonged the time course of odor responses by slowing the receptor current onset (Fig. 5 C and D).
Fig. 5.
Changes in the sensitivity and response kinetics by adaptation. (A) Incremental responses in Or22a-expressing OSNs to 35-ms pulses of ethyl propionate with no background (Top) or 1 mM (Middle) and 4 mM (Bottom) ethyl propionate backgrounds. (B) The dose–response relationship for incremental responses. The peak amplitudes of the incremental responses are plotted against concentrations, with black, red, and green symbols representing data for no background, and 1 and 4 mM ethyl propionate backgrounds, respectively. The background-induced responses are similar to those in Fig. 4 A and C. (C) Changes in the response kinetics by adaptation. Normalized small responses in experiments with and without a 4 mM ethyl propionate background. (D) The collective time-to-peak values for weak responses with and without a 4 mM ethyl propionate background.
Voltage and Calcium Dependence of Adaptation.
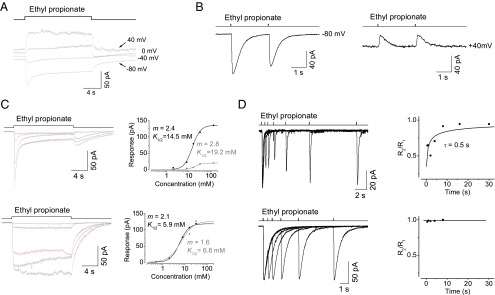
Another striking property of adaptation in Drosophila Or-expressing OSNs was its sensitivity to changes in holding potential. The receptor current strongly adapted at a holding potential of −80 mV, but the adaptation was abolished at a holding potential of +40 mV; specifically, the odor-induced receptor current reversed in polarity and then rose and was maintained at a steady level during the 15-s stimulation at a holding potential of +40 mV (Fig. 6A). When examined via the paired-pulse protocol, the second pulse triggered a smaller receptor current at −80 mV, but not at +40 mV (Fig. 6B).
Fig. 6.
The voltage and Ca2+ dependence of adaptation. (A) The voltage dependence of step adaptation. Odor stimulation: 20-s steps of 50 mM ethyl propionate. Holding potentials are as indicated. (B) The voltage dependence of paired-pulse responses. Left and right panels were obtained from the same OSN. Odor stimulation: 35-ms pulses of 50 mM ethyl propionate at an interval of 4 s. Holding potentials at −80 mV (Left) and +40 mV (Right). Note the abolishment of the reduction in second-pulse–triggered receptor current at +40 mV. (C) The Ca2+ dependence of step adaptation. The top and bottom panels are from the same OSN. Adaptation (Left, Top) is abolished after removing extracellular Ca2+ (Left, Bottom), with traces for 8 mM ethyl propionate marked in red. (Right) The dose–response relationships at a transient peak and in the steady state are represented with black and gray symbols, respectively. (D) The Ca2+ dependence of paired-pulse adaptation. Top and bottom panels were obtained from the same OSN. Odor stimulation: 35-ms pulses of 50 mM ethyl propionate. Note that adaptation (Left, Top) is abolished when extracellular Ca2+ is removed (Left, Bottom). The response ratio at transient peaks between the two pulses plotted against the intervals (Right).
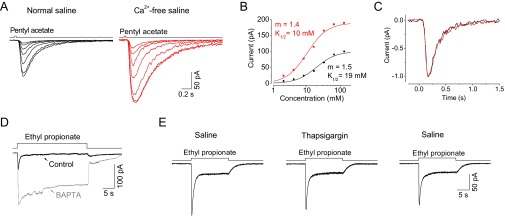
The voltage dependence of adaptation may be explained by a desensitization triggered by Ca2+ entry through nonselective cation transduction channels, which have a reversal potential of approximately −10 mV as noted above (Fig. 1E). To investigate a role for Ca2+ in OSN adaptation, we first removed all extracellular Ca2+. In the absence of extracellular Ca2+, the receptor current rose and then remained at the same steady level during long-step stimulations, indicating that adaptation had been abolished (Fig. 6C, Bottom). The removal of extracellular Ca2+ also increased the receptor current induced by odor steps. For example, the receptor current induced by 8 mM ethyl propionate was threefold larger in the absence of extracellular Ca2+ (Fig. 6C). Interestingly, a similar increase in response amplitude also occurred for a receptor current triggered by brief pulses (Fig. S3 A–C). When extracellular Ca2+ was removed, adaptation induced by paired pulses was also abolished, even at negative membrane potentials (Fig. 6D). Furthermore, when BAPTA was dialyzed through the electrode into the recorded OSNs to buffer intracellular Ca2+, adaptation was also abolished (Fig. S3D), further demonstrating that Ca2+ plays a key role in the observed adaptation. Finally, we found that Ca2+ released from calcium stores in the endoplasmic reticulum (ER) did not contribute to odor adaptation because similar adaptation existed when the calcium store was depleted via the inhibition of ER Ca2+-ATPase activity with thapsigargin (Fig. S3E).
Fig. S3.
Effects of Ca2+ on odor responses in Or22a-expressing OSNs. (A) Ca2+ effects on brief-pulse responses. The dose–response families of a recorded Or22a-expressing OSN in normal saline (Left), and Ca2+-free saline (Right). (B) Normalized dose–response relationships from the response families, with black and red fit representing data from normal saline and Ca2+-free saline, respectively. (C) Normalized response waveforms of small responses in both normal saline and Ca2+-free saline, represented in black and red, respectively. (D) BAPTA effects on adaptation. Using whole-cell patch-clamp recordings, receptor currents of the recorded Or22a-expressing OSN to 30-s step of 50 mM ethyl propionate were recorded with (black trace) and without (gray trace) 10 mM BAPTA in the recording pipette. Note the abolishment of adaptation in the presence of BAPTA. (E) Adaptation induced by 15-s steps of 50 mM ethyl propionate before (Left), during (Middle), and after washing out (Right) the application of 2 µM thapsigargin in the recorded Or22a-expressing OSN.
Extension of the Operating Range and Adjustment of Signaling Gain by Adaptation.
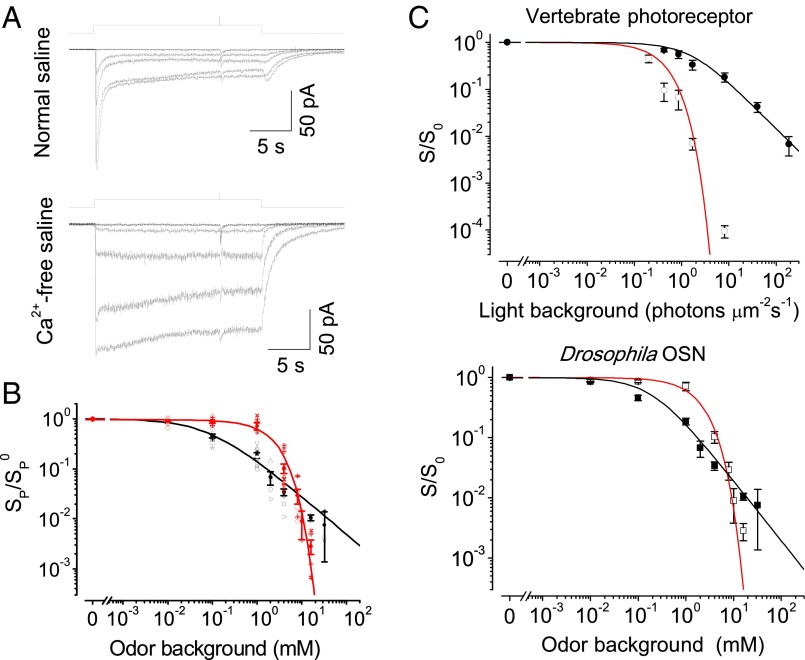
As shown above, Ca2+ entry during an odor response attenuated odor sensitivity, enabling OSNs to avoid saturation. Next, we quantified the changes in sensitivity and operating range during adaptation by adopting a standard protocol used for retinal photoreceptors (29, 40). Specifically, we measured the incremental sensitivity of OSNs to a brief stimulation superimposed on a background stimulus. The OSNs were adapted with an odor step and tested with a superimposed odor pulse (Fig. 7A). The pulse concentration was adjusted to trigger a receptor current at ∼10% of the saturated response. The odor sensitivity SPB, defined as the peak response amplitude divided by the incremental odor pulse concentration, progressively decreased with background concentration CB. Interestingly, we found that the relationship between SPB and CB was in accordance with the well-known Weber–Fechner relation, SPB/Sp = 1/[1 + (CB/C0)], where Sp is the sensitivity in the absence of an odor background, and C0 is the background concentration that halves the OSN’s sensitivity (Fig. 7B). After abolishing adaptation by removing extracellular Ca2+, the odor sensitivity was higher than that predicted by the Weber–Fechner relation at low backgrounds but dropped sharply at high backgrounds, with a saturating exponential relationship between SPB and CB. Thus, as shown in Fig. 7B, adaptation in Drosophila Or-expressing OSNs extends the operating range of odor concentration by selectively reducing sensitivity at low backgrounds but increasing it at high backgrounds compared with sensitivity in the absence of adaptation. This dynamic sensitivity change differs from the monotonic sensitivity increase incurred upon light adaptation in vertebrate photoreceptors (Fig. 7C) (40).
Fig. 7.
Gain control during adaptation. (A) Incremental sensitivity under background adaptation. The top and bottom panels were obtained from the same OSN. Odor backgrounds: 20-s steps of ethyl propionate at various concentrations. Incremental pulses: 35-ms pulses of ethyl propionate at various concentrations. Normal saline (Top) and Ca2+-free saline (Bottom). (B) The normalized odor sensitivity against background concentrations. Black and red symbols represent data in the presence and absence of extracellular Ca2+, respectively. The fit in black represents the Weber–Fechner relation, SPB/Sp = 1/[1 + (CB/C0)] with C0 = 0.09 mM; the fit in red represents the saturating exponential function, SPB/Sp = exp(−kCB), where k is a constant of 0.4 mM−1. (C) Model results and experimental data. (Top) The normalized photosensitivity of vertebrate photoreceptors, with data points extracted from figure 2b in ref. 40. (Bottom) The same data as that shown in B for Drosophila OSNs. The red curves are fit with S/S0 = exp(−u0/KNA), and the black curves are fit with S/S0 = exp[−u0/(K0 +α u0)] × [K0/(K0 + α u0)], with parameters specified in Fig. S4.
To understand the effects of adaptation on response sensitivity, we developed a general phenomenological model of sensory adaptation (see Supporting Information for details of the model). Our model revealed two opposing effects of adaptation: desensitization and alleviation of saturation (to maintain high sensitivity). At high backgrounds, alleviating saturation dominates over desensitization; therefore, adaptation leads to a higher sensitivity. At low backgrounds, the balance between these effects depends on the strength of adaptation. For a strongly adaptive system such as Drosophila Or-expressing OSNs, the desensitization effect of adaptation is relatively strong and leads to a lower sensitivity at low odor concentrations as shown in Fig. 7C. However, for a weakly adaptive system such as retinal rod photoreceptors, the desensitization effect is relatively weak, and adaptation increases sensitivity at a low background light intensity (Fig. 7C). The trade-off between these two adaptation effects quantitatively explains the difference in sensitivity change between vertebrate photoreceptors and Drosophila Or-expressing OSNs (Fig. 7C and Discussion).
Discussion
Despite the popularity of Drosophila as a model organism in olfactory research, the cellular signaling and physiology of its OSNs remain largely unclear. An attempt has previously been made to obtain patch-clamp recordings of single Drosophila OSNs (41), but the low yield of this blind recording approach, which does not enable visualization of the recorded OSNs, and the absence of robust odor responses have been major drawbacks. The technical challenge of developing a targeted recording method is mainly imposed by the small size of the peripheral olfactory organs, the cuticle encasing the olfactory tissue, and the sheath cells wrapping the OSNs. Here, we developed an antennal preparation by cutting the third antenna into transverse slices to expose the somata of OSNs. With this preparation, we were able to perform patch-clamp recordings of single visualized Drosophila OSNs after mechanically removing the surrounding sheath cells.
In this study, we provided a comprehensive characterization of the basic properties of odor-induced receptor currents in Drosophila OSNs. For brief-pulse stimulations, we found that the Or-mediated receptor current had a longer response latency and time-to-peak compared with the Ir responses. When examined during exposure to long-step odors, the receptor current of Drosophila Ir-expressing OSNs did not adapt. In contrast, Drosophila Or-expressing OSNs strongly adapted, consistently with the LFP studies using either EAG or SSR (19, 24, 42). Furthermore, Drosophila Or-expressing OSNs with a sensory dendrite inside or outside the sensillar cavity exhibited similar adaptation, indicating that the cellular mechanisms intrinsic to OSNs rather than lymph-related factors produce the adaptation. Interestingly, Drosophila Or-expressing OSNs share similar adaptation features with vertebrate OSNs, despite possible differences in odor transduction mechanisms (43). For example, as in vertebrate OSNs (37, 44), the steady-state receptor current in Drosophila Or-expressing OSNs during adaptation can reach less than 20% of the transient peak response. Also similarly to vertebrate OSNs (37, 38), adaptation reduced the sensitivity and slowed the response kinetics of Drosophila Or-expressing OSNs, consistently with LFP studies using SSR (24). Furthermore, we found that adaptation in Drosophila Or-expressing OSNs was mediated by the Ca2+ influx during odor responses, as is that in vertebrate OSNs (36, 38).
What are the molecular targets of Ca2+ effects during odor adaptation? In vertebrate OSNs, several mechanisms have been proposed, including the desensitization of transduction channels, phosphorylation of odorant receptors, inhibition of adenylyl cyclase, and potentiation of phosphodiesterase activity (37, 38, 45). Recent molecular genetic studies have revealed, however, that adaptation in vertebrate OSNs is more complex than previously understood (46, 47). To date, the odor transduction mechanism in Drosophila OSNs remains unclear, although heterologous studies have suggested novel and controversial mechanisms (43). However, the observed differences in responses kinetics and adaptation properties between Or- and Ir-OSNs suggest that the two types of neurons may use distinct odor transduction mechanisms. Alternatively, if both Or and Ir were ionotropic, the activation of Or and Ir would have to be different. If G-protein signaling is involved in generating odor responses in Drosophila Or-expressing OSNs, the targets of Ca2+ modulation could be any component ranging from Ors to transduction channels as in vertebrate OSNs. Otherwise, Ors/Orco would be the target of Ca2+ modulation during adaptation in Drosophila Or-expressing OSNs. Future studies are required to fully elucidate the mechanisms of Ca2+-mediated adaptation in Drosophila OSNs as well as in vertebrate OSNs.
Odor sensitivity in Drosophila Or-expressing OSNs adapts to the odor background in accordance with the Weber–Fechner relation, which is well known for describing the adaptation of retinal photoreceptors (40, 48). In the absence of extracellular Ca2+, the Weber–Fechner relation no longer held and was replaced by a saturating exponential relationship with a concomitant reduction in the operating range. Interestingly, the two mathematical relationships intersect at a background concentration, revealing that adaptation selectively reduces sensitivity at a low background but increases sensitivity at a high background. This dynamic adjustment of sensitivity during adaptation is in great contrast to the long-held view that adaptation always increases sensitivity as has been established for vertebrate photoreceptors (40, 48).
Motivated by the observed difference in sensitivity change, we developed a general mathematical framework to understand the response of sensory systems with and without adaptation (Supporting Information). The results of our model reveal a trade-off between two adaptation effects: (i) desensitization to reduce odor sensitivity and (ii) alleviation of saturation to increase sensitivity. At high backgrounds, adaptation prevents the sensory system from reaching saturation, in which sensitivity drops exponentially. As a result, adaptation leads to a higher sensitivity in all sensory systems at high backgrounds (Fig. 7C). At low backgrounds, the sensory system is not close to saturation; thus, the desensitization effect of adaptation can become dominant. For a strongly adaptive system such as the Drosophila Or-expressing OSNs, this desensitization effect is strong and leads to a lower sensitivity at low background odor concentrations. In contrast, in retinal rod photoreceptors, adaptation increases sensitivity at all background light intensities (40, 48). This difference can be explained by the relatively weak adaptation of retinal photoreceptors; thus, the increase in sensitivity caused by the alleviation of saturation dominates desensitization at all backgrounds (Supporting Information). Similarly to Drosophila Or-expressing OSNs, sensory neurons in many other modalities, such as hair cells (49), exhibit strong adaptation. Thus, understanding of the dynamic sensitivity adjustment by odor adaptation in Drosophila may serve as a guiding principle for understanding the effects of adaptation in other senses. With respect to modeling, it remains a challenge to understand the general effects of adaptation according to the underlying molecular mechanisms in sensory neurons (50), as has been done in the simpler case of bacterial chemotaxis (51).
The ability of strong adaptation by Ors would allow Drosophila to track odor changes over a broad range of concentrations and to detect other odors even in the presence of certain background odor. In contrast, without the ability to adapt, Irs are better suited for detecting absolute odor concentrations, allowing Drosophila to efficiently locate food, mates, or predators.
Materials and Methods
Fly Stocks.
All flies were raised on standard cornmeal agar medium, under 60% humidity and a 12-h light/12-h dark cycle at 25 °C. The Orco-Gal4, Or47a-Gal4, Or22a-Gal4, and Orco2 flies were obtained from the Bloomington Stock Center and had been deposited by Dr. Leslie B. Vosshall, Rockefeller University, New York. UAS-mCD8-GFP flies were obtained from the same center and had been deposited by Dr. Liqun Luo, Stanford University, Stanford, CA. Ir84a-Gal4 was a gift from Dr. Richard Benton, University of Lausanne, Lausanne, Switzerland.
Preparation.
Young adult flies (1–4 d after eclosion) were immobilized on ice. The decapitated heads were transferred into dissection solution. The third segment of an antenna was isolated and cut into three transverse pieces (slices), each ∼50 µm thick. The antennal slice was stabilized in the recording chamber with vacuum grease, with one cut end facing up and continuously bath-perfused with 95% O2/5% (vol/vol) CO2-bubbled Drosophila saline. Each OSN in the antenna projects a dendrite from the cell body into a sensillum. On average, ∼20 intact OSNs could be found in the top layer of a slice, whereas the others (∼10) typically had damaged dendrites, cell bodies, or sensilla. Below the top layer at the open end, most OSNs were intact. Patch-clamp recordings could be made from OSNs in the top three layers.
Solutions and Electrical Recordings.
Drosophila saline contained the following (in mM): 158 NaCl, 3 KCl, 4 MgCl2, 1.5 CaCl2, 26 NaHCO3, 1 NaH2PO4, 5 N-tri(hydroxymethyl)-methyl-2-aminoethane-sulfonic acid (TES), 10 d-glucose, 17 sucrose, and 5 trehalose, bubbled with 95% O2/5% CO2 (pH 7.4). The osmolality was ∼400 mOsm and has been found to be critical for the health of OSNs and for eliciting stable odor responses (up to 2 h). The dissection solution was made by replacing NaHCO3, NaH2PO4, and TES in Drosophila saline with 5 mM 4-(2-hydroxyethl)-1-piperazineethanesulfonic acid (Hepes) and 27 mM NaCl (pH 7.4, adjusted with NaOH), bubbled with oxygen. All chemicals, including odors, were obtained from Sigma-Aldrich. Odors were freshly dissolved in Drosophila saline daily within their water solubility. The pH of odor solutions was not corrected. For example, the pH values of 1 and 50 mM butyric acid solutions are ∼7.10 and 4.40, respectively. Tetraethyl ammonium chloride (TEA), 4-aminopyridine (4-AP), and thapsigargin were also obtained from Sigma-Aldrich, and tetrodotoxin (TTX) was obtained from Alomone Labs. All reagents were directly dissolved in Drosophila saline, with the exception of thapsigargin, which was dissolved in dimethyl sulfoxide (DMSO).
OSNs in the antennal slice were visualized on an upright microscope (Scientifica), with infrared–differential interference contrast (IR-DIC) optics and a 60× water-immersion objective (Olympus). The image was captured with an IR-CCD (DAGE-MTI) and displayed on a television monitor (Sony). Patch-clamp recordings were made with MultiClamp 700B (Molecular Devices). The patch electrodes were made from borosilicate glass (WPI) with a P-97 puller (Sutter). The OSNs in the Drosophila antenna are small, with cell bodies of only 3–5 µm in diameter, requiring a recording pipette tip of ∼0.2 µm and a resistance of ∼20 MΩ when filled with intracellular saline (in mM: 185 K-gluconate, 5 NaCl, 2 MgCl2, 0.1 CaCl2, 1 EGTA, 10 Hepes; pH 7.4; ∼390 mOsm). Typically, a seal of 2–8 GΩ between OSN membrane and a patch-clamp pipette could be obtained. For perforated patch-clamp recordings, amphotericin B was dissolved in DMSO, then diluted with intracellular saline to a final concentration of 200 µg/mL, and backfilled into the recording pipette. For whole-cell patch-clamp recordings, GTP-Tris (0.5 mM) and Mg-ATP (4 mM) were added to the intracellular saline. For cell-attached recordings, the recording pipette was filled with dissection solution. To measure the current–voltage relationship, voltage-sensitive Na channels and K channels were blocked by a mixture of TTX (50 nM), TEA (10 mM), and sometimes also 4-AP (10 mM). In experiments requiring the removal of extracellular Ca2+, the perfusion saline was composed of the following (in mM): 158 NaCl, 3 KCl, 7.6 MgCl2, 26 NaHCO3, 1 NaH2PO4, 10 EGTA, 5 TES, 10 d-glucose, 17 sucrose, and 5 trehalose, bubbled with 95% O2/5% CO2 (pH 7.4). Current and voltage signals were digitized and recorded with Digidata 1440A and pClamp 10.2 (Molecular Devices), filtered at 2 kHz, and sampled at 5 kHz. Recorded currents were low-pass filtered at 200 Hz (unless stated otherwise) for display, introducing a ∼3-ms peak delay compared with low-pass filtering at 2 kHz. The voltage was clamped at −80 mV unless stated otherwise. Measured voltages were corrected for a liquid junction potential.
Odor Stimulation.
Rapid solution changes were produced by translating the interface between the two flowing solution streams across the recorded OSN with an electronic stepper (Warner Instruments) attached to a three-barrel tube (Warner Instruments), with the tip positioned ∼50 µm away from an antennal slice. Each barrel was connected to an 8-to-1 manifold. Thus, a total of 24 solutions could be used. The solution flow was driven by gravity at a speed of ∼16 mm⋅s−1 at the tubing tip and was controlled by solenoid valves (The Lee Company) and a valve controller (AutoMate Scientific). The inner width of each square barrel of the perfusion tubing was 600 µm, emitting a solution readily covering the antennal slice (∼50 µm thick, ∼40 µm wide, and ∼70 µm long).
Dendrite-Out Preparation.
Typically, the dendrite of an OSN consists of an inner dendrite housed in the antennae and an outer dendrite in the sensillum. The outer (or sensory) dendrite is thus immersed in sensillar lymph. To rule out contributions of factors in the sensillar lymph to the odor responses of OSNs, we developed a dendrite-out preparation. Before patch-clamp recording, the sensory dendrite of a targeted OSN was pulled out from its sensillar cavity, by using a glass pipette with a tip opening of ∼2 µm to suck the inner dendrites and then mechanically pull out the sensory dendrites from the sensillar cavity. As a result, the sensory dendrite of the targeted OSN was immersed in the bath perfusion solution rather than in the sensillar lymph.
Measurements of Response Latency.
The time delay between the voltage trigger driving the electronic stepper and the appearance of an electrical response is due to the following: (i) the time required for the stepper-driven mechanical translation of the three-barrel tubing, (ii) the travel time for the odor solution to reach the antennal slice, (iii) the time required for odorant molecules to penetrate the pores in the sensillum and reach the OSN dendrite by diffusion, and (iv) the time delay in olfactory transduction (i.e., response latency). The first two delays were measured to be 28 ms, based on liquid-junction current measurements (Fig. S1G). The third delay has been estimated by others to be ∼5 ms (1). Thus, the response latency (tlatency) is obtained by subtracting 33 ms (28 + 5 ms) from the total response delay.
A Mathematical Model of Adaptation
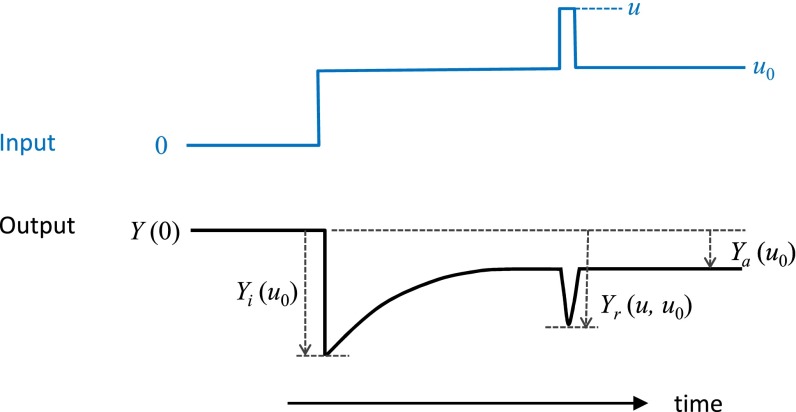
We developed a general phenomenological model of adaptation to understand the competing effects of adaptation on response sensitivity. Here, the input is u and the output is Y. In addition to its dependence on the input, the output Y also depends on some internal (intracellular) variable X, which changes with the external input (stimulus) and allows the system to adapt.
After a sudden change of stimulus, from u = 0 to u = u0 > 0, the output exhibits a large initial response Yi(u0) before it relaxes to a steady-state value Ya(u0). Adaptation occurs if |Ya(u0) − Y(0)| < |Yi(u0) − Y(0)|, where Y(0), the baseline activity at u = 0, is set to zero for convenience. See Fig. S4 for an illustration.
Fig. S4.
Definition of parameters in the theoretical model of sensory adaptation. Input parameters: u0, u; output parameters: Y(0), Yi(u0), Yr(u, u0), and Ya(u0).
In the adapted steady state, the internal parameter X depends on the external stimulus u0, i.e., X = Xa(u0). Now, for a sudden change of the input from u0 to an arbitrary value of u, we can assume the slow variable X is unchanged, and the output will depend on both u and Xa(u0): Y (u, Xa(u0)). This assumption is valid if we are only interested in the responses to the small and fast changes of the input from u0. The response of a cell, which is adapted to the background u0, to a new stimulus u is defined as Yr(u, u0) ≡ Y (u, Xa(u0)) (Fig. S4).
We hypothesize that the effect of adaptation roughly corresponds to a rescaling of the input variable:
| [S1] |
where Ka(u0) is the rescaling factor that is an increasing function of u0 and F is a monotonically increasing response function. The meaning of this hypothesis is intuitively clear. For a change in input from u = 0 to u = u0, the initial response Yi(u0) = F(u0/Ka(0)), and the adapted output is Ya(u0) = F(u0/Ka(u0)). As Ka(u0) > Ka(0), the adapted output is smaller than the initial response, i.e., the system adapts.
The sensitivity can then be computed directly from this hypothesis:
| [S2] |
from which we obtain the relative sensitivity (relative to the sensitivity at u0 = 0, i.e., S0 ≡ S(0)):
| [S3] |
In an adaptive system, Ka(u0) increases as u0 increases. Given that the adapted response reaches an asymptotic constant as u → ∞, we expect that Ka(u0) should increase linearly with u0 asymptotically as u0 → ∞. For simplicity, we assume the following:
| [S4] |
where K0 and α are constants that differ across sensory systems. Note that the form of Ka(u0) may be more complex in the real system. In this simple model of adaptation, K0 provides a basic scale for stimulus strength (either concentration or intensity). For a very large stimulus strength u0 >> K0, the adapted response Ya(u0) → F(α−1). The parameter α characterizes the strength of adaptation. Perfect adaptation corresponds to α → ∞, where Ka = ∞ and the adapted response is always F(0) independent of u0. The case of no adaptation corresponds to α = 0, where Ka remains a constant independent of u0.
Now, we have a simple phenomenological description of the adaptive response parameterized by the nonlinear response function F, and the two parameters K0 and α characterize the effect of adaptation on the internal states and, consequently, the responses of the cells. The detailed form of F(z) depends on the details of the response mechanism. In general, F(z) increases with z in a nonlinear manner. For the Drosophila OSNs and photoreceptors (40), F(z) should saturate to a finite value as z → ∞. The responses of both the adaptive cells and the perturbed cells without adaptation (i.e., calcium clamped) can be described by the same model by using different parameters. For cells without adaptation, Ka is independent of u0. Setting it to a constant Kna, we obtain the following:
| [S5] |
where the subscript “na” means no adaptation. Because the experimentally measured Rna seems to follow an exponential decay function (Fig. 7B) (40), we can approximate F(z) as follows:
| [S6] |
where F0 is a scale factor. Note that F(z) may be fitted by other nonlinear functions, such as the Hill function. Using this exponential form, we have the following:
| [S7] |
In principle, the characteristic value Kna for nonadaptive cells is not the same as K0 for adaptive cells.
Using the functional form of F, we can also determine the relative sensitivity of the adaptive cells:
| [S8] |
It is easy to see that the nonadaptive case corresponds simply to α = 0. For large values of u0 >> K1 ≡ K0/α, we have the following:
| [S9] |
which decreases as 1/u0 in agreement with the Weber–Fechner law.
From our model, it is clear that whether these two relative response curves, Rna and Ra, cross each other depends on the value of α. For the Drosophila Or-expressing OSNs, the adaptation is strong, so α is large, which leads to K1 < K0, and, therefore, the possibility of these two curves crossing. (Note that correspondence of the parameters is as follows: C0 → K1 and k → Kna−1, from the notation used in the main text to what is used here.) For weaker adaptive systems, such as photoreceptors (40), α will be smaller, and the two curves will not cross, i.e., Ra > Rna is always true.
Quantitatively, the crossover can be understood as follows. For a very large stimulus u0 >> Kna, K0, the inequality Ra > Rna is always true because Rna decays exponentially whereas Ra decays only as 1/u0 following the Weber–Fechner law. For very small backgrounds, u0 << Kna, K0, we have the following:
| [S10] |
Therefore, when α > K0/Kna − 1, we have Rna > Ra for small u0, and the two relative sensitivities cross over at some intermediate background u∗; when α < K0/Kna − 1, Rna < Ra is true for the full range of backgrounds and there is no crossover.
All response data for both adaptive and nonadaptive cells (i.e., with calcium clamping) can be fitted by this simple model with only three parameters: K0, α, and Kna. Their values can be estimated from the experimental data. For the olfactory system, the estimated parameters are as follows: α = 3.5, K0 = 0.9 mM, and Kna = 2 mM. It is straightforward to see that α > K0/Kna − 1 is satisfied and, therefore, that the two sensitivity curves have a crossover. In other words, adaptation reduces the sensitivity at low backgrounds but increases it at high backgrounds. For the photoreceptors (40), these values are as follows: α = 1.2, K0 = 4.0, Kna = 0.38. It is also clear that α < K0/Kna − 1 and there is no crossover; in other words, adaptation simply increases the sensitivity at all backgrounds. By implementing Eqs. S7 and S8, the experimental data derived from Drosophila Or-expressing OSNs and retinal photoreceptors are nicely reproduced (Fig. 7C).
The Number of Odorant Molecules Hitting the Sensillum Between SSR and Slice Recordings
SSR Recordings (21).
Twenty microliters of diluted odor (in solvent) was loaded on a 0.5 × 4-cm filter paper, which was inserted into a syringe. A 500-ms puff of the syringe air at a speed of 125 mL/min was injected to a main airstream of 2 L/min (from a tubing of 5-mm diameter) for stimulation. The antenna was 1 cm away from the tubing.
Odorant molecules hitting the sensillum in SSR.
where Ac is the collecting area of a sensillum; Vair, the speed of airflow; Tair, stimulus, the stimulus duration; Cair, odor concentrations contacting the sensillum, which depend on many factors, including the odor types, solvent types, odor dilution in solvent, amount of odors loaded on the filter paper, temperature, time for odor emission from the filter paper, volume of the syringe, odor dilution in the main air stream, etc.; and NA, 6.02*1023 molecules per mol.
Parameters in SSR.
-
i)
Vair: 1,800 mm/s (corresponding to a flow of 2 L/min + 125 mL/min = 2.125 L/min, via a tubing of 5-mm diameter);
-
ii)
Tair, stimulus: 500 ms;
-
iii)
Cair: unknown (see above, difficult to be quantified).
Antennal Slice Recordings (Our Current Manuscript).
Odorant molecules hitting the sensillum in slice recordings.
Parameters in slice recordings.
-
i)
Vsolution: 16 mm/s (corresponding to a flow of 0.35 mL/min via a 600× 600-μm square tubing);
-
ii)
Tsolution,stimulus: 35 ms;
-
iii)
Csolution: the concentrations used in the slice recordings.
Thus,
Cair is difficult to be quantified as shown above; thus, an exact comparison of the odorant molecules between recordings is difficult to make.
However,
Therefore, the faster airflow and longer stimulation time in SSR need to be considered when comparing odorant molecules between preparations. Although the exact value of Cair (thus, the ratio of Cair/Csolution) is unknown, we expected that it is smaller than 1/1,600, but not too much off from this number. However, the absorption of odorant molecules into sensillum lymph (i.e., the effective odorant molecules stimulating OSNs) remains unclear (52, 53). We recorded single sensillum from an isolated intact antenna that was perfused, and stimulated as the slice preparation. The sensitivity (for example, ab3A neuron) was approximately twofold higher than that in the antennal slice. Hence, the sensitivity in slice recordings may not be too much off from that in SSR. When stimulated with an optimized odor, the OSN sensitivity is higher. For example, in the slice preparation, we found that Or56a-expressing OSNs responded to geosmin at a submicromolar range.
Acknowledgments
We thank King-Wai Yau, Jeremy Nathans, Trevor D. Lamb, Christopher J. Potter, and Karl-Ernst Kaissling for discussions; Wendy W.-S. Yue and Minmin Luo for comments on the manuscript; and Alex Kolodkin, Adrienne Dubin, Baruch Minke, and Vikas Bhandawat for technical advice and help. The work was supported by National Natural Science Foundation of China Grant 31471053, the Ministry of Education [the Young Thousand Talent Program (D.-G.L.)], the Peking–Tsinghua Center for Life Sciences, the Center for Quantitative Biology, the State Key Laboratory of Membrane Biology, and the College of Life Sciences at Peking University. Y.T. was supported by NIH Grant R01GM081747, and Y.S. was supported by National Natural Science Foundation of China Grant 31471024 and Natural Science Foundation of Zhejiang Province (Grant Z15C090001).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1518329113/-/DCSupplemental.
References
- 1.Kaissling KE. Chemo-electrical transduction in insect olfactory receptors. Annu Rev Neurosci. 1986;9:121–145. doi: 10.1146/annurev.ne.09.030186.001005. [DOI] [PubMed] [Google Scholar]
- 2.Prasad BC, Reed RR. Chemosensation: Molecular mechanisms in worms and mammals. Trends Genet. 1999;15(4):150–153. doi: 10.1016/s0168-9525(99)01695-9. [DOI] [PubMed] [Google Scholar]
- 3.Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413(6852):211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- 4.Ache BW, Young JM. Olfaction: Diverse species, conserved principles. Neuron. 2005;48(3):417–430. doi: 10.1016/j.neuron.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Kleene SJ. The electrochemical basis of odor transduction in vertebrate olfactory cilia. Chem Senses. 2008;33(9):839–859. doi: 10.1093/chemse/bjn048. [DOI] [PubMed] [Google Scholar]
- 6.Ma M. Odor and pheromone sensing via chemoreceptors. Adv Exp Med Biol. 2012;739:93–106. doi: 10.1007/978-1-4614-1704-0_6. [DOI] [PubMed] [Google Scholar]
- 7.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 8.Benton R. Chemical sensing in Drosophila. Curr Opin Neurobiol. 2008;18(4):357–363. doi: 10.1016/j.conb.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Su CY, Menuz K, Carlson JR. Olfactory perception: Receptors, cells, and circuits. Cell. 2009;139(1):45–59. doi: 10.1016/j.cell.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117(7):965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125(1):143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 12.Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15(17):1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 13.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15(17):1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 14.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136(1):149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abuin L, et al. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69(1):44–60. doi: 10.1016/j.neuron.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silbering AF, et al. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J Neurosci. 2011;31(38):13357–13375. doi: 10.1523/JNEUROSCI.2360-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rytz R, Croset V, Benton R. Ionotropic receptors (IRs): Chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem Mol Biol. 2013;43(9):888–897. doi: 10.1016/j.ibmb.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Keil TA, Steiner C. Morphogenesis of the antenna of the male silkmoth. Antheraea polyphemus, III. Development of olfactory sensilla and the properties of hair-forming cells. Tissue Cell. 1991;23(6):821–851. doi: 10.1016/0040-8166(91)90034-q. [DOI] [PubMed] [Google Scholar]
- 19.Störtkuhl KF, Hovemann BT, Carlson JR. Olfactory adaptation depends on the Trp Ca2+ channel in Drosophila. J Neurosci. 1999;19(12):4839–4846. doi: 10.1523/JNEUROSCI.19-12-04839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqi O. Neurogenetics of olfaction in Drosophila melanogaster. Trends Genet. 1987;3:137–142. [Google Scholar]
- 21.Clyne P, Grant A, O’Connell R, Carlson JR. Odorant response of individual sensilla on the Drosophila antenna. Invert Neurosci. 1997;3(2-3):127–135. doi: 10.1007/BF02480367. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrino M, Nakagawa T, Vosshall LB. Single sensillum recordings in the insects Drosophila melanogaster and Anopheles gambiae. J Vis Exp. 2010;36:1–5. doi: 10.3791/1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benton R, Dahanukar A. Electrophysiological recording from Drosophila olfactory sensilla. Cold Spring Harb Protoc. 2011;2011(7):824–838. doi: 10.1101/pdb.prot5630. [DOI] [PubMed] [Google Scholar]
- 24.Nagel KI, Wilson RI. Biophysical mechanisms underlying olfactory receptor neuron dynamics. Nat Neurosci. 2011;14(2):208–216. doi: 10.1038/nn.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaissling KE. Single unit and electroantennogram recordings in insect olfactory organs. In: Spielman AI, Brand JG, editors. Experimental Cell Biology of Taste and Olfaction. Current Techniques and Protocols. CRC; Boca Raton, FL: 1995. pp. 361–377. [Google Scholar]
- 26.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43(5):703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Firestein S, Picco C, Menini A. The relation between stimulus and response in olfactory receptor cells of the tiger salamander. J Physiol. 1993;468:1–10. doi: 10.1113/jphysiol.1993.sp019756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhandawat V, Reisert J, Yau KW. Signaling by olfactory receptor neurons near threshold. Proc Natl Acad Sci USA. 2010;107(43):18682–18687. doi: 10.1073/pnas.1004571107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo DG, Kefalov V, Yau KW. Phototransduction in retinal rods and cones. In: Masland RH, Albridght TD, editors. The Senses—A Comprehensive Reference. Vol 1. Elsevier; New York: 2008. pp. 269–301. [Google Scholar]
- 30.Cao LH, Luo DG, Yau KW. Light responses of primate and other mammalian cones. Proc Natl Acad Sci USA. 2014;111(7):2752–2757. doi: 10.1073/pnas.1400268111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su CY, Menuz K, Reisert J, Carlson JR. Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature. 2012;492(7427):66–71. doi: 10.1038/nature11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao CA, Ignell R, Carlson JR. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J Neurosci. 2005;25(37):8359–8367. doi: 10.1523/JNEUROSCI.2432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurahashi T, Lowe G, Gold GH. Suppression of odorant responses by odorants in olfactory receptor cells. Science. 1994;265(5168):118–120. doi: 10.1126/science.8016645. [DOI] [PubMed] [Google Scholar]
- 34.Kaissling KE, Zack Strausfeld C, Rumbo ER. Adaptation processes in insect olfactory receptors. Mechanisms and behavioral significance. Ann N Y Acad Sci. 1987;510:104–112. doi: 10.1111/j.1749-6632.1987.tb43475.x. [DOI] [PubMed] [Google Scholar]
- 35.Getahun MN, Wicher D, Hansson BS, Olsson SB. Temporal response dynamics of Drosophila olfactory sensory neurons depends on receptor type and response polarity. Front Cell Neurosci. 2012;6:54. doi: 10.3389/fncel.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurahashi T, Shibuya T. Ca2+-dependent adaptive properties in the solitary olfactory receptor cell of the newt. Brain Res. 1990;515(1-2):261–268. doi: 10.1016/0006-8993(90)90605-b. [DOI] [PubMed] [Google Scholar]
- 37.Leinders-Zufall T, Ma M, Zufall F. Impaired odor adaptation in olfactory receptor neurons after inhibition of Ca2+/calmodulin kinase II. J Neurosci. 1999;19(14):RC19. doi: 10.1523/JNEUROSCI.19-14-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reisert J, Matthews HR. Adaptation of the odour-induced response in frog olfactory receptor cells. J Physiol. 1999;519(Pt 3):801–813. doi: 10.1111/j.1469-7793.1999.0801n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boccaccio A, Lagostena L, Hagen V, Menini A. Fast adaptation in mouse olfactory sensory neurons does not require the activity of phosphodiesterase. J Gen Physiol. 2006;128(2):171–184. doi: 10.1085/jgp.200609555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthews HR, Murphy RL, Fain GL, Lamb TD. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988;334(6177):67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- 41.Dubin AE, Harris GL. Voltage-activated and odor-modulated conductances in olfactory neurons of Drosophila melanogaster. J Neurobiol. 1997;32(1):123–137. doi: 10.1002/(sici)1097-4695(199701)32:1<123::aid-neu11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 42.Martelli C, Carlson JR, Emonet T. Intensity invariant dynamics and odor-specific latencies in olfactory receptor neuron response. J Neurosci. 2013;33(15):6285–6297. doi: 10.1523/JNEUROSCI.0426-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakagawa T, Vosshall LB. Controversy and consensus: Noncanonical signaling mechanisms in the insect olfactory system. Curr Opin Neurobiol. 2009;19(3):284–292. doi: 10.1016/j.conb.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Firestein S, Shepherd GM, Werblin FS. Time course of the membrane current underlying sensory transduction in salamander olfactory receptor neurones. J Physiol. 1990;430:135–158. doi: 10.1113/jphysiol.1990.sp018286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385(6618):725–729. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- 46.Song Y, et al. Olfactory CNG channel desensitization by Ca2+/CaM via the B1b subunit affects response termination but not sensitivity to recurring stimulation. Neuron. 2008;58(3):374–386. doi: 10.1016/j.neuron.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cygnar KD, Collins SE, Ferguson CH, Bodkin-Clarke C, Zhao H. Phosphorylation of adenylyl cyclase III at serine1076 does not attenuate olfactory response in mice. J Neurosci. 2012;32(42):14557–14562. doi: 10.1523/JNEUROSCI.0559-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pugh EN, Jr, Nikonov S, Lamb TD. Molecular mechanisms of vertebrate photoreceptor light adaptation. Curr Opin Neurobiol. 1999;9(4):410–418. doi: 10.1016/S0959-4388(99)80062-2. [DOI] [PubMed] [Google Scholar]
- 49.Peng AW, Effertz T, Ricci AJ. Adaptation of mammalian auditory hair cell mechanotransduction is independent of calcium entry. Neuron. 2013;80(4):960–972. doi: 10.1016/j.neuron.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Palo G, et al. Common dynamical features of sensory adaptation in photoreceptors and olfactory sensory neurons. Sci Rep. 2013;3:1251. doi: 10.1038/srep01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tu Y. Quantitative modeling of bacterial chemotaxis: Signal amplification and accurate adaptation. Annu Rev Biophys. 2013;42:337–359. doi: 10.1146/annurev-biophys-083012-130358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaissling KE. Flux detectors versus concentration detectors: Two types of chemoreceptors. Chem Senses. 1998;23(1):99–111. doi: 10.1093/chemse/23.1.99. [DOI] [PubMed] [Google Scholar]
- 53.Zhou Y, Wilson RI. Transduction in Drosophila olfactory receptor neurons is invariant to air speed. J Neurophysiol. 2012;108(7):2051–2059. doi: 10.1152/jn.01146.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]