Significance
Although it has been established for over 100 years that Lewy bodies (LBs) represent the major pathological hallmark of Parkinson's disease (PD), we still do not know why these fibrillar intraneuronal inclusions of α-synuclein (α-Syn) protein form, or how they contribute to disease progression. One of the major causes underlying this gap in knowledge is the lack of animal models that reproduce the formation of fibrillar LB-like inclusions. In this study, we show that the absence of human α-Syn (hα-Syn) fibrillization into LBs in mice can be attributed to interactions between hα-Syn and its endogenously expressed mouse α-Syn homologue. Moreover, we provide well-characterized primary neuronal and in vivo models that recapitulate the main molecular feature of PD, bona fide α-Syn fibrillization.
Keywords: Parkinson’s disease, alpha-synuclein, aggregation
Abstract
Lewy bodies (LBs) are intraneuronal inclusions consisting primarily of fibrillized human α-synuclein (hα-Syn) protein, which represent the major pathological hallmark of Parkinson's disease (PD). Although doubling hα-Syn expression provokes LB pathology in humans, hα-Syn overexpression does not trigger the formation of fibrillar LB-like inclusions in mice. We hypothesized that interactions between exogenous hα-Syn and endogenous mouse synuclein homologs could be attenuating hα-Syn fibrillization in mice, and therefore, we systematically assessed hα-Syn aggregation propensity in neurons derived from α-Syn–KO, β-Syn–KO, γ-Syn–KO, and triple-KO mice lacking expression of all three synuclein homologs. Herein, we show that hα-Syn forms hyperphosphorylated (at S129) and ubiquitin-positive LB-like inclusions in primary neurons of α-Syn–KO, β-Syn–KO, and triple-KO mice, as well as in transgenic α-Syn–KO mouse brains in vivo. Importantly, correlative light and electron microscopy, immunogold labeling, and thioflavin-S binding established their fibrillar ultrastructure, and fluorescence recovery after photobleaching/photoconversion experiments showed that these inclusions grow in size and incorporate soluble proteins. We further investigated whether the presence of homologous α-Syn species would interfere with the seeding and spreading of α-Syn pathology. Our results are in line with increasing evidence demonstrating that the spreading of α-Syn pathology is most prominent when the injected preformed fibrils and host-expressed α-Syn monomers are from the same species. These findings provide insights that will help advance the development of neuronal and in vivo models for understanding mechanisms underlying hα-Syn intraneuronal fibrillization and its contribution to PD pathogenesis, and for screening pharmacologic and genetic modulators of α-Syn fibrillization in neurons.
The aggregation of proteins into fibrillar structures is a key hallmark of many neurodegenerative disorders. In Parkinson's disease (PD), α-synuclein (α-Syn), a predominantly presynaptic protein involved in the regulation of neurotransmitter release, abnormally fibrilizes and forms intraneuronal inclusions termed “Lewy bodies” (LBs) (1, 2). So far, the mechanisms underlying LB formation remain poorly understood, and the impact of LB presence on neuronal viability remains controversial, in part due to the lack of animal models recapitulating α-Syn fibrillization into LB-like inclusions in the brain.
Because patients with familial history of parkinsonism were found carrying either multiplications or point mutations of the α-Syn gene SNCA (2), most animal models of PD have been generated by overexpressing WT human α-Syn (hα-Syn) or mutant forms linked to familial PD (3). Strikingly, although rodent models expressing hα-Syn do not recapitulate the formation of fibrillar LBs within dopaminergic neurons, hα-Syn overexpression in Drosophila led to dramatic neuronal loss accompanied with LB-like structures comprising fibrillar α-Syn (4). Drosophila, unlike rodents, do not express an endogenous α-Syn homolog, implying that hα-Syn fibril formation could be more favorable in models lacking endogenous α-Syn expression. Subsequent experiments in mice supported this suggestion, as hα-Syn transgenic (Tg) mice lacking endogenous mouse α-Syn (mα-Syn) expression exhibited exacerbated pathology compared with WT counterparts (5), and manifested fibrillar/granular accumulations in the olfactory bulb (6). Interestingly, in vitro test-tube experiments also showed that small amounts of mα-Syn directly inhibit the fibrillization of purified hα-Syn protein in solution (7).
Despite these interesting observations, it remained unclear whether endogenously expressed synuclein homologs directly inhibit hα-Syn aggregation in neurons, and whether ectopic hα-Syn expression in their absence would allow de novo hα-Syn fibrillization events. Therefore, we systematically evaluated the propensity of hα-Syn to aggregate on synuclein-KO backgrounds. Using a battery of biochemical and imaging techniques, we demonstrate that in cultured primary neurons and brains of mα-Syn–KO mice, overexpressed hα-Syn aggregates readily into inclusions that are similar to LBs in terms of solubility, immunoreactivity, and amyloidogenicity, and represent a bona fide fibrillization process as revealed by serial-section transmission electron microscopy (ssTEM), live imaging, and response to pharmacological aggregation inhibitors. Similarly, primary neurons lacking expression of β-Syn (β-Syn−/−) or of all three homologs (α-, β-, and γ-Syn) also manifest enhanced hα-Syn aggregation, thereby suggesting that endogenous synuclein homologs may represent natural regulators of abnormal α-Syn aggregation. To dissect potential mechanisms underlying this phenomenon, we performed immunoprecipitation and surface plasmon resonance (SPR) experiments, which showed that mα-Syn interacts preferentially with aggregated hα-Syn preformed fibrils (PFFs) rather than with monomers. Importantly, in vitro aggregation experiments and in vivo assessment of cross-seeding propensities of hα-Syn and mα-Syn showed that the observed cross-species interactions attenuate seeding and spreading of aggregates. These findings possibly explain why current rodent PD models expressing hα-Syn do not exhibit pronounced hα-Syn fibrillization, and provide models that reproduce a critical pathological feature of the disease: the de novo formation of fibrillar hα-Syn aggregates.
Results
Induction of hα-Syn Inclusion Formation in SNCA−/− Primary Neurons.
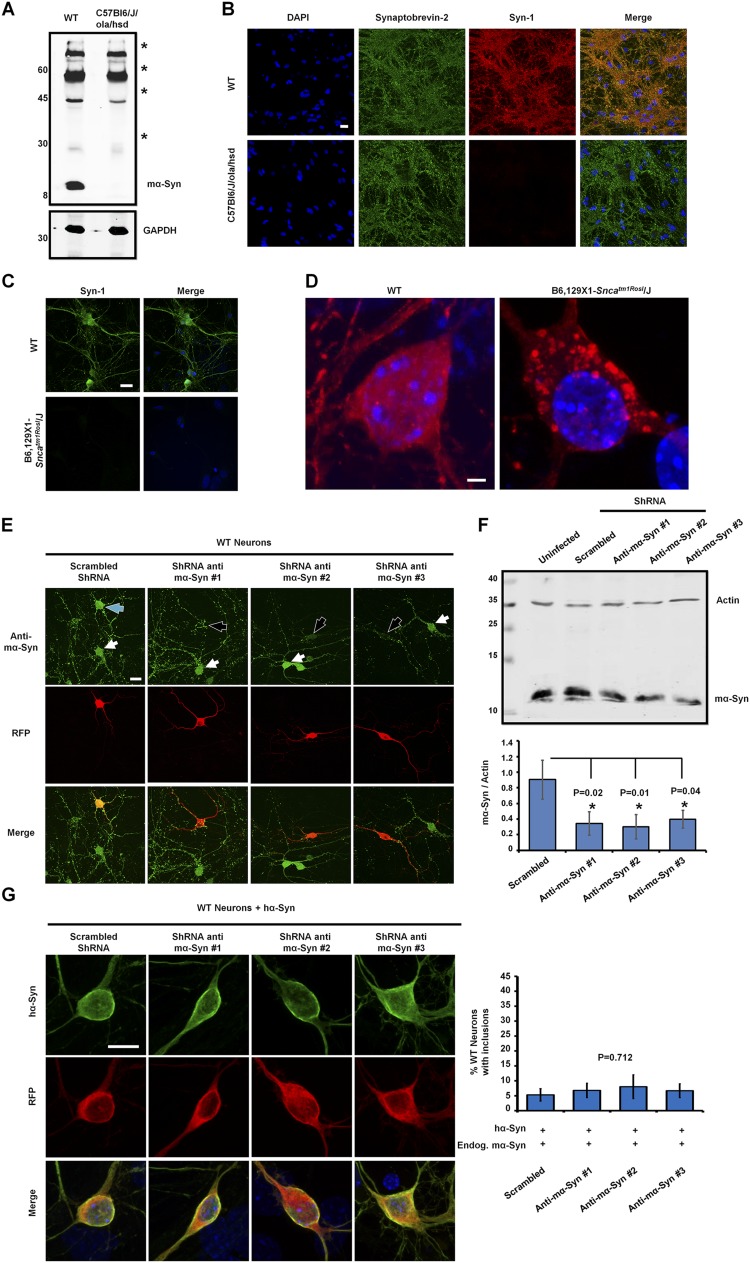
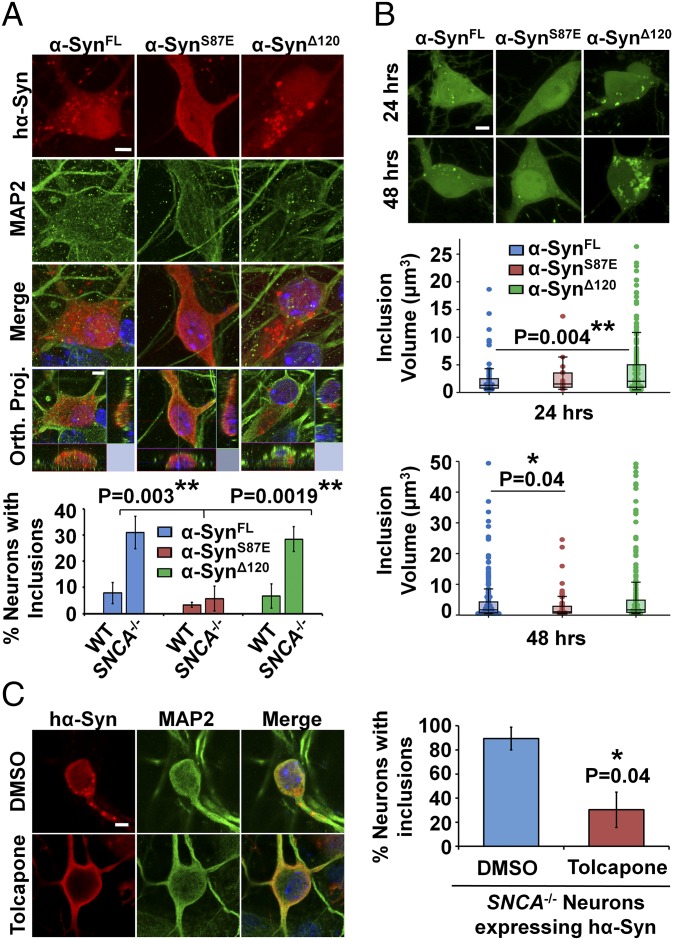
Several studies have reported that overexpressed hα-Syn in mouse primary neurons exhibits diffuse localization without forming discreet inclusions (8–11). To assess whether the absence of mα-Syn would affect this distribution, we examined the localization of transiently expressed hα-Syn in primary neurons derived from C57Bl6/J/ola/hsd mice (SNCA−/−) lacking expression of mα-Syn (Fig. S1 A and B) (12). Interestingly, although hα-Syn exhibited the previously reported diffuse distribution in WT neurons, a significant proportion of the SNCA−/− neurons developed spheroid-like inclusions that were consistently observed in close association with nuclear and cytoplasmic membranes (Fig. 1A and Movie S1). A similar phenotype was observed in primary neurons derived from another SNCA−/− mouse strain (B6,129 × 1-Sncatm1Rosl/J) generated by targeted deletion of two exons of the mα-Syn gene (Fig. S1 C and D) (13), thereby establishing that the formation of inclusions is directly linked to the specific loss of mα-Syn expression. In line with this finding, re-expression of mα-Syn in SNCA−/− neurons significantly reduced the formation of intraneuronal hα-Syn inclusions, and restored the diffuse distribution of hα-Syn in most neurons (Fig. 1B).
Fig. S1.
The total abolition of mα-Syn expression is necessary to promote the formation of hα-Syn inclusions. (A and B) The absence of mα-Syn expression in C57Bl6/J/ola/hsd mice was established by Western blot analysis of brain homogenates (A) and by immunocytochemistry of primary neurons (B), which showed no mα-Syn staining in synaptic terminals of KO neurons. (The asterisks in A indicate nonspecific signals that are detected equally in WT and SNCA−/− brains.) (C and D) Similarly, immunocytochemistry established the loss of mα-Syn expression in primary neurons derived from B6,129 × 1-Sncatm1Rosl/J mice (C) and revealed that overexpressed hα-Syn forms inclusions when expressed in B6,129 × 1-Sncatm1Rosl/J primary neurons (D). (E) Immunocytochemistry using an anti–mα-Syn–specific antibody (green) shows that WT neurons transfected with plasmids encoding anti–mα-Syn ShRNA (having RFP as a marker of transfection; black arrows) show reduced levels of mα-Syn as compared with nontransfected neurons (white arrows). In contrast, neurons expressing scrambled shRNA (blue arrow) do not show any change in mα-Syn levels. (F) Biochemical analysis of WT neurons infected with lentiviruses shows that the levels of endogenous mα-Syn are decreased significantly with anti–mα-Syn shRNA expression as compared with the expression of scrambled shRNA (n = 3 independent experiments). One-way ANOVA and Scheffé post hoc analysis were applied to obtain P values. (G) WT neurons coexpressing hα-Syn with anti–mα-Syn shRNA (having RFP as a marker of transfection) exhibit diffuse localization of hα-Syn similar to that seen when scrambled shRNA is expressed. Quantification of the formation of inclusions (>1 µm) in 25 neurons per condition (mean ± SD, n = 3 independent experiments) reveals no significant differences across conditions (P = 0.712, one-way ANOVA and Scheffé post hoc analysis). (Scale bars: 20 µm in B, C, and E, 2 µm in D, and 10 µm in G.)
Fig. 1.
The absence of mα-Syn promotes hα-Syn aggregation in primary neurons. (A) hα-Syn exhibits diffuse distribution in WT neurons and forms inclusions (>1 µm) in cell bodies and neurites in a significant proportion of SNCA−/− neurons. β3-Tubulin staining was performed to reveal neurons, and merged images show orthogonal Z-stack projections (Orth. Proj.). (B) Significantly fewer inclusions (>1 µm) are observed in SNCA−/− neurons with restored mα-Syn expression. (C) Significantly less monomeric hα-Syn is detected in nonionic detergent-soluble (Det. Sol.) fractions of lentivirally infected SNCA−/− neurons than in WT counterparts (n = 4 independent experiments). In contrast, nonionic detergent-insoluble (Det. Insol.) fractions of infected SNCA−/− neurons show more monomeric and HMW hα-Syn species, comparable to the detergent-insoluble fractions of WT neurons treated with hα-Syn PFFs. Actin was used to control for equal protein loading. In B and C, hα-Syn and mα-Syn were differentially revealed using the Syn-211 and D37A6 antibodies, respectively. In A–C, the Mann–Whitney test was applied to obtain P values. In A and B, 25 neurons per condition were quantified (n = 3 independent experiments). (D) Nine different anti–α-Syn antibodies (epitopes in Table S1) detect inclusions in SNCA−/− neurons transfected with hα-Syn. (E) hα-Syn inclusions in transfected SNCA−/− neurons are ThS-positive, phosphorylated at S129, and ubiquitinated. Colocalization was confirmed by assessing regression coefficients (R2). (Scale bars: 10 µm in A and B and 5 µm in D and E.)
To investigate whether the total abolishment of mα-Syn expression is necessary to promote hα-Syn inclusion formation, we assessed whether decreasing mα-Syn levels in WT neurons via shRNA-mediated silencing is sufficient to promote this process. Transient expression of three different vectors encoding shRNA hairpin loops showed efficient silencing of mα-Syn in transfected neurons as compared to neurons transfected with scrambled shRNA sequences (Fig. S1E), and biochemical analysis of lentivirally infected neurons showed ∼50% reduction in mα-Syn expression (Fig. S1F). Notably however, mα-Syn silencing did not promote the formation of hα-Syn inclusions in WT neurons (Fig. S1G), suggesting that even low levels of mα-Syn are sufficient to attenuate inclusion formation.
hα-Syn Inclusions Reproduce Key LB Features and Exhibit Fibrillar Ultrastructure.
Several cellular models manifesting α-Syn accumulation into inclusions in human cell lines have been reported, upon α-Syn overexpression alone (14–16), coexpression with synphilin-1 (17), exposure to proteasome inhibitors (9), or application of oxidative/nitrative insults (18, 19). Nevertheless, inclusions observed under these conditions typically do not reproduce all key LB features, including decreased solubility, hyperphosphorylation at Serine-129 (pS129) (20), ubiquitination (21), thioflavin-S (ThS) binding, and fibrillar ultrastructural organization (1). Therefore, we assessed whether the hα-Syn inclusions observed in SNCA−/− neurons fulfill these pathological features.
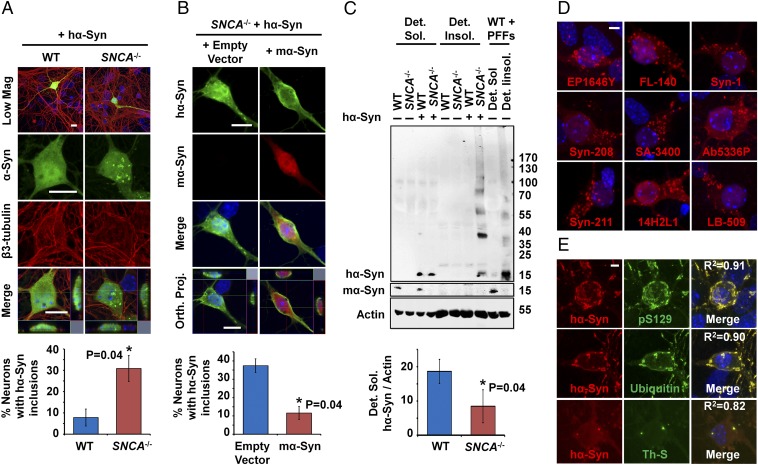
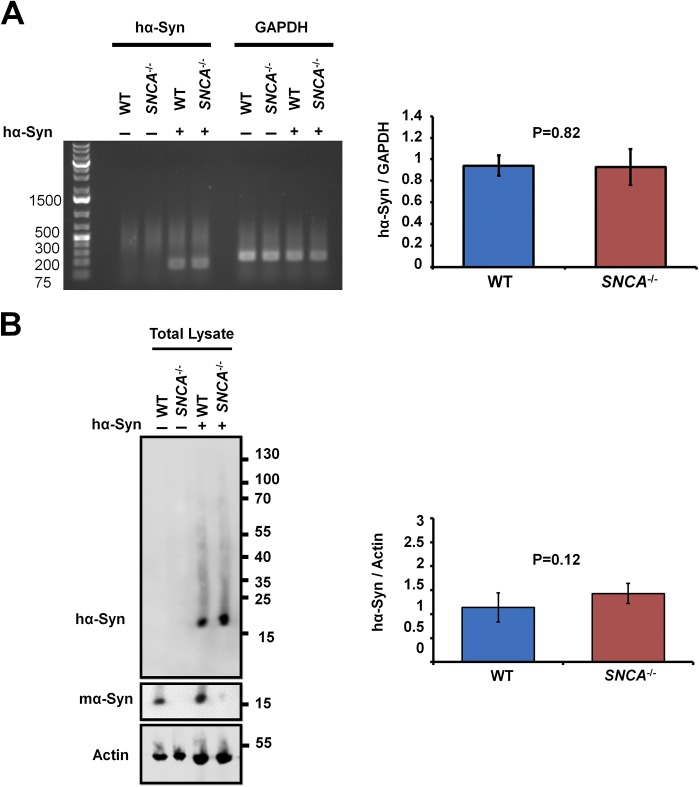
To determine whether the formation of inclusions is linked to decreased hα-Syn solubility, WT or SNCA−/− neurons were infected with lentiviruses encoding hα-Syn and were then fractionated into nonionic detergent-soluble and nonionic detergent-insoluble fractions. Using this assay, lentivirally infected SNCA−/− neurons showed significantly less monomeric hα-Syn in detergent-soluble fractions compared to WT counterparts (Fig. 1C). Interestingly, this effect was not caused by decreased total hα-Syn expression in SNCA−/− neurons, because similar total hα-Syn RNA and protein levels were observed in unfractionated WT and SNCA−/− neurons (Fig. S2 A and B). In contrast, the decrease in soluble monomeric hα-Syn was concomitant with the appearance of high-molecular-weight (HMW) hα-Syn species in detergent-insoluble fractions of lentivirally infected SNCA−/− neurons (Fig. 1C). To validate our fractionation protocol, we treated WT neurons with insoluble hα-SynAlexa633 PFFs (characterized in Fig. S3), which showed a strong signal almost exclusively within the detergent-insoluble fractions (Fig. 1C), as previously reported (22). Taken together, these results suggest that abolishing mα-Syn expression enhances the aggregation propensity of hα-Syn in primary neurons, as reflected by decreased solubility and the formation of insoluble HMW hα-Syn aggregates.
Fig. S2.
Similar total mRNA and protein levels of hα-Syn are expressed in WT and SNCA−/− neurons. Seven days postinfection with viruses encoding hα-Syn (+) or empty vector (−), WT and SNCA−/− primary neurons were lysed, and then RNA (A) or total proteins (B) were extracted. (A) Semiquantitative RT–PCR using specific primers against hα-Syn or GAPDH (housekeeping gene for normalization) followed by agarose gel electrophoresis demonstrates that no significant differences in hα-Syn mRNA levels are detected between WT and SNCA−/− primary neurons. (B) Western blotting of neurons lysed and scraped directly into loading buffer shows that no significant differences in total protein levels of hα-Syn are detected across conditions. hα-Syn and mα-Syn were differentially revealed using the Syn211 and D37A6 antibodies, respectively, and actin was used to control for equal protein loading. The Mann–Whitney test was applied to obtain P values. Three independent experiments were performed.
Fig. S3.
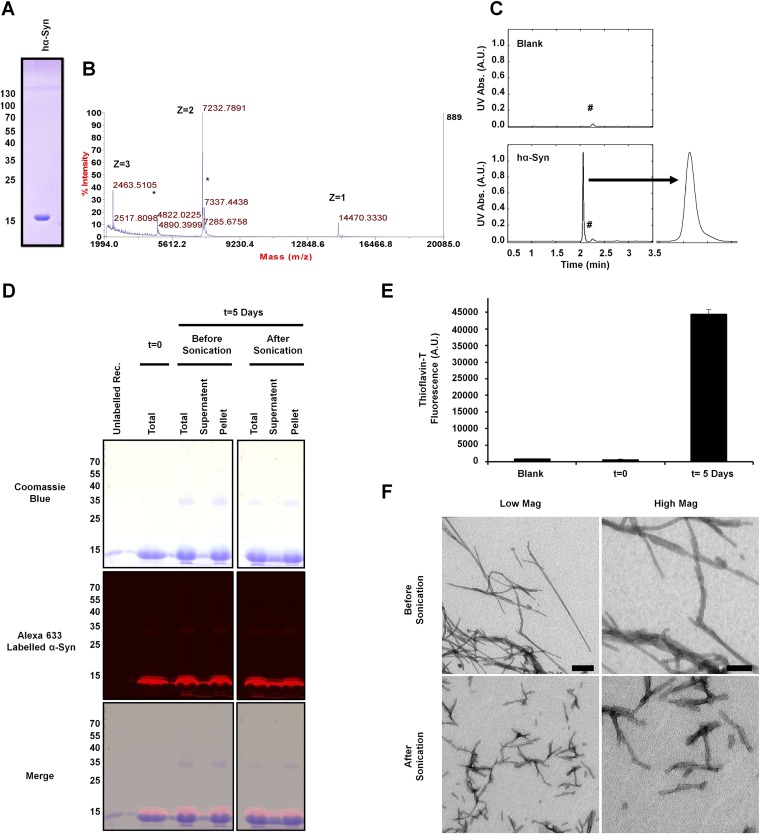
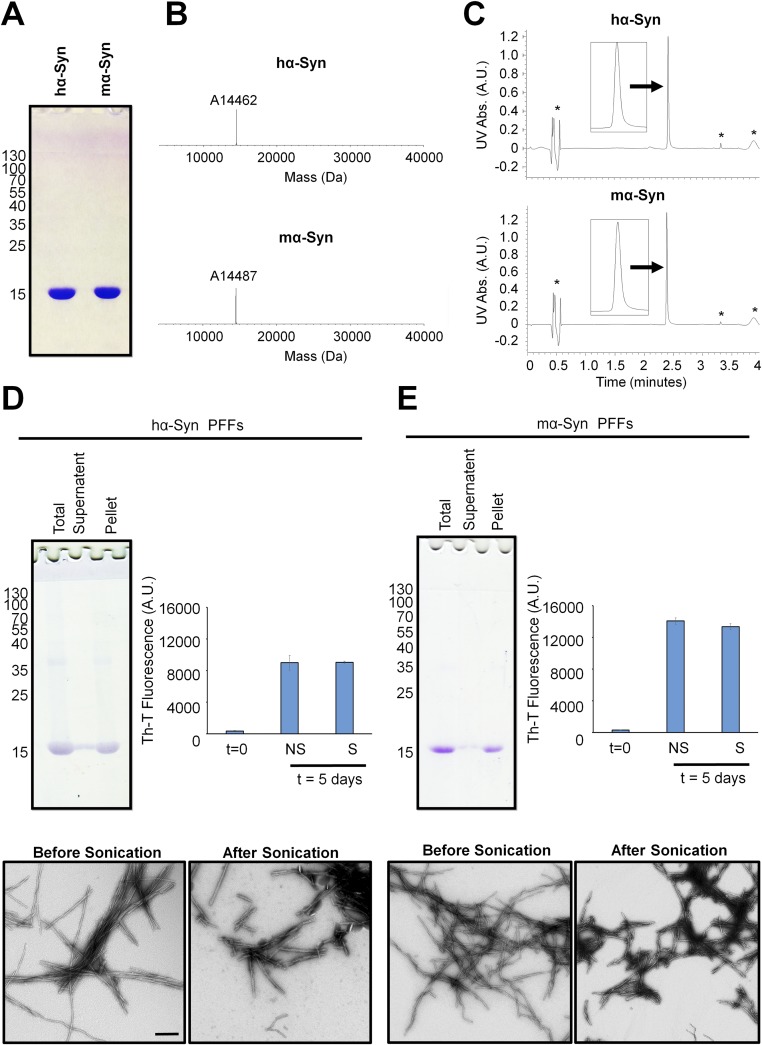
Characterization of hα-SynAlexa-633 PFFs. (A–C) Purified recombinantly expressed hα-Syn was first characterized by SDS PAGE, which showed that the protein runs as a single monomeric band (A), by ESI-MS analysis, which showed that the protein has the correct mass (expected mass 14,461 Da) (B), and by UPLC analysis, which showed a single sharp, symmetrical peak (C). The asterisks in B indicate the sinapinic acid matrix adduct observed by ESI-MS analysis, and the hashtags in C indicate a small peak observed by UPLC in the blank and samples. (D) Alexa Fluor-633–labeled PFFs generated by incubating monomeric hα-Syn with 10% labeled hα-SynAlexa-633 for 5 d at 37 °C with shaking were characterized by Coomassie gel, which showed that the PFFs sediment upon centrifugation (present mostly in the pellet rather than the supernatant) and are fluorescent upon gel scanning at 680 nm. Similar results were obtained after brief sonication of PFFs, with an increased presence of soluble monomers in the supernatant fraction. (E) Assessment of ThT binding (mean ± SD) showed that PFFs formed after 5 d of incubation comprise amyloid structure, unlike the initial protein solution before incubation. (F) TEM analysis validated the fibrillar nature of produced proteins and showed that sonication led to the generation of small fibrillar fragments. (Scale bars: 200 nm and 100 nm in the left and right panels, respectively.)
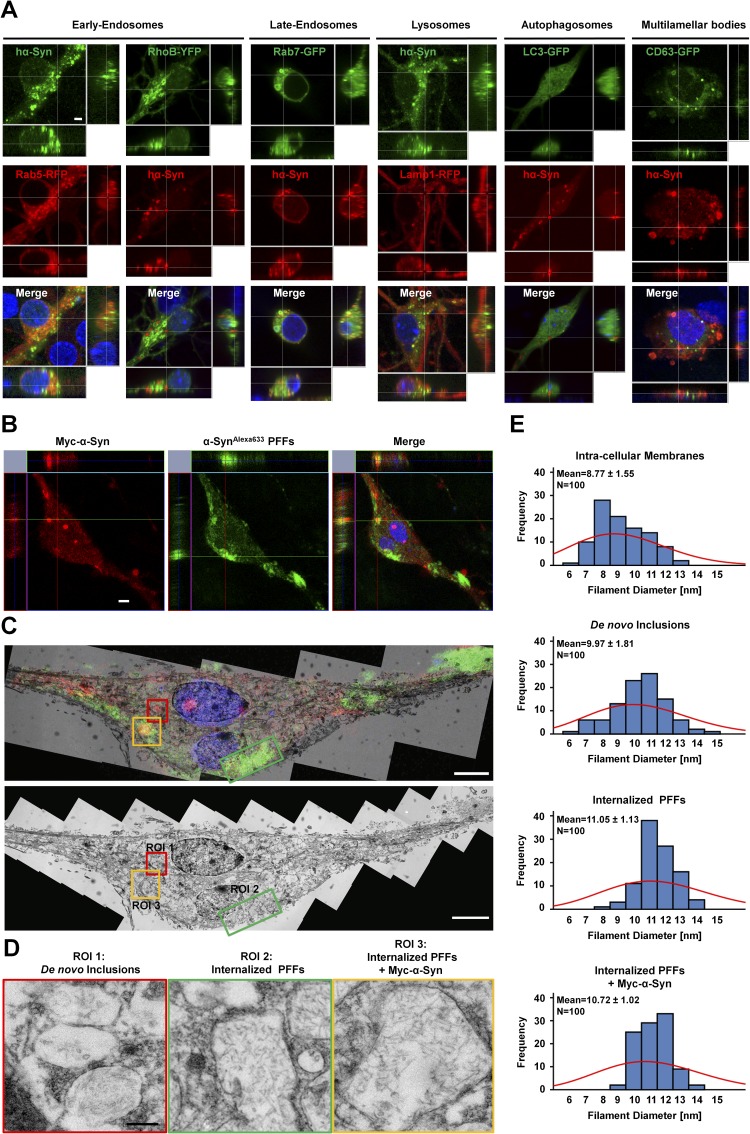
One possibility is that the inclusions in SNCA−/− neurons represent aggregated hα-Syn that is contained within vesicles of the endo-lysosomal pathway. Therefore, we systematically assessed the colocalization of inclusions with specific markers for early endosomes [Ras-related protein (Rab) 5 and Ras homolog gene family member B (RhoB)], late endosomes (Rab7), lysosomes [lysosomal-associated membrane protein 1 (Lamp1)], autophagosomes [microtubule-associated proteins 1A/1B light chain 3 (LC3)], and multilamellar bodies [CD63 antigen (CD63)]. As shown in Fig. S4A, although some hα-Syn inclusions showed weak partial colocalization with cotransfected markers of late endosomes (Rab7-GFP) and lysosomes (Lamp1-RFP) indicating potential degradation by this pathway, most of the inclusions showed no colocalization with any of the cotransfected markers.
Fig. S4.
Characterization of hα-Syn aggregates by immunofluorescence and electron microscopy. (A) Immunocytochemistry shows partial weak colocalization of hα-Syn inclusions with cotransfected markers of late endosomes (Rab7-GFP) and lysosomes (Lamp1-RFP), early endosomes (RhoB-YFP and Rab5-RFP), auto-phagosomes (LC3-GFP), and multilamellar bodies (GFP-CD63). (Scale bar: 2 µm.) (B) SNCA−/− neurons were transfected to express Myc–hα-Syn and 24 h later were treated with hα-SynAlexa633 PFFs for an additional 24 h. Immunocytochemistry using an anti-Myc antibody was performed; then stained neurons were imaged by confocal microscopy. hα-SynAlexa633 PFFs are shown in green to allow better visualization in following panels. (C) The two adjacent neurons imaged by fluorescent microscopy were reprocessed and imaged by TEM. (Upper) To correlate structures observed by ssTEM and fluorescence, single-plane images were superimposed. (Lower) A single-plane TEM image is shown with designated ROIs that comprise de novo inclusions composed of Myc–hα-Syn alone (ROI1), internalized hα-SynAlexa633 PFFs alone (ROI2), or mixtures of hα-SynAlexa633 PFFs and Myc–hα-Syn (ROI3). (D) High-resolution images of inclusions in ROI1, ROI2, and ROI3. (E) Quantification of filament diameter (n = 100 per condition) shows that the overall distribution of membrane diameters is different from that of filaments observed in de novo inclusions and from that of internalized PFFs (± Myc–hα-Syn). A frequency histogram with Poisson distribution (red) is shown, together with mean ± SD values. (Scale bars: 5 µm in B and C and 250 nm in D.)
Next, we assessed whether the inclusions formed in SNCA−/− neurons are readily detectable using common LB probes, namely, antibodies against α-Syn (1), pS129–α-Syn (20), and ubiquitin (21), as well as thioflavin-S (23). As shown in Fig. 1D, immunostaining using nine different anti–α-Syn antibodies with epitopes spanning most of the α-Syn sequence all revealed bright spheroid intraneuronal inclusions, thereby establishing that these structures indeed comprise full-length hα-Syn. Moreover, dual immunofluorescence analysis showed that the inclusions are also hyperphosphorylated at S129 and are ubiquitinated, and costaining with thioflavin-S indicated that the inclusions could comprise cross–β-sheet fibrillar content (Fig. 1E).
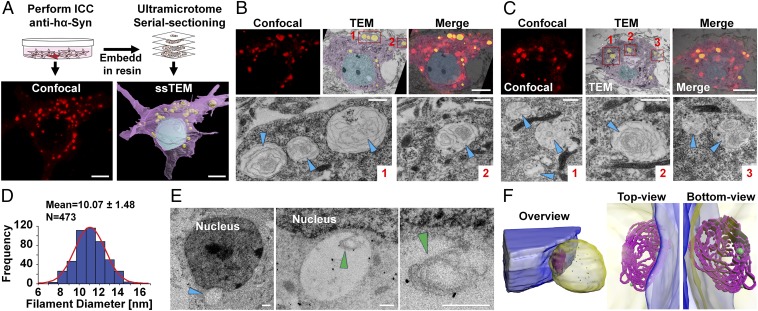
To establish whether the hα-Syn inclusions comprise the characteristic fibrillar ultrastructure observed in LBs, we performed correlative light and electron microscopy (CLEM) which allows ultrastructural examination of neurons previously identified by confocal microscopy as containing fluorescent inclusions (Fig. 2 A–C). Seventy-two hours posttransfection, hα-Syn inclusions were detected in close association with nuclear and cytoplasmic membranes or near mitochondria but were rarely found surrounded by a uniform lipid bilayer, thereby further ruling out the possibility that the inclusions are vesicular in nature. Remarkably, high-magnification ssTEM of the correlated inclusions revealed intertwining whirls of filamentous structures in most analyzed inclusions. Individual measurements of these structures showed that the filaments have an average diameter ranging from 7–13 nm (Fig. 2D), which is similar to that of fibrils within genuine LBs (24), and thereby suggests that hα-Syn could be aggregating into fibrils within these inclusions.
Fig. 2.
Ultrastructure of hα-Syn inclusions in SNCA−/− neurons. (A) For CLEM analysis, a stained neuron was imaged by confocal microscopy and then was reprocessed and imaged by TEM, resulting in a high-resolution 3D acquisition of the neuron. For illustration, the nucleus is rendered in blue, the cytosol in purple, and inclusions in yellow. (Scale bars: 5 µm.) (B) To correlate structures observed by ssTEM and fluorescence, single-plane images were superimposed. High-resolution images of inclusions (red boxes 1 and 2) are shown in the lower panel, with arrowheads indicating filamentous structures. (Scale bars: 5 µm in the upper panel and 2 μm in the lower panel.) (C) Same analysis as in B on different confocal and ssTEM planes from the same neuron. (D) Most filaments observed by ssTEM (n = 473) have a width ranging from 7–13 nm. The frequency histogram and normal curve (in red) are shown, as well as values of mean ± SD. (E) Immunogold labeling of SNCA−/− neurons expressing hα-Syn with the ab-6176 anti-synuclein antibody establishes that filaments are positive for synuclein. Different magnifications of the inclusion (blue arrowhead) and filaments (green arrowheads) are shown. (Scale bar: 1 µm in the left panel and 125 nm in the middle and right panels.) (F) A 3D model of the inclusion (blue arrowhead in E) reveals a mesh of intertwining filamentous structures (in purple) with gold particles reacting with the inner and outer portions. The nucleus is rendered in blue, the inclusion in yellow, and immunogold particles in green. Different views of the inclusion are shown.
To rule out the possibility that these flexible filamentous structures could represent membranes that have been entrapped within inclusions, we performed an additional CLEM experiment in which we treated neurons exhibiting de novo aggregates (composed of Myc–hα-Syn) with hα-SynAlexa633 PFFs for 24 h and then, within adjacent neurons, compared the ultrastructure and diameter of de novo filaments (comprising only Myc–hα-Syn), internalized PFFs, mixtures of both, and intracellular membranes (Fig. S4 B and C). As shown in Fig. S4 D and E, although intracellular membranes showed a broad distribution of diameters (7–12 nm with a mean average of 8.7 nm), internalized PFFs and mixtures of de novo formed filaments and PFFs showed a much more defined width distribution of 10–13 nm and a higher mean diameter of ∼11 nm. Interestingly, although filaments in de novo inclusions exhibited a broader width distribution compared to PFFs and mixtures (7–13 nm), with some of the filaments having a low diameter that could classify them as membranous, the great majority (∼75%) were in the range of 10–13 nm, within the narrow distribution of PFFs.
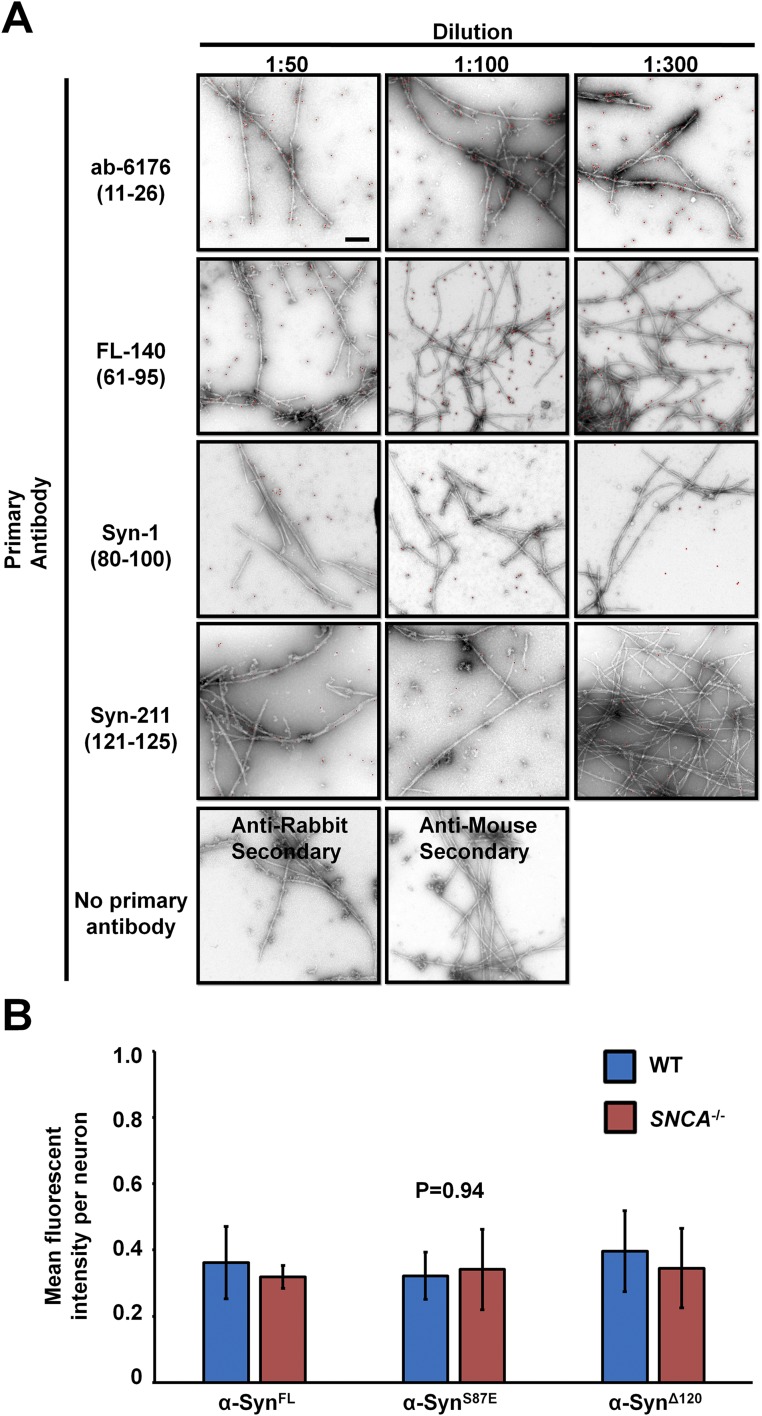
To determine whether hα-Syn is the constituent of the observed fibrillar structures, we sought to assess their reactivity with antibodies against α-Syn by immunogold labeling. First, we probed the ability of four different antibodies that have different epitopes against the N terminus, C terminus, and non-Abeta component (NAC) region of α-Syn to detect hα-Syn PFFs. Notably, we found that polyclonal anti–PAN-Syn antibodies such as ab-6176 and FL-140 detect PFFs much more readily than monoclonal hα-Syn–specific antibodies (Fig. S5A). Therefore, we assessed the reactivity of the fibrillar structures observed within hα-Syn–expressing SNCA−/− neurons to the ab-6176 antibody. Importantly, 3D reconstruction of ssTEM images clearly showed immunogold particles reacting with outer and inner portions of the filaments detected within inclusions (Fig. 2 E and F), thereby confirming the presence of synuclein within these structures.
Fig. S5.
Two separate control experiments compared the detection of PFFs with different anti-synuclein antibodies and the expression of the three α-Syn variants (WT, S87E, and 1-120) in SNCA−/− neurons. (A) hα-Syn PFFs were immunogold-labeled using different antibodies against synuclein: ab-6176, FL-140, Syn-1, and Syn-211 (respective epitopes in amino acid sequence are shown in parentheses) and then were negatively stained and imaged by TEM. Three different concentrations of primary antibodies were used: 1:50 (Left), 1:100 (Center), and 1:300 (Right), together with controls (primary antibodies were omitted). Colloidal gold particles are overlaid with red circles of the same size for better visualization. (Scale bar: 200 nm.) (B) Quantification of the mean fluorescent intensity per neuron (mean ± SD; n = 25 neurons per condition; three independent experiments) showed that no significant differences in fluorescence levels (P = 0.94; one-way ANOVA and Scheffé post hoc analysis) were detected across conditions.
Taken together, these findings demonstrate that the inclusions observed in SNCA−/− neurons exhibit similarities to LBs in terms of solubility and immunoreactivity, and demonstrate signs of bona fide fibrillization events. However, the filaments do not have an orderly arrangement with a dense core, as typically observed in brainstem LBs, but are embedded among an electron-translucent medium, which is probably composed of sequestered soluble α-Syn protein. Because the filaments are not organized as in authentic LBs, they may recapitulate early stages of LB formation that may need more time or additional factors to mature and remodel into more organized LB-like structures.
hα-Syn Inclusions in SNCA−/− Neurons Grow in Size and Incorporate Soluble α-Syn.
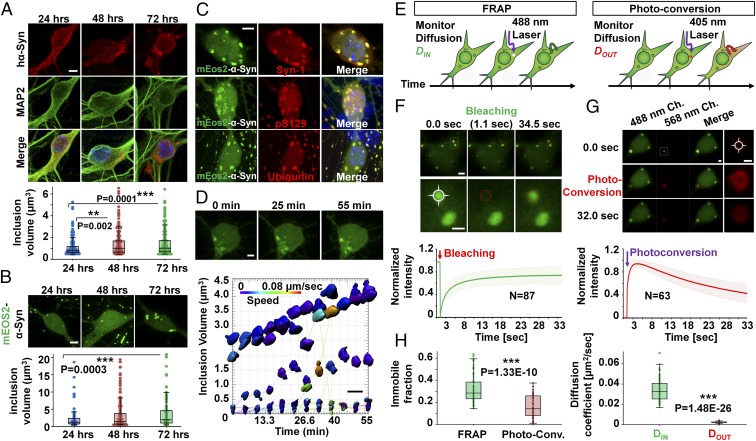
We then investigated mechanisms underlying hα-Syn inclusion growth over time using fluorescence microscopy. In SNCA−/− neurons transiently expressing hα-Syn, inclusions were detected 24, 48, and 72 h posttransfection, and the size of inclusions appeared to increase over time as assessed by quantifying 3D-rendered inclusion volumes (Fig. 3A). To investigate the kinetics of inclusion growth in real time, we performed fluorescence live-imaging experiments using hα-Syn that is N-terminally tagged to mEOS2, a fluorescent protein that is photoconvertible from bright green (506 nm) to orange-red (584 nm) when excited at near-UV wavelengths (25). As shown in Fig. 3B, somatic and neuritic mEOS2–α-Syn inclusions were detected 24 h posttransfection in SNCA−/− neurons and continued to grow in volume over 72 h. Moreover, the detected inclusions were reactive with antibodies against total α-Syn, ubiquitin, and pS129–α-Syn (Fig. 3C), suggesting that the fusion protein reproduces the aggregation properties of untagged hα-Syn and could be used to investigate the kinetics of inclusion formation in neurons.
Fig. 3.
hα-Syn inclusions in SNCA−/− neurons grow and incorporate soluble hα-Syn. (A) Immunofluorescence and 3D rendering of inclusions reveals a significant increase in inclusion volume at 48 and 72 h posttransfection in 25 MAP2+ neurons per condition. (B) Similarly, mEOS2–α-Syn inclusions (n = 50 per condition) show a significant increase in inclusion volume 72 h posttransfection. (C) mEOS2–α-Syn inclusions are immunopositive for total α-Syn (Syn-1), pS129–α-Syn (WAKO), and ubiquitin. (D) mEOS2–Syn inclusions are immobile and grow in volume during ∼1 h of live imaging. (Scale bars: 5 µm in A–C and 2 µm in D.) (E) FRAP and photoconversion experiments allow monitoring rates of Din and Dout of inclusions, respectively. (F and G, Upper) Fluorescence before, during, and after inclusion photobleaching (F) or photoconversion (G) is shown. Higher magnification images of yellow boxes are shown, with crossed circles denoting areas of photobleaching (F) or photoconversion (G). (Lower) Average plots (± SD) of normalized FRAP or photoconversion recovery curves (n = 87 or 63 inclusions, respectively). (Scale bars: 2 µm at lower magnification and 1 µm at higher magnification.) Ch, channel. (H) Values for immobile fractions and diffusion coefficients obtained from photoconversion curves (n = 50 inclusions) are significantly lower than those obtained by FRAP. In A, B, and H, the Mann–Whitney test was applied to obtain P values.
Short-interval (∼1 h) confocal live imaging followed by 3D surface rendering showed that the volume of individual inclusions increased over time, with very few reversible fusion events noted (Fig. 3D). Moreover, analysis of the speed of moving particles showed that most detected inclusions are immobile (Fig. 3D), thereby ruling out the possibility of inclusion growth being caused mainly by the fusion of small, motile aggregates. Notably, the percent increase in volume was variable among different inclusions, with some doubling their volume within 50 min and others showing modest changes. This observation suggests that the growth rate of inclusions is not linear and constant over time, and is not necessarily similar for the whole population of inclusions within the same neuron.
To determine whether mEOS2–α-Syn inclusions incorporate soluble hα-Syn over time, we performed fluorescence recovery after photobleaching (FRAP) and photoconversion live-imaging experiments (Fig. 3E), which allow assessing the rates of protein diffusion into (DIN) and out of (DOUT) inclusions respectively, and determining the relative proportion of immobile protein within inclusions (immobile fraction, IF). In FRAP experiments we photobleached individual inclusions and monitored the recovered fluorescence from diffusing soluble cytosolic protein. As shown in Fig. 3F and Movie S2, FRAP measurements showed the recovery of fluorescent signal within photobleached inclusions, and monoexponential fitting of FRAP plots from ∼90 different inclusions allowed the estimation of DIN and IF. The average value for DIN was very low (∼0.03 µm2/s), consistent with previous results for immobilized α-Syn–tetracysteine inclusions in SH-SY5Y cells (0.03–0.04 µm2/s) (26), suggesting the presence of a structure that impedes free diffusion of soluble hα-Syn. In line with this notion, ∼32% of the protein was estimated to be found within the IF, thereby establishing that inclusions comprise immobilized proteins that are unable to equilibrate with the cytoplasmic pool, likely because they are bound or sequestered in a dense compact structure. The presence of high amounts of soluble mobile protein (∼68%) within inclusions is in line with our ssTEM data showing filamentous structures embedded within a bulk of electron-translucent milieu, which is probably sequestered soluble hα-Syn protein.
To assess the DOUT, we used the photoconvertible property of mEOS2, where we photoconverted individual inclusions by near-UV irradiation and monitored the decrease in photoconverted fluorescence intensity within inclusions. The decay in fluorescence would reflect outward diffusion of proteins from the inclusions to the cytosol. As expected, irradiation of inclusions with a 405-nm laser resulted in rapid photoconversion of 488-nm mEOS2–α-Syn fluorescence to 568 nm (Fig. 3G and Movie S3). Strikingly, although exponential fitting of decay curves from ∼50 different neurons revealed a slightly lower estimation of the IF than obtained from FRAP experiments (16 ±10%), the estimated DOUT (0.0022 ± 0.0007 µm2/s) was almost 16-fold lower than the DIN (Fig. 3H). This result suggests that, within the same time period, significantly more protein enters into inclusions than diffuses out. As such, our findings together suggest that the hα-Syn aggregates in SNCA−/− neurons incorporate soluble protein into stable structures, most likely the fibrils observed by ssTEM.
Inclusion Formation in SNCA−/− Neurons Is an Aggregation-Driven Process.
To establish whether the formation of hα-Syn inclusions is an aggregation-driven process, we assessed whether altering the propensity of hα-Syn for aggregation affects the formation and/or growth of neuronal inclusions. Two modified hα-Syn proteins, a C-terminally truncated variant (at position 120) that has been shown to aggregate more readily than full-length α-Syn in vitro and in vivo (27), and a variant with the S87E substitution that inhibits hα-Syn aggregation in similar models (28, 29), were expressed in WT or SNCA−/− neurons. As shown in Fig. 4A, although similar proportions of SNCA−/− neurons expressing full-length (α-SynFL) or truncated (α-SynΔ120) hα-Syn developed inclusions, the S87E aggregation-deficient variant exhibited mostly diffuse distribution. This effect was not caused by a difference in protein expression levels among the three proteins, because similar mean fluorescence levels per neuron were detected across conditions (Fig. S5B). As expression of the α-SynΔ120 mutant did not affect the number of neurons forming inclusions, we investigated whether the kinetics of mEOS2–α-Syn inclusion growth are affected by this modification (Fig. 4B). Surface reconstruction of inclusions showed that at 24 h posttransfection mEOS2–α-SynΔ120 formed significantly larger inclusions than mEOS2–α-SynWT. In contrast, the few inclusions formed by the mEOS2–α-SynS87E mutant were much smaller compared to mEOS2–α-SynWT inclusions at 48 h.
Fig. 4.
hα-Syn inclusion formation in SNCA−/− neurons reflects an aggregation-driven process. (A) Significantly fewer SNCA−/− neurons expressing α-SynS87E develop inclusions (>1 µm) than their α-SynFL and SynΔ120 counterparts (mean ± SD, n = 3 independent experiments with 25 neurons per condition). (B) Live imaging and 3D rendering of inclusions (50 neurons per condition) show that mEOS2–α-SynΔ120 forms significantly larger inclusions 24 h posttransfection than mEOS2–α-SynWT, and that the mEOS2–α-SynS87E mutant forms smaller inclusions at 48 h in SNCA−/− neurons. (C) Significantly fewer tolcapone-treated SNCA−/− neurons expressing hα-Syn (25 neurons per condition) develop inclusions (>1 µm) than DMSO-treated controls (mean ± SD, n = 3 independent experiments). In A the one-way ANOVA and Scheffé post hoc analysis were applied, and in B and C the Mann–Whitney test was applied to obtain P values. Orth. Proj., orthogonal projection. (Scale bars: 5 µm.)
Furthermore, we investigated whether treatment with a pharmacological α-Syn aggregation inhibitor, tolcapone (30), would affect the formation of inclusions in this model. Remarkably, tolcapone treatment reduced the formation of inclusions by ∼60% and restored the diffuse distribution of hα-Syn in SNCA−/− neurons (Fig. 4C). Together, these results demonstrate that the formation of hα-Syn inclusions in SNCA−/− neurons is an aggregation-driven process, as inclusion formation was significantly inhibited both by the introduction of a single amino acid substitution known to inhibit hα-Syn’s aggregation propensity, and by treatment with a known aggregation inhibitor.
Endogenous β-Syn KO Is a Natural hα-Syn Aggregation Inhibitor in Neurons.
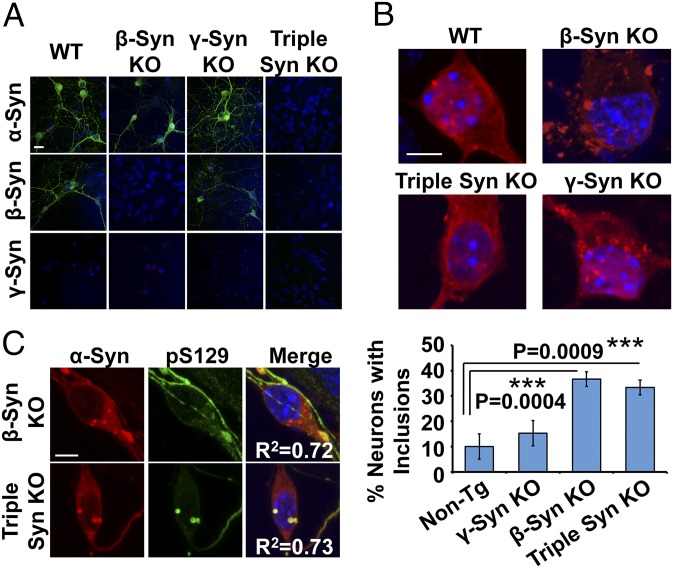
Our results suggest that homologous members of the synuclein family could act as natural physiological inhibitors of abnormal α-Syn aggregation. To test this hypothesis, we compared hα-Syn’s propensity for aggregation in primary neurons derived from mice lacking expression of β-Syn (β-Syn KO) (31), γ-Syn (γ-Syn KO) (32), or all three synucleins (triple KO) (33). Loss of α- and β-Syn in corresponding KO cultures was validated by immunocytochemistry (Fig. 5A); γ-Syn expression was not detectable in any of the cultures, probably because of its low expression levels in hippocampal neurons, as previously reported (34). Remarkably, when we assessed the distribution of exogenously expressed hα-Syn across the different cultures (Fig. 5B), we found that β-Syn–KO and triple-KO neurons similarly develop α-Syn inclusions, unlike WT controls and γ-Syn–KO neurons that mostly exhibit diffuse hα-Syn distribution. Importantly, the inclusions formed in β-Syn–KO and triple-KO neurons were also hyperphosphorylated at S129, thus exhibiting a key pathological LB-like phenotype (Fig. 5C). These findings suggest that, unlike endogenous γ-Syn, the other two homologs, α-Syn and β-Syn, have similar inhibitory effects on ectopic hα-Syn aggregation. Our observation that similar percentages of β-Syn–KO and triple-KO neurons develop inclusions (∼35%) rules out the presence of a synergistic effect for the deletion of both proteins.
Fig. 5.
Endogenous β-Syn naturally acts as an inhibitor of hα-Syn aggregation in primary neurons. (A) Immunocytochemistry validates the loss of α- and β-Syn expression in α-Syn–KO, β-Syn–KO, and triple Syn–KO neurons compared to WT neurons. (B) Significantly more β-Syn–KO neurons and triple-KO neurons (one-way ANOVA and Scheffé post hoc analysis) develop inclusions (>1 µm in diameter) compared to non-Tg neurons (mean ± SD, n = 3 independent experiments, 20 neurons per condition). (C) Inclusions formed in β-Syn–KO and triple Syn–KO neurons are pS129–α-Syn–positive. Colocalization was confirmed by assessing regression coefficients (R2). (Scale bars: 20 µm in A and 5 µm in B and C.)
The Absence of mα-Syn Promotes Specific Aggregation of hα-Syn in Vivo.
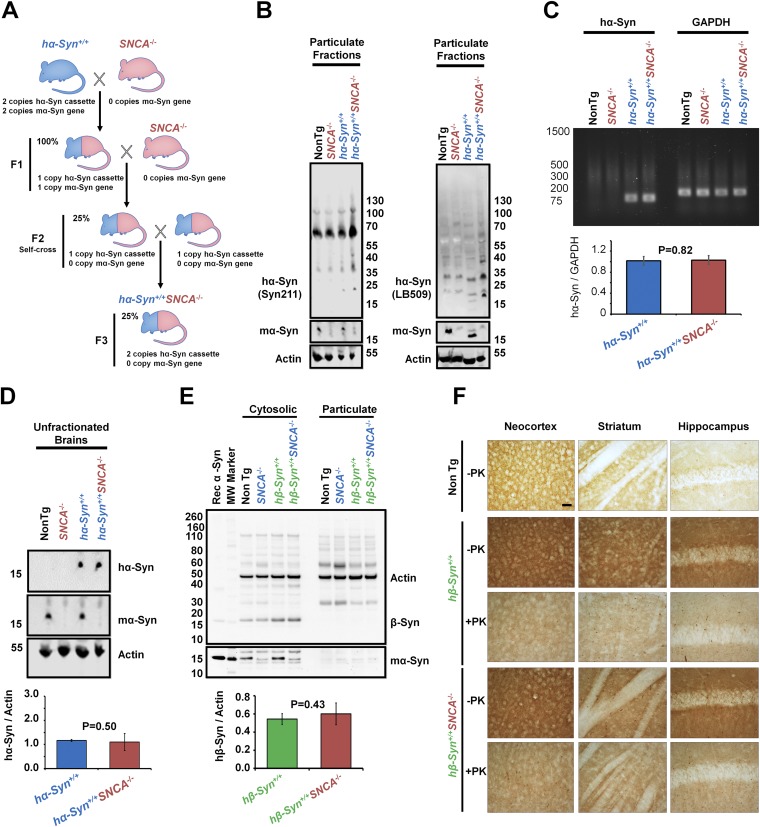
To test whether the presence of mα-Syn affects the aggregation propensity of hα-Syn in the brain, we crossed mice lacking endogenous mα-Syn (SNCA−/−) (13) with mice carrying two copies of hα-Syn (hα-Syn+/+) (Fig. S6A) (35) to generate (hα-Syn+/+SNCA−/−) mice. Biochemical fractionation of brain homogenates into cytosolic (soluble) and particulate (insoluble) protein fractions was performed to assess whether these mice exhibit changes in hα-Syn aggregation and solubility, compared to hα-Syn+/+ controls. In line with our previous data, less monomeric hα-Syn was detected in cytosolic fractions derived from hα-Syn+/+SNCA−/− mice than in cytosolic fractions derived from hα-Syn+/+ counterparts (Fig. 6A). This decrease in monomeric hα-Syn was concomitant with the appearance of HMW hα-Syn species within particulate fractions, which were detected using three different antibodies against hα-Syn, thereby establishing their specificity (Fig. 6A and Fig. S6B). Moreover, the decrease in soluble hα-Syn was not the result of decreased total hα-Syn expression in hα-Syn+/+SNCA−/− mice compared to hα-Syn+/+ counterparts, as similar total hα-Syn mRNA and protein levels were observed in unfractionated brains of both genotypes (Fig. S6 C and D). These findings suggest that hα-Syn aggregates readily and becomes less soluble in the absence of mα-Syn in Tg mouse brains.
Fig. S6.
Generation and characterization of Tg mice expressing hα-Syn or hβ-Syn in the absence of mα-Syn. (A) Schematic representation of the strategy used to generate Tg mice expressing hα-Syn in the absence of mα-Syn. First, Tg mice expressing hα-Syn (hα-Syn+/+; blue) under the PDGFβ promoter (35) were crossed with SNCA−/− mice (red) (13) to generate heterozygotes (F1). These mice then were backcrossed with SNCA−/− mice before being self-crossed to generate Tg mice expressing two copies of hα-Syn in the absence of any mα-Syn expression (hα-Syn+/+SNCA−/−, F3). (B–D) Brains of age-matched hα-Syn+/+ or hα-Syn+/+ SNCA−/− Tg mice (with non-Tg and SNCA−/− mice as controls) were lysed. Then, protein from particulate fractions (B), total RNA (C), or total proteins in unfractionated brains (D) were extracted. (B) Western blotting of particulate fractions using two different antibodies against hα-Syn [Syn-211 (Left) and LB-509 (Right)] shows increased HMW species in detergent-insoluble fractions of hα-Syn+/+ SNCA−/− Tg brains, compared with hα-Syn+/+ mice and non-Tg and SNCA−/− controls. (C) Semiquantitative RT-PCR using specific primers against hα-Syn or GAPDH (housekeeping gene for normalization) followed by agarose gel electrophoresis demonstrates that no significant differences in hα-Syn mRNA levels were detected between hα-Syn+/+ and hα-Syn+/+ SNCA−/− Tg mice brains. (D) Western blotting of unfractionated brains (homogenized and then centrifuged at 5,000 × g for 5 min) of hα-Syn+/+ or hα-Syn+/+ SNCA−/− mice showed that no significant differences in hα-Syn total protein levels were noted between the two conditions. hα-Syn and mα-Syn were differentially revealed using the Syn211 and D37A6 antibodies, respectively, and actin was used to control for equal protein loading. (E) hβ-Syn+/+ and hβ-Syn+/+SNCA−/− mice exhibit similar β-Syn levels in cytosolic fractions. β-Syn was not detected in particulate counterparts. Loss of mα-Syn expression in SNCA−/− mice is verified using the α-Syn–specific antibody SA-3400 and actin to assure equal protein loading. The Mann–Whitney test was applied in C–E to obtain P values, and three independent experiments were performed. (F) Compared with non-Tg mice, the brains of both hβ-Syn+/+ and hβ-Syn+/+SNCA−/− mice show increased terminal distribution of β-Syn in the neocortex, striatum, and hippocampus, that weakens upon proteinase K (PK) treatment. No hβ-Syn inclusions were noted across conditions. (Scale bar: 100 µm.)
Fig. 6.
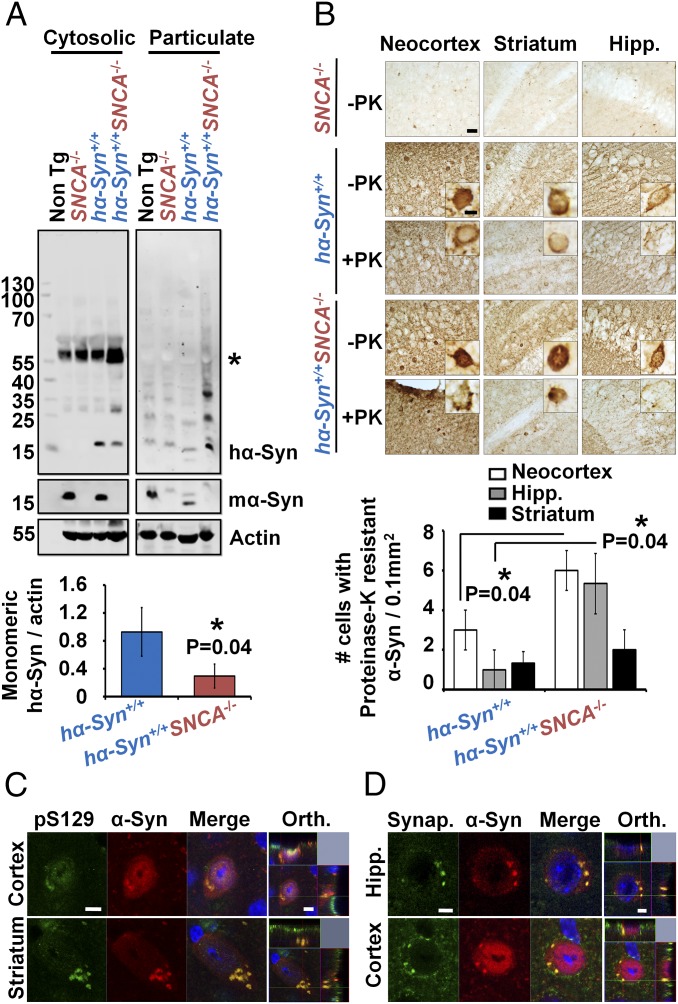
The absence of mα-Syn promotes hα-Syn aggregation in transgenic mice. (A) Significantly less monomeric hα-Syn is detected in cytosolic fractions from hα-Syn+/+SNCA−/− mice than in hα-Syn+/+ counterparts (n = 3 independent experiments), concomitant with the appearance of HMW hα-Syn species in particulate fractions. Loss of mα-Syn expression in SNCA−/− mice was verified using the mα-Syn–specific antibody D37A6, and actin was used to control for equal protein loading. The asterisk indicates a nonspecific band in cytosolic fractions. (B) Significantly more proteinase K (PK)-resistant hα-Syn inclusions/0.1 mm2 are detected in the neocortex and hippocampus (Hipp.) of hα-Syn+/+SNCA−/− mice than in hα-Syn+/+ mice (n = 3 mice per condition). (Scale bars: 100 µm in overviews and 15 µm in insets.) In A and B the Mann–Whitney test was applied to obtain P values. (C and D) Double-positive hα-Syn inclusions for total α-Syn and pS129 α-Syn (C) or synaptophysin (D) are detected in the cortex, striatum, and hippocampus of hα-Syn+/+SNCA−/− mice. Orthogonal (Orth.) Z-stack projections verify the intracellular nature of inclusions. (Scale bars: 5 µm.)
To evaluate further the extent of hα-Syn aggregation in mouse brains, we performed immunohistochemical analyses on sections treated with proteinase K, which allows selective visualization of highly structured proteinase K resistant aggregates. Consistent with previous studies (35), hα-Syn+/+ mice showed abundant intraneuronal α-Syn immunoreactive inclusions in the cortex, hippocampus, and striatum, some of which were proteinase K resistant (Fig. 6B). Strikingly, neurons from hα-Syn+/+SNCA−/− mice showed higher immunoreactivity to anti–α-Syn antibodies and exhibited a significant increase in proteinase K-resistant inclusions in the cortex and hippocampus (Fig. 6B). Moreover, double immunolabeling showed that these inclusions are phosphorylated at S129 and exhibit strong reactivity for the synaptic vesicle protein synaptophysin (Fig. 6 C and D), both of which are markers of LB pathology (20, 36). Taken together, these findings demonstrate that the aggregation propensity of hα-Syn is enhanced in the absence of mα-Syn in vivo, as reflected by decreased hα-Syn solubility and accumulation into proteinase K-resistant inclusions.
To assess whether abolishing mα-Syn expression would promote the aggregation of human β-Syn (hβ-Syn), the considerably less aggregation-prone homolog of hα-Syn, we crossed Tg mice expressing hβ-Syn (37) with SNCA−/− mice to generate hβ-Syn+/+SNCA−/− mice. Biochemical fractionation revealed that the absence of mα-Syn did not affect the levels of soluble hβ-Syn or cause the appearance of insoluble hβ-Syn species (Fig. S6E). In addition, immunohistochemical analysis showed that hβ-Syn was localized mostly within neuronal terminals and did not form any inclusions in the brains of hβ-Syn+/+ mice, as previously reported (37), or in the brains of hβ-Syn+/+SNCA−/− mice (Fig. S6F). These results show that α-Syn ablation does not promote the aggregation of hβ-Syn in vivo.
mα-Syn Interacts with Aggregated hα-Syn Species and Attenuates Seeding and Spreading.
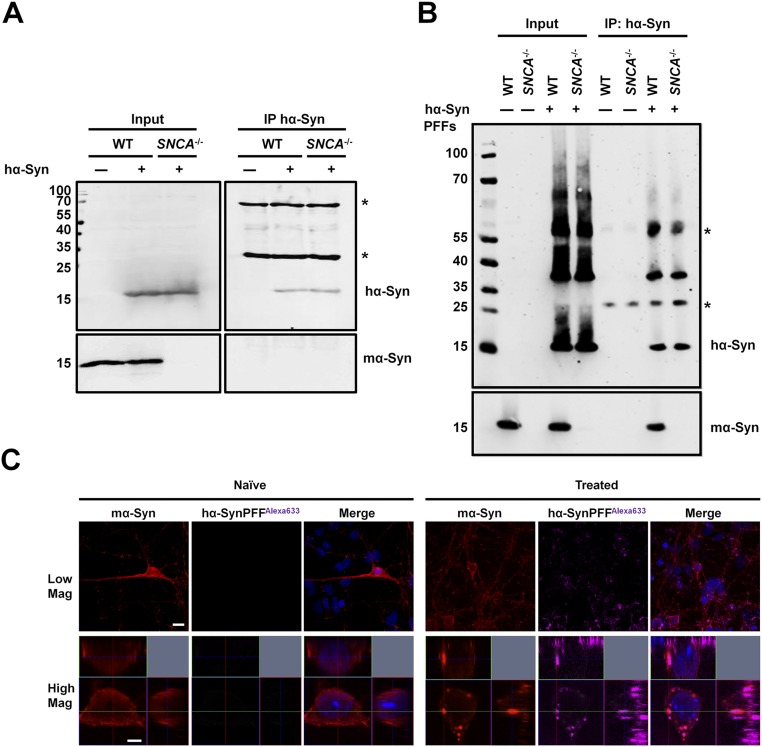
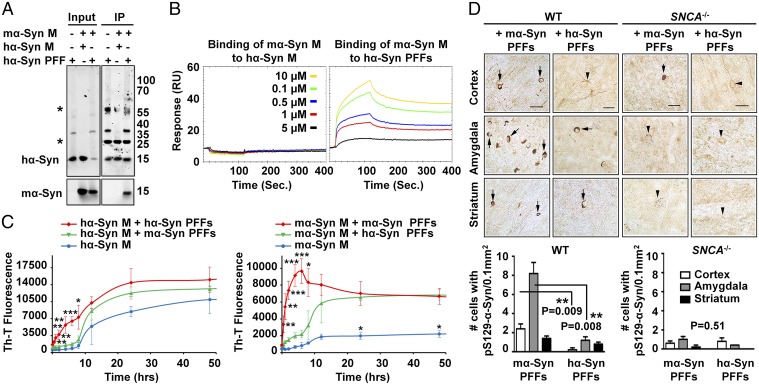
To investigate the mechanism by which mα-Syn affects hα-Syn aggregation, we first assessed whether overexpressed hα-Syn interacts directly with endogenous mα-Syn in WT primary neurons. Notably, we did not detect any interaction between the two proteins at the monomer level by coimmunoprecipitation (Fig. S7A). To investigate whether mα-Syn interacts with multimeric/aggregated hα-Syn species rather than hα-Syn monomers, we assessed the interaction of mα-Syn monomers with equimolar amounts of hα-Syn monomers or hα-Syn PFFs (characterized in Fig. S8). Intriguingly, mα-Syn was coimmunoprecipitated only when it was mixed with hα-Syn PFFs and not with hα-Syn monomers (Fig. 7A), indicating that monomeric mα-Syn might interact preferentially with hα-Syn PFFs in vitro. To confirm these findings and to obtain a quantitative assessment of the interaction between mα-Syn monomers and hα-Syn PFFs by an independent readout, we performed SPR measurements. Briefly, saturating amounts of either hα-Syn monomers or hα-Syn PFFs were immobilized by amine coupling to separate CM5 chips, and then increasing concentrations of mα-Syn monomers were flushed onto the chips. As shown in Fig. 7B, and in line with our immunoprecipitation experiments, no interaction was noted between injected mα-Syn monomers and immobilized hα-Syn monomers, even at the highest tested concentration for mα-Syn (10 µM). In contrast, mα-Syn monomers interacted readily with sonicated hα-Syn PFFs in a dose-dependent manner, even at concentrations as low as 0.1 µM.
Fig. S7.
Endogenous mα-Syn interacts with fibrillar rather than monomeric hα-Syn species in WT primary neurons. (A) WT or SNCA−/− primary neurons expressing hα-Syn were lysed, and hα-Syn was immunoprecipitated. Although hα-Syn was pulled down successfully, no mα-Syn was detected, thus ruling out the presence of a strong interaction between the two proteins in primary neurons. Western blotting of input solutions established similar protein levels before immunoprecipitation. (B) WT primary neurons treated with 0.1 µM sonicated hα-Syn PFFs for 3 d were lysed, and hα-Syn was immunoprecipitated. Endogenous mα-Syn was detected in the immunoprecipitated sample comprising hα-Syn PFFs. PFF-treated SNCA−/− primary neurons and naive WT and SNCA−/− neurons were used as controls. Western blotting of input solutions established similar protein levels before immunoprecipitation. The asterisks in A and B indicate the heavy and light chains of the antibody used for immunoprecipitation. (C) Immunolabeling of WT primary neurons treated with 0.1 µM sonicated hα-SynAlexa-633 PFFs for 24 h shows that internalized PFFs colocalize with endogenous mα-Syn. Orthogonal projections of Z stacks verify the intracellular nature of inclusions, and WT neurons treated with PBS (naive neurons) were used as controls. (Scale bars: 10 µm and 2 µm for low- and high-magnification images, respectively.)
Fig. S8.
Characterization of hα-Syn PFFs and mα-Syn. (A–C) Purified recombinantly expressed hα-Syn and mα-Syn monomers were characterized by SDS PAGE, which showed that the proteins run as single monomeric bands (A), by LC-MS analysis, which showed that the proteins have the correct mass (expected masses 14,461 Da for hα-Syn and 14,485 Da for mα-Syn) (B), and by UPLC analysis, which showed single sharp, symmetrical peaks for both proteins (C). The asterisks in C indicate peaks observed by UPLC in the blank and in samples. (D) Monomeric hα-Syn was incubated for 5 d at 37 °C with shaking to form PFFs that were sonicated and then characterized by Coomassie gel, which showed that sonicated PFFs sediment into the pellet upon centrifugation (Upper Left); by assessment of ThT binding (mean ± SD), which showed that nonsonicated (NS) and sonicated (S) PFFs comprise the amyloid structure, unlike the initial protein solution before incubation (t = 0) (Upper Right); and by TEM analysis (Lower), which validated the fibrillar nature of the PFFs and showed that sonication led to the generation of small fibrillar fragments. (E) The same analysis as shown in D but performed for mα-Syn. A.U., arbitrary units. (Scale bar: 200 nm.)
Fig. 7.
mα-Syn interacts with aggregated hα-Syn species and attenuates seeding and spreading. (A) Following immunoprecipitation (IP) of hα-Syn, mα-Syn monomers (M) were detected only in the sample comprising the mixture of mα-Syn M with hα-Syn PFFs, and not with hα-Syn M. The asterisks indicate the heavy and light chains of the antibody used for IP. (B) A dose-dependent response (interaction) was noted by SPR between immobilized hα-Syn PFFs and injected mα-Syn M, but not between the injected mα-Syn M and immobilized hα-Syn M. (C) Mixtures of α-Syn PFFs and monomers of the same species show significantly higher ThT binding at early time points than seen with α-Syn monomers alone or with PFFs of different species (n = 3 independent experiments). Mean ± SD values are shown. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way ANOVA and Scheffé post hoc analysis). (D) Significantly more pS129–α-Syn inclusions (arrows) are observed in the cingulate cortex and amygdala of WT mice injected with mα-Syn PFFs (n = 5 mice per condition) than in counterparts injected with hα-Syn PFFs or in SNCA−/− mice injected with hα-Syn/mα-Syn PFFs, which show diffuse staining (arrowheads). The Mann–Whitney test was applied to obtain P values. (Scale bar: 100 µm.)
To investigate whether endogenous mα-Syn similarly interacts with aggregated hα-Syn species in primary neurons, we treated WT neurons with sonicated hα-Syn PFFs and assessed interaction by immunoprecipitation and immunofluorescence analyses. Importantly, and in line with our in vitro results, mα-Syn coimmunoprecipitated with pulled-down hα-Syn PFFs (Fig. S7B). Moreover, confocal imaging revealed that internalized hα-Syn PFFs colocalize with endogenous mα-Syn (Fig. S7C), which redistributes from a diffuse somatic localization into defined puncta as previously described (22). Together, these results show that mα-Syn interacts preferentially with aggregated hα-Syn PFFs in vitro and in neurons.
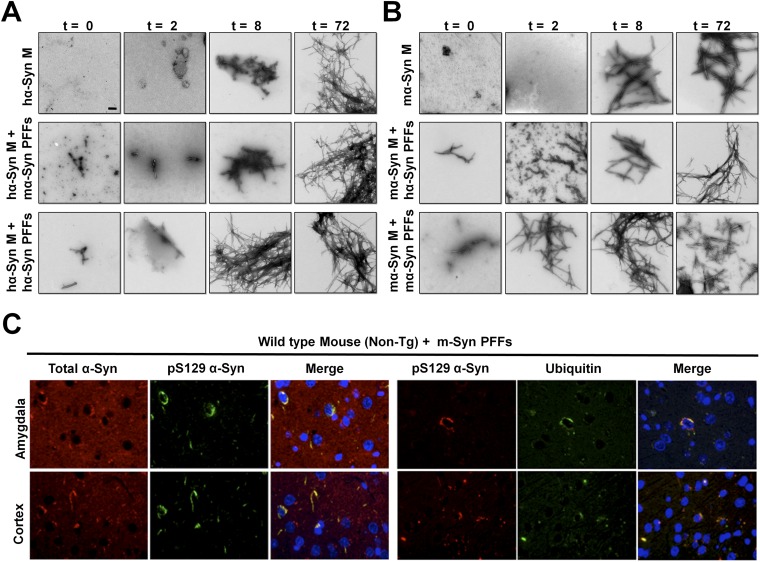
Next, we explored whether such an interaction between α-Syn PFFs and α-Syn monomers from different species would seed or, alternatively, attenuate progressive monomer aggregation. To do so, we assessed the aggregation kinetics of hα-Syn or mα-Syn monomers in the presence of either hα-Syn PFFs or mα-Syn PFFs (characterized in Fig. S8). Strikingly, assessment of thioflavin-T (ThT) binding and the formation of fibrillar structures by TEM showed that the seeding efficiency is significantly higher when hα-Syn monomers and PFFs are of the same species (Fig. 7C and Fig. S9 A and B). For instance, whereas the lag phase was eliminated in the mixture of hα-Syn monomers and hα-Syn PFFs, resulting in the appearance of fibrillar structures at 8 h of incubation, the aggregation profile of the mixture of hα-Syn monomers and mα-Syn PFFs was similar to that of hα-Syn alone, in which only oligomeric species were formed at 8 h of incubation. Interestingly, the mixture of mα-Syn monomers with mα-Syn PFFs showed the most dramatic elimination of the lag phase, with fibrils formed after only 2 h of incubation. Here again, although the addition of hα-Syn PFFs to mα-Syn monomers resulted in higher ThT plateau values than seen with mα-Syn monomers alone, it failed to eliminate the lag phase and resulted in the formation of oligomeric species at 2 h. Taken together, these findings strongly show that the aggregation kinetics of α-Syn are significantly accelerated when the seeds and monomers are from the same species, and that the presence of mixed species of monomers and PFFs results in reduced seeding capacity and slower aggregation kinetics.
Fig. S9.
α-Syn PFFs preferentially seed the aggregation of monomeric α-Syn of the same species in vitro and in vivo. (A and B) Recombinant hα-Syn (A) or mα-Syn (B) monomers (M) were incubated either alone (20 µM) or with 10% sonicated hα-Syn PFFs or mα-Syn PFFs. TEM analysis of samples at 0, 2, 8, and 72 h shows that mixtures of α-Syn PFFs and monomers of the same species start forming fibrils at earlier time points than α-Syn monomers alone or mixtures with PFFs of different species. (Scale bar: 200 nm.) (C) mα-Syn PFFs were injected into the striatum of WT (nontransgenic) mice, and α-Syn pathology was assessed in different brain regions 1 mo postinjection. Double-immunolabeling for total α-Syn and pS129–α-Syn or pS129–α-Syn and ubiquitin shows that the somatic inclusions found in the amygdala and cortex of WT mice injected with mα-Syn PFFs are phosphorylated at S129 and are ubiquitinated.
To evaluate whether the seeding and spreading potential of α-Syn PFFs is similarly affected by the presence of PFFs and endogenous monomers of the same species in the brain, we injected mα-Syn PFFs or hα-Syn PFFs into the striatum of WT (non-Tg) or SNCA−/− mice (as controls) and then compared the seeding and spreading propensity in different brain regions 1 month postinjection. Strikingly, we observed the most pronounced spreading of α-Syn pathology only when mα-Syn PFFs were injected into WT mice expressing endogenous mα-Syn (Fig. 7D); in these mice mα-Syn accumulated into somatic inclusions that were ubiquitinated and hyperphosphorylated at S129 in the cortex and amygdala (Fig. S9C). In contrast, hα-Syn PFFs induced significantly less pathology at these regions in WT mice at the same time point, and SNCA−/− mice showed very limited overall pathology following injection with either hα-Syn PFFs or mα-Syn PFFs. These findings are consistent with the significantly reduced seeding efficiency of hα-Syn PFFs in the presence of monomeric mα-Syn in vitro; this reduced seeding efficiency may allow more time for natural inhibitory or clearance mechanisms to interfere with the seeding and spreading of exogenous hα-Syn PFFs in mouse brains.
Discussion
The aggregation of α-Syn into LBs has been directly linked to the pathogenesis of PD (2). The mechanism of formation and the precise effect of LBs on neuronal physiology and viability remains poorly understood, mostly because of the lack of animal and cellular models exhibiting de novo α-Syn fibrillization into LB-like structures. Although doubling α-Syn expression is sufficient to cause PD in humans, attempts to reproduce molecular PD pathology based on α-Syn overexpression in various animal models have not been successful. This lack of success could imply the presence of cellular factors that inhibit hα-Syn aggregation in these models. In this study, we sought to systematically examine whether endogenous α-Syn homologs attenuate hα-Syn aggregation in mouse neurons. We found that a substantial fraction of SNCA−/− neurons expressing hα-Syn develop inclusions that exhibit key LB-like features, including S129 hyperphosphorylation, ubiquitination, and ThS reactivity. Importantly, this phenomenon reflected de novo hα-Syn fibrillization, as revealed by ssTEM following both CLEM and immunogold-labeling experiments. To the best of our knowledge, none of the previously published cellular models that rely solely on the overexpression of hα-Syn have reported inlcusions that exhibit these features altogether.
Multiple lines of evidence from many of our experiments further converged to establish that the formation of inclusions in SNCA−/− neurons is an aggregation-driven process. Overexpression of the aggregation-incompetent mutant S87E or treatment with the pharmacological α-Syn aggregation inhibitor tolcapone significantly reduced the formation of inclusions. In contrast, overexpression of the aggregation-prone truncated hα-Syn variant (1-120) resulted in the formation of larger inclusions than seen with the WT protein. In all cases in which we observed increased inclusion formation, we also observed decreased hα-Syn solubility and the appearance of HMW aggregates, concomitant with the loss of monomers. Moreover, time-lapse live imaging coupled with FRAP and photoconversion experiments showed in real time that the inclusions grow in size and readily incorporate soluble hα-Syn protein. Interestingly however, when we explored the toxic potential of these inclusions, we found that the aggregates formed do not provoke increased toxicity (SI Discussion and Fig. S10). This finding is consistent with previous studies suggesting that intracellular hα-Syn aggregates may be protective in cellular models (38, 39). Further studies are underway to determine whether these aggregates exhibit a similar role under conditions of cellular stress, such as treatment with mediators of oxidative stress or inhibitors of protein degradation, or other molecules known to contribute to PD pathogenesis.
Fig. S10.
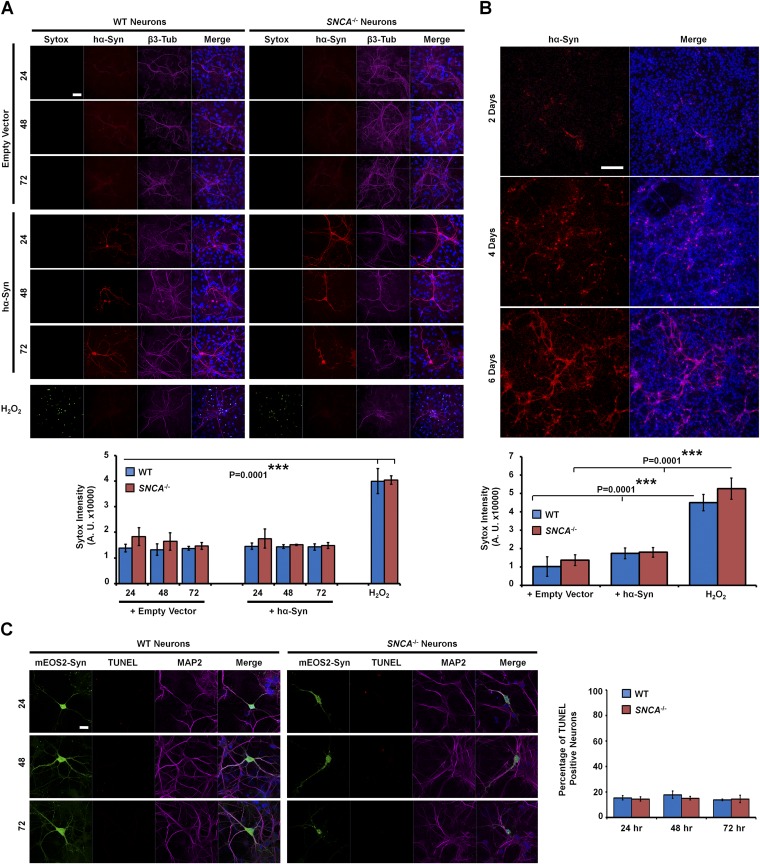
hα-Syn inclusions are not toxic to SNCA−/− neurons. (A, Upper) The SYTOX Green dye exclusion assay was used to assess the amounts of dead neurons upon transfection with empty vector or hα-Syn for 24, 48, and 72 h. Neurons were stained with the Syn-211 antibody and with the anti–β3-tubulin antibody to reveal transfected and total neurons, respectively. Neurons treated with 200 μM H2O2 for 24 h were used as positive controls showing toxicity (i.e., SYTOX Green uptake). (Lower) Quantification of SYTOX Green fluorescence intensity over time reveals no significant differences across conditions other than the highly significant increase upon H2O2 treatment. (B, Upper) Immunocytochemistry using the Syn-211 antibody shows that lentiviral infection mediates most prominent expression of hα-Syn at 6 d postinfection. (Lower) The SYTOX Green dye exclusion assay shows no significant differences in SYTOX Green uptake between WT and SNCA−/− neurons expressing hα-Syn or the empty vector control, unlike neurons treated with 200 μM H2O2, which exhibit prominent uptake of SYTOX Green. In A and B, one-way ANOVA and Scheffé post hoc analysis were applied to obtain P values. Three independent experiments were performed. (C, Left) Representative images showing that WT and SNCA−/− neurons expressing mEOS2–α-Syn are not TUNEL+ at any time point after transfection. MAP2 staining was used to identify neurons. (Right) Quantification of 75 neurons per condition (n = 2 independent experiments) reveals similar low percentages of TUNEL+ WT and SNCA−/− neurons expressing mEOS2–α-Syn. (Scale bars: 50 µm in A, 200 µm in B, and 20 µm in C.)
Having established that SNCA−/− primary neurons are more permissive of hα-Syn aggregation in culture, we sought to validate this finding in vivo. Two previous independent studies had suggested that the expression of hα-SynΔ120 or hα-SynA53T in Tg mice lacking mα-Syn expression leads to the formation of fibrillar inclusions within the substantia nigra or spinal cord (5, 6). Therefore, we directly compared the aggregation propensity of hα-Syn in brains of mice expressing or lacking endogenous mα-Syn. Our data showed that hα-Syn exhibits enhanced aggregation propensity in the absence of mα-Syn, as reflected by decreased solubility, enhanced detection of HMW oligomeric species, and the formation of abundant proteinase K-resistant and pS129 hyperphosphorylated inclusions. To rule out the possibility that knocking out mα-Syn promotes nonspecific aggregation, we conducted similar studies on hβ-Syn, the α-Syn homolog that is significantly less prone to aggregate. As expected, knocking out mα-Syn did not affect the levels, distribution, or staining pattern of hβ-Syn in Tg mice.
Our observation that the reintroduction of mα-Syn in SNCA−/− neurons significantly attenuates the formation of hα-Syn inclusions suggests that the mouse homolog might directly inhibit hα-Syn aggregation. Intriguingly, although we could not detect a prominent interaction between hα-Syn and mα-Syn at the monomeric level in neurons, we consistently observed that monomeric mα-Syn preferentially interacts with sonicated PFFs in vitro and in neurons. Moreover, we found that this interaction does not promote progressive seeding and spreading of pathology in vitro or in mouse brains in vivo. As such, endogenous mα-Syn might directly inhibit hα-Syn fibrillization in WT primary neurons by stabilizing oligomeric species and capping the terminals of early fibrillar structures formed by hα-Syn. This hypothesis is in line with the early study by Rochet et al. (7) showing that mα-Syn inhibits the fibrillization of hα-Syn by stabilizing oligomers on the pathway to amyloid formation in vitro. Moreover, it is consistent with literature reports demonstrating that the propagation and spreading of α-Syn pathology is prominent when the injected PFFs and host-expressed monomers are from the same species. In most of these studies, seeding and spreading of α-Syn pathology was observed when mα-Syn PFFs were injected into non-Tg mice expressing mα-Syn (40–42) or when hα-Syn PFFs were injected into Tg mice expressing human α-Syn protein (43–48).
An attractive general implication of our findings is that the attenuation of aggregation by homologous proteins could represent a generic biological phenomenon. This proposition is consistent with multiple studies reporting decreased aggregation kinetics in the presence of homologous amyloidogenic proteins underlying different diseases. For instance, the key peptides involved in the formation of amyloid plaques in Alzheimer’s disease, Aβ40 and Aβ42, were shown to inhibit each other’s aggregation reciprocally in vitro in a concentration-dependent manner (49). Moreover, mutant hemoglobin β-chain polymerization in sickle cell anemia was shown to be attenuated upon increasing ratios of its γ-chain homolog (50). Likewise, in transthyretin (TTR) amyloid diseases, murine/human TTR homologs form heterotetramers that impair amyloid formation (51, 52), and aggregation in vivo was achieved mostly in Tg mice expressing TTR mutants on an endogenous TTR-KO background (52). Finally, the aggregation kinetics of the islet amyloid polypeptide (IAPP) in bulk solution was reduced dramatically by the presence of its rat homolog (53, 54).
In light of these findings, we investigated whether this phenomenon could be extended to the other endogenous hα-Syn homologs, β-Syn and γ-Syn. Interestingly, we found that β-Syn–KO cultures also developed hα-Syn inclusions that were hyperphosphorylated at S129. In contrast, the distribution of hα-Syn was not affected when expressed in γ-Syn–KO neurons, because the levels of γ-Syn in WT hippocampal neurons were already very low and therefore could not be reduced drastically in γ-Syn–KO neurons. These results are also in line with previous reports showing that β-Syn inhibits α-Syn aggregation in vitro (55, 56) as well as in vivo; double-Tg mice coexpressing both hα-Syn and hβ-Syn exhibit ameliorated motor deficits and lower α-Syn accumulation compared to Tg mice expressing hα-Syn alone (37, 57). However, our results further suggest that the total levels or ratio of both endogenous α- and β-Syn could be critical in protecting against hα-Syn aggregation. Once this level drops below a certain threshold (in either single or triple KOs), the aggregation of hα-Syn into LB-like inclusions becomes progressive. Supporting this proposition is the observation that intermediate reduction of mα-Syn levels by shRNA did not promote the formation of hα-Syn inclusions in WT neurons.
In summary, we have shown that two endogenous members of the synuclein family, mα-Syn and mβ-Syn, attenuate the aggregation of overexpressed hα-Syn in mouse neurons. Taken together with the multitude of studies that have reported the inhibition of aggregation of other amyloidogenic proteins by their homologues (such as Aβ, TTR, hemoglobin, and IAPP), this observation could reflect a more generic biological phenomenon in which endogenous homologs act as natural inhibitors of abnormal aggregation. This phenomenon could explain why most current rodent PD models relying on hα-Syn overexpression in WT mice do not show prominent hα-Syn fibrillization. As such, understanding the interactions between homologous proteins and the effect of such interactions on protein aggregation and amyloid formation could facilitate the generation of novel cellular and in vivo models of protein misfolding. The ability to assess the dynamics of inclusion formation quantitatively in our neuronal model provides unique opportunities to elucidate molecular and cellular determinants that influence the mechanisms of α-Syn fibrillization in living neurons, and to identify pathways that modulate this process and contribute to neurodegeneration. Further studies using this model could make it possible to identify novel drugs for the treatment of PD based on modulating α-Syn aggregation and interneuronal spreading, or enhancing the degradation and clearance of toxic α-Syn aggregates.
SI Discussion
To investigate the consequence of hα-Syn aggregation into fibrillar structures on the viability of the SNCA−/− neurons, we used the SYTOX Green vital dye (Invitrogen) and the TUNEL (Roche) marker of apoptosis as readouts. Notably, hα-Syn expression for 24, 48, or 72 h did not provoke neuronal death in WT neurons, as previously reported (8, 38, 59), or in SNCA−/− primary cultures (Fig. S10A), indicating that hα-Syn expression is not toxic to these neurons per se. As the relatively low transfection efficiency of neurons could mask subtle effects, we infected the neurons with lentiviruses that mediate more efficient neuronal transduction of hα-Syn, with maximal expression occurring 6 d postinfection (Fig. S10B). Again, however, assessment of cellular death using the SYTOX Green assay revealed no significant differences between WT and SNCA−/− neurons infected with hα-Syn or with the empty vector control, in contrast to the 24-h H2O2 treatment that induced cellular death and prominent staining with SYTOX Green (Fig. S10B). A possible explanation of this result is that only a fraction of SNCA−/− neurons exhibit inclusions, and hence the effects could be diluted by non-inclusion–forming neurons. To address this possibility, we counted individual WT and SNCA−/− neurons expressing mEOS2–α-Syn undergoing apoptotic death as identified by the TUNEL assay. Although inclusion-bearing SNCA−/− neurons expressing mEOS2–α-Syn appeared to have less defined neurites, similar low percentages of TUNEL+ mEOS2–α-Syn–expressing neurons were observed across the different cultures (Fig. S10C). Thus, these results together intriguingly rule out hα-Syn aggregation into fibrillar LB-like inclusions as being predominantly toxic to cultured primary hippocampal neurons at the tested time points.
Materials and Methods
Full experimental details are provided in SI Materials and Methods.
Approval of Animal Experimentation.
All animal experiments were performed in accordance with guidelines and regulations of the Ecole Polytechnique Fédérale de Lausanne (EPFL), Cardiff University, and University of California San Diego, and were approved by the Swiss Federal Veterinary Office.
Primary Neuron Culture Preparation, Transfection, Infection, and Tolcapone Treatment.
Primary hippocampal and cortical neuronal cultures were prepared from postnatal day 0 C57BL/6JRccHsd (WT; Harlan Laboratories), C57BL/6JOlaHsd (α-Syn KO; Harlan Laboratories) (12), B6,129 × 1-Sncatm1Rosl/J (α-Syn–KO; Jackson Laboratories) (13), β-Syn–KO (31), γ-Syn–KO (58), or α-β-γ-Syn–KO (33) mice as previously described (10). Briefly, dissected tissues were dissociated with papain and triturated using a glass pipette. After centrifugation at 400 × g for 2 min, cells were plated in minimum essential medium (MEM)/10% (vol/vol) horse serum onto poly-l-lysine (Sigma)–coated coverslips [12 mm glass (VWR International) for immunocytochemistry or 13 mm plastic (Thermanox, Nunc) for immunogold staining and TEM] at 1.5 × 105 cells/mL, or at 3 × 105 cells/mL in 35-mm confocal dishes (World Precision) for live imaging, or in 10-cm dishes (BD Biosciences) for biochemical analysis. Medium was changed after 4 h to Neurobasal/ B27 medium, and neurons were treated with Arabinosylcytosine (AraC; Sigma) after 6 d in vitro (DIV) to stop glial division. At 7 DIV, neurons were either transiently transfected for up to 3 d using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions or were infected with lentiviruses at a multiplicity of infection (MOI) of 10 for up to 7 d. When indicated, at 24 h posttransfection, neurons were treated with 20 µM tolcapone (Sigma) for an additional 24 h before immunocytochemistry.
SI Materials and Methods
Plasmids, shRNA Sequences, and Lentiviral Production.
The generation of the plasmid encoding mEOS2–α-Syn has been described previously (10). Briefly, hα-Syn cDNA was amplified by PCR and cloned into the pmEOS2-C1 plasmid (minus the start codon) to generate the mEOS2–α-SynFL fusion construct. The mEOS2–α-SynS87E and the mEOS2–α-SynΔ120 mutants were produced using the QuikChange site-directed mutagenesis system (Stratagene) to introduce the S87E mutation or a stop codon at position 120, respectively.
The plasmids encoding the fluorescent markers for early endosomes (RhoB-YFP) and autophagosomes (LC3-GFP) were obtained from BD Biosciences, Clontech. The plasmid encoding GFP-CD63 was kindly provided by Remy Sadoul, Université Joseph Fourier, Grenoble, France, plasmids encoding fluorescently tagged markers of the endo-lysosomal pathway (Rab5-RFP, Rab7-GFP, and Lamp1-RFP) were kindly provided by Darren Moore, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, the pRK172–mα-Syn plasmid was kindly provided by Virginia Lee, University of Pennsylvania, Philadelphia, and the pcDNA6–hα-Syn and pT7-7–hα-Syn plasmids were kindly provided by Peter Lansbury, Harvard Medical School, Boston. The V16C mutation then was introduced into the pT7-7–hα-Syn plasmid using the QuikChange site-directed mutagenesis system (Stratagene) to allow site-specific fluorescent labeling.
Coding sequences (ORFs) of hα-SynFL, hα-SynS87E, hα-SynΔ120, or mα-Syn were individually subcloned by Amsbio into a CMV-driven lentivector. A null control sequence (200 bp) was cloned in the same vector as a negative control. Three shRNA sequences against mα-Syn (NM_001042451) were also designed and cloned by Amsbio into a lentiviral-H1-shRNA expression vector comprising RFP-Puromycin as a dual marker. A control shRNA sequence having minimal off-target effects was cloned into the same vector:
shRNA mα-Syn sequence #1: ATATAGCTGCTGCCACTGGCT
shRNA mα-Syn sequence #2: GGAAGGAATCCTGGAAGACAT
shRNA mα-Syn sequence #3: GTCCTCTATGTAGGTTCCAAA
shRNA scrambled sequence: GTCTCCACGCGCAGTACATTT
Lentiviral particles were produced and titered by Amsbio using the P24 antigen-capture assay kit (catalog no. 5421; Advanced BioScience Laboratories, Inc.) in HEK 293T cells or via RFP fluorescent cell counting at 72 h posttransduction on HT1080 cells for shRNA-encoding viruses. Viral titers ranged from 4.88 × 106 to 6.82 × 106 infectious units (ifu)/mL.
Recombinant Protein Expression and Purification.
As previously described (10), BL21(DE3) cells were first transformed with pT7-7–hα-Syn, pRK172–mα-Syn, or pT7-7–hα-SynV16C and then were grown in LB medium at 37 °C. After induction with 1 mM 1-thio-β-d-galactopyranoside (AppliChem) for 4 h, sonication was performed to lyse and pellet the bacteria. The supernatant obtained after centrifugation at 48,000 × g (20 min) was boiled for 5 min and recentrifuged for 20 min. The resulting supernatant was purified by anion exchange (HiPrep 16/10 Q FF; GE Healthcare Life Sciences) followed by gel filtration (HiLoad 26/60 Superdex 200; GE Healthcare Life Sciences), reverse-phase C4 HPLC (Proto 300 C4, 20 mm i.d. × 250 mm, 10 µm average bead diameter; Higgins Analytical) and finally was lyophilized. Purity was established by loading 1 µg of protein on 15% (vol/vol) SDS/PAGE gels, staining with Coomassie stain (Life Technologies) for 5 min, and then destaining with boiled water until distinct bands appeared. To determine the exact mass and to rule out the presence of other contaminants, mass spectrometry and ultra-high performance liquid chromatography (UPLC) were performed as explained below.
Mass Spectrometry and UPLC.
As previously described (60), proteins were analyzed by LC-MS using either an LTQ system (Thermo Scientific) or an LCQ Fleet mass spectrometer. Protein solutions were desalted before mass spectrometry analysis by reversed-phase chromatography using either a Hypersil GOLD C18 column (4.6 × 150 mm, 5 μm) (Thermo Scientific) on the LCQ Fleet System or a Poroshell 300SB C3 column (1.0 × 75 mm, 5 μm) (Agilent Technologies) on the LTQ system. In both cases, 10-μL samples were injected into the column, and a linear gradient from 5–95% of solvent B against solvent A was deployed to elute proteins over 10 min, with solvent A comprising 0.1% formic acid in ultrapure water and solvent B consisting of 0.1% formic acid in acetonitrile. Flow rates were 500 μL/min (LTQ System) and 300 μL/min (LCQ Fleet System). MagTran software (Amgen Inc.) was used to deconvolve charge states.
UPLC analysis of proteins was performed on an Acquity UPLC BEH300 C18, 1.7-µm pore size, 2.1 × 100 mm column (Waters) or an Acquity UPLC BEHC4, 1.7-µm pore size, 2.1 × 100 mm column (Waters), with a linear gradient of 10−90% of solvent B against solvent A over 4 min [solvent A was water/0.1% trifluoroacetic acid (TFA), and solvent B was acetonitrile/0.1% TFA].
Preparation of hα-SynAlexa633–Labeled Protein.
Recombinant hα-SynV16C was dissolved in 200 mM Tris, pH 7.0 (Trizma hydrochloride solution; Sigma) at a final concentration of 100 µM. After two equivalents (final concentration 200 µM) of Tris(2-carboxyethyl)-phosphine hydrochloride (TCEP) (Sigma-Aldrich) were added to the protein solution followed by incubation for 5 min at room temperature, five equivalents (500 µM) of Alexa-Fluor 633 maleimide (Pierce) were added to the solution for 1 h, resulting in 95% hα-SynV16C-Alexa633 as shown by electrospray ionization (ESI)-MS. To separate the free dye from the labeled hα-SynAlexa633, PD10 (GE Healthcare) gel filtration columns were used according to the manufacturer’s instructions. Briefly, after the column was equilibrated with 50 mL of H2O, 25 mL of the protein solution was added and then was eluted with H2O. Collected hα-Syn fractions were identified by SDS/PAGE and Coomassie staining (Life Technologies), and by MALDI-TOF-MS and ESI-MS analysis to verify the correct mass of the labeled protein. The hα-SynAlexa633 fractions then were combined and lyophilized.
Preparation and Characterization of PFFs.
To prepare hα-SynAlexa633–labeled PFFs, a 300-µM solution comprising unlabeled recombinant hα-Syn with 10% hα-SynAlexa633 was prepared by dissolving lyophilized proteins in 50 mM Tris and 150 mM NaCl, pH 7.5. The resulting solution was centrifuged at 14,000 × g (10 min at 4 °C), and the supernatant then was incubated at 37 °C under constant agitation at 850 rpm on an orbital shaker for 5 d. The generated hα-Syn fibrils were sonicated briefly (40% amplitude, one pulse for 5 s) and then were aliquoted and stored at −20 °C. To prepare unlabeled hα-Syn or mα-Syn PFFs, recombinant proteins were processed similarly and were incubated at similar concentrations and incubation conditions.
Amyloid formation (before and after sonication) was assessed by ThT binding, remaining soluble protein content, and TEM. ThT fluorescence reading (excitation at 450 nm, emission at 485 nm) was performed using a Bucher Analyst AD plate reader after protein samples were incubated with ThT (10 μM, in 50 mM glycine, pH 8.5) in black 384-well plates (Nunc). To determine the remaining soluble protein content, after 25-μL aliquots were centrifuged at 20,000 × g (10 min, 4 °C) to pellet insoluble aggregates, the supernatant was resolved on 15% (vol/vol) SDS/PAGE gels that were stained with Coomassie stain (Life Technologies). To analyze the ultrastructure of α-Syn PFFs, 3.5 μL of the samples was applied onto glow-discharged Formvar/carbon-coated 200-mesh copper grids (Electron Microscopy Sciences) for 60 s. The grid was then blotted with filter paper, washed twice with ultrapure water and once with staining solution [uranyl formate 0.7% (wt/vol)], and then stained for 30 s, blotted, and dried by vacuum suction. Imaging was carried out on a Tecnai Spirit BioTWIN electron microscope (FEI) operated at 80 kV and equipped with a LaB6 gun and a 4 k × 4 k FEI Eagle CCD camera.
Monitoring in Vitro Aggregation Kinetics.
As previously described, lyophilized proteins of monomeric recombinant hα-Syn or mα-Syn were dissolved in 50 mM Tris and 150 mM NaCl, pH 7.5, and filtered through a 100-KDa MW-cutoff filter to remove potential aggregates. These proteins were incubated at 20 µM either alone or with 10% hα-Syn or mα-Syn PFFs at 37 °C under constant agitation at 850 rpm for 3 d. Amyloid formation in samples taken at different time points was monitored by ThT binding and TEM, as described above.
SPR Detection of Interactions Between hα-Syn and mα-Syn.
SPR experiments were performed using a Biacore X100 instrument with a CM5 sensor chip (GE Healthcare). hα-Syn monomers (ligand 1) or hα-Syn PFFs (ligand 2) were immobilized through amine coupling chemistry to separate CM5 chips, using standard ligand immobilization protocols from Biacore. In each chip, both flow cells (Fc1 and Fc2) were activated for 7 min with a 1:1 mixture of 0.1 M N-hydroxysuccinimide (NHS) and 0.4 M 3-N,N-dimethyla-mino (EDC) at a flow rate of 5 µL/min. Each ligand was injected at 5 µL/min in a solution of 50 mM Tris and 150 mM NaCl, pH 7.5 (TBS) at a concentration of 20 µM on the activated sensor surface 2 (Fc2); then 1 M ethanolamine-HCl (pH 8.5) was added to block the unreacted NHS-esters. The sensor surface 1 (Fc1) was activated/deactivated without ligand for its use as a reference surface for subtraction of nonspecific signal effects. The running buffer for all experiments was TBS. mα-Syn monomers were diluted in running buffer at 0.1, 0.5, 1, 5, and 10 µM and were injected in increasing concentrations over the two ligands, with one duplicate of lower concentration (5 µM) after the highest analyte concentration. The contact time for binding and the dissociation time were 450 s. All binding cycles were performed at 25 °C. Regeneration using 1 M NaOH followed by 1.5 M glycine/HCl buffer was applied to the sensor surfaces between runs. Interaction data were analyzed using the Biacore X100 Evaluation Software (GE Healthcare) and Scrubber 2.0 software.
Immunocytochemistry and Imaging of Primary Neurons.
As previously described (10), primary neurons were washed with PBS, fixed with 4% (vol/vol) paraformaldehyde in PBS, and then incubated in blocking buffer [3% (wt/vol) BSA, 0.02% saponin in PBS] for 1 h at room temperature. For ThS staining, fixed cells were incubated with 0.05% ThS for 15 min and were differentiated with 70% (vol/vol) ethanol before blocking. Coverslips were incubated overnight at 4 °C in blocking buffer comprising primary antibodies (Table S1). Subsequently, coverslips were washed five times with PBS and incubated in blocking buffer comprising appropriate Alexa Fluor-conjugated secondary antibodies (Invitrogen) as well as the nuclear stain DAPI (1:2,500) for 1 h at room temperature. After five washes with PBS, coverslips were washed once with double-distilled water and then were mounted on glass slides with DABCO mounting medium (Sigma-Aldrich). Neurons were imaged using a LSM700 confocal microscope (Carl Zeiss). Image analysis and 3D surface reconstruction were performed using the Zen 2012 SP1 (black edition; Carl Zeiss) and Imaris software, respectively.
Table S1.
Antibodies used in this study
| Antigen | Antibody name/catalog number | Epitope | Source |
| hα-Syn | Syn-211/sc-12767 | 121-125 | Santa Cruz |
| Syn-208 | 89-110 | Virginia Lee* | |
| 14H2L1 | 117-125 | Life Technologies | |
| LB-509/18-0215 | 115-122 | Life Technologies | |
| hα-Syn + mα-Syn | Syn-1/610787 | 80-100 | BD Laboratory |
| SA3400 | 117-131 | Enzo Life Sciences | |
| Ab5336P | 108-120 | Millipore | |
| mα-Syn | D37A6 | Cell Signaling | |
| pS129 α-Syn | WAKO/014-20281 | WAKO | |
| MJF-R13/ab-168381 | Abcam | ||
| Other synucleins | β-Syn/sc-136452 | Santa Cruz | |
| γ-Syn | Homemade | ||
| EP-1646Y (α- β-Syn) | 1-50 | Gentex | |
| FL-140 (α-, β-, γ-Syn)/sc10717 | 61-95 | Santa Cruz | |
| ab-6176 (α-, β,- γ-Syn) | 11-26 | Abcam | |
| Markers | Actin/ab-6276 | Abcam | |
| β3-tubulin | Santa Cruz | ||
| Synaptophysin/D35E4 | Cell Signaling | ||
| Synaptobrevin-2/D601A | Cell Signaling | ||
| Ubiquitin/042691GS | Millipore | ||
| MAP2/188 003 | Synaptic Systems |
University of Pennsylvania, Philadelphia.
CLEM Analysis of Primary Neurons.
SNCA−/− neurons were transfected to express hα-Syn for 72 h. In a separate condition, SNCA−/− neurons were transfected to express Myc–hα-Syn for 24 h and then were treated with hα-SynAlexa633 PFFs for an additional 24 h. In both cases, the neurons were plated on FluoroDishes (World Precision), fixed with 4% (vol/vol) formaldehyde, and then stained using either the Syn-211 antibody (in the condition expressing hα-Syn) or the anti-Myc antibody (for the Myc–hα-Syn + hα-SynAlexa633 PFF-treated condition) using the immunocytochemistry protocol described above. Stained neurons were imaged by fluorescent confocal microscopy, and their location on the FluoroDish was established relative to scratches previously made at the bottom of the glass using a diamond-tipped pen. The neurons then were processed for TEM and embedded in resin. First, they were fixed with 2.5% (vol/vol) glutaraldehyde (Electron Microscopy Sciences) and 2% (vol/vol) paraformaldehyde (Electron Microscopy Sciences) in 0.1 M phosphate buffer, pH 7.4, for 2 h, washed extensively in 0.1 M cacodylate buffer, pH 7.4, and postfixed with 1% osmium tetroxide (Electron Microscopy Sciences) in 0.1 M cacodylate buffer pH 7.4 for 1 h. After washing with double-distilled water, cells were stained en block with 1% uranyl acetate aqueous solution (Electron Microscopy Sciences) for 1 h and washed in double-distilled water for 5 min, followed immediately by dehydration in graded alcohol series (2 × 50%, 70%, 90%, 95%, and 2 × absolute ethanol) for 3 min each. Dehydrated cells were infiltrated with Durcupan resin (Electron Microscopy Sciences) diluted with ethanol at 1:1 for 30 min, at 2:1 for 30 min, and twice with pure Durcupan for 30 min each. Finally, after the 2-h incubation in Durcupan resin, the FluoroDish was filled with fresh Durcupan resin (1 mm above the cells) and polymerized at 65 °C overnight to cure the resin completely. The hard resin was removed from the FluoroDish by immersing the dish alternately in hot (60 °C) water followed by liquid nitrogen. A laser microdissection microscope (Leica Microsystems) was used to mark the position of selected neurons (previously imaged by fluorescent confocal microscopy) on the resin surface to help cut out the cells from resin disk and to facilitate the trimming process. The selected neuron was cut from the disk using a single-edged razor blade and was glued with superglue to a blank resin block. The cutting face was trimmed using a Leica Ultracut UCT microtome (Leica Microsystems) and a glass knife. Serial sections (50- to 60-nm thick) were cut with a diamond knife (DiATOME) and collected onto 2-mm single-slot copper grids (Electron Microscopy Sciences) coated with Formvar support film. Sections were contrasted with uranyl acetate and lead citrate and examined using a Tecnai Spirit TEM (FEI) operating at an acceleration voltage of 80 kV and equipped with a bottom-mounted 4 × 4 k Eagle CCD camera controlled by TIA acquisition software (FEI). For inclusion colocalization, the basis was (i) to identify the exact neuron imaged by confocal microscopy and subsequently by TEM, particularly to correlate the overall shape of the neuron by TEM and fluorescence; (ii) to correlate the location of the nucleus by fluorescence and TEM to use its shape as an internal guide-mark within the neuron; and then (iii) to correlate the fluorescently labeled α-Syn inclusion within high-resolution TEM images as accurately as possible. It is important to note that the difference in the thickness of each single optical slice obtained by confocal imaging (∼300 nm) and the physical thickness of each ultrathin section imaged by TEM (60 nm per section) is the limiting factor for exact and precise alignment. Because each confocal optical slice is ∼5× thicker than the ultrathin section imaged by TEM, the confocal image always will account for fluorescence from more structures (i.e., from structures above or below the ultrathin section, which need to be verified case by case) than observed in a single TEM section.
Immunogold Labeling and Negative Staining of hα-Syn PFFs.
Five microliters of each sample was applied onto glow-discharged Formvar/carbon-coated 200-mesh copper grids and adsorbed for 60 s. To minimize unspecific binding of the primary antibody, grids were incubated on two drops of blocking solution [10 mM PBS with 0.2% calf BSA (BSAc)] for 30 s each and then with primary antibodies for 40 min. Grids were washed again on two drops of blocking solution for 30 s each and then were incubated with secondary antibodies (either goat anti-rabbit or goat anti-mouse) coupled with 10-nm colloidal gold particles) diluted 1:50 in the blocking solution for 40 min. Then, grids were washed in two drops of PBS and were incubated for 1 min on a drop of 1% aqueous solution of glutaraldehyde to stabilize bound antibodies. Finally, grids were washed three times on a clean drop of ultrapure water and negatively stained with 2% (wt/vol) aqueous uranyl acetate for 45 s, blotted, and air-dried. Imaging was carried out on a Tecnai Spirit BioTWIN electron microscope (FEI) operated at 80 kV and equipped with a LaB6 gun and a 4 × 4 k FEI Eagle CCD camera.
Pre-Embedding Immunolabeling and TEM of Primary Neurons.
To preserve antigenicity, cells were fixed with 2% (vol/vol) paraformaldehyde (Electron Microscopy Sciences) and 0.1% glutaraldehyde (Electron Microscopy Sciences) in 0.1 M phosphate buffer (PB) at pH 7.4 for 1 h. Aldehyde-reactive groups were inactivated with 0.1% sodium borohydrate in 0.1 M PB for 15 min. After a thorough washing with 0.1 M PB, cells were cryoprotected with 2% (vol/vol) glycerol and 20% (vol/vol) DMSO in 0.1 M PB. The plasma membrane was permeabilized using two rounds of freezing/thawing cycles in liquid nitrogen. Cells then were incubated for 1 h in blocking solution containing 0.01 M PBS at pH 7.4, 0.2% BSAc, 0.05% Triton X-100, and 5% (vol/vol) normal goat serum and were washed twice for 10 min each in 0.01 M PBS with 0.2% BSAc and 0.02% Triton X-100. Neurons then were incubated with the rabbit primary antibody anti–PAN-Syn 6176 at a dilution of 1:50 overnight at 4 °C in blocking solution containing 0.01 M PBS (pH 7.4), 0.2% BSAc, 0.02% Triton X-100. The excess of primary antibody was removed by washing three times in 0.01 M PBS at pH 7.4 containing 0.2% BSAc for 5 min each wash. Then neurons were incubated with secondary antibody (goat anti-rabbit) coupled with 0.8-nm Nanogold particles (Aurion) at a dilution of 1:50 in 0.01 M PBS at pH 7.4 and 0.2% BSAc for 4 h at room temperature. Excess secondary antibody was removed by washing three times in 0.01 M PBS at pH 7.4 containing 0.2% BSAc for 5 min each wash and twice for 10 min each in 0.1 M PB at pH 7.4. The antibodies were stabilized by 30-min fixation with 2.5% (vol/vol) glutaraldehyde (Electron Microscopy Sciences) in 0.1 M PB at pH 7.4. The ultra-small Nanogold particles were silver-enhanced (R-Gent SE-EM Silver Enhancement Reagents; Aurion) for 1 h at room temperature in the dark, and staining was monitored by light microscopy. After washing with double-distilled water and 0.1 M PB, cells were postfixed with 0.05% osmium tetroxide (Electron Microscopy Sciences) in 0.1 M PB for 15 min and were washed in double-distilled water for 5 min followed immediately by dehydration in a graded alcohol series (2 × 50%, 70%, 90%, 95%, and 2 × absolute ethanol) for 3 min each. Dehydrated cells were infiltrated with Durcupan resin diluted with ethanol at 1:1 for 30 min, at 2:1 for 30 min, and twice with pure Durcupan for 30 min each. Finally, after 2 h incubation in fresh Durcupan resin, the coverslips were placed with grown cells faced down on 1-mm-thick silicone rings placed on a glass slide (coated with mold-separating agent) and filled with fresh resin. This sandwich was polymerized at 65 °C overnight to cure the resin completely. The coverslips were removed from the hard resin by immersing the polymerized resin disk alternately into hot (60 °C) water followed by liquid nitrogen until the coverslip parted. Block preparation, ultramicrotomy, and TEM imaging were performed as described above in CLEM Analysis of Primary Neurons.
3D Reconstruction of TEM Images.
Serial images throughout the immunogold-labeled inclusions, as well as the neuron reconstruction in the CLEM experiment, were combined into image stacks and aligned using the FIJI program (fiji.sc/Fiji). Segmentation of the feature of interest and 3D reconstructions were done using the TrakEM2 plugin for FIJI, and final 3D models were visualized using the Blender program (v.2.69; www.blender.org/).
Live Imaging, FRAP, and Photoconversion Experiments.
All live-imaging experiments were performed using the LSM 700 inverted point scanning confocal microscope (Carl Zeiss) equipped with a stage maintained at 37 °C, using a Plan-Apochromat 40×/1.3 Oil differential interference contrast M27 objective. When noted, 3D surface reconstruction of Z-stacks was performed using Imaris software to assess the increase in inclusion volume and number over time.
For FRAP and photoconversion experiments, transfected neurons were first identified using a 488-nm laser (0.5% transmission) and a 515- to 565-nm bandpass filter. Then a circular region of interest (ROI) (r = 0.45 µm) representing an individual inclusion was either photobleached using the same diode laser at 488 nm (50% transmission, 50 iterations) or photoconverted using a diode laser at 405 nm (50% transmission, 50 iterations). For each experiment, time-lapse imaging of the 488-nm channel (alone for FRAP or with the 568 channel for photoconversion) was performed, with an interval of around 0.1 s and with the pinhole maximally opened (460 µm). Three hundred fifty images for FRAP and photoconversion (frame size 128 × 128 pixels, 0.179 × 0.179 µm, eight bits, pixel dwell: 2.55 µs) were acquired, 10 of which were acquired before bleaching.
FRAP and Photoconversion Data Analysis.
FRAP and photoconversion data analysis was performed as previously described (61, 62). Datasets from different neurons were analyzed using Zen 2012 SP1 (black editionl Carl Zeiss) and Fiji (by Image J) software to obtain intensity plots at each time point t at the bleached ROI I(t) and of the entire neuron T(t), which was used to normalize FRAP and photoconversion curves as per the following formulas: Irel(t)= [I(t)/Imax] [Tmax/T(t)] for FRAP and Irel(t)= [I(t)/Imax]/[Tmax/T(t)] for photoconversion, with Tmax and Imax representing maximum fluorescence intensities of the entire neuron and of the bleached ROI, respectively. Postbleach values (for FRAP) and prebleach values (for photoconversion) were set to zero to facilitate comparison of the curves and monoexponential fitting (done using the R statistical coding script) using the following equations:
For FRAP: Irel(t) = (1 − IF) (1 − e–koff t) where IF is the immobile fraction and koff is the off rate of binding; for photoconversion: Irel(t) = IF + (MF)(e–t/τ), where MF is the mobile fraction, and τ is the time constant for the decay of fluorescent intensity. The half time τ1/2 then was determined using the following formulas:
For FRAP: τ1/2 = (ln 0.5)/−koff; for photoconversion: τ1/2 = 0.69 τ.
The diffusion coefficient D was finally calculated from τ1/2 values: D = (0.88) (W2/4 τ1/2), with W being the radius of the bleached ROI.
Protein Extraction and Immunoblotting of Primary Neurons.
Seven days after lentiviral infection, neurons (in 10-cm dishes) were washed once with PBS. To extract total protein content, neurons were directly scraped in 1× loading buffer [1.5% (wt/vol) SDS (SERVA Electrophoresis GmbH), 10% (vol/vol) glycerol (Fisher Scientific), 62.5 mM Tris(hydroxymethyl)aminomethane (Biosolve) (pH 6.8), 0.0025% Bromophenol blue (Aldrich), and 10% (vol/vol) β-mercaptoethanol (Sigma)] and were boiled for 10 min at 95 °C. To perform sequential extraction of proteins, neurons were scraped into ice-cold lysis buffer (20 mM Trizma base, 150 mM NaCl, 1 mM EDTA, 0.25% Nonidet P-40, 0.25% Triton X-100, pH 7.4) containing protease and phosphatase inhibitors. Lysates were incubated for 20 min on ice and were cleared by centrifugation at 14,000 × g for 20 min at 4 °C. Resulting supernatants comprise nonionic detergent-soluble fractions. Pellets then were resuspended in lysis buffer containing 5% (wt/vol) SDS and were briefly sonicated (60% amplitude, 3 s on/off for 15 s). The resulting solutions comprise nonionic detergent-insoluble fractions.
After sample concentrations of soluble or insoluble fractions were determined using the BCA protein assay kit from Thermo Scientific, equal protein amounts were separated on Novex 4–12% (vol/vol) Bis-Tris gels (Life Technologies) and transferred to a nitrocellulose membrane using a semidry transfer cell from Bio-Rad. The membrane was blocked for 1 h at room temperature with Odyssey blocking buffer (Li-COR Biosciences GmbH) diluted 1:3 in PBS and was probed with primary antibodies (Table S1) in blocking buffer at 4 °C overnight. After three washes in PBST [PBS 0.01% (vol/vol) Tween-20 (Fluka)], the membrane was incubated with Alexa Fluor (680 or 800 nm)-conjugated secondary antibodies (Invitrogen or Li-COR Biosciences GmbH) at room temperature for 1 h, washed three times with PBST, and scanned using a Li-COR scanner at a wavelength of 700 or 800 nm.
Immunoprecipitation Experiments.
To assess the interaction of recombinant proteins, solutions of recombinant mα-Syn were mixed with hα-Syn monomers or hα-Syn PFFs (2.5 μM each) in a solution of 50 mM Tris, 150 mM NaCl, pH 7.5. Solutions of hα-Syn PFFs alone (2.5 μM) were included as controls. Then 50 μL of each of the samples was incubated with 1 μg of primary antibody (Syn-211) overnight at 4 °C. To assess interactions in primary neurons, cells were lysed in RIPA buffer [20 mM Trizma base, 150 mM NaCl, 1 mM EDTA, 1% (vol/vol) Nonidet P-40, 0.5% (wt/vol) sodium-deoxycholate, 0.1% (wt/vol) SDS, 50 mM NaF, pH 7.4] containing protease inhibitors, incubated for 30 min with agitation 4 °C, and then cleared by centrifugation at 14,000 × g for 20 min at 4 °C. Then 1,000 μg of each sample was incubated with 1 μg of primary antibody (Syn-211) overnight at 4 °C. In both cases, 50 μL of Dynabeads Protein G (Invitrogen) was washed with PBS and then incubated with each of the samples overnight at 4 °C. The beads were collected using a DynaMag magnet (Invitrogen), washed three times with PBS-Tween 0.01%, and incubated with 40 μL 1× loading buffer at 95 °C for 10 min. A DynaMag magnet (Invitrogen) was used to separate the beads again, and then the supernatants were electrophoresed on Novex 4–12% (vol/vol) Bis-Tris gels (Life Technologies) for Western blotting as discussed above.
SYTOX Green and TUNEL Cytotoxicity Assays.
To assess membrane integrity in neurons, the SYTOX Green dye exclusion assay (Molecular Probes, Invitrogen) was performed according to the manufacturer’s instructions and as previously described (10). Briefly, the SYTOX Green dye was diluted (1:15,000) in the medium of neurons plated and transfected in 96-well clear-bottomed black plates (Corning) and then incubated for 30 min at 37 °C in the dark. Neurons subsequently were washed twice with PBS; then the fluorescence intensity per well was measured using the Infinite M200-Pro plate reader (excitation: 487 nm; emission: 519 nm) after the addition of 100 µL of PBS per well. Neurons were fixed, and immunocytochemistry was performed as described above. To investigate presence of apoptotic neurons, the TUNEL assay was performed. Fixed transfected neurons first were washed twice with PBS and then were permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate buffer for 2 min on ice. After two washes with PBS, neurons were washed with MTBS buffer (66 mM NaCl, 100 mM Tris⋅HCl, pH 7.4) and subsequently were incubated for 1 h at 37 °C with the TUNEL reaction mix (Roche) according to the manufacturer’s recommendations. Then, the neurons were washed twice with PBS and once with MTBS and were blocked for immunocytochemistry as described above.
Generation of Tg Mice Lacking mα-Syn Expression.
Tg mice expressing hα-Syn under the regulatory control of the human PDGF B chain promoter were generated as previously described (35). Early on, these mice (line D) develop intraneuronal inclusions, dopaminergic deficits, and motor impairments (35). To eliminate endogenous mα-Syn expression in these mice, we crossed them first with SNCA−/− mice (13) to generate heterozygotes and then backcrossed these heterozygotes with SNCA−/− mice to remove the remaining copy of mα-Syn. The resulting mice were self-crossed to generate Tg mice expressing two copies of hα-Syn in the absence of any mα-Syn expression (hα-Syn+/+SNCA−/−).
To generate Tg mice expressing hβ-Syn in the absence of endogenously expressed mα-Syn, Tg mice expressing hβ-Syn under the control of the mThy-1 cassette (37) were crossed with SNCA−/− mice (13) to generate hβ-Syn+/+SNCA−/− mice using a strategy similar to that discussed above. Genomic DNA was extracted from tail biopsies and analyzed with PCR amplification to verify transgene presence in all animals (35).
Processing and Biochemical Analysis of Transgenic Mice Brains.
Anesthetized mice were killed by transcardiac saline perfusion, and brains were removed and divided sagittally. For protein or RNA analysis, the right hemibrain was frozen in isopentane cooled in a Shandon Lipshaw Histobath (NesLab) and stored at −80 °C. For immunohistochemical analysis, the left hemibrain was fixed in 4% (vol/vol) paraformaldehyde at 4 °C for 48 h and then was sectioned sagittally at 40 μm using a Vibratome 2000 (Leica) as described previously (35).
Brain homogenates were separated into total, cytosolic, and particulate fractions as previously described (35). Briefly, frozen brain tissue was placed in cold PDGF buffer (1 mM Hepes, 5 mM benzamidine, 2 mM 2-mercaptoethanol, 3 mM EDTA, 0.5 mM magnesium sulfate, 0.05% sodium azide, pH 8.8) comprising phosphatase and protease inhibitors and then was homogenized for 1 min on ice. Clearing lysates by centrifugation at 5,000 × g for 5 min at 4 °C resulted in supernatants that comprise total protein content. To fractionate the brains further, the same supernatants were ultracentrifuged at 100,000 × g for 1 h at 4 °C. The resulting supernatants comprise soluble cytosolic fractions. The pellets were resuspended and sonicated in PDGF buffer and comprise insoluble particulate fractions.
After sample concentrations were determined using the BCA protein assay kit from Thermo Scientific, equal protein amounts were heated for 10 min at 70 °C and were separated by gel electrophoresis on 4–12% (vol/vol) BisTris SDS/PAGE gels (Invitrogen). After proteins were transferred to nitrocellulose membranes using the wet transfer system (Bio-Rad), immunoblots were blocked for 1 h with 10% (wt/vol) BSA in PBS and were incubated overnight at 4 °C with primary antibodies (Table S1). Membranes were processed using a chemiluminescence kit (Western Lightning Chemiluminescence Reagent Plus; Perkin-Elmer) and were imaged using a VersaDoc system (Bio-Rad).
Immunohistochemical Analysis of Tg Mice Brains.
Immunohistochemical analysis was performed on serially sectioned, free-floating, vibratome sections of mouse brains. Sections were washed with PBS before and after being pretreated with 10% (vol/vol) H2O2 (Sigma) in PBS for 20 min at room temperature. Then sections were treated with proteinase K (1 μg/mL) (Invitrogen) in 5× TE buffer [50 mM Tris⋅HCl (pH 7.4), 5 mM EDTA] for 8 min at room temperature. An alternative set of sections did not receive proteinase K treatment and was washed in PBS alone. All sections then were washed with PBS and incubated for 1 h in blocking solution containing 10% (vol/vol) normal goat (or normal horse) serum (Vector Laboratories). Sections were incubated overnight with primary antibodies (Table S1) at 4 °C, washed with PBS, and reincubated with biotinylated secondary antibodies (Vector Laboratories) for 2 h at room temperature. Sections then were rinsed in PBS and subsequently were incubated in avidin–biotin complex (ABC Elite; Vector Laboratories) for 45 min. Finally, sections were rinsed again with PBS, incubated in PBS containing 3′-diaminobenzidine tetrahydrochloride (DAB) (Sigma) and 0.001% H2O2 (Sigma) to reveal staining, rinsed with H2O, mounted on glass slides, dehydrated, cleared with xylene, and coverslipped.
Immunofluorescence analysis was performed as previously described (28). Briefly, slices were washed with PBS, incubated in 10% (vol/vol) H2O2 for 30 min at room temperature, and then blocked in a solution of 3% (wt/vol) BSA and 0.1% Triton X-100 in PBS for 1 h at room temperature. Then, slices were incubated overnight at 4 °C in blocking buffer comprising primary antibodies (Table S1), washed with PBS, and subsequently incubated for 1 h at room temperature with secondary antibodies conjugated to Alexa Fluor-488 or Alexa Fluor-568 (Invitrogen). Slices finally were washed and mounted on glass slides using DABCO mounting solution (Sigma-Aldrich). Sections were imaged using a Zeiss LSM700 confocal microscope.
Semiquantitative RT–PCR of Primary Neurons and Brain Homogenates.
RNA was extracted from primary neurons infected with lentiviruses for 7 d or from frozen brain tissues of Tg mice using the RNeasy kit (Qiagen) following the manufacturer’s instructions. Semiquantitative RT-PCR was run using the Transcriptor One-Step kit (Roche Diagnostic) using the following primers:
GAPDH: Fwd 5′-CAGGTTGTCTCCTGCGACTT-3′, Rev 5′-GGCCTCTCTTGCTCAGTGTC-3′
hα-Syn: Fwd 5′- AGGGAGCATTGCAGCAGC-3′, Rev 5′- GTTCGTAGTCTTGATACCCTTCCTCAG-3′
The PCR products were loaded onto an agarose gel (1%) and were stained with SYBR Safe (Life Technologies), and a 1-kb Plus DNA ladder (Fermentas) was used to estimate the size of the bands. Images of the agarose gels were acquired with a GENi Imager (Syngene, Biolabo Scientific).
Stereotaxic Injection of α-Syn PFFs in Mice and Immunohistochemical Analysis.
Three-month-old C57BL6/C3H F1 or SNCA−/− mice (63) obtained from Jackson Laboratories were anesthetized with ketamine hydrochloride (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and were stereotaxically injected in one hemisphere with sonicated recombinant hα-Syn or mα-Syn PFFs (5 μg) in the dorsal neostriatum (coordinates: +0.2 mm relative to bregma, +2.0 mm from midline, +2.6 mm beneath the dura). Injections were performed using a 10-μL syringe (Hamilton) at a rate of 0.1 μL per min (2.5 μL total per site) with the needle in place for 5 min at each target. Animals (five mice per condition) were killed at 30 d postinjection by overdose with ketamine/xylazine; brains were removed after transcardial perfusion with PBS and were fixed overnight in neutral buffered formalin (Fisher Scientific) before being serially sectioned with the vibratome.
Immunohistochemistry was performed on 40-μm-thick serial sections. Coronal sections were stained using cDAB (Vector Laboratories) as a chromogen, or double immunolabeling was performed using appropriate fluorescent secondary antibodies conjugated to Alexa Fluor-488 or -568 (Invitrogen).
Statistical Analysis.
Data are presented as mean ± SD, and statistical analyses were performed using StatView v. 5.0 (SAS Institute) and SPSS (IBM) software. To determine statistical significance between two groups, P values were calculated using a two-tailed Mann–Whitney u test. For multigroup comparisons, a one-way ANOVA was performed followed by Scheffé post hoc analysis. P values less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Nathalie Jordan and Celine Vocat for help in preparing primary cultures, plasmid cloning, and production of recombinant proteins; Mr. Samuel Kilchenmann for expert help in conducting and analyzing surface plasmon resonance (SPR) experiments; Dr. Anne-Laure Mahul and Dr. Issa Issac for helping with blinded quantification of some experiments; Mr. Anass Chiki for help with protein characterization; Mr. Maroun Bou Sleiman for help with statistical analysis; Prof. Virginia Lee for kindly providing the Syn-208 antibody; and Mrs. Dania Al Labban and Dr. Juan Reyes for their critical evaluation of the manuscript. This work was supported by the Ecole Polytechnique Fédérale de Lausanne (EPFL), the Swiss National Science Foundation, and Wellcome Trust Grant 075615/Z/04/z (to V.L.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512876113/-/DCSupplemental.
References
- 1.Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 2.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14(1):38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson’s disease. Neuron. 2010;66(5):646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404(6776):394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 5.Cabin DE, et al. Exacerbated synucleinopathy in mice expressing A53T SNCA on a Snca null background. Neurobiol Aging. 2005;26(1):25–35. doi: 10.1016/j.neurobiolaging.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Tofaris GK, et al. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1-120): Implications for Lewy body disorders. J Neurosci. 2006;26(15):3942–3950. doi: 10.1523/JNEUROSCI.4965-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rochet JC, Conway KA, Lansbury PT., Jr Inhibition of fibrillization and accumulation of prefibrillar oligomers in mixtures of human and mouse alpha-synuclein. Biochemistry. 2000;39(35):10619–10626. doi: 10.1021/bi001315u. [DOI] [PubMed] [Google Scholar]
- 8.Petrucelli L, et al. Parkin protects against the toxicity associated with mutant alpha-synuclein: Proteasome dysfunction selectively affects catecholaminergic neurons. Neuron. 2002;36(6):1007–1019. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- 9.McLean PJ, Kawamata H, Hyman BT. Alpha-synuclein-enhanced green fluorescent protein fusion proteins form proteasome sensitive inclusions in primary neurons. Neuroscience. 2001;104(3):901–912. doi: 10.1016/s0306-4522(01)00113-0. [DOI] [PubMed] [Google Scholar]
- 10.Fares MB, et al. The novel Parkinson’s disease linked mutation G51D attenuates in vitro aggregation and membrane binding of α-synuclein, and enhances its secretion and nuclear localization in cells. Hum Mol Genet. 2014;23(17):4491–4509. doi: 10.1093/hmg/ddu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalaf O, et al. The H50Q mutation enhances α-synuclein aggregation, secretion, and toxicity. J Biol Chem. 2014;289(32):21856–21876. doi: 10.1074/jbc.M114.553297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Specht CG, Schoepfer R. Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neurosci. 2001;2:11–19. doi: 10.1186/1471-2202-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abeliovich A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 14.Tabrizi SJ, et al. Expression of mutant alpha-synuclein causes increased susceptibility to dopamine toxicity. Hum Mol Genet. 2000;9(18):2683–2689. doi: 10.1093/hmg/9.18.2683. [DOI] [PubMed] [Google Scholar]
- 15.Opazo F, Krenz A, Heermann S, Schulz JB, Falkenburger BH. Accumulation and clearance of alpha-synuclein aggregates demonstrated by time-lapse imaging. J Neurochem. 2008;106(2):529–540. doi: 10.1111/j.1471-4159.2008.05407.x. [DOI] [PubMed] [Google Scholar]
- 16.Tofaris GK, Layfield R, Spillantini MG. alpha-synuclein metabolism and aggregation is linked to ubiquitin-independent degradation by the proteasome. FEBS Lett. 2001;509(1):22–26. doi: 10.1016/s0014-5793(01)03115-5. [DOI] [PubMed] [Google Scholar]
- 17.Engelender S, et al. Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions. Nat Genet. 1999;22(1):110–114. doi: 10.1038/8820. [DOI] [PubMed] [Google Scholar]
- 18.Lee HJ, Shin SY, Choi C, Lee YH, Lee SJ. Formation and removal of alpha-synuclein aggregates in cells exposed to mitochondrial inhibitors. J Biol Chem. 2002;277(7):5411–5417. doi: 10.1074/jbc.M105326200. [DOI] [PubMed] [Google Scholar]
- 19.Paxinou E, et al. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J Neurosci. 2001;21(20):8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujiwara H, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4(2):160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa M, et al. Phosphorylated alpha-synuclein is ubiquitinated in alpha-synucleinopathy lesions. J Biol Chem. 2002;277(50):49071–49076. doi: 10.1074/jbc.M208046200. [DOI] [PubMed] [Google Scholar]
- 22.Volpicelli-Daley LA, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin DJ, Lee VM, Trojanowski JQ. Parkinson’s disease dementia: Convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci. 2013;14(9):626–636. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998;95(11):6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinney SA, Murphy CS, Hazelwood KL, Davidson MW, Looger LL. A bright and photostable photoconvertible fluorescent protein. Nat Methods. 2009;6(2):131–133. doi: 10.1038/nmeth.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberti MJ, Jovin TM, Jares-Erijman E. Confocal fluorescence anisotropy and FRAP imaging of α-synuclein amyloid aggregates in living cells. PLoS One. 2011;6(8):e23338. doi: 10.1371/journal.pone.0023338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oueslati A, Fournier M, Lashuel HA. Role of post-translational modifications in modulating the structure, function and toxicity of alpha-synuclein: Implications for Parkinson’s disease pathogenesis and therapies. Prog Brain Res. 2010;183:115–145. doi: 10.1016/S0079-6123(10)83007-9. [DOI] [PubMed] [Google Scholar]
- 28.Paleologou KE, et al. Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions. J Neurosci. 2010;30(9):3184–3198. doi: 10.1523/JNEUROSCI.5922-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oueslati A, Paleologou KE, Schneider BL, Aebischer P, Lashuel HA. Mimicking phosphorylation at serine 87 inhibits the aggregation of human α-synuclein and protects against its toxicity in a rat model of Parkinson’s disease. J Neurosci. 2012;32(5):1536–1544. doi: 10.1523/JNEUROSCI.3784-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Giovanni S, et al. Entacapone and tolcapone, two catechol O-methyltransferase inhibitors, block fibril formation of alpha-synuclein and beta-amyloid and protect against amyloid-induced toxicity. J Biol Chem. 2010;285(20):14941–14954. doi: 10.1074/jbc.M109.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandra S, et al. Double-knockout mice for alpha- and beta-synucleins: Effect on synaptic functions. Proc Natl Acad Sci USA. 2004;101(41):14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchman VL, et al. Persyn, a member of the synuclein family, has a distinct pattern of expression in the developing nervous system. J Neurosci. 1998;18(22):9335–9341. doi: 10.1523/JNEUROSCI.18-22-09335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anwar S, et al. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J Neurosci. 2011;31(20):7264–7274. doi: 10.1523/JNEUROSCI.6194-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Henning Jensen P, Dahlström A. Differential localization of alpha-, beta- and gamma-synucleins in the rat CNS. Neuroscience. 2002;113(2):463–478. doi: 10.1016/s0306-4522(02)00143-4. [DOI] [PubMed] [Google Scholar]
- 35.Masliah E, et al. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: Implications for neurodegenerative disorders. Science. 2000;287(5456):1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura M, et al. Synaptophysin and chromogranin A immunoreactivities of Lewy bodies in Parkinson’s disease brains. Brain Res. 1994;634(2):339–344. doi: 10.1016/0006-8993(94)91940-2. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto M, Rockenstein E, Mante M, Mallory M, Masliah E. beta-Synuclein inhibits alpha-synuclein aggregation: A possible role as an anti-parkinsonian factor. Neuron. 2001;32(2):213–223. doi: 10.1016/s0896-6273(01)00462-7. [DOI] [PubMed] [Google Scholar]
- 38.Ko LW, Ko HH, Lin WL, Kulathingal JG, Yen SH. Aggregates assembled from overexpression of wild-type alpha-synuclein are not toxic to human neuronal cells. J Neuropathol Exp Neurol. 2008;67(11):1084–1096. doi: 10.1097/NEN.0b013e31818c3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka M, et al. Aggresomes formed by alpha-synuclein and synphilin-1 are cytoprotective. J Biol Chem. 2004;279(6):4625–4631. doi: 10.1074/jbc.M310994200. [DOI] [PubMed] [Google Scholar]
- 40.Luk KC, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masuda-Suzukake M, et al. Pathological alpha-synuclein propagates through neural networks. Acta Neuropathol Commun. 2014;2:88–99. doi: 10.1186/s40478-014-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paumier KL, et al. Intrastriatal injection of pre-formed mouse α-synuclein fibrils into rats triggers α-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol Dis. 2015;82:185–199. doi: 10.1016/j.nbd.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bétemps D, et al. Alpha-synuclein spreading in M83 mice brain revealed by detection of pathological α-synuclein by enhanced ELISA. Acta Neuropathol Commun. 2014;2:29–42. doi: 10.1186/2051-5960-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luk KC, et al. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med. 2012;209(5):975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sacino AN, et al. Brain injection of α-synuclein induces multiple proteinopathies, gliosis, and a neuronal injury marker. J Neurosci. 2014;34(37):12368–12378. doi: 10.1523/JNEUROSCI.2102-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peelaerts W, et al. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522(7556):340–344. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- 47.Sacino AN, et al. Intramuscular injection of α-synuclein induces CNS α-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc Natl Acad Sci USA. 2014;111(29):10732–10737. doi: 10.1073/pnas.1321785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sacino AN, et al. Induction of CNS α-synuclein pathology by fibrillar and non-amyloidogenic recombinant α-synuclein. Acta Neuropathol Commun. 2013;1(1):38–51. doi: 10.1186/2051-5960-1-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasegawa K, Yamaguchi I, Omata S, Gejyo F, Naiki H. Interaction between A beta(1-42) and A beta(1-40) in Alzheimer’s beta-amyloid fibril formation in vitro. Biochemistry. 1999;38(47):15514–15521. doi: 10.1021/bi991161m. [DOI] [PubMed] [Google Scholar]
- 50.Eaton WA, Hofrichter J. The biophysics of sickle cell hydroxyurea therapy. Science. 1995;268(5214):1142–1143. doi: 10.1126/science.7539154. [DOI] [PubMed] [Google Scholar]
- 51.Hammarström P, Schneider F, Kelly JW. Trans-suppression of misfolding in an amyloid disease. Science. 2001;293(5539):2459–2462. doi: 10.1126/science.1062245. [DOI] [PubMed] [Google Scholar]
- 52.Sousa MM, et al. Evidence for early cytotoxic aggregates in transgenic mice for human transthyretin Leu55Pro. Am J Pathol. 2002;161(5):1935–1948. doi: 10.1016/S0002-9440(10)64469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao P, Meng F, Abedini A, Raleigh DP. The ability of rodent islet amyloid polypeptide to inhibit amyloid formation by human islet amyloid polypeptide has important implications for the mechanism of amyloid formation and the design of inhibitors. Biochemistry. 2010;49(5):872–881. doi: 10.1021/bi901751b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sellin D, Yan LM, Kapurniotu A, Winter R. Suppression of IAPP fibrillation at anionic lipid membranes via IAPP-derived amyloid inhibitors and insulin. Biophys Chem. 2010;150(1-3):73–79. doi: 10.1016/j.bpc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Uversky VN, et al. Biophysical properties of the synucleins and their propensities to fibrillate: Inhibition of alpha-synuclein assembly by beta- and gamma-synucleins. J Biol Chem. 2002;277(14):11970–11978. doi: 10.1074/jbc.M109541200. [DOI] [PubMed] [Google Scholar]
- 56.Park JY, Lansbury PT., Jr Beta-synuclein inhibits formation of alpha-synuclein protofibrils: A possible therapeutic strategy against Parkinson’s disease. Biochemistry. 2003;42(13):3696–3700. doi: 10.1021/bi020604a. [DOI] [PubMed] [Google Scholar]
- 57.Fan Y, et al. Beta-synuclein modulates alpha-synuclein neurotoxicity by reducing alpha-synuclein protein expression. Hum Mol Genet. 2006;15(20):3002–3011. doi: 10.1093/hmg/ddl242. [DOI] [PubMed] [Google Scholar]
- 58.Robertson DC, et al. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J Neurochem. 2004;89(5):1126–1136. doi: 10.1111/j.1471-4159.2004.02378.x. [DOI] [PubMed] [Google Scholar]
- 59.Cookson MR. alpha-Synuclein and neuronal cell death. Mol Neurodegener. 2009;4:9–22. doi: 10.1186/1750-1326-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fauvet B, et al. Characterization of semisynthetic and naturally Nα-acetylated α-synuclein in vitro and in intact cells: Implications for aggregation and cellular properties of α-synuclein. J Biol Chem. 2012;287(34):28243–28262. doi: 10.1074/jbc.M112.383711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tenreiro S, et al. Phosphorylation modulates clearance of alpha-synuclein inclusions in a yeast model of Parkinson’s disease. PLoS Genet. 2014;10(5):e1004302. doi: 10.1371/journal.pgen.1004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoi H, et al. A monomeric photoconvertible fluorescent protein for imaging of dynamic protein localization. J Mol Biol. 2010;401(5):776–791. doi: 10.1016/j.jmb.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 63.Klivenyi P, et al. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis. 2006;21(3):541–548. doi: 10.1016/j.nbd.2005.08.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.