Significance
The Toll-like receptor 4 (TLR4)/myeloid differentiation factor 2 (MD-2) complex recognizes lipopolysaccharide (LPS) on Gram-negative bacteria to induce an innate immune response. Neoseptins, chemically synthesized peptidomimetics that bind and activate the mouse TLR4 (mTLR4)/MD-2 complex independent of LPS, were discovered through unbiased screening and reverse genetic studies, and improved by chemical modification. NMR and X-ray crystallography of the TLR4/MD-2/Neoseptin-3 complex determined the mechanism by which Neoseptin-3 activates mTLR4/MD-2 and triggers myeloid differentiation primary response gene 88- and Toll-interleukin 1 receptor domain-containing adaptor inducing IFN-beta-dependent signaling. Neoseptin-3 binds as a dimer within the hydrophobic pocket of MD-2, contacting residues distinct from those contacted by LPS or lipid A, yet triggering a conformational change very similar to that elicited by LPS or lipid A. Natural peptides might conceivably produce similar effects.
Keywords: neoseptins, peptidomimetic compounds, innate immunity, proinflammatory response, crystal structure
Abstract
Structurally disparate molecules reportedly engage and activate Toll-like receptor (TLR) 4 and other TLRs, yet the interactions that mediate binding and activation by dissimilar ligands remain unknown. We describe Neoseptins, chemically synthesized peptidomimetics that bear no structural similarity to the established TLR4 ligand, lipopolysaccharide (LPS), but productively engage the mouse TLR4 (mTLR4)/myeloid differentiation factor 2 (MD-2) complex. Neoseptin-3 activates mTLR4/MD-2 independently of CD14 and triggers canonical myeloid differentiation primary response gene 88 (MyD88)- and Toll-interleukin 1 receptor (TIR) domain-containing adaptor inducing IFN-beta (TRIF)-dependent signaling. The crystal structure mTLR4/MD-2/Neoseptin-3 at 2.57-Å resolution reveals that Neoseptin-3 binds as an asymmetrical dimer within the hydrophobic pocket of MD-2, inducing an active receptor complex similar to that induced by lipid A. However, Neoseptin-3 and lipid A form dissimilar molecular contacts to achieve receptor activation; hence strong TLR4/MD-2 agonists need not mimic LPS.
Toll-like receptors (TLRs) are innate immune receptors that serve as sensors of microbial molecules including lipopolysaccharide (LPS) or its precursor lipid A, lipopeptides, flagellin, and nucleic acids. In response to TLR engagement, rapid induction of proinflammatory signaling ensues, beginning with myeloid differentiation primary response gene 88 (MyD88)- or Toll-interleukin 1 receptor (TIR) domain-containing adaptor inducing IFN-beta (TRIF)-dependent recruitment of kinases and ubiquitin ligases that activate MAP kinases (MAPKs), NF-κB, and IFN regulatory factors (IRF) (1). These transcriptional regulators induce cytokines, chemokines, and costimulatory molecules that activate other receptors to promote the innate immune response.
Numerous microbial ligands have been implicated as activators of TLR4, TLR9, TLR2, and other TLRs (2). A wide variety of molecules of endogenous origin have also been reported to engage TLRs, particularly TLR4 and TLR2, and activate them in the absence of microbial challenge (3–18). These latter reports have engendered speculation that host-derived molecules, by directly stimulating TLRs, might sometimes trigger sterile inflammation (19). Concerns as to the purity and hence the true identity of the ligands notwithstanding, the reported promiscuity of TLRs raises questions concerning the manner in which molecules structurally unrelated to the bona fide microbial ligands might productively engage a signaling receptor.
To address whether and how structurally disparate molecules might trigger biological responses through known innate immune receptors while minimizing the possibility of microbial ligand contamination, we screened a library of synthetic peptidomimetic compounds (20) for stimulatory activity in primary mouse peritoneal macrophage cultures, measuring tumor necrosis factor (TNF) secretion as an indicator of activation. Here we describe a molecule identified through this screen and its interaction with the TLR4/MD-2 complex of mice.
Results
Neoseptin-3 Induces TNFα, IL-6, and IFN-β Production.
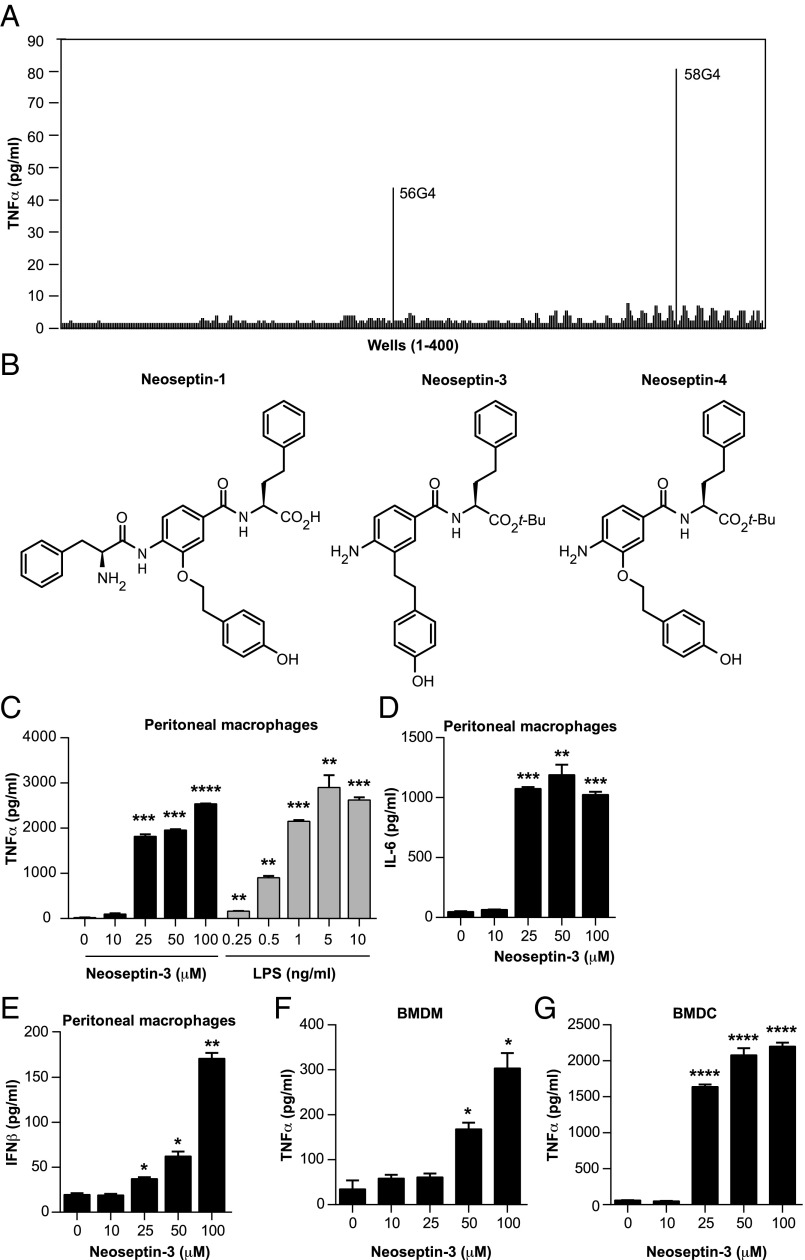
Among ∼90,000 compounds tested for their ability to activate TNFα biosynthesis in wild-type mouse peritoneal macrophages, we identified only two 20-compound mixtures exhibiting weak stimulatory activity (Fig. 1A). Individual compound testing of the strongest of these pools assigned activity to a single molecule, termed Neoseptin-1. Chemical modification of Neoseptin-1 combined with structure–activity relationship (SAR) studies produced structurally simpler, much stronger and approximately equipotent agonists, respectively designated Neoseptin-3 and Neoseptin-4 (Fig. 1B). Neoseptin-3, which induced TNFα production by macrophages in a concentration-dependent manner (Fig. 1C), was selected for more detailed studies.
Fig. 1.
Neoseptin-3 induces TNFα, IL-6, and IFN-β secretion in different mouse cells. (A) Screen of peptidomimetic molecules (400 wells, 20 compounds per well; ref. 40) for stimulation of TNFα production by mouse peritoneal macrophages, a subset of the full set of compounds examined. (B) Chemical structures of Neoseptin-1, -3, and -4. (C–E) TNFα (C), IL-6 (D), or IFN-β (E) in the supernatants of mouse peritoneal macrophages after treatment with Neoseptin-3 or LPS for 4 h. (F and G) TNFα in the supernatants of mouse BMDM (F) or BMDC (G) after treatment with Neoseptin-3 for 4 h. In C–G, the means of triplicate samples are plotted; P values were determined by Student’s t test and represent the significance of differences between responses of unstimulated cells and stimulated cells. Results in C–G are representative of two independent experiments (error bars represent SEM). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
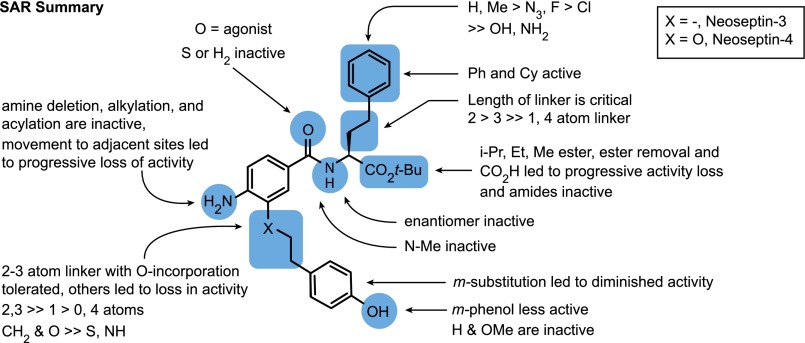
Further SAR analysis indicated that few chemical substitutions were compatible with retention of biological activity (Fig. S1). Even subtle modifications such as the substitution of fluorine for hydrogen at the para position of the phenyl ring, or transfer of the amino group of the aniline ring to an adjacent position led to a dramatic loss of activity. However, some of the modified compounds could antagonize Neoseptin-3.
Fig. S1.
Summary of Neoseptin-3 SAR analysis.
In vitro dose–response experiments demonstrated an EC50 of 18.5 μM for Neoseptin-3. Despite lower potency, Neoseptin-3 efficacy approximated that of LPS in promoting macrophage TNFα production (Fig. 1C). Neoseptin-3 also activated IL-6 and IFN-β production in a dose-dependent manner (Fig. 1 D and E). Responses to Neoseptin-3 were similar for mouse bone marrow-derived macrophages (BMDM) and bone marrow-derived dendritic cells (BMDC) (Fig. 1 F and G). These data indicate that Neoseptin-3 stimulates production of type I IFN and proinflammatory cytokines.
Neoseptin-3 Activates NF-κB, MAPK, and TANK-Binding Kinase 1 Signaling.
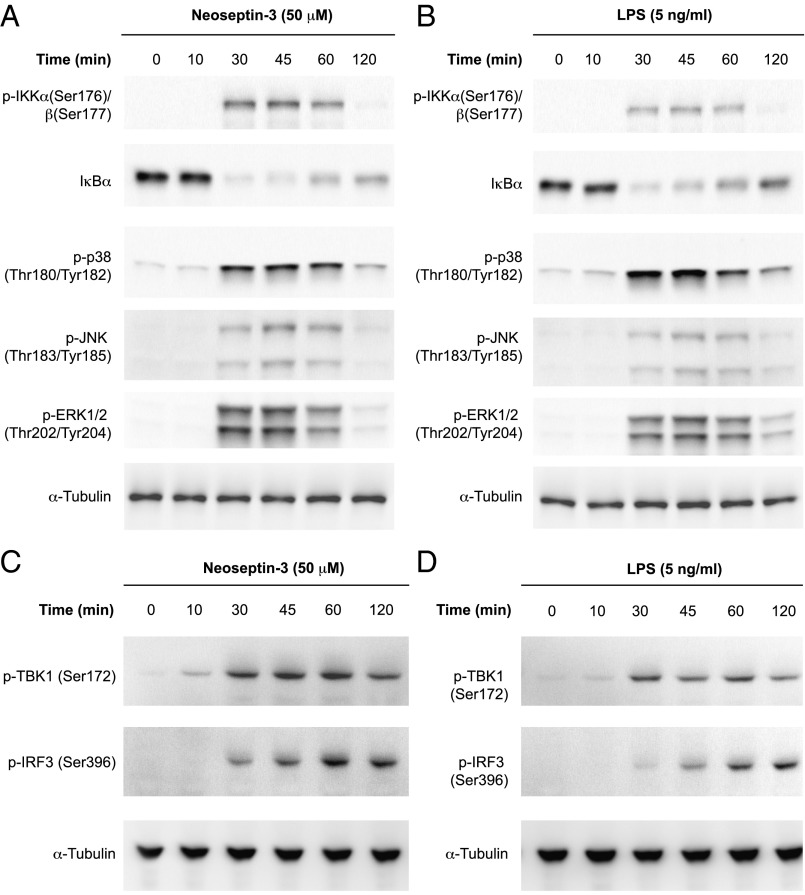
TLR signaling induces type I IFN and proinflammatory cytokine production dependent on NF-κB, MAPKs, and IRFs, and we evaluated these signaling pathways after Neoseptin-3 stimulation of macrophages. Neoseptin-3 induced phosphorylation of IκB kinases α (IKKα), IKKβ, p38, c-Jun N-terminal kinase (JNK), and ERK, and degradation of IκBα, consistent with activation of MAPK and canonical NF-κB signaling (Fig. 2A). TANK-binding kinase 1 (TBK1) and IRF3 phosphorylation also increased in response to Neoseptin-3 (Fig. 2C). Notably, the time course of these signaling events was similar when activated by Neoseptin-3 or by LPS (Fig. 2 B and D).
Fig. 2.
Neoseptin-3 activates NF-κB, MAPK, and TBK1. Mouse peritoneal macrophages were treated with Neoseptin-3 (A and C) or LPS (B and D). Lysates were collected at the indicated times after treatment for immunoblot analysis with the indicated antibodies. Results are representative of two independent experiments.
Neoseptin-3 Targets the Mouse TLR4/MD-2 Complex.
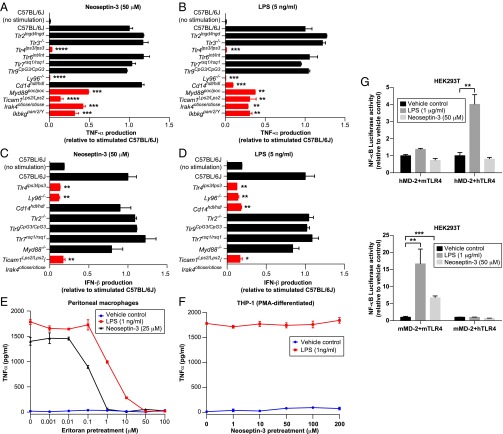
To determine the molecular target of Neoseptin-3, we analyzed its effects on peritoneal macrophages from wild-type C57BL/6J mice and mice deficient in TLR signaling components. LPS served as a control and was compared with Neoseptin-3 in its ability to induce TNFα and IFN-β production (Fig. 3 A–D). Induction of TNFα by Neoseptin-3 was completely abrogated in TLR4- or MD-2–deficient macrophages, and dramatically reduced in macrophages from MyD88, TRIF, interleukin-1 receptor-associated kinase 4 (IRAK4), and IKKγ mutant mice (Fig. 3A). Only CD14-deficient macrophages exhibited distinct responses to the two molecules, producing TNFα in response to Neoseptin-3 but not LPS. Neither Neoseptin-3 nor LPS required TLR2, TLR3, TLR6, TLR7, or TLR9 to induce TNFα. Similarly, IFN-β production in response to either Neoseptin-3 or LPS was dependent on TLR4, MD-2, and TRIF (Fig. 3C), whereas LPS, but not Neoseptin-3, additionally required CD14 (Fig. 3D). These data suggested that Neoseptin-3 targets the TLR4/MD-2 complex. Pretreatment with Eritoran, a pharmacological antagonist of TLR4, blocked production of TNFα by macrophages stimulated with either LPS or Neoseptin-3 (Fig. 3E), strongly supporting the interpretation that TLR4/MD-2 is the direct target of Neoseptin-3.
Fig. 3.
Neoseptin-3 activates mouse TLR4/MD-2. (A–D) TNFα (A and B) or IFN-β (C and D) in the supernatants of mouse peritoneal macrophages of the indicated genotypes after treatment with Neoseptin-3 (A and C) or LPS (B and D) (n = 3 mice per genotype). Cytokine levels were normalized to those of stimulated C57BL/6J cells. P values were determined by Student’s t test and represent the significance of differences between responses of stimulated C57BL/6J cells and stimulated cells of mutant genotypes; red bars indicate those with statistically significant differences. (E) TNFα in the supernatants of mouse peritoneal macrophages pretreated with the TLR4 antagonist Eritoran for 1 h, followed by addition of vehicle, Neoseptin-3 (25 μM), or LPS (1 ng/mL) for another 4 h. (F) TNFα in the supernatants of PMA-differentiated human THP-1 cells pretreated with Neoseptin-3 for 1 h, followed by addition of vehicle or LPS (1 ng/mL) for another 4 h. (G) NF-κB–dependent luciferase activity in HEK293T cells transiently expressing mouse or human TLR4 plus mouse or human MD-2 and stimulated with Neoseptin-3 (50 μM) or LPS (1 μg/mL). Data were normalized to luciferase activity measured in cells treated with vehicle. P values were determined by Student’s t test. In E–G, the means of triplicate samples are plotted. All results are representative of two independent experiments. In A–G, error bars represent SEM. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
In contrast to mouse macrophages, human macrophage-like cells generated by phorbol 12-myristate 13-acetate (PMA) treatment of the THP-1 monocyte line failed to respond to Neoseptin-3 (Fig. 3F). Moreover, unlike lipid IVa, an antagonist of LPS in human cells (21, 22), Neoseptin-3 failed to antagonize LPS stimulation of THP-1 cells (Fig. 3F). An NF-κB–dependent luciferase reporter in HEK293T cells could only be activated by Neoseptin-3 when mouse TLR4 (mTLR4)/mouse MD-2 (mMD-2) were coexpressed, whereas LPS activated the reporter in cells coexpressing mTLR4/mMD-2 or human TLR4/human MD-2 (hMD-2) (Fig. 3G). These data suggest that Neoseptin-3 specifically engages and activates mouse TLR4/MD-2, but cannot activate human TLR4/MD-2 or mixed heterodimers.
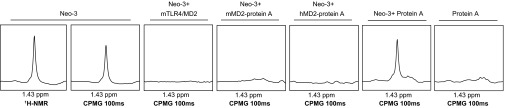
Direct interaction between Neoseptin-3 and highly purified TLR4/MD-2 complexes was demonstrated in vitro by NMR spectroscopy. In Carr Purcell Meiboom Gill (CPMG) experiments (23), Neoseptin-3 alone showed a relaxation time greater than 100 ms, which was reduced upon addition of mMD-2, hMD-2, or mTLR4/mMD-2, consistent with binding of Neoseptin-3 to h- or mMD-2 or the mTLR4/MD-2 complex (Fig. 4). We concluded that the biologically relevant molecular target for Neoseptin-3 is the TLR4/MD-2 complex.
Fig. 4.
NMR spectroscopy of Neoseptin-3 with mTLR4/MD-2. One-dimensional 1H-NMR spectra of the methyl regions of Neoseptin-3 alone, with mTLR4/MD-2, with mouse MD-2/protein A, or with human MD-2/protein A. Controls were Neoseptin-3 plus protein A and protein A alone. A CPMG sequence was applied for 100 ms (CPMG 100ms) as indicated.
Two Neoseptin-3 Molecules Bind to the Hydrophobic Pocket of MD-2 and Induce Agonistic Dimerization of Two mTLR4/MD-2 Complexes.
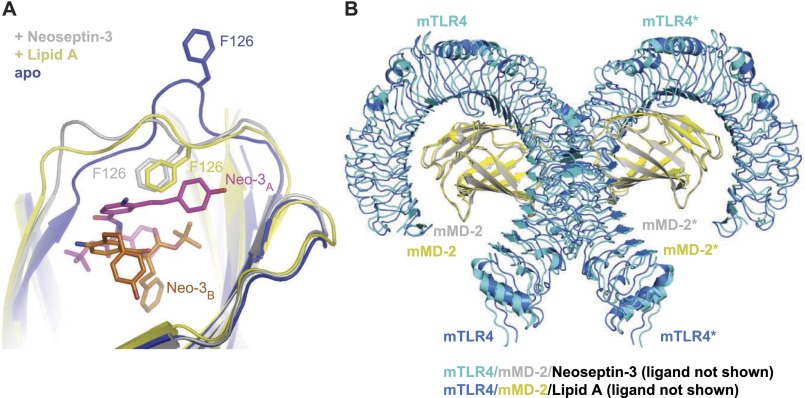
We determined the crystal structure of the mTLR4/MD-2 complex in apo form, in complex with lipid A, and in complex with Neoseptin-3 (Table S1). The overall conformation of the 1:1 mTLR4/MD-2 heterodimer is similar in all three structures [the root mean square deviations (rmsd) between the Cα atoms of these structures are <0.5 Å]. As expected, the apo form of mTLR4/MD-2 is a monomeric 1:1 complex in solution and does not adopt the active dimer conformation in the crystal. Both lipid A and Neoseptin-3 induce formation of a dimer consisting of two mTLR4/MD-2 heterodimers arranged symmetrically in an “m” shape as observed in the previously reported structures of TLR4/MD-2 bound to LPS (24, 25). Despite completely different chemical structures, Neoseptin-3 and lipid A induce similar local conformational changes in MD-2 around the bound ligand, and a nearly identical dimerization interface between the two mTLR4/MD-2 heterodimers (Fig. S2). In particular, the MD-2 Phe126 loop region undergoes a conformational change similar to that observed in the lipid A complexed structure (Fig. S2A). Similar conformational changes occur in LPS- or lipid IVa-bound mouse TLR4/MD-2 structures and in the LPS-bound human TLR4-MD-2 structure (24, 25). By convention, we distinguish the second TLR4 and MD-2 subunits of the active heterotetrameric complex with an asterisk.
Table S1.
Data collection and refinement statistics (molecular replacement)
| Parameters | mTLR4/MD-2 | mTLR4/MD-2/Neoseptin-3 | mTLR4/MD-2/Lipid A |
| Data collection | |||
| Space group | P43212 | P21 | P21 |
| Cell dimensions | |||
| a, b, c, Å | 127.96, 128.13, 277.01 | 70.75, 148.56, 131.72 | 66.84, 145.74, 136.20 |
| α, β, γ, ° | 90.00, 89.97, 90.00 | 90.00, 98.30, 90.00 | 90.00, 100.67, 90.00 |
| Resolution, Å | 50.0–2.90 | 50.0–2.55 | 50.0–2.70 |
| Rmerge (I) | 0.083 (1.000) | 0.104 (0.959) | 0.058 (1.000) |
| I/σ(I) | 27.05 (1.58) | 11.69 (1.15) | 22.39 (1.02) |
| Completeness, % | 99.6 (99.8) | 99.6 (97.5) | 96.2 (80.1) |
| Redundancy | 7.9 (7.9) | 3.8 (3.3) | 4.0 (3.4) |
| Refinement | |||
| Resolution, Å | 48.7–3.00 | 42.4–2.57 | 40.0–2.69 |
| No. of reflections | 44,498 | 71,127 | 52,861 |
| Rwork/Rfree | 25.8/31.7 | 19.7/24.4 | 20.0/27.2 |
| No. atoms | |||
| Protein | 11,874 | 11,977 | 11,911 |
| Ligand | 0 | 140 | 242 |
| Water | 0 | 342 | 235 |
| B-factors | |||
| Protein | 55.34 | 35.70 | 34.85 |
| Ligand | — | 18.61 | 56.69 |
| Water | — | 25.89 | 32.28 |
| rmsd | |||
| Bond length, Å | 0.011 | 0.016 | 0.015 |
| Bond angles, ° | 1.690 | 1.363 | 1.796 |
Values in parentheses are for highest resolution shells.
Fig. S2.
Neoseptin-3 and lipid A induce similar local conformational changes in MD-2 and a similar active dimerization interface between two mTLR4/MD-2 heterodimers. (A) Superposition of mMD-2 in mTLR4/MD-2/Neoseptin-3, mTLR4/MD-2/lipid A, and the apo mTLR4/MD-2 complexes showing the similar local conformational changes in the Phe126 loop region. (B) Superposition of the mTLR4/MD-2/Neoseptin-3 and mTLR4/MD-2/lipid A active dimers.
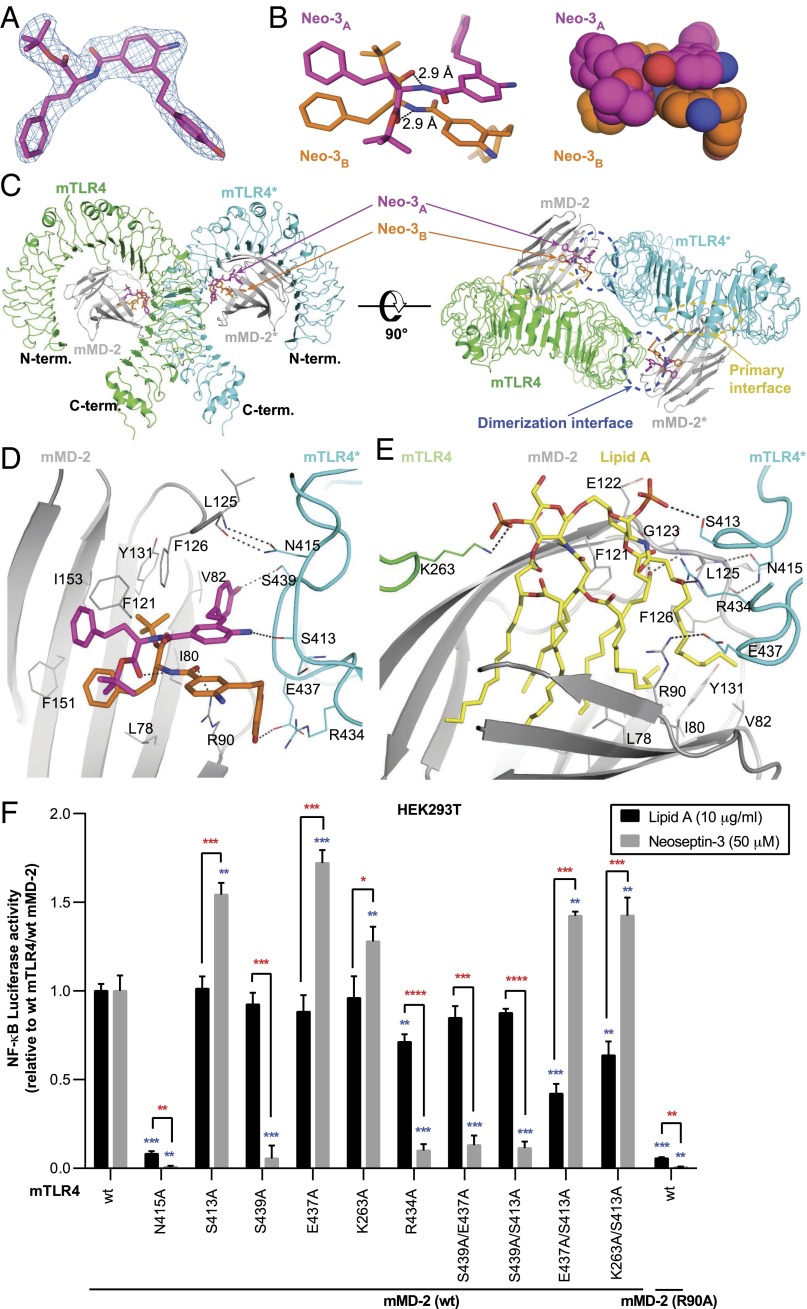
Unexpectedly, the electron density map of the mTLR4/MD-2/Neoseptin-3 structure revealed that two Neoseptin-3 molecules, designated Neo-3A and Neo-3B, bound to each 1:1 mTLR4/MD-2 heterodimer. The configurations of the two bound Neoseptin-3 molecules were resolved unambiguously in the clear electron density map (Fig. 5A). The two Neoseptin-3 molecules are packed tightly against each other at the central aniline rings and amide bond regions (Fig. 5B). Two hydrogen bonds are also formed between the NH group of the amide bond of one Neoseptin-3 molecule and the ester carbonyl group of the other (Fig. 5B).
Fig. 5.
Structure of mTLR4/MD-2/Neoseptin-3 complex. (A) 2Fo-Fc electron density map of one Neoseptin-3 molecule (Neo-3A) in the complex. The contour level of the density is 1.0σ. (B) Stick (Left) and atomic sphere representations (Right) of Neo-3A and Neo-3B bound to mTLR4/MD-2 complex. (C) Orthogonal views of the overall structure of mTLR4/MD-2/Neoseptin-3. (D) Enlarged view of the dimerization interface showing interactions of Neoseptin-3 with MD-2 and mTLR4*. (E) Enlarged view of the dimerization interface showing interactions of lipid A with MD-2 and mTLR4*. In B, D, and E, dashed lines represent hydrogen bonds. (F) NF-κB–dependent luciferase activity in HEK293T cells transiently expressing mTLR4 and mMD-2 bearing the indicated mutations and stimulated with Neoseptin-3 (50 μM) or lipid A (10 μg/mL). Data were normalized to luciferase activity measured in stimulated cells expressing wild-type (wt) mTLR4 and mMD-2. P values were determined by Student’s t test. P values represent the significance of differences between responses of cells expressing the two wild-type proteins versus cells expressing a given mutant protein and stimulated with the same ligand (blue asterisks); or the significance of differences between responses to stimulation with lipid A versus Neoseptin-3 for cells expressing a given mutant protein (red asterisks). The means of triplicate samples are plotted. Results are representative of two independent experiments (error bars represent SEM). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
Each Neoseptin-3 molecule interacts with different regions of MD-2 and TLR4* and adopts a distinct conformation. The Neoseptin-3 molecules are bound to MD-2 close to the entrance of the hydrophobic pocket and facilitate the formation of the agonistic dimerization interface with mTLR4* (Fig. 5C). At this dimerization interface, Neo-3A forms two specific hydrogen bonds with mTLR4*: one between the phenol hydroxyl group and the side chain of residue Ser439*, and the other between the amino group of the central aniline and the main chain carbonyl of Ser413* (Fig. 5D). Neo-3B forms one hydrogen bond between its phenol hydroxyl group and Glu437* of mTLR4*, and another hydrogen bond between its amide carbonyl group and MD-2 residue Arg90 (Fig. 5D). The phenol ring of Neo-3A is nearly completely buried and forms part of the hydrophobic core of the dimerization interface. The terminal phenol group of Neo-3B is also an essential part of the active dimerization interface and is sandwiched between the planar guanidinium groups of Arg434* of mTLR4* and Arg90 of MD-2. The resulting dimerization interface also includes two hydrogen bonds between MD-2 Leu125 backbone atoms and the side chain of mTLR4* Asn415, as is observed in the crystal structures of all active TLR4/MD2 dimers (24, 25).
Structure-based site-directed mutagenesis of mTLR4 and mMD-2 confirmed the importance of specific interactions observed in the mTLR4/MD-2/Neoseptin-3 complex (Fig. 5F and Fig. S3). In particular, mutations of mTLR4 Ser439 and Arg434 severely reduced NF-κB–dependent reporter activation in response to Neoseptin-3. Although the mTLR4 Ser439Ala mutation did not affect lipid A-induced activation significantly, the Arg434Ala mutation reduced the response to lipid A by ∼30% (Fig. 5F). These results are consistent with the observation that mTLR4 Ser439 and Arg434 interact intimately with Neoseptin-3 (Fig. 5D) but do not have close contacts with lipid A (Fig. 5E). Mutations of mTLR4 Glu437 and Lys263 unexpectedly increased responsiveness to Neoseptin-3, but did not affect lipid A responsiveness. These two residues form hydrogen bonds with Neoseptin-3 and lipid A, respectively (Fig. 5 D and E), but the mutagenesis data suggest that these hydrogen bonds might not be critical for ligand binding. Two residues, Asn415 of mTLR4 and Arg90 of mMD-2 are important for both Neoseptin-3 and lipid-A (Fig. 5 D and E), as mutations of these two residues essentially abolished the responsiveness to both Neoseptin-3 and lipid A (Fig. 5F).
Fig. S3.
Expression of mutant mTLR4 and mMD-2 proteins in HEK293T cells. Lysates of the same cells used for measurements of luciferase activity in Fig. 5F were also subjected to immunoblotting with the indicated antibodies.
Neoseptin-3 Activates mTLR4/MD-2 Through a Structural Mechanism Different From That of LPS.
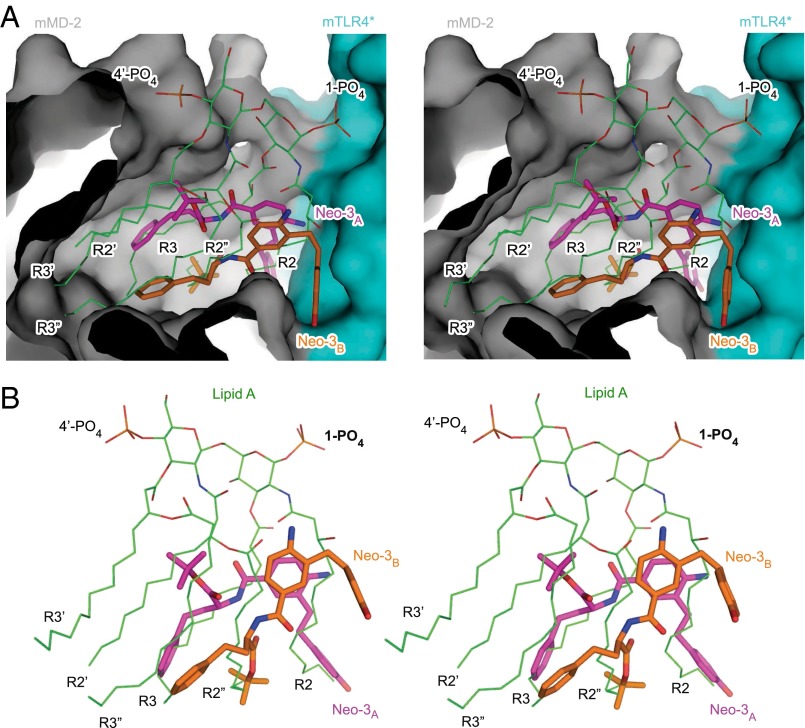
The different chemical structures and sizes of Neoseptin-3 and LPS (or lipid A) translate to distinct modes of receptor binding. The t-butyl ester group and the benzene ring of both Neoseptin-3 molecules reside within the hydrophobic pocket of MD-2, occupying less than half the total volume of the pocket (Fig. 6A). The t-butyl group of Neo-3B is deeply buried and, together with the two terminal benzene rings of Neo-3A and Neo-3B, forms many hydrophobic contacts with MD-2 (Fig. 5D). Comparison of the bound Neoseptin-3 and lipid A revealed that the t-butyl group of Neo-3B overlaps with the R2′′ chain of lipid A, whereas the two terminal benzene rings from the two Neoseptin-3 molecules overlap with the R3 chains (Fig. 6B); these groups anchor the two Neoseptin-3 molecules to MD-2 near the dimerization interface. On the other hand, the two phenol rings of Neo-3A and Neo-3B are outside the hydrophobic pocket of MD-2 but become largely buried upon dimerization with mTLR4*, providing the core interface between MD-2 and mTLR4*. Whereas the Neo-3A phenol ring overlaps with the lipid A R2 chain, the Neo-3B phenol ring shows no overlap with lipid A. The phenol ring of Neo-3B is sandwiched between the side chains of mMD-2 Arg90 and mTLR4* Arg434*, creating a key contact area between MD-2 and TLR4* that is not present in the TLR4/MD-2/lipid A complex. The sites occupied by the other three acyl chains (R3′, R2′, and R3′′) and the two phosphoglucosamine moieties of lipid A remain vacant in the mTLR4/MD-2/Neoseptin-3 structure. Therefore, a number of hydrogen bonds between lipid A phosphoglucosamine moieties and TLR4/MD-2 amino acids are not present in the Neoseptin-3 complex. Overall, through electrostatic and hydrophobic interactions largely distinct from those induced by LPS or lipid A, Neoseptin-3 induced an active 2:2 mTLR4/MD-2 dimer conformation virtually identical to that induced by LPS or lipid A.
Fig. 6.
The different binding modes of Neoseptin-3 and lipid A to mTLR4/MD-2. (A) Stereoview of the bound Neoseptin-3 and lipid A within the hydrophobic pocket of MD-2 (gray) at the dimerization interface with mTLR4* (cyan). The MD-2 in the two complex structures have been superimposed to give the relative positioning of the bound Neoseptin-3 and lipid A. The molecular surfaces of MD-2 and mTLR4* in the Neoseptin-3 complex are shown. Lipid A is shown as thin lines with carbon atoms colored green, whereas Neoseptin-3 molecules are shown as thick sticks. (B) Stereoview of Neoseptin-3 and lipid A as bound to mTLR4/MD-2 showing the overlapping of different chemical groups in the two molecules.
Discussion
Using an unbiased approach, we identified a weak inducer of macrophage TNF biosynthesis, optimized it through SAR studies, and identified it as a highly efficacious and specific agonist for the mouse TLR4/MD-2 complex. NMR and crystallographic data indicate that binding of the ligand, Neoseptin-3, modulates the conformation of MD-2 and facilitates active TLR4/MD-2 dimer formation, presumably initiating a transmembrane conformational change that triggers adapter recruitment and signaling. Neoseptin-3 exhibits no structural similarity to lipid A, the active moiety of LPS molecules. Nonetheless, it closely mimics the action of lipid A, eliciting nearly the same conformational change in the receptor complex. This fact in itself suggests the likelihood that non-LPS ligands of natural origin, including polypeptides, might be capable of activating the TLR4/MD-2 complex, rendering LPS-mimetic effects without an LPS-like structure.
Our structural data reveal how activation of mTLR4/MD-2 is achieved by Neoseptin-3. Remarkably, two ligand molecules bind to the receptor in conjunction with one another in an asymmetrical manner. To our knowledge, such a ligand binding mode is unique among all ligand–protein interactions that have been characterized so far. The Neoseptins are endowed with structural features that promote their own noncovalent dimerization when bound to the TLR4/MD-2 receptor complex, and structural features that promote an activating conformational change on the part of the TLR4/MD-2 receptor complex itself. We noticed that Neo-3A has a lower average crystallographic B-factor relative to Neo-3B (14.4 vs. 22.9 Å2), suggesting that Neo-3A may be more ordered and binds more tightly in the receptor complex. This may bear on the temporal sequence of binding, with Neo-3A binding first before Neo-3B and helping to stabilize Neo-3B through π-stacking interactions as well as hydrogen bonding. The requirement for binding of two Neoseptin-3 molecules along with an overall smaller molecular size compared with lipid A may account for the failure of Neoseptin-3 to antagonize LPS-mediated activation of human TLR4/MD-2. Although our in vitro binding assay shows that Neoseptin-3 binds to human MD-2 and possibly to the human TLR4/MD-2 complex as well, we do not yet understand why Neoseptin-3 fails to elicit cytokine responses in THP-1 cells. One possibility is that Neoseptin-3 fails to induce agonistic dimerization of human TLR4/MD-2 in a manner analogous to that of lipid IVa (25, 26).
The crystal structure of the mTLR4/MD-2/Neoseptin-3 complex explains thoroughly the sensitivity to chemical substitutions observed during SAR analysis of Neoseptin-3 (Fig. S1). For example, substitutions at the amine group on the aniline ring, the amide carbonyl group, or the hydroxyl group on the phenol ring all resulted in a loss of activity because they abrogated hydrogen bonding between these groups of Neoseptin-3 and mTLR4/MD-2. Modification of the t-butyl ester group to an isopropyl, ethyl, methyl ester, or carboxylic acid resulted in progressive loss of activity. This observation may reflect the fact that the hydrophobic cavity of MD-2 is sufficiently large to accommodate a t-butyl group at this position and a smaller or polar group at the same position would be much less favorable. Unlike lipid A, whose six acyl chains and a number of carbonyl groups fill the hydrophobic pocket of MD-2, the two Neoseptin-3 molecules occupy less than half of the hydrophobic pocket of MD-2. Nevertheless the interactions between Neoseptin-3 and MD-2, primarily hydrophobic but also including a specific hydrogen bond between Neo-3B and Arg90 of MD-2, are sufficiently strong and specific to anchor the ligand near the entrance of the hydrophobic pocket of MD-2 for dimerization with mTLR4*. Like lipid A, Neoseptin-3 does not merely induce conformational change of the receptor, but participates in creating the dimerization interface. However, the details of this interface differ substantially for Neoseptin-3 versus lipid A at the atomic level. The interactions between Neoseptin-3 and mTLR4* are primarily mediated by the two phenol groups of the two Neoseptin-3 molecules, through both hydrophobic and π-stacking interactions as well as specific hydrogen bonds. In the case of LPS or lipid A, the interface between the ligand and TLR4* is primarily mediated by the R2 acyl chain, and hydrogen bonds between TLR4* and the R2-OH and 1-PO4 groups.
The acyl chains of LPS are believed to bind to the hydrophobic pocket of CD14 (27), which aids in the delivery of LPS to TLR4/MD-2 (28, 29). The requirement of CD14 for cytokine responses to LPS but not Neoseptin-3 is consistent with this view. It has also been proposed that CD14 prompts the internalization of the TLR4/MD-2 complex and thus permits activation of intracellular signaling, including the activation of the TRIF/TRAM pathway (30). However, insofar as Neoseptin-3 is CD14 independent, this model might be reexamined. Indeed, recent reports similarly documented dissociation between CD14, TLR4 internalization, and TRIF-dependent signaling in response to stimulation with chemically synthesized substituted pyrimido[5,4-b]indoles or a mouse monoclonal TLR4/MD-2 antibody (31–33).
The determination of the crystal structures of two chemically unrelated TLR4 agonists bound to TLR4/MD-2 revealed certain shared characteristics of such ligands. First, the agonist must contain groups capable of mediating the formation of the activating dimerization interface between MD-2 and mTLR4*. Neoseptin-3 and lipid A achieve this via distinct functional/chemical groups and different ligand-protein contacts. Second, tight and specific interaction with the hydrophobic pocket of MD-2 is needed to anchor the ligand properly, though it is not necessary for the ligand to fill the entire pocket of MD-2. These two aspects should be the main target areas for future development of novel and more potent ligands as potential modulators of innate immunity. As for Neoseptin-3, further optimization will be based on structure guided modification of Neo-3A and Neo-3B, and covalently linked derivatives thereof.
We identified two mutations in mTLR4 (Ser413Ala and Glu437Ala) that enhance mTLR4/MD-2 responses to a ligand that does not exist in nature. We infer that naturally occurring mutations of TLR4 or MD-2 could allow normally noninteracting or nonstimulatory molecules to become receptor agonists. Conversely, it is possible that mutations within host genes might create “neo-ligands” for TLRs (34). Such mutations might generally be disfavored, manifested as lethality or as autoinflammatory or autoimmune diseases. However, some peptides or other molecules of endogenous origin might act as agonists for the TLR4/MD-2 complex in conformity with the rules elaborated above, perhaps helping to initiate sterile inflammation to contribute to the repair of damaged tissues (35). As our results demonstrate, two peptidomimetic molecules, each of a small molecular size (<500 Da), can jointly elicit activation of a TLR4/MD-2 complex. High-resolution structural characterization of proposed endogenous agonists, similar to those detailed here, will be essential to determine whether and how they are capable of activating individual TLRs.
Materials and Methods
Mice.
C57BL/6J, Tlr2−/−, and Tlr3−/− mice were purchased from The Jackson Laboratory. Ly96−/− (MD-2−/−) mice were from RIKEN BioResource Center. Tlr4lps3/lps3, Tlr2lngd/lngd, Tlr6int/int, Tlr7rsq1/rsq1, Tlr9CpG3/CpG3, Cd14hdl/hdl, Myd88poc/poc, Ticam1Lps2/Lps2, Irak4otiose/otiose, and Ikbkgpanr2/Y mice were generated on a pure C57BL/6J background by ENU mutagenesis and are described at mutagenetix.utsouthwestern.edu. All experimental procedures using mice were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Southwestern Medical Center, and were conducted in accordance with institutionally approved protocols and guidelines for animal care and use. All of the mice were maintained at the University of Texas Southwestern Medical Center in accordance with institutionally approved protocols.
Synthesis of Neoseptin-3.
Fisher esterification of 3-hydroxy-4-nitrobenzoic acid (cat. H2SO4, MeOH, reflux, 18 h, 99% yield) afforded methyl 3-hydroxy-4-nitrobenzoate, from which the aryl triflate was prepared (Tf2O, Et3N, CH2Cl2, 0 °C, 18 h, 79% yield). Sonogashira cross-coupling of the triflate with [(4-triisopropylsilyloxy)phenyl]acetylene (PdCl2(PPh3)2, CuI, Et3N/DMF, Bu4NI, 70 °C), silyl ether cleavage, and concurrent methyl ester hydrolysis (LiOH·H2O, THF/MeOH/H2O 4:1:1, 25 °C, 12 h, 81% yield over two steps) gave 3-((4-hydroxyphenyl)ethynyl)-4-nitrobenzoic acid. Carbodiimide-mediated coupling of the benzoic acid with l-HoPhe-OtBu (EDCI·HCl, HOAt, 2,6-lutidine, DMF, 25 °C, 12 h, 72% yield) provided (S)-tert-butyl 2-(3-((4-hydroxyphenyl)ethynyl)-4-nitrobenzamido)-4-phenylbutanoate. Alkyne hydrogenation with concurrent nitro group reduction via Pearlman’s catalyst (H2, Pd(OH)2/C, EtOAc, 25 °C, 12 h, 94% yield) yielded Neoseptin-3.
Isolation of Peritoneal Macrophages, BMDM, BMDC, and Cell Culture.
Thioglycollate-elicited macrophages were recovered 4 d after i.p. injection of 2 mL of BBL thioglycollate medium, brewer modified [4% (wt/vol); BD Biosciences] by peritoneal lavage with 5 mL of PBS. The peritoneal macrophages were cultured in DMEM cell culture medium [DMEM containing 10% (vol/vol) FBS (Gemini Bio Products), 1% penicillin and streptomycin (Life Technologies)] at 37 °C and 95% air/5% CO2. Murine BMDMs were collected by flushing bone marrow cells from femurs and tibiae of mice. These cells were cultured for 7 d in DMEM cell culture medium containing 10% (vol/vol) conditioned medium from L929 cells. For BMDCs, bone morrow cells were cultured in Petri dishes in 10 mL of DMEM cell culture medium containing 10 ng/mL murine GM-CSF (R&D Systems). On day 3 of culture, this was replaced with fresh GM-CSF medium. Loosely adherent cells were transferred to a fresh Petri dish and cultured for an additional 4 d.
THP-1 (ATCC) cells were differentiated by treatment with 100 nM PMA (Sigma) in RPMI cell culture medium [RPMI containing 10% (vol/vol) FBS (Gemini Bio Products), 1% penicillin and streptomycin (Life Technologies)] for 24 h. After that, cells were washed with PBS and cultured in fresh RPMI cell culture medium for 24 h before use in experiments. HEK293T cells (ATCC) were cultured in DMEM cell culture medium.
Measurement of Cytokine Production.
Cells were seeded onto 96-well plates at 1 × 105 cells per well and stimulated with Neoseptin-3 [dissolved in DMSO; final DMSO concentrations (≤0.2%) were kept constant in all experiments] or ultra-pure LPS (dissolved in H2O, Enzo Life Sciences) for 4 h. Mouse TNFα, IL-6, or IFN-β, or human TNFα in the supernatants were measured by ELISA kits according to the manufacturer’s instructions (eBioscience and PBL Assay Science). Pretreatment with Eritoran (Eisai) or Neoseptin-3 was for 1 h at the indicated concentrations. Unless otherwise indicated, mouse cells were from wild-type C57BL/6J mice.
Luciferease Assay.
HEK293T cells were transfected with an NF-κB–dependent luciferase reporter plasmid (Clontech) using Lipofectamine 2000 (Life Technologies) and clones with stable expression were selected by culture in DMEM containing puromycin (Life Technologies). Cells were cotransfected with constructs for mouse or human TLR4 plus mouse or human MD-2, and 2 d later were stimulated with 50 μM Neoseptin-3 or 1 μg/mL LPS for 6 h. Cells were lysed, and luciferase activity was measured using the Steady-Glo Luciferase Assay System (Promega).
Constructs.
cDNAs encoding TLR4 and MD-2 (human and mouse) were amplified using standard PCR techniques and subsequently inserted into mammalian expression vector pcDNA3-C-V5 (V5 tag at the C terminus of encoded protein) using the In-Fusion HD Cloning Kit (Clontech).
Mutagenesis.
Point mutations were introduced into mTLR4 and mMD-2 by standard site-directed mutagenesis. Luciferase assays were conducted as described above except that stimulation was with 50 μM Neoseptin-3 or 10 μg/mL lipid A for 6 h.
Western Blotting.
Peritoneal macrophages (1 × 106 per well) were stimulated in 12-well plates with Neoseptin-3 (50 μM) or LPS (5 ng/mL) for the indicated times and lysed directly in sample buffer (Sigma). Cell lysates were separated by SDS/PAGE and transferred to nitrocellulose membranes. Membranes were probed with the following antibodies: phospho-IKKα (Ser176)/IKKβ (Ser177), IκBα, phospho-p38 (Thr180/Tyr182), phospho-JNK (Thr183/Tyr185), phospho-ERK1/2 (Thr202/Tyr204), phospho-TBK1 (Ser172), phospho-IRF3 (Ser396) (Cell Signaling Technology), and α-tubulin (Sigma).
Protein Expression, Purification, and Crystallization.
The hybrid construct of mouse TLR4 (residues 26–544) fused with hagfish variable lymphocyte receptor (VLR) (residues 126–200) was cloned into plasmid pAcGP67a. The mouse MD-2 (residues 19–160) fused to a protein A tag was cloned into a second pAcGP67a plasmid. The mTLR4VLR and MD-2-Protein A constructs were coexpressed in Hi5 insect cells (Invitrogen) and purified by IgG Sepharose (GE Healthcare) affinity chromatography. The eluted mTLR4VLR/MD-2-protein A was buffer exchanged to a buffer containing 20 mM Hepes pH 7.5, 40 mM NaCl. The protein A tag was removed by TEV cleavage and partially deglycosylated using PNGase F (New England BioLabs) at room temperature for 3 h and then 4 °C overnight. The tag-free mTLR4VLR/MD-2 complex (termed mTLR4/MD-2 hereafter and in the main text as they are functionally interchangeable) was further purified by ion exchange (HiTrap Q) and gel filtration (Superdex 200 16/60) chromatography. The final protein buffer contained 25 mM Hepes pH 8.0, 75 mM NaCl (Buffer A). The purified mTLR4/MD-2 complex was concentrated to 24 mg/mL.
To reconstitute the mTLR4/M-D2/lipid A complex, 2 mg/mL mouse TLR4/MD-2 protein was incubated with 206 μM Escherichia coli lipid A (Re mutant) (Enzo Life Sciences) and 0.05% Triton X-100 at 37 °C for 2 h. The mTLR4/MD-2/lipid A complex was then purified by gel filtration chromatography (Superdex 200 16/60) in Buffer A and concentrated to 8 mg/mL. To reconstitute the mTLR4/MD-2/Neoseptin-3 complex, 2 mg/mL mTLR4/MD-2 protein was incubated with 1 mM Neoseptin-3 dissolved in 20% (vol/vol) DMSO at room temperature for 4 h. The Neoseptin-3 precipitates were spun down. The complex was then buffer exchanged to Buffer A by a PD-10 desalting column (GE Healthcare) to remove DMSO and excess Neoseptin-3. The mTLR4/MD-2/Neoseptin-3 complex was concentrated to 12 mg/mL.
mTLR4/MD-2 and mTLR4/MD-2/lipid A crystals were grown with a hanging-drop vapor diffusion method by mixing 1 μL of protein with 1 μL of crystallization solution. The optimized crystallization conditions for each complex are summarized in Table S2. After 1 wk, the mTLR4/MD-2 and mTLR4/MD-2/lipid A crystals were flash-frozen in liquid nitrogen in different cryoprotectant solutions (Table S2). mTLR4/MD-2/Neoseptin-3 crystals were grown with the same hanging-drop vapor diffusion method by first mixing 1.5 μL of protein with 1.5 μL of crystallization solution (Table S2) and then microseeded with mTLR4/MD-2/lipid A crystal seeds. The crystals appeared overnight and, after 10 d, the crystals were flash-frozen in liquid nitrogen in cryoprotectant (Table S2).
Table S2.
Crystallization conditions
| Protein complex | Crystallization solution | Cryoprotectant |
| mTLR4/MD-2 | 0.1 M Bicine pH 7.5, 12% PEG 10,000 | 0.1 M Bicine pH 7.5, 0.05 M NaCl, 18% PEG 10,000, 35% ethylene glycol |
| mTLR4/MD-2/Lipid A | 0.1 M Tris pH 8.0, 0.2 M LiCl, 14% PEG 8,000, 2.5% PEG 400 | 0.1 M Tris pH 8.0, 0.2 M LiCl, 18% PEG 8,000, 0.05 M NaCl, 30% sucrose |
| mTLR4/MD-2/Neoseptin-3 | 0.1 M Tris pH 8.0, 0.2 M LiCl, 12% PEG 8,000 | 0.1 M Tris pH 8.0, 0.2 M LiCl, 16% PEG 8,000, 0.05 M NaCl, 30% sucrose |
Data Collection and Structure Determination.
Diffraction data were collected at Beamlines BM19 and ID19 of Advance Photon Source, Argonne National Laboratory. The data were indexed, integrated, and scaled using the HKL3000 package (36). The initial phases for the apo mTLR4/MD-2, mTLR4/MD-2/lipid A, and mTLR4/MD-2/Neoseptin-3 complexes were determined by the molecular replacement method using the program PHASER (37). The published mouse TLR4/MD-2 structure [Protein Data Bank (PDB) ID code 2Z64] was used as a search model for apo mTLR4/MD-2. Later the refined structure of apo mTLR4/MD-2 was used as a search model for mTLR4/MD-2/lipid A and mTLR4/MD-2/Neoseptin-3 complex structure determinations. The electron densities for Neoseptin-3 and lipid A were evident from the early stages of refinement and the atomic models of Neoseptin-3 and lipid A were built into the electron density map unambiguously. The manual model building was performed with COOT (38) and the crystallographic refinement was performed with Refmac5 (39). The data collection and refinement statistics for all three structures are summarized in Table S1.
In Vitro Binding Assay of Neoseptin-3 to TLR4/MD-2 by NMR Spectroscopy.
One-dimensional 1H-NMR spectra of the methyl regions of Neoseptin-3 were acquired at 25 °C on a Varian INOVA 600 spectrometer equipped with a cold probe. Neoseptin-3 (20 μM) in the absence and presence of 10 μM protein [mouse TLR4/MD-2 complex, mouse MD-2-protein A, human MD-2-protein A, or protein A (MP Biomedicals)] or protein A alone was dissolved in a buffer containing 70% (wt/vol) PBS, 20% (wt/vol) D2O, 10% (wt/vol) d6-DMSO (Cambridge Isotope Laboratories). To each sample was applied a Carr Purcell Meiboom Gill (CPMG) sequence for 100 ms (CPMG 100ms). The CPMG sequence allows signal relaxation during the 100-ms delay and leads to almost complete relaxation of the signal from large size molecules. The signal of Neoseptin-3 alone changed little during the 100-ms delay because of its very small size. Addition of large size proteins to Neoseptin-3 resulted in complete loss of the signal indicating that Neoseptin-3 binds to the much higher molecular weight protein in solution.
Statistical Analyses.
Data represent means ± SEM in all graphs depicting error bars. The statistical significance of differences between experimental groups was determined using GraphPad Prism 6 and the indicated statistical tests. P values are indicated by *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. P ≤ 0.05 was considered statistically significant.
Acknowledgments
We thank Ian Wilson for the initial design of the mouse TLR4VLR and MD2-protein A hybrid constructs for coexpression of the complex; Jose Rizo-Rey for help with the NMR experiments; Diana Tomchick and Srinivasan Raghunathan for help with X-ray data collection and structure determination; and Peter Jurek and Anne Murray for assistance with manuscript preparation. Results shown in this report are derived from work performed at Argonne National Laboratory, Structural Biology Center at Advanced Photon Source. Argonne is operated by UChicago Argonne, LLC, for the US Department of Energy, Office of Biological and Environmental Research under Contract DE-AC02-06CH11357. This work was supported by NIH/National Institute of General Medical Sciences (NIGMS) Grant R01GM104496 (to H.Z.) and NIH/National Institute of Allergy and Infectious Diseases (NIAID) Grants U24 AI082657 (to B.B. and D.L.B.) and U19 AI100627 (to B.B.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org [PDB ID codes 5HG3 (mTLR4/MD2), 5HG4 (mTLR4/MD2/Neoseptin-3), and 5HG6 (mTLR4/MD2/lipid A)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1525639113/-/DCSupplemental.
References
- 1.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 2.Uematsu S, Akira S. Toll-like receptors and innate immunity. In: Bauer S, Hartmann G, editors. Handbook of Experimewntal Pharmacology. Vol. 183. Springer; Berlin: 2008. pp. 1–20. [Google Scholar]
- 3.Roelofs MF, et al. Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol. 2006;176(11):7021–7027. doi: 10.4049/jimmunol.176.11.7021. [DOI] [PubMed] [Google Scholar]
- 4.Yu M, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26(2):174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 5.Vabulas RM, et al. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277(17):15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 6.Vabulas RM, et al. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277(23):20847–20853. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- 7.Vabulas RM, et al. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276(33):31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 8.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167(5):2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 9.Termeer C, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195(1):99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang P, Cox CJ, Alvarez KM, Cunningham MW. Cutting edge: Cardiac myosin activates innate immune responses through TLRs. J Immunol. 2009;183(1):27–31. doi: 10.4049/jimmunol.0800861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HS, et al. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27(2):321–333. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Schaefer L, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115(8):2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogl T, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13(9):1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 14.Biragyn A, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298(5595):1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 15.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168(10):5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 16.Midwood K, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15(7):774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 17.Walton KA, et al. Receptors involved in the oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine-mediated synthesis of interleukin-8. A role for Toll-like receptor 4 and a glycosylphosphatidylinositol-anchored protein. J Biol Chem. 2003;278(32):29661–29666. doi: 10.1074/jbc.M300738200. [DOI] [PubMed] [Google Scholar]
- 18.Okamura Y, et al. The extra domain A of fibronectin activates toll-like receptor 4. J Biol Chem. 2001;276(13):10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 19.Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 20.Whitby LR, Boger DL. Comprehensive peptidomimetic libraries targeting protein-protein interactions. Acc Chem Res. 2012;45(10):1698–1709. doi: 10.1021/ar300025n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saitoh S, et al. Lipid A antagonist, lipid IVa, is distinct from lipid A in interaction with Toll-like receptor 4 (TLR4)-MD-2 and ligand-induced TLR4 oligomerization. Int Immunol. 2004;16(7):961–969. doi: 10.1093/intimm/dxh097. [DOI] [PubMed] [Google Scholar]
- 22.Golenbock DT, Hampton RY, Qureshi N, Takayama K, Raetz CR. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem. 1991;266(29):19490–19498. [PubMed] [Google Scholar]
- 23.Rizo J, Chen X, Araç D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 2006;16(7):339–350. doi: 10.1016/j.tcb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Park BS, et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458(7242):1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 25.Ohto U, Fukase K, Miyake K, Shimizu T. Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc Natl Acad Sci USA. 2012;109(19):7421–7426. doi: 10.1073/pnas.1201193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316(5831):1632–1634. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- 27.Kim JI, et al. Crystal structure of CD14 and its implications for lipopolysaccharide signaling. J Biol Chem. 2005;280(12):11347–11351. doi: 10.1074/jbc.M414607200. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Q, Akashi S, Miyake K, Petty HR. Lipopolysaccharide induces physical proximity between CD14 and toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J Immunol. 2000;165(7):3541–3544. doi: 10.4049/jimmunol.165.7.3541. [DOI] [PubMed] [Google Scholar]
- 29.da Silva Correia J, Soldau K, Christen U, Tobias PS, Ulevitch RJ. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. transfer from CD14 to TLR4 and MD-2. J Biol Chem. 2001;276(24):21129–21135. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- 30.Zanoni I, et al. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147(4):868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajaiah R, Perkins DJ, Ireland DD, Vogel SN. CD14 dependence of TLR4 endocytosis and TRIF signaling displays ligand specificity and is dissociable in endotoxin tolerance. Proc Natl Acad Sci USA. 2015;112(27):8391–8396. doi: 10.1073/pnas.1424980112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi T, et al. Novel synthetic toll-like receptor 4/MD2 ligands attenuate sterile inflammation. J Pharmacol Exp Ther. 2014;350(2):330–340. doi: 10.1124/jpet.114.214312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan M, et al. Identification of substituted pyrimido[5,4-b]indoles as selective Toll-like receptor 4 ligands. J Med Chem. 2013;56(11):4206–4223. doi: 10.1021/jm301694x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beutler B. Neo-ligands for innate immune receptors and the etiology of sterile inflammatory disease. Immunol Rev. 2007;220(1):113–128. doi: 10.1111/j.1600-065X.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- 35.Bianchi ME. DAMPs, PAMPs and alarmins: All we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 36.Minor W, Cymborowski M, Otwinowski Z, Chruszcz M. HKL-3000: The integration of data reduction and structure solution--from diffraction images to an initial model in minutes. Acta Crystallogr D Biol Crystallogr. 2006;62(Pt 8):859–866. doi: 10.1107/S0907444906019949. [DOI] [PubMed] [Google Scholar]
- 37.McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Likelihood-enhanced fast translation functions. Acta Crystallogr D Biol Crystallogr. 2005;61(Pt 4):458–464. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- 38.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 39.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53(Pt 3):240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 40.Shaginian A, et al. Design, synthesis, and evaluation of an alpha-helix mimetic library targeting protein-protein interactions. J Am Chem Soc. 2009;131(15):5564–5572. doi: 10.1021/ja810025g. [DOI] [PMC free article] [PubMed] [Google Scholar]