Significance
Associating environmental cues with their outcomes occurs through multiple strategies relying on different neural substrates. Unpredicted reward evokes dopamine release, which also develops to predictive cues, suggesting that predictive dopamine signals arise only after extensive pairings of cues with appetitive outcomes. However, recent work suggests that dopamine may also contribute to model-based learning, which does not require that cues and their appetitive outcomes be experienced in tandem. Taking advantage of the appetitive value of a hypertonic sodium solution, which radically and reversibly changes with physiological state, we show that dopamine differentially encodes hypertonic NaCl depending on sodium balance independent of prior experience. Conversely, dopamine only encoded a NaCl cue after extensive, state-dependent experience, firmly supporting dopamine’s role in experience-dependent learning.
Keywords: nucleus accumbens, dopamine, voltammetry, learning, motivation
Abstract
Phasic dopamine signaling participates in associative learning by reinforcing associations between outcomes (unconditioned stimulus; US) and their predictors (conditioned stimulus; CS). However, prior work has always engendered these associations with innately rewarding stimuli. Thus, whether dopamine neurons can acquire prediction signals in the absence of appetitive experience and update them when the value of the outcome changes remains unknown. Here, we used sodium depletion to reversibly manipulate the appetitive value of a hypertonic sodium solution while measuring phasic dopamine signaling in rat nucleus accumbens. Dopamine responses to the NaCl US following sodium depletion updated independent of prior experience. In contrast, prediction signals were only acquired through extensive experience with a US that had positive affective value. Once learned, dopamine prediction signals were flexibly expressed in a state-dependent manner. Our results reveal striking differences with respect to how physiological state shapes dopamine signals evoked by outcomes and their predictors.
Reconciling differences between anticipated and experienced outcomes is fundamental for how an organism learns about the world. A key component of temporal difference (TD) learning models is the reward prediction error (RPE) term (1, 2), which is thought to be represented by phasic activity of midbrain dopamine neurons (3–5). Indeed, conditioned stimulus (CS)-related dopamine activity correlates with multiple behavioral indices of learning (6–8), and phasic dopamine signaling is sufficient to drive CS-unconditioned stimulus (US) learning (9).
In much of the supportive empirical work, food- or fluid-restricted animals first experience and then learn to anticipate an innately appetitive US (e.g., sucrose, juice, water). Thus, the US always has an inherent caloric, nutritive, or positive affective value to the organism. Consequently, it is uncertain whether dopamine neurons can acquire CS-US associations without first experiencing the US as a reward. Resolving this question is critical, because the striatal underpinnings of goal-directed behavior may encompass both RPE and experience-independent, model-based strategies (10, 11). One way to delineate dopamine’s role in these different learning strategies would be to promote associations between a CS and a neutral or normally avoided US whose affective value could be manipulated and then determine the experience dependency of dopamine CS responses.
Sodium appetite is an ideal platform on which to address this question. Sodium depletion induces a powerful sodium hunger and radically but reversibly alters the rewarding value of hypertonic NaCl solutions (12, 13). The appetite is highly selective for sodium and manifests independent of prior experience with either sodium solutions or sodium deficiency (14, 15). Therefore, sodium appetite facilitates the delivery of a US (hypertonic NaCl) that is rewarding only in a specific physiological state. We measured phasic dopamine signaling in the nucleus accumbens (NAc) of rats while delivering a hypertonic NaCl solution directly into the oral cavity (intraoral) while rats were under different physiological states. We found that dopamine responses to the NaCl US were state-dependent and used this feature to investigate how physiological state influenced acquisition and expression of NaCl CS-US associations. In contrast to the US, dopamine responses to the NaCl CS depended on an interaction between experience and physiological state. Our data suggest that dopamine neurons only signal reward predictions after extensive and direct, state-dependent experience with an appetitive US and, moreover, that reward prediction signals are expressed in a state-dependent manner, a finding most consistent with TD models.
Results
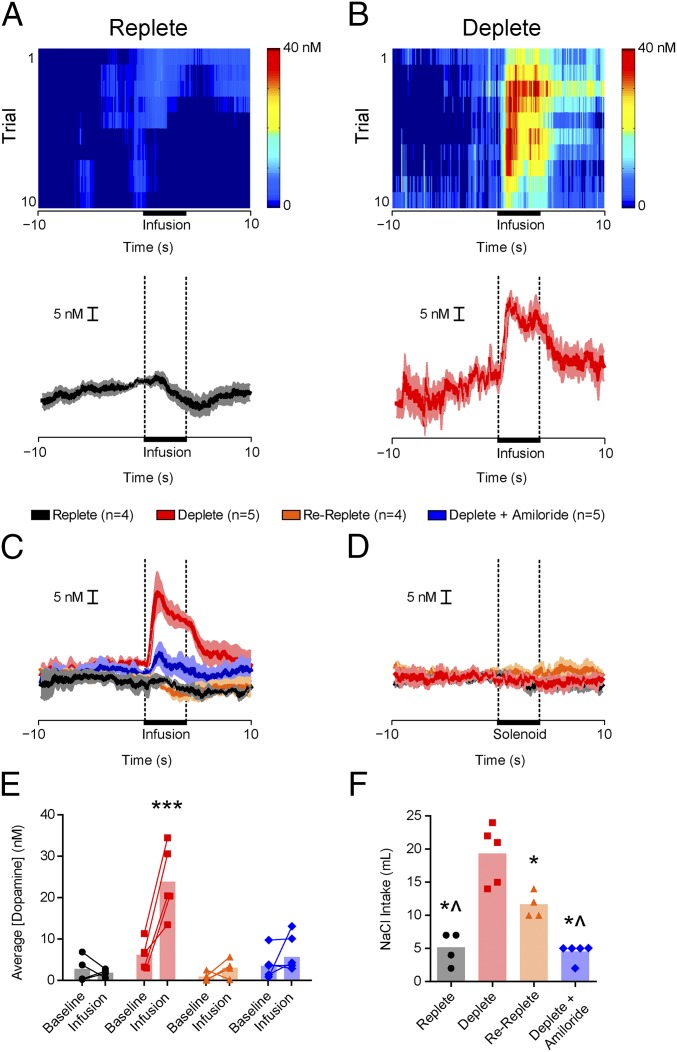
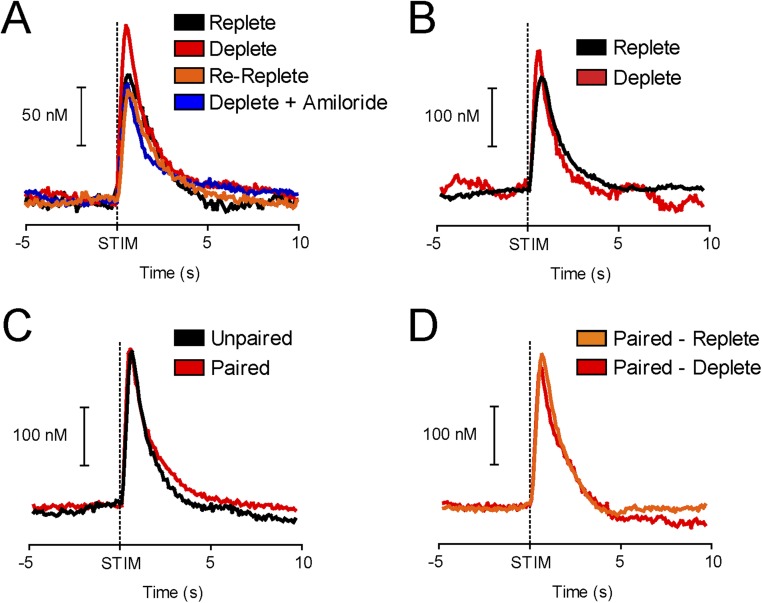
Sodium appetite renders normally avoided hypertonic NaCl positively reinforcing (13). Given the link between phasic dopamine and positive reinforcement (16, 17), we first examined whether sodium appetite regulates the unconditioned dopamine response to hypertonic NaCl. We measured NAc dopamine with fast-scan cyclic voltammetry (FSCV) while delivering brief (4 s) intraoral infusions of 0.45 M NaCl to naive rats. The 0.45 M concentration was selected to maximize the ability to transform a normally avoided US into a powerful appetitive stimulus. We tested four groups of rats in different states of sodium balance: Replete (n = 4), Deplete (n = 5), Re-Replete (n = 4, sodium-depleted but allowed to restore sodium balance for 48 h before testing), and Deplete + Amiloride [n = 5, deplete but received 0.45 M NaCl in amiloride (100 μM)]. Intraoral NaCl evoked phasic dopamine release only in Deplete rats (two-way ANOVA: epoch × group interaction: F3,14 = 10.17, P < 0.001; post hoc: Deplete infusion: P < 0.001 vs. all comparisons; Fig. 1 A–C and E). Importantly, a dopamine response was absent in Re-Replete rats, indicating the response depends on physiological state at the time of NaCl exposure. Furthermore, the dopamine response to intraoral NaCl was taste-dependent. Amiloride, which blocks lingual sodium channels and disrupts NaCl intake induced by depletion (Fig. S1), attenuated NaCl-evoked dopamine (Fig. 1C; Deplete + Amiloride). The dopamine response was unconditioned because (i) it was evident on the first infusion in NaCl naive Deplete rats (Fig. 1B and Fig. S2 A and B) and (ii) the sound associated with NaCl delivery (solenoid valve click) in the absence of intraoral NaCl did not evoke dopamine release (Fig. 1D). Following depletion, sodium appetite was probed by measuring overnight intake of 0.45 M NaCl (one-way ANOVA: F3,14 = 27.92, P < 0.0001; post hoc: Deplete: P < 0.05 vs. all other groups, Re-Replete: P < 0.05 vs. Replete and Deplete + Amiloride; Fig. 1F). Additional experiments suggested the lateral hypothalamus encoded NaCl taste in a state-dependent manner upstream of ventral tegmental area (VTA) dopamine neurons (Fig. S3).
Fig. 1.
Phasic dopamine signaling evoked by hypertonic NaCl depends on a taste-by-state interaction. (A, Top) Trial-by-trial (y axis) heat plot from a representative Replete rat depicting NAc dopamine concentration during 20 s (x axis) surrounding 4-s intraoral infusions of 0.45 M NaCl. (A, Bottom) Dopamine concentration from the 10 trials (mean ± SEM). (B) Same as in A in a Deplete rat. (C) Average dopamine concentration (±SEM) evoked by intraoral NaCl across groups (group details are provided in Results): Replete (n = 4, black), Deplete (n = 5, red), Re-Replete (n = 4, orange), and Deplete + Amiloride (n = 5, blue). (D) Average dopamine (±SEM) evoked by solenoid click (no intraoral NaCl delivered). (E) Average dopamine concentration during baseline (−4 to 0 s) vs. intraoral infusion (0.1–4 s). There were no differences during baseline, and intraoral NaCl evoked dopamine selectively in Deplete rats (infusion vs. all comparisons, ***P < 0.001). (F) Overnight NaCl intake following depletion (Deplete, Re-Replete, and Deplete + Amiloride) or control (Replete) treatment. *Significantly different from Deplete (at least P < 0.05); ^significantly different from Re-Replete (at least P < 0.05). Opaque bars represent the mean.
Fig. S1.
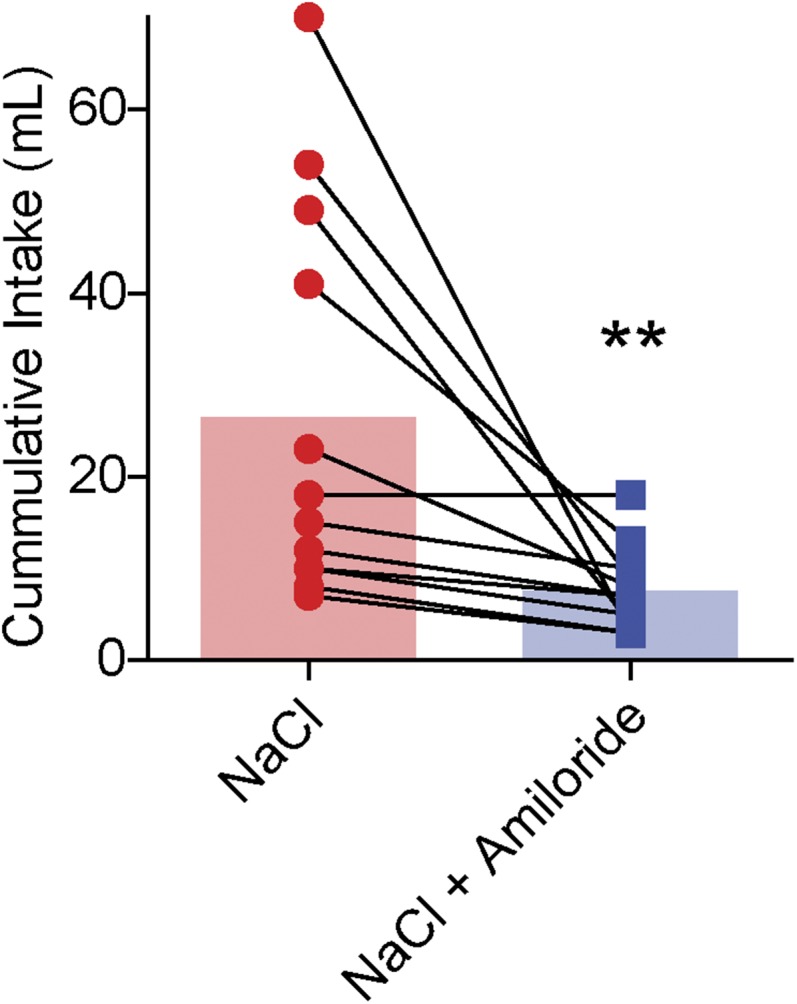
One hundred micromolar amiloride disrupts overnight intake of 0.45 M NaCl in sodium-depleted rats (related to Fig. 1). Previous work indicates that amiloride, an epithelial sodium channel blocker, disrupts consumption of hypertonic NaCl solutions by sodium-deficient rats (SI Experimental Procedures). We confirmed this result for 0.45 M NaCl by inducing sodium appetite in rats (n = 12) on two separate days spaced 1 wk apart. Following 24-h sodium depletion, rats were given ad libitum home cage access to either 0.45 M NaCl alone or 0.45 M NaCl mixed with 100 μM amiloride in a counterbalanced fashion. Deplete rats consumed significantly less 0.45 M NaCl in the presence of 100 μM amiloride compared with 0.45 M NaCl alone (W = 66, **P < 0.01; Wilcoxon matched pairs test). Thus, even at NaCl concentrations as high as 0.45 M, 100 μM amiloride is sufficient to disrupt the ability of sodium-depleted rats to identify and exploit sources of sodium.
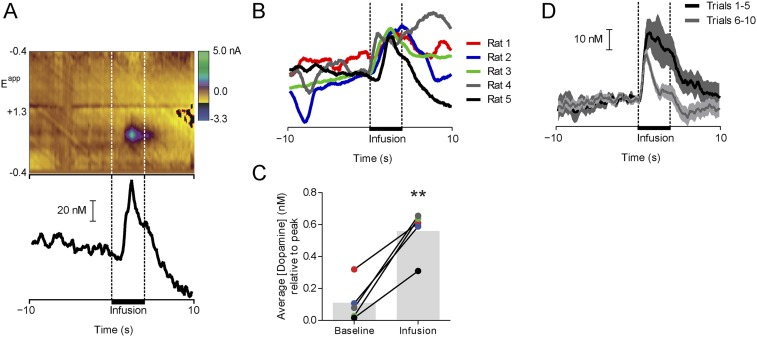
Fig. S2.
Intraoral NaCl infusion evokes phasic dopamine release on the first trial in sodium-depleted rats, and the response diminishes across the session (related to Fig. 1 and Fig. S3). (A) Representative electrochemical data from the first intraoral NaCl infusion in a Deplete rat. (Top) Single-trial color plot depicts current changes (color) as a function of electrode potential (y axis) during the 10 s before and after the first intraoral NaCl infusion (x axis) in a representative Deplete rat. Dopamine [identified by its oxidation (∼0.6 V, green feature)] was transiently evoked following the intraoral infusion. (Bottom) Dopamine concentration extracted from the electrochemical data above. (B) Dopamine concentration (normalized to the peak concentration measured in the trial) during the 10 s before to 10 s after the first intraoral infusion of 0.45 M NaCl in sodium-depleted rats. Each trace plots the first trial response from an individual rat (n = 5). Colors signify individual rats. Data in A come from rat 5. (C) Average dopamine concentration (relative to peak) taken from the data in A during the 4 s before (baseline) compared with the 4 s after intraoral infusion. The colors of individual data points correspond to rats in B. **Significant difference between the baseline and infusion epochs (paired t test: t4 = 6.61, P < 0.01). (D) Average dopamine concentration (±SEM) evoked by the first five intraoral infusions of 0.45 M NaCl (black) compared with the last five infusions (gray) in sodium-depleted rats (n = 5 rats). Averaging across the infusion window (0.1–4 s) for all trials revealed a significant decrease in the magnitude of dopamine evoked by NaCl across the session (paired t test: t24 = 2.15, P < 0.05).
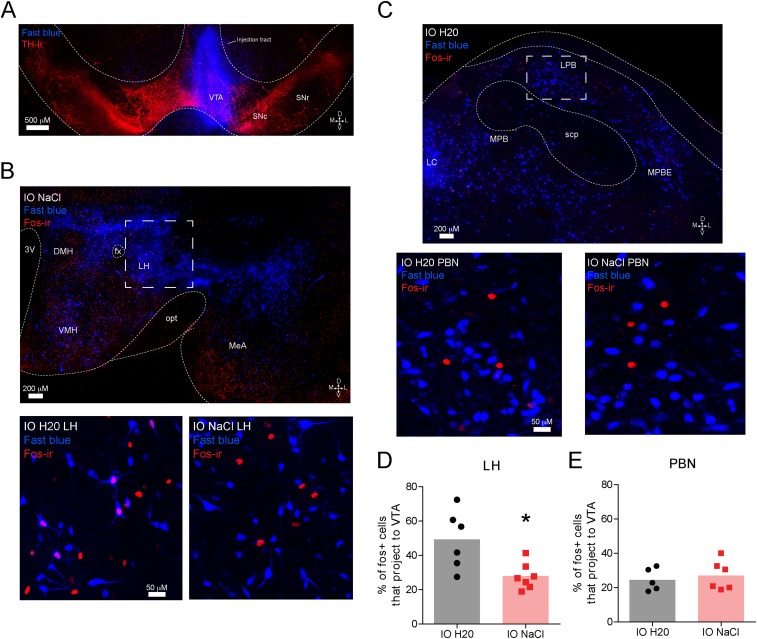
Fig. S3.
Sodium-depleted rats that received intraoral infusions of NaCl have less Fos immunoreactivity in VTA projecting neurons from the LH, but not the PBN, compared with sodium-depleted rats that received intraoral distilled H2O (related to Fig. 1). Interestingly, we observed a significant decrease in the magnitude of dopamine release evoked by intraoral NaCl across the session in sodium-depleted rats (Fig. S2). This result suggested that the input (or inputs) providing NaCl-related information to VTA dopamine neurons becomes quiescent as rats recovered lost sodium. To investigate this possibility, we injected the VTA with Fast Blue [250 nL, 2% (vol/vol) in distilled water], a fluorescent retrograde tracer (n = 7 rats). Ten days later, all rats were sodium-depleted for 24 h. The behavioral session was identical to the unconditioned intraoral infusion session used in the voltammetry experiments presented in Fig. 1. Sodium-depleted rats received 30 intraoral infusions of either 0.45 M NaCl (n = 4) or distilled water (n = 3). Given that the magnitude of NaCl-evoked dopamine release decayed across the infusion session (Fig. S2C), we reasoned that a VTA input capable of driving dopamine responses to NaCl might be differentially activated by sodium depletion with (intraoral NaCl) or without (intraoral distilled water) partial repletion. One hour following sodium depletion, all rats were perfused and brains were stored in cryoprotectant in preparation for sectioning, immunohistochemistry, and confocal microscopy. We focused our analyses on the PBN and the LH because both are critical for sodium appetite (52) and are positioned at different stages of integrating visceral and gustatory signals. (A) Confocal image (20×) of a representative VTA injection site depicting tyrosine hydroxylase (TH) immunoreactivity (red) and the injection site of Fast Blue (blue). D, dorsal; L, lateral; M, medial; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; V, ventral. (B, Top) Confocal image (20×) at the level of the hypothalamus in a sodium-depleted rat that received intraoral NaCl. The image depicts retrograde labeling of VTA projecting neurons (blue) and Fos immunoreactivity (red). DMH, dorsomedial hypothalamus; fx, fornix; IO, intraoral; MeA, medial amygdala; opt, optic tract; 3V, third ventricle; VMH, ventromedial hypothalamus. The dashed box indicates the approximate LH region of interest (ROI) used for quantification in D. (B, Bottom) Confocal image (20×) zoomed in to the LH ROI in a representative sodium-depleted rat that received intraoral H2O (Left) or intraoral NaCl (Right). Note the absence of colabeling following intraoral NaCl. (C, Top) Confocal image (20×) at the level of the lateral PBN from a rat that received intraoral H2O. The image depicts retrograde labeling of VTA projecting neurons (blue) and Fos immunoreactivity (red). LC, locus coeruleus; MPB, medial parabrachial region; MPBE, medial parabrachial external region; scp, superior cerebellar peduncles. The dashed box indicates the approximate LPB ROI used for quantification in E. (C, Bottom) Confocal image (20×) zoomed in on the LPB ROI in a representative sodium-deficient rat that received intraoral H20 (Left) or intraoral NaCl (Right). (D) Graph depicts the percentage of Fos-positive LH neurons that project to the VTA in sodium-depleted rats that received intraoral H2O (black, n = 3 rats, two LH slices per rat) vs. intraoral NaCl (red, n = 4 rats, two LH slices from three rats, one LH slice from one rat). Given the variability in tracer uptake and transport, as well as Ab staining, each slice was treated as an independent observation. Rats that received intraoral NaCl had a significantly smaller proportion of Fos-positive cells that projected to the VTA compared with rats that received intraoral H2O (unpaired t test: t11 = 3.0, *P < 0.05). The shaded bar in the background indicates the mean; data from individual LH slides are represented by individual dots. (E) Same as in D but for LPB neurons that project to the VTA [intraoral H2O (black, n = 3 rats, two LPB slices from two rats, one LPB slice from one rat) vs. intraoral NaCl (red, n = 4 rats, three PBN slices from three rats, one PBN slice from one rat)]. One slice was removed from each group due to poor hind-brain tracer expression. No differences were observed in the LPB (unpaired t test: t9 = 0.54, P > 0.05). Thus, VTA LH neurons may drive phasic dopamine responses to NaCl in sodium-depleted rats, and this drive may weaken as repletion occurs. Moreover, these data indicate that the transformation in NaCl encoding relevant to dopamine signaling likely occurs after initial stages of central visceral and gustatory processing because no differences were observed in the PBN. Interestingly, LH lesions disrupt sodium consumption in sodium-depleted animals, even after animals have recovered from the adipsia and anorexia typically induced by such lesions (53). These results raise the possibility that the deficit in sodium consumption following LH lesions may result from the elimination of LH drive to VTA dopamine neurons.
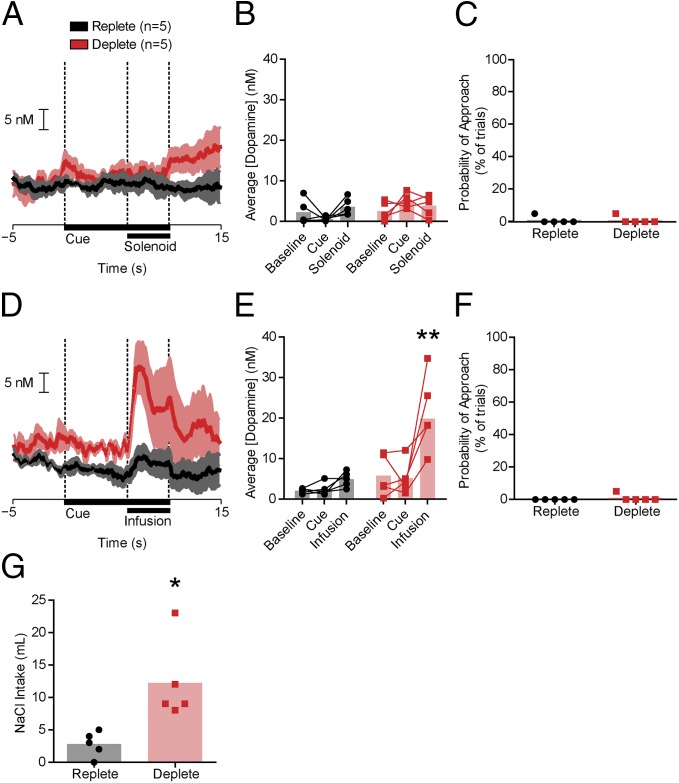
We then took advantage of the state dependency of the dopamine response to NaCl to investigate how the mesolimbic dopamine system acquires information about outcome-predictive stimuli. Rats with normal sodium balance (n = 10) received daily conditioning sessions where a CS (light/lever combination) was presented just before the NaCl US for seven sessions. Rats were then tested under Deplete (n = 5) or Replete (n = 5) conditions. Recordings were first made during presentations of the CS alone (i.e., in extinction). Our goal was to determine if the CS would evoke a dopamine spike when Deplete rats first experienced the CS while sodium deficient but had yet to experience the NaCl US in the new physiological state. Despite ample experience with the CS-US pairing, the NaCl CS did not evoke phasic dopamine release during extinction in either Deplete or Replete rats (two-way ANOVA: epoch: F2,16 = 0.98, P = 0.10; treatment: F1,16 = 4.0, P = 0.07; interaction: F2,16 = 2.92, P = 0.39; Fig. 2 A and B). Moreover, neither group exhibited conditioned-approach behavior (Fig. 2C). We next began a within-session reinstatement period in which the CS was paired with the NaCl US. The NaCl US evoked phasic dopamine release selectively in Deplete rats during reinstatement (two-way ANOVA: epoch × group interaction: F2,16 = 4.55, P < 0.05; post hoc: Deplete infusion vs. baseline or CS, both P < 0.01; Replete, no significant differences; Fig. 2 D and E). Deplete rats consumed significantly more postsession NaCl than Replete rats (unpaired t test: t9 = 3.22, P < 0.05; Fig. 2G). Thus, even after 7 d of CS-US training while sodium-replete, both NAc dopamine signaling (Fig. 2 A, B, D, and E) and the behavior (Fig. 2 C and F) of Deplete rats closely resembled subjects with no prior CS training with an appetitive US.
Fig. 2.
Seven days of CS-US training while sodium-replete does not condition responses to a sodium CS. (A) Average dopamine concentration (±SEM) during extinction for Replete (n = 5, black) and Deplete (n = 5, red) rats. (B) Neither the CS nor the solenoid click evoked dopamine during extinction compared with baseline. (C) Approach behavior during extinction. (D) Average traces of dopamine concentration (±SEM) during reinstatement. (E) Intraoral NaCl significantly increased dopamine concentration in Deplete rats. Deplete, infusion vs. baseline or CS (**P < 0.01); Replete, no significant differences (all comparisons, P > 0.05). (F) Approach behavior during reinstatement. (G) Postrecording session sodium intake was elevated in deplete rats (*P < 0.05). Opaque bars represent the mean.
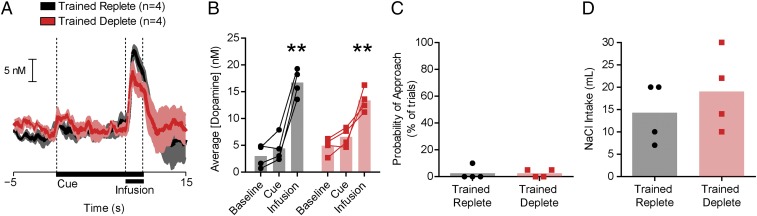
The previous experiment suggested that the acquisition of dopamine reward predictions requires that the predicted outcome first be experienced as appetitive. Thus, we tested whether a single day of NaCl CS-US training while rats were sodium deficient would condition dopamine and/or behavioral responses to a NaCl cue. One group of rats was depleted 24 h before a single NaCl CS-US training session (n = 4, Trained Deplete), whereas another was depleted and allowed to recover for 48 h before training (n = 4, Trained Replete). Twenty-four hours after depletion, all rats were given overnight access to 0.45 M NaCl to confirm sodium appetite. Postdepletion NaCl consumption did not differ between groups (unpaired t test: t6 = 0.55, P = 0.60). Thus, by the time of the recording session, both groups had equivalent experience with sodium depletion, CS-US training, and NaCl exposure, although one group had CS-US training paired and the other had training unpaired with sodium deficiency. Twenty-four hours before the test session, Trained Replete and Trained Deplete rats were again depleted of sodium. The following day, dopamine measurements were made with FSCV. A single CS-US training session while sodium deficient was insufficient to condition a dopamine response to the NaCl CS (Fig. 3). In contrast, the US evoked phasic dopamine release regardless of training history (two-way ANOVA: epoch: F2,12 = 107.6, P < 0.0001; training history: F1,12 = 0.04, P = 0.83; interaction: F2,12 = 7.13, P < 0.01; post hoc: both groups infusion > baseline, cue epochs, all at least P < 0.01, no difference from baseline during cue epoch for either group; Fig. 3 A and B). In addition, the CS failed to evoke conditioned-approach behavior (Fig. 3C). Sodium appetite at the time of dopamine measurements was probed by measuring overnight intake of 0.45 M NaCl, which confirmed a sodium appetite in both groups (unpaired t test: t6 = 0.85, P = 0.42; Fig. 3D). Thus, a single training session that paired a CS with an appetitive US was insufficient for the development of a dopamine reward prediction signal.
Fig. 3.
One day of sodium CS-US training while deplete is insufficient to condition a dopamine response to the sodium CS. (A) Average dopamine concentration (±SEM) for Trained Replete (n = 4, black) and Trained Deplete (n = 4, red) rats. (B) NaCl US, but not CS, evoked phasic dopamine release regardless of training history. Dopamine concentration during the infusion was significantly greater than both the baseline and cue periods in both groups (**P < 0.01). Dopamine concentration during the CS was not greater than baseline for either group. (C) Neither group approached the cue lever during the CS. (D) Postrecording session NaCl consumption did not differ between groups.
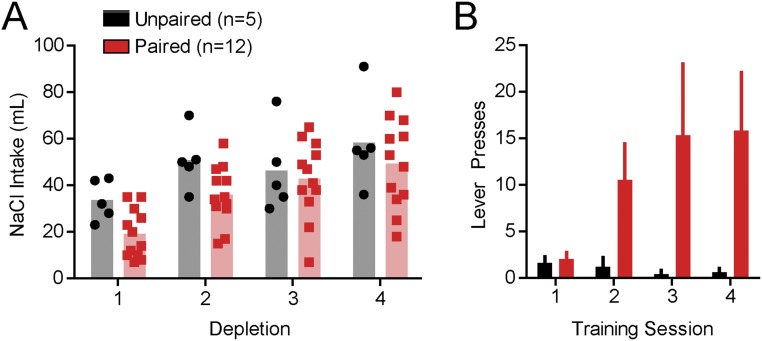
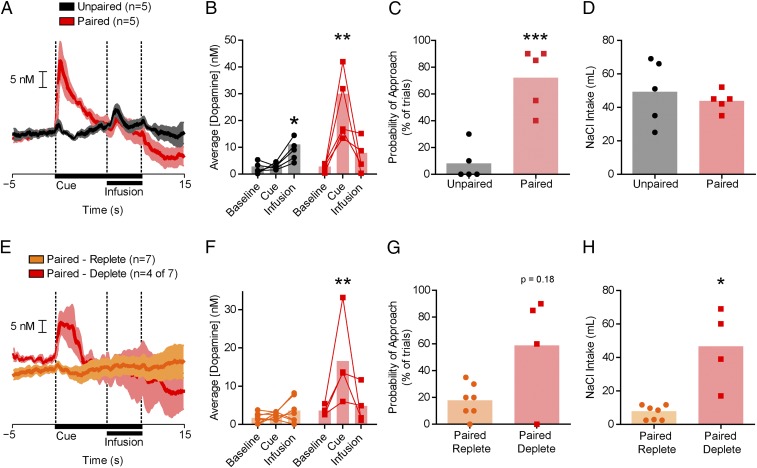
We next explored the possibility that extensive experience with the appetitive features of the predicted US is essential to condition dopamine reward prediction signals. We sodium-depleted two groups of rats four times and conducted four CS-US training sessions. For one group, training was always conducted while sodium deficient (Paired, n = 12). The other group was trained preceding/after recovery from depletion (Unpaired, n = 5; SI Experimental Procedures). During training, Paired rats developed preliminary signs of conditioned-approach behavior (Fig. S4). After training, we sodium-depleted a subset of Paired rats (n = 5 of 12) and all Unpaired rats 24 h before the FSCV recording session. The CS evoked dopamine release only in Paired rats, whereas the NaCl US, but not the CS, evoked dopamine release in Unpaired rats (two-way ANOVA epoch × training history interaction: F2,16 = 10.33, P < 0.01; post hoc: Paired, CS vs. baseline or infusion, both P < 0.01; Unpaired, infusion vs. baseline or CS, both P < 0.05; Fig. 4 A and B). Only Paired rats exhibited conditioned-approach behavior (unpaired t test: t9 = 5.39, P < 0.001; Fig. 4C). Both groups consumed NaCl after the recording session, eliminating attribution of these differences to sodium appetite at the time of dopamine measurements (Welch’s corrected t test: t4 = 0.60, P > 0.05; Fig. 4D).
Fig. S4.
Multiple days of sodium depletion and sodium CS-US training produce differential effects on sodium intake and sodium CS approach behavior depending on training history (related to Fig. 4). We sodium-depleted two groups of rats four times and conducted CS-US training either while rats were deplete (Paired, n = 12) or before depletion/following recovery (Unpaired, n = 5; SI Experimental Procedures). Thus, both groups of rats had the same experience with depletion and training, but the physiological state in which training occurred was manipulated between groups. (A) Over the course of training, both groups demonstrated an increased preference for hypertonic NaCl in the overnight test [significant effect of day (1–4): F3,45 = 20.64, P < 0.0001; no effect of training history (Unpaired, Paired): F1,45 = 2.32, P > 0.05; no interaction: F3,45 = 1.13, P > 0.05]. Our results are consistent with previous work showing that sodium intake in depleted rats increases over the course of multiple depletions (54). Importantly, it also demonstrates that the groups did not differ with respect to sodium preference before the FSCV recording session. (B) Only Paired rats began to interact with the cue lever during cue presentations. Pressing the lever had no impact on the occurrence of intraoral NaCl infusion. Group differences were not significant, but not all lever interactions (paw touching, bites, or licks) triggered a lever press. The recording sessions were video recorded to characterize Pavlovian conditioned-approach behaviors more accurately.
Fig. 4.
CS-US associations are acquired and expressed in a state-dependent manner. (A) Average dopamine concentration (±SEM) for a subset of Paired (n = 5 of 12, red) and Unpaired (n = 5, black) rats. (B) Sodium CS evoked phasic dopamine release only in Paired rats. Paired, CS vs. baseline or infusion (**P < 0.01); Unpaired, infusion vs. baseline or CS (*P < 0.05). (C) Conditioned-approach behavior for Paired and Unpaired rats (***P < 0.001). (D) Postrecording session sodium intake did not differ between Paired and Unpaired rats. (E) Average dopamine concentration (±SEM) in Paired rats tested while replete (Paired-Replete; n = 7 of 12, orange) or deplete (Paired-Deplete; second recording obtained in four of seven rats, red). (F) NaCl CS evoked dopamine only in Paired-Deplete rats. Paired-Deplete CS vs. baseline (**P < 0.01); Paired-Replete, no significant differences (all comparisons, P > 0.05). (G) Conditioned-approach behavior for Paired-Replete vs. Paired-Deplete rats (P = 0.18). (H) Postrecording session NaCl intake for Paired-Replete vs. Paired-Deplete rats (*P < 0.05). Opaque bars represent the mean.
Because physiological state influenced the acquisition of dopamine prediction signals, we next sought to determine whether physiological state would affect their expression. We first tested Paired rats (n = 7, trained as above) in the absence of sodium need (Paired-Replete) and later obtained a second recording from a subset of these same animals while they were sodium-deficient (Paired-Deplete, n = 4 of 7). In absence of sodium need (Paired-Replete), the CS did not evoke dopamine release. However, 2 d later, once sodium appetite was induced (Paired-Deplete), the sodium CS evoked a large dopamine response (two-way ANOVA epoch × state interaction: F2,18 = 6.23, P < 0.01; post hoc: Paired-Replete, no significant differences; Paired-Deplete, CS vs. baseline, P < 0.01; Fig. 4 E and F). In the Paired-Deplete condition, rats tended to show more conditioned-approach behavior compared with Paired-Replete (Mann–Whitney U test, P = 0.18; Fig. 4G). Moreover, NaCl consumption following the recording session was significantly elevated in the Paired-Deplete condition relative to Paired-Replete condition (Welch’s corrected t test: t3 = 3.31, P < 0.05; Fig. 4H), thereby confirming sodium appetite. Thus, using the same group of rats, we show that the dopamine response to the CS is flexibly expressed based on physiological state.
SI Experimental Procedures
Intraoral Cannula Surgery.
Under ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) anesthesia, rats were implanted with intraoral cannulas (24, 40). Each cannula consisted of a ∼6-cm length of PE6 (Scientific Commodities, Inc.) tubing that was flanged at one end with a Teflon washer. Intraoral cannulas were inserted just lateral to the first maxillary molar until the Teflon washer rested flush against the molar. The other end was exteriorized out of a small (∼5 mm) incision at the top of the scalp and held in place with a second Teflon washer. Rats were permitted at least 1 wk to recover from intraoral cannula surgery before the start of experiments. Intraoral cannulas were flushed with distilled water every other day to maintain patency.
FSCV Surgery.
Following recovery from intraoral cannula implantation or behavioral training, rats were anesthetized with ketamine hydrochloride (100 mg/kg, i.p.) and xylazine hydrochloride (10 mg/kg, i.p.). All implants were targeted relative to Bregma using the rat brain atlas of Paxinos and Watson (41). An intracranial guide cannula (Bioanalytical Systems) was implanted dorsal to the right NAc core [+1.3 mm anteroposterior (AP), +1.5 mm medial-lateral (ML), −2.5 mm dorsoventral (DV)]. Next, a bipolar-stimulating electrode (0.20-mm diameter; Plastics One) was lowered into the VTA (−5.2 mm AP, +1.0 mm ML, −8.2 mm DV from the brain surface). Lastly, a chlorinated silver reference electrode was placed in the left forebrain. Stainless-steel skull screws and dental cement secured implants to the skull.
Amiloride Disrupts Intake of 0.45 M NaCl in Sodium-Depleted Rats.
Oral application of amiloride blocks epithelial sodium channels (ENaCs) and interferes with sodium taste perception (42–44). Previous work indicates that 100 μM amiloride is sufficient to disrupt hypertonic sodium intake in depleted animals (23). Thus, we sought to confirm that 100 μM amiloride was sufficient to disrupt the ability of depleted animals to identify and consume 0.45 M NaCl (Fig. S1). For this experiment, we depleted naive rats (n = 12) of sodium for 24 h as described above on two separate occasions spaced 1 wk apart. Following overnight sodium depletion, rats were returned to their home cage and given overnight ad libitum access to distilled water and either 0.45 M NaCl or 0.45 M NaCl adulterated with 100 μM amiloride (counterbalanced across the two depletions). Intake of both water and NaCl solutions was measured 24 h after the start of the home cage test.
Intraoral Infusion Sessions.
All experiments involving uncued intraoral infusions were conducted in naive rats (n = 18 across four groups) (Fig. 1 and Fig. S2). The start of the behavioral session was indicated by the illumination of the house light and onset of white noise that persisted throughout the session. Each FSCV recording session consisted of 10 intraoral infusion trials. To confirm that dopamine responses were driven by intraoral infusions and not by the opening of the solenoid, in a subset of rats, we also randomly presented trials in which the solenoid opened but no fluid was delivered. During an infusion trial, the solenoid valve opened for 4 s to deliver 200 μL of 0.45 M NaCl through the intraoral cannula. A variable intertrial interval (range: 30–90 s; mean: 60 ± 8.2 s) followed each trial to prevent anticipation of NaCl solution delivery. After data collection, rats were disconnected from the head stage and returned to their home cage. Uncued intraoral infusion sessions were conducted in four separate groups of rats as follows. Replete rats (n = 4) received vehicle injections 24 h before FSCV recording, and thus were tested in a positive state of sodium balance. Deplete rats (n = 5) were depleted of sodium as described above for 24 h before the FSCV recording session. Re-Replete rats (n = 4) were depleted of sodium but allowed to recover for 48 h before the FSCV recording session. Deplete + Amiloride rats (n = 5) were depleted of sodium for 24 h but received intraoral infusions of 0.45 M NaCl in 100 μM ENaC blocker amiloride.
Identifying VTA Projecting Neurons That Differentially Express Fos in Sodium-Depleted Rats That Received Intraoral NaCl vs. Distilled Water.
Rats (n = 7) were anesthetized and implanted with intraoral cannulas, as well as injected unilaterally with 250 nL of Fast Blue [2% (vol/vol) in distilled water] in the right VTA (AP = −5.8, ML = +0.7, DV = −8.4 from the skull surface) (Fig. S3). Injections were made through a custom-made 32-gauge stainless injector connected to a 10-μL Hamilton syringe in 100-nL increments at a rate of 100 nL⋅min−1 (5-min wait between injections). Following the final injection, we waited an additional 10 min before removing the injector. The incision was sutured, and animals were returned to their home cage for recovery. Ten days after Fast Blue injection, rats were sodium-depleted as described above. Following 24-h sodium depletion, rats were placed in the operant chamber and connected to the intraoral infusion line. Rats then received 30 intraoral infusions of either 0.45 M NaCl (n = 4) or distilled water (n = 3) in a manner identical to the FSCV recording sessions. One hour following the intraoral infusion session, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg) and transcardially perfused with PBS (0.1 M PBS) followed by 4% (wt/vol) paraformaldehyde in PBS. Forty-micrometer brain sections were immunoprocessed as previously described (45, 46). Sections were incubated overnight at 4 °C in blocking solution containing rabbit anti-Fos (1:500, PC38; Millipore) or sheep tyrosine hydroxylase (1:500, P40101-150; Pel Freeze). The following day, sections were incubated in fluorescence-conjugated donkey anti-rabbit IgG Alexa Fluor 647 secondary Ab (1:800; Jackson Immuno) for 2 h in darkness. Brain sections were mounted and coverslipped, and z-stack and tiled images were captured on a Zeiss LSM 710 confocal microscope using a 20× objective. Sections containing the lateral hypothalamus (LH) or parabrachial nucleus (PBN) were examined on the side of Fast Blue injection. Fast Blue-containing and Fos-immunoreactive cells were counted using Fiji (47) by three experimenters (two were blinded to treatment condition). The numbers of Fast Blue-positive, Fos-positive, and colabeled cells were highly correlated across the three counters (all at least r = 0.70). Here, we report the average of the three counts for each slice. For the LH, we quantified a 700 × 700-pixel square centered on the lateral edge of the fornix (an example of the counting area is shown in Fig. S3). We examined the LH because it sends dense projections to VTA dopamine neurons (48) and lesions to this area impair sodium appetite (49). For the PBN, we counted a 550 × 400-pixel rectangle that was centered on the densest mass of Fast Blue-positive cells within the dorsolateral region [lateral parabrachial region (LPB)]. The LPB was selected because it is one of the earliest stages of gustatory processing and lesions of the dorsal LPB impair sodium appetite (50), as well as the ability of rewarding tastes to evoke dopamine release in the NAc (51).
Pavlovian Conditioning.
Rats were trained to associate the concurrent extension of a lever and illumination of a cue light (CS) with intraoral delivery of 0.45 M NaCl (Figs. 2–4). Across all experiments, the light/lever cue lasted 10 s, whereas the exact parameters of solenoid opening/fluid delivery varied across experiments (described below). The house light and white noise remained on throughout the sessions. For all conditioning experiments, the CS onset signaled the beginning of a trial and the duration of the CS was 10 s. A variable intertrial interval (range: 30–90 s, mean: 60 ± 8.2 s) followed all trials to prevent anticipation of the cue. In total, we conducted three conditioning experiments described below.
Conditioning Experiment 1.
All training was conducted while rats were sodium-replete. Six seconds after the onset of the CS, the solenoid was opened for 4 s, and it was closed at the offset of the 10-s CS. Thus, in experiment 1, we delivered 200 μL of 0.45 M NaCl per trial (30 trials per session, ∼6 mL total; Fig. 2). Rats (n = 10 total) were trained in this manner for 5 d and then surgically prepared for FSCV. After returning to presurgery body weight, rats were retrained for 2 d before the FSCV recording session. Twenty-four hours before the FSCV recording session, a subset of rats was depleted of sodium (n = 5, Deplete), whereas the remaining rats were given control injections (n = 5, Replete). Thus, all rats learned about the sodium CS-US association while sodium-replete and were then tested under either deplete or replete conditions. During the FSCV recording session, rats were first tested in extinction (CS was followed by solenoid opening, but no NaCl was delivered), to test for the presence of a CS response without first allowing rats to experience NaCl infusions while deplete. Following 20 extinction trials, we reinstated NaCl delivery during the cue and presented another 20 trials to test for the presence of a CS and/or US response. Rats were returned to their home cage after the FSCV recording session, and ad libitum intake of 0.45 M NaCl was measured over the subsequent 24 h.
Conditioning Experiment 2.
Before a single training session, all rats (n = 8) were depleted of sodium for 24 h. One group of rats was trained while sodium-depleted (n = 4, Trained Deplete) (Fig. 3). A second group of rats was trained 2 d later after being allowed to recover lost sodium through ad libitum access to 0.45 M NaCl for 24 h (n = 4, Trained Replete). Thus, rats had equivalent experience with sodium depletion but learned about the sodium CS-US association in different states of sodium balance. During each trial, the solenoid was opened for 2 s, and it closed at CS offset to deliver 100 μL of 0.45 M NaCl per trial (60 trials per session). Note that the volume of a single intraoral infusion was halved from experiment 1 to deliver the same total volume of 0.45 M NaCl (∼6 mL) during a session while doubling the number of trials in a single session. Comparing US-evoked dopamine in Fig. 2 against Fig. S4 suggests this change had a negligible impact on our results. After the training session, both groups were returned to their home cage, and trained deplete rats were given ad libitum access to 0.45 M NaCl for 24 h. Two days after training, rats were surgically prepared for FSCV. Following recovery, all rats were depleted of sodium and an FSCV recording session was conducted 24 h later while rats were sodium-depleted. Thus, trained deplete rats were tested in the same physiological state in which they learned about the sodium CS-US association, whereas trained replete rats were not. The FSCV recording session was identical to training except that we only presented 20 trials to minimize potential learning effects during the session. As above, rats were returned to their home cage after the session, and we measured ad libitum intake of 0.45 M NaCl after 24 h.
Conditioning Experiment 3a.
For experiments 3a and 3b, all rats (n = 17) experienced a total of four conditioning sessions and four 24-h sodium depletions before the FSCV recording session (Fig. 4 A–D and Fig. S4). Trial timing was identical to experiment 1 (4-s US starting 6 s after CS onset, 200 μL per trial, 30 trials per session). For one group of rats (n = 5, Paired), training always took place 24 h after sodium depletion while rats were sodium-depleted. For Unpaired rats (n = 5), training sessions occurred −2, −1, +2, or +3 d relative to depletion in a counterbalanced fashion. Thus, in unpaired rats, the training schedule dissociated sodium need from sodium CS-US training while still equating experience with sodium depletion. Following each training session (Paired) or 24 h after the start of sodium depletion (Unpaired), rats were given ad libitum access to 0.45 M NaCl, and intake was measured after 24 h. After the fourth training session, rats were surgically prepared for FSCV and allowed to recover. Both groups were depleted of sodium before the FSCV recording session, and 20 trials were presented during the session.
Conditioning Experiment 3b.
A second subset of paired rats (n = 7) was given vehicle injections and housed in wire-bottom cages with ad libitum access to standard chow and water 24 h before the recording session (Fig. 4 E–H and Fig. S4). Thus, in experiment 3b, rats were trained while sodium-depleted but were tested while in a state of normal sodium balance (Paired-Replete). As above, we presented 20 trials during the FSCV recording session; afterward, rats were given ad libitum access to 0.45 M NaCl, and intake was measured after 24 h. The day after the FSCV recording session, the same rats were depleted of sodium for 24 h and a second FSCV recording session was attempted (Paired-Deplete). Electrodes were lowered to the same recording depth as the original session in an attempt to capture a similar NAc release site across the 2 d. We obtained high-quality second recordings (good signal-to-noise ratio, free from electrical artifacts) from four of seven rats. Following the FSCV recording session, rats were returned to their home cage and given ad libitum access to 0.45 M NaCl, and intake was measured after 24 h.
Chemometric Analysis.
Following the recording session, we electrically stimulated the VTA at 120 μA with a range of frequencies (30–60 Hz) and pulse numbers (5, 8, 10, 20, and 24) to evoke a range of dopamine concentrations in the NAc. Background-subtracted cyclic voltammograms from the dopamine evoked by these stimulations were later used for chemometric analysis to extract dopamine concentration from electrochemical data as described previously (39).
Verification of Cannula Placements and Recording Sites.
Following completion of experiments, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg) and a small electrolytic lesion was made at the voltammetry recording site using a polyimide-insulated stainless-steel electrode (A-M Systems, Inc.). Rats were then transcardially perfused with cold 0.01 M PBS, followed by 10% (vol/vol) buffered formalin solution (Sigma, Inc.). Brains were removed and stored in formalin for 24 h, and then transferred to 30% (wt/vol) sucrose in 0.1 M phosphate buffer. All brains were sectioned at 50 μm on a cryostat. NAc sections were mounted, and lesion locations were verified using light microscopy in conjunction with the rat brain atlas of Paxinos and Watson (41). All recordings were confirmed to be from the NAc core (Fig. S6).
Fig. S6.
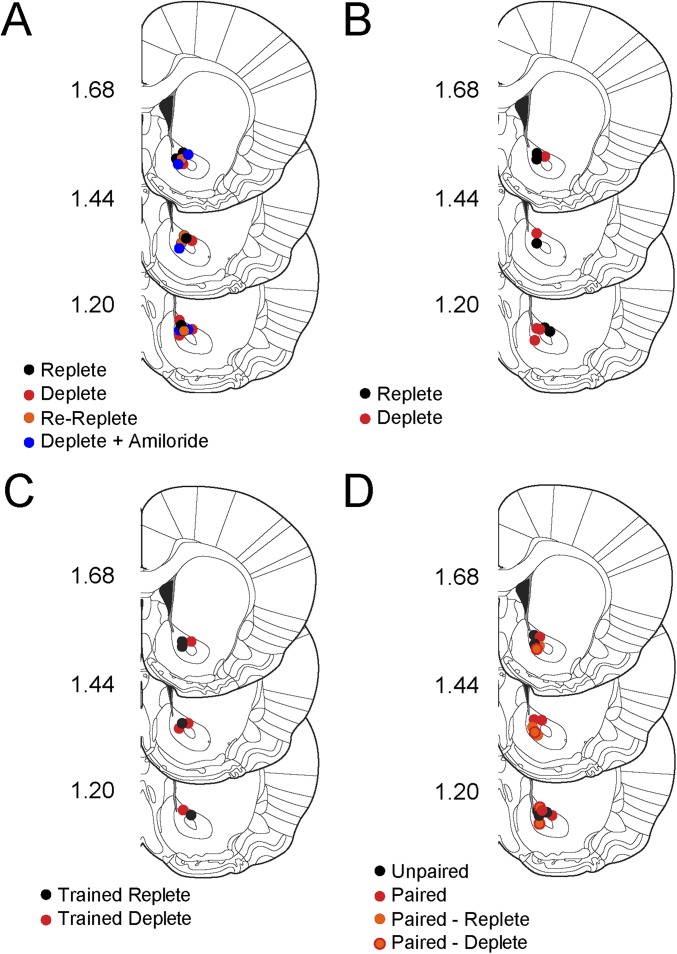
Summary of NAc recording sites from all experiments (related to Figs. 1–4). Slides were adapted from the rat brain atlas of Paxinos and Watson (41). Recording sites were marked with an electrolytic lesion, brains were removed and sliced on a freezing cryostat, and lesion location was visualized with light microscopy (SI Experimental Procedures). (A) Dots represent approximate locations of FSCV electrodes from rats that received intraoral infusions of NaCl in different states of sodium balance. Experimental data are presented in Fig. 1. Each color defines a group: Black indicates Replete (n = 4), red indicates Deplete (n = 5), orange indicates Re-Replete (n = 4), and blue indicates Deplete + Amiloride (n = 5). (B) Approximate recording locations from rats that were trained to associate a CS with the NaCl US while sodium-replete for 7 d and then either depleted of sodium (Deplete, red, n = 5) or given control injections (Replete, black, n = 5). Experimental data are presented in Fig. 2. (C) Approximate recording locations in rats that were trained to associate a CS with the NaCl US for 1 d while either sodium-replete (black, n = 4) or sodium-deplete (red, n = 4). Experimental data are presented in Fig. 3. (D) Approximate recording locations from rats that received NaCl CS-US training and depletion for 4 d in an unpaired (black, n = 5) or paired (red, n = 5) fashion. Orange dots represent approximate recording locations from paired rats that were tested only while sodium-replete (n = 3 of 7). Orange dots with red borders mark recording locations in paired rats that were tested first while replete and then tested 2 d later while sodium-depleted (n = 4 of 7). Experimental data are presented in Fig. 4.
Behavioral Data Analysis.
Body weight loss, 0.45 M NaCl intake, and water intake were recorded for all sodium depletions. These values were averaged and compared between relevant groups. During conditioning, we tracked the number of lever presses that occurred during CS. Importantly, not all lever interactions (licks, bites, or paw contact with lever) registered as lever presses; therefore, we recorded the FSCV recording sessions. Video-recordings were used to quantify approach behavior during all conditioning FSCV recording sessions by measuring the percentage of trials in which rats came into contact with the lever during the CS.
Discussion
Appetitive and nonpreferred/aversive stimuli differentially modulate dopamine signaling (18–20). In turn, the presence or absence of a phasic increase in dopamine in response to a primary stimulus can differentially drive learning about predictive cues and reinforce goal-directed behavior (9). We leveraged the fact that the appetitive qualities of a hypertonic NaCl solution strongly depend on physiological state. We found that the NaCl US evoked phasic dopamine release only in Deplete rats and this dopamine release did not require prior US experience (Fig. 1 and Fig. S2). Importantly, the response to the US was taste- and state-dependent. In contrast, the mesolimbic system acquired information about the CS only through extensive and direct, state-dependent experience with the US (Figs. 2–4 A–D). Once the NaCl CS-US association was learned, the phasic dopamine response to the CS was flexibly expressed according to physiological state (Fig. 4 E and F). The results have broad implications for how predictive dopamine signals are acquired, updated, and expressed.
The “real-time” responses of dopamine neurons to unconditioned affective stimuli have been visited (21), and revisited (19), yet considerable debate remains (22). Here, using intraoral delivery, we show in naive rats that dopamine release in the NAc core is robustly evoked when NaCl is appetitive but unchanged when it would be avoided. This differential encoding of the same stimulus was independent of prior learning or experience but dependent on physiological state and the ability to detect the sodium ion in solution, both of which are prerequisites for the avid consumption of hypertonic NaCl (23). It is notable that we did not observe a change in dopamine concentration following intraoral NaCl infusions in Replete rats. Previous studies demonstrated that innately or learned aversive stimuli (e.g., quinine, sucrose previously paired with LiCl to induce a conditioned taste aversion) suppress dopamine release (24, 25). However, concentrations of NaCl similar to the concentration used here (0.5 M) evoke a mixture of appetitive and aversive taste reactivity that switches to entirely appetitive following sodium depletion (26). Moreover, whether dopamine neurons encode nonpreferred/noxious/aversive stimuli with decreases, no change, or increases in firing rate may depend on anatomical location in the midbrain (18, 27) and projection target (20, 28). Thus, it remains possible that dopamine terminal fields outside the NAc core may yield different patterns of release. In addition, higher concentrations of NaCl (>0.5 M) may have yielded different results because these concentrations have been shown to recruit amiloride-insensitive taste pathways typically activated by aversive, nonsodium taste stimuli [bitter and sour (29)]. Still, our results demonstrate instant updating of dopamine responses to primary stimuli without need for prior experience. Sodium appetite is a long-studied, striking example of goal-directed behavior. Sodium-deficient animals avidly consume concentrated sodium solutions compared with animals with no sodium deficit. Moreover, the expression of sodium appetite is unlikely to depend on postingestive experience (14). We hypothesize that the ability of sodium taste to drive neuronal responses that support behavioral reinforcement (16, 17) is highly adaptive and helps to ensure rapid and immediate sodium consumption without need for postingestive learning.
Phasic dopamine responses to reward-predictive cues are arguably a fundamental brain signal, with evidence supporting their existence in mice (19), rats (3), monkeys (18), and humans (30). Cue-evoked dopamine signals serve to invigorate goal-directed behaviors aimed at the impending reward (31). Unlike our results with the NaCl US, dopamine reward prediction signals did not instantaneously update, and therefore did not simply reflect the change in the affective value of the US (Fig. 2). Instead, for dopamine responses to develop to a predictive cue, animals had to experience the CS-US pairing under conditions in which the US was appetitive (Fig. 4). Moreover, the pairings between a CS and an appetitive outcome must be extensive (Fig. 3). Previous work suggested a correlation between cue-evoked dopamine release and the development of conditioned-approach behavior (6). We found a similar relationship that further supports a role for dopamine in promoting learned approach behavior.
Our data reveal striking differences with respect to how previous experience and physiological state interact to modulate dopamine prediction signals. Given that sodium-deficient animals will consume hypertonic NaCl without needing to learn that the solution will relieve their deficit (14), it is notable that following a change in physiological state, we failed to observe instant updating of the value of the cue in either approach behavior or the phasic dopamine response (Fig. 2). The lack of instant behavioral updating contrasts with both an older report (13) and a recent report (11). Importantly, there were many methodological differences between the current work and previous work, including sodium depletion strategies, sex, and NaCl concentration. Given that the higher salt concentrations used in the previous work would also have activated sour and bitter taste receptors (29), taste-mediated, experience-independent learning may not rely on sodium ion transduction; instead, it may rely on other pathways. However, the most striking differences relate to training history. In both previous reports, rats underwent some form of pretraining where they learned cue- or response-outcome associations for a nonsodium US (sucrose, water). It is also critical to note that neither study measured phasic dopamine signaling, and thus cannot speak to dopamine prediction signals. We show that both behavior and the dopamine response to a NaCl CS are flexibly expressed with physiological state, but only after multiple days of training under deplete conditions.
FSCV combined with sodium appetite enabled us to conclude that acquisition of dopamine reward prediction signals is consistent with RPE models rather than model-based strategies. Work in nonhuman primates has shown that dopamine RPEs are modulated by an external context that dictates the likelihood a given trial will be rewarded (32). The authors explained the modulation using a TD model that featured a context parameter. Our data therefore reflect the ability of a subject’s internal context (physiological state) to modulate RPE expression once it has been learned. Moreover, we have previously shown that physiological state (e.g., hunger and associated hormones) augments the magnitude of dopamine responses to primary rewards (33) and their predictors (34). Thus, physiological state powerfully augments the magnitude, acquisition, and expression of reward-related responses in the mesolimbic system. A recent study in humans found evidence for both model-based and model-free learning strategies in the striatum (10). Because this work used functional MRI, it was unknown which striatal inputs carried model-based vs. model-free information. Our results strongly suggest that during initial learning, mesolimbic dopamine does not contribute to model-based encoding at the level of the ventral striatum.
In contrast to the experience-based acquisition of CS-US associations, the absence of a dopamine CS response in Paired-Replete rats during their first need-free session (Fig. 4 E and F) is inconsistent with a standard model-free account of how dopamine reward prediction signals are expressed. One potential explanation is that physiological state acts similar to a discriminative stimulus and “gates” the expression of a learned association. The predictive values of model-free discriminative stimuli are normally learned through experiencing different combinations of states and their associated outcomes. However, Paired-Replete rats never before experienced intraoral sodium infusions or predictive cues in a need-free condition. Thus, physiological state may act as a discriminative stimulus through a model-based process allowing rats to infer the change in value of NaCl upon the first CS exposure in a need-free state, and this inference was reflected by the lack of a dopamine response to the CS in a low-value context.
In sum, our data suggest that differential encoding of primary affective stimuli (here, the same taste stimulus) by dopamine neurons can manifest independent of learning. In contrast, dopamine neurons only acquire cue-outcome associations through direct and extended experience with primary stimuli that have positive affective value. Moreover, once these associations are formed, they are expressed as a function of their current value to the organism. Our findings parallel recent studies in Drosophila demonstrating that dopamine neurons facilitate the formation and expression of nutrient-related memories in a state-dependent manner (35, 36), suggesting a highly conserved process. Thus, different physiological states give rise to unique subjective experiences, which can have profound influences on brain substrates of associative learning.
Experimental Procedures
Subjects.
Male Sprague–Dawley rats (Charles River) weighing 375–475 g were used. This study reports data obtained from 60 rats. Most attrition resulted from loss of patency of the intraoral cannula or the inability to lower a recording electrode successfully into the NAc on test days. Because we were unable to obtain a recording from these animals, we omitted all of their data from inclusion in the study. Rats were individually housed with lights on from 7:00 AM to 7:00 PM and tested during the light phase. Animal care and use was in accordance with the NIH Guide for the Care and Use of Laboratory Animals (37), and was approved by the Institutional Animal Care and Use Committee at the University of Illinois at Chicago.
Sodium Depletion Protocol.
Sodium appetite was induced by two injections of furosemide (10 mg/kg, s.c.; Sigma) spaced 1 h apart. Diuresis was verified by weight loss of ≥20 g in the 1 h following the second injection. During the 24-h depletion, rats were housed in wire-bottom cages to prevent consumption of urine and had ad libitum access to a sodium-deficient diet (Teklad Sodium Deficient Diet; Harlan) and distilled water. Vehicle-treated rats were also housed in wire-bottom cages but given regular chow and water. Upon being returned to their home cage, rats were given ad libitum 24-h access to 0.45 M NaCl to restore lost sodium and ad libitum access to normal chow and water. Overnight water and NaCl intake, as well as body weight, were recorded during the 24-h repletion period.
Apparatus.
All sessions took place in a standard operant box (Med Associates) inside a sound-insulated chamber. An infusion line from a syringe containing 0.45 M NaCl was attached to a solenoid valve (flow rate = 50 μL⋅s−1; The Lee Company) and suspended outside the sound-insulated chamber. The infusion line passed through a commutator (Crist Instruments) and was connected to the rats’ intraoral cannula. Before all sessions, the intraoral cannula was flushed with distilled water to ensure patency. For experiments involving amiloride, intraoral cannulas were first flushed with 100 μM amiloride in distilled water to ensure epithelial sodium channels were blocked in advance of the first intraoral infusion.
FSCV Protocol.
FSCV in awake and behaving rats and analyte identification and quantification have been extensively described previously (38). Briefly, a micromanipulator containing a glass-insulated carbon fiber (∼75 µm; Goodfellow USA) (recording) electrode was inserted into the NAc guide cannula. The recording electrode was then lowered into NAc and locked into place. An FSCV head stage (University of Washington Electronics and Materials Engineering Shop) was used to tether the rat, apply voltage changes, and measure resultant current changes. The electrode was held at −0.4 V and ramped in a triangular fashion (−0.4 to +1.3 to −0.4 V, 400 V⋅s−1; “scan”). While recording, scans were applied at 10 Hz. To verify that the recording location supported phasic dopamine release, electrical stimulation was delivered to the VTA (24 pulses, 60 Hz, 120 μA). If this electrical stimulation failed to evoke dopamine release, the recording electrode was advanced 0.16 mm and the process was repeated. Once a stable release site was confirmed (Figs. S5 and S6), the experimental session began. After the recording session, electrodes were removed; rats were disconnected from the head stage and returned to their home cage.
Fig. S5.
In experiments where differences in phasic dopamine release were observed, all recording sites support phasic dopamine release (related to Figs. 1, 2, and 4). Before and after all experimental sessions, we electrically stimulated the VTA (60 Hz, 120 μA, 24 monophasic pulses). Critically, all recording sites supported electrically evoked phasic dopamine release, thereby reducing the possibility that differences in phasic signaling we observed arose from electrodes that were poorly positioned to capture phasic signals. (A) Average phasic dopamine spike during 5 s before to 10 s after electrical stimulation (STIM) of the VTA in rats that received intraoral infusions of NaCl in different states of sodium balance (SEM omitted for clarity because error bars overlap). Experimental data are presented in Fig. 1. Black indicates Replete (n = 4), red indicates Deplete (n = 5), orange indicates Re-Replete (n = 4), and blue indicates Deplete + Amiloride (n = 5). (B) Same as in A but for rats that were trained to associate a CS with the NaCl US while sodium-replete for 7 d and then either depleted of sodium (Deplete, red, n = 5) or given control injections (Replete, black, n = 5). Experimental data are presented in Fig. 2. (C) Same as in A but for rats that received NaCl CS-US training and depletion for 4 d in an unpaired (black, n = 5) or paired (red, n = 5) fashion. (D) Same as in A but for paired rats that were tested while sodium-replete (n = 7) and then again when sodium-deplete (n = 4 of 7). Experimental data from C and D are presented in Fig. 4.
FSCV Data Analysis.
Electrochemical data were recorded during the entire session. Individual trials were background-subtracted, and dopamine concentration surrounding the opening of the solenoid (intraoral infusion sessions) or the CS onset (conditioning sessions) was extracted from voltammetric data using principal component analysis (39). For intraoral infusion experiments, we calculated the average dopamine concentration during the 4-s baseline immediately before infusion (−4 to 0 s) and compared this concentration with the average dopamine concentration evoked by infusion of NaCl (0.1–4 s; Fig. 1). For conditioning experiments, we calculated the average dopamine concentration during the 2-s baseline period immediately before CS onset (−2 to 0 s) and compared this concentration with the average dopamine concentration evoked during the first 2 s of the CS (0.1–2 s) and the first 2 s of the subsequent intraoral infusion (6–8 s in Figs. 2 and 4 and 8–10 s in Fig. 3).
Statistical Analysis.
For intraoral infusions, dopamine concentration evoked during the epoch of interest was compared using a two-way [epoch (baseline, infusion) × treatment (Replete, Deplete, Re-Replete, Deplete + Amiloride)] ANOVA. For conditioning experiments, dopamine concentration was compared using a two-way ANOVA with main effects of epoch (baseline, cue, infusion) and treatment (Replete, Deplete; Fig. 2), 1-d training (Trained Replete, Trained Deplete; Fig. 3), training history (Paired, Unpaired; Fig. 4), or testing (Paired-Replete, Paired-Deplete; Fig. 4) conditions. One-way ANOVAs and Tukey’s honest significant difference post hoc tests were used where appropriate. NaCl intake and measures of approach behavior were compared separately using a two-tailed unpaired t test, Welch’s corrected t test, the Mann–Whitney U test, or Wilcoxon’s matched pairs test (two groups) or one-way ANOVA (more than two comparisons). Statistical analyses were performed using GraphPad 5.0 (Prism, Inc.), MATLAB (MathWorks), or SPSS Version 20.0 (IBM).
Acknowledgments
We thank Dr. John H. R. Maunsell for helpful comments and Pawel Bujakowski for assistance with video scoring. This work was supported by NIH Grants R01 DA025634 (to M.F.R.), K01 DA033380 (to J.E.M.), R01 DA038168 (to G.D.S.), and T32 MH093315 (to J.A.M.) and by the University of Illinois at Chicago Dean’s Scholar Fellowship (to J.J.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1519643113/-/DCSupplemental.
References
- 1.Rescorla RA, Wagner AR. 1972. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement, Classical Conditioning II Current Research and Theory (Appleton-Century-Crofts, New York), pp 64–99.
- 2.Sutton RS, Barto AG. Toward a modern theory of adaptive networks: Expectation and prediction. Psychol Rev. 1981;88(2):135–170. [PubMed] [Google Scholar]
- 3.Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10(8):1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- 4.Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1(4):304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- 5.Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci. 1996;16(5):1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuber GD, et al. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321(5896):1690–1692. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan W-X, Brown J, Dudman JT. Neural signals of extinction in the inhibitory microcircuit of the ventral midbrain. Nat Neurosci. 2013;16(1):71–78. doi: 10.1038/nn.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412(6842):43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg EE, et al. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16(7):966–973. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans’ choices and striatal prediction errors. Neuron. 2011;69(6):1204–1215. doi: 10.1016/j.neuron.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson MJF, Berridge KC. Instant transformation of learned repulsion into motivational “wanting”. Curr Biol. 2013;23(4):282–289. doi: 10.1016/j.cub.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richter CP. Increased salt appetite in adrenalectomized rats. Am J Physiol. 1936;115:155–161. [Google Scholar]
- 13.Quartermain D, Miller NE, Wolf G. Role of experience in relationship between sodium deficiency and rate of bar pressing for salt. J Comp Physiol Psychol. 1967;63(3):417–420. doi: 10.1037/h0024611. [DOI] [PubMed] [Google Scholar]
- 14.Handal PJ. Immediate acceptance of sodium salts by sodium deficient rats. Psychon Sci. 1965;3(1):315–316. [Google Scholar]
- 15.Geran LC, Spector AC. Anion size does not compromise sodium recognition by rats after acute sodium depletion. Behav Neurosci. 2004;118(1):178–183. doi: 10.1037/0735-7044.118.1.178. [DOI] [PubMed] [Google Scholar]
- 16.Tsai H-C, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324(5930):1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witten IB, et al. Recombinase-driver rat lines: Tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72(5):721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459(7248):837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482(7383):85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerner TN, et al. Intact-Brain Analyses Reveal Distinct Information Carried by SNc Dopamine Subcircuits. Cell. 2015;162(3):635–647. doi: 10.1016/j.cell.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379(6564):449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- 22.Fiorillo CD. Two dimensions of value: Dopamine neurons represent reward but not aversiveness. Science. 2013;341(6145):546–549. doi: 10.1126/science.1238699. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein IL, Hennessy CJ. Amiloride-sensitive sodium channels and expression of sodium appetite in rats. Am J Physiol. 1987;253(2 Pt 2):R371–R374. doi: 10.1152/ajpregu.1987.253.2.R371. [DOI] [PubMed] [Google Scholar]
- 24.Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11(12):1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF. Encoding of aversion by dopamine and the nucleus accumbens. Front Neurosci. 2012;6:137. doi: 10.3389/fnins.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berridge KC, Flynn FW, Schulkin J, Grill HJ. Sodium depletion enhances salt palatability in rats. Behav Neurosci. 1984;98(4):652–660. doi: 10.1037//0735-7044.98.4.652. [DOI] [PubMed] [Google Scholar]
- 27.Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA. 2009;106(12):4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76(Pt B):351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oka Y, Butnaru M, von Buchholtz L, Ryba NJP, Zuker CS. High salt recruits aversive taste pathways. Nature. 2013;494(7438):472–475. doi: 10.1038/nature11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442(7106):1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wassum KM, Ostlund SB, Loewinger GC, Maidment NT. Phasic mesolimbic dopamine release tracks reward seeking during expression of Pavlovian-to-instrumental transfer. Biol Psychiatry. 2013;73(8):747–755. doi: 10.1016/j.biopsych.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakahara H, Itoh H, Kawagoe R, Takikawa Y, Hikosaka O. Dopamine neurons can represent context-dependent prediction error. Neuron. 2004;41(2):269–280. doi: 10.1016/s0896-6273(03)00869-9. [DOI] [PubMed] [Google Scholar]
- 33.Cone JJ, McCutcheon JE, Roitman MF. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci. 2014;34(14):4905–4913. doi: 10.1523/JNEUROSCI.4404-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cone JJ, Roitman JD, Roitman MF. Ghrelin regulates phasic dopamine and nucleus accumbens signaling evoked by food-predictive stimuli. J Neurochem. 2015;133(6):844–856. doi: 10.1111/jnc.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin S, et al. Neural correlates of water reward in thirsty Drosophila. Nat Neurosci. 2014;17(11):1536–1542. doi: 10.1038/nn.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huetteroth W, et al. Sweet taste and nutrient value subdivide rewarding dopaminergic neurons in Drosophila. Curr Biol. 2015;25(6):751–758. doi: 10.1016/j.cub.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Committee on Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. Natl Inst Health; Bethesda: 1996. DHHS Publ No (NIH) 85-23. [Google Scholar]
- 38.Fortin SM, Cone JJ, Ng-Evans S, McCutcheon JE, Roitman MF. Sampling phasic dopamine signaling with fast-scan cyclic voltammetry in awake, behaving rats. Curr Protoc Neurosci. 2015;70:7.25.1–7.25.20. doi: 10.1002/0471142301.ns0725s70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heien MLAV, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76(19):5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- 40.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143(2):263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 41.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th Ed. Elsevier; London: 2007. pp. 547–612. [Google Scholar]
- 42.Schiffman SS, Lockhead E, Maes FW. Amiloride reduces the taste intensity of Na+ and Li+ salts and sweeteners. Proc Natl Acad Sci USA. 1983;80(19):6136–6140. doi: 10.1073/pnas.80.19.6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223(4634):403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- 44.Spector AC, Guagliardo NA, St John SJ. Amiloride disrupts NaCl versus KCl discrimination performance: Implications for salt taste coding in rats. J Neurosci. 1996;16(24):8115–8122. doi: 10.1523/JNEUROSCI.16-24-08115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15(8):1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73(6):1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watabe-Uchida M, Zhu L, Ogawa SKK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74(5):858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 49.Dayawansa S, Peckins S, Ruch S, Norgren R. Parabrachial and hypothalamic interaction in sodium appetite. Am J Physiol Regul Integr Comp Physiol. 2011;300(5):R1091–R1099. doi: 10.1152/ajpregu.00615.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scalera G, Spector AC, Norgren R. Excitotoxic lesions of the parabrachial nuclei prevent conditioned taste aversions and sodium appetite in rats. Behav Neurosci. 1995;109(5):997–1008. [PubMed] [Google Scholar]
- 51.Hajnal A, Norgren R. Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav. 2005;84(3):363–369. doi: 10.1016/j.physbeh.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 52.Geerling JC, Loewy AD. Central regulation of sodium appetite. Exp Physiol. 2008;93(2):177–209. doi: 10.1113/expphysiol.2007.039891. [DOI] [PubMed] [Google Scholar]
- 53.Wolf G. Effect of Dorsolateral Hypothalamic Lesions on Sodium Appetite Elicited By Desoxycorticosterone and By Acute Hyponatremia. J Comp Physiol Psychol. 1964;58(3):396–402. doi: 10.1037/h0048232. [DOI] [PubMed] [Google Scholar]
- 54.Sakai RR, Fine WB, Epstein AN, Frankmann SP. Salt appetite is enhanced by one prior episode of sodium depletion in the rat. Behav Neurosci. 1987;101(5):724–731. doi: 10.1037//0735-7044.101.5.724. [DOI] [PubMed] [Google Scholar]