Significance
Growth hormone-releasing hormone (GHRH) antagonist MIA-602 reduces hyperlipidemia in rats with type 1 diabetes (T1D). Elevated triglyceride-rich lipoprotein (TRL) and LDL levels correlate with renal and cardiovascular disease in T1D. Activity of GLP-1 in the intestine to lower TRL, glucagon, and postprandial glucose levels is impaired in T1D subjects. Expression of GHRH receptor was upregulated in the small intestine, involved in chylomicron synthesis in T1D rats. MIA-602 restored GLP-1 actions on hyperlipidemia and hyperglucagonemia in T1D rats and reduced generation of Apo-B48 induced by oleic acid in intestinal epithelial cells in vitro in a GLP-1–dependent manner. MIA-602 significantly improved proteinuria and vasorelaxation capacity in T1D rats. These findings unravel a previously unidentified pathway in T1D mediated by GHRH associated with impaired GLP-1 signaling and hyperlipidemia.
Keywords: GHRH, type 1 diabetes, dyslipidemia, GLP-1 signaling, kidney damage
Abstract
Dyslipidemia associated with triglyceride-rich lipoproteins (TRLs) represents an important residual risk factor for cardiovascular and chronic kidney disease in patients with type 1 diabetes (T1D). Levels of growth hormone (GH) are elevated in T1D, which aggravates both hyperglycemia and dyslipidemia. The hypothalamic growth hormone-releasing hormone (GHRH) regulates the release of GH by the pituitary but also exerts separate actions on peripheral GHRH receptors, the functional role of which remains elusive in T1D. In a rat model of streptozotocin (STZ)-induced T1D, GHRH receptor expression was found to be up-regulated in the distal small intestine, a tissue involved in chylomicron synthesis. Treatment of T1D rats with a GHRH antagonist, MIA-602, at a dose that did not affect plasma GH levels, significantly reduced TRL, as well as markers of renal injury, and improved endothelial-dependent vasorelaxation. Glucagon-like peptide 1 (GLP-1) reduces hyperglucagonemia and postprandial TRL, the latter in part through a decreased synthesis of apolipoprotein B-48 (ApoB-48) by intestinal cells. Although plasma GLP-1 levels were elevated in diabetic animals, this was accompanied by increased rather than reduced glucagon levels, suggesting impaired GLP-1 signaling. Treatment with MIA-602 normalized GLP-1 and glucagon to control levels in T1D rats. MIA-602 also decreased secretion of ApoB-48 from rat intestinal epithelial cells in response to oleic acid stimulation in vitro, in part through a GLP-1–dependent mechanism. Our findings support the hypothesis that antagonizing the signaling of GHRH in T1D may improve GLP-1 function in the small intestine, which, in turn, diminishes TRL and reduces renal and vascular complications.
Dyslipidemia frequently accompanies type 1 diabetes (T1D) and represents an important component of the disease, imposing cardiovascular risk and correlating with renal dysfunction (1, 2). Current clinical approaches directed toward diabetic dyslipidemia, including changes in lifestyle, stringent glycemic control, lipid lowering therapy, or combinations thereof, offer limited benefit, thus emphasizing the need for the development of novel therapies.
Therapy with statins reduces major cardiovascular events largely through reduction of low-density lipoprotein (LDL) cholesterol (3). Still, an important residual cardiovascular risk, which is independent of LDL cholesterol levels, remains (4–8). Chylomicrons (CMs), chylomicron remnants (CMRs), and very low-density lipoproteins (VLDLs), cumulatively known as triglyceride-rich lipoproteins (TRLs), contribute significantly to postprandial lipemia (9). Increased TRL levels represent an important additional risk factor for atherosclerosis (10), particularly in subjects with diabetes or the metabolic syndrome (11).
Glucagon-like peptide 1 (GLP-1), an incretin hormone secreted in the small intestine, promotes postprandial insulin release, thereby reducing blood glucose levels (12). Endogenous GLP-1 also reduces postprandial glucagon secretion through direct actions on pancreatic islet cells, thus diminishing hepatic glucose output (13). GLP-1 analogs are used in the treatment of type 2 diabetes, leading not only to improvements in glycemic control but also to reductions in CM biogenesis, systemic inflammation, and endothelial dysfunction (14–16). However, in T1D patients, a progressive elevation of postprandial glucagon, along with GLP-1 and plasma glucose, has been observed (17), suggesting impaired GLP-1 signaling or, alternatively, the presence of other dominant pathways blunting GLP-1 pathways.
Hypersecretion of growth hormone (GH) has been demonstrated to impair metabolic control in T1D patients by increasing circulating glucose and lipids (18–21). The release of GH by the pituitary is predominantly regulated by hypothalamic growth hormone-releasing hormone (GHRH). However, receptors for GHRH are also expressed in extrapituitary sites and were shown to be independently involved in various physiological and pathological events (22–24). Whether the GHRH receptor is up-regulated in the small intestine in the context of T1D, and whether its activation plays a role in the impairment of GLP-1 signaling and in the disease process, however, are still unknown.
Here, we examined the expression of peripheral GHRH receptors during the development of streptozotocin (STZ)-induced T1D in rats. Additionally, we assessed the effects of s.c. administered GHRH receptor antagonist, MIA-602 (23), on the metabolic profile, endothelial vasoreactivity, and renal injury. Our results demonstrate up-regulated expression of GHRH receptors in the small intestine in T1D. Moreover, the GHRH antagonist, MIA-602, restored the levels of GLP-1 to normal, blunted dyslipidemia and hyperglucagonemia, and improved vasorelaxation and kidney function in fed T1D animals. MIA-602 blunted secretion of apolipoprotein B-48 (ApoB-48) from rat primary intestinal epithelial cells in response to oleic acid challenge, in part through restoration of GLP-1 signaling.
These findings demonstrate a previously unrecognized role for GHRH signaling in the complications of dyslipidemia and hyperglucagonemia associated with T1D.
Results
Expression of GHRH Receptor Is Increased in the Small Intestine of T1D Rats.
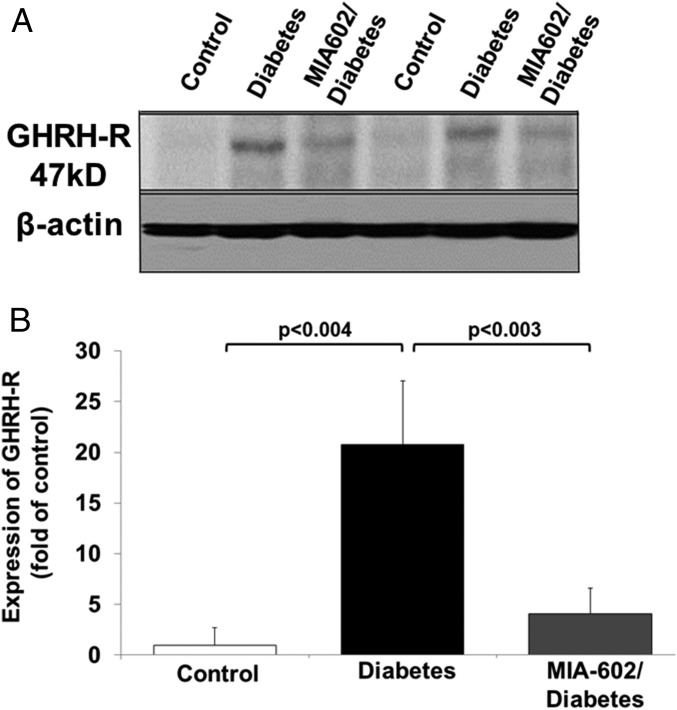
We induced T1D in Wistar rats (male, 320–350 g) with i.p. injection of a single dose of STZ (50 mg/kg body weight). The expression of GHRH receptors, the nominative pituitary phenotype and its bioactive splice variant, SV-1 receptor, has been demonstrated in several peripheral tissues, including lung, heart, intestine, colon, and kidney (23, 24). However, the potential functional role of GHRH receptors in the small intestine, a tissue crucially involved in CM synthesis (25), has not been investigated during T1D. The entire small intestine was removed from rats, and its length was measured from the pylorus to the ileocecal junction. Averaged values were as follows: control, 96 ± 17.1 cm; diabetes, 155.8 ± 10.6 cm; and MIA-602–diabetes, 157.3 ± 7.6 cm. The entire intestine was then divided into four segments, and the third and fourth distal segments (jejunum–ileum) were included for the protein evaluation. As shown in a representative Western blot experiment in Fig. 1 A and B, a significantly increased expression of the GHRH receptors (>20-fold) was detected in homogenates of jejunal–ileal segments of the distal small intestine of T1D rats after 14 wk of diabetes. This was compared with nondiabetic controls, using a polyclonal rabbit antibody reacting with rat GHRH receptor and with its splice variant 1. Upon the development of hyperglycemia (>300 mg/dL plasma glucose), which usually occurred 3 d post-STZ injection, rats were treated with a GHRH receptor antagonist, MIA-602 (23) (25 μg/kg, s.c.) or with vehicle, three times a week for 14 wk of diabetes in total. GHRH has been shown to increase the expression of the GHRH receptor via the cAMP/PKA/CREB pathway (24, 26, 27). Treatment with GHRH antagonist significantly blunted GHRH receptor expression (by approximately fivefold) in the jejunum–ileum of diabetic rats (Fig. 1).
Fig. 1.
GHRH receptor expression in rat small intestine. (A) Representative Western blot assessing GHRH receptor protein expression in homogenates from rat small intestine isolated from nondiabetic controls, type 1 diabetic, or MIA-602/diabetic rats, using a polyclonal rabbit antibody reacting with rat GHRH receptor and its splice variant 1 receptor (SV-1) (Abcam). (B) Densitometric analysis of GHRH receptor expression in control, diabetic, and MIA-602/diabetic rats (n = 5 per group).
GHRH Antagonist Reduces Dyslipidemia in T1D Rats.
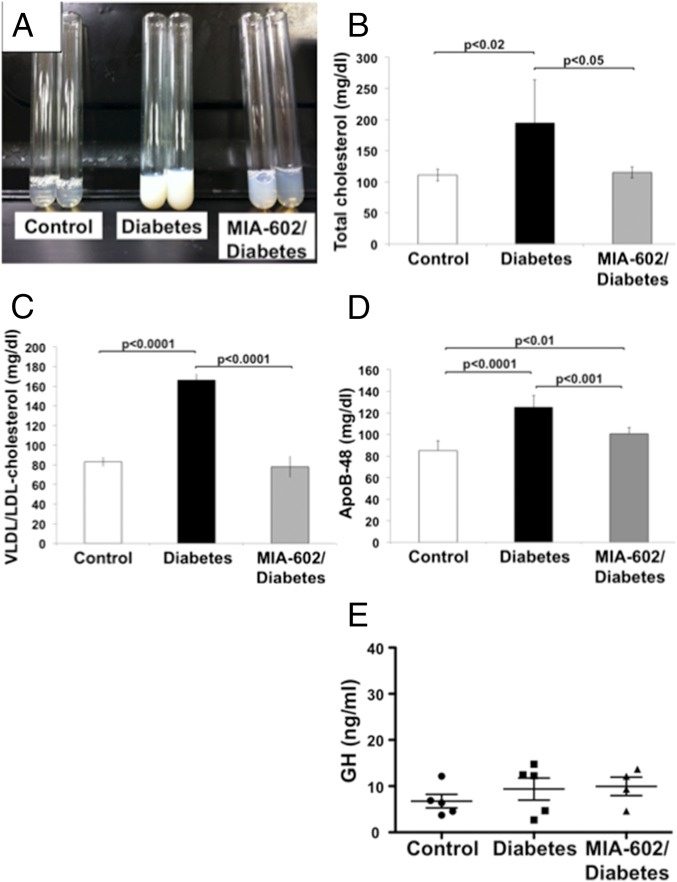
Our observation on the increased expression of GHRH receptors in the small intestine, as well as the relationship of the intestine to CM synthesis, prompted us to investigate the effects of GHRH on lipid metabolism during T1D. To specifically test the effects of the GHRH antagonist, MIA-602, on metabolic and hormonal profiles, we did not administer insulin during the study period. This avoided potentially confounding influences on CM assembly in the enterocyte, on lipoprotein lipase activity in the vasculature of fat and muscle tissue (28, 29), on hepatic uptake of CMs or VLDL remnants (30), and on intraislet glucagon secretion (31). As shown in Table S1 and SI Results, treatment with MIA-602 did not affect intake of food or water, or 24-h urine volume, in T1D rats. Moreover, treatment with MIA-602 did not affect body weight at any point during the study. By contrast, we detected a significant reduction in lipemic plasma, which was visually apparent (Fig. 2A), in T1D rats treated with MIA-602. Total cholesterol levels (Fig. 2B) and VLDL/LDL-cholesterol (Fig. 2C) were also lower in the animals treated with MIA-602 compared with vehicle-treated STZ diabetic animals. The reduced VLDL/LDL-cholesterol fraction may have resulted from diminished de novo hepatic synthesis of fatty acids, reduced esterification of fatty acids from TRL remnant hepatic uptake, or improvement in TRL clearance (32–34). Plasma triglyceride levels were significantly increased in diabetic rats (89.3 ± 4.2 mg/dL), compared with the control group (84.7 ± 1.2 mg/dL; P < 0.05 vs. STZ), and this was significantly blunted by MIA-602 treatment (82.3 ± 2.8 mg/dL; P < 0.01 vs. STZ; n = 6 per group). As shown in Fig. 2D, treatment with MIA-602 blunted plasma levels of ApoB-48. A limitation of these findings is that in rat serum ApoB-48 is not an exclusive marker for intestinal lipoproteins as it is in humans (35). Indeed, diabetic rats accumulate both intestinal and hepatic ApoB-48 in serum. As such, although we show an in vitro effect of GHRH antagonist on intestinal generation of ApoB-48, the in vivo effects cannot be interpreted to exclusively reflect intestinal lipoprotein production. Notably, treatment with MIA-602, at the dose used, did not significantly impact the plasma GH levels in T1D rats [control nondiabetic (6.7 ± 3.3 ng/mL), STZ (9.4 ± 5.3 ng/mL), and MIA-602/STZ groups (10.0 ± 4.0 ng/mL)] (Fig. 2E). These results emphasize the role of GHRH signaling in dyslipidemia during T1D, independent of its effects on GH generation.
Table S1.
Food and water intake, 24-h urine volume, and body weight from rats in metabolic cages
| Exp. group | Food intake, g/d | Water intake, mL/d | 24-h urine volume, mL/d | Body weight, g |
| Control | 11.8 ± 8.7 | 31.7 ± 9.0 | 17.3 ± 9.8 | 637.1 ± 29.7 |
| Diabetes | 38.2 ± 6.2* | 144.3 ± 24.3† | 107.7 ± 25.8† | 320.1 ± 86.8† |
| MIA-602/diabetes | 32.9 ± 16.6‡ | 121.9 ± 37.5* | 84.5 ± 25.9* | 336.3 ± 59.7† |
P < 0.005 vs. control.
P < 0.0005 vs. control.
P < 0.05 vs. control.
Fig. 2.
GHRH antagonist MIA-602 reduces dyslipidemia in T1D rats. (A) MIA-602 reduces lipemia in T1D rats (representative picture). (B) MIA-602 reduces total cholesterol levels in plasma of T1D rats (n = 5 per group in control, MIA-602/diabetes, and n = 8 per group in diabetes). (C) MIA-602 blunts increase in VLDL/LDL fraction in plasma from diabetic rats (n = 5 per group in control, MIA-602/diabetes, and n = 8 per group in diabetes). (D) Significant reduction in plasma ApoB-48 levels upon treatment with MIA-602 in T1D rats (n = 7 per group). (E) MIA-602 treatment (25 μg/kg, s.c., three times a week for 14 wk) does not affect plasma GH levels in T1D rats (n = 5 per group).
GHRH Impairs GLP-1 Signaling in T1D.
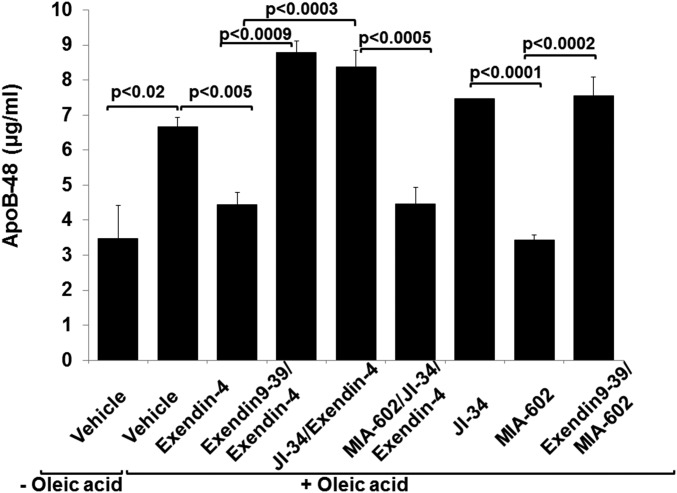
Increased levels of ApoB-48 lipoprotein during T1D may result from increased intestinal production of CM and/or decreased clearance of CMR. Activation of the GLP-1 receptor reduces postprandial triglyceride levels, in part by decreasing intestinal synthesis of ApoB-48, thus inhibiting CM assembly in enterocytes (14, 15). Western blot data indicated an augmented expression of GLP-1 receptor in distal small intestine (2.6 times that of control) and liver (3.6 times that of control) in T1D rats (Fig. 3A). This was unaffected by MIA-602 treatment (Fig. 3A). MIA-602 diminished intestinal GHRH receptor expression (Fig. 1 A and B), lipemia, and ApoB-48 plasma levels in T1D rats (Fig. 2 A and D), suggesting that it might have reduced apolipoprotein production by enterocytes. To support this hypothesis, we compared effects of a GHRH agonist, JI-34, with GHRH antagonist, MIA-602, on Apo-B48 generation induced by oleic acid (0.5 mM) in rat small intestinal epithelial cells (IEC-6) (ATCC CRL-1592). GHRH agonist, JI-34 (1 μM), did not modify the expression of either GLP-1 receptor (Fig. 3 B and C), GHRH receptor, or SV-1 receptor (Fig. 3 B, D, and E) in these cells, after up to 24 h of treatment. To mimic treatment with the GHRH antagonist MIA-602 in our in vivo T1D model, we evaluated its effect in the absence of insulin on the secretion of ApoB-48 from cells exposed to oleic acid in vitro, in the presence or absence of a GLP-1 agonist. Intestinal epithelial cells respond to oleic acid treatment (0.5 mM) by releasing increased amounts of ApoB-48 lipoproteins in the medium (6.67 ± 0.26 µg/mL; P < 0.025), compared with cells treated with medium alone (3.48 ± 0.98 µg/mL) (Fig. 4). The GLP-1 agonist exendin-4 (10 nM) significantly reduced the release of ApoB-48 (4.44 ± 0.36 µg/mL; P < 0.01) in oleic acid-treated IECs (Fig. 4). This action of exendin-4 on ApoB-48 release was abrogated by pretreating the cells with either the GLP-1 receptor antagonist exendin 9–39 (100 nM; 8.78 ± 0.34 µg/mL) or with the GHRH agonist, JI-34 (8.38 ± 0.46 µg/mL) (Fig. 4). GHRH antagonist, MIA-602, restored the protective effect of exendin-4 in the presence of JI-34 (4.47 ± 0.46 µg/mL). These data indicated that GHRH can impair GLP-1 signaling in IECs. We also investigated whether GHRH increases ApoB-48 secretion in the absence of GLP-1 agonist. As shown in Fig. 4, treatment with the agonist, JI-34 (1 μM), slightly but significantly increased (7.47 ± 0.005 µg/mL; P < 0.05), whereas the antagonist, MIA-602 (1 μM), significantly reduced secretion of ApoB-48 (3.43 ± 0.15 µg/mL; P < 0.0004), compared with cells challenged with oleic acid alone. The GLP-1 receptor antagonist, exendin 9–39, completely abrogated this MIA-602 effect on ApoB-48 secretion (7.56 ± 0.53 µg/mL; P < 0.0002). Taken together, these results indicate that the inhibitory action of MIA-602 on the generation of ApoB-48 in IECs is at least partially mediated through restoration of GLP-1 signaling.
Fig. 3.
GHRH agonist does not modulate expression of GLP-1-R and GHRH-R in T1D rats. (A and B) Representative immunoblots and (A and C–E) densitometric analysis for GLP-1 receptor, GHRH receptor, and SV-1 in vivo in T1D rats (A) (n = 5 per group) and in IEC-6 (B–E) following JI-34 treatment (1 μM) for 1 and 24 h, in the absence or presence of insulin (5 μg/mL).
Fig. 4.
GHRH agonist increases and GHRH antagonist decreases ApoB-48 secretion in oleic acid-treated IEC-6. One-hour pretreatment with GHRH agonist JI-34 (1 μM) increases ApoB-48 secretion in rat small intestinal epithelial cells (IEC-6) (ATCC CRL-1592) grown to confluence in six-well plates, in the absence or presence of GLP-1 agonist exendin-4 (3 h, 10 nM) and oleic acid (3 h, 0.5 mM) (n = 6 per group). GHRH antagonist MIA-602 (1 μM) abrogates secretion of ApoB-48 by itself or in the presence of JI-34 and exendin-4 in rat IEC, in a GLP-1–dependent manner, because this effect is blunted upon addition of the GLP-1 receptor antagonist exendin 9–39 (100 nM) (n = 6 per group).
Effects of GHRH Antagonist on Plasma Glucose and the Glucose Regulatory Hormones.
We next investigated the effects of MIA-602 on plasma glucose and glucose regulatory hormones. Blood glucose levels were similar among T1D rats in the nonfasting state, when treated with vehicle or with MIA-602 in the absence of exogenous insulin administration (Table S2 and SI Results). The destruction of pancreatic beta cells by STZ was evidenced by the complete loss of endogenous insulin and amylin. Amylin is colocalized and cosecreted with insulin in the granules within pancreatic beta cells (Table S2). Consistent with findings in T1D patients (17), plasma levels of both glucagon and GLP-1 were significantly higher in vehicle-treated T1D rats compared with control nondiabetic rats, indicating that secretion of GLP-1 is not impaired in T1D. Levels of GLP-1 and glucagon were reduced toward normal values in T1D rats treated with MIA-602 (Table S2 and SI Results), suggesting that MIA-602 modulated glucagon secretion from pancreatic alpha cells independently of intraislet insulin.
Table S2.
Plasma glucose and glucose regulatory hormones
| Exp. group | Glucose, mg/dL | Insulin, pg/mL | Amylin, pg/mL | Glucagon, pg/mL | GLP-1, pg/mL |
| Control | 265.8 ± 71 | 2,976.1 ± 4,788.3 | 31.7 ± 9.0 | 73.0 ± 33.9 | 30.7 ± 17.6 |
| Diabetes | 641 ± 37.5* | ND | ND | 190.7 ± 66.4† | 164.4 ± 68.4‡ |
| MIA-602 diabetes | 603 ± 137.4† | ND | ND | 55.9 ± 34.3§ | 48.7 ± 25.2¶ |
ND, nondetectable.
P < 0.0001 vs. control.
P < 0.05 vs. control.
P < 0.03 vs. control.
P < 0.02 vs. diabetes.
P < 0.05 vs. diabetes.
GHRH Antagonist Reduces Kidney Damage in T1D.
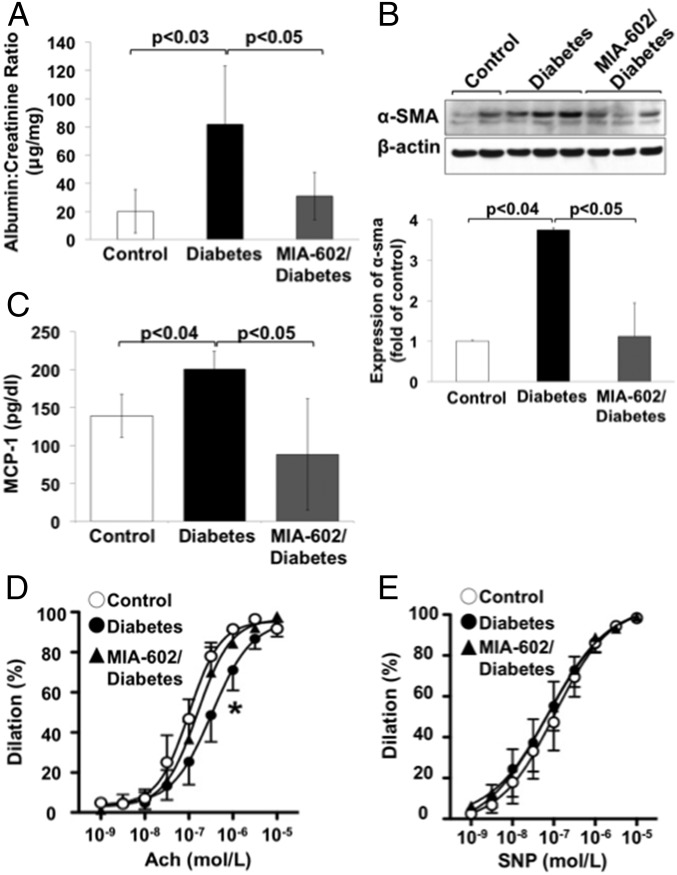
Both dyslipidemia and hyperglycemia were shown to induce nephropathy through oxidative and inflammatory mechanisms in diabetic humans and rodents (36–38). Because treatment with MIA-602 significantly improved dyslipidemia in T1D rats, we evaluated its effect on proteinuria and on expression of α-smooth muscle actin (α-sma), a marker of renal fibrosis; both of these are indicative of kidney injury. We found that both proteinuria (expressed as the albumin/creatinine ratio) and α-sma expression in kidney cortex (detected by Western blotting in homogenates) (39) were significantly increased in vehicle-treated T1D rats, compared with controls and were reduced by MIA-602 treatment (Fig. 5 A and B). Notably, the bioactive GHRH splice variant 1 receptor is expressed in fibroblasts (39), suggesting that instigation of the GHRH receptor may directly promote renal fibroblast activation.
Fig. 5.
GHRH antagonist reduces kidney damage and endothelial dysfunction in T1D rats. (A) Measurement of proteinuria in urine from control, T1D, and MIA-602/T1D rats, expressed as the albumin/creatinine ratio (n = 5 per group). (B, Upper) Representative immunoblot of α-sma expression (a marker of fibroblast activation and renal fibrosis) in homogenates of kidney cortex. (Lower) Densitometric analysis demonstrates a significant reduction in α-sma expression in MIA-602/diabetic rats (n = 5 per group). (C) MIA-602 treatment of T1D rats reduces plasma MCP-1 plasma levels (measured using MILLIPLEX MAP Rat Metabolic Hormone Magnetic Bead Panel, Metabolism Multiplex Assay; RMHMAG-84K; EMD Millipore). (D) Improvement in endothelial-dependent vasodilation from acetylcholine (ACh) in aortic rings of T1D rats treated with MIA-602 (n = 5 per group; *P < 0.05 vs. control group). (E) Endothelial-independent vasodilator responses to the NO donor sodium nitroprusside (SNP).
MIA-602 Improves Vascular Function in T1D.
CMs and CMRs were demonstrated to increase the generation of the proinflammatory and proatherogenic chemokine, monocyte chemoattractant protein 1 (MCP-1), in vascular endothelial cells (40). Therefore, we analyzed MCP-1 levels in the serum. Induction of T1D resulted in a significant increase of MCP-1 serum levels; this was reduced by treatment with MIA-602 (Fig. 5C). Both dyslipidemia and increased MCP-1 plasma levels represent risk factors for vascular endothelial dysfunction. We detected a significant impairment in endothelial-dependent vasodilation by acetylcholine (ACh) in thoracic aortas of vehicle-treated T1D rats (Fig. 5D) (EC50 diabetic: 5.815 × 10−7 ± 8.972 × 10−8 M; EC50 control: 1.50 × 10−7 ± 2.09 × 10−8 M). This was abrogated by treatment with MIA-602 (EC50 MIA-602/diabetic: 2.04 × 10−7 ± 2.20 × 10−8 M). Responses to the endothelial-independent vasodilator sodium nitroprusside (SNP) were similar among the three groups of animals (Fig. 5E). These findings suggest that interference with GHRH signaling improves endothelial function, a harbinger of cardiovascular risk, in T1D.
SI Results
MIA-602 Treatment Does Not Affect Body Weight, Food or Water Intake in T1D Rats.
To avoid potentially confounding influences on chylomicron assembly in the enterocyte, on lipoprotein lipase activity in the vasculature of fat and muscle tissue, on hepatic uptake of chylomicrons or VLDL remnants, and on intraislet glucagon secretion, we did not administer insulin during the study period of the experiments in diabetic rats. As shown in Table S1, treatment with MIA-602 did not affect intake of food or water, or 24-h urine volume, in T1D rats. Moreover, treatment with MIA-602 did not affect body weight at any point during the study (Table S1). All animal experiments were approved by the Institutional Animal Care and Use Committee at Augusta University.
Effects of MIA-602 on Plasma Glucose and the Glucose Regulatory Hormones.
We investigated the effects of MIA-602 on plasma glucose and glucose regulatory hormones. Blood glucose levels were similar among T1D rats in the nonfasting state, when treated with vehicle or with MIA-602 in the absence of exogenous insulin administration (Table S2). The absence of endogenous insulin and amylin indicated the complete destruction of pancreatic beta cells by streptozotocin (STZ) (Table S2). Consistent with findings in T1D subjects, plasma levels of both glucagon and GLP-1 were significantly higher in vehicle-treated T1D rats compared with control nondiabetic rats, indicating that secretion of GLP-1 is not impaired in T1D. MIA-602 treatment normalized levels of GLP-1 and glucagon toward normal values in T1D rats (Table S2), suggesting that MIA-602 modulated glucagon secretion from pancreatic alpha cells independently of intraislet insulin.
Discussion
T1D affects nearly 2 in 1,000 juveniles in the United States (41). Cardiovascular disease, the leading cause of morbidity and mortality in T1D patients, is caused by a complex interplay of metabolic risk factors, including hyperglycemia, dyslipidemia, and kidney disease (1). CMs, CMRs, and VLDLs, collectively known as TRLs, are increasingly recognized for their role in diabetic atherogenesis (42–45). Here, we provide a previously unidentified insight into mechanisms that regulate TRL production in T1D. We demonstrate an up-regulated expression of GHRH receptors in the small intestine of T1D rats, in conjunction with dysregulated GLP-1 signaling. Using a cell culture model, we show that GHRH receptor signaling modulates ApoB-48 production by small intestine cells in a GLP-1–dependent manner.
Type 1 diabetic patients were shown to exhibit elevated GH levels and exaggerated GH response to GHRH (46, 47), which in turn may contribute to dyslipidemia (48). Besides stimulating GH production in the pituitary gland, GHRH also exerts peripheral effects through full-length pituitary type receptors and splice variant 1 receptors that are expressed in various organs, including lung, heart, stomach, small intestine, colon, and kidney (22–24). Various functions of peripheral GHRH receptors remain to be fully elucidated. Here, we demonstrate that expression of GHRH receptors in small intestine, a tissue crucially involved in CM synthesis (25), is up-regulated in T1D. Moreover, s.c. treatment with a GHRH antagonist, MIA-602, significantly reduced plasma levels of LDL, VLDL, and ApoB-48 lipoprotein in T1D rats. We considered the possibility that the GHRH antagonist MIA-602 could favorably modulate lipid metabolism by reducing the production of GH (48). However, MIA-602, with the treatment regimen used (25 μg/kg, s.c., three times a week for 14 wk), did not affect plasma levels of GH in T1D rats. This result is consistent with patient data, suggesting that circulating GHRH levels are not relevant in dysregulation of GH in T1D (49). Our findings demonstrate that the GHRH antagonist MIA-602, at least in this experimental T1D model, can improve lipid profiles without affecting GH production.
The small intestine plays a crucial role in regulating the rate of production of CMs in both the fed and fasting states (25). Insulin influence in the intestine can reduce levels of ApoB48 and can stimulate lipoprotein lipase activity in control animals (50, 51). However, oxidative stress, T1D, fructose feeding, and inflammation can each trigger dysregulation of intestinal insulin signaling and lipoprotein lipase deficiency, which can cause exaggerated lipogenesis and lipoprotein synthesis (28, 29, 50, 51). This, in turn, can lead to an accumulation of both intestinal (CMs) and hepatic (VLDL) lipoproteins and their remnants. Because MIA-602 significantly improved lipemia, this raises the possibility that it also improved the activity of lipoprotein lipase and TRL clearance, in addition to inhibiting ApoB-48 secretion. Insulin is absent in our STZ-induced T1D rat model; therefore, this action of MIA-602 cannot be due to an enhancement of insulin activity. It might potentially be accomplished by increasing the action of gastric inhibitory polypeptide (GIP), an intestinal hormone known to increase lipoprotein lipase expression (52). However, plasma levels of GIP were not increased in T1D rats treated with MIA-602 (46.7 ± 9.6 pg/mL), compared with vehicle-treated diabetic animals (85.7 ± 37.5 pg/mL; not significant vs. MIA/STZ).
The incretin GLP-1 lowers levels of TRL in the intestine and reduces glucagon levels (53). However, plasma levels of both GLP-1 and glucagon have been reported to be elevated in T1D patients (17). These data suggest that T1D patients exhibit impaired GLP-1 signaling and thus may not benefit from GLP-1–based therapies. Despite increased plasma levels of GLP-1 and a stronger expression of the GLP-1 receptor in small intestine, T1D rats exhibited elevated glucagon levels, suggesting impaired GLP-1 signaling. Treatment with the GHRH antagonist MIA-602 reduced plasma levels of GLP-1, glucagon, and TRL.
Results from in vitro experiments using primary rat small intestinal epithelial cells treated with oleic acid show that the GHRH agonist JI-34 impairs the action of the GLP-1 receptor agonist exendin-4 on secretion of ApoB-48. By contrast, the GHRH antagonist MIA-602 significantly reduced ApoB-48 levels, an effect that was blunted by the specific GLP-1 receptor antagonist exendin 9–39. These outcomes were not associated with changes in the expression of either GHRH or GLP-1 receptors in the intestinal epithelial cells. These findings suggest that activation of GHRH receptors blunts the effects of GLP-1 signaling on the release of ApoB-48. Our data suggest that antagonizing GHRH signaling has the capacity to improve GLP-1 signaling in T1D rats in vivo. Besides directly affecting ApoB-48 secretion in small intestinal epithelial cells, GLP-1 has also been proposed to inhibit CM production via melanocortin-4 receptors, thus establishing a brain–gut axis (54). We have not excluded the possibility that MIA-602 may also modulate lipoprotein production through this pathway.
Plasma triglyceride levels predict incident albuminuria in T1D subjects and rodents (36, 55). Diabetic albuminuria involves several pathogenic mechanisms, including disruption of the glomerular barrier as well as proximal tubular injury. Impaired function of glomerular endothelial barriers involves disruption of the glycocalyx by reactive oxygen species, which are themselves induced in T1D by hyperlipidemia and/or hyperglycemia (37). Lipid profiles were significantly improved in T1D rats treated with MIA-602. We hypothesize that this has partially contributed to the significant improvement in proteinuria in T1D rats treated with MIA-602. Alternatively, the GHRH antagonist might have acted through a direct renal mechanism, as by improving microvascular barrier function. This is unlikely, however, because we have previously shown that, in lung microvascular endothelial cells, MIA-602 slightly decreased, whereas GHRH agonists strongly enhanced, barrier function (24). We also observed a significant reduction of α-sma, a marker of fibroblast activation and renal fibrosis in kidney cortex of T1D rats treated with MIA-602. Taken together, these results indicate renoprotective activities, in addition to the lipid-lowering effect, of GHRH antagonists in T1D.
Endothelial dysfunction is an important hallmark of cardiovascular morbidity and mortality in T1D subjects. Dyslipidemia associated with enhanced TRL is an important risk factor for cardiovascular disease, because it induces the generation of proinflammatory and proatherogenic mediators such as MCP-1 (56). Treatment with MIA-602 both improved endothelial function and reduced plasma MCP-1 levels in T1D rats. In addition, MIA-602 appeared to restore metabolic responsiveness to GLP-1 in these animals. GLP-1, aside from reducing glucagon levels and improving dyslipidemia, was also shown to improve vasorelaxation responses by restoring nitric oxide (NO) bioavailability in renal arteries of hypertensive rats (57). Whether such a mechanism contributed to the beneficial effects of MIA-602 on aortic endothelial function in T1D rats remains to be determined.
Based on our results, we hypothesize that GHRH signaling is at least partially involved in the impairment of GLP-1 signaling in T1D, both in the presence and absence of insulin. This, in turn, contributes to dyslipidemia, nephropathy, and endothelial dysfunction. The role of GHRH signaling in T1D, however, appears to be complex, as we previously demonstrated that synthetic GHRH agonists can enhance viability of pancreatic beta cells in a STZ-induced mouse model and thus might be useful as an adjunctive therapy for islet cell transplantation (27). For the majority of patients who live to adulthood with T1D, inhibition of GHRH signaling could potentially emerge as a promising therapeutic approach to ameliorate the dyslipidemia, kidney damage, and cardiovascular disease risk associated with this disease.
Materials and Methods
In vitro vascular functional studies were performed by placing vessel rings of thoracic aorta as obtained from each of the groups, in a small vessel myograph (Danish Myo Technology). Vessels were precontracted with phenylephrine (10−6 M). Endothelial-dependent and -independent relaxation responses in vessels were tested with progressive concentrations of ACh or the nitric oxide donor SNP, respectively. The vasorelaxant responses are expressed as percent decreases from phenylephrine-induced maximal contraction. All animal experiments were approved by the Institutional Animal Care and Use Committee at Augusta University.
All other information is listed in SI Materials and Methods.
SI Materials and Methods
Cell Culture.
Rat small intestinal epithelial cells (IEC-6) (ATCC CRL-1592) were grown in six-well plates in DMEM supplemented with 10% (vol/vol) FBS (heat-inactivated FBS; Gibco/Invitrogen), 1 mM sodium pyruvate, 2 mM l-alanyl-l-glutamine (GLUTAMAX I), 5 μg/mL insulin (Sigma-Aldrich), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a 5% (vol/vol) CO2 atmosphere. For all experiments, cells up to passage 6 were used for all experiments.
Peptide Analogs Preparation.
GHRH agonist JI-34 and GHRH antagonist MIA-602 were synthesized in the laboratory of A.V.S. (23, 24) and dissolved initially in DMSO before dilution with incubation medium. The final concentration of DMSO in the medium never exceeded 0.1% (vol/vol).
T1 Diabetes Animal Model.
Wistar rats (male, 200–250 g; Envigo RMS) were rendered diabetic with a single dose of STZ (50 mg/kg body weight, i.p.). Once rats became hyperglycemic (>350 mg/dL plasma glucose, usually 3–4 d post STZ injection), they were divided into two groups, with and without treatment with the GHRH antagonist MIA-602 (25 μg/kg per dose, s.c.) administered three times a week for 14 wk after establishment of diabetes. Untreated animals received vehicle alone with the same regimen. Upon completion of the 14-wk period, animals were placed in metabolic cages for 3 d for adaptation, allowing them free movement, water, and food intake. During the entire experiment, rats were fed regular rat chow (Teklad Diet) and received water ad libitum. On the third day in the metabolic cages, food and water intake was monitored for a period of 24 h. Twenty-four-hour urine specimens were also collected, centrifuged at 400 × g for 5 min for particulate removal, aliquoted, and stored at −80 °C. Animals were then sacrificed in the morning around 10:00 AM in a fed state, under deep anesthesia with isoflurane, by exsanguination, followed by removal of vital organs. Plasma was separated from heparinized blood samples, aliquoted, and saved at −80 °C for further analysis. Rats were handled according to high ethical and scientific standards for laboratory animals, and our protocol was reviewed and approved by the Institutional Animal Care and Use Committee at the Medical College of Georgia at Augusta University.
Assessment of ApoB-48 Lipoprotein Secretion by Cultured Intestinal Epithelial Cells.
Upon confluence, cells were washed twice with serum-free DMEM, upon which fresh serum-free medium, supplemented with or without 5 μg/mL insulin was added. After 2 h of equilibration, cells were pretreated for 1 h with the GHRH receptor agonist JI-34 (1 μM; dissolved in DMSO), whereas control cells received vehicle alone, followed by addition of the GLP-1 receptor agonist (Exendin-4; 10 nM; Sigma-Aldrich). After 3 h, cells were treated with 0.5 mM oleic acid (OA) complexed to BSA (Sigma-Aldrich) for 3 h, to allow for lipid loading into ApoB-48 lipoproteins. Additional cells were preincubated or not for 1 h with the GLP-1 receptor antagonist Exendin 9–39 (100 nM; Sigma-Aldrich), or the GHRH antagonist MIA-602 (1 μM; dissolved in DMSO). Upon completion of treatment, medium was collected, centrifuged at 1,000 × g for 3 min, and saved at −80 °C for further analysis.
Determination of Metabolites.
ApoB-48 lipoprotein was measured in plasma samples from rats and in culture medium supernatant from IECs by the use of a commercial ELISA kit (MyBioSource).
The VLDL/LDL Cholesterol Assay kit (Abcam), the Total Cholesterol Assay Kit (Wako), and the PicoProbe Triglyceride Quantification Assay Kit (Abcam) were used for analysis of VLDL/LDL fractions, total cholesterol, and triglycerides in plasma samples.
Albumin and creatinine levels in urine were analyzed by the use of Nephrat II and Creatinine Companion (Exocell) assay kits, respectively.
Chemokines and glucose regulatory hormones (MCP-1, insulin, amylin, GIP, and glucagon) were analyzed using the MILLIPLEX MAP Rat Metabolic Hormone Magnetic Bead Panel, Metabolism Multiplex Assay (RMHMAG-84K; EMD Millipore). Levels of growth hormone (GH) were assay with Rat Growth Hormone ELISA kit (EMD Millipore).
Glucose levels were measured in blood by AlphaTRAK Blood Glucose Monitoring System.
Western Blotting.
Liver was removed and cortex was separated from the kidneys, snap-frozen in liquid nitrogen, and stored at −70 °C until processed. Frozen liver and kidney cortex were homogenized in RIPA lysis buffer containing protease and phosphatase inhibitor mixtures (Sigma), with a bead disruptor (Mini-Beadbeater-1) for protein extraction. The entire small intestine was removed, and its length was measured from the pylorus to the ileocecal junction. The intestine was divided into four segments. The third and fourth distal segments comprising the jejunum–ileum were flushed with PBS to eliminate luminal contents, and then washed four times with cold PBS under gentle agitation, for 10 min each. Tissue samples were drained of excess buffer by placing them for 1 min on surgical gauze, and subsequently snap-frozen in liquid nitrogen, and stored at −70 °C until processed. Frozen small intestine was homogenized in a modified RIPA buffer containing 1% Triton X-100, with a bead disruptor (Mini-Beadbeater-1). Samples were incubated for 10 min on ice, then sonicated on ice (three cycles, 10 s each), and centrifuged at 10,000 × g for 15 min for protein extraction. Equal amounts of protein (50 μg) were subjected to electrophoresis in 10% (wt/vol) SDS/PAGE and subsequently transferred to PVDF membrane (Bio-Rad). Proteins were detected with the following antibodies: GHRH receptor (Abcam), GLP-1 receptor (Santa Cruz), α-sma (Sigma-Aldrich), or β-actin (Sigma-Aldrich). Data obtained from Western blots were processed by ImageJ quantification software.
Statistical Analysis.
All data are expressed as mean ± SD. Comparisons between different groups were performed with the use of a two-tailed, unpaired Student t test. Program GraphPad Prism, version 5.0, was used for statistical analysis of vasorelaxation studies. A value of P < 0.05 was considered significant.
Acknowledgments
We thank Dr. Ronald Goldberg (Division of Endocrinology, Diabetes and Metabolism, Diabetes Research Institute, University of Miami Miller School of Medicine) and Dr. Monty Krieger (Department of Biology, Massachusetts Institute of Technology) for critically reading the manuscript. This work was supported by a Pilot Study Research Program Intramural Grant from the Office of the Vice President for Research at the Medical College of Georgia, Augusta University (to M.J.R.), as well as by the Medical Research Service of Veterans Affairs Department (A.V.S.), Departments of Pathology and Medicine, Division of Hematology/Oncology, University of Miami, Miller School of Medicine (A.V.S.), the South Florida Veterans Affairs Foundation for Research and Education (A.V.S.), and the L. Austin Weeks Family Endowment for Research (N.L.B.).
Footnotes
Conflict of interest statement: A.V.S. and N.L.B. are coinventors on patents on GHRH antagonists, assigned to the University of Miami and Department of Veterans Affairs.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1525520113/-/DCSupplemental.
References
- 1.Katz M, Giani E, Laffel L. Challenges and opportunities in the management of cardiovascular risk factors in youth with type 1 diabetes: Lifestyle and beyond. Curr Diab Rep. 2015;15(12):119. doi: 10.1007/s11892-015-0692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuttle KR, et al. Diabetic kidney disease: A report from an ADA Consensus Conference. Diabetes Care. 2014;37(10):2864–2883. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baigent C, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 4.Castro Cabezas M, Verseyden C, Meijssen S, Jansen H, Erkelens DW. Effects of atorvastatin on the clearance of triglyceride-rich lipoproteins in familial combined hyperlipidemia. J Clin Endocrinol Metab. 2004;89(12):5972–5980. doi: 10.1210/jc.2003-031329. [DOI] [PubMed] [Google Scholar]
- 5.Ahn CH, Choi SH. New drugs for treating dyslipidemia: Beyond statins. Diabetes Metab J. 2015;39(2):87–94. doi: 10.4093/dmj.2015.39.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller M, et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51(7):724–730. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 7.Chapman MJ, et al. European Atherosclerosis Society Consensus Panel Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur Heart J. 2011;32(11):1345–1361. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klop B, Proctor SD, Mamo JC, Botham KM, Castro Cabezas M. Understanding postprandial inflammation and its relationship to lifestyle behaviour and metabolic diseases. Int J Vasc Med. 2012;2012:947417. doi: 10.1155/2012/947417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbons GF. Assembly and secretion of hepatic very-low-density lipoprotein. Biochem J. 1990;268(1):1–13. doi: 10.1042/bj2680001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botham KM, Wheeler-Jones CP. Postprandial lipoproteins and the molecular regulation of vascular homeostasis. Prog Lipid Res. 2013;52(4):446–464. doi: 10.1016/j.plipres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Deedwania P, et al. Treating to New Targets Investigators Reduction of low-density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: Analysis of the Treating to New Targets study. Lancet. 2006;368(9539):919–928. doi: 10.1016/S0140-6736(06)69292-1. [DOI] [PubMed] [Google Scholar]
- 12.Fonseca VA. Ongoing clinical trials evaluating the cardiovascular safety and efficacy of therapeutic approaches to diabetes mellitus. Am J Cardiol. 2011;108(3) Suppl:52B–58B. doi: 10.1016/j.amjcard.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Freyse EJ, et al. Blood glucose lowering and glucagonostatic effects of glucagon-like peptide I in insulin-deprived diabetic dogs. Diabetes. 1997;46(5):824–828. doi: 10.2337/diab.46.5.824. [DOI] [PubMed] [Google Scholar]
- 14.Sivertsen J, Rosenmeier J, Holst JJ, Vilsbøll T. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat Rev Cardiol. 2012;9(4):209–222. doi: 10.1038/nrcardio.2011.211. [DOI] [PubMed] [Google Scholar]
- 15.Hein GJ, Baker C, Hsieh J, Farr S, Adeli K. GLP-1 and GLP-2 as yin and yang of intestinal lipoprotein production: Evidence for predominance of GLP-2-stimulated postprandial lipemia in normal and insulin-resistant states. Diabetes. 2013;62(2):373–381. doi: 10.2337/db12-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg RB, Holman R, Drucker DJ. Clinical decisions. Management of type 2 diabetes. N Engl J Med. 2008;358(3):293–297. doi: 10.1056/NEJMclde0708469. [DOI] [PubMed] [Google Scholar]
- 17.Fredheim S, et al. The influence of glucagon on postprandial hyperglycaemia in children 5 years after onset of type 1 diabetes. Diabetologia. 2015;58(4):828–834. doi: 10.1007/s00125-014-3486-3. [DOI] [PubMed] [Google Scholar]
- 18.Hansen AP, Johansen K. Diurnal patterns of blood glucose, serum free fatty acids, insulin, glucagon and growth hormone in normals and juvenile diabetics. Diabetologia. 1970;6(1):27–33. doi: 10.1007/BF00425888. [DOI] [PubMed] [Google Scholar]
- 19.Press M, Tamborlane WV, Sherwin RS. Importance of raised growth hormone levels in mediating the metabolic derangements of diabetes. N Engl J Med. 1984;310(13):810–815. doi: 10.1056/NEJM198403293101302. [DOI] [PubMed] [Google Scholar]
- 20.Campbell PJ, Bolli GB, Cryer PE, Gerich JE. Pathogenesis of the dawn phenomenon in patients with insulin-dependent diabetes mellitus. Accelerated glucose production and impaired glucose utilization due to nocturnal surges in growth hormone secretion. N Engl J Med. 1985;312(23):1473–1479. doi: 10.1056/NEJM198506063122302. [DOI] [PubMed] [Google Scholar]
- 21.Williams RM, et al. The effects of a specific growth hormone antagonist on overnight insulin requirements and insulin sensitivity in young adults with type 1 diabetes mellitus. Diabetologia. 2003;46(9):1203–1210. doi: 10.1007/s00125-003-1175-8. [DOI] [PubMed] [Google Scholar]
- 22.Christodoulou C, et al. Expression of growth hormone-releasing hormone (GHRH) and splice variant of GHRH receptors in normal mouse tissues. Regul Pept. 2006;136(1-3):105–108. doi: 10.1016/j.regpep.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Pozsgai E, et al. The effect of a novel antagonist of growth hormone releasing hormone on cell proliferation and on the key cell signaling pathways in nine different breast cancer cell lines. Int J Oncol. 2011;39(4):1025–1032. doi: 10.3892/ijo.2011.1098. [DOI] [PubMed] [Google Scholar]
- 24.Lucas R, et al. Agonist of growth hormone-releasing hormone reduces pneumolysin-induced pulmonary permeability edema. Proc Natl Acad Sci USA. 2012;109(6):2084–2089. doi: 10.1073/pnas.1121075109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao C, Dash S, Morgantini C, Lewis GF. New and emerging regulators of intestinal lipoprotein secretion. Atherosclerosis. 2014;233(2):608–615. doi: 10.1016/j.atherosclerosis.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 26.Mayo KE, Godfrey PA, Suhr ST, Kulik DJ, Rahal JO. Growth hormone-releasing hormone: Synthesis and signaling. Recent Prog Horm Res. 1995;50:35–73. doi: 10.1016/b978-0-12-571150-0.50007-x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, et al. Beneficial effects of growth hormone-releasing hormone agonists on rat INS-1 cells and on streptozotocin-induced NOD/SCID mice. Proc Natl Acad Sci USA. 2015;112(44):13651–13656. doi: 10.1073/pnas.1518540112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pritchard KA, Jr, Patel ST, Karpen CW, Newman HA, Panganamala RV. Triglyceride-lowering effect of dietary vitamin E in streptozocin-induced diabetic rats. Increased lipoprotein lipase activity in livers of diabetic rats fed high dietary vitamin E. Diabetes. 1986;35(3):278–281. doi: 10.2337/diab.35.3.278. [DOI] [PubMed] [Google Scholar]
- 29.Ferreira LD, Huey PU, Pulford BE, Ishii DN, Eckel RH. Sciatic nerve lipoprotein lipase is reduced in streptozotocin-induced diabetes and corrected by insulin. Endocrinology. 2002;143(4):1213–1217. doi: 10.1210/endo.143.4.8723. [DOI] [PubMed] [Google Scholar]
- 30.Laatsch A, et al. Insulin stimulates hepatic low density lipoprotein receptor-related protein 1 (LRP1) to increase postprandial lipoprotein clearance. Atherosclerosis. 2009;204(1):105–111. doi: 10.1016/j.atherosclerosis.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Liang X, Wang S. Intra-islet glucagon secretion and action in the regulation of glucose homeostasis. Front Physiol. 2013;3:485. doi: 10.3389/fphys.2012.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo Q, Avramoglu RK, Adeli K. Intestinal assembly and secretion of highly dense/lipid-poor apolipoprotein B48-containing lipoprotein particles in the fasting state: Evidence for induction by insulin resistance and exogenous fatty acids. Metabolism. 2005;54(5):689–697. doi: 10.1016/j.metabol.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Lewis GF. Fatty acid regulation of very low density lipoprotein production. Curr Opin Lipidol. 1997;8(3):146–153. doi: 10.1097/00041433-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Vatner DF, et al. Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc Natl Acad Sci USA. 2015;112(4):1143–1148. doi: 10.1073/pnas.1423952112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Windmueller HG, Spaeth AE. Regulated biosynthesis and divergent metabolism of three forms of hepatic apolipoprotein B in the rat. J Lipid Res. 1985;26(1):70–81. [PubMed] [Google Scholar]
- 36.Kiss E, et al. Lipid droplet accumulation is associated with an increase in hyperglycemia-induced renal damage: Prevention by liver X receptors. Am J Pathol. 2013;182(3):727–741. doi: 10.1016/j.ajpath.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 37.Marcovecchio ML, et al. Prevalence of abnormal lipid profiles and the relationship with the development of microalbuminuria in adolescents with type 1 diabetes. Diabetes Care. 2009;32(4):658–663. doi: 10.2337/dc08-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh A, et al. Reactive oxygen species modulate the barrier function of the human glomerular endothelial glycocalyx. PLoS One. 2013;8(2):e55852. doi: 10.1371/journal.pone.0055852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dioufa N, et al. Acceleration of wound healing by growth hormone-releasing hormone and its agonists. Proc Natl Acad Sci USA. 2010;107(43):18611–18615. doi: 10.1073/pnas.1013942107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domoto K, et al. Chylomicron remnants induce monocyte chemoattractant protein-1 expression via p38 MAPK activation in vascular smooth muscle cells. Atherosclerosis. 2003;171(2):193–200. doi: 10.1016/j.atherosclerosis.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 41.Beauchamp G, Haller MJ. Can we prevent type 1 diabetes? Curr Diab Rep. 2015;15(11):86. doi: 10.1007/s11892-015-0658-6. [DOI] [PubMed] [Google Scholar]
- 42.Havel RJ. Triglyceride-rich lipoproteins and plasma lipid transport. Arterioscler Thromb Vasc Biol. 2010;30(1):9–19. doi: 10.1161/ATVBAHA.108.178756. [DOI] [PubMed] [Google Scholar]
- 43.Parhofer KG. Interaction between glucose and lipid metabolism: More than diabetic dyslipidemia. Diabetes Metab J. 2015;39(5):353–362. doi: 10.4093/dmj.2015.39.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van de Woestijne AP, van der Graaf Y, Westerink J, Nathoe HM, Visseren FL. Effect of statin therapy on incident type 2 diabetes mellitus in patients with clinically manifest vascular disease. Am J Cardiol. 2015;115(4):441–446. doi: 10.1016/j.amjcard.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Andersson C, Lyass A, Larson MG, Robins SJ, Vasan RS. Low-density-lipoprotein cholesterol concentrations and risk of incident diabetes: Epidemiological and genetic insights from the Framingham Heart Study. Diabetologia. 2015;58(12):2774–2780. doi: 10.1007/s00125-015-3762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobs ML, Nathoe HM, Blankestijn PJ, Stijnen T, Weber RF. Growth hormone responses to growth hormone-releasing hormone and clonidine in patients with type I diabetes and in normal controls: Effect of age, body mass index and sex. Clin Endocrinol (Oxf) 1996;44(5):547–553. doi: 10.1046/j.1365-2265.1996.713534.x. [DOI] [PubMed] [Google Scholar]
- 47.Catalina PF, Mallo F, Andrade MA, García-Mayor RV, Diéguez C. Growth hormone (GH) response to GH-releasing peptide-6 in type 1 diabetic patients with exaggerated GH-releasing hormone-stimulated GH secretion. J Clin Endocrinol Metab. 1998;83(10):3663–3667. doi: 10.1210/jcem.83.10.5153. [DOI] [PubMed] [Google Scholar]
- 48.Lombardi G, et al. The cardiovascular system in growth hormone excess and growth hormone deficiency. J Endocrinol Invest. 2012;35(11):1021–1029. doi: 10.3275/8717. [DOI] [PubMed] [Google Scholar]
- 49.Foot AB, Davidson K, Edge JA, Wass JA, Dunger DB. The growth hormone releasing hormone (GHRH) response to a mixed meal is blunted in young adults with insulin-dependent diabetes mellitus whereas the somatostatin response is normal. Clin Endocrinol (Oxf) 1990;32(2):177–183. doi: 10.1111/j.1365-2265.1990.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 50.Federico LM, Naples M, Taylor D, Adeli K. Intestinal insulin resistance and aberrant production of apolipoprotein B48 lipoproteins in an animal model of insulin resistance and metabolic dyslipidemia: Evidence for activation of protein tyrosine phosphatase-1B, extracellular signal-related kinase, and sterol regulatory element-binding protein-1c in the fructose-fed hamster intestine. Diabetes. 2006;55(5):1316–1326. doi: 10.2337/db04-1084. [DOI] [PubMed] [Google Scholar]
- 51.Veilleux A, et al. Intestinal lipid handling: Evidence and implication of insulin signaling abnormalities in human obese subjects. Arterioscler Thromb Vasc Biol. 2014;34(3):644–653. doi: 10.1161/ATVBAHA.113.302993. [DOI] [PubMed] [Google Scholar]
- 52.Eckel RH, Fujimoto WY, Brunzell JD. Gastric inhibitory polypeptide enhanced lipoprotein lipase activity in cultured preadipocytes. Diabetes. 1979;28(12):1141–1142. doi: 10.2337/diab.28.12.1141. [DOI] [PubMed] [Google Scholar]
- 53.Watts GF, Chan DC. Novel insights into the regulation of postprandial lipemia by glucagon-like peptides: Significance for diabetes. Diabetes. 2013;62(2):336–338. doi: 10.2337/db12-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farr S, et al. Central nervous system regulation of intestinal lipoprotein metabolism by glucagon-like peptide-1 via a brain-gut axis. Arterioscler Thromb Vasc Biol. 2015;35(5):1092–1100. doi: 10.1161/ATVBAHA.114.304873. [DOI] [PubMed] [Google Scholar]
- 55.Bjornstad P, et al. Plasma triglycerides predict incident albuminuria and progression of coronary artery calcification in adults with type 1 diabetes: The Coronary Artery Calcification in Type 1 Diabetes Study. J Clin Lipidol. 2014;8(6):576–583. doi: 10.1016/j.jacl.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen YG, et al. Molecular signatures differentiate immune states in type 1 diabetic families. Diabetes. 2014;63(11):3960–3973. doi: 10.2337/db14-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu L, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012;60(3):833–841. doi: 10.1161/HYPERTENSIONAHA.112.195115. [DOI] [PubMed] [Google Scholar]