Significance
Glial cell line-derived neurotrophic factor (GDNF) growth factor induces spermatogonial stem cells to proliferate in culture to produce progenitor spermatogonia. Sertoli cell GDNF was thought responsible for this in the testis. We showed that testosterone triggers GDNF secretion by peritubular myoid (PM) cells in culture and that undifferentiated spermatogonia (spermatogonial stem cells and progenitors) co-cultured with testosterone-treated PM cells and transplanted to sterile mice restored spermatogenesis. To determine if PM cell GDNF is essential to maintain the undifferentiated spermatogonial population, we made mice lacking the Gdnf gene in PM cells. The number of undifferentiated spermatogonia was severely depleted in 2-wk-old mice and adults were infertile. This is the first study, to our knowledge, to show that the undifferentiated spermatogonial pool cannot be maintained without GDNF from PM cells.
Keywords: spermatogonial stem cell, stem cell niche, male fertility, spermatogenesis, conditional gene targeting
Abstract
Spermatogonial stem cells (SSCs) are a subpopulation of undifferentiated spermatogonia located in a niche at the base of the seminiferous epithelium delimited by Sertoli cells and peritubular myoid (PM) cells. SSCs self-renew or differentiate into spermatogonia that proliferate to give rise to spermatocytes and maintain spermatogenesis. Glial cell line-derived neurotrophic factor (GDNF) is essential for this process. Sertoli cells produce GDNF and other growth factors and are commonly thought to be responsible for regulating SSC development, but limited attention has been paid to the role of PM cells in this process. A conditional knockout (cKO) of the androgen receptor gene in PM cells resulted in male infertility. We found that testosterone (T) induces GDNF expression in mouse PM cells in vitro and neonatal spermatogonia (including SSCs) co-cultured with T-treated PM cells were able to colonize testes of germ cell-depleted mice after transplantation. This strongly suggested that T-regulated production of GDNF by PM cells is required for spermatogonial development, but PM cells might produce other factors in vitro that are responsible. In this study, we tested the hypothesis that production of GDNF by PM cells is essential for spermatogonial development by generating mice with a cKO of the Gdnf gene in PM cells. The cKO males sired up to two litters but became infertile due to collapse of spermatogenesis and loss of undifferentiated spermatogonia. These studies show for the first time, to our knowledge, that the production of GDNF by PM cells is essential for undifferentiated spermatogonial cell development in vivo.
The seminiferous epithelium is separated by tight junctions between Sertoli cells into a luminal compartment containing spermatocytes and spermatids and a basal compartment containing spermatogonial stem cells (SSCs) and spermatogonia. The basal compartment is bounded above and on the sides by Sertoli cells and below by the basement membrane of the seminiferous tubule and a layer of peritubular myoid (PM) cells. SSCs are thought to reside in a microenvironmental niche in the basal compartment, where extrinsic cues influence their decision to either self-renew or enter the pathway of spermatogonial development (1, 2). They are a minor fraction of the undifferentiated spermatogonia in the basal compartment. The other undifferentiated spermatogonia (progenitors) give rise to differentiating spermatogonia that proliferate mitotically to progress on a developmental pathway toward becoming spermatocytes (3, 4). Our current understanding of the progression of SSCs to differentiating spermatogonia comes mainly from cell kinetic studies, germ cell transplantation assays, and the use of molecular markers that identify different populations of spermatogonia.
The leading model for spermatogonial development specifies that when SSCs divide, they either self-renew by becoming two type A-single (As) spermatogonia or give rise to type A-paired (Apr) spermatogonia connected by an intercellular bridge to become undifferentiated spermatogonia (5–7). The pairs continue to divide to form short chains of bridge-connected undifferentiated type A-aligned (Aal) spermatogonia, and these in turn divide to form longer chains of differentiating (type A1, A2, A3, intermediate, and B) spermatogonia.
Although SSCs are single cells, not all As spermatogonia are likely to be SSCs. There are ∼35,000 As spermatogonia in the testes of adult mice (8), but only about 3,000 of these have the ability to regenerate spermatogenesis when transplanted to germ cell-depleted testes (9). Although there are no generally accepted molecular markers specific for SSCs, potential candidates are inhibitor of DNA binding 4 (ID4) and paired box 7 (PAX7), which are expressed in minor subsets of As spermatogonia (10–12). However, it remains to be reported if ID4 and PAX7 are coexpressed in the same subset of As spermatogonia. SSCs also share molecular markers with undifferentiated spermatogonia, including Nanos2, Gfra1, Zbtb16, Bcl6b, and THY1(13–17). In addition, differentiating spermatogonia have characteristic molecular markers, including Ngn3, Nanos3, Spo11, and KIT (18–22). These molecular markers have been proven to be valuable tools for monitoring the presence or absence of different populations of spermatogonia.
A conditional knockout (cKO) of the androgen receptor (Ar) gene in PM cells resulted in progressive loss of spermatogonia beginning at postnatal d 21, leading to disorganization of the seminiferous epithelium and infertility (23). This strongly suggested that androgens regulate genes in PM cells whose products are essential for SSC maintenance. In other studies, mice heterozygous for a global mutation in the gene for glial cell-derived neurotrophic factor (Gdnf) had reduced stem cell reserves, whereas mice with a transgene overexpressing GDNF experienced an overaccumulation of undifferentiated spermatogonia (24). GDNF also was reported to be critical for development of SSCs in vitro and their ability to restore spermatogenesis after transplantation to the testes of germ-cell-depleted mice (25, 26). This led us to hypothesize that regulation of GDNF production in PM cells by testosterone (T) is essential for SSC maintenance. In studies to test this hypothesis, we determined that PM cells isolated from adult mice and treated in vitro with T produce GDNF but not when untreated. We also found that SSCs from neonatal mice co-cultured with T-treated PM cells and transplanted to testes of germ cell-depleted mice restored spermatogenesis but not when they were co-cultured with untreated PM cells (10). These results supported the hypothesis but did not rule out the possibilities that PM cells or Sertoli cells produce other factors in vivo in addition to GDNF that are responsible for SSC self-renewal and differentiation. In these studies, we generated mice with a cKO of the Gdnf gene in PM cells to test the hypothesis that the production of GDNF by PM cells is essential for the in vivo development of undifferentiated spermatogonia.
Results
Disruption of the Gdnf Gene in PM Cells.
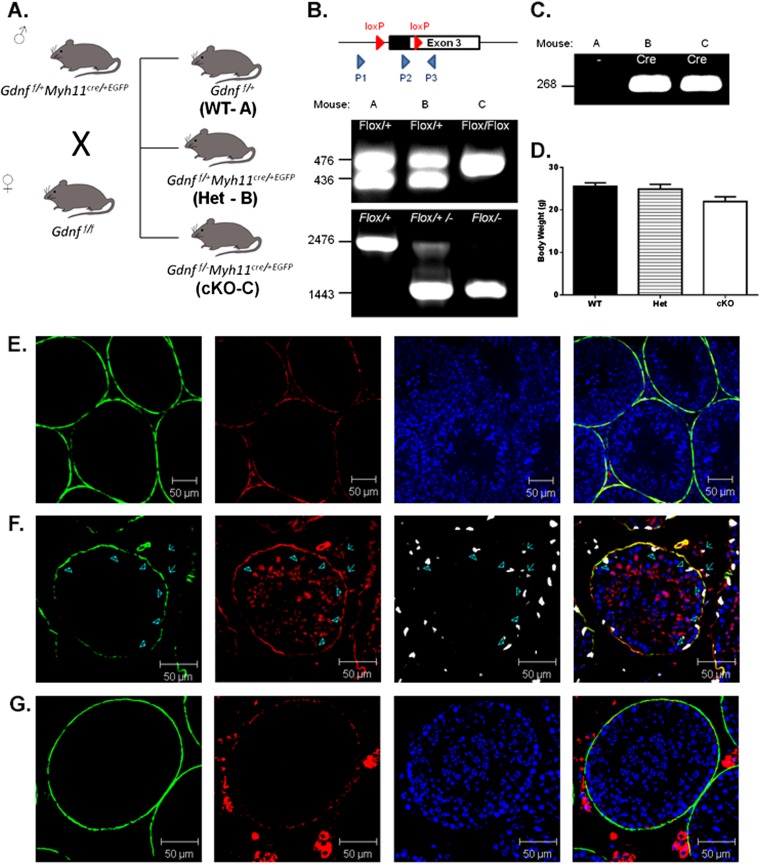
We tested the hypothesis that the production of GDNF by PM cells in vivo was essential for development of undifferentiated spermatogonia by generating mice with a conditional deletion of one allele (Het) or both alleles (cKO) of the Gdnf gene in PM cells. This was done by crossing mice with exon 3 of the Gdnf gene flanked by LoxP sites with Myh11-cre,-EGFP transgenic mice (Fig. S1). The Myh11-cre was effective at disrupting the Ar gene in PM cells (23). We confirmed that MYH11 is present in PM cells by immunostaining (Fig. S1E, green) and that Myh11-cre,-EGFP is expressed in PM cells and not at detectable levels in GATA4-containing Sertoli cells (Fig. S1F, arrow heads). The efficacy of the Myh11-cre,-EGFP mice for targeting floxed genes in PM cells was verified by crossing them with (flox/stop/flox) ROSA-dtTomato mice and determining that Tomato protein is expressed in PM cells (Fig. S1G, red). Expression also was seen of MYH11 in blood vessels (Fig. S1F, red) and of dtTomato in Leydig cells (Fig. S1G, red), but GDNF was not expressed at detectable levels in those cells.
Fig. S1.
Generation of mice with a conditional disruption of the Gdnf gene (cKO) in PM cells. (A) Breeding scheme to generate cKO mice. The offspring generated by crossing male Gdnf f/+:Myh11cre/+,EGFP and female Gdnff/f mice were designated WT (Gdnff/+), Het (Gdnf f/+:Myh11cre/+,EGFP), and cKO (Gdnf f/–:Myh11cre/+,EGFP). (B) Genotyping was performed by PCR with the P2 and P3 primer set to identify floxed (∼476 bp) and WT (∼436 bp) alleles and with P1 and the P3 primer set to identify WT (2,476 bp band only), Het (2,476 bp and 1,443 bp bands), and cKO (1,443 bp band only) alleles. (C) The presence of the Myh11cre/+,EGFP transgene was determined by PCR (268 bp band). (D) There were no significant differences in body weights between adult WT, Het, and cKO males. (E) The expression of MYH11 in PM cells was confirmed by immunostaining with rabbit anti-mouse MYH11 and Alexa Fluor 546 donkey anti-rabbit (red) antibodies, along with use of a mouse monoclonal antibody to the PM cell molecular marker ACTA2 (green) and nuclear staining with DAPI (blue). (F) The expression of Myh11-cre,-EGFP in PM cells was seen by immunostaining with rabbit antiserum to GFP and Alexa Fluor 546 donkey anti-rabbit antiserum (red), but not seen in Sertoli cells (arrow heads), labeled with goat antiserum to GATA4 and Alexa Fluor 647 donkey anti-goat antiserum (white). ACTA2 was labeled in PM cells (green). (G) Expression of Myh11-cre,-EGFP in PM cells was confirmed by crossing Myh11-cre/+,-EGFP mice with (flox/stop/flox) dtTomato transgenic mice. Deletion of the stop codon resulted in expression of dtTomato, as detected by immunostaining with rabbit antiserum to dtTomato and Alexa Fluor 546 donkey anti-rabbit (red). ACTA2 antibody conjugated with FITC (green) was used to label PM cells, and nuclei were stained with DAPI (blue).
Effects of cKO on Fertility and Testis.
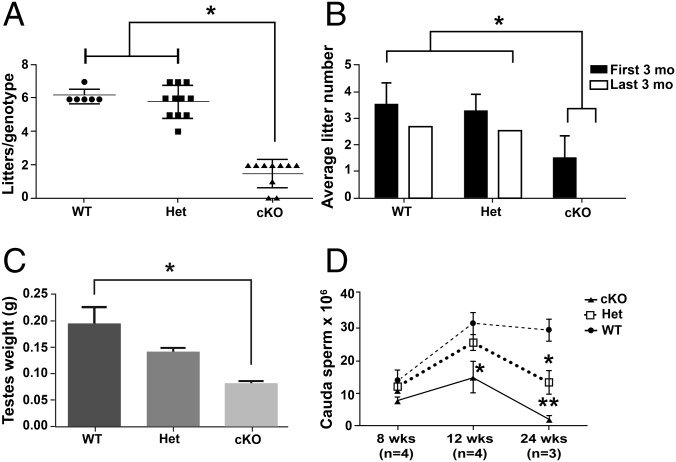
The role of GDNF production by PM cells was examined first by determining the effect of the Gdnf cKO on male fertility. Eight-week-old wild-type (WT), Het, and cKO males were mated continuously for 6 mo with one WT female each, and the numbers of litters sired by WT males (6.17 ± 0.71) and Het males (5.82 ± 0.98) were not significantly different, whereas the number of litters sired by cKO males was significantly lower (1.5 ± 0.85) (Fig. 1A). The WT and Het males sired 4–7 litters and were fertile throughout the mating trial, whereas the cKO males sired 0–2 litters and were fertile only during the first 3 mo (Fig. 1B), suggesting an age-dependent loss of fertility in cKO males. However, the sizes of the litters sired by WT (6.54 ± 0.48), Het (7.43 ± 0.52), and cKO (5.8 ± 1.05) males were not significantly different.
Fig. 1.
Effects of targeted disruption of the Gdnf gene in PM cells on male reproductive function. (A) The number of litters sired by WT, Het, and cKO males in a 6 mo breeding study. (B) The average number of litters sired by WT, Het, and cKO males in the first and second 3-mo period of the breeding study. (C) The weights of testes from 8-wk-old WT, Het, and cKO mice. (D) The number of sperm released from the cauda epididymides of 8-, 12-, and 24-wk-old WT, Het, and cKO males. Each bar and data point represents the mean ± SEM. *P < 0.05, **P < 0.01.
To identify possible underlying causes for the loss of fertility in cKO males, other male reproductive system parameters were examined. At 8 wk, testis weights were significantly lower in cKO than in Het and WT males (Fig. 1C), but there were no differences in body weights (Fig. S1D). In addition, the sperm numbers in the cauda epididymis of 8-, 12-, and 24-wk-old WT, Het, and cKO males were determined (Fig. 1D). There were no significant differences at 8 wk, but by 12 wk sperm numbers were significantly lower in cKO males than in WT and Het males. By 24 wk, the sperm numbers were dramatically lower for cKO males and significantly lower for Het males than for WT males. These results led us to hypothesize that the loss of fertility with age was due to disruption of spermatogonial development.
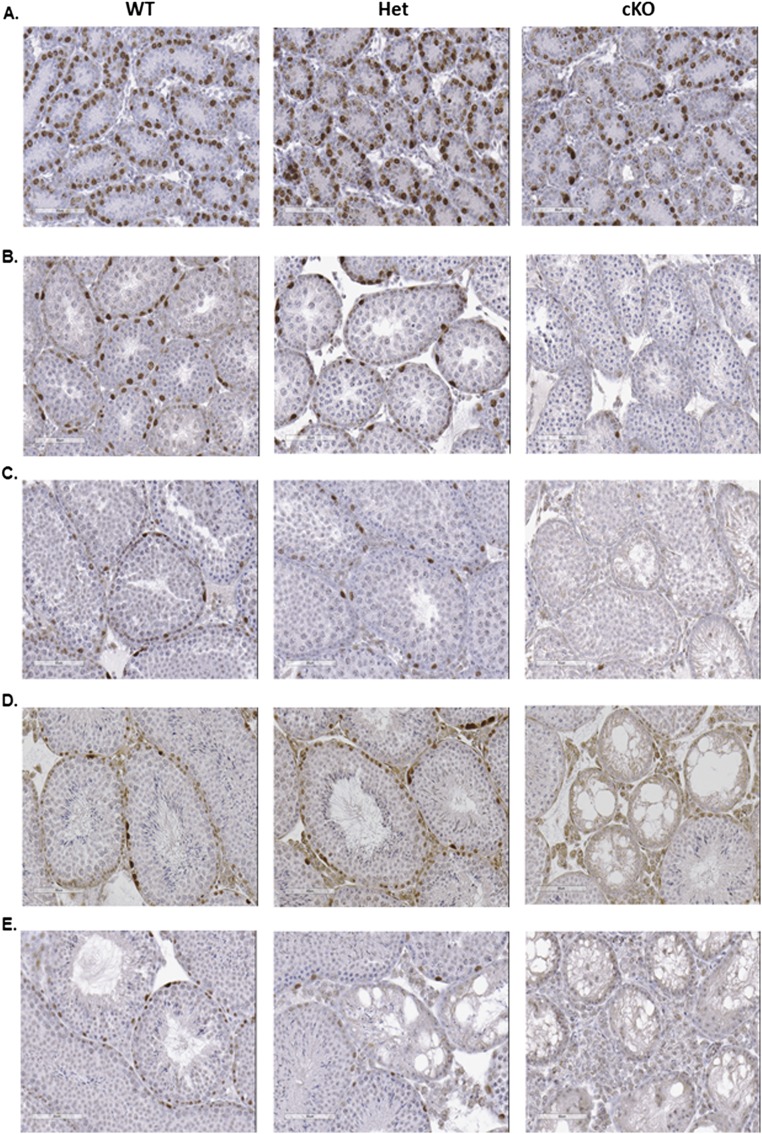
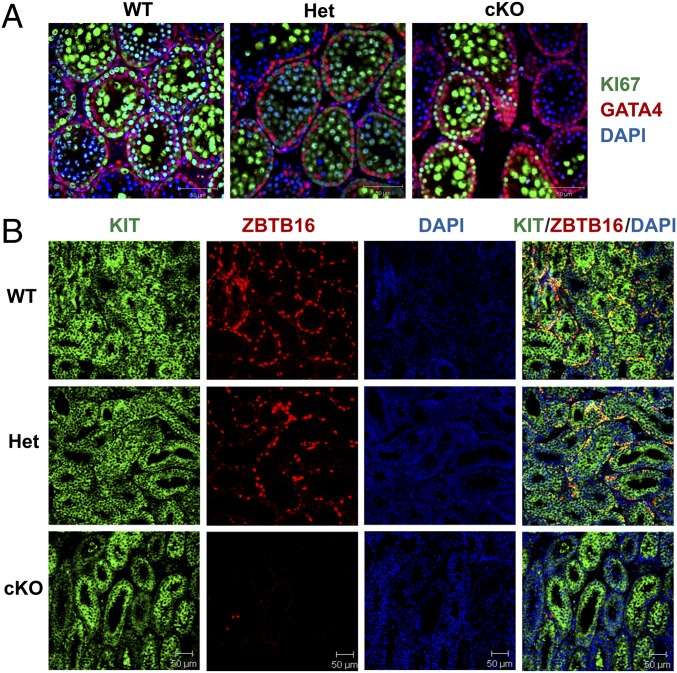
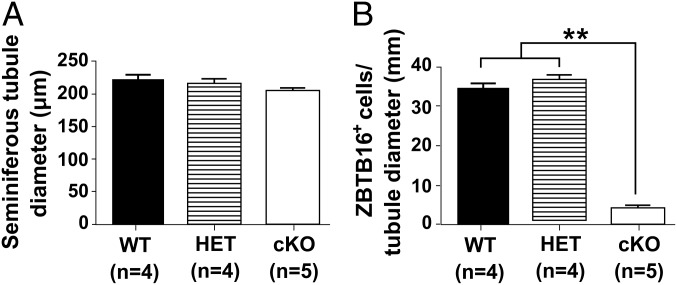
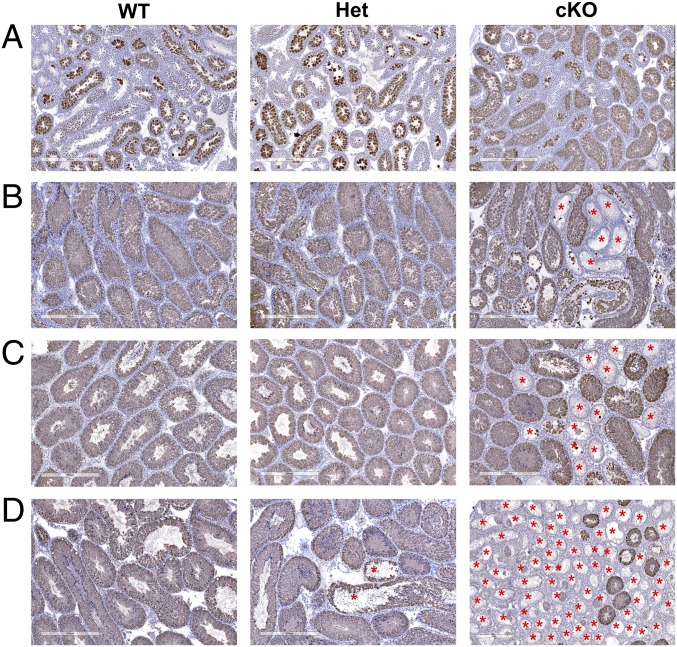
To test this hypothesis, sections of testes from 1- to 12-wk-old WT, Het, and cKO males were immunostained for ZBTB16 (Table S1), a marker for undifferentiated spermatogonia (15). Differences were not apparent at 1 wk (Fig. S2A), but at 2 wk some seminiferous tubules in cKO males lacked spermatogonia (Fig. S2B). This increased at 4 wk (Fig. S2C) and was more pronounced at 8 wk (Fig. S2D). By 12 wk, some tubules in Het mice lacked undifferentiated spermatogonia, and spermatogenesis was severely defective in most tubules in cKO mice (Fig. S2E). In addition, sections of testes from 2-wk-old WT, Het, and cKO mice were immunostained with antibodies to GATA4, a marker for Sertoli cells, and to KI67 (Table S1), a marker for cell proliferation (27–29). All tubules in WT and Het mice contained KI67-labeled germ cells, whereas some tubules in cKO mice lacked KI67-labeled germ cells (Fig. 2A). In addition, sections of testes from 2-wk-old mice were co-immunostained with antibodies to ZBTB16 and to KIT (Table S1), a marker for differentiated spermatogonia (22). There were little apparent differences in the KIT staining in WT, Het, and cKO mice (Fig. 2B), but the number of ZBTB16-positive germ cells per tubule cross-section was dramatically lower in cKO testes compared with the HET or WT testes (Figs. 2B and 3B). There were no differences in the diameter of seminiferous tubules of WT, Het, and cKO mice (Fig. 3A). Similar results were seen in sections immunostained for HSPA2 (Table S1), a marker for meiotic and postmeiotic spermatogenic cells (30, 31). An occasional cKO tubule cross-section showed a lack of HSPA2-positive germ cells at 2 wk (Fig. 4A), more tubules lacked HSPA2-positive germ cells at 4 and 8 wk (Fig. 4 B and C), and most tubules lacked HSPA2-positive germ cells (Sertoli cell only) at 12 wk (Fig. 4D). Possible interpretations of these results were that the low number was either due to apoptosis of spermatogonia or to a failure to repopulate the spermatogonial pool in cKO testes.
Table S1.
Antibodies
| Name of antibody | Source and catalog no. | Species | Dilution |
| Primary | |||
| ACTA2 | Sigma-Aldrich, A5228 (Clone 1A4) | Mouse, monoclonal | 1:200 |
| ACTA2-FITC | Sigma-Aldrich, F3777 (Clone 1A4) | Mouse, monoclonal | 1:200 |
| dtTomato | Clontech, 632496 | Rabbit, polyclonal | 1:500 |
| GATA4 | Santa Cruz, SC-1237 | Goat, polyclonal | 1:300 |
| GDNF (d-20) | Santa Cruz, SC-328 | Rabbit, polyclonal | 1:200 |
| GFP | Life Technologies, A11122 | Rabbit, polyclonal | 1:200 |
| HSPA2 | Eddy laboratory, Allen et al. PMID 3352605 | Rabbit, polyclonal | 1:1,000 |
| KI67 | Abcam, ab15580 | Rabbit, polyclonal | 1:200 |
| KIT | Abcam, ab65525 (Clone 2B8) | Rat, monoclonal | 1:100 |
| SOX9 | Cosmo Bio Co. Japan, KAL-KO608 | Rabbit, polyclonal | 1:200 |
| ZBTB16 (PLZF, H-300) | Santa Cruz, SC-22839 | Rabbit, polyclonal | 1:200 |
| Secondary | |||
| Alexa Fluor 488, Donkey anti-mouse IgG | Life Technologies, A21202 | Donkey, polyclonal | 1:200 |
| Alexa Fluor 546, Donkey anti-rabbit IgG | Life Technologies, A10040 | Donkey, polyclonal | 1:200 |
| Alexa Fluro 647, Donkey anti-goat IgG | Life Technologies, A11056 | Donkey, polyclonal | 1:200 |
| DAB staining | |||
| VECTSTAIN Elite ABC Kit (Rabbit IgG) | Vector Laboratories, PK-6106 | ||
| ImmPACT DAB Peroxidase Substrate | Vector Laboratories, SK-4105 | ||
| Vybrant apoptosis Assay Kit 6 (V23200) | |||
| Annexin V + Alexa Fluor 350 | Life Technology | ||
| PI | Life Technology | ||
Fig. S2.
ZBTB16 immunostaining of undifferentiated spermatogonia in testes from WT, Het, and cKO mice at 1, 2, 4, 8, and 12 wk. (A) No obvious differences are seen at 1 wk. (B) Fewer ZBTB16-positive cells were present in cKO testes compared to Het and WT at 2 wk. (C–E) ZBTB16-positive cells were rarely observed at 4 (C), 8 (D), and 12 (E) wk in cKO testes. Disrupted tubules were observed in 4- to 12-wk testes. [Scale bars: A, 400 µm; and B–F, 300 µm.]
Fig. 2.
Scanning confocal microscopy images of sections of testes from 2-wk-old WT, Het, and cKO mice immunostained for markers of spermatogonia, Sertoli cells, and cellular proliferation. (A) Sections immunostained for markers of Sertoli cells (GATA4) and for cellular proliferation (KI67). (B) Sections immunostained for markers of differentiating spermatogonia (KIT) and for SSCs and undifferentiated spermatogonia (ZBTB16), counterstained for DNA (DAPI).
Fig. 3.
Tubule diameters and numbers of undifferentiated spermatogonia in WT, Het, and cKO mice at 2 wk. (A) Seminiferous tubule cross-section diameter (µm). (B) The number of ZBTB16-positive cells per tubule diameter (mm). Each bar represents the mean ± SEM. ANOVA analysis with Tukey test. **P < 0.01. (Scale bar: 50 μm.)
Fig. 4.
Sections of testes from WT, Het, and cKO mice immunostained for HSPA2, a marker for meiotic and postmeiotic spermatogenic cells. (A) Two-week-old mice. (B) Four-week-old mice. (C) Eight-week-old mice. (D) Twelve-wk-old mice. (Scale bar: 300 μm.)
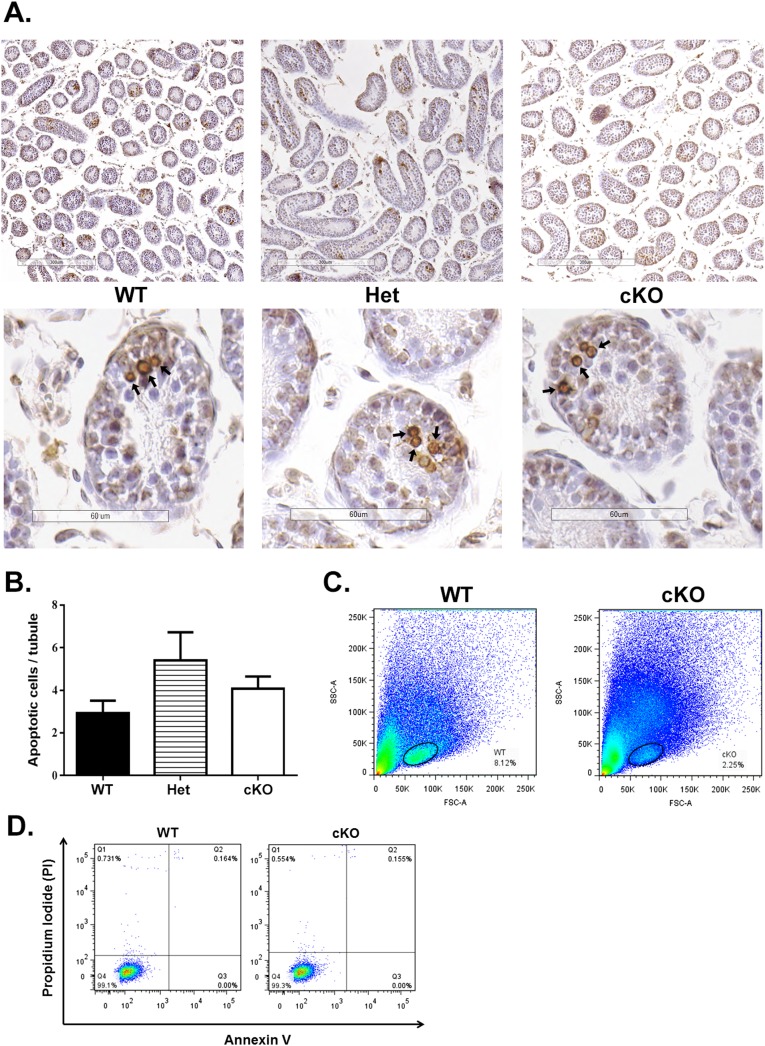
TUNEL labeling and flow cytometry were used to test these possibilities. There were no significant differences in the frequency of TUNEL-labeled apoptotic cells per tubule cross-section in testes of 2-wk-old WT, Het, and cKO mice (Fig. S3 A and B). The gated area for analyzing apoptosis of undifferentiated spermatogonia by flow cytometry was set using previously reported criteria (10, 32) and captured 6.44 ± 1.23% of cells in WT and 3.37 ± 0.56% of cells in cKO samples (Fig. S3C). When an antibody to Annexin V (ANXA5) (Table S1) (33) was used to label the early apoptotic cell population (Q4), differences were not significant between WT (0.06 ± 0.09%) and cKO (0.08 ± 0.1%) testes (Fig. S3 C and D). Similar results were seen for the late apoptotic cells population (Q2) of WT (0.06 ± 0.09%) and cKO mice (0.1 ± 0.08%). These results suggest that the reduction of differentiated spermatogonia was not due to apoptosis but rather the result of a preceding reduced population of undifferentiated spermatogonia.
Fig. S3.
Apoptosis analysis of testes of 2-wk-old mice. (A) TUNEL labeling of sections of testes. Spermatogenic cells undergoing apoptosis were seen in some tubules in WT, Het, and cKO mice (arrows). (B) There were no significant differences in the number of TUNEL-labeled germ cells per tubule cross-section in WT, Het, and cKO mice. (C) A representative forward and side scatter dot plot with the gated area in WT and cKO. The gated area in WT has 6.44 ± 1.23% cells, with 3.37 ± 0.56% cells in cKO. (D) Example of flow cytometry analysis of gated cells from WT and cKO mice with the separation based on labeling with Alexa Fluro 350-conjugated Annexin V and PI. Annexin V fluorescence is plotted against PI fluorescence for the cells stained either with Annexin V, PI, or both. Each fraction includes the percentage of the cell populations in WT and cKO that did not show significant differences. Q1, dead cells (generated during the cell processing procedure); Q2, late apoptotic cells (or necrotic cells); Q3, live cells; Q4, early apoptotic cells. [Scale bars: A, 300 μm (Upper) and 60 μm (Lower).]
Effects of cKO on Gdnf mRNA and GDNF Protein Expression.
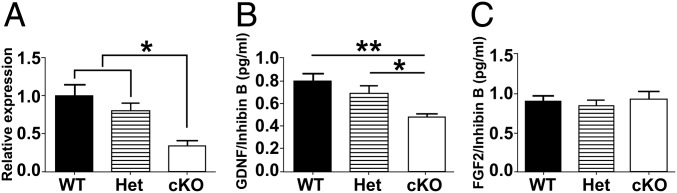
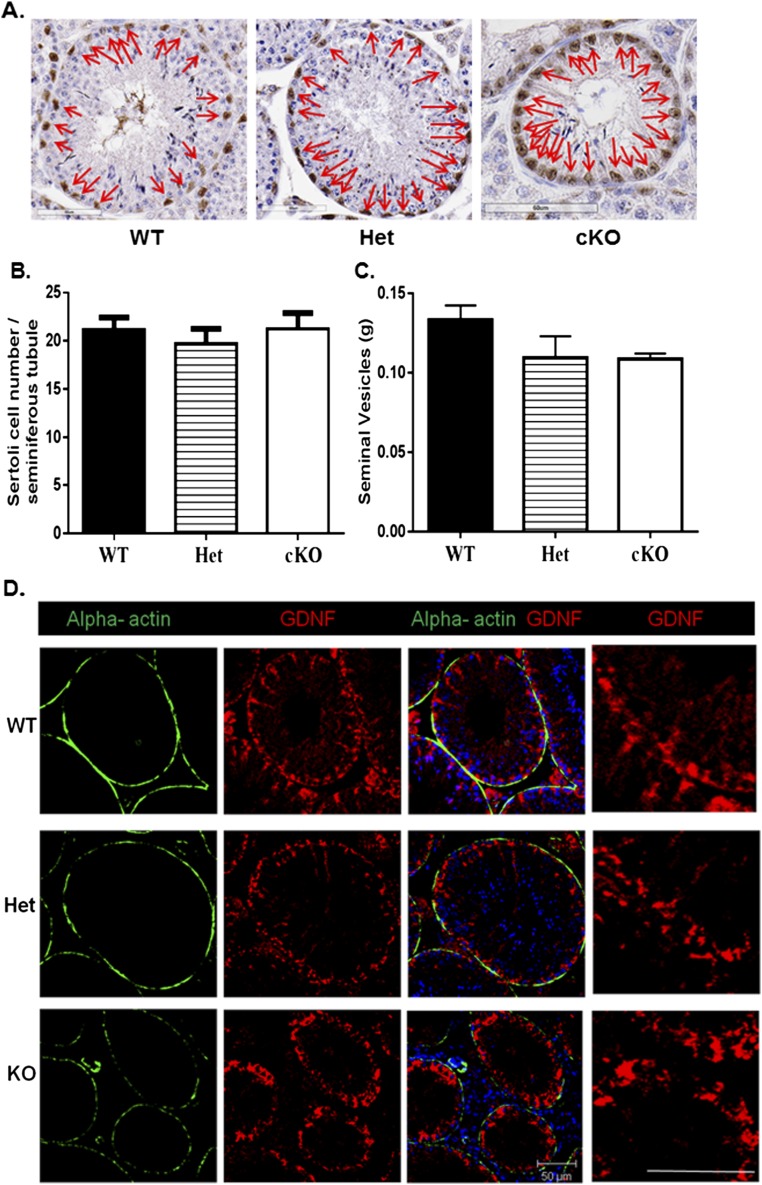
The previous findings suggested that a lack of GDNF production by PM cells in cKO mice disrupts developmental progression of undifferentiated spermatogonia. To examine this possibility, we first used quantitative PCR (qPCR) to compare Gdnf (Table S2) mRNA levels in testes from 8-wk-old WT, Het, and cKO mice. The Gdnf steady-state levels were significant lower in cKO mice compared with the levels in WT and Het mice (Fig. 5A). To determine if this was due to a reduction in Sertoli cell numbers, sections were immunostained for SOX9 (Fig. S4A), a marker for Sertoli cells (34). When SOX9-positive cells were counted and normalized to the total number of tubules per section, no significant differences in Sertoli cell numbers were found between WT, Het, and cKO mice (Fig. S4B). In addition, ELISAs were used to measure the GDNF protein concentration in testes of WT, Het, and cKO mice. When the GDNF ELISA results were normalized to the ELISA levels for inhibin beta-B (INHBB), an indicator of Sertoli cell numbers and function (35), the GDNF levels in cKO testes were found to be reduced by about 40% compared with WT testes (Fig. 5B). Also, when sections of testes from 12-wk-old mice were immunostained, the GDNF signal seemed similar in Sertoli cells in WT, Het, and cKO testes; appeared to be reduced in PM cells in Het testes compared with WT testes; and were not detectable in PM cells in cKO testes (Fig. S4D). Furthermore, seminal vesicle weights were not significantly different between WT, Het, and cKO mice, implying that their androgen levels were comparable (Fig. S4C). These results suggested that production of GDNF by Sertoli cells and of androgens by Leydig cells were not disrupted in cKO mice and that decreases in Gdnf mRNA and GDNF protein levels in the testis were the direct result of disruption of the Gdnf gene in PM cells.
Table S2.
PCR primers
| Gene name | Primer sequences |
| Gdnf | Forward (F), CGCTGACCAGTGACTCCAATA |
| Reverse (R), AGTTAAAACGCACCCCCGAT | |
| Spo11 | F, TGCCTGCCTTCACAATAGACAA |
| R, ATGGACAATACTTTCAGAATCA | |
| Gapdh | F, GCCGGTGCTGAGTATGTCGT |
| R, ATCACGCCACAGCTTTCCAGAGGG | |
| P1 | CCAGCCAACCAACCAACCAACCAACCACTA |
| P2 | ACTGGATAGGAGTGAGAACAGCCGTCTGA |
| P3 | GTCCAGAATCAACCACCAAGCGTCCC |
| Cre | F, GGACATGTTCAGGGATCGCCAGGCG |
| R, GCACAACCAGTGAAACAGCATTGCTG |
Fig. 5.
The Gdnf mRNA and GDNF and FGF2 protein levels in testes of 8-wk-old mice. (A) The Gdnf mRNA levels in WT, Het, and cKO mice relative to Sox9 levels. (B) The GDNF protein levels in WT, Het, and cKO mice relative to INHBB levels. (C) The FGF2 protein levels in WT, Het, and cKO mice relative to INHBB levels. ANOVA analysis with Tukey test. *P < 0.05, **P < 0.01.
Fig. S4.
Sertoli cell numbers, seminal vesicle weights, and the GDNF expression of 8-wk-old WT, Het, and cKO mice. (A) Sertoli cell nuclei (arrows) were immunostained with rabbit anti-mouse SOX9 antibody. (B) The numbers of Sertoli cells per tubule cross-sections of WT, Het, and cKO were not significantly different. (C) The seminal vesicle weights of WT, Het, and cKO mice were not significantly different. (D) The expression of GDNF in Sertoli cells of WT, Het, and cKO mice. Sections were immunostained with rabbit antiserum to GDNF and Alexa Fluor 546 donkey anti-rabbit antiserum (red) and with FITC-conjugated mouse monoclonal antibody to ACTA2 (green) to determine which cell types contained GDNF. GDNF was localized to Sertoli cells and PM cells in WT and Het mice but only in Sertoli cells in cKO mice. Nuclei were stained with DAPI. (Scale bar: 50 µm.)
Effects of cKO on Genes Involved in Spermatogonial Development.
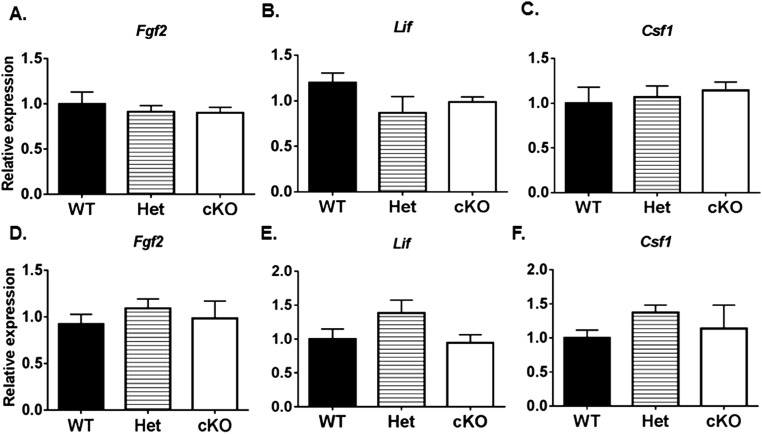
We determined if the cKO of Gdnf in PM cells alters the expression of certain other genes involved in SSC development. FGF2 is expressed by Sertoli cells and was reported to enhance the ability of GDNF to maintain SSC proliferation in vitro (26, 32, 36, 37). However, no significant differences were detected by ELISA in FGF2 protein levels in testes of 8-wk-old WT, Het, and cKO mice (Fig. 5C) or in Fgf2 (Table S3) mRNA levels in testes of 1- and 4-wk-old WT, Het, and cKO mice (Fig. S5 A and B). The steady-state transcript levels for other growth factors (Table S3) indicated to be beneficial to SSC self-renewal in vitro, including Lif (produced by Leydig cells) (38, 39) and Csf1 (produced by Leydig cells and PM cells) (40), were not significantly different in testes of 1- and 4-wk-old WT and cKO mice (Fig. S5 A and B).
Table S3.
PCR primers
| Gene name | Taqman primer gene ID |
| Fgf2 | Mm00433287_m1 |
| Zbtb16 | Mm01176868_m1 |
| Bcl6b | Mm00455912_g1 |
| Lif | Mm00434762_g1 |
| Csf1 | Mm00432686_m1 |
| Nanos2 | Mm02525720_s1 |
| Gfra1 | Mm00439086_m1 |
| Kit | Mm00445212_m1 |
| Nanos3 | Mm00808138_m1 |
| Sox9 | Mm00448840_m1 |
Fig. S5.
Steady-state levels of genes for growth factors in testes from WT, Het, and cKO mice relative to Gapdh. Expression at 1 wk for (A) Fgf2, (B) Lif, and (C) Csf1. Expression at 4 wk for (D) Fgf2, (E) Lif, (C) Csf1, and (F) Kit.
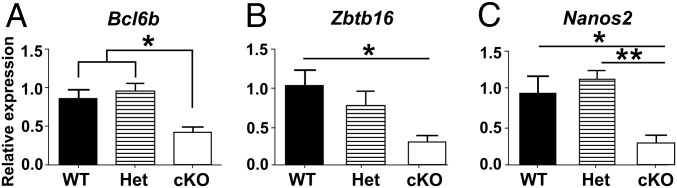
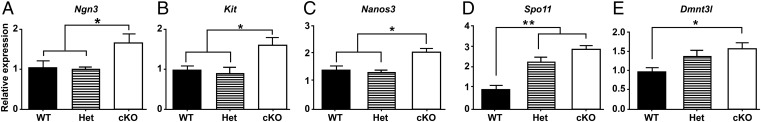
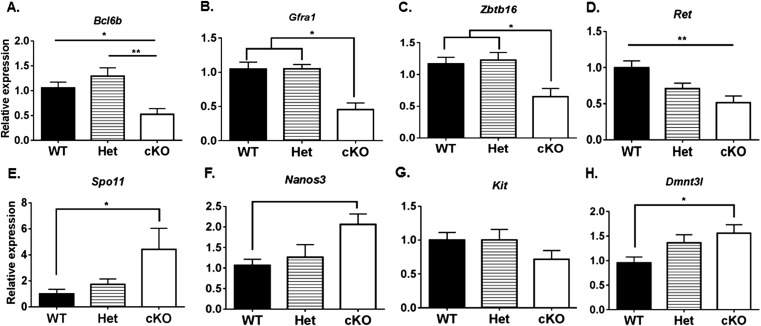
We also evaluated the expression of genes in testes of 1-wk-old WT, Het, and cKO mice for markers of SSCs and undifferentiated spermatogonia (Tables S2 and S3), including Bcl6b (16), Zbtb16 (15, 41), and Nanos2 (42), and for markers of differentiating spermatogonia, including Ngn3 (18, 43), Kit (21), Nanos3 (44), Spo11 (45), and Dnmt3l (46). The expression levels of markers of undifferentiated spermatogonia were significantly lower in cKO mice than in WT or Het mice (Fig. 6), whereas the markers for differentiated spermatogonia were significantly lower in WT than in cKO mice (Fig. 7). This suggests that most spermatogonia in the testes of 1-wk-old cKO mice are differentiating spermatogonia. In addition, we determined the steady-state levels of expression of genes in 4-wk-old WT, Het, and cKO mice for markers of undifferentiated spermatogonia (Bcl6b, Gfra1, Zbtb16, and Ret) and differentiating spermatogonia (Spo11, Nanos3, Kit, and Dnmt3l). The markers for undifferentiated spermatogonia were significantly lower at 4 wk in cKO mice than in WT mice (Fig. S6 A–D), whereas there were no significant differences for most markers for differentiated spermatogonia in cKO and WT mice (Fig. S6 E–H). The exception was a higher level of expression of Spo11 in cKO than in WT mice, indicating a higher spermatocyte-to-spermatogonia ratio at 4 wk in cKO than in WT or Het mice. These results suggest that the initial fertility in a few cKO males and subsequent collapse in spermatogenesis in all cKO males are preceded by a decline in the level of undifferentiated spermatogonia but not in the level of differentiated spermatogonia in testes of 1-wk-old cKO mice or of spermatocytes in 4-wk-old cKO mice.
Fig. 6.
The mRNA levels for markers of undifferentiated spermatogonia in testes of WT, Het, and cKO 1-wk-old mice relative to Sox9 levels. (A) Bcl6b. (B) Zbtb16. (C) Nanos2. ANOVA analysis with Tukey test. *P < 0.05, **P < 0.01.
Fig. 7.
The mRNA levels for markers of differentiated spermatogonia in testes of WT, Het, and cKO 1-wk-old mice relative to Sox9 levels. (A) Ngn3. (B) Kit. (C) Nanos3. (D) Spo11. (E) Dnmt3l. ANOVA analysis with Tukey test. *P < 0.05.
Fig. S6.
Steady-state levels of molecular markers for undifferentiated and differentiated spermatogonia in testes from 4-wk-old WT, Het, and cKO mice. Expression levels of markers for undifferentiated spermatogonia genes (A) Bcl6b, (B) Gfra1, (C) Zbtb16, and (D) Ret. Expression levels of markers for differentiated spermatogonia: (E) Spo11, (F) Nanos3, (G) Kit, and (H) Dnmt3l. *P < 0.05.
Lack of Effects of cKO on Undifferentiated Spermatogonia.
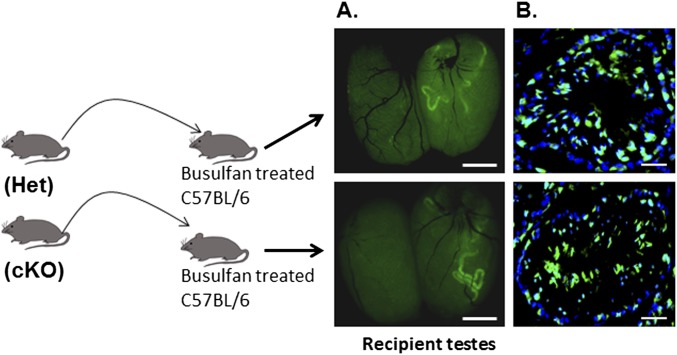
A defining characteristic of SSCs is their ability to establish colonies of undifferentiated spermatogonia and to reestablish spermatogenesis when transplanted to testes of mice depleted of germ cells. To determine if the cKO had a direct effect on the development potential of SSCs, testicular cells from neonatal Het and cKO mice carrying the Myh11-cre,-EGFP transgene were transplanted into the testes of germ cell-depleted recipient mice. When testes of recipient mice were examined 11 wk later, colonies of GFP-expressing spermatogenic cells derived from Het and cKO donors were seen (Fig. S7A). Sections of testes receiving germ cells from Het and cKO donor mice immunostained with an antibody to GFP contained elongated spermatids expressing Myh11-cre,-EGFP (Fig. S7B), indicating that the SSCs from Het and cKO mice were able to restore spermatogenesis in germ cell-depleted testes and that neonatal SSCs do not require GDNF from PM cells to acquire their developmental potential.
Fig. S7.
Scheme for testing the ability of undifferentiated spermatogonia from neonatal Het and cKO mice to repopulate the testes of germ cell-depleted mice following transplantation. (A) Testes of mice that received GFP-expressing (Myh11-cre,-EGFP) germ cells 11 wk earlier from Het (Upper panel) or cKO (Lower panel) mice imaged under a dissecting microscope with fluorescence optics. (B) Cryostat sections of testes of mice that received EGFP-expressing germ cells 11 wk earlier from Het (Upper panel) or cKO (Lower panel) mice immunostained with an antibody to GFP and counterstained with DAPI. (Scale bar: 50 µm.)
Discussion
We generated mice with a cKO in the Gdnf gene in PM cells to test the hypothesis that GDNF produced by PM cells is essential for development of undifferentiated spermatogonia. The cKO resulted in a loss of fertility, with cKO males siring 0–2 litters during the first 3 mo and no litters during the second half of a 6-mo mating study. There was a significant reduction by 8 wk in testis weight in cKO males compared with WT males due to a collapse of spermatogenesis, with most seminiferous tubules lacking ZBTB16-positive undifferentiated spermatogonia and HSPA2-positive spermatocytes and spermatids by 12 wk of age. This resulted in a significant reduction in the number of sperm in the cauda epididymis of 12-wk-old cKO males and a paucity of sperm in the cauda epididymis of 24-wk-old cKO males. These results supported the hypothesis and encouraged further studies to determine the underlying cause of the infertility.
The first wave of spermatogenesis in the mouse starts 2–3 d after birth with gonocytes dividing and giving rise to SSCs and undifferentiated spermatogonia, continues with the expansion of the undifferentiated spermatogonial population, and progresses to undifferentiated spermatogonia giving rise to differentiating spermatogonia beginning at 6 d of age (47, 48). SSCs share molecular markers with undifferentiated spermatogonia (13–17), and these markers were expressed at significantly higher levels at 1 wk after birth in the testes of WT mice than in cKO mice. However, molecular markers for differentiated spermatogonia (18–22) were present at significantly higher levels at 1 wk after birth in cKO mice than in WT mice. In addition, germ cells containing the ZBTB16 marker for SSCs and undifferentiated spermatogonia (15) were detected by immunohistochemistry in 2-wk-old WT mice, but rarely in cKO males, and some of their tubules lack proliferating germ cells. We interpret these results to indicate that SSCs and undifferentiated spermatogonia generate differentiated spermatogonia in the first wave of spermatogenesis in both WT and cKO mice but that the undifferentiated spermatogonial pool is not replenished in cKO mice. In addition, markers for differentiating spermatogonia and spermatocytes were expressed at comparable levels in testes of 4-wk-old WT and cKO mice, suggesting that spermatogonial and spermatocyte differentiation proceeds on schedule during the first wave of spermatogenesis in cKO mice. These results provide strong evidence that GDNF produced by PM cells is required to replenish the undifferentiated spermatogonial pool but not for generating the first wave of spermatogonial differentiation or for the further development of spermatogenic cells.
Earlier studies provided evidence that SSCs were sensitive to changes in the level of GDNF in the testis. Mice with a global targeted disruption of one allele of the Gdnf gene (global Het) were fertile but showed depletion of stem cell reserves, whereas transgenic mice with global overexpression of GDNF showed accumulation of undifferentiated spermatogonia (24). Disruption of both alleles (global KO) of the genes for GDNF or for either the RET or GDNFR1 subunits of the GDNF receptor resulted in disruption of renal and neuronal development and early postnatal death (49–52). In addition, 30–35% of Gdnf global Het mice had renal defects (49, 53). In vitro studies since have demonstrated that GDNF regulates expression of genes in SSCs that influence their self-renewal and differentiation (1, 3, 54). Because GDNF has a critical role in multiple developmental processes in addition to spermatogenesis, it remained uncertain whether the perturbations of SSC development in vivo were due to direct effects on SSCs or indirect effects of total GDNF levels. In the present study with PM cell Gdnf Het and cKO males, the following was observed: (i) a modest reduction in testis weight in Het males at 8 wk and a collapse in spermatogenesis in some tubules in Het males at 12 wk; (ii) a significant reduction in the number of sperm in the cauda epididymis in 12- and 24-wk-old Het males; (iii) a significant reduction in Gdnf mRNA and GDNF protein levels in the testes of Het and cKO mice, with protein levels being reduced about 20% in Het mice and 40% in cKO mice; (iv) a lack of GDNF detectable by confocal microscopy in PM cells of cKO mice; (v) an absence of obvious changes in GDNF levels in Sertoli cells of Het or cKO mice; and (vi) SSCs from neonatal Het and cKO males were able to repopulate the testes of germ cell-depleted mice. In addition, seminal vesicle weights and Sertoli cell numbers were comparable in WT and cKO mice, suggesting intact endocrine function. This confirms and extends the earlier findings that GDNF levels influence SSC development and demonstrates that GDNF levels must be kept within a relatively narrow range in the testis for SSC maintenance. A novel finding of the present study is that the production of GDNF by PM cells is essential for ensuring that optimal GDNF levels exist for repopulating the undifferentiated spermatogonial pool.
Although these studies show the importance of GDNF production by PM cells in vivo, previous in vitro studies suggested that other factors might be required as well. Colony stimulating factor 1 (CSF1) is produced by Leydig and PM cells and enhanced the self-renewal of SSCs in vitro in the presence of GDNF (40), as did fibroblast growth factor 2 (FGF2) (26, 37, 55), whose transcripts were identified in Sertoli cells (56, 57). Sertoli cells are recognized to have a key role in forming the niche and in determining the number of niches and SSCs in the testis (58). In addition, an androgen-stimulated factor produced by rat PM cells, referred to as peritubular factor that modulates Sertoli cell function (PModS), positively influenced production of transferrin (TRF), androgen binding protein (ABP, SCGB1B27), inhibin (59), and other proteins by Sertoli cells (60, 61). The activity was contained in 56–59-kDa fractions of PM cell-conditioned medium, but its molecular identity remains unknown. It also was found that PM cells produced neuregulin, Sertoli cells expressed receptors for neuregulin, and recombinant neuregulin stimulated TRF and ABP production by Sertoli cells (62). Furthermore, PM cells secrete IGF1 and FGF2, and these also were able to stimulate TRF and ABP production by Sertoli cells (62). However, these studies were performed in vitro, and GDNF is the only factor shown so far to influence SSCs in vivo. Although FGF2 was reported to regulate Gdnf expression levels in Sertoli cells (63), changes in FGF2 protein or Fgf2 mRNA levels were not seen in Gdnf cKO testes. Disruption of the Ar gene in Sertoli cells resulted in infertility due to arrest of spermatogenesis in the meiotic (64, 65) or postmeiotic phases (66), suggesting that androgen-regulated processes in Sertoli cells are not essential for spermatogonia. Nevertheless, Sertoli cells are likely to be key players in the network of cell–cell interactions and in the production by neighboring cells of factors that define and regulate the microenvironment of the SSC niche. The generation of mice with a cKO of genes for GDNF or other factors in Sertoli cells or in other cells in the testis will be needed to determine their roles in regulating SSC development in vivo.
Previous studies demonstrated that (i) cKO of the Ar gene in PM cells resulted in infertility (23), (ii) androgen response elements (AREs) were present in the promoter region of the Gdnf gene in mice (67), (iii) GDNF was essential for SSC maintenance in vitro (1, 9, 26), and (iv) PM cells isolated from human testes produced GDNF (68). We recently reported that (i) PM cells in adult mice produce GDNF, (ii) isolated PM cells from adult mice produce GDNF in response to T treatment, and (iii) SSCs co-cultured with T-treated PM cells are significantly more effective at repopulating the testes of germ cell-depleted mice following transplantation than SSCs co-cultured with untreated PM cells (10). The results of our previous and present studies have led to the identification of a model for the direct endocrine role in the regulation of spermatogonial cell development, one in which T acts through PM cells to modulate the level of GDNF in the testis niche to optimize renewal of the undifferentiated spermatogonial pool. This represents a novel and significant new paradigm for the extrinsic regulation of spermatogonial development. It remains to be determined if GDNF acts in vivo to influence the SSC self-renewal/differentiation switch, to regulate the proliferation of undifferentiated spermatogonia, or to modulate a combination of these processes.
Materials and Methods
Mice with a floxed Gdnf allele [Gdnff/+;B6.129S1(Cg)-Gdnftm1.1Neas/J, JAX stock no. 014097] and transgenic mice expressing cre recombinase driven by the Myh11 promoter [B6.Cg-Tg(Myh11-cre,-EGFP)2Mik/J, JAX stock no. 007742] were used. All animal procedures were approved in advance by the National Institute of Environmental Health Sciences Animal Care and Use Committee. Further details on generating the PM cell Gdnf cKO mice and the procedures used in these studies are described in SI Materials and Methods.
SI Materials and Methods
Animals.
Male Myh11-cre/+,-EGFP mice was crossed with female Gdnf f/+ mice (Fig. S1A) to produce male mice with a PM cell-specific deletion of one allele of Gdnf (Het). Male Gdnff/+:Myh11-cre/+,-EGFP mice were crossed with female Gdnf f/f mice to generate Gdnf f/–:Myh11-cre/+,-EGFP mice with both alleles of the Gdnf gene disrupted in PM cells (Gdnf cKO) (Fig. S1 B and C). Myh11-cre/+,-EGFP mice were crossed with dtTomato mice [B6;129S6-Gt(ROSA)26Sortm9(CAG–tdTomato)Hze/J, stock no. 007908, The Jackson Laboratory] carrying a transgene with a floxed stop codon (flox/stop/flox) in the promoter to determine where Myh11-cre/+,-EGFP is expressed (Fig. S2C).
PCR and Real-Time qPCR.
Mouse genotypes were determined by PCR on samples prepared using the Qiagen DNeasy blood and tissue kit to extract DNA from tail biopsies. Littermates were genotyped using primers 1 (P1) and P3 (Table S2) to identify WT, Het, and cKO mice (Fig. S1 A–C).
RNA isolation and PCR procedures used were described previously (69). Relative mRNA expression levels for most genes were determined by qPCR using Taqman primers. The PCR reactions for Taqman primer followed the manufacturer’s recommendations. The Gdnf, Spo11, and Gapdh RT-PCR reactions contained 1 µL cDNA, 12.5 µL of Applied Biosystems Power SYBR Green Master Mix, 0.5 µL (stock concentration 20 pmol/µL) of each forward and reverse primer, and 11.5 µL double-distilled water. The reaction was performed at 94 °C for 2 min and then for 40 cycles at 94 °C for 20 s, 60 °C for 20 s, and 75 °C for 30 s. The sequences of Gdnf primers and the ID numbers for Taqman primers used for other genes are listed in Tables S2 and S3.
Immunohistochemistry and Histology.
Testes with the tunica albuginea removed were fixed in either 4% (vol/vol) paraformaldehyde (PFA) (Electron Microscopy Sciences) for TUNEL labeling and cryostat sections or in Bouin’s solution (HT10132, Sigma-Aldrich) for histology and immunohistochemistry. Paraffin sections (8 µm thick) were processed as described previously (69). Slides were incubated at 4 °C overnight with primary antibodies (Table S1), washed three times in PBS, and secondary antibodies were applied at room temperature (RT) for 1 h. Prolong Gold mounting medium containing DAPI (Life Technologies) was applied. After 4% (vol/vol) PFA fixation, the cryostat section samples were processed through sucrose/PBS [10%, 15%, and 12% (vol/vol)] solutions for 30 min each and embedded in optimal cutting temperature (OCT) compound. Cryostat sections (10 μm thick) were collected on slides and thawed at RT. The antibodies and the dilutions used for these studies are listed in Table S1. The immunofluorescence images were captured using a Zeiss LSM 510 UV Meta confocal microscope.
Antibodies to SOX9 and HSPA2 were used to label Sertoli cells and meiotic and postmeiotic germ cells, respectively, followed by detection with the VECTASTAIN Elite ABC kit and ImmPACT DAB (Table S1) chromogen. Slides for histological analysis were stained with hematoxylin and eosin by immersion in hematoxylin (MHS-16, Sigma-Aldrich) for 20 s, washed with tap water three times, and immersed in eosin solution (HT110216, Sigma-Aldrich) for 30 s. They were then dehydrated through 70%, 95%, and 100% (vol/vol) ethanol and xylene solutions for 1 min each before coverslips were applied. Images of histology and immunostained slides were captured using an Aperio Scanscope XT Digital Slide Scanner followed with an Image Scope (Leica Biosystems) to analyze images.
TUNEL Staining.
After paraffin removal, sections were immersed in a solution of 3% (vol/vol) hydrogen peroxide in methanol for 15 min to block endogenous peroxidase. After three washes in PBS, the slides were microwaved in 10 mM citrate buffer for 1 min. The In Situ Cell Death Detection Kit–peroxidase (no. 11684817910, Roche) was used according to the supplier’s directions with minor changes for optimization. Briefly, after the enzyme solution was mixed with the labeling solution, a 1:2 dilution was performed with TUNEL dilution buffer (no. 11966006001, Roche) before being applied to the slides. The slides were incubated at 37 °C for 1 h in a humidified chamber, washed three times in PBS, and blocked with 3% (wt/vol) BSA in PBS at RT for 20 min. A 1:2 dilution of peroxidase converter was applied to the slide and incubated at room temperature for 30 min. The DAB chromogen (sk-4104, Vector Laboratory) was used to identify apoptotic cells. The TUNEL-positive cells were counted in 10 tubule cross-sections in each of 10 randomly selected tissue sections, with a total of 100 tubule cross-sections being counted per biological replicate. The results of WT, Het, and cKO were analyzed from each group of three individual biological replicates (n = 3). The images were captured using an Aperio Scanscope XT Digital Slide Scanner following with an Image Scope (Leica Biosystems) to analyze images.
ELISA.
GDNF, FGF2, and INHBB levels were determined according to the manufacturers’ recommendations, using ELISA kits from Promega (G7621), Abcam (ab100670), and Sigma-Aldrich (RAB0325), respectively, with proteins extracted from testes of 8-wk-old mice. Testes with the tunica albuginea removed were placed in 200 μL extraction buffer (Dulbecco’s PBS) and protease inhibitor mixture (Roche, no. 11873580001) in 1.5 mL Eppendorf tubes and homogenized with a pestle on ice for 15 strokes. Extraction buffer was added to bring the volume to 500 μL, and the sample was centrifuged at 1,000 × g for 10 min at 4 °C. The supernatant was collected and the protein concentration was determined using a BCA protein assay kit (Thermo Scientific). GDNF and INHBB levels were assayed using 100 µg of total protein per 100 µL total sample volume per well in a 96-well plate. FGF2 levels were assayed using 50 µg of total protein per 100 µL total sample volume per well in a 96-well plate.
Flow Cytometry.
Testicular cells (106) were incubated with the Vybrant apoptosis Assay Kit 6 (V23200, Life technologies) containing antibody to Annexin V (ANX5A) and propidium iodide (PI) to label apoptotic cells. The cell gating and data analysis were performed as described previously (10). The labeling procedure followed the manufacturer’s protocol. A BD LSRII Flow Cytometer (BD Biosciences) was used to determine the cell populations, and FLOWJO software was used to analyze the percentage of positive cells (10). The designation of each fraction (Q1 to Q4) was based on Vermes et al. (69) with minor modifications of the negative and positive control gate setting.
Germ Cell Transplantation.
Dispersed testicular cells from neonatal Het and cKO mice were transplanted into the testes of busulfan-treated C57BL/6J mice as described previously (69). This was to determine if there were detrimental effects of deleting one of both Gdnf alleles in neonatal PM cells on the ability of transplanted SSCs to repopulate recipient testes. The germ cells derived from Het and cKO mice were detected directly by fluorescence microscopy or with an antibody to GFP by their expression of EGFP from the Myh11-cre/+,-EGFP transgene.
Sperm Collection.
The cauda epididymis from both testes were collected in 1.5 mL Eppendorf tubes containing 500 µL PBS, three incisions were made in each cauda epididymis, and the tissues were incubated at room temperature for 15 min to allow the sperm to swim out. The sperm concentrations were determined using a hemocytometer and expressed as the average of triplicate counts.
Statistical Analysis.
Results are presented as mean ± SEM. The statistical analysis used AVONA in the Prism 6 Graphpad software, and the significant differences between means were determined by the Tukey test.
Acknowledgments
The authors thank Yukitomo Arao and Bart Phillips for critical reviews of the manuscript, and Paula Brown, Gina Goulding, Linwood Koonce, and Maria Sifre for expert technical support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517994113/-/DCSupplemental.
References
- 1.Hofmann MC. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol. 2008;288(1-2):95–103. doi: 10.1016/j.mce.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Rooij DG, van Beek ME. Computer simulation of the rodent spermatogonial stem cell niche. Biol Reprod. 2013;88(5):131. doi: 10.1095/biolreprod.113.108639. [DOI] [PubMed] [Google Scholar]
- 3.Caires K, Broady J, McLean D. Maintaining the male germline: Regulation of spermatogonial stem cells. J Endocrinol. 2010;205(2):133–145. doi: 10.1677/JOE-09-0275. [DOI] [PubMed] [Google Scholar]
- 4.Chan F, et al. Functional and molecular features of the Id4+ germline stem cell population in mouse testes. Genes Dev. 2014;28(12):1351–1362. doi: 10.1101/gad.240465.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oakberg EF. Spermatogonial stem-cell renewal in the mouse. Anat Rec. 1971;169(3):515–531. doi: 10.1002/ar.1091690305. [DOI] [PubMed] [Google Scholar]
- 6.Huckins C, Oakberg EF. Morphological and quantitative analysis of spermatogonia in mouse testes using whole mounted seminiferous tubules, I. The normal testes. Anat Rec. 1978;192(4):519–528. doi: 10.1002/ar.1091920406. [DOI] [PubMed] [Google Scholar]
- 7.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21(6):776–798. [PubMed] [Google Scholar]
- 8.Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290(2):193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 9.Nagano MC. Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol Reprod. 2003;69(2):701–707. doi: 10.1095/biolreprod.103.016352. [DOI] [PubMed] [Google Scholar]
- 10.Chen LY, Brown PR, Willis WB, Eddy EM. Peritubular myoid cells participate in male mouse spermatogonial stem cell maintenance. Endocrinology. 2014;155(12):4964–4974. doi: 10.1210/en.2014-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aloisio GM, et al. PAX7 expression defines germline stem cells in the adult testis. J Clin Invest. 2014;124(9):3929–3944. doi: 10.1172/JCI75943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oatley MJ, Kaucher AV, Racicot KE, Oatley JM. Inhibitor of DNA binding 4 is expressed selectively by single spermatogonia in the male germline and regulates the self-renewal of spermatogonial stem cells in mice. Biol Reprod. 2011;85(2):347–356. doi: 10.1095/biolreprod.111.091330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sada A, Hasegawa K, Pin PH, Saga Y. NANOS2 acts downstream of glial cell line-derived neurotrophic factor signaling to suppress differentiation of spermatogonial stem cells. Stem Cells. 2012;30(2):280–291. doi: 10.1002/stem.790. [DOI] [PubMed] [Google Scholar]
- 14.He Z, Jiang J, Hofmann MC, Dym M. Gfra1 silencing in mouse spermatogonial stem cells results in their differentiation via the inactivation of RET tyrosine kinase. Biol Reprod. 2007;77(4):723–733. doi: 10.1095/biolreprod.107.062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buaas FW, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36(6):647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 16.Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem. 2007;282(35):25842–25851. doi: 10.1074/jbc.M703474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubota H, Avarbock MR, Brinster RL. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod. 2004;71(3):722–731. doi: 10.1095/biolreprod.104.029207. [DOI] [PubMed] [Google Scholar]
- 18.Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci USA. 2006;103(25):9524–9529. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki H, Sada A, Yoshida S, Saga Y. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev Biol. 2009;336(2):222–231. doi: 10.1016/j.ydbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Romanienko PJ, Camerini-Otero RD. Cloning, characterization, and localization of mouse and human SPO11. Genomics. 1999;61(2):156–169. doi: 10.1006/geno.1999.5955. [DOI] [PubMed] [Google Scholar]
- 21.Sorrentino V, Giorgi M, Geremia R, Besmer P, Rossi P. Expression of the c-kit proto-oncogene in the murine male germ cells. Oncogene. 1991;6(1):149–151. [PubMed] [Google Scholar]
- 22.Pellegrini M, et al. ATRA and KL promote differentiation toward the meiotic program of male germ cells. Cell Cycle. 2008;7(24):3878–3888. doi: 10.4161/cc.7.24.7262. [DOI] [PubMed] [Google Scholar]
- 23.Welsh M, Saunders PT, Atanassova N, Sharpe RM, Smith LB. Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J. 2009;23(12):4218–4230. doi: 10.1096/fj.09-138347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng X, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287(5457):1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 25.Nagano M, Ryu BY, Brinster CJ, Avarbock MR, Brinster RL. Maintenance of mouse male germ line stem cells in vitro. Biol Reprod. 2003;68(6):2207–2214. doi: 10.1095/biolreprod.102.014050. [DOI] [PubMed] [Google Scholar]
- 26.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2004;101(47):16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Western PS, Miles DC, van den Bergen JA, Burton M, Sinclair AH. Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells. 2008;26(2):339–347. doi: 10.1634/stemcells.2007-0622. [DOI] [PubMed] [Google Scholar]
- 28.Shinohara T, Avarbock MR, Brinster RL. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 1999;96(10):5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson R, Schaible K, Heasman J, Wylie C. Expression of the homophilic adhesion molecule, Ep-CAM, in the mammalian germ line. J Reprod Fertil. 1999;116(2):379–384. doi: 10.1530/jrf.0.1160379. [DOI] [PubMed] [Google Scholar]
- 30.Dix DJ, et al. Developmentally regulated expression of Hsp70-2 and a Hsp70-2/lacZ transgene during spermatogenesis. Dev Biol. 1996;174(2):310–321. doi: 10.1006/dbio.1996.0076. [DOI] [PubMed] [Google Scholar]
- 31.Rosario MO, Perkins SL, O’Brien DA, Allen RL, Eddy EM. Identification of the gene for the developmentally expressed 70 kDa heat-shock protein (P70) of mouse spermatogenic cells. Dev Biol. 1992;150(1):1–11. doi: 10.1016/0012-1606(92)90002-x. [DOI] [PubMed] [Google Scholar]
- 32.Kanatsu-Shinohara M, et al. Reconstitution of mouse spermatogonial stem cell niches in culture. Cell Stem Cell. 2012;11(4):567–578. doi: 10.1016/j.stem.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Henriksén K, Parvinen M. Stage-specific apoptosis of male germ cells in the rat: Mechanisms of cell death studied by supravital squash preparations. Tissue Cell. 1998;30(6):692–701. doi: 10.1016/s0040-8166(98)80088-8. [DOI] [PubMed] [Google Scholar]
- 34.Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122(9):2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- 35.Barakat B, O’Connor AE, Gold E, de Kretser DM, Loveland KL. Inhibin, activin, follistatin and FSH serum levels and testicular production are highly modulated during the first spermatogenic wave in mice. Reproduction. 2008;136(3):345–359. doi: 10.1530/REP-08-0140. [DOI] [PubMed] [Google Scholar]
- 36.Kanatsu-Shinohara M, Takashima S, Shinohara T. Transmission distortion by loss of p21 or p27 cyclin-dependent kinase inhibitors following competitive spermatogonial transplantation. Proc Natl Acad Sci USA. 2010;107(14):6210–6215. doi: 10.1073/pnas.0914448107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishii K, Kanatsu-Shinohara M, Toyokuni S, Shinohara T. FGF2 mediates mouse spermatogonial stem cell self-renewal via upregulation of Etv5 and Bcl6b through MAP2K1 activation. Development. 2012;139(10):1734–1743. doi: 10.1242/dev.076539. [DOI] [PubMed] [Google Scholar]
- 38.Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70(5):841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- 39.Kanatsu-Shinohara M, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69(2):612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 40.Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development. 2009;136(7):1191–1199. doi: 10.1242/dev.032243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costoya JA, et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36(6):653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- 42.Sada A, Suzuki A, Suzuki H, Saga Y. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science. 2009;325(5946):1394–1398. doi: 10.1126/science.1172645. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida S, et al. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol. 2004;269(2):447–458. doi: 10.1016/j.ydbio.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 44.Kusz K, et al. NANOS3 gene mutations in men with isolated sterility phenotype. Mol Reprod Dev. 2009;76(9):804. doi: 10.1002/mrd.21070. [DOI] [PubMed] [Google Scholar]
- 45.Romanienko PJ, Camerini-Otero RD. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell. 2000;6(5):975–987. doi: 10.1016/s1097-2765(00)00097-6. [DOI] [PubMed] [Google Scholar]
- 46.Liao HF, et al. DNMT3L promotes quiescence in postnatal spermatogonial progenitor cells. Development. 2014;141(12):2402–2413. doi: 10.1242/dev.105130. [DOI] [PubMed] [Google Scholar]
- 47.Bellvé AR, Millette CF, Bhatnagar YM, O’Brien DA. Dissociation of the mouse testis and characterization of isolated spermatogenic cells. J Histochem Cytochem. 1977;25(7):480–494. doi: 10.1177/25.7.893996. [DOI] [PubMed] [Google Scholar]
- 48.Bellvé AR, et al. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977;74(1):68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pichel JG, et al. GDNF is required for kidney development and enteric innervation. Cold Spring Harb Symp Quant Biol. 1996;61:445–457. [PubMed] [Google Scholar]
- 50.Moore MW, et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382(6586):76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 51.Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367(6461):380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 52.Enomoto H, et al. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21(2):317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- 53.Sánchez MP, et al. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382(6586):70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 54.Yang QE, Oatley JM. Spermatogonial stem cell functions in physiological and pathological conditions. Curr Top Dev Biol. 2014;107:235–267. doi: 10.1016/B978-0-12-416022-4.00009-3. [DOI] [PubMed] [Google Scholar]
- 55.Kanatsu-Shinohara M, et al. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72(4):985–991. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 56.Mullaney BP, Skinner MK. Growth factors as mediators of testicular cell-cell interactions. Baillieres Clin Endocrinol Metab. 1991;5(4):771–790. doi: 10.1016/s0950-351x(10)80014-x. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi S, et al. Effect of recombinant basic fibroblast growth factor (bFGF) on fibroblast-like cells from human rotator cuff tendon. Tohoku J Exp Med. 2002;198(4):207–214. doi: 10.1620/tjem.198.207. [DOI] [PubMed] [Google Scholar]
- 58.Oatley MJ, Racicot KE, Oatley JM. Sertoli cells dictate spermatogonial stem cell niches in the mouse testis. Biol Reprod. 2011;84(4):639–645. doi: 10.1095/biolreprod.110.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skinner MK, McLachlan RI, Bremner WJ. Stimulation of Sertoli cell inhibin secretion by the testicular paracrine factor PModS. Mol Cell Endocrinol. 1989;66(2):239–249. doi: 10.1016/0303-7207(89)90036-1. [DOI] [PubMed] [Google Scholar]
- 60.Skinner MK, Tung PS, Fritz IB. Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol. 1985;100(6):1941–1947. doi: 10.1083/jcb.100.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skinner MK, Fritz IB. Identification of a non-mitogenic paracrine factor involved in mesenchymal-epithelial cell interactions between testicular peritubular cells and Sertoli cells. Mol Cell Endocrinol. 1986;44(1):85–97. doi: 10.1016/0303-7207(86)90109-7. [DOI] [PubMed] [Google Scholar]
- 62.Hoeben E, Swinnen JV, Heyns W, Verhoeven G. Heregulins or neu differentiation factors and the interactions between peritubular myoid cells and Sertoli cells. Endocrinology. 1999;140(5):2216–2223. doi: 10.1210/endo.140.5.6712. [DOI] [PubMed] [Google Scholar]
- 63.Simon L, et al. Common and distinct factors regulate expression of mRNA for ETV5 and GDNF, Sertoli cell proteins essential for spermatogonial stem cell maintenance. Exp Cell Res. 2007;313(14):3090–3099. doi: 10.1016/j.yexcr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 64.De Gendt K, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA. 2004;101(5):1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang C, et al. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci USA. 2004;101(18):6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131(2):459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- 67.Lamberti D, Vicini E. Promoter analysis of the gene encoding GDNF in murine Sertoli cells. Mol Cell Endocrinol. 2014;394(1-2):105–114. doi: 10.1016/j.mce.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Spinnler K, Köhn FM, Schwarzer U, Mayerhofer A. Glial cell line-derived neurotrophic factor is constitutively produced by human testicular peritubular cells and may contribute to the spermatogonial stem cell niche in man. Hum Reprod. 2010;25(9):2181–2187. doi: 10.1093/humrep/deq170. [DOI] [PubMed] [Google Scholar]
- 69.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184(1):39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]