Significance
Inflammation is involved in the pathogenesis of many chronic diseases. Many deleterious effects of inflammation are mediated through increased production of proinflammatory mediators, known as cytokines and chemokines. Many current therapies for these diseases involve blocking single proinflammatory mediators, such as TNF, using parenteral administration of recombinant binding proteins. We demonstrate here that a genetic modification in the mouse that increases the expression of an endogenous antiinflammatory protein, tristetraprolin (TTP), results in protection against mouse models for several human inflammatory diseases, including rheumatoid arthritis, psoriasis, and multiple sclerosis, presumably by decreasing the production of proinflammatory cytokines. Our results suggest that increasing TTP expression may be an effective therapeutic strategy in the treatment of certain inflammatory diseases.
Keywords: AU-rich elements, mRNA stability, inflammation, deadenylation
Abstract
Tristetraprolin (TTP) is an inducible, tandem zinc-finger mRNA binding protein that binds to adenylate-uridylate–rich elements (AREs) in the 3′-untranslated regions (3′UTRs) of specific mRNAs, such as that encoding TNF, and increases their rates of deadenylation and turnover. Stabilization of Tnf mRNA and other cytokine transcripts in TTP-deficient mice results in the development of a profound, chronic inflammatory syndrome characterized by polyarticular arthritis, dermatitis, myeloid hyperplasia, and autoimmunity. To address the hypothesis that increasing endogenous levels of TTP in an intact animal might be beneficial in the treatment of inflammatory diseases, we generated a mouse model (TTPΔARE) in which a 136-base instability motif in the 3′UTR of TTP mRNA was deleted in the endogenous genetic locus. These mice appeared normal, but cultured fibroblasts and macrophages derived from them exhibited increased stability of the otherwise highly labile TTP mRNA. This resulted in increased TTP protein expression in LPS-stimulated macrophages and increased levels of TTP protein in mouse tissues. TTPΔARE mice were protected from collagen antibody-induced arthritis, exhibited significantly reduced inflammation in imiquimod-induced dermatitis, and were resistant to induction of experimental autoimmune encephalomyelitis, presumably by dampening the excessive production of proinflammatory mediators in all cases. These data suggest that increased systemic levels of TTP, secondary to increased stability of its mRNA throughout the body, can be protective against inflammatory disease in certain models and might be viewed as an attractive therapeutic target for the treatment of human inflammatory diseases.
Tristetraprolin (TTP) is the prototype of a small family of RNA binding proteins that can bind to adenylate-uridylate (AU)–rich elements (AREs) in the 3′-UTR (3′UTR) of its target mRNAs and promote their rapid turnover (1, 2). TTP-deficient mice developed a chronic systemic inflammatory syndrome (3) that was prevented by interfering with the action of TNF (3–5). Tnf mRNA was then identified as a direct target of TTP-mediated destabilization (4, 6); its increase in stability in the TTP KO mice leads to the commensurate overproduction of TNF protein (4, 5).
TTP mRNA expression exhibits a pattern characteristic of immediate-early response genes in several cell types, with low-to-undetectable levels of expression under basal conditions, and a rapid and transient induction upon stimulation (4, 7, 8). The transient nature of this induction is largely due to the instability of the TTP mRNA itself, part of which is thought to be due to AREs located within the 3′UTR of TTP mRNA (4, 8, 9). Indeed, TTP has been suggested to bind to its own AREs and autoregulate its expression through a negative feedback loop (9). Although expression of TTP protein in these systems is also rapidly inducible, the protein is more stable than the mRNA after induction, often persisting at high levels for several hours (7, 10).
The severe systemic inflammatory phenotype of the TTP KO mice identified TTP as an endogenous antiinflammatory protein. We have long wondered whether increasing endogenous levels of TTP in an intact animal might protect against the development of immune and inflammatory diseases. Early attempts to accomplish this using transgenic delivery of TTP resulted in embryonic lethality, presumably due to unregulated overexpression. To test our hypothesis in a different way, we generated a novel knock-in mouse model in which an instability motif in the 3′UTR of TTP mRNA was deleted in the mouse genome. We anticipated that, by deleting these instability elements, TTP mRNA would be stabilized under physiological conditions, and this would result in modest increases in TTP mRNA and protein levels that were still under the control of the endogenous genetic locus.
These mice, termed TTPΔARE mice, appear normal but exhibit increased TTP mRNA stability, as well as increased levels of TTP protein in their tissues. They were strikingly resistant to the development of three models of experimental immune-mediated inflammatory diseases. These data suggest that treatments leading to increased endogenous TTP levels could be a promising therapeutic approach in immune and inflammatory human diseases.
Results
Generation of TTPΔARE Mice.
TTPΔARE mice were generated as described in SI Materials and Methods. Briefly, 136 bases (bases 1564–1699 of GenBank accession no. NM_011756) (depicted in red in Fig. S1A), comprising an AU-rich region of the TTP mRNA 3′UTR, were deleted in the mouse genome. The WT allele, the targeting construct, and the mutant allele are shown schematically in Fig. S1B. The WT, heterozygous, and homozygous TTPΔARE mice could be identified readily by PCR genotyping of tail DNA (Fig. S1C).
Fig. S1.
Generation and characterization of TTPΔARE mice. (A) Shown is the Zfp36 (TTP) mRNA 3′UTR sequence from the stop codon to the end of the transcript; the polyadenylation signal is underlined. The segment deleted in the TTPΔARE allele is highlighted in red. (B) Schematic diagrams of the WT Zfp36 allele, the design of the targeting construct, and the targeted allele after Cre-based excision. The targeting construct contained the mutated exon 2 with a 136-base deletion in the 3′UTR. (C) PCR analysis of genomic DNA from WT, heterozygous, and homozygous TTPΔARE mice. The locations of the two primers (P1 and P2) used for genotyping are shown in B. (D) Body weights of male (n = 6) and female (n = 4) mice of the indicated genotypes at 8–12 wk of age. (E) Serum levels of triglycerides (Tgl), cholesterol (Chl), HDL, LDL, and (F) alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) (n = 4), all measured at 8–12 wk of age. Data are represented as mean ± SEM.
The body weights of adult (12-wk-old) TTPΔARE mice were similar to those of sex-matched WT littermates (Fig. S1D). Serum lipid levels were comparable between WT and TTPΔARE mice (Fig. S1E), as were the serum activities of several liver enzymes (Fig. S1F). Histopathological (Fig. S2) and immunohistochemical (Fig. S3) examination of major tissues revealed no obvious anatomical or cellular abnormalities in adult TTPΔARE mice. Similarly, complete blood counts (Table S1) and bone marrow cell counts (Table S2) did not reveal any evident abnormalities in adult TTPΔARE mice. These data suggest that genetic deletion of 136 bases in the TTP transcript did not result in any apparent physiological or developmental defects in the mice used in this study.
Fig. S2.
Histopathological analysis (H&E) of tissues of TTP∆ARE and WT mice. Representative images from each tissue are shown (n = 4).
Fig. S3.
Immunohistochemical analysis of liver, spleen, and thymus of TTP∆ARE and WT mice. The following primary antibodies were used to show the presence of various populations of immune cells: Ly6G: Neutrophils, CD3: T cells, CD45: Leucocytes, Pax5: B cells, and F4/80: Macrophages. Representative images from each tissue are shown (n = 4).
Table S1.
Evaluation of the peripheral blood counts (complete blood counts) of WT and TTPΔRE mice
| Complete blood counts | WT (n = 5) | TTPΔARE (n = 5) |
| RBC, 106/mL | 10.45 ± 0.23 | 10.74 ± 0.19 |
| Hemoglobin, g/dL | 15.46 ± 0.39 | 15.88 ± 0.27 |
| Hematocrit, % | 48.42 ± 1.36 | 50.74 ± 0.53 |
| Mean corpuscular volume, fL | 46.32 ± 0.31 | 47.30 ± 0.46 |
| Mean corpuscular hemoglobin, pg | 14.78 ± 0.05 | 14.80 ± 0.07 |
| Mean corpuscular hemoglobin concentration, g/dL | 31.92 ± 0.16 | 31.28 ± 0.30 |
| Reticulocytes, 106/μL | 0.45 ± 0.01 | 0.43 ± 0.02 |
| WBC, 103/μL | 9.42 ± 1.10 | 9.68 ± 0.98 |
| Neutrophils, 103/μL | 1.22 ± 0.62 | 2.06 ± 0.38 |
| Lymphocytes, 103/μL | 7.88 ± 0.87 | 7.18 ± 0.80 |
| Eosinophils, 103/μL | 0.20 ± 0.03 | 0.30 ± 0.03 |
| Monocytes, 103/μL | 0.10 ± 0.0 | 0.14 ± 0.02 |
| Platelets, 103/μL | 889.8 ± 21.5 | 812.6 ± 44.5 |
No P values were significant between the two groups.
Table S2.
Evaluation of the number of cells present in the bone marrows of WT and TTPΔRE mice
| Bone marrow differential cell counts | WT (n = 5) | TTPΔARE (n = 5) | P value |
| Erythrocyte maturation index | 0.11 ± 0.03 | 0.11 ± 0.007 | NS |
| Granulocyte maturation index | 0.54 ± 0.05 | 0.56 ± 0.02 | NS |
| Myeloid:erythroid precursors ratio | 2.06 ± 0.13 | 1.86 ± 0.20 | NS |
| Rubriblasts, % counts | 0.64 ± 0.13 | 0.86 ± 0.12 | NS |
| Prorubricytes, % counts | 1.48 ± 0.41 | 1.52 ± 0.22 | NS |
| Rubricytes, % counts | 11.28 ± 0.50 | 11.64 ± 0.83 | NS |
| Metarubricytes, % counts | 8.08 ± 1.14 | 9.0 ± 1.03 | NS |
| Myeloblasts, % counts | 0.36 ± 0.07 | 0.74 ± 0.16 | NS |
| Promyelocytes, % counts | 0.76 ± 0.15 | 1.04 ± 0.22 | NS |
| Neutrophilic myelocytes, % counts | 13.36 ± 1.08 | 11.40 ± 0.73 | NS |
| Neutrophilic metamyelocytes, % counts | 7.92 ± 0.71 | 9.90 ± 0.40 | 0.04 |
| Neutrophilic bands, % counts | 1.80 ± 0.15 | 2.50 ± 0.35 | NS |
| Neutrophilic segments (% counts) | 16.24 ± 1.34 | 12.74 ± 0.96 | NS |
| Eosinophilic myelocytes, % counts | 0.98 ± 0.21 | 1.58 ± 0.28 | NS |
| Eosinophilic metamyelocytes, % counts | 0.52 ± 0.15 | 0.84 ± 0.19 | NS |
| Eosinophilic bands, % counts | 0.56 ± 0.12 | 0.56 ± 0.16 | NS |
| Eosinophilic segments, % counts | 1.26 ± 0.23 | 0.54 ± 0.20 | 0.04 |
| Lymphocytes, % counts | 29.96 ± 2.14 | 31.86 ± 1.16 | NS |
| Monocytes, % counts | 1.96 ± 0.17 | 1.52 ± 0.32 | NS |
| Macrophages, % counts | 1.00 ± 0.25 | 0.90 ± 0.19 | NS |
| Megakaryocytes, % counts | 0.12 ± 0.04 | 0.08 ± 0.04 | NS |
| Plasma cells, % counts | 0.50 ± 0.09 | 0.22 ± 0.14 | NS |
| Mitotic figures, % counts | 1.16 ± 0.20 | 0.64 ± 0.14 | NS |
NS, not significant.
Effect of the TTPΔARE Mutation on TTP mRNA Expression, TTP mRNA Stability, and TTP Protein Expression in Cells and Mouse Tissues.
The effect of the homozygous TTPΔARE mutation on TTP mRNA expression was examined in cultured primary bone marrow-derived macrophages (BMDM). Under unstimulated conditions, TTP mRNA levels were increased approximately threefold in the TTPΔARE cells (Fig. 1A). They increased dramatically in cells of both genotypes after LPS stimulation, but TTP mRNA levels were significantly elevated in the TTPΔARE cells compared with WT cells at 3 h (Fig. 1B). To test TTP mRNA stability, we stimulated TTP gene transcription in BMDM with LPS, or mouse embryonic fibroblasts (MEF) with 10% (vol/vol) FBS, for 1 h in each case, followed by treatment with actinomycin D to inhibit transcription, and quantitated the remaining RNA at various times. Under these conditions, TTP mRNA degraded significantly more slowly in both the TTPΔARE BMDM (Fig. 1C) and the MEF (Fig. 1D). These data demonstrate the increased stability of the mutant TTP mRNA in these cell types.
Fig. 1.
TTP mRNA expression and stability, and TTP protein expression in primary cells and tissues derived from homozygous TTPΔARE mice. (A) Relative levels of TTP mRNA under basal (i.e., unstimulated) conditions in serum-deprived BMDMs (n = 3–4). (B) Time course of expression of TTP mRNA before and after stimulation (LPS; 1 µg/mL) in BMDMs. Data are expressed as a percentage of WT at 1 h (n = 3–4). (C) TTP mRNA decay in BMDMs and (D) MEFs. BMDMs or MEFs were stimulated with LPS (1 µg/mL) or 10% FBS (vol/vol), respectively, for 1 h, followed by treatment with actinomycin D. Percent remaining mRNA was measured by real-time RT-PCR (n = 4). The insets show semilogarithmic decay plots of the same data, analyzed by nonlinear regression. The approximate half-lives were: WT (BMDMs), ∼32 min; TTPΔARE (BMDMs), ∼80 min; WT (MEFs), ∼28 min; TTPΔARE (MEFs), ∼54 min. (E) TTP protein levels in BMDMs under basal and LPS- (1 µg/mL) stimulated conditions. Tubulin was used as a loading control. (F) TTP protein expression in mouse tissues. The lane labeled TTP KO contains an equal amount of protein from the respective TTP KO mouse tissue, included as a negative control. Actin was used as a loading control. Statistical analysis was performed by two-tailed Student’s t test for A, C, and D and by two-way ANOVA for B. Error bars represent SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
TTP protein was readily detectable in unstimulated conditions in the TTPΔARE BMDM but not in the WT BMDM (Fig. 1E). Furthermore, TTP protein levels were elevated compared with WT at all times after LPS stimulation in the TTPΔARE cells (Fig. 1E). Most importantly, TTP protein levels were increased in liver, spleen, and thymus from TTPΔARE mice (Fig. 1F).
Effect of the TTPΔARE Mutation on Potential TTP Targets in TTPΔARE BMDM and Intact Mice.
We next analyzed the expression of known or suspected TTP targets in LPS-stimulated BMDM. For Tnf, Il1b, and Cxcl2 mRNAs, the levels in the TTPΔARE cells were significantly lower than WT at 6 h, although there was a general pattern of decreased expression at 3 h as well (Fig. S4A). There were no significant differences at any time point for Il10, Il6, and Il23a mRNAs, although there was a trend toward decreased expression at 3 and 6 h in the TTPΔARE cells (Fig. S4A). In cultures of BMDM cells stimulated by LPS, IL-10 concentrations were significantly lower in the medium of TTPΔARE cells at both 8 and 24 h, whereas levels of IL-1B and IL-6 were unchanged (Fig. S4B). Surprisingly, levels of TNF and chemokine (C-X-C motif) ligand 2 (CXCL2) were significantly increased at 24 h in the medium of the TTPΔARE cells (Fig. S4B).
Fig. S4.
Expression of known or suspected TTP targets in LPS-stimulated BMDMs. (A) Cells were either left unstimulated or were stimulated with LPS (1 µg/mL), RNA was extracted, and real-time RT-PCR was performed using transcript-specific primers for Tnf, Il1b, Cxcl2, Il10, Il6, and Il23a mRNAs. Values were normalized to Gapdh mRNA, and the fold changes were calculated relative to basal levels (n = 4). Statistical analysis was performed by two-way ANOVA (**P < 0.01, ***P < 0.001). (B) Cells were either left unstimulated or were stimulated with LPS (1 µg/mL); cell culture supernatants were collected and assayed for the indicated cytokines using 5-plex Milliplex MAP mouse cytokine/chemokine magnetic bead panel (EMD Millipore). Data were normalized to the total RNA (n = 4). Statistical analysis was performed by an unpaired Student’s t test for each time point (*P < 0.05, **P < 0.01, ***P < 0.001).
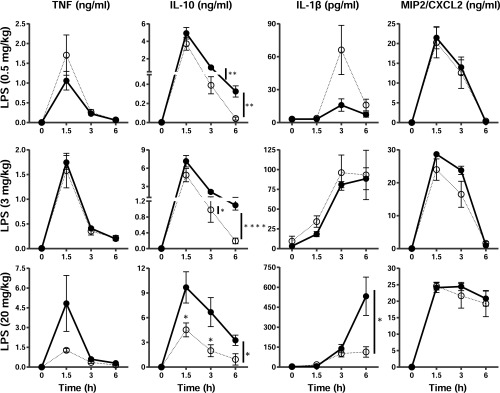
We then injected WT and TTPΔARE mice with three different doses of LPS and measured the serum levels of TNF, IL10, IL-1B, and CXCL2 in response. In myeloid cell-deficient TTP KO mice, the serum TNF response to LPS was a sensitive indicator of TTP deficiency, with serum levels increasing by more than 100-fold over WT after low-dose LPS injections (11). In the present study, after injection of LPS at 0.5, 3, or 20 mg/kg body weight, the average serum concentrations of TNF were not significantly different between the WT and the TTPΔARE mice, either during the peak increase at 1.5 h or at 3 and 6 h (Fig. S5). At the highest dose of LPS, there was a trend toward lower TNF levels at 1.5 h that did not achieve statistical significance. Serum levels of IL10 were no different between the two genotypes 1.5 h after 0.5 and 3 mg/kg LPS but were significantly lower at 1.5 h in the TTPΔARE mice injected with 20 mg/kg and decreased significantly more rapidly after that point with all three doses of LPS (Fig. S5). IL-1B levels were significantly lower in the TTPΔARE mice at 6 h after 20 mg/kg LPS, whereas the levels of CXCL2 did not differ significantly between the two genotypes at any of the three doses of LPS tested.
Fig. S5.
Expression of TNF, IL-10, IL-1B, and CXCL2 in mouse serum in response to LPS injections. WT and TTPΔARE mice were injected i.p. with LPS at 0.5, 3, or 20 mg/kg body weight, as indicated, and then blood was collected at the indicated times and serum was assayed for cytokines (n = 5 or 6). Closed circles with solid lines represent WT and open circles with dotted lines represent TTPΔARE. Statistical analysis was performed by two-tailed unpaired Student’s t test. Error bars represent SEM; *P < 0.05, **P < 0.01, ****P < 0.0001.
Effect of the TTPΔARE Mutation on Collagen Antibody-Induced Arthritis.
Because of the recent appreciation that TTP exerts modulatory effects on transcripts involved in several aspects of the immune system (3, 4, 12), we investigated the effect of the systemic TTP overexpression found in the TTPΔARE mice on the severity of certain models of immune and inflammatory diseases. We first evaluated the susceptibility of the TTPΔARE mice to collagen antibody-induced arthritis (CAIA). When the WT and TTPΔARE mice were taken through this protocol, the body weights of animals from both genotypes decreased in parallel after LPS; although the WT mice did not recover their original body weights, the TTPΔARE animals were able to gain back almost 90% (wt/wt) of their preinjection body weight by day 9 (Fig. 2A). Clinical arthritis scores (13) in the same animals demonstrated rapid increases from day 6 in the WT mice, but no or minimal clinical disease in the TTPΔARE mice (Fig. 2B). Of the 13 TTPΔARE animals that received the collagen antibody, 6 showed very minimal swelling on day 7, but none showed any external signs of redness, marked swelling, or ankylosis of the tarsal joints, all of which were observed in all 14 of the WT animals tested, from day 6 to day 9.
Fig. 2.
Effect of the homozygous TTPΔARE mutation on CAIA. (A) Shown are the percent body weight changes and (B) clinical arthritis scores over a period of 9 d of CAIA (n = 14, WT; n = 13,TTPΔARE). (C) Representative H&E-stained sections of tarsal joints from WT and TTPΔARE mice. Arrows indicate the presence of intense inflammatory synovial cellular infiltrates in and around the joint spaces in the WT mice, but not in the TTPΔARE mice. (D) Histopathology scores were determined for three lesions (i.e., subacute periarticular inflammation, synovial cell hyperplasia, and fibrosis). Severity was graded on a four-point scale. Mean severity scores are shown (n = 7). (E) Serum levels of G-CSF and (F) IL6 from days 0, 7, and 9 of CAIA induction (n = 7). Statistical analysis was performed by two-tailed Student’s t test at each time point for A, B, E, and F and each lesion in D. Error bars represent SEM; *P < 0.05, **P < 0.01.
Histopathological analysis of the paws and other limb joints showed signs of disease in all seven WT mice that were assessed, with more than three or four joints affected per mouse. In contrast, only two of the seven TTPΔARE mice showed histological signs of disease, with only one or two joints affected per mouse. The WT mice exhibited inflammatory cell infiltrates, particularly neutrophils with a few macrophages, in the joint space, as well as marked synovial hyperplasia, cartilage necrosis, bone erosion, and fibrosis; none of these lesions was present in the TTPΔARE mice (Fig. 2C). The histopathology scores for the four joints were consistently higher in WT mice compared with TTPΔARE mice, in which the four joints were almost entirely normal, with the exception of one joint in one mouse, which exhibited minimal signs of arthritis (Fig. 2D).
Serum cytokine analysis revealed significantly decreased levels of granulocyte-colony stimulating factor (G-CSF) and IL6 on day 7 following CAIA induction in the TTPΔARE mice (Fig. 2 E and F). The levels of 23 other cytokines/chemokines tested were found to be very low or undetectable. NanoString gene expression profiling (14) of the whole-joint RNA showed that 49 inflammatory transcripts (of the 248 total transcripts tested) were significantly down-regulated in the joints from the TTPΔARE mice compared with WT joints (Table S3). Of these 49, at least 5 are known or suspected TTP targets (2) (i.e., Ccl2, Ccl20, Cxcl1, Il1β, and Il6 mRNAs), and another 11 have typical TTP binding sites within their 3′UTRs. These data demonstrate that the homozygous TTPΔARE animals were largely protected against CAIA, possibly due to the relative down-regulation of inflammatory mediators.
Table S3.
Transcripts down-regulated in joints from TTPΔARE mice compared with joints from WT mice
| Transcript | WT (mean ± SEM) | TTPΔARE (mean ± SEM) | P value | Known or suspected TTP targets or transcripts with potential TTP binding sites |
| C1qa | 1,519 ± 154 | 890 ± 83 | 0.005 | |
| C1qb | 2,720 ± 326 | 1,613 ± 138 | 0.010 | |
| C3ar1 | 1,090 ± 104 | 579 ± 58 | 0.001 | One 9-mer in mouse |
| C4a | 2,289 ± 148 | 1,768 ± 110 | 0.018 | |
| C7 | 269 ± 32 | 165 ± 31 | 0.044 | |
| Ccl19 | 295 ± 49 | 151 ± 17 | 0.020 | |
| Ccl2 | 527 ± 105 | 115 ± 41 | 0.004 | Known or strongly suspected TTP target |
| Ccl20 | 11 ± 3.2 | 3.4 ± 1.2 | 0.048 | Known or strongly suspected TTP target |
| Ccl7 | 249 ± 48 | 48 ± 16 | 0.002 | |
| Ccl8 | 979 ± 162 | 319 ± 77 | 0.004 | |
| Ccr1 | 212 ± 39 | 80 ± 16 | 0.011 | |
| Ccr2 | 914 ± 172 | 297 ± 77 | 0.008 | One moderately conserved 7-mer |
| Cfb | 939 ± 142 | 510 ± 109 | 0.037 | |
| Cfl1 | 4,759 ± 468 | 3,456 ± 212 | 0.029 | |
| Cxcl1 | 61 ± 17 | 7.5 ± 3.3 | 0.012 | Known or strongly suspected TTP target |
| Cxcl10 | 40 ± 8.3 | 11.5 ± 4.4 | 0.013 | |
| Cxcl3 | 31.2 ± 9.9 | 2.0 ± 0.4 | 0.014 | One 9-mer highly conserved, one 8-mer moderately conserved |
| Cxcl5 | 356 ± 106 | 34.1 ± 11.1 | 0.013 | One 8-mer in mouse |
| Cxcr1 | 7.5 ± 2.0 | 2.8 ± 0.3 | 0.043 | |
| Cysltr1 | 65.1 ± 13.5 | 27.5 ± 7.4 | 0.034 | |
| H2-Eb1 | 2,228 ± 295 | 1,142 ± 165 | 0.009 | |
| Hdac4 | 524 ± 46 | 296 ± 9.7 | 0.0008 | |
| Il10rb | 538 ± 54 | 378 ± 19 | 0.019 | |
| Il1b | 420 ± 97 | 102 ± 29 | 0.011 | Known or strongly suspected TTP target |
| Il1r1 | 962 ± 118 | 527 ± 42 | 0.006 | Two 7-mers moderately conserved |
| Il6 | 38 ± 12 | 5.6 ± 2.1 | 0.027 | Known or strongly suspected TTP target |
| Itgb2 | 969 ± 115 | 671 ± 36 | 0.034 | One 8-mer moderately conserved |
| Limk1 | 128 ± 4.2 | 104 ± 5.1 | 0.004 | |
| Mafg | 229 ± 6.0 | 174 ± 18 | 0.017 | One 7-mer highly conserved |
| Map3k5 | 249 ± 14 | 184 ± 21 | 0.029 | |
| Mmp3 | 4,465 ± 1061 | 786 ± 221 | 0.006 | |
| Mmp9 | 2,339 ± 420 | 576 ± 69 | 0.002 | One 9-mer in mouse |
| Mrc1 | 1,073 ± 129 | 700 ± 85 | 0.037 | One 7-mer moderately conserved, one 8-mer in mouse, rat |
| Myd88 | 331 ± 42 | 192 ± 19 | 0.013 | |
| Oas1a | 115 ± 18 | 67.7 ± 9.8 | 0.041 | |
| Oas2 | 34.8 ± 6.3 | 8.2 ± 3.5 | 0.004 | |
| Pdgfa | 517 ± 27.9 | 412 ± 32.4 | 0.034 | |
| Plcb1 | 140 ± 8.8 | 93.2 ± 15.3 | 0.024 | One 8-mer, one 7-mer both highly conserved |
| Ptger4 | 105.4 ± 8.8 | 60.6 ± 13.0 | 0.017 | One 7-mer, one-8 mer both highly conserved |
| Stat2 | 211.4 ± 13.4 | 158.6 ± 16.9 | 0.034 | |
| Stat3 | 1,605 ± 108.9 | 1,223 ± 47.5 | 0.009 | |
| Tgfbr1 | 681.9 ± 53.8 | 520.1 ± 41.4 | 0.038 | |
| Tlr1 | 106.6 ± 18.9 | 26.3 ± 11.5 | 0.004 | |
| Tlr2 | 166.6 ± 22.4 | 59.3 ± 15.4 | 0.002 | |
| Tlr6 | 87.5 ± 12.2 | 53.6 ± 8.4 | 0.046 | |
| Tlr8 | 194.7 ± 28.2 | 109.3 ± 18.7 | 0.030 | |
| Traf2 | 173.9 ± 12.5 | 126.7 ± 11.5 | 0.020 | |
| Trem2 | 214.0 ± 24.0 | 112.0 ± 7.7 | 0.002 | |
| Tyrobp | 3,747 ± 500 | 2,085 ± 169 | 0.010 |
Shown are the means ± SEM of the normalized counts obtained for the 49 transcripts (of the 248 measured) that were significantly down-regulated in the TTPΔARE compared with WT mice in the NanoString assay. Statistical analysis was performed by two-tailed unpaired Student’s t test. The rightmost column indicates either that a particular transcript has been demonstrated or strongly suggested as a TTP target transcript in previous studies, or contains potential TTP binding sites [(7-mer (UAUUUAU), 8-mer (UAUUUAUU or UUAUUUAU) or 9-mer (UUAUUUAUU)] in its 3′UTR. Also indicated is the conservation of these potential binding sites in other mammals.
Imiquimod-Induced Dermatitis in WT and TTPΔARE Mice.
We next evaluated the response to imiquimod (IMQ)-induced dermatitis, a commonly used model of human psoriasis (15). IMQ-containing cream was applied daily for five consecutive days to the shaved backs of 7- to 9-wk-old mice. Clinical signs of psoriasis-like dermatitis, particularly erythema, scaling, and thickening, were observed by approximately day 2 or 3. The increase in skinfold thickness was significantly lower in the TTPΔARE mice than in the WT mice (Fig. 3A).
Fig. 3.
Inflammation in IMQ-induced dermatitis. (A) Increase in skinfold thickness, measured as the difference between skin fold thickness on day 0 and day 6 (n = 7–8). (B) Representative H&E staining of untreated (Left, control) or IMQ-treated (Middle and Right, IMQ) skin from mice of the indicated genotypes. a, acanthosis; d, dermal infiltration; pu, pustule. The right panel shows higher-power magnifications of the inset in the middle panel, demonstrating more infiltrating cells in the WT compared with the TTPΔARE section. (C) Shown are Ly6G immunostained sections from control (Left) and IMQ-treated (Right) mice of the indicated genotypes (n = 4–5). (D) Ly6G positive cells in Fig. 4C were quantitated as the number of cells in one high-power field (400×) in an area of maximal infiltration from each animal (n = 4–5). (E) Histopathology scores were determined for five lesions, namely, epidermal hyperplasia, hyperkeratosis, parakeratosis, parakeratotic inflammation, and dermal inflammation. Severity was graded on a four-point scale. Each mean score is depicted by a black line and each point represents data from one animal (n = 7–8). (F) NanoString gene expression analysis was performed on RNA isolated from affected skin. Shown are the normalized counts for the 11 transcripts that were significantly down-regulated in the TTPΔARE group compared with the WT group (n = 6). Statistical analysis was performed by two-tailed unpaired Student’s t test for A, D, E, and F. Error bars represent SEM; *P < 0.05, **P < 0.01.
Skin pathology resembling psoriasis, particularly thickening of the epidermis (acanthosis and hyperkeratosis), was observed in both the WT and the TTPΔARE mice; however, cellular infiltration into the dermis was significantly decreased in the TTPΔARE mice (Fig. 3B, Middle and Right). Moreover, epidermal neutrophilic abscesses/pustules were absent in the TTPΔARE mice (Fig. 3B, Middle). In addition, neutrophil infiltration into the dermis was markedly decreased in the TTPΔARE mice (Fig. 3 C and D). Overall, the WT mice showed more severe epidermal hyperplasia, parakeratosis, and dermal inflammation, whereas the TTPΔARE mice showed more pronounced hyperkeratosis (Fig. 3E). NanoString gene expression profiling of day-6 skin RNA demonstrated 11 transcripts that were significantly down-regulated in TTPΔARE compared with WT skin (Fig. 3F). Four of the 11 transcripts are known or strongly suspected TTP targets (i.e., Cxcl1, Il12b, Il1a, and Myc). The remaining seven, C1qa, Cxcl3, Cxcr1, Cysltr1, Il1r1, Ptgir, and Tnfaip3, although not known to be direct TTP targets, also play critical roles in inflammation. These results suggest that the TTPΔARE mice, although still susceptible to IMQ-induced dermatitis, exhibited significantly decreased inflammation compared with WT mice.
Effect of the TTPΔARE Mutation on Experimental Autoimmune Encephalomyelitis.
Finally, we assessed the effect of the TTPΔARE mutation on one type of experimental autoimmune encephalomyelitis (EAE), a mouse model for human multiple sclerosis (16). We used the C57BL/6-MOG35-55 version of EAE, which has been described recently as the “gold standard” animal model for multiple sclerosis (17). In this experiment, the entire population of WT (n = 11) and homozygous TTPΔARE (n = 10) mice was given the encephalitogenic stimulus without knowledge of their genotype, with the genotype code broken only at the end of the experiment. WT mice exhibited progressive weight loss beginning on approximately day 12, whereas the TTPΔARE mice did not lose body weight, on average (Fig. 4A). The WT mice, but not the TTPΔARE mice, began exhibiting clinical signs of EAE on approximately day 10. By day 14, 8 of the 11 WT mice exhibited clinical signs of EAE, and an additional WT mouse was killed because of a maximal clinical score of 5.0 on day 13. In contrast, none of the 10 TTPΔARE mice exhibited clinical signs of EAE by day 14. By day 20, another WT mouse was killed because it reached a near-maximal clinical score, and the remaining nine of nine mice exhibited severe clinical signs (clinical scores between 2.0 and 4.0) of EAE. Four of the 10 TTPΔARE mice also exhibited clinical signs of EAE by day 20; however, the signs exhibited by TTPΔARE mice were of mild severity (clinical score between 0.5 and 2.0) (Fig. 4B). Another WT mouse was killed on day 27. By day 30, 8 of the original 11 WT mice remained, all of which exhibited clinical signs of severe EAE, but 10 of the 10 original TTPΔARE mice remained, of which only 5 mice exhibited clinical signs of mild EAE. The mean cumulative disease scores and the mean maximal disease scores for the TTPΔARE mice were significantly lower than those of the WT mice (Fig. 4C). These data demonstrate that the TTPΔARE mice were markedly resistant to the induction of this model of EAE compared with the WT mice.
Fig. 4.
Effect of the TTPΔARE mutation on EAE. (A) Shown are means ± SEM of the percent body weight loss and (B) clinical disease scores observed over a period of 30 d during the course of EAE (n = 11,WT; n = 10,TTPΔARE). (C) Shown are data on the prevalence, the cumulative disease scores (mean and median), and the maximal disease scores (mean and median) for the WT and TTPΔARE groups. Cumulative EAE scores were calculated by summing daily scores for each mouse across the designated time course of disease. Maximal scores were calculated as the most severe EAE score for each mouse. Mice that did not exhibit EAE had a score of zero for the cumulative and maximal scores, and these scores were included in the group average. Pairwise comparisons were analyzed by two-tailed t tests. Cumulative scores, P = 0.003; maximal scores, P = 0.0018.
SI Materials and Methods
Generation of TTPΔARE Knock-in Mice.
TTPΔARE knock-in mice containing a 136-bp (1564–1699; GenBank accession no. NM_011756) deletion in the 3′UTR of Zfp36 locus were generated at Ozgene using standard embryonic stem cell targeting techniques. The targeting construct was generated from C57BL/6 genomic DNA and cloned into the Ozgene PacF vector containing a PGK-neomycin selection cassette flanked by Flp recombinase target sites. The targeting vector (Fig. 1B) contained a WT exon 2 and the 5′ loxP site, a 5′ homology arm containing PshAI and NsiI sites for genomic screening, and a 3′ homology arm containing the mutant (136-bp deletion) exon 2, the 3′ loxP site and PvuII and EcoRV sites for genomic screening. All of the fragments in the targeting vector were generated by PCR amplification from C57BL/6 genomic DNA and confirmed by restriction enzyme digestion and sequencing. C57BL/6 ES cells were electroporated with the PmeI linearized targeting vector. Correctly targeted ES cells were identified by Southern analysis using a 5′ probe with a PshAI digestion and a 3′ probe with a PvuII digestion. Both probes lie outside the targeting vector. A neo probe with XbaI digestion was used to identify random integrations. Deletion of the PGK-neomycin cassette and the WT exon 2 was performed at Ozgene through breeding with a Cre deleter mouse. Mice produced from breeding with a Cre deleter mouse were heterozygous knock-in for the mutant exon 2 containing the 136-bp deletion. Heterozygous mice were bred to Taconic C57BL/6N mice to remove the Cre transgene and then intercrossed to generate homozygous knock-in mice for the mutant exon 2. Offspring were routinely genotyped by PCR using primer 1 (P1), 5′-CGTCTCCCCATCTTCAATCGT-3′ and primer 2 (P2), 5′-CAACCCCCCCCAAAAAATAGA-3′. These primers amplified a 780-bp endogenous Zfp36 WT allele and a 644-bp mutant Zfp36 knock-in (containing mutant exon 2) allele (Fig. 1C). PCR conditions were: 93 °C for 2 min followed by 30 cycles of 93 °C for 30 s, 58 °C for 30 s, and 72 °C for 1 min, with a final extension of 72 °C for 10 min. All experiments were performed on homozygous knock-in mice (TTPΔARE homozygotes) for the mutant exon 2. The controls used were littermate C57BL/6N mice obtained either from intercrossing TTPΔARE/+ heterozygous mice or C57BL/6N WT mice (obtained from the previous TTPΔARE/+ heterozygous matings).
Mouse Disease Models.
CAIA was induced in male mice between 8–12 wk of age by injecting them with 5 mg of an arthritogenic anti-type II collagen mAb mixture by i.v. (3 mg) and i.p. injections (2 mg) on day 0. On day 3, the mice received an i.p. injection of 50 µg of Escherichia coli-derived LPS. Mice were weighed and monitored daily, followed by killing on day 9, and paws and joints were collected for RNA and histology. Clinical scores were determined for each paw based on the A13 scale (13). Scores from four paws were added to produce a final score for each mouse.
For the induction of IMQ-induced dermatitis, male mice between 7–9 wk of age received a daily dose of 62.5 mg of commercially available Aldara cream containing 5% (wt/wt) IMQ on the shaved dorsal skin for five consecutive days, as described before (36). Control mice were shaved but otherwise left untreated. Skinfold thickness was measured with digital vernier calipers. Tissue was collected for analysis on day 6.
EAE was induced as described previously (37). Briefly, male and female mice between 17–18 wk of age were immunized with 200 µg MOG35–55 in CFA emulsion by s.c. injections across the lower back. Each mouse received three separate injections (∼33 µL per injection) for a total injection volume of 100 µL per mouse. These mice also received 200 ng of pertussis toxin by i.p. injection, at the time of immunization and 2 d postimmunization. Animals were monitored for loss of body weight and clinical signs associated with EAE daily through day 30. The scale used to score the clinical signs of classical EAE has been described previously (37).
Reagents.
LPS (E. coli serotype 055:B5) and actinomycin D were purchased from Sigma-Aldrich. Collagen antibody was purchased from Chondrex Inc. IMQ cream [5% (wt/wt)] (Medicis Pharmaceutical Corp.) was obtained from Triangle Compounding Pharmacy. Synthetic MOG35–55 peptide was obtained from The University of North Carolina Microprotein Sequencing & Peptide Synthesis Facility. Incomplete Freund’s adjuvant and heat-killed Mycobacterium tuberculosis H37Ra were obtained from BD Diagnostic Systems. Pertussis toxin was purchased from EMD Millipore.
Cell Culture and Treatments.
BMDMs.
Littermate WT and TTPΔARE mice, either males or females, between the ages of 8–12 wk were killed by CO2 inhalation, and bone marrow cells were isolated from the femurs as described before (11). Briefly, cells were cultured in RPMI medium 1640 (Life Technologies) supplemented with 10% (vol/vol) FBS (HyClone), 15 mM Hepes, 2 mM l-glutamine (Life Technologies), 100 U/mL penicillin (Life Technologies), and 100 µg/mL streptomycin (Life Technologies), along with 30% (vol/vol) L929 cell conditioned medium. Culture medium was replaced with fresh medium every 3 d. Macrophages were fully differentiated by day 8–10, at which time they were harvested and seeded onto either 100-mm or 60-mm cell culture dishes for subsequent experiments. A day before the experiment, the cells were subjected to serum starvation with RPMI medium 1640 containing only 1% FBS (vol/vol), and were kept in this medium for at least 16 h followed by stimulation with 1 µg/mL LPS (E. coli serotype 055:B5; Sigma) for the indicated times.
MEFs.
Mice heterozygous for the KI allele were crossed to generate embryos of all three possible genotypes, that is, WT, heterozygous TTPΔARE, or homozygous TTPΔARE. Primary MEFs were isolated from embryos at day 15.5 of gestation. Tail DNA from the embryos was used to determine the genotype of each embryo. MEFs were isolated as described before (11) and were maintained in DMEM (Life Technologies) containing 10% (vol/vol) FBS (HyClone), 100 U/mL penicillin (Life Technologies), 100 µg/mL streptomycin (Life Technologies), and 2 mM l-glutamine (Life Technologies). Cells were used between passages two and four. One day before the experiment, MEFs at ∼60–70% confluence were subjected to serum starvation with DMEM containing only 0.5% FBS (vol/vol) for at least 16 h, after which the cells were stimulated with fresh DMEM containing 10% FBS (vol/vol) for the indicated times.
mRNA Decay Assays.
BMDMs or MEFs were serum-starved overnight, stimulated with LPS (1 µg/mL) or FBS (10% vol/vol), respectively, for 1 h, followed by addition of actinomycin D (5 µg/mL) to inhibit transcription for the indicated times. Total RNA was isolated, cDNA was synthesized, and the transcript abundance was determined by real-time quantitative RT-PCR.
RNA Isolation and Quantitative RT-PCR.
Total cellular RNA from BMDMs or MEFs was isolated using the GE Healthcare Illustra RNAspin MiniRNA Isolation Kit according to the manufacturer’s instructions (GE Healthcare). The RNA content and purity were determined by measuring absorbance at 260 and 260/280 nm, respectively, on Tecan’s Infinite 200 PRO NanoQuant using i-control software (Tecan). Transcript levels for TTP and cytokines were determined by real-time quantitative RT-PCR. Briefly, first-strand cDNAs were synthesized using oligo(dT)12–18 primers and SuperScript III Reverse Transcriptase (Invitrogen). Quantitative real-time RT-PCR was performed using either SYBR Green or the predesigned primer-probe sets available from Applied Biosystems on the ABI Prism 7900 Sequence Detection System (Applied Biosystems). The ct values were normalized to Gapdh mRNA internal controls. The expression levels were calculated according to the 2−ΔΔct method (38). To amplify cDNA of mouse Il1b, Cxcl2, Il6, and Il23a, we used TaqMan Universal PCR Master Mix (Applied Biosystems) and predesigned TaqMan assays for the Il1b transcript (FAM/MGB probe Mm01336189_m1), the Cxcl2 mRNA (FAM/MGB probe Mm00436450_m1), the Il6 mRNA (FAM/MGB probe Mm00446190_m1), the Il23a mRNA (FAM/MGB probe Mm00518984_m1), and a mouse Gapdh mRNA control (catalog no. 4352932E), according to the manufacturer’s instructions. Real-time PCR for Tnf and Il10 transcripts was performed using SYBER Green qPCR Master Mix and primer pairs: Tnf, forward 5′-CCCTCACACTCAGATCATCTTCT-3′, reverse 5′-GCTACGACGTGGGCTACAG-3; Il10, forward 5′-GCTCTTACTGACTGGCATGAG-3′, reverse 5′-CGCAGCTCTAGGAGCATGTG-3′.
Cell/Tissue Lysis and Immunoblotting.
Cells and tissues were lysed in RIPA buffer [50 mM Tris (pH 8.0), 150 mM NaCl, 1% Nonidet P-40, 5 mM EDTA, and 5% (vol/vol) glycerol] containing Roche protease inhibitor mixture (Roche Diagnostics) and phosphatase inhibitors (10 mM sodium fluoride and 1 mM sodium orthovanadate). Cells were scraped and tissues were mechanically homogenized using a Tekmar Tissumizer. Insoluble material was removed from both cell and tissue lysates by centrifugation (13,000 × g, 10 min, 4 °C) and the protein concentration of the supernatants was determined using the Bradford assay (Bio-Rad). Equivalent amounts of denatured protein were separated on 10% Criterion Tris⋅HCl precast gels (Bio-Rad) and transferred onto nitrocellulose membranes. Membranes were then incubated overnight at 4 °C with a rabbit antiserum raised against a recombinant mouse TTP–maltose binding protein fusion (10) or its preimmune serum, followed by an incubation with horseradish peroxidase-conjugated goat anti-rabbit IgG (Bio-Rad). SuperSignal West Pico chemiluminescent substrate (Pierce) was used as a detection reagent.
Histopathology.
CAIA.
Paws and joints fixed in 10% (vol/vol) neutral-buffered formalin were decalcified in Decal STAT (Decal Chemical Corp.) for 1–2 h and embedded in paraffin. Sections were stained with H&E and examined by light microscopy. Histological analyses of bone, cartilage, and synovium was performed and compared between the saline-injected and collagen antibody-injected animals and within the two genotypes (i.e., WT and TTPΔARE). Subacute periarticular arthritis, synovial cell hyperplasia, and fibrosis were assessed in all of the joints available per limb. The severity of arthritis was graded on a scale of 1–4 as follows: 1 = minimal (1–5%), 2 = mild (6–30%), 3 = moderate (31–80%), and 4 = marked (above 80%) based on the percent involvement of joint affected. Severity was based on the observations made in two sections per paw.
IMQ-induced dermatitis.
Paraffin blocks of skin sections were prepared using routine methods. Sections were prepared and stained with H&E and examined by light microscopy in a blinded fashion. The lesions noted were epidermal hyperplasia, hyperkeratosis, parakeratosis, parakeratotic inflammation, and dermal inflammation. Severity of the lesions was graded on a scale of 1–4 as follows: 1= minimal, 2 = mild, 3 = moderate, and 4 = marked. Severity was based on the observations made in two sections per tissue.
Immunohistochemical Staining of Skin Sections for Ly6G.
Formalin-fixed, paraffin-embedded tissue sections were deparaffinized in xylene and rehydrated through graded ethanol. Enzyme-induced epitope retrieval was performed using Carezyme II for 3 min at 37 °C. Endogenous peroxidase blocking was done by immersing the sections in 3% (vol/vol) H2O2 for 15 min. Nonspecific sites were blocked by incubating slides with 10% normal rabbit serum (Jackson ImmunoResearch) for 20 min, and endogenous biotin and avidin binding sites were blocked using the avidin-biotin blocking kit (Vector Laboratories). The sections were then incubated with rat polyclonal anti-Ly6G primary antibody (BioLegend) at a dilution of 1:500 for 60 min at room temperature, followed by biotin-linked secondary rabbit anti-rat IgG antibody (Vector Laboratories) at a dilution of 1:500 for 30 min at room temperature. Labeling was done with the Vectastain RTU Kit Label (Vector Laboratories) for 30 min at room temperature and the antigen–antibody complex was visualized using 3-diaminobenzidine chromogen (Dako). Quantification of staining was performed by a pathologist in a blinded fashion. Semiquantitative scoring was performed on a scale of 0–4. Both the local intensity of the infiltration and the uniformity and diffuseness of cell infiltrates within the dermis were taken into account while scoring. Scoring was based on an assessment of the total number of Gr1+ cells in the dermis, epidermis, and parakeratotic material.
Analysis of Serum Cytokine Levels.
Blood was obtained through retroorbital bleeding on days 0, 7, and 9 for the CAIA study and at basal, 1.5 h, 3 h, and 6 h for LPS-induced endotoxemia. Serum was prepared and stored at −20 °C until use. Cytokines/chemokines were measured using either a 25-plex or a 5-plex Milliplex MAP mouse cytokine/chemokine magnetic bead panel (EMD Millipore) with a reader (LiquiChip 200; Qiagen) and the Luminex XPonent 3.1 software (Luminex Corp.) for data analysis.
NanoString Analysis.
Gene expression analysis was carried out using a preassembled CodeSet that measures 248 inflammation related mouse transcripts (nCounter GX Mouse Inflammation Kit v2 152061), using the NanoString nCounter Analysis System (NanoString Technologies). Analysis and normalization of the raw NanoString data were conducted using nSolver Analysis Software 2.0. Raw counts were normalized to six internal reference transcripts: Cltc, Gapdh, Gusb, Hprt, Pgk1, and Tubb5 mRNAs. Background counts were estimated using the average counts of eight negative control probes in every reaction.
Statistical Analysis.
All values are represented as mean ± SEM. Data were analyzed and statistics performed using GraphPad Prism software using Student’s unpaired two-tailed t test (for comparing two groups) and ANOVA (for comparing three or more groups). P values of less than 0.05 were considered significant.
Discussion
The severe inflammatory syndrome seen in the TTP KO mice led to the identification of Tnf mRNA as a major target of TTP (4, 6). As a result, the emphasis in most follow-up studies has been on the involvement of TTP in innate immunity. More recently, several studies have highlighted the importance of other aspects of the immune response in the pathogenesis of the TTP deficiency syndrome. For example, mice in which TTP was deleted only from myeloid cells did not exhibit the TTP deficiency syndrome, although they were hypersensitive to small amounts of injected LPS (11), suggesting the involvement of other cell types in the development of the syndrome. In another study, genetic ablation of genes in the IL17/IL23 axis protected mice against the development of the TTP deficiency syndrome, even in the setting of normal TNF pathways and responses (12). These recent studies highlight the concept that TTP deficiency in an intact animal affects many arms of the immune system and is a true systemic disease.
Since the earliest discovery of TTP as an endogenous antiinflammatory protein, we have been interested in the concept of attempting to increase levels or activities of TTP in animals, and potentially in humans, as a possible treatment for inflammatory conditions. Our initial attempts to accomplish this involved transgenic mice in which TTP was overexpressed using strong general promoters; these invariably led to embryonic lethality. In a different approach to increasing TTP expression without loss of its normal physiological regulation, we developed the knock-in TTPΔARE mouse described in this study, in which an ARE instability element was genetically removed from TTP mRNA. Our hope was that this germ-line mutation would lead to stabilization of TTP mRNA and overexpression of the protein throughout the body, without preconceived biases about which cell types should be preferentially targeted. Similar genetic approaches to the stabilization of ARE-containing mRNAs have been used in the cases of TNF (18) and IFN-γ (19), among others.
Remarkably, the TTPΔARE mice appeared phenotypically normal, despite elevated and stabilized TTP mRNA in cells derived from them, and increased levels of TTP protein in several tissues. We then tested the hypothesis that the increased “whole-body” levels of TTP might protect the mice against inflammation in four distinct experimental models that involved different aspects of the immune system. First, we tested the response of the TTPΔARE mice to LPS endotoxemia. Somewhat surprisingly, the TTPΔARE mice did not exhibit decreased levels of TNF in the serum after the i.p. injection of three different doses of LPS. However, serum levels of IL10, whose mRNA is also a known TTP target (20), were lower in the mutant mice than in the WT mice at the highest LPS dose and returned more rapidly to baseline levels in the TTPΔARE mice than in the WT mice at all LPS doses. These results suggest that the acute TNF response to LPS was not affected by further increasing endogenous levels of TTP, which are already massively induced in myeloid cells by LPS in parallel with TNF (11).
The second disease model tested was CAIA, often used as an experimental model of human rheumatoid arthritis (21). We found that TTPΔARE mice were essentially completely protected against the development of CAIA. The mechanism of this protection is likely to involve the decreased stability of proinflammatory cytokine mRNAs in many cells and tissues. For example, we found that serum levels of G-CSF and IL-6 were significantly reduced in TTPΔARE mice 7 d after the start of the CAIA protocol. Il-6 mRNA has been suggested previously to be a target of TTP (22), whereas, to our knowledge, Csf1 mRNA, encoding G-CSF, has not been identified as a direct target. However, the 3′UTR of Csf1 mRNA in the mouse contains two sequence elements that form the core of ideal TTP binding sites, UAUUUAU, and several other closely related sequences; many of these, including the two core 7-mers, are widely conserved among mammals, including humans. G-CSF levels have been shown to be elevated in TTP KO mouse plasma (23), and G-CSF (24) KO mice are protected from collagen-induced arthritis. G-CSF is known for its role in maintaining homeostatic levels of granulocytes (25), and G-CSF administration results in neutrophil production (26). G-CSF also enhances neutrophil trafficking in the synovium, and neutrophil depletion arrests the progression of arthritis (27), suggesting that G-CSF plays a critical role in mediating inflammatory arthritis by promoting the trafficking of neutrophils into the synovium. It seems reasonable to suggest that, among other proinflammatory pathways affected by the increased levels of TTP, the decreased levels of G-CSF may have resulted in decreased manifestations of CAIA.
Gene expression profiling of whole-joint RNA revealed decreases in the levels of at least five known or suspected TTP targets in the TTPΔARE mice, whereas at least 11 other significantly down-regulated transcripts contained potential TTP binding sites and encode proteins that play critical roles in inflammation. The remaining 33 down-regulated transcripts encode components of the complement pathway, toll-like receptor signaling, cytokines/chemokines and their receptors, and enzymes that break down extracellular matrix, supporting the idea that increased levels of TTP can directly and perhaps indirectly regulate immune response transcripts and protect against inflammation.
We also explored the response of the TTPΔARE mice to IMQ-induced dermatitis. We found that the TTPΔARE mice had less severe epidermal hyperplasia and strikingly reduced neutrophil infiltration. A previous study demonstrated that neutrophil infiltration was considerably reduced in IMQ-induced dermatitis in Il1r1 KO mice (28). Interestingly, neutrophil depletion has been shown to ameliorate the severity of disease in this model of psoriasis (29), and clinical studies have demonstrated disease remission by drug-induced agranulocytosis, supporting a critical pathogenic role of neutrophils in psoriasis (30). We found that Il1r1 mRNA expression was significantly reduced in skin from the TTPΔARE mice, suggesting that decreased IL-1R1 signaling may have resulted in reduced dermal neutrophilic infiltration. Four other known or suspected TTP targets were also decreased in the TTPΔARE mice skin: Cxcl1, Il12a, Il1a, and Myc mRNAs, and seven additional significantly reduced transcripts, including C1qa, Cxcl3, Cxcr1, Cysltr1, Il1r1, Ptgir, and Tnfaip3 mRNAs, encode important proinflammatory proteins. These data support the idea that increased TTP levels exert a beneficial effect on the course of this disease model by targeting elevated levels of certain cytokines, some of which are direct TTP targets and others that may represent indirect responders.
The fourth disease model tested was EAE (16). Many types of observation have suggested critical roles for cytokines and chemokines, many of whose transcripts are TTP targets, in the pathogenesis of both EAE and human multiple sclerosis (31, 32). Similar to the results obtained with CAIA, the homozygous TTPΔARE mice were strikingly resistant to active EAE induction, presumably due to decreased expression of inflammatory mediators in the blood and CNS following immunization.
Taken together, our results support the hypothesis that moderately elevated levels of TTP throughout the body can have beneficial effects in some mouse models of immune and inflammatory disease. Current therapy of several human diseases of this type involves antibody-based molecules that bind directly to the relevant cytokine, such as anti-TNF compounds (33); these are produced by expensive recombinant DNA techniques and, because of their protein nature, require parenteral administration. Our data support the concept that therapies aimed at increasing levels or activities of TTP in cells throughout the body might benefit patients with these conditions. One possible approach to this is gene therapy; indeed, a previous study demonstrated protective effects of adenovirus-delivered TTP in an experimental periodontitis model (34). Other approaches might involve cell-based therapies, such as hematopoietic stem cell transplantation of genetically modified cells (35). Ideally, small molecules could be identified that, when administered orally, could cause elevations of TTP throughout the body. If such compounds could be identified that had minimal toxicity, it seems reasonable to suggest that they might be useful therapeutically in the treatment of certain chronic inflammatory diseases.
Materials and Methods
TTPΔARE knock-in mice with a 136-bp deletion of an AU-rich region of the Zfp36 3′UTR were generated at Ozgene using C57BL/6 embryonic stem cells and standard targeting techniques. Adult male homozygous mutant mice were injected intraperitoneally with three different doses of LPS as acute models of inflammation. They were also subjected to the following models of inflammatory disease: CAIA (13), IMQ-induced dermatitis (36), and the myelin oligodendrocyte glycoprotein MOG35-55 peptide-induced model of EAE (37). Detailed materials and methods are contained in SI Materials and Methods.
All animal procedures were conducted according to US Public Health Service policy on the humane care and use of laboratory animals. The National Institute of Environmental Health Sciences Institutional Animal Care and Use Committee approved all animal procedures used in this study. The East Carolina University Institutional Animal Care and Use Committee approved the animal protocol used to induce EAE.
Acknowledgments
We thank Dr. Dave Brar for performing the multiplex cytokine assays, Debra King and Page Myers for technical assistance, Drs. Dori Germolec and Michael Fessler for useful comments on the manuscript, and the Histology and Immunohistochemistry Core and the Molecular Genomics Core for help with histology and NanoString assays, respectively. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1519906113/-/DCSupplemental.
References
- 1.Blackshear PJ. Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem Soc Trans. 2002;30(Pt 6):945–952. doi: 10.1042/bst0300945. [DOI] [PubMed] [Google Scholar]
- 2.Brooks SA, Blackshear PJ. Tristetraprolin (TTP): Interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim Biophys Acta. 2013;1829(6-7):666–679. doi: 10.1016/j.bbagrm.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor GA, et al. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4(5):445–454. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- 4.Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281(5379):1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 5.Carballo E, Blackshear PJ. Roles of tumor necrosis factor-alpha receptor subtypes in the pathogenesis of the tristetraprolin-deficiency syndrome. Blood. 2001;98(8):2389–2395. doi: 10.1182/blood.v98.8.2389. [DOI] [PubMed] [Google Scholar]
- 6.Lai WS, et al. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol Cell Biol. 1999;19(6):4311–4323. doi: 10.1128/mcb.19.6.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai WS, Parker JS, Grissom SF, Stumpo DJ, Blackshear PJ. Novel mRNA targets for tristetraprolin (TTP) identified by global analysis of stabilized transcripts in TTP-deficient fibroblasts. Mol Cell Biol. 2006;26(24):9196–9208. doi: 10.1128/MCB.00945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai WS, Stumpo DJ, Blackshear PJ. Rapid insulin-stimulated accumulation of an mRNA encoding a proline-rich protein. J Biol Chem. 1990;265(27):16556–16563. [PubMed] [Google Scholar]
- 9.Tchen CR, Brook M, Saklatvala J, Clark AR. The stability of tristetraprolin mRNA is regulated by mitogen-activated protein kinase p38 and by tristetraprolin itself. J Biol Chem. 2004;279(31):32393–32400. doi: 10.1074/jbc.M402059200. [DOI] [PubMed] [Google Scholar]
- 10.Cao H, Tuttle JS, Blackshear PJ. Immunological characterization of tristetraprolin as a low abundance, inducible, stable cytosolic protein. J Biol Chem. 2004;279(20):21489–21499. doi: 10.1074/jbc.M400900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu LQ, Stumpo DJ, Blackshear PJ. Myeloid-specific tristetraprolin deficiency in mice results in extreme lipopolysaccharide sensitivity in an otherwise minimal phenotype. J Immunol. 2012;188(10):5150–5159. doi: 10.4049/jimmunol.1103700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molle C, et al. Tristetraprolin regulation of interleukin 23 mRNA stability prevents a spontaneous inflammatory disease. J Exp Med. 2013;210(9):1675–1684. doi: 10.1084/jem.20120707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khachigian LM. Collagen antibody-induced arthritis. Nat Protoc. 2006;1(5):2512–2516. doi: 10.1038/nprot.2006.393. [DOI] [PubMed] [Google Scholar]
- 14.Geiss GK, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26(3):317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 15.Flutter B, Nestle FO. TLRs to cytokines: Mechanistic insights from the imiquimod mouse model of psoriasis. Eur J Immunol. 2013;43(12):3138–3146. doi: 10.1002/eji.201343801. [DOI] [PubMed] [Google Scholar]
- 16.Miller SD, Karpus WJ. Experimental autoimmune encephalomyelitis in the mouse. Curr Protoc Immunol. 2007;78(15.1):15.1.1–15.1.18. doi: 10.1002/0471142735.im1501s77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Nun A, et al. From classic to spontaneous and humanized models of multiple sclerosis: Impact on understanding pathogenesis and drug development. J Autoimmun. 2014;54:33–50. doi: 10.1016/j.jaut.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: Implications for joint and gut-associated immunopathologies. Immunity. 1999;10(3):387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 19.Hodge DL, et al. IFN-gamma AU-rich element removal promotes chronic IFN-gamma expression and autoimmunity in mice. J Autoimmun. 2014;53:33–45. doi: 10.1016/j.jaut.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoecklin G, et al. Genome-wide analysis identifies interleukin-10 mRNA as target of tristetraprolin. J Biol Chem. 2008;283(17):11689–11699. doi: 10.1074/jbc.M709657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nandakumar KS, Holmdahl R. Efficient promotion of collagen antibody induced arthritis (CAIA) using four monoclonal antibodies specific for the major epitopes recognized in both collagen induced arthritis and rheumatoid arthritis. J Immunol Methods. 2005;304(1-2):126–136. doi: 10.1016/j.jim.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Zhao W, Liu M, D’Silva NJ, Kirkwood KL. Tristetraprolin regulates interleukin-6 expression through p38 MAPK-dependent affinity changes with mRNA 3′ untranslated region. J Interferon Cytokine Res. 2011;31(8):629–637. doi: 10.1089/jir.2010.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan IM, et al. Deletion of tristetraprolin caused spontaneous reactive granulopoiesis by a non-cell-autonomous mechanism without disturbing long-term hematopoietic stem cell quiescence. J Immunol. 2011;186(5):2826–2834. doi: 10.4049/jimmunol.1002806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawlor KE, et al. Critical role for granulocyte colony-stimulating factor in inflammatory arthritis. Proc Natl Acad Sci USA. 2004;101(31):11398–11403. doi: 10.1073/pnas.0404328101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieschke GJ, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84(6):1737–1746. [PubMed] [Google Scholar]
- 26.Roberts AW. G-CSF: A key regulator of neutrophil production, but that’s not all! Growth Factors. 2005;23(1):33–41. doi: 10.1080/08977190500055836. [DOI] [PubMed] [Google Scholar]
- 27.Eyles JL, et al. A key role for G-CSF-induced neutrophil production and trafficking during inflammatory arthritis. Blood. 2008;112(13):5193–5201. doi: 10.1182/blood-2008-02-139535. [DOI] [PubMed] [Google Scholar]
- 28.Tortola L, et al. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J Clin Invest. 2012;122(11):3965–3976. doi: 10.1172/JCI63451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumida H, et al. Interplay between CXCR2 and BLT1 facilitates neutrophil infiltration and resultant keratinocyte activation in a murine model of imiquimod-induced psoriasis. J Immunol. 2014;192(9):4361–4369. doi: 10.4049/jimmunol.1302959. [DOI] [PubMed] [Google Scholar]
- 30.Toichi E, Tachibana T, Furukawa F. Rapid improvement of psoriasis vulgaris during drug-induced agranulocytosis. J Am Acad Dermatol. 2000;43(2 Pt 2):391–395. doi: 10.1067/mjd.2000.103264. [DOI] [PubMed] [Google Scholar]
- 31.Axtell RC, Steinman L. Gaining entry to an uninflamed brain. Nat Immunol. 2009;10(5):453–455. doi: 10.1038/ni0509-453. [DOI] [PubMed] [Google Scholar]
- 32.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10(5):514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 33.Taylor PC, Feldmann M. Anti-TNF biologic agents: Still the therapy of choice for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):578–582. doi: 10.1038/nrrheum.2009.181. [DOI] [PubMed] [Google Scholar]
- 34.Patil CS, et al. Targeting mRNA stability arrests inflammatory bone loss. Mol Ther. 2008;16(10):1657–1664. doi: 10.1038/mt.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cieri N, et al. Adoptive immunotherapy with genetically modified lymphocytes in allogeneic stem cell transplantation. Immunol Rev. 2014;257(1):165–180. doi: 10.1111/imr.12130. [DOI] [PubMed] [Google Scholar]
- 36.van der Fits L, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 37.Islam SM, Curtis AD, 2nd, Taslim N, Wilkinson DS, Mannie MD. GM-CSF-neuroantigen fusion proteins reverse experimental autoimmune encephalomyelitis and mediate tolerogenic activity in adjuvant-primed environments: Association with inflammation-dependent, inhibitory antigen presentation. J Immunol. 2014;193(5):2317–2329. doi: 10.4049/jimmunol.1303223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]