Mechanism by which AAT entry into CD4+ T cells block NF-κB activation and HIV-1 replication.
Keywords: clathrin-dependent endocytosis, endosome, lysosome, HIV-1, NF-κB, IκBα
Abstract
In α1-antitrypsin–deficient HIV patients, an accelerated decline of CD4+ T cell numbers is observed, suggesting that α1-antitrypsin is a potential endogenous HIV inhibitor. In infected T lymphocytes, α1-antitrypsin potently blocks NF-κB activation and HIV-1 replication by directly interacting with IκBα in the cytosol, thereby altering its ubiquitination pattern. However, the mechanism of α1-antitrypsin entry into the cytosol, where IκBα locates, remains unclear. In the present study, we investigated the mechanism of α1-antitrypsin internalization in CD4+ T cells. Thus, primary CD4+ T cells were infected with HIV-1 and then incubated with α1-antitrypsin to detect its internalization. We found that CD4+ T cells internalized α1-antitrypsin through a clathrin-dependent endocytosis process. Next, intracellular α1-antitrypsin exerted the inhibitory effect on NF-κB activation and HIV-1 replication. On primary CD4+ T cells, α1-antitrypsin interacted with low-density lipoprotein receptor-related protein 1 to initiate the internalization. Inside CD4+ T lymphocytes, α1-antitrypsin was transported from the endosome to the lysosome and then released into the cytosol, where it is possible for α1-antitrypsin to directly interact with IκBα. These results together suggest that α1-antitrypsin internalization is a clathrin-dependent and low-density lipoprotein receptor-related protein 1–mediated endocytosis process. Internalized α1-antitrypsin is transported through the endosome–lysosome–cytosol routine to interact with cytosolic IκBα and block NF-κB activation and HIV-1 replication.
Introduction
α1-Antitrypsin (AAT) is one of the most abundant serine protease inhibitors in the blood that belongs to the serpin superfamily. In the human body, AAT protects tissues by inhibiting the activity of serine proteases, including neutrophil elastase, cathepsin G, and proteinase-3 [1]. Recently, clinical studies have demonstrated that AAT might be an endogenous suppressor of HIV-1 and that pre-existing AAT deficiency is associated with accelerated HIV/AIDS progression [2, 3]. A number of in vitro studies have revealed that AAT potently inhibits HIV-1 replication by blocking the activation of the NF-κB signaling pathway [4–7]. Moreover, virus inhibitory peptide, the 20-residue C-proximal subfragment of AAT, also inhibits HIV-1 entry into the host cell by interacting with the gp41 fusion peptide and blocking HIV-1 infection [8]. Our previous study has demonstrated that AAT inhibits HIV-1 replication in infected CD4+ T cells. The inhibitory effect of AAT is mediated by blocking NF-κB activation through directly interacting with IκBα, thereby altering its ubiquitination pattern [7]. However, it is unclear how AAT enters CD4+ T lymphocytes, making it possible for AAT to directly interact with IκBα in the cytosol and thereby inhibit NF-κB activation and HIV-1 replication.
To take up extracellular molecules, cells usually use the well-defined endocytosis process, including clathrin-dependent and clathrin-independent endocytosis [9]. For the clathrin-dependent endocytosis process, which occurs in almost all cell types, plasma membrane proteins are specifically recognized by adaptor proteins and then packed into clathrin-coated vesicles for transport into the host cell [10]. Slightly differently, clathrin-independent endocytosis can occur in many different forms, which have been less studied [11, 12]. Recent studies have reported that AAT is internalized through an endocytosis process in endothelial cells [13, 14]. However, to our best knowledge, no report has shown the mechanism of AAT internalization in primary CD4+ T lymphocytes, which is the main target of HIV-1 in the human body.
In the present study, we investigated the mechanism of AAT internalization and transportation in primary CD4+ T cells and clarify how AAT interacted with IκBα to mediate the inhibition of NF-κB activation and HIV-1 replication. Our results have demonstrated that AAT interacts with LRP1 on primary CD4+ T cells, which then mediate AAT internalization through a clathrin-dependent endocytosis process. In these cells, internalized AAT is translocated from the endosome to the lysosome and then released into the cytosol, where it is possible for AAT to interact with cytosolic IκBα and thereby inhibit NF-κB activation and HIV-1 replication.
MATERIALS AND METHODS
HIV-1 virus preparation
HIV-1 stock for infection was prepared by propagating HIV-1IIIB in neoplastic H9 cells. The virus was harvested at the time when p24 and/or reverse transcriptase production had reached a peak. Next, the virus was pooled, filtered through a 0.45 µm membrane, and stored in aliquots in liquid nitrogen until used. Our HIV-1IIIB preparations typically contained 1 µg/ml p24 and 1 × 106 cpm reverse transcriptase activity.
CD4+ T cell isolation and infection
CD4+ T cells were prepared from healthy, HIV-negative PBMCs using the CD4+ T cell isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany), as described previously [15]. Isolated CD4+ T cells were then activated with PHA (5 μg/ml; Sigma-Aldrich, St. Louis, MO, USA) for 3 d in CM consisting of RPMI 1640 medium with HEPES (20 mM), nonessential amino acid (1%), l-glutamine (2 mM), 10% heat-inactivated and defined FBS (Gibco, Invitrogen, Carlsbad, CA, USA), and gentamicin (50 µg/ml; Life Technologies, Carlsbad, CA). After PHA stimulation, CD4+ T cells were incubated in CM containing IL-2 (20 U/ml; NIH AIDS Reference and Reagent Program; Hoffmann-La Roche, Inc., Nutley, NJ, USA) for 2 h at 37°C. For HIV-1 infection, activated CD4+ T cells were centrifuged to remove the culture medium. Next, the cells were resuspended in HIV-1IIIB stock (50,000 cpm reverse transcriptase activity per 106 cells) and incubated at 37°C for 2 h. After infection, unbound virus was removed by washing 5 times with PBS. The infected CD4+ T cells were then cultured in CM with 20% FBS and IL-2 (20 U/ml), as described in each experiment. The purity of CD4+ T cells is usually more than 98%, which was confirmed by flow cytometry assay.
Macrophage preparation
Macrophages were prepared from PBMCs from the whole blood of healthy, HIV-negative donors. In brief, macrophages were prepared by culturing isolated PBMCs in CM with 10% human serum (Gemini Bio-Products, West Sacramento, CA, USA). After 7 d of incubation, attached macrophages were collected and incubated in CM with 20% FBS for further experiments. The purity of the macrophages is usually more than 95%, which was confirmed by flow cytometry assay.
QRT-PCR assay for HIV-1 viral RNA production
Cell-free supernatant from the culture system was collected, and HIV-1 viral RNA was isolated using the QIAamp viral RNA mini Kit (Qiagen, Hilden, Germany) as directed by the manufacturer. Isolated viral RNA was then reverse-transcripted into cDNA using random primer (Invitrogen) and SuperScript III reverse transcriptase (Invitrogen), as directed by the manufacturer. Next, cDNA was used as a template to run qPCR [forward primer (nt 3696 in HXB2): 5′-TGGGTACCAGCACACAAAGG-3′, reverse primer (nt 3850): 5′-ATCACTAGCCATTGCTCTCCAAT-3′, probe: ATTGGAGGAAATGAAC-MBG (FAM labeled)] with TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA), as directed by the manufacturer. The qPCR conditions were as follows: 50°C × 2 min (1×), 95°C × 10 min (1×), followed by 60 cycles of 95°C × 15 s and 60°C × 1 min. A standard curve was prepared using known concentrations (i.e., copy numbers) of ACH-2 DNA to determine the number of copies of viral RNA present in the cultures. The primers were used at 900 nM and the probe at 250 nM.
ELISA assay for HIV-1 p24 production
Cell-free supernatant from the culture system was collected, and p24 production was detected using the HIV-1 p24 antigen ELISA kit (ZeptoMetrix Corp., Buffalo, NY, USA) in accordance with the manufacturer’s instructions. In brief, lysed viral particles were added to the washed plate with an antibody specific for p24 antigen. After incubating for 2 h at 37°C, the plate was washed. Next, a reconstituted HIV-1 p24 detector antibody was added and incubated at 37°C. After 1 h of incubation, the plate was washed, and a second antibody with HRP was added. After incubating for 30 min at 37°C, the substrate was added to react for 30 min more, and then the reaction was stopped. The absorbance at 450 nm was detected.
Protein extraction
The cells was collected and washed 3 times with ice cold PBS as described previously [16]. Buffer A (10 mM Tris-HCl [pH 7.4], 1.5 mM MgCl2, 10 mM KCl, 2 mM DTT, 0.1% Triton X-100, 2.5 mM NaH2PO4, 1 mM Na3VO4, 1 mM NaF, 1 mM PMSF, protease inhibitor cocktail; Roche, Indianapolis, IN, USA) was added to the cell pellet and gently inverted. The mixture was then incubated on ice for 15 min and centrifuged at 250 g for 5 min at 4°C to discard the supernatant. Buffer A was added to the cell pellet again, and the cell pellet was resuspended with a small gauge needle by drawing 5 times. Next, the mixture was centrifuged at 8000 g for 20 min at 4°C, and the supernatant (cytosolic proteins) was collected. To extract nuclear proteins, the pellet after cytosolic protein extraction was resuspended in buffer B (20 mM Tris-HCl [pH 7.4], 1.5 mM MgCl2, 420 mM KCl, 0.2 mM EDTA, 2 mM DTT, 1% Igepal CA-630, 25% [vol/vol] glycerol, 2.5 mM NaH2PO4, 1 mM Na3VO4, 1 mM NaF, 1 mM PMSF, protease inhibitor cocktail; Roche) and disrupted with a small gauge needle by drawing 5 times. The mixture was then agitated gently at 4°C for 50 min and centrifuged at 16,000 g for 5 min to collect the supernatant (nuclear protein). The nuclear and cytosolic proteins were stored at −80°C for further use.
For whole-cell protein extraction, the cells were collected and washed 3 times with ice cold PBS. Radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.4, 1% CHAPS, 250 mM NaCl, 0.5% Triton X-100, 1% Igepal CA-630, 1 mM DTT, 1 mM Na3VO4, 1 mM NaF, 1 mM PMSF, 4 mM EDTA, protease inhibitor cocktail; Roche) was added to the cell pellet and vortexed for 60 s. Next, the mixture was incubated on ice for 45 min and homogenized with a small gauge needle by drawing 3 times. After homogenizing, the mixture was centrifuged at 14,000 g for 10 min at 4°C to collect the supernatant (whole-cell proteins). Whole-cell proteins were also stored at −80°C for further use.
Western blot assay
Whole-cell proteins, nuclear proteins, or cytoplasm proteins were mixed with 4 × loading buffer [250 mM Tris-HCl, pH 8.8, 4% (wt/vol) SDS, 4% (vol/vol) β-mercaptoethanol, 40% (vol/vol) glycerol, 0.01% (wt/vol) bromphenol blue] and incubated for 20 min at room temperature. Next, the mixture was incubated at 100°C for 5 min. The proteins were then separated by SDS-PAGE and transferred to Hybond-P membrane (Amersham Life Science, Arlington Heights, IL). After blocking in TBS with 5% nonfat milk for 2 h at room temperature, the membrane was incubated with the primary antibody [rabbit anti-human p105/p50 (Abcam, Cambridge, United Kingdom); rabbit anti-human p65 (Abcam); rabbit anti-human β-actin (Abcam); goat anti-human H2B (Santa Cruz Biotechnology, Santa Cruz, CA, USA); rabbit anti-human H2B (Abcam); goat anti-human AAT (Bethyl Inc., Montgomery, TX, USA); mouse anti-human clathrin (Abcam); mouse anti-human LRP1 (Abcam); rat anti-human LAMP1 (Abcam); rat anti-human LAMP 2 (Abcam); rabbit anti-human Rab4 (Abcam); or rabbit anti-human Rab5 enzyme (Abcam)] in TBS with 5% nonfat milk for 2 h at room temperature. Next, the membrane was washed 3 times in TBS with 0.1% Tween-20 and 5% nonfat milk. After washing, the membrane was incubated with HRP-linked secondary antibody (anti-rabbit IgG-HRP, anti-goat IgG-HRP, anti-rat IgG-HRP, or anti-mouse IgG-HRP; all from Chemicon International, Temecula, CA, USA) in TBS with 5% nonfat milk for 2 h at room temperature. Finally, the membrane was washed in TBS with 0.1% Tween-20 and 5% nonfat milk and then developed. Semiquantitation was performed with a phosphorimaging scanner.
Immunoprecipitation assay
Proteins were incubated with protein A/G agarose beads at 4°C to remove the nonspecific binding proteins. After 1 h of incubation, the mixture was centrifuged to collect the supernatant. Antibody was then added to the supernatant to incubate at 4°C for 2 h. Next, a protein A/G bead was added to incubate for another 1 h at 4°C. The mixture was then centrifuged to collect the bead, which was washed for 3 times with lysis buffer and mixed with 4× loading buffer to boil for 5 min. Next, the mixture was centrifuged for 5 min at 3000 g at 4°C. The supernatant was collected to detect the proteins of interest using Western blot assay.
Alexa Fluor 488 conjugation of AAT
AAT was conjugated with Alexa Fluor 488 dye in accordance with the protocol in the Alexa Fluor 488 protein labeling kit (Invitrogen). In brief, Alexa Fluor 488 carboxylic acid, TFP ester, bis(triethylammonium salt) was added to AAT and then incubated at room temperature. After 1 h of incubation, labeled AAT was separated and collected by size-exclusion chromatography. Next, the level of Alexa Fluor 488 on AAT was detected following the protocol provided.
Confocal microscopy
The cells were treated with Alexa Fluor 488-labeled AAT as shown in each experiment. Uninternalized AAT was then removed by treating the cells with trypsin and washing 3 times in PBS. Next, the cells were fixed with 4% paraformaldehyde. The cells were then plated on the glass, and green fluorescence was detected using a confocal microscope (Olympus, Center Valley, PA). The image was constructed by overlaying multiple pictures taken at different position in the z-axle.
RNA interference
The cells were incubated in CM with 10% FBS. One day before siRNA transfection, the cells were grown in the medium without antibiotics. On siRNA transfection, specific or control siRNA was diluted into Opti-MEM I (Life Technologies) reduced serum medium without serum and mixed gently. DNA-Lipofectamine 2000 (Life Technologies) was also diluted into Opti-MEM I reduced serum medium without serum and mixed gently. After 5 min of incubation at room temperature, diluted siRNA and DNA-Lipofectamine 2000 were mixed and incubated for another 20 min at room temperature. Next, the mixture was added to the cells. After 6 h of incubation at 37°C, the medium was removed, and normal CM with serum and antibiotics was applied. The transfected cells were used for the protein expression assay and other experiments.
Membrane receptor identification
The cells were washed with cold PBS for 3 times and then incubated with AAT for 1 h at 4°C. Excess AAT was washed off, and 3 mM DSP (Thermo Fisher Scientific, Pittsburgh, PA, USA) was added to stabilize the interaction between AAT and membrane proteins for 30 min. The reaction was then stopped by 10 to 20 mM Tris buffer, and membrane proteins were extracted using the membrane protein extraction kit (BioVision Inc., San Francisco, CA, USA) following the protocol provided. Next, isolated membrane proteins were incubated with AAT antibody to precipitate proteins interacting with AAT. Precipitated proteins were then separated using SDS-PAGE, and the specific protein bands were cut and digested with the sequencing grade-modified trypsin (Promega, Madison, WI, USA). The digested peptides were then identified by HPLC-MS using the protocol described in the next sections.
In-gel digestion for peptide mass fingerprinting assay
Isolated proteins were separated by SDS-PAGE, and the appropriate gel bands were cut out for mass spectrometry assay. In brief, extracted gel bands were washed with 50% acetonitrile and 50 mM ammonium bicarbonate and then dried to be reduced with DTT and alkylated with iodoacetamide in 100 mM ammonium bicarbonate. Next, gel bands were digested with Promega sequencing grade modified trypsin for 24 h, and the reaction was then stopped by formic acid. Next, digested gel bands were sonicated for 20 min and centrifuged to collect the digested peptides. The peptides were then analyzed using the HPLC-MS system (API QSTAR pulsar I LC/MS System; Applied Biosystems; or LTQ XL Orbitrap LC/MS System; Thermo Fisher Scientific). The proteins were identified by searching the specific mass spectrum in the protein database (Mascot).
In-gel FASP or FASP for peptide mass fingerprinting assay
Isolated proteins were separated by SDS-PAGE. The appropriate gel bands were cut out and transferred to a P1000 tip. After smashing into a tube by centrifugation, the gel pieces were loaded into a P200 tip and briefly centrifuged. The shredded gels were then treated with 50% acetonitrile, 4 M urea, and 50 mM Tris buffer (pH 8.5) for 25 min and transferred to a 10 kDa filter tube to remove the solution. After centrifugation, shredded gel was treated with 8 M urea, 100 mM Tris, and 0.1 M DTT buffer (pH 8.5). After DTT treatment, the gel pieces were treated with 8 M urea, 100 mM Tris, and 50 mM iodoacetamide buffer (pH 8.5). Next, the gel pieces were incubated with 50 mM ammonium bicarbonate and then digested with Promega sequencing grade modified trypsin for 24 h. The digestion reaction was stopped by formic acid. The peptides were analyzed on the HPLC-MS system [API QSTAR pulsar I LC/MS System (Applied Biosystems) or LTQ XL Orbitrap LC/MS System (Thermo Fisher Scientific)]. The protein was identified by searching the specific mass spectrum in the protein database (Mascot).
Statistical analysis
An analysis of the data was performed using the paired Student’s t test. Values of P ≤ 0.05 were considered significant. Every experiment was repeated at least 3 times on different donors. Unless specifically stated, the error bars indicate the standard errors of the mean (sem).
Studies using human cells or tissues
Studies using blood- or tissue-derived cells obtained from humans were reviewed and approved by an appropriate institutional review committee (in accordance with the NIH and Brigham Young University guidelines).
RESULTS
In CD4+ T lymphocytes, AAT blocks NF-κB activation by directly interacting with cytosolic IκBα to alter its ubiquitination pattern and thereby inhibits HIV-1 replication [7]. Because AAT is not expressed in CD4+ T cells [7], we postulated that AAT might be transported into CD4+ T cells to exert its inhibitory effect on NF-κB activation and HIV-1 replication. Primary CD4+ T cells were therefore incubated with 5 mg/ml Alexa Fluor 488 dye-labeled AAT to detect the internalization of AAT by confocal microscopy. Because AAT concentration of 5 mg/ml occur in subjects with inflammation and are thus physiologically relevant and this concentration of AAT significantly inhibits NF-κB activation and HIV-1 replication [7], we selected this concentration for the remainder of our experiments. As expected, the internalization of AAT was detected by confocal microscopy in HIV-1-infected CD4+ T cells (Fig. 1). When uninfected CD4+ T cells were treated with same amount of labeled AAT, AAT internalization was also observed (Supplemental Fig. 1). These results suggest that AAT internalization in CD4+ T cells is independent of HIV-1 infection.
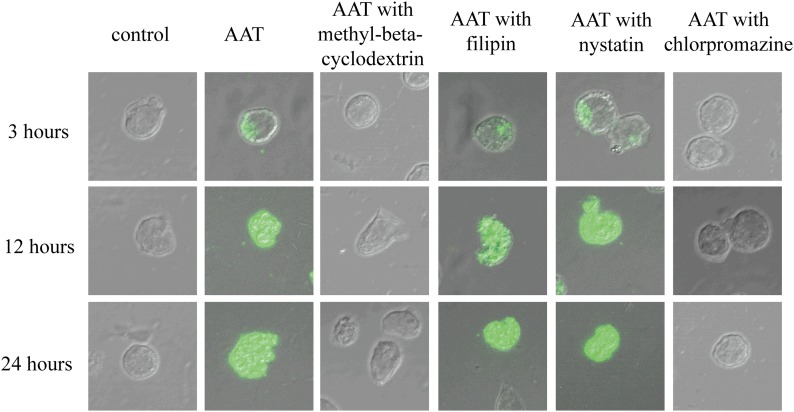
Figure 1. HIV-1-infected CD4+ T cells internalized AAT through a clathrin-dependent endocytosis process.
Activated primary CD4+ T cells were infected with HIV-1IIIB and then cultured with the presence or absence of methyl-β-cyclodextrin, filipin or nystatin, or chlorpromazine (all 20 µg/ml) for 1 h. Next, these T cells were incubated with the presence or absence of 5 mg/ml Alexa Fluor 488-labeled AAT. After 3, 12, or 24 h of incubation, the cells were collected, fixed, and imaged with a confocal microscope (×600).
Because most mammalian cells uptake intracellular particles or proteins through an endocytosis process [17], we reasoned that AAT internalization might be also achieved through an endocytosis process. Infected CD4+ T cells were therefore pretreated with an endocytosis inhibitor (methyl-β-cyclodextrin) and then cultured with labeled AAT to detect AAT internalization. As expected, methyl-β-cyclodextrin pretreatment blocked AAT internalization (Fig. 1), suggesting that AAT internalization was mediated through an endocytosis process in CD4+ T cells. In cells, the endocytosis process can usually be divided into 2 major categories: clathrin-dependent and clathrin-independent endocytosis [9]. Thus, we also sought to investigate whether AAT internalization was dependent or independent of clathrin. Infected CD4+ T cells were pretreated with clathrin-dependent endocytosis inhibitor (chlorpromazine) or caveolae-dependent endocytosis inhibitor (filipin or nystatin) to detect AAT internalization. To our surprise, chlorpromazine pretreatment blocked AAT internalization but filipin or nystatin pretreatment had no obvious effect on this process (Fig. 1). In uninfected CD4+ T cells, similar results were also obtained (Supplemental Fig. 1). Thus, these results together indicate that AAT internalization is a clathrin-dependent endocytosis process in CD4+ T cells and that this process is independent of HIV-1 infection.
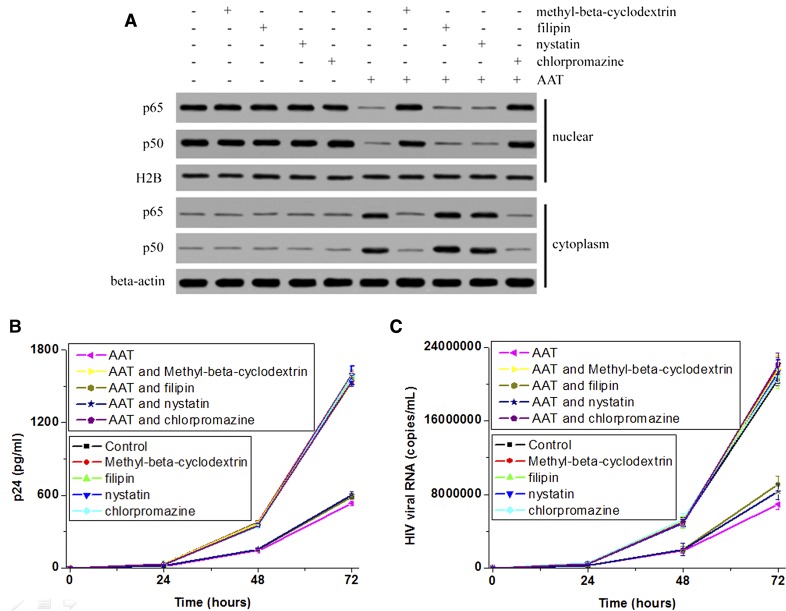
Next, we studied whether intracellular or extracellular AAT exerted its inhibitory effect on NF-κB activation and HIV-1 replication in CD4+ T cells. Because NF-κB activation is mediated through IκBα degradation and NF-κB nuclear translocation in T lymphocytes [18], the distribution of NF-κB (a dimer of p65 and p50 in CD4+ T cells) in the cytosol and nucleus was detected to determine its activation by Western blotting. HIV-1 replication was also measured by detecting viral RNA and p24 production in the supernatant. The results revealed that AAT significantly blocked p65 and p50 nuclear translocation and viral RNA and p24 production in activated primary CD4+ T cells (P < 0.05; Fig. 2). Methyl-β-cyclodextrin or chlorpromazine pretreatment reversed the inhibitory effect of AAT on these responses (Fig. 2). However, filipin or nystatin pretreatment had no obvious effect on the inhibitory effect of AAT (Fig. 2). Moreover, methyl-β-cyclodextrin, chlorpromazine, filipin, and nystatin had no obvious effect on p65 and p50 nuclear translocation and viral RNA and p24 production in CD4+ T cells (Fig. 2). Thus, these results suggest that only intracellular exogenous AAT exerted the inhibitory effect on NF-κB activation and HIV-1 replication.
Figure 2. Endocytosis inhibitor blocked the inhibitory effect of AAT on NF-κB activation and HIV-1 replication.
Activated primary CD4+ T cells were infected with HIV-1IIIB and then cultured with the presence or absence of methyl-β-cyclodextrin, filipin or nystatin, or chlorpromazine for 1 h. Next, these T cells were incubated with the presence or absence of AAT. After 24 h of incubation, the cells were collected to extract the cytosolic and nuclear proteins. The distribution of p50 and p65 (A) was detected by Western blot. β-Actin and H2B were used as loading controls. The supernatant from 0, 24, 48, or 72 h of incubation was also collected to detected HIV-1 replication by measuring viral p24 (B) and RNA (C).
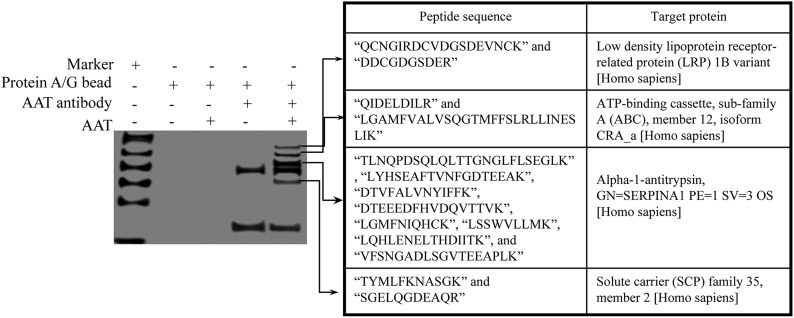
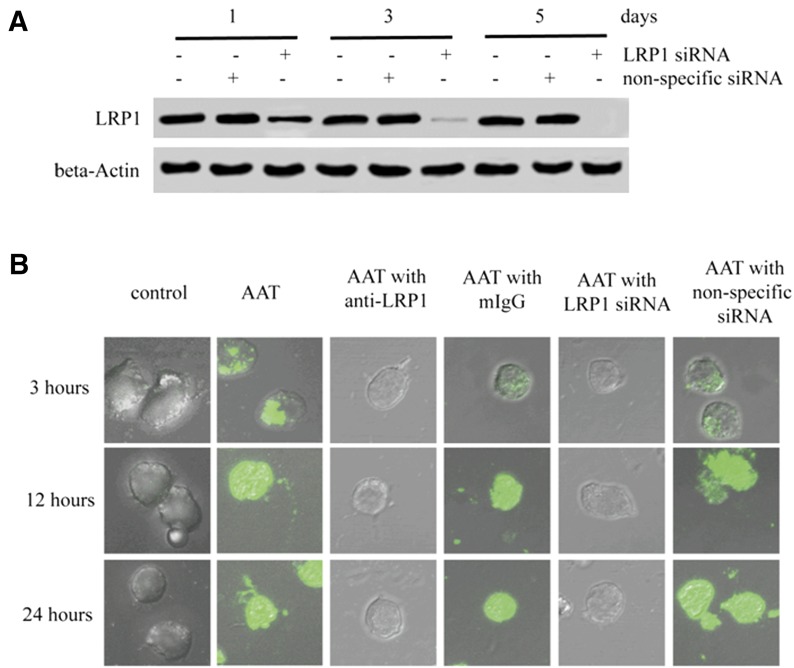
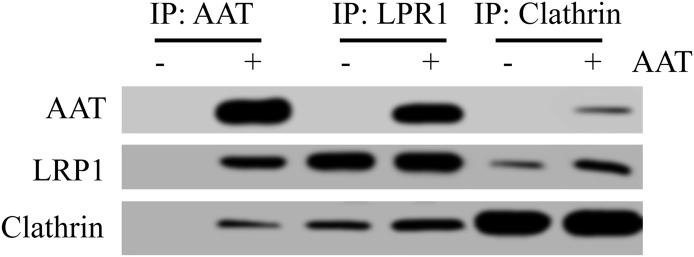
Usually, the endocytosis process is mediated through the interaction between the membrane receptor and ligand [17]. We next sought to identify whether AAT also interacted with a specific membrane receptor on CD4+ T cells to mediate its internalization. Activated CD4+ T cells were therefore infected with HIV-1IIIB and then cocultured with AAT. To stabilize the interaction between AAT and the potential membrane receptor, DSP was also added to extract the whole cell proteins, and AAT was then precipitated using AAT antibody. The membrane proteins interacting with AAT were identified by the peptide mass fingerprinting assay using the LC-MS/MS system. As expected, AAT interacted with several membrane proteins on CD4+ T cells, including LRP1, ATP-binding cassette protein, and solute carrier protein (Fig. 3). Among these proteins, LRP1 is the most promising candidate [19, 20]. To test whether LRP1 played an essential role in AAT internalization, LRP1 was knocked down with LRP1 siRNA to monitor AAT internalization in these T lymphocytes. The results showed that LRP1 siRNA efficiently blocked LRP1 expression and inhibited AAT internalization in CD4+ T cells (Fig. 4). LRP1 siRNA also blocked the inhibitory effect of AAT on p65/p50 nuclear translocation and viral RNA and p24 production (Supplemental Fig. 2). Moreover, AAT internalization was also inhibited specifically when the CD4+ T cells were treated with LRP1 blocking antibody, which further confirmed that LRP1 was the target that interacted with AAT to mediate its internalization (Fig. 4B). Because AAT internalization in CD4+ T cells was a clathrin-dependent endocytosis process, we also sought to determine whether LRP1 associated with clathrin to mediate AAT internalization. To achieve this goal, we treated AAT-treated CD4+ T lymphocytes with DSP to stabilize the interaction between the proteins and then precipitated LPR1, AAT, or clathrin to detect the interaction among these proteins. As expected, a strong association was detected among AAT, LRP1, and clathrin in the CD4+ T cells (Fig. 5). Thus, these results suggest that LRP1 on CD4+ T cells might interact with AAT to mediate its internalization through a clathrin-dependent endocytosis process.
Figure 3. AAT interacted with membrane proteins on CD4+ T cells.
Activated primary CD4+ T cells were infected with HIV-1IIIB and then cultured with the presence or absence of AAT for 2 h. Unbound AAT was then removed, and the membrane proteins were extracted from these cells. Next, AAT antibody was added to precipitate the proteins interacting with AAT. Precipitated proteins were then separated by SDS-PAGE, and the specific proteins interacting with AAT were collected and identified by fingerprint mass spectrometry.
Figure 4. LRP1-mediated AAT internalization in CD4+ T cells.
Activated primary CD4+ T cells were treated with LRP1 siRNA or non-specific siRNA. After 1, 3, or 5 d of incubation, the cells were collected and lysed to detect LRP1 expression by Western blot (A). β-Actin was used a loading control. Moreover, activated CD4+ T cells with or without 3 d of siRNA treatment were infected with HIV-1IIIB and then incubated with the presence or absence of Alexa Fluor 488-labeled AAT, 5 µg/ml LRP1 antibody, or 5 µg/ml mouse IgG for 3, 12, or 24 h. The internalization of AAT was then detected by confocal microscope (B) (×600).
Figure 5. AAT was associated with LRP1 and clathrin in CD4+ T cells.
Activated primary CD4+ T cells were infected with HIV-1IIIB and then cultured with the presence or absence of AAT. After 3 h of incubation, the cells were treated with DSP to stabilize the protein interaction and then lysed to extract whole cell proteins. AAT antibody, LRP1 antibody, or clathrin antibody was then added to the lysis to precipitate proteins. The interaction among AAT, LRP1, and clathrin were detected by Western blot.
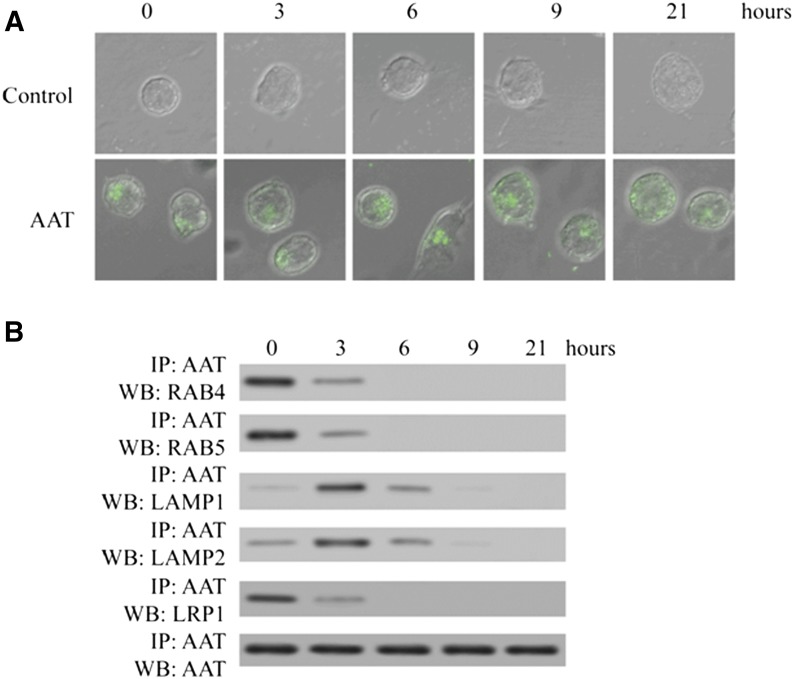
Usually, cells take up extracellular molecules through receptor-mediated clathrin-dependent endocytosis process, and internalized molecules are then transported from the endosome to the lysosome [17]. In the lysosome, internalized molecules are degraded, recycled back to the cell surface, trafficked to organelles, or released into the cytosol [21, 22]. Because AAT interacts directly with cytosolic IκBα to block NF-κB activation and HIV-1 replication in CD4+ T cells [7], we also sought to investigate how AAT was transported in CD4+ T cells to make it possible that AAT directly interacted with IκBα in the cytosol. To achieve this goal, infected CD4+ T cells were treated with Alexa Fluor 488 dye-labeled AAT for 3 h, and then uninternalized AAT was removed. The cytosolic location of AAT was detected by confocal microscopy. The results demonstrated that AAT was initially confined to some specific cellular compartments and then distributed homogenously in the cytosol (Fig. 6A). When DSP was added to these CD4+ T cells to stabilize the possible protein interactions and AAT was then precipitated, we found that AAT was associated with LRP1 and endosome proteins, Rab4 and Rab5, during the first several hours. Next, AAT was found to be associated with lysosome proteins, Lamp1 and Lamp2. After 6 h of incubation, we could not detect the association between AAT and endosome and lysosome proteins, but AAT was distributed evenly in the cytosol (Fig. 6). Thus, these results suggest that internalized AAT is transported from the endosome to the lysosome and then released into the cytosol.
Figure 6. AAT was transported through the routine of endosome–lysosome–cytosol.
Activated primary CD4+ T cells were infected with HIV-1IIIB and then cultured with the presence or absence of Alexa Fluor 488-labeled AAT. After 3 h of incubation, uninternalized AAT was removed, and the cells were incubated for another 0, 3, 6, 9, or 21 h. AAT internalization was detected by confocal microscope (×600) (A). Furthermore, after 0, 3, 6, 9, or 21 h of incubation, the cells were also treated with DSP to stabilize the protein interaction and then lysed to extract whole cell proteins. Next, AAT antibody was added to the cell lysate to precipitate the proteins interacting with AAT. LRP1, RAB4, RAB5, LAMP1, AAT, and LAMP2 were detected by Western blot (B).
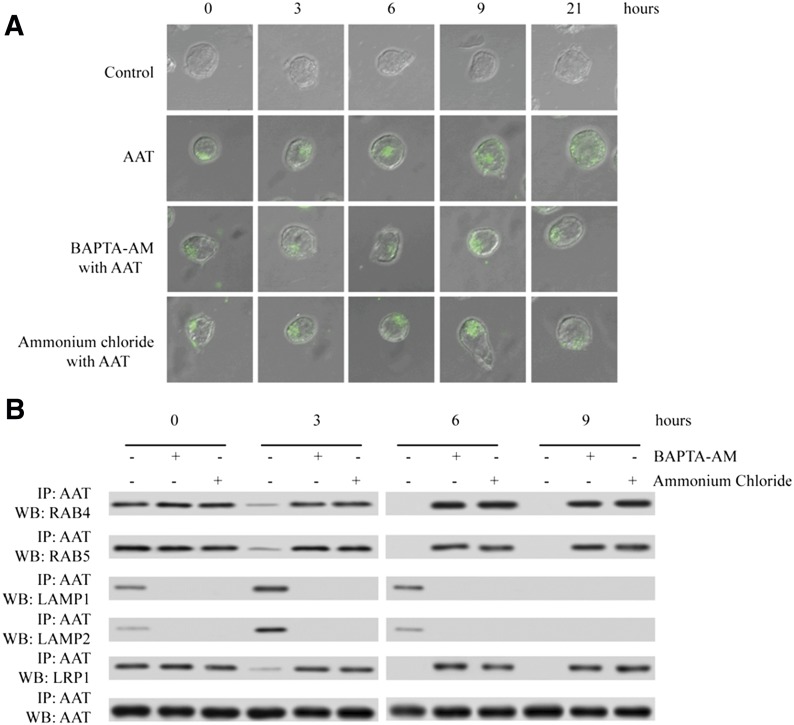
To confirm that AAT is transported from the endosome to the lysosome and then released into the cytosol, we also treated infected CD4+ T cells with BAPTA-AM or ammonium chloride to block the fusion of endosomes with lysosomes, which is a calcium- and pH-dependent process [17]. Next, we monitored AAT transportation, p65/p50 nuclear translocation, and HIV-1 replication. The results revealed that the endosome–lysosome fusion inhibitor BAPTA-AM and ammonium chloride blocked the homogenous distribution of AAT and confined AAT in specific cellular compartments (Fig. 7A). As expected, after BAPTA-AM and ammonium chloride treatment, we also found that AAT was always associated with LRP1 and endosome proteins Rab4 and Rab5, not the lysosome proteins Lamp1 and Lamp2 (Fig. 7B). Moreover, BAPTA-AM and ammonium chloride treatment also blocked the inhibitory effect of AAT on p65/p50 nuclear translocation and HIV-1 replication (Supplemental Fig. 3). Therefore, these results together suggest that LRP1 and clathrin could mediate AAT internalization and that internalized AAT is transported from the endosome to the lysosome and then released into the cytosol, where AAT could directly interact with IκBα to alter its ubiquitination pattern and thereby inhibit NF-κB activation and HIV-1 replication.
Figure 7. Lysosome inhibitor, NH4Cl, and endosome-lysosome fusion inhibitor, BAPTA-AM, suppressed the transportation of AAT from the endosome to the lysosome.
Activated primary CD4+ T cells were infected with HIV-1IIIB and then cultured with the presence or absence of Alexa Fluor 488-labeled AAT. After 3 h of incubation, unbound AAT was removed, and the cells were treated with the presence or absence of 10 mM NH4Cl or 10 µM BAPTA-AM for another 0, 3, 6, 9, or 21 h. AAT internalization was detected by confocal microscope (×600) (A). The cells after 0, 3, 6, and 9 h of incubation were also treated with DSP to stabilize the protein interaction, and these cells were then lysed to extract the whole cell proteins. AAT antibody was added to the lysate to precipitate the proteins interacting with AAT. LRP1, RAB4, RAB5, LAMP1, AAT, and LAMP2 were detected by Western blot (B).
DISCUSSION
Although it is clear that AAT inhibits NF-κB activation and HIV-1 replication by interacting with cytosolic IκBα and thereby altering its ubiquitination pattern in CD4+ T cells [7], it is unknown how AAT enters into CD+ T cells and makes it possible for AAT to directly interact with IκBα. In the present study, we found that the general endocytosis inhibitor, methyl-β-cyclodextrin, blocked AAT internalization in CD4+ T cells. AAT internalization was also blocked by the clathrin-dependent endocytosis inhibitor chlorpromazine. Moreover, methyl-β-cyclodextrin and chlorpromazine also eliminated the inhibitory effect of AAT on NF-κB activation and HIV-1 replication. These results indicate that, in CD4+ T cells, AAT internalization is a clathrin-dependent endocytosis process and only intercellular AAT exerts the inhibitory effect on NF-κB activation and HIV-1 replication. In endothelial cells, intracellular uptake of AAT is also a clathrin-dependent endocytosis process, rather than diffusion [14, 23]. However, the study by Aldonyte et al has shown that AAT internalization is caveolae-dependent owing to the identification of AAT in the caveolar fraction of the plasma membrane in endothelial cells [13]. In the present study, we found that caveola-mediated endocytosis inhibitor, filipin or nystatin, did not block AAT internalization in CD4+ T cells. The functional studies showed that caveola pathway inhibitors also did not interfere with the inhibitory effect of AAT on NF-κB activation and HIV-1 replication. Although we cannot rule out the contributions of non-clathrin- or non-caveola mediated endocytosis in AAT uptake, the profound effect of a specific inhibitory strategy using chlorpromazine indicated that AAT was primarily internalized via the clathrin-mediated endocytosis process.
In mammalian cells, clathrin-dependent endocytosis occurs in almost all cell types. This process mediates the internalization of numerous essential nutrients and factors, such as the cholesterol-laden LDL particle that binds to the LDL receptor and the iron-laden transferrin that binds to the transferrin receptor [24, 25]. In the present study, we found that AAT interacted with LRP1 on CD4+ T cells. AAT internalization could be blocked by LRP1 siRNA and LRP1 antibody. Moreover, the inhibitory effect of AAT on NF-κB activation and HIV-1 replication was also eliminated by LRP1 siRNA and LRP1 blocking antibody. When AAT, LRP1, or clathrin was precipitated, we could also detect the association among AAT, LRP1, and clathrin. Previously, several in vitro biochemical studies suggested that a SEC receptor recognizes and interacts with AAT, which then mediates the internalization of AAT in some carcinoma cell lines [26, 27]. In the present study, we found that LRP1, one of the SEC receptors, plays an essential role in AAT internalization in CD4+ T cells. To the best of our knowledge, this is the first report showing the direct interaction between AAT and LRP1 on the cell membrane. When primary macrophages were treated with LRP1 siRNA to detect the internalization of AAT, the results showed that LRP1 siRNA treatment also blocked AAT internalization in these macrophages (Supplemental Fig. 4), which further confirmed the results that the take up of AAT was mediated through an LRP1-dependent endocytosis process and that this process is not T cell-specific. Moreover, in vitro biochemical studies also reported that free AAT would be internalized via SEC/LRP-mediated endocytosis only when AAT formed complexes with trypsin [20, 28]. However, in the present study, we detected AAT internalization in CD4+ T cells without the presence of trypsin. The possible explanation for the difference was that AAT might interact with some trypsin-like targets on the membrane of CD4+ T cells or in the culture medium to mediate its internalization. Another explanation is that the AAT–trypsin complex was not necessary for AAT internalization at all. No matter which explanation is correct, it is safe to conclude that AAT internalization is predominantly fulfilled through an LRP1-mediated clathrin-dependent endocytosis process in primary CD4+ T cells.
In cells, receptor-mediated endocytosis via clathrin-coated pits is a shared pathway used for the internalization of a variety of ligand–receptor complexes, including LRP and ligand [17]. Although uncertainty still exists about many aspects of the endocytic pathways, the general transportation routine for internalized protein is from the endosome to the lysosome [29–31]. In the present study, we found that AAT associated with the endosome at the initial stage of internalization and then translocated in the lysosome. The endosome–lysosome fusion inhibitor blocked the transportation of AAT from the endosome to the lysosome and eliminated the inhibitory effect of intracellular AAT on NF-κB activation and HIV-1 replication. In cells, the most common fates of internalized molecules are to be degraded or recycled back to the cell surface; however, many alternate fates, such as trafficking to organelles such as the Golgi apparatus or translocation into the cytosol, also occur [32, 33]. In the present study, we found that AAT escaped from the lysosome and then distributed evenly around the cytosol, where it was possible for AAT to directly interact with IκBα and thereby change the polyubiquitination pattern of IκBα. Although it remains to be clarified how AAT escapes from the lysosome and then is distributed evenly around the cytosol, the process by which proteins escape from the lysosome does occur in many mammalian cells [34–38]. Thus, in CD4+ T cells, it is reasonable that clathrin and LRP1 mediate AAT internalization and that internalized AAT is transported from the endosome to the lysosome. Next, lysosomal AAT is released into the cytosol, where AAT interacts with cytosolic IκBα and alters its polyubiquitination pattern to mediate the inhibition of NF-κB activation and HIV-1 replication. Although it is rational to believe that AAT is released from the lysosome into the cytosol, investigation of the accurate molecular mechanism is still necessary in the future and might provide us with some novel insights into the general endocytosis process.
In patients, the amount change of AAT in the whole blood has a close relationship with HIV/AIDS progression, and pre-existing AAT deficiency is associated with accelerated HIV/AIDS progression [2–4]. In the present study, we found that AAT internalization was a clathrin-dependent and LRP1-mediated endocytosis process in CD4+ T cells. Internalized AAT was transported from the endosome to the lysosome and then released into the cytosol. In the cytosol, AAT directly interacted with cytosolic IκBα to alter its polyubiquitination pattern and blocked NF-κB activation and HIV-1 replication. Because of the clinical availability of AAT and its inhibitory effect on HIV-1 replication, the study of this molecule in the HIV/AIDS setting will help the community understand the mechanism accurately, and this study could also be highly informative for future biologic drug designs for HIV/AIDS treatment.
AUTHORSHIP
X.Z. performed the experiments. X.Z., Z.L., L.S., J.Y., and G.F.B. designed experiments and analyzed data. X.Z. and Z.L. wrote the manuscript. X.Z., Z.L., L.S., J.Y., and G.F.B. edited the manuscript.
ACKNOWLEDGMENTS
This work was partially supported by U.S. Public Health Service Grant AI91517 from the U.S. National Institutes of Health (NIH) National Institute of Allergy and Infectious Disease (to G.F.B.) and by a Brigham Young University grant from the College of Physical and Mathematical Sciences (to G.F.B.). This work was also partially supported by International Science and Technology Cooperation Project of China Grant 2015DFR31060 (to Z.L.); the Science and Technology Program of Hainan Province Grants KJHZ2015-24 and ZDZX2013023 (to Z.L.); and the central government finance support for enhancing the comprehensive strengths of universities in Midwest China. The work was also partially supported by the NIH National Cancer Institute (NCI) Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the reviews or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. Funding for open access was provided by NIH NCI Contract No. HHSN261200800001E. The authors thank Drs. Karen J. Merrell, John T. Prince, Richard A. Lempicki, Tomozumi Imamichi, and Ryan Taylor for their technical advice and help.
Glossary
- AAT
α1-antitrypsin
- CM
complete tissue culture medium
- DSP
dithiobis succinimidyl propionate
- FASP
filter-aided sample preparation
- HPLC-MS
high-performance liquid chromatography-mass spectrometry system
- LRP1
low-density lipoprotein receptor-related protein 1
- qPCR
quantitative polymerase chain reaction
- QRT-PCR
quantitative real-time polymerase chain reaction
- SEC
serpin–enzyme complex
- siRNA
small interfering RNA
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.Carrell R. W. (1986) Alpha 1-antitrypsin: molecular pathology, leukocytes, and tissue damage. J. Clin. Invest. 78, 1427–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potthoff A. V., Münch J., Kirchhoff F., Brockmeyer N. H. (2007) HIV infection in a patient with alpha-1 antitrypsin deficiency: a detrimental combination? AIDS 21, 2115–2116. [DOI] [PubMed] [Google Scholar]
- 3.Bryan C. L., Beard K. S., Pott G. B., Rahkola J., Gardner E. M., Janoff E. N., Shapiro L. (2010) HIV infection is associated with reduced serum alpha-1-antitrypsin concentrations. Clin. Invest. Med. 33, E384–E389. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro L., Pott G. B., Ralston A. H. (2001) Alpha-1-antitrypsin inhibits human immunodeficiency virus type 1. FASEB J. 15, 115–122. [DOI] [PubMed] [Google Scholar]
- 5.Congote L. F. (2007) Serpin A1 and CD91 as host instruments against HIV-1 infection: are extracellular antiviral peptides acting as intracellular messengers? Virus Res. 125, 119–134. [DOI] [PubMed] [Google Scholar]
- 6.Hayes V. M., Gardiner-Garden M. (2003) Are polymorphic markers within the alpha-1-antitrypsin gene associated with risk of human immunodeficiency virus disease? J. Infect. Dis. 188, 1205–1208. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X., Shapiro L., Fellingham G., Willardson B. M., Burton G. F. (2011) HIV replication in CD4+ T lymphocytes in the presence and absence of follicular dendritic cells: inhibition of replication mediated by α-1-antitrypsin through altered IκBα ubiquitination. J. Immunol. 186, 3148–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Münch J., Ständker L., Adermann K., Schulz A., Schindler M., Chinnadurai R., Pöhlmann S., Chaipan C., Biet T., Peters T., Meyer B., Wilhelm D., Lu H., Jing W., Jiang S., Forssmann W. G., Kirchhoff F. (2007) Discovery and optimization of a natural HIV-1 entry inhibitor targeting the gp41 fusion peptide. Cell 129, 263–275. [DOI] [PubMed] [Google Scholar]
- 9.Marsh M., Helenius A. (2006) Virus entry: open sesame. Cell 124, 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty G. J., McMahon H. T. (2009) Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902. [DOI] [PubMed] [Google Scholar]
- 11.Mayor S., Pagano R. E. (2007) Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 8, 603–612. [DOI] [PubMed] [Google Scholar]
- 12.Sandvig K., Torgersen M. L., Raa H. A., van Deurs B. (2008) Clathrin-independent endocytosis: from nonexisting to an extreme degree of complexity. Histochem. Cell Biol. 129, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldonyte R., Hutchinson T. E., Jin B., Brantly M., Block E., Patel J., Zhang J. (2008) Endothelial alpha-1-antitrypsin attenuates cigarette smoke induced apoptosis in vitro. COPD 5, 153–162. [DOI] [PubMed] [Google Scholar]
- 14.Sohrab S., Petrusca D. N., Lockett A. D., Schweitzer K. S., Rush N. I., Gu Y., Kamocki K., Garrison J., Petrache I. (2009) Mechanism of α-1 antitrypsin endocytosis by lung endothelium. FASEB J. 23, 3149–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thacker T. C., Zhou X., Estes J. D., Jiang Y., Keele B. F., Elton T. S., Burton G. F. (2009) Follicular dendritic cells and human immunodeficiency virus type 1 transcription in CD4+ T cells. J. Virol. 83, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X., Li J., Yang W. (2014) Calcium/calmodulin-dependent protein kinase II regulates cyclooxygenase-2 expression and prostaglandin E2 production by activating cAMP-response element-binding protein in rat peritoneal macrophages. Immunology 143, 287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee S., Ghosh R. N., Maxfield F. R. (1997) Endocytosis. Physiol. Rev. 77, 759–803. [DOI] [PubMed] [Google Scholar]
- 18.Zhou X., Yang W., Li J. (2006) Ca2+- and protein kinase C-dependent signaling pathway for nuclear factor-kappaB activation, inducible nitric-oxide synthase expression, and tumor necrosis factor-α production in lipopolysaccharide-stimulated rat peritoneal macrophages. J. Biol. Chem. 281, 31337–31347. [DOI] [PubMed] [Google Scholar]
- 19.Poller W., Willnow T. E., Hilpert J., Herz J. (1995) Differential recognition of α 1-antitrypsin-elastase and α 1-antichymotrypsin-cathepsin G complexes by the low density lipoprotein receptor-related protein. J. Biol. Chem. 270, 2841–2845. [DOI] [PubMed] [Google Scholar]
- 20.Kounnas M. Z., Church F. C., Argraves W. S., Strickland D. K. (1996) Cellular internalization and degradation of antithrombin III-thrombin, heparin cofactor II-thrombin, and α 1-antitrypsin-trypsin complexes is mediated by the low density lipoprotein receptor-related protein. J. Biol. Chem. 271, 6523–6529. [DOI] [PubMed] [Google Scholar]
- 21.Cuervo A. M., Dice J. F. (1998) Lysosomes, a meeting point of proteins, chaperones, and proteases. J. Mol. Med. 76, 6–12. [DOI] [PubMed] [Google Scholar]
- 22.Turk B., Stoka V., Rozman-Pungercar J., Cirman T., Droga-Mazovec G., Oresić K., Turk V. (2002) Apoptotic pathways: involvement of lysosomal proteases. Biol. Chem. 383, 1035–1044. [DOI] [PubMed] [Google Scholar]
- 23.Hinrichsen L., Harborth J., Andrees L., Weber K., Ungewickell E. J. (2003) Effect of clathrin heavy chain- and alpha-adaptin-specific small inhibitory RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J. Biol. Chem. 278, 45160–45170. [DOI] [PubMed] [Google Scholar]
- 24.Brodsky F. M., Chen C. Y., Knuehl C., Towler M. C., Wakeham D. E. (2001) Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17, 517–568. [DOI] [PubMed] [Google Scholar]
- 25.Schmid S. L. (1997) Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu. Rev. Biochem. 66, 511–548. [DOI] [PubMed] [Google Scholar]
- 26.Joslin G., Fallon R. J., Bullock J., Adams S. P., Perlmutter D. H. (1991) The SEC receptor recognizes a pentapeptide neodomain of alpha 1-antitrypsin-protease complexes. J. Biol. Chem. 266, 11282–11288. [PubMed] [Google Scholar]
- 27.Joslin G., Wittwer A., Adams S., Tollefsen D. M., August A., Perlmutter D. H. (1993) Cross-competition for binding of alpha 1-antitrypsin (alpha 1 AT)-elastase complexes to the serpin-enzyme complex receptor by other serpin-enzyme complexes and by proteolytically modified alpha 1 AT. J. Biol. Chem. 268, 1886–1893. [PubMed] [Google Scholar]
- 28.Perlmutter D. H., Joslin G., Nelson P., Schasteen C., Adams S. P., Fallon R. J. (1990) Endocytosis and degradation of α 1-antitrypsin-protease complexes is mediated by the serpin-enzyme complex (SEC) receptor. J. Biol. Chem. 265, 16713–16716. [PubMed] [Google Scholar]
- 29.Maxfield F. R., McGraw T. E. (2004) Endocytic recycling. Nat. Rev. Mol. Cell Biol. 5, 121–132. [DOI] [PubMed] [Google Scholar]
- 30.Grant B. D., Donaldson J. G. (2009) Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 10, 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turk B., Turk V. (2009) Lysosomes as “suicide bags” in cell death: myth or reality? J. Biol. Chem. 284, 21783–21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eskelinen E. L., Tanaka Y., Saftig P. (2003) At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 13, 137–145. [DOI] [PubMed] [Google Scholar]
- 33.Luzio J. P., Pryor P. R., Bright N. A. (2007) Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8, 622–632. [DOI] [PubMed] [Google Scholar]
- 34.Feldstein A. E., Werneburg N. W., Canbay A., Guicciardi M. E., Bronk S. F., Rydzewski R., Burgart L. J., Gores G. J. (2004) Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology 40, 185–194. [DOI] [PubMed] [Google Scholar]
- 35.Guicciardi M. E., Deussing J., Miyoshi H., Bronk S. F., Svingen P. A., Peters C., Kaufmann S. H., Gores G. J. (2000) Cathepsin B contributes to TNF-α-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Invest. 106, 1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werneburg N. W., Guicciardi M. E., Bronk S. F., Gores G. J. (2002) Tumor necrosis factor-alpha-associated lysosomal permeabilization is cathepsin B dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G947–G956. [DOI] [PubMed] [Google Scholar]
- 37.Werneburg N. W., Guicciardi M. E., Bronk S. F., Kaufmann S. H., Gores G. J. (2007) Tumor necrosis factor-related apoptosis-inducing ligand activates a lysosomal pathway of apoptosis that is regulated by Bcl-2 proteins. J. Biol. Chem. 282, 28960–28970. [DOI] [PubMed] [Google Scholar]
- 38.Werneburg N., Guicciardi M. E., Yin X. M., Gores G. J. (2004) TNF-alpha-mediated lysosomal permeabilization is FAN and caspase 8/Bid dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G436–G443. [DOI] [PubMed] [Google Scholar]