Abstract
Major histocompatibility complex class II molecules present peptides from an extracellular source of antigens to CD4+ T lymphocytes. The class II-associated invariant chain affects this role of alpha and beta polypeptides by restriction of peptide loading to endocytic vesicles. Up to now no specific portion of the invariant chain has been defined as the class II binding site. We constructed recombinant invariant chain genes and inspected association of the mutant invariant chains with class II polypeptides. Here we demonstrate that an extracytoplasmic sequence of the invariant chain (aa 81-109) that is only 23 residues away from the transmembrane region is essential for contact with class II polypeptides, whereas the remaining C-terminal part is dispensable for binding. The sequence of invariant-chain-derived peptides that were eluted from class II molecules is contained in this segment and may define the class II binding site of the invariant chain. The membrane-proximal position of this region suggests that the invariant chain and invariant-chain-derived peptides isolated from class II molecules bind to a domain distinct from the class II pocket.
Full text
PDF
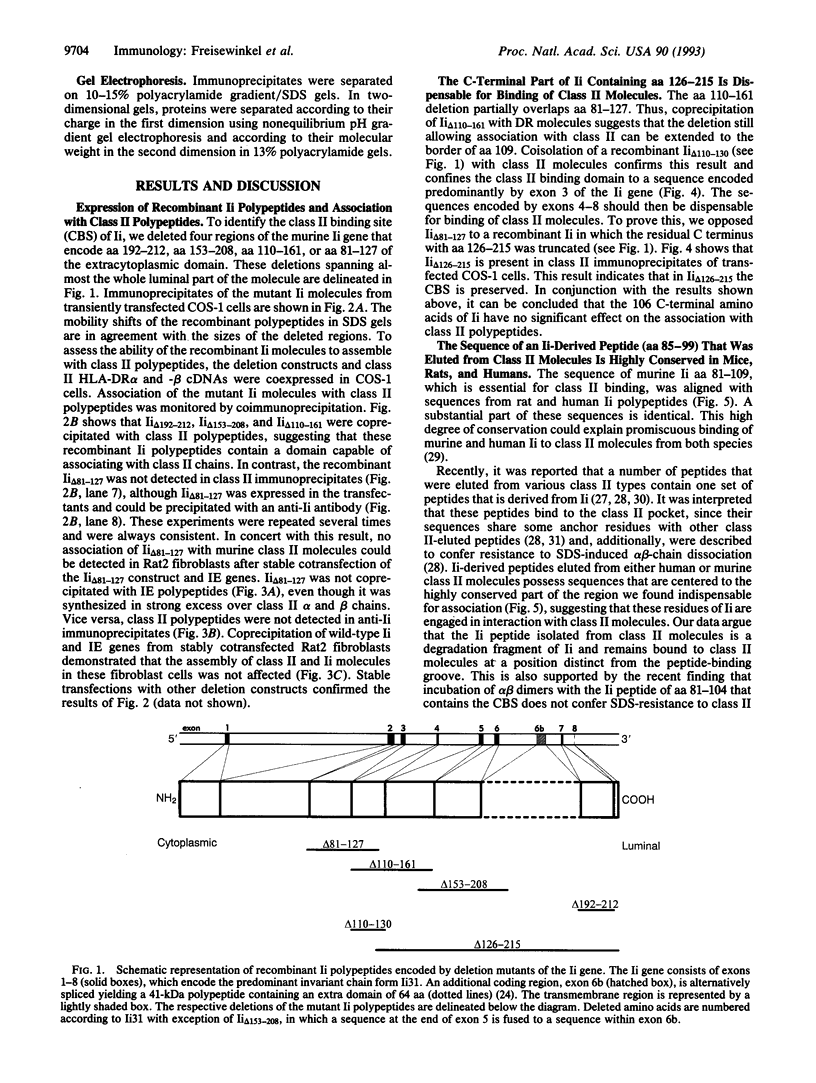

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bikoff E. K., Huang L. Y., Episkopou V., van Meerwijk J., Germain R. N., Robertson E. J. Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+ T cell selection in mice lacking invariant chain expression. J Exp Med. 1993 Jun 1;177(6):1699–1712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J. S., Cresswell P. Role for intracellular proteases in the processing and transport of class II HLA antigens. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3975–3979. doi: 10.1073/pnas.85.11.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron D. J., Engelman E. G., Benike C. J., McDevitt H. O. Ia antigens on alloreactive T cells in man detected by monoclonal antibodies. Evidence for synthesis of HLA-D/DR molecules of the responder type. J Exp Med. 1980 Aug 1;152(2 Pt 2):127s–136s. [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G., Lane W. S., Gorga J. C., Stern L. J., Vignali D. A., Strominger J. L. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature. 1992 Aug 27;358(6389):764–768. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- Claesson L., Larhammar D., Rask L., Peterson P. A. cDNA clone for the human invariant gamma chain of class II histocompatibility antigens and its implications for the protein structure. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7395–7399. doi: 10.1073/pnas.80.24.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Glimcher L. H., Polla B. S., Poljak A., Morton C. C., McKean D. J. Murine class II (Ia) molecules associate with human invariant chain. J Immunol. 1987 Mar 1;138(5):1519–1523. [PubMed] [Google Scholar]
- Henkes W., Syha J., Reske K. Nucleotide sequence of rat invariant gamma chain cDNA clone pLR gamma 34.3. Nucleic Acids Res. 1988 Dec 23;16(24):11822–11822. doi: 10.1093/nar/16.24.11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. F., Michel H., Dickinson T. A., Shabanowitz J., Cox A. L., Sakaguchi K., Appella E., Grey H. M., Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992 Jun 26;256(5065):1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- Karp D. R., Jenkins R. N., Long E. O. Distinct binding sites on HLA-DR for invariant chain and staphylococcal enterotoxins. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9657–9661. doi: 10.1073/pnas.89.20.9657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjer-Nielsen L., Perera J. D., Boyd L. F., Margulies D. H., McCluskey J. The extracellular domains of MHC class II molecules determine their processing requirements for antigen presentation. J Immunol. 1990 Apr 15;144(8):2915–2924. [PubMed] [Google Scholar]
- Koch N., Lauer W., Habicht J., Dobberstein B. Primary structure of the gene for the murine Ia antigen-associated invariant chains (Ii). An alternatively spliced exon encodes a cysteine-rich domain highly homologous to a repetitive sequence of thyroglobulin. EMBO J. 1987 Jun;6(6):1677–1683. doi: 10.1002/j.1460-2075.1987.tb02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch N., Sandrin M. S., Hämmerling G. J., Tada N., Kimura S., Hämmerling U. Monoclonal antibodies detect polymorphic determinants shared by I-A and I-E antigens. Mol Immunol. 1984 Apr;21(4):293–299. doi: 10.1016/0161-5890(84)90100-7. [DOI] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. Signal recognition particle-dependent membrane insertion of mouse invariant chain: a membrane-spanning protein with a cytoplasmically exposed amino terminus. J Cell Biol. 1986 Jun;102(6):2169–2175. doi: 10.1083/jcb.102.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotteau V., Teyton L., Peleraux A., Nilsson T., Karlsson L., Schmid S. L., Quaranta V., Peterson P. A. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990 Dec 13;348(6302):600–605. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- Marks M. S., Cresswell P. Invariant chain associates with HLA class II antigens via its extracytoplasmic region. J Immunol. 1986 Apr 1;136(7):2519–2525. [PubMed] [Google Scholar]
- O'Sullivan D. M., Noonan D., Quaranta V. Four Ia invariant chain forms derive from a single gene by alternate splicing and alternate initiation of transcription/translation. J Exp Med. 1987 Aug 1;166(2):444–460. doi: 10.1084/jem.166.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesando J. M., Graf L. Differential expression of HLA-DR, -DQ, and -DP antigens on malignant B cells. J Immunol. 1986 Jun 1;136(11):4311–4318. [PubMed] [Google Scholar]
- Peterson M., Miller J. Antigen presentation enhanced by the alternatively spliced invariant chain gene product p41. Nature. 1992 Jun 18;357(6379):596–598. doi: 10.1038/357596a0. [DOI] [PubMed] [Google Scholar]
- Peterson M., Miller J. Invariant chain influences the immunological recognition of MHC class II molecules. Nature. 1990 May 10;345(6271):172–174. doi: 10.1038/345172a0. [DOI] [PubMed] [Google Scholar]
- Rath S., Lin R. H., Rudensky A., Janeway C. A., Jr T and B cell receptors discriminate major histocompatibility complex class II conformations influenced by the invariant chain. Eur J Immunol. 1992 Aug;22(8):2121–2127. doi: 10.1002/eji.1830220824. [DOI] [PubMed] [Google Scholar]
- Riberdy J. M., Newcomb J. R., Surman M. J., Barbosa J. A., Cresswell P. HLA-DR molecules from an antigen-processing mutant cell line are associated with invariant chain peptides. Nature. 1992 Dec 3;360(6403):474–477. doi: 10.1038/360474a0. [DOI] [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature. 1990 Jun 14;345(6276):615–618. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. Proteolysis of the class II-associated invariant chain generates a peptide binding site in intracellular HLA-DR molecules. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3150–3154. doi: 10.1073/pnas.88.8.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche P. A., Teletski C. L., Karp D. R., Pinet V., Bakke O., Long E. O. Stable surface expression of invariant chain prevents peptide presentation by HLA-DR. EMBO J. 1992 Aug;11(8):2841–2847. doi: 10.1002/j.1460-2075.1992.tb05351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., Hong S. C., Barlow A., Janeway C. A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991 Oct 17;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., al-Ramadi B. K., Rothbard J., Janeway C. A., Jr Truncation variants of peptides isolated from MHC class II molecules suggest sequence motifs. Nature. 1992 Oct 1;359(6394):429–431. doi: 10.1038/359429a0. [DOI] [PubMed] [Google Scholar]
- Singer P. A., Lauer W., Dembić Z., Mayer W. E., Lipp J., Koch N., Hämmerling G., Klein J., Dobberstein B. Structure of the murine Ia-associated invariant (Ii) chain as deduced from a cDNA clone. EMBO J. 1984 Apr;3(4):873–877. doi: 10.1002/j.1460-2075.1984.tb01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B., Pessara U., Lin R. H., Habicht J., Grez M., Koch N. A role of Ia-associated invariant chains in antigen processing and presentation. Cell. 1989 Feb 24;56(4):683–689. doi: 10.1016/0092-8674(89)90590-4. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Suzuki T., Taniguchi M., Shinohara N. Monoclonal anti-Ia murine alloantibodies crossreactive with the Ia-homologues of other mammalian species including humans. Transplantation. 1983 Dec;36(6):712–718. doi: 10.1097/00007890-198336060-00025. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]