Abstract
Despite described benefits of aerobic high-intensity interval exercise (HIIE), the acute responses during different HIIE modes and associated health risks have only been sparsely discovered in heart disease patients. Therefore, the aim of this study was to investigate the acute responses for physiological parameters, cardiovascular and inflammatory biomarkers, and catecholamines yielded by two different aerobic HIIE protocols compared to continuous exercise (CE) in phase III cardiac rehabilitation. Eight cardiac patients (7 with coronary heart disease, 1 with myocarditis; 7 males, 1 female; age: 63.0 ± 9.4 years; height: 1.74 ± 0.05 m; weight: 83.6 ± 8.7 kg), all but one treated with ß-blocking agents, performed a maximal symptom-limited incremental exercise test (IET) and three different exercise tests matched for mean load (Pmean) and total duration: 1) short HIIE with a peak workload duration (tpeak) of 20 s and a peak workload (Ppeak) equal to the maximum power output (Pmax) from IET; 2) long HIIE with a tpeak of 4 min, Ppeak was corresponding to the power output at 85 % of maximal heart rate (HRmax) from IET; 3) CE with a target workload equal to Pmean of both HIIE modes. Acute metabolic and peak cardiorespiratory responses were significantly higher during long HIIE compared to short HIIE and CE (p < 0.05) except HRpeak which tended to be higher in long HIIE than in short HIIE (p = 0.08). Between short HIIE and CE, no significant difference was found for any parameter. Acute responses of cardiovascular and inflammatory biomarkers and catecholamines didn’t show any significant difference between tests (p > 0.05). All health-related variables remained in a normal range in any test except NT-proBNP, which was already elevated at baseline. Despite a high Ppeak particularly in short HIIE, both HIIE modes were as safe and as well tolerated as moderate CE in cardiac patients by using our methodological approach.
Key points.
High-intensity interval exercise (HIIE) with short peak workload durations (tpeak) induce a lower acute metabolic and peak cardiorespiratory response compared to intervals with long tpeak despite higher peak workload intensities and identical mean load. No significant difference for any physiological parameter was found between short HIIE and CE.
Between short HIIE, long HIIE, and CE, no significant difference was found in the increase (or decrease, respectively,) of health related markers such as cardiovascular biomarkers, catecholamines, or inflammatory parameters during exercise.
During all exercise modes, all risk markers remained in a normal range except for NT-proBNP which was, however, already elevated at baseline.
Short HIIE, long HIIE, and CE were safely performed by patients with CHD or myocarditis in cardiac rehabilitation by using our methodological approach to exercise prescription. This approach included the prescription of exercise intensities with respect to LTP1, LTP2, and Pmax as well as a conscious setting of Pmean at a moderate level (80 % of PLTP2). Importantly, all exercise modes were matched for Pmean and exercise duration in order to enable a comparison of the three protocols.
Key words: intermittent exercise, exercise prescription, acute effects, health-related markers, heart disease patients
Introduction
Exercise training is accepted as a fundamental non-pharmacological intervention strategy in cardiac rehabilitation (Normandin et al., 2013). For training therapy, extensive research looking at the individual dose-effect relationship should be standard practice as it is for pharmacological interventions (Church and Blair, 2009). The participation of vigorous-intensity aerobic exercise (Haskell et al., 2007) as well as high- to severe-intensity aerobic interval exercise (Mezzani et al., 2012) is encouraged by current guidelines and recommendations. The various beneficial effects yielded by aerobic high-intensity interval training in heart disease patients are well documented (Juneau et al., 2014; Warburton et al., 2005). In particular, maximal oxygen uptake (VO2max), which has been noted as the best single predictor of death among cardiac patients (Kavanagh et al., 2002), was found to be improved through high-intensity interval exercise (HIIE). These improvements were observed to be similar (Conraads et al., 2015) or even greater compared to moderate continuous exercise training (Arena et al., 2013; Haykowsky et al., 2013; Wisloff et al., 2007). Therefore, aerobic high-intensity interval training has been reported to be an effective alternate to conventional continuous endurance training in cardiac rehabilitation (Gibala et al., 2012; Guiraud et al., 2012).
However, since the peak workload in aerobic HIIE is usually higher than in moderate continuous exercise (moderate CE), the acute physiological responses and, consequently, the potential risk of adverse events, may be increased during HIIE (Arena et al., 2013; Rognmo et al., 2012; Keteyian, 2012; Thompson et al., 2007). Though Rognmo et al. (2012) found low event rates during both moderate CE (1 event per 129456 exercise hours) and HIIE (1 event per 23182 exercise hours), Keteyian (2012) argued that an exploratory interpretation of these data might be that moderate CE is safer than HIIE. As emphasized by Arena et al. (2013) and our own working group (Tschakert and Hofmann, 2013), both the adverse event risk and the achieved beneficial effects may vary between different HIIE protocols (Mezzani et al., 2012). More precisely, health risks and training adaptations are caused by the acute physiological responses yielded by particular interval protocols depending on the setting of the single HIIE determinants (intensity and duration of the peak workload and recovery phases and the resulting mean load, respectively). Therefore, it is highly relevant to discover which HIIE prescription is most suitable for cardiac patients with respect to both safety and efficiency (Arena et al., 2013; Gibala et al., 2012).
With respect to the particular setting of HIIE, the fundamental findings of Astrand et al. (1960) and Saltin et al. (1976) revealed that a great amount of work at high intensities can be obtained with clear submaximal circulatory and respiratory load by an appropriate application of short work periods. These findings were supported by recent investigations in healthy individuals (Tschakert et al., 2015) and patients with type 1 diabetes mellitus (Moser et al., 2015).
The number of studies investigating the acute response to aerobic HIIE in cardiac patients, however, is rather small. The impact of different HIIE protocols on the acute physiological response was investigated in chronic heart failure (CHF) patients (Meyer et al., 1996; 2012) and in subjects with stable coronary heart disease (CHD) (Guiraud et al., 2010). Meyer et al. (1996) did not find essential differences in the acute metabolic, cardiac, and hormonal response between the HIIE modes, whereas Guiraud et al. (2010) and Meyer et al. (2012) found short HIIE to be more effective with respect to the time to exhaustion, the time spent near VO2max, and rating of perceived exertion (RPE) compared to long intervals. Cardiac risk markers such as cardiac troponin T (cTnT) and creatine phosphokinase MB (CK MB) which are biomarkers of cardiac injury, and B-type natriuretic peptide (NT-proBNP), a marker for cardiomyocyte stress (heart failure), as well as Lipoprotein-associated phospholipase A2 (Lp-PLA2), a platelet-activating factor, were not measured.
Few studies have investigated the acute physiological response yielded by certain HIIE protocols compared to CE in cardiac patients. No significantly different response for cardiac biomarkers (Benda et al., 2015; Normandin et al., 2013), endothelial microparticles (Guiraud et al., 2012; 2013), hemodynamic markers and arteriovenous O2-difference (Gayda et al., 2012; Meyer et al., 1998), respiratory markers (Normandin et al., 2013), C-reactive protein (Normandin et al., 2013), and RPE (Normandin et al., 2013) were observed between HIIE and CE, despite markedly higher peak intensities in HIIE. In these studies, HIIE and CE were isocaloric (Benda et al., 2015; Guiraud et al., 2012; 2013; Normandin et al., 2013) or matched for mean load (Meyer et al., 1998). However, no HIIE protocols with peak workload durations longer than 1 min were applied in these investigations. Meyer et al. (2012) used longer peak workload durations of 90 s for intermittent exercise but did not compare HIIE vs. CE.
The question arises which HIIE protocol is most convenient for cardiac patients. Guiraud et al. (2010) and Meyer et al. (2012) suggested HIIE protocols with short tpeak and passive recovery to be more convenient than HIIE with long tpeak and active recovery. Benda et al. (2015) and Guiraud et al. (2011, 2012) suggested highly intense exercise stimuli to the peripheral muscles without great cardiovascular stress to be possible by using intermittent exercise with short bouts of work followed by short recovery periods. In line with that, Meyer et al. (2012) and Conraads et al. (2015) suggested long intervals, such as the 4 x 4 min model, to be problematic since patients can hardly sustain 4 min intervals at high intensities. In contrast, Arena et al. (2013) favors HIIE with long tpeak similar to the “Norwegian” model which is frequently applied in scientific studies and practice in patients suffering from different chronic diseases (Helgerud et al., 2010; Rognmo et al., 2004; Tjonna et al., 2008; Wisloff et al., 2007). However, as emphasized by Normandin et al. (2013), the acute physiological responses to the 4 x 4 interval mode, or to similar HIIE protocols applying a longer tpeak (Warburton et al., 2005), were not published.
Therefore, the aim of our study was to investigate the acute response for metabolic, cardiorespiratory, and plasma parameters such as cardiovascular and inflammatory markers and catecholamines, in patients undergoing phase III cardiac rehabilitation. The test protocols which were applied in the study included short (20 s) and long (4 min) HIIE and moderate CE matched for mean load and total duration.
We hypothesized that during short HIIE, mean and peak values for lactate (La) as well as peak values for heart rate (HR) and oxygen uptake (VO2) will be similar to CE but significantly lower compared to long HIIE despite a markedly higher Ppeak in short HIIE. However, we hypothesized that the exercise-induced changes in the concentration of cardiovascular biomarkers, catecholamines, and inflammatory markers will not significantly differ between CE, short HIIE, and long HIIE, indicating that all three exercise tests can be performed safely.
Methods
Eight patients undergoing phase III cardiac rehabilitation (7 males, 1 female; age: 63.0 ± 9.4 years; height: 1.74 ± 0.05 m; weight: 83.6 ± 8.7 kg; VO2max: 21.6 ± 7.6 ml·kg-1·min-1) participated in this study. Preceding coronary incidences (> 8 weeks) included coronary heart disease (CHD; n = 7) and myocarditis without CHD (n = 1). Left ventricular ejection fraction (LVEF) was ≥ 45 % in all subjects. Seven of eight patients were treated with ß-blocking agents during the study (Table 1). The experimental protocol was approved by the ethics commission of the Medical University of Graz, Austria (EK decision number 23-397 ex 10/11). The test design, and potentially associated health risks, were explained to all subjects who gave their written informed consent before participating in the study. They were familiar with the cycle ergometer exercise in the cardiac rehabilitation center (ZARG, Graz, Austria) where all tests were performed within the rehabilitation program under medical supervision.
Table 1.
1. Baseline clinical characteristics and medication (n = 8). Results are presented as row values (% of the sample).
| Cardiac etiology | |
|---|---|
| CHD | 7 (87.5 %) |
| Bypass | 1 (12.5 %) |
| PCI | 3 (37.5 %) |
| Myocardial infarction | 1 (12.5 %) |
| Myocarditis | 1 (12.5 %) |
| Medication | |
| Beta blocker | 7 (87.5 %) |
| ACE inhibitors | 7 (87.5 %) |
| Statins | 6 (75 %) |
| Anti platelet agents | 7 (87.5 %) |
| Proton pump inhibitors | 7 (87.5 %) |
CHD, coronary heart disease; PCI, percutaneous coronary intervention; ACE, angiotensin-converting enzyme.
Experimental design
At the beginning of the study, the subjects performed a maximal symptom-limited incremental exercise test (IET) in order to assess the maximum aerobic power output (Pmax), VO2max, and maximum heart rate (HRmax), as well as the first and second lactate turn point (LTP1, LTP2) referring to the three phase model of metabolism (Hofmann and Tschakert, 2011) and to the Lactate Shuttle Theory by Brooks (Brooks, 2009). LTP1 and LTP2 were accordant with the first (VT1) and second ventilatory threshold (VT2) and were used for exercise intensity prescription for HIIE and CE.
Then, the participants performed three specific exercise tests: short HIIE (A), long HIIE (B), and CE (C). Importantly, all exercise tests were matched for mean load (Pmean) and total exercise duration. The testing sessions were randomly assigned and interspersed by at least 2 days.
Incremental Exercise Test (IET)
The IET started with a resting period of 1 min (0 W) and a subsequent warm-up phase at 10 Watt (W) also for 1 min. Then, the power output was increased by 10 W per minute until (symptom-limited) exhaustion according to the standard protocol of the Austrian Society of Cardiology (Wonisch et al., 2008). A 3 min cool-down period at 10 W finalized the IET.
LTP1 and LTP2 were determined by means of computer-aided linear regression break point analysis. LTP1 was defined as the first increase of La above baseline level, and LTP2 was defined as the second abrupt increase of La between LTP1 and Pmax.
Short and long High-Intensity Interval Exercise (HIIE) and Continuous Exercise (CE)
After the IET, the participants performed three specific exercise tests (short HIIE, long HIIE, and CE) in randomized order. For each of these exercise tests, the periods for rest (0 W for 3 min), warm-up (consisting of 3 workload steps of 1 min each with increasing intensity until PLTP2 was reached), and cool-down (5 min passive recovery, 0 W) were the same. The specific exercise protocols were matched for mean load, which was purposefully set at 80 % of the power output at LTP2, and for total exercise duration (28 min). The other exercise components were prescribed as follows (Figure 1): A) short HIIE with a tpeak of 20 sec, a Ppeak at 100 % of Pmax from IET, a recovery workload (Prec) 10 % below the power output at LTP1, and a calculated recovery duration (trec) (39 ± 6 s); B) long HIIE (according to Wisloff et al. (2007) but modified) with a tpeak of 4 min, a Ppeak corresponding to the power output at 85 % of HRmax (74 % of Pmax) from IET, a trec of 3 min, and a calculated Prec (95 ± 20 % of PLTP1); and C) CE with a target workload equal to Pmean of both HIIE protocols (80 % of PLTP2 equating to 57 % of Pmax from IET). The participants were permitted to cycle at a cadence of 70-90 revolutions per minute (rpm), and each subject completed all tests with the same rpm.
Figure 1.
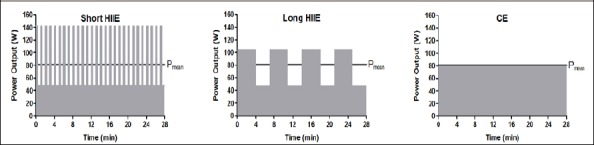
Prescription of the three specific exercise protocols (short HIIE, long HIIE, and CE) matched for mean load and exercise duration. Consequently, the total work performed during the 28 min (dark area) was also equal between tests, but Ppeak was markedly higher in short HIIE. HIIE, high-intensity interval exercise; CE, continuous exercise.
The equation Pmean = (Ppeak · tpeak + Prec · trec) / (tpeak + trec) (Tschakert and Hofmann, 2013) was used and transformed in order to calculate trec for short HIIE and Prec for long HIIE.
Measurements
An electrocardiogram (Cardiosoft v6.51 GE Healthcare, GE Healthcare, UK) was obtained from each subject during all tests and supervised by an experienced physician. Pulmonary gas-exchange variables were collected continuously during all tests by breath-by-breath measurement and were averaged over 5 s periods (MetaMax 3B, Cortex, Germany). The maximum oxygen uptake (VO2max) was defined as the highest mean value for VO2 over 30 s. Heart rate data, averaged over 5 s periods, were also obtained during all tests via chest belt telemetry (PE 4000, Polar Electro, Kempele, Finland). Blood lactate concentrations were obtained from capillary blood samples taken from ear lobes during all tests. For IET, capillary blood samples were taken at the end of rest and warm-up, at the end of each workload step, and at the end of the cool-down period. For each of the three exercise tests, capillary blood samples were taken at rest and at the end of the warm-up phase, after 4, 7, 11, 14, 18, 21, 25, and 28 min of the specific exercise protocol, and after the 5 min cool-down phase.
Venous blood withdrawal from the cubital vein was done before each test and directly after the specific exercise protocol. The blood samples were collected in specific blood tubes and centrifuged (~3000 rpm, 10 min) to separate the plasma, which was immediately frozen in aliquots at -80° C for longer storage and subsequent analysis.
Data analysis procedures
The determination of individual turn points during IET (LTP1, LTP2, VT1, VT2) was accomplished by means of a computer-supported linear regression turn point model within defined regions of interest (ROI) (Hofmann and Tschakert, 2011). ROI for LTP1 (and VT1) was between La (and VE) at first workload and La (and VE) at 70 % of Pmax, ROI for LTP2 (and VT2) was between La (and VE) at LTP1 and La (and VE) at Pmax (Hofmann et al., 2001).
The concentrations of capillary blood lactate were evaluated via the fully enzymatic-amperometric method (Biosen S-line, EKF diagnostics, Barleben, Germany).
Plasma concentrations of the following markers were determined and compared with reference values by the Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz: creatine phosphokinase (CK) was measured enzymatically (Roche Diagnostics, Mannheim, Germany) on a cobas® 8000 modular analyzer from Roche. C-reactive protein (CRP) was measured by immunoturbidimetry (Roche Diagnostics, Mannheim, Germany) on a cobas® 8000 modular analyzer from Roche. N-terminal pro B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT), and interleukin 6 (IL-6) were determined by electrochemiluminescence (Roche Diagnostics, Mannheim, Germany). Lipoprotein-associated phospholipase A2 (Lp-PLA2) activity was measured with the PLAC® test (diaDexus Inc., South San Francisco, USA) on an Olympus AU640 (Olympus Diagnostika, Hamburg, Germany). Adiponectin (Immundiagnostik AG, Bensheim, Germany) and leptin (DRG Instruments GmbH, Marburg, Germany) were measured by ELISA according to the manufacturer’s instructions. Homoarginine (hArg), asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) were measured by high-performance liquid chromatography (HPLC). The concentrations of free noradrenaline (NA), adrenaline (ADR), and dopamine (DOP) were detected using an amperometrical detector (RECIPE, Munich, Germany) and specialized software (ClarityTM, DataApex, Prague, Czech Republic).
Statistical analysis
All data are presented as mean ± standard deviation (SD) and were analyzed by means of SPSS (IBM SPSS Statistics 23). Normal distribution of variables and variables’ paired differences were assessed by the Kolmogorov-Smirnov test.
A one-way ANOVA with repeated measures (within factors) was conducted in order to determine the effects of the different exercise regimes on the acute response of La, HR, and gas exchange variables. When the analysis revealed a significant difference, post-hoc paired t-tests with least significant difference (LSD) were used to locate the origin of the significant difference.
A two-way repeated measures ANOVA design (2x3) was used to test the differences in the increase (or decrease) of plasma parameters during exercise (factor time: pre, post) between the three test protocols (factor test: CE, short HIIE, and long HIIE). The interaction between both factors was taken into account. If an overall significance could be detected for one or both of the factors, post-hoc t-tests with LSD were conducted for clarification. A p-value < 0.05 was considered as significant.
Results
Incremental exercise test
Figure 2 shows the performance curves for lactate and heart rate during the incremental exercise test. The lactate performance curve showed three phases of blood lactate appearance and two corresponding turn points (LTP1, LTP2). The first and second lactate turn point was significantly related to the first (VT1) and second ventilatory threshold (VT2) with no significant difference in power output (LTP1: 51.8 ± 10.4 W; VT1: 52.3 ±13.8 W; LTP2: 101.1 ± 30.1 W; VT2: 99.8 ± 31.2 W).
Figure 2.
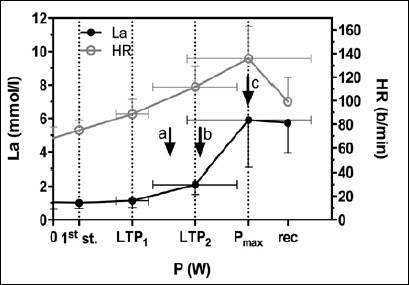
Performance curves for lactate and heart rate during the incremental exercise test (IET). The three phases of lactate metabolism are separated by the first (LTP1) and second lactate turn point (LTP2). Values are means ± SD. Exercise intensities for both HIIE modes and CE were set with respect to PLTP1, PLTP2, and Pmax from IET. Arrow a represents Pmean for all three exercise protocols; arrow b represents Ppeak for long HIIE; arrow c represents Ppeak for short HIIE. La, blood lactate; HR, heart rate; 1st st., first workload step; Pmax, maximum power output; rec, recovery.
CE, short HIIE, and long HIIE
Metabolic and cardiorespiratory parameters:
All subjects completed each exercise session. As shown in Table 2, mean and peak lactate values were significantly higher during long HIIE than during short HIIE and CE (p < 0.05). A lactate steady state (LaSS) (no La increase higher than 1.0 mmol·l-1 for the last 20 min of the specific exercise protocol) was reached in each test (Figure 3A).
Table 2.
Mean and peak values for P, La, HR, and VO2 during CE, short HIIE (20 s), and long HIIE (4x4 min) compared to the according values at Pmax or LTP2 and VT2 from IET. Values are means (±SD).
| Peak values | CE | HIIE 20s | HIIE 4x4min | at Pmax (IET) | |
|---|---|---|---|---|---|
| P (W) | 80.9 (24.0) | 142.4 (44.9)* | 105.3 (51.8)*‡ | 142.4 (44.9) | |
| La (mmol·l-1) | 3.63 (1.73) | 3.90 (1.98) | 5.54 (2.93)*‡ | 5.90 (2.82) | |
| HR (b·/min-1) | 111.3 (18.0) | 116.3 (22.8) | 124.0 (24.9)* | 135.8 (27.1) | |
| VO2 (l·min-1) | 1.53 (.25) | 1.63 (.36) | 1.87 (.61)*‡ | 1.78 (.60) | |
| Mean values | CE | HIIE 20s | HIIE 4x4min | at LTP2 (IET) | at VT2 (IET) |
| P (W) | 80.9 (24.0) | 80.9 (24.0) | 80.9 (24.0) | 101.1 (30.1) | 99.8 (31.2) |
| La (mmol·l-1) | 3.20 (1.58) | 3.35 (1.74) | 4.80 (2.55)*‡ | 2.39 (.98) | 2.34 (1.03) |
| HR (b·min-1) | 103.6 (17.2) | 106.2 (20.8) | 109.1 (20.5) | 111.7 (17.8) | 110.7 (18.5) |
| VO2 (l·min-1) | 1.40 (.24) | 1.46 (.34) | 1.48 (.34) | 1.36 (.28) | 1.36 (.29) |
P power output; La, blood lactate concentration; HR, heart rate; VO2, oxygen uptake; CE, continuous exercise; HIIE, high-intensity interval exercise; LTP2, second lactate turn point; VT2, second ventilatory threshold; Pmax, maximum power output; IET, incremental exercise test.
*, significant difference to CE.
‡, significant difference to short HIIE.
Figure 3.
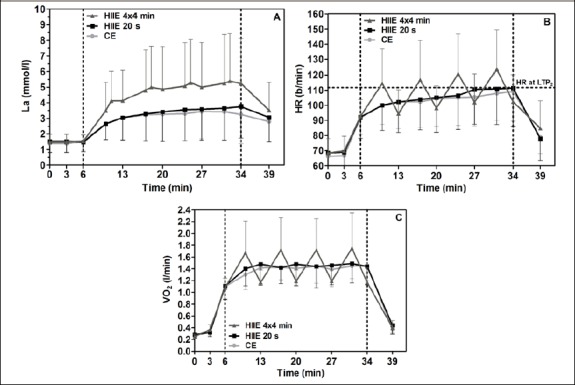
Acute response for La (A), HR (B), and VO2 (C) to CE, short HIIE, and long HIIE. Values are means ± SD. The specific exercise modes were conducted from min 6 to min 34 after a standardized warm up phase. La, blood lactate; HR, heart rate; VO2, oxygen uptake; HIIE, high-intensity interval exercise; CE, continuous exercise; LTP2, second lactate turn point from the incremental exercise test.
In long HIIE, HRpeak was significantly higher than in CE (p < 0.05) and tended to be higher than in short HIIE (p = 0.08). HRpeak during long HIIE was also markedly higher compared to the heart rate at LTP2 in the IET (111.7 ± 17.7 b/min). Peak VO2 values were significantly higher in long HIIE compared to short HIIE and CE (p < 0.05). As expected, no significant differences for HRmean and VO2mean were found between CE, short HIIE, and long HIIE.
Between short HIIE and CE, no significant difference was found for any physiological parameter (Table 2).
Plasma parameters:
Cardiac biomarkers: Between CE, short HIIE, and long HIIE, no significant differences were found in the exercise-induced increase (or decrease) of NT-proBNP, hs-cTnT, CK MB, and Lp-PLA2 (p > 0.05) (Figure 4A-D).
Figure 4.
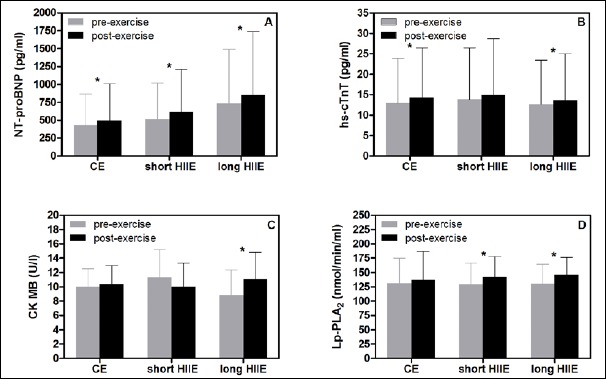
Comparison of baseline vs. post-exercise values of cardiac biomarkers NT-proBNP (A), hs-cTnT (B), CK MB (C), and Lp-PLA2 (D) for CE, short HIIE, and long HIIE. Between test protocols, no significant difference was found for the exercise-induced increase (or decrease, respectively) of cardiac biomarkers. HIIE, high-intensity interval exercise; CE, continuous exercise; NT-proBNP, N-terminal pro B-type natriuretic peptide; hs-cTnT, high-sensitivity cardiac troponin T; CK MB, creatine phosphokinase MB; Lp-PLA2, Lipoprotein-associated phospholipase A2. *, significant difference between pre- vs. post-exercise value (p < 0.05).
The differences between pre- and post-exercise values for each single exercise protocol are presented in Figure 4A-D.
Catecholamines: The increase (or decrease) of the free catecholamines NA, ADR, and DOP during exercise was not significantly different between CE, short HIIE, and long HIIE (p > 0.05) (Table 3).
Table 3.
Pre- and post-exercise values for different plasma parameters in CE, short HIIE (20 s), and long HIIE (4x4 min). Values are means (±SD).
| CE | CE | HIIE 20s | HIIE 20s | HIIE 4x4min | HIIE 4x4min | |
|---|---|---|---|---|---|---|
| Plasma Parameters | pre-exercise | post-exercise | pre-exercise | post-exercise | pre-exercise | post-exercise |
| CK (U·l-1) | 109.3 (48.1) | 117.1 (51.1)* | 128.3 (77.4) | 139.4 (77.5)* | 124.6 (64.2) | 135.3 (67.2)* |
| CRP (mg·l-1) | 3.6 (3.4) | 3.8 (3.5)* | 2.8 (2.5) | 3.0 (2.6) | 2.8 (1.6) | 3.0 (1.7)* |
| IL-6 (pg·ml-1) | 4.5 (2.2) | 5.4 (2.2)* | 4.5 (1.7) | 5.8 (2.3)* | 5.2 (3.2) | 6.6 (4.0)* |
| Adiponectin (µg·ml-1) | 7.5 (5.1) | 7.8 (5.3)* | 7.2 (5.4) | 7.8 (5.8)*† | 7.1 (4.6) | 7.3 (4.6)* |
| Leptin (ng·ml-1) | 7.2 (4.6) | 6.9 (3.8) | 5.2 (2.1) | 5.3 (2.1) | 5.9 (2.5) | 6.0 (2.4) |
| hArg (µmol·l-1) | 1.7 (.7) | 1.7 (.7) | 1.8 (.7) | 1.8 (.7) | 1.6 (.6) | 1.6 (.6) |
| ADMA (µmol·l-1) | .7 (.2) | .7 (.2) | .7 (.2) | .7 (.2) | .7 (.2) | .7 (.2) |
| SDMA (µmol·l-1) | .8 (.3) | .7 (.3) | .8 (.3) | .9 (.5) | .8 (.3) | .8 (.3) |
| ADR (pg·ml-1) | 84.7 (54.9) | 127.7 (46.9)* | 102.1 (93.5) | 166.4 (87.6)* | 83.6 (38.2) | 139.4 (67.5) |
| NA (pg·ml-1) | 1011.3 (438.8) | 1764.2 (947.9)* | 955.8 (602.8) | 2057.2 (1488.0)* | 892.0 (395.8) | 1723.8 (888.1)* |
| DOP (pg·ml-1) | 302.9 (207.1) | 300.1 (188.4) | 370.8 (218.4) | 371.6 (119.4) | 332.1 (241.8) | 360.2 (256.0) |
CK, creatine phosphokinase; CRP, C-reactive protein; IL-6, interleukin 6; hArg, homo arginine; ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; ADR, free adrenaline; NA, free noradrenaline; DOP, free dopamine; CE, continuous exercise; HIIE, high-intensity interval exercise.
*, significant difference to pre-exercise value.
†, significantly higher increase from baseline to post-exercise value compared to CE and long HIIE.
Significant differences (p < 0.05) between pre-exercise vs. post-exercise values were found for NA and ADR in all tests (Table 3).
Other plasma parameters: Between CE, short HIIE, and long HIIE, no significant differences were found in the increase (or decrease) of CK, vascular parameters such as ADMA, SDMA, and hArg, both inflammatory markers CRP and IL-6, and leptin (p > 0.05). Only adiponectin showed a significantly higher increase during short HIIE compared to CE and long HIIE (p < 0.05) (Table 3).
Between pre- and post-exercise values, a significant difference (p < 0.05) in each test was found for CK, CRP, IL-6, and adiponectin (Table 3).
Discussion
In this study, we investigated the acute response for health-related variables such as cardiac biomarkers, inflammatory parameters, and catecholamines induced by moderate CE vs. short and long HIIE in patients with CHD or myocarditis undergoing phase 3 cardiac rehabilitation.
The major finding of our study was that although the acute metabolic and peak-cardiorespiratory responses were higher during long HIIE compared to short HIIE and CE, the exercise-induced increase (or decrease, respectively) of any health-related plasma parameters was not significantly different between the three test protocols, and all exercise modes were performed safely. The methodological approach for exercise prescription may be a critical factor in this population.
Methodological approach
Exercise should be prescribed individually and as accurately as possible. Homogeneous relative exercise stimuli as well as acute physiological responses across subjects should be induced. Therefore, exercise intensities are recommended to be prescribed with respect to objective and individual submaximal (LTP1, LTP2 or VT1, VT2) and maximal markers (Pmax) assessed during an incremental exercise test (Hofmann and Tschakert, 2011; Mezzani et al., 2012). Intensity prescriptions by means of fixed percentages of HRmax or VO2max have been shown to result in heterogeneous relative exercise stimuli and, consequently, in a wide range of acute physiological responses across subjects (Mezzani et al., 2012; Scharhag-Rosenberger et al., 2010). In our study, all exercise intensities were prescribed with respect to LTP1, LTP2, or Pmax except for Ppeak for long HIIE which was set at the power output at 85 % of HRmax according to the original Norwegian 4x4 HIIE mode (Wisloff et al., 2007).
Second, CE and both HIIE protocols were matched for mean load and total duration in our study (Figure 1). This allows a consistent comparison and interpretation of obtained results (Moser et al., 2015; Tschakert and Hofmann, 2013; Tschakert et al., 2015) as it is when isocaloric exercise modes are applied (Benda et al., 2015; Normandin et al., 2013).
Since the mean load determines the average cardiorespiratory response and strongly influences the acute metabolic response as well (Tschakert and Hofmann, 2013; Tschakert et al., 2015), Pmean was set consciously in order to allow for better control and regulation of the acute physiological response (Tschakert and Hofmann, 2013). Pmean was set moderately (at 80 % of PLTP2) to avoid high cardiac and metabolic stress. In the original Norwegian 4x4 min model (Helgerud et al., 2007; Rognmo et al., 2004; Wisloff et al., 2007), the mean load has not been considered.
Acute metabolic and cardiorespiratory responses
If the power output is above the second lactate turn point (LTP2) and the second ventilatory threshold (VT2) respectively, the blood lactate concentration increases with exercise duration (Beneke et al., 2011). In patients treated with beta blockers, who typically show a non-regular heart rate performance curve, a peak power output at 85% of HRmax might correspond to workloads above LTP2 (Hofmann et al., 2001, 2005; Wonisch et al., 2003). With respect to that, the acute metabolic response (Lamean, Lapeak) in this study was significantly higher during long HIIE compared to short HIIE and CE, despite a markedly higher Ppeak and a significantly shorter trec in short HIIE. A LaSS was reached in each exercise protocol, even in long 4x4 HIIE. However, the LaSS during long HIIE was probably facilitated by the moderate Pmean but was still near the maximal LaSS (Figure 3A).
The acute mean cardiorespiratory response (HRmean, VO2mean) did not significantly differ between CE, short HIIE, and long HIIE since Pmean for each test was equal. However, during the 4x4 min HIIE, the HR markedly oscillated between work and recovery phases (Figure 3B) reflecting a temporarily elevated cardiac stress during the peak workload periods. In short HIIE, the HR oscillation was clearly smaller due to the short tpeak of 20 s resulting in peak HR values similar to those during CE. This is remarkable given the high Ppeak in short HIIE (corresponding to Pmax from IET). As a consequence, the peak cardiorespiratory response (HRpeak, VO2peak) was significantly higher during long HIIE compared to short HIIE and CE. Our results are in accordance with the fundamental findings of Astrand et al. (1960) and Saltin et al. (1976) and support the results of Meyer et al. (1997), who have successfully applied HIIE in clinical populations.
Plasma parameters
We measured hs-cTnT and CK MB, which are risk markers of cardiac damage, NT-proBNP, a risk factor of cardiac insufficiency, and Lp-PLA2, a platelet-activating factor reported to be associated with many traditional risk factors for cardiovascular disease such as cardiac death, myocardial infarction, acute coronary syndromes and ischemic stroke. None of these biomarkers showed significantly different acute increases (or decreases) between short and long HIIE and CE (Figure 4A-D). This is in accordance with the studies of Benda et al. (2015), Normandin et al. (2013), and Guiraud et al. (2011).
Importantly, during all tests the post-exercise concentrations of hs-cTnT, CK MB, and Lp-PLA2 were not clinically relevant excluding myocardial injuries during any exercise test. The levels of NT-proBNP clearly exceeded the normal range (up to 150 pg·ml-1 for females and up to 100 pg·ml-1 for males) already at baseline and further increased during exercise (Figure 4A). However, the NT-proBNP levels remained below the age-specific cut-off value of 900 pg·ml-1 for 50-75 years of age (Gaggin and Januzzi, 2013).
In addition, no significantly different increase (or decrease) between the three exercise regimes was found for all other plasma parameters such as free catecholamines (NA, ADR, and DOP), inflammatory markers (CRP, IL-6), vascular markers (ADMA, SDMA, hArg), a marker for overall muscle damage (CK), and a metabolic parameter (leptin). Only the acute response of adiponectin, another metabolic marker regulating insulin sensitivity and glucose homeostasis, was shown to depend on exercise mode as it was significantly higher during short HIIE compared to CE and long HIIE. None of these biomarkers showed exercise-induced levels which were clinically relevant (Table 3). This shows that no elevated risks, such as inflammatory reactions (Pedersen, 2009), atherosclerotic processes (Kayacelebi et al., 2015), or myocardial/skeletal muscle damage, were induced.
Our results are in line with findings of several other authors. Meyer et al. (1996; 1997) did not find significantly different increases for catecholamines between different HIIE protocols, and between CE and HIIE, in patients with chronic heart failure. However, in these studies, Pmean was not matched. A recent study by Moser et al. (2015), in patients with type 1 diabetes mellitus, also revealed a similar catecholamine response during aerobic short HIIE and Pmean-matched CE. Studies by Guiraud et al. (2012, 2013) in CHD patients revealed no significant increases of endothelial microparticles (EMP) during short HIIE and isocaloric CE, which are specific biological markers associated with endothelial dysfunction. Most importantly, our findings support the results of other studies which revealed that aerobic HIIE was well tolerated and did not induce significant arrhythmias, severe or prolonged ischemia, or abnormal blood pressure responses in cardiac patients (Guiraud et al., 2011; Gayda et al., 2012; Meyer et al., 1996; 1997; 1998; Meyer et al., 2012; Normandin et al., 2013).
However, in most of these HIIE studies, peak workload durations were no longer than 15 s up to a maximum of 60 s except the HIIE protocol used by Meyer et al. (2012) which included a tpeak of 90 s. In contrast, the 4x4 HIIE model, which is frequently applied in both healthy individuals (Helgerud et al., 2007) and patients (Rognmo et al., 2004; 2012; Wisloff et al., 2007), includes long work phases of 4 min. However, studies of Laursen et al. (2005), Helgerud et al. (2007), and Ronnestad et al. (2015) revealed similar or even superior training effects yielded by short vs. long HIIE in healthy subjects. Additionally, HIIE with long work phases up to 4 min was clearly shown to induce significantly higher acute metabolic and peak cardiorespiratory responses compared to Pmean-matched short HIIE and CE in healthy subjects (Astrand et al., 1960; Saltin et al., 1976; Tschakert et al., 2015; Wiewelhove et al., 2015). Similar results were recently found by Peake et al. (2014). Meyer et al. (2012), Conraads et al (2015), and Keteyian (2012) suggested that the 4x4 HIIE may be highly demanding, even more so for heart disease patients, especially if Ppeak is set at 90 or even 95 % of HRmax (Wisloff et al., 2007). Furthermore, Normandin et al. (2013) and Keteyian (2012) critically noted that the acute physiological response to this protocol was not investigated.
Another aspect of HIIE prescription that has to be considered is the setting of Prec. Guiraud et al. (2010) and Meyer et al. (2012) found passive recovery to be more convenient than active recovery in patients with CHD and chronic heart failure, respectively. However, HIIE protocols with passive vs. active recovery were not matched by total work or mean load. In our study, we incorporated active recovery phases during both HIIE modes since we wanted to adopt the original 4 x 4 min HIIE protocol (including active recovery) as far as possible. Blood lactate clearance was found to be greater during active recovery compared to passive recovery (Bangsbo et al. 1994); however, this topic is controversially discussed. In addition, passive instead of active recovery phases with the same mean load results in markedly shorter recovery phases. In short-stage HIIE, trec may be too short to allow a sufficient resynthesis of creatine phosphate leading to a more pronounced anaerobic lactic metabolism (Tschakert and Hofmann, 2013). A comparison of HIIE protocols with passive vs. active recovery phases matched for mean load and total duration is required.
By using our methodological approach for exercise prescription, we could clearly show that even long HIIE with a tpeak of 4 min (and a Ppeak corresponding to the power output at 85 % of HRmax from IET) as well as short HIIE with a Ppeak according to Pmax from IET were as safe and as well tolerated as moderate CE in patients with CHD or myocarditis.
Future perspectives
Further methodological studies are required to determine if aerobic HIIE is also safe for heart disease patients (CHD patients as well as chronic heart failure patients) when Ppeak (of long HIIE) or Pmean (of long and short HIIE) is higher than in our study. Mechanistic HIIE studies with a consistent methodological approach are also necessary in patients with other chronic diseases such as diabetes mellitus type 1 and type 2, the metabolic syndrome, chronic obstructive pulmonary disease (COPD), or cancer. By all means, we recommend prescribing the Pmean consciously for a better control and prediction of acute cardiorespiratory and metabolic responses.
On the basis of methodological studies looking at the acute responses, training intervention studies are highly relevant to investigate the short- and long-term effects of different HIIE modes vs. Pmean-matched CE. If short intervals lead to similar training adaptations as long intervals, short HIIE is suggested to be the preferable HIIE mode since it is associated with lower metabolic and peak cardiorespiratory demands. In addition, it is still unclear to what extent systemic adaptations and peripheral muscular adaptations contribute to the overall aerobic training effect (increase of VO2max) yielded by different HIIE regimes.
Limitations
A limit of this study was that plasma parameters were measured only before and at the end of exercise but not over a period of 24 or 48 hours after exercise. Another limit of the study was the small number of participants (n = 8). However, an a-priory power analysis revealed the statistical power to be sufficient for our experimental design. Furthermore, since our small sample was composed only of patients with CHD or myocarditis, our results are not generalizable to all heart disease patients. It is unknown if the obtained results also apply to patients with lower functional capacity, particularly in the long intervals.
Conclusion
Our study revealed that, in cardiac rehabilitation, the acute metabolic and peak-cardiorespiratory response was higher during long HIIE compared to short HIIE and CE. However, both HIIE regimes did not yield significantly higher acute responses for cardiac and cardiovascular biomarkers, inflammatory markers, and catecholamines compared to conventional moderate CE. In addition, the exercise-induced levels of health-related markers remained within a normal range except for NT-proBNP which was already elevated at baseline. Despite markedly higher peak workloads during the intervals, long and short HIIE were as safe as moderate CE in patients with CHD or myocarditis by using our methodological approach for exercise prescription. However, subjects with lower functional capacity may not tolerate HIIE with long intervals and active recovery. Exercise prescription needs to be personalized taking this fact into account.
Both the acute responses for health related markers and training adaptations induced by different HIIE protocols are of high relevance for cardiac patients as well as those suffering from other chronic diseases. Therefore, in the field of intermittent exercise, further methodological investigations and training intervention studies, with a consistent approach to the exercise prescription, are required.
Acknowledgements
There are no funding sources for the present study and no financial, consultant, institutional and other relationships that might lead to bias or a conflict of interest. We declare that the experiments comply with the current laws of Austria, the country in which the experiments were performed.
Biographies

Gerhard TSCHAKERT
Employment
Ass.-Professor at the University of Graz, Austria. Institute of Sports Science Exercise Physiology, Training & Training Therapy Research Group
Degree
Dr. rer. nat.
Research interests
Exercise physiology, exercise prescription, exercise testing, performance diagnostics, and training therapy
E-mail: gerhard.tschakert@uni-graz.at

Julia Maria KRÖPFL
Employment
Postdoctoral Researcher at ETH Zurich, Switzerland. Institute of Human Movement Sciences and Sport
Exercise Physiology Lab
Degree
PhD
Research interests
Adult stem and progenitor cells, heart disease, vascular remodelling, exercise physiology
E-mail: julia.kroepfl@hest.ethz.ch

Alexander MUELLER
Employment
Research Assistant at the University of Graz, Austria
Institute of Sports Science
Exercise Physiology, Training & Training Therapy Research Group
Degree
Mag. rer. nat.
Research interests
Exercise physiology, exercise testing, performance diagnostics, training and training therapy
E-mail: alexander.mueller@edu.uni-graz.at

Hanns HARPF
Employment
Chief and Medical Director at the Center for Ambulatory Rehabilitation Graz (ZARG), Austria
Degree
Dr. med. for internal medicine, sports medicine
Research interests
Coronary artery disease, training therapy, exercise testing
E-mail: ordination@harpf.at

Leonhard HARPF
Employment
Deputy chief and deputy medical director at the Center for Ambulatory Rehabilitation Graz (ZARG), Austria
Degree
Dr. med. for internal medicine, cardiology
Research interests
Coronary artery disease, training therapy, exercise testing
E-mail: handy@leonhard-harpf.at

Heimo TRANINGER
Employment
Deputy chief and therapeutic manager at the Center for Ambulatory Rehabilitation Graz (ZARG), Austria
Degree
Mag. rer. nat.
Research interests
Exercise physiology, training therapy, exercise testing, performance diagnostic and training
E-mail: heimo.traninger@its.at

Sandra WALLNER-LIEBMANN
Employment
Professor at the Institute for Pathophysiology and Immunology, Medical University of Graz, Graz, Austria
Degree
Dr. rer. nat.
Research interests
Nutrition and Metabolism
E-mail: sandra.wallner@medunigraz.at

Tatjana STOJAKOVIC
Employment
Assoc. Professor at the Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Austria
Degree
Dr. rer. nat.
Research interests
Lipid and lipoprotein metabolism, epidemiology of cardiovascular diseases
E-mail: tatjana.stojakovic@medunigraz.at

Hubert SCHARNAGL
Employment
Assoc. Professor at the Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Austria
Degree
PhD
Research interests
Lipid and lipoprotein metabolism, epidemiology of cardiovascular diseases
E-mail: hubert.scharnagl@medunigraz.at

Andreas MEINITZER
Employment
Analytical Method Development, Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Austria
Degree
Mag. rer. nat. Dr. scient. med.
Research interests
Analytical chemistry, drug analysis, medical biochemistry biomarkers of cardiovascular disease, epidemiology.
E-mail: andreas.meinitzer@medunigraz.at

Patriz PICHLHOEFER
Employment
Public Health Worker/Coordinator, Market town of Vorau in cooperation with the federal state government of Styria, Austria
Degree
Mag. rer. nat.
Research interests
Public health and training therapy
E-mail: gesundheit@vorau.gv.at

Peter HOFMANN
Employment
Professor at the University of Graz, Austria. Institute of Sports Science Exercise Physiology, Training & Training Therapy Research Group
Degree
Dr. rer. nat.
Research interests
Exercise testing and performance diagnostics, exercise prescription and training, training therapy
E-mail: peter.hofmann@uni-graz.at
References
- Arena R., Myers J., Forman D.E., Lavie C.J., Guazzi M. (2013) Should high-intensity-aerobic interval training become the clinical standard in heart failure? Heart Failure Reviews 18(1), 95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrand I., Astrand P.O., Christensen E.H., Hedman R. (1960) Intermittent muscular work. Acta Physiologica Scandinavica 48, 448-453. [DOI] [PubMed] [Google Scholar]
- Bangsbo J., Graham T., Johansen L., Saltin B. (1994) Muscle lactate metabolism in recovery from intense exhaustive exercise: impact of light exercise. Journal of Applied Physiology 77, 1890-1895. [DOI] [PubMed] [Google Scholar]
- Benda N.M.M., Eijsvogels T.M.H. Van Dijk A.P.J. Hopman M.T.E. Thijssen D.H.J. (2015) Changes in BNP and cardiac troponin I after high-intensity interval and endurance exercise in heart failure patients and healthy controls. International Journal of Cardiology 184, 426-427. [DOI] [PubMed] [Google Scholar]
- Beneke R., Leithaeuser R.M., Ochentel O. (2011) Blood lactate diagnostics in exercise testing and training. International Journal of Sports Physiology and Performance 6(1), 8-24. [DOI] [PubMed] [Google Scholar]
- Brooks G.A. (2009) Cell-cell and intracellular lactate shuttles. Journal of Physiology 587, 5591-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church T.S., Blair S.N. (2009) When will we treat physical activity as a legitimate medical therapy...even though it does not come in a pill? British Journal of Sports Medicine 43(2), 80-81. [DOI] [PubMed] [Google Scholar]
- Conraads V.M., Pattyn N., De Maeyer C., Beckers P.J., Coeckelberghs E., Cornelissen V.A., Denollet J., Frederix G., Goetschalckx K., Hoymans V.Y., Possemiers N., Schepers D., Shivalkar B., Voigt J.-U., Van Craenenbroeck E.M., Vanthees L. (2015) Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: The SAINTEX-CAD study. International Journal of Cardiology 179, 203-210. [DOI] [PubMed] [Google Scholar]
- Gaggin H.K., Januzzi J.L., Jr. (2013) Biomarkers and diagnostics in heart failure. Biochimica et Biophysica Acta 1832(12), 2442-2450. [DOI] [PubMed] [Google Scholar]
- Gayda M., Normandin E., Meyer P., Juneau M., Haykowsky M., Nigam A. (2012) Central hemodynamic responses during acute high-intensity interval exercise and moderate continuous exercise in patients with heart failure. Applied Physiology, Nutrition, and Metabolism 37(6), 1171-1178. [DOI] [PubMed] [Google Scholar]
- Gibala M.J., Little J.P., MacDonald M.J., Hawley J.A. (2012) Physiological adaptations to low-volume, high-intensity interval training in health and disease. Journal of Physiology 590, 1077-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiraud T., Juneau M., Nigam A., Gayda M., Meyer P., Mekary S., Paillard F., Bosquet L. (2010) Optimization of high intensity interval exercise in coronary heart disease. European Journal of Applied Physiology 108(4), 733-740. [DOI] [PubMed] [Google Scholar]
- Guiraud T., Nigam A., Juneau M., Meyer P., Gayda M., Bosquet L. (2011) Acute responses to high-intensity intermittent exercise in CHD patients. Medicine and Science in Sports and Exercise 43(2), 211-217. [DOI] [PubMed] [Google Scholar]
- Guiraud T., Nigam A., Gremeaux V., Meyer P., Juneau M., Bosquet L. (2012) High-intensity interval training in cardiac rehabilitation. Sports Medicine 42(7), 587-605. [DOI] [PubMed] [Google Scholar]
- Guiraud T., Gayda M., Juneau M., Bosquet L., Meyer P., Theberge-Julien G., Galinier M., Nozza A., Lambert J., Rheaume E., Tardif J.C., Nigam A. (2013) A single bout of high-intensity interval exercise does not increase endothelial or platelet microparticles in stable, physically fit men with coronary heart disease. The Canadian Journal of Cardiology 29(10), 1285-1291. [DOI] [PubMed] [Google Scholar]
- Haskell W.L., Lee I.M., Pate R.R., Powell K.E., Blair S.N., Franklin B.A., Macera C.A., Heath G.W., Thompson P.D., Bauman A. (2007) Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise 39, 1423-1434. [DOI] [PubMed] [Google Scholar]
- Haykowsky M.J., Timmons M.P., Kruger C., McNeely M., Taylor D.A., Clark A.M. (2013) Meta-analysis of aerobic interval training on exercise capacity and systolic function in patients with heart failure and reduced ejection fractions. The American Journal of Cardiology 111(10), 1466-1469. [DOI] [PubMed] [Google Scholar]
- Helgerud J., Hoydal K., Wang E., Karlsen T., Berg P., Bjerkaas M., Simonsen T., Helgesen C., Hjorth N., Bach R., Hoff J. (2007) Aerobic high-intensity intervals improve VO2max more than moderate training. Medicine and Science in Sports and Exercise 39(4), 665-671. [DOI] [PubMed] [Google Scholar]
- Helgerud J., Bjorgen S., Karlsen T., Husby V.S., Steinshamn S., Richardson R.S., Hoff J. (2010) Hyperoxic interval training in chronic obstructive pulmonary disease patients with oxygen desaturation at peak exercise. Scandinavian Journal of Medicine and Science in Sports 20(1), e170-176. [DOI] [PubMed] [Google Scholar]
- Hofmann P., von Duvillard S.P., Seibert F.J., Pokan R., Wonisch M., Lemura L.M., Schwaberger G. (2001) %HRmax target heart rate is dependent on heart rate performance curve deflection. Medicine and Science in Sports and Exercise 33, 1726-1731. [DOI] [PubMed] [Google Scholar]
- Hofmann P., Wonisch M., Pokan R., Schwaberger G., Smekal G., von Duvillard S.P. (2005) Beta1-adrenoceptor mediated origin of the heart rate performance curve deflection. Medicine and Science in Sports and Exercise 37(10), 1704-1709. [DOI] [PubMed] [Google Scholar]
- Hofmann P., Tschakert G. (2011) Special needs to prescribe exercise intensity for scientific studies. Cardiology Research and Practice (Electronic Resource) 15, 209302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneau M., Hayami D., Gayda M., Lacroix S., Nigam A. (2014) Provocative issues in heart disease prevention. The Canadian Journal of Cardiology 30(12 Suppl), 401-409. [DOI] [PubMed] [Google Scholar]
- Kavanagh T., Mertens D.J., Hamm L.F., Beyene J., Kennedy J. (2002) Prediction of long-term prognosis in 12169 men referred for cardiac rehabilitation. Circulation 106, 666-671. [DOI] [PubMed] [Google Scholar]
- Kayacelebi A.A., Langen J., Weigt-Usinger K., Chobanyan-Jürgens K., Mariotti F., Schneider J.Y., Rothmann S., Froelich J.C., Atzler D., Choe C.U., Schwedhelm E., Huneau J.F., Luecke T., Tsikas D. (2015) Biosynthesis of homoarginine (hArg) and asymmetric dimethylarginine (ADMA) from acutely and chronically administered free L-arginine in humans. Amino Acids 47(9), 1893-1908. [DOI] [PubMed] [Google Scholar]
- Keteyian S.J. (2012) Swing and a miss or inside the park home run: Which fate awaits high intensity exercise training? Circulation 126(12), 1431-1433. [DOI] [PubMed] [Google Scholar]
- Laursen P.B., Shing C.M., Peake J.M., Coombes J.S., Jenkins D.G. (2005) Influence of high-intensity interval training on adaptations in well-trained cyclists. Journal of Strength and Conditioning Research 19(3), 527-533. [DOI] [PubMed] [Google Scholar]
- Meyer K., Samek L., Schwaibold M., Westbrook S., Hajric R., Lehmann M., Eßfeld D., Roskamm H. (1996) Physical responses to different modes of interval exercise in patients with chronic heart failure - application to exercise training. European Heart Journal 17, 1040-1047. [DOI] [PubMed] [Google Scholar]
- Meyer K., Samek L., Schwaibold M., Westbrook S., Hajric R., Beneke R., Lehmann M., Roskamm H. (1997) Interval training in patients with severe chronic heart failure - analysis and recommendation for exercise procedures. Medicine and Science in Sports and Exercise 29(3), 306-312. [DOI] [PubMed] [Google Scholar]
- Meyer K., Foster C., Georgakopoulos N., Hajric R., Westbrook S., Ellestad A., Tilman K., Fitzgerald D., Young H., Weinstein H., Roskamm H. (1998) Comparison of left ventricular function during interval versus steady-state exercise training in patients with chronic congestive heart failure. The American Journal of Cardiology 82(11), 1382-1387. [DOI] [PubMed] [Google Scholar]
- Meyer P., Normandin E., Gayda M., Billon G., Guiraud T., Bosquet L., Fortier A., Juneau M., White M., Nigam A. (2012) High-intensity interval exercise in chronic heart failure: protocol optimization. Journal of Cardiac Failure 18(2), 126-133. [DOI] [PubMed] [Google Scholar]
- Mezzani A., Hamm L.F., Jones A.M., McBride P.E., Moholdt T., Stone J.A., Urhausen A., Williams M.A. (2012) Aerobic exercise intensity assessment and prescription in cardiac rehabilitation. A joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation, and the Canadian Association of Cardiac Rehabilitation. Journal of Cardiopulmonary Rehabilitation and Prevention 32, 327-350. [DOI] [PubMed] [Google Scholar]
- Moser O., Tschakert G., Mueller A., Groeschl W., Pieber T.R., Obermayer-Pietsch B., Koehler G., Hofmann P. (2015) Effects of high-intensity interval exercise versus moderate continuous exercise on glucose homeostasis and hormone response in patients with Type 1 Diabetes Mellitus using novel ultra-long-acting insulin. PLoS One (Electronic Resource) 10(8), e0136489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normandin E., Nigam A., Meyer P., Juneau M., Guiraud T., Bosquet L., Mansour A., Gayda M. (2013) Acute responses to intermittent and continuous exercise in heart failure patients. The Canadian Journal of Cardiology 29, 466-471. [DOI] [PubMed] [Google Scholar]
- Peake J.M., Tan S.J., Markworth J.F., Broadbent J.A., Skinner T.L., Cameron-Smith D. (2014) Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. American Journal of Physiology, Endocrinology and Metabolism 307(7), E539-552. [DOI] [PubMed] [Google Scholar]
- Pedersen B.K. (2009) Edward F. Adolph distinguished lecture: muscle as an endocrine organ: IL-6 and other myokines. Journal of Applied Physiology 107, 1006-1014. [DOI] [PubMed] [Google Scholar]
- Rognmo O., Hetland E., Helgerud J., Hoff J., Slordahl SA. (2004) High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. European Journal of Cardiovascular Prevention and Rehabilitation 11, 216-222. [DOI] [PubMed] [Google Scholar]
- Rognmo O., Moholdt T., Bakken H., Hole T., Molstad P., Myhr N.E., Grimsmo J., Wisloff U. (2012) Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 126(12), 1436-1440. [DOI] [PubMed] [Google Scholar]
- Ronnestad B.R., Hansen J., Vegge G., Tonnessen E., Slettalokken G. (2015) Short intervals induce superior training adaptations compared with long intervals in cyclists - An effort-matched approach. Scandinavian Journal of Medicine and Science in Sports 25(2), 143-151. [DOI] [PubMed] [Google Scholar]
- Saltin B., Essen B., Pedersen P.K. (1976) Intermittent exercise: its physiology and some practical applications. Medicine and Sport 9, 23-51. [Google Scholar]
- Scharhag-Rosenberger F., Meyer T., Gaeßler N., Faude O., Kindermann W. (2010) Exercise at given percentages of VO2max: heterogeneous metabolic response between individuals. Journal of Science and Medicine in Sport 13(1), 74-79. [DOI] [PubMed] [Google Scholar]
- Thompson P.D., Franklin B.A., Balady G.J., Blair S.N., Corrado D., Mark Estes N.A., III, Fulton J.E., Gordon N.F., Haskell W.L., Link M.S., Maron B.J., Mittleman M.A., Pelliccia A., Wenger N.K., Willich S.N., Costa F. (2007) Exercise and acute cardiovascular events: Placing the risks into perspective: A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 115, 2358-2368. [DOI] [PubMed] [Google Scholar]
- Tjonna A.E., Lee S.J., Rognmo O., Stolen T.O., Bye A., Haram P.M., Loennechen J.P., Al-Share Q.Y., Skogvoll E., Slordahl S.A., Kemi O.J., Naijar S.M., Wisloff U. (2008) Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: A pilot study. Circulation 118(4), 346-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschakert G., Hofmann P. (2013) High-intensity intermittent exercise: methodological and physiological aspects. International Journal of Sports Physiology and Performance 8(6), 600-610. [DOI] [PubMed] [Google Scholar]
- Tschakert G., Kroepfl J., Mueller A., Moser O., Groeschl W., Hofmann P. (2015) How to regulate the acute physiological response to “aerobic” high-intensity interval exercise. Journal of Sports Science and Medicine 14, 29-36. [PMC free article] [PubMed] [Google Scholar]
- Warburton D.E.R., McKenzie D.C., Haykowsky M.J., Taylor A., Shoemaker P., Ignaszewski A.P., Chan S.Y. (2005) Effectiveness of high-intensity interval training for the rehabilitation of patients with coronary artery disease. The American Journal of Cardiology 95, 1080-1084. [DOI] [PubMed] [Google Scholar]
- Wiewelhove T., Fernandez-Fernandez J., Raeder C., Meyer T., Kellmann M., Pfeiffer M., Ferrauti A. (2015) Acute responses and muscle damage in different high-intensity interval running protocols. The Journal of Sports Medicine and Physical Fitness, in press. [PubMed] [Google Scholar]
- Wisloff U., Stoylen A., Loennechen J.P., Bruvold M., Rognmo O., Haram P.M., Tjonna A.E., Helgerud J., Slordahl S.A., Lee S.J., Videm V., Bye A., Smith G.L., Najjar S.M., Ellingson O., Skjaerpe T. (2007) Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115, 3086-3094. [DOI] [PubMed] [Google Scholar]
- Wonisch M., Hofmann P., Fruhwald F.M., Kraxner W., Hoedl R., Pokan R., Klein W. (2003) Influence of beta-blocker use on percentage of target heart rate exercise prescription. European Journal of Cardiovascular Prevention and Rehabilitation 10(4), 296-301. [DOI] [PubMed] [Google Scholar]
- Wonisch M., Berent R., Klicpera M., Laimer H., Marko C., Pokan R., Schmid P., Schwann H. (2008) Practice guidelines for exercise testing. Austrian Journal of Cardiology 15(Suppl A), 3-17. (In German: English abstract). [Google Scholar]


