Altered eosinophil content identified in adipose tissue of CCR2-deficient mice, associated with changes in macrophage polarization and type-2 cytokine expression.
Keywords: inflammation, obesity, interleukin 5
Abstract
Adipose tissue (AT) inflammation during obesity is mediated by immune cells and closely correlates with systemic insulin resistance. In lean AT, eosinophils are present in low but significant numbers and capable of promoting alternative macrophage activation in an IL-4/IL-13-dependent manner. In WT mice, obesity causes the proportion of AT eosinophils to decline, concomitant with inflammation and classical activation of AT macrophages. In this study, we show that CCR2 deficiency leads to increased eosinophil accumulation in AT. Furthermore, in contrast to WT mice, the increase in eosinophils in CCR2−/− AT is sustained and even amplified during obesity. Interestingly, a significant portion of eosinophils is found in CLSs in AT of obese CCR2−/− mice, which is the first time eosinophils have been shown to localize to these inflammatory hot spots. CCR2−/− bone marrow precursors displayed increased expression of various key eosinophil genes during in vitro differentiation to eosinophils, suggesting a potentially altered eosinophil phenotype in the absence of CCR2. In addition, the proportion of eosinophils in AT positively correlated with local expression of Il5, a potent eosinophil stimulator. The increase in eosinophils in CCR2−/− mice was detected in all white fat pads analyzed and in the peritoneal cavity but not in bone marrow, blood, spleen, or liver. In AT of CCR2−/− mice, an increased eosinophil number positively correlated with M2-like macrophages, expression of the Treg marker Foxp3, and type 2 cytokines, Il4, Il5, and Il13. This is the first study to link CCR2 function with regulation of AT eosinophil accumulation.
Introduction
CCR2 is a member of the CC-chemokine family of receptors that is primarily known for its ability to bind CCL2, leading to chemotaxis of leukocytes [1, 2]. CCR2 is also known to be a receptor for additional chemokines, including CCL7, CCL8, CCL13, and CCL16 (reviewed in ref. [3]). CCR2 is expressed on the surface of leukocytes from multiple lineages, including monocytes, macrophages, lymphocytes, and granulocytes, and its expression is tightly regulated, differing from 1 cell type to another [4]. Leukocytes from mice with a gene-targeted CCR2 deficiency show no differences in rolling velocity but do have reduced adhesion to the endothelium and decreased extravasation into inflamed tissues in response to CCL2 [5]. Furthermore, CCR2−/− mice have a severe reduction in the number of circulating Ly6Chi inflammatory monocytes as a result of impaired egress from the bone marrow [6]. The loss of circulating inflammatory monocytes in CCR2−/− mice decreases macrophage accumulation at sites of acute inflammation, such as thioglycollate-induced peritonitis [7, 8]. Given the potent role of CCR2 in monocyte chemotaxis, CCR2−/− mice have been used previously to study the role of macrophages in chronic inflammatory diseases, such as atherosclerosis and obesity [6, 9].
It is established that obesity leads to immune system-driven chronic inflammation that promotes insulin resistance and type 2 diabetes in rodents and humans (reviewed in ref. [10]). This inflammation is characterized by an influx of inflammatory immune cells into metabolic tissues, such as AT [11, 12]. The role of CCR2 in leukocyte accumulation during chronic inflammation, such as obesity, is not yet clear. Based on the near absence of circulating Ly6Chi monocytes in CCR2−/− mice [6], it was anticipated that AT macrophage accumulation would be severely blunted. However, we [8] and others [9] have shown that AT macrophage accumulation in obese CCR2−/− mice is only mildly decreased, and this reduction is only apparent after long periods of HFD feeding. No effects of CCR2 deficiency on macrophage accumulation have been reported at earlier periods of HFD feeding when initiation of macrophage recruitment occurs. Moreover, tissues of CCR2−/− mice display diminished type 1 [7] and increased type 2 immune responses [13]. Increased expression of IL-4 and “M2” macrophage markers arginase (Arg1) and Ym1 (Chil3) characterizes the enhanced type 2 response [8, 13]. Additionally, we found previously that hematopoietic CCR2 deficiency leads to aberrant accumulation of a unique myeloid cell population in AT of obese mice [8]. In the current report, we identify these cells as eosinophils and further characterize their contribution to AT inflammation in the lean and obese state.
Eosinophils were only recently discovered in AT and have already been found to play an important function in sustaining alternative activation of AT macrophages [14–16]. AT eosinophils were shown to decrease in obesity, concomitant with M1-like polarization of the AT macrophages. Additionally, mice with systemically elevated eosinophils were protected against developing obesity-related insulin resistance, whereas mice with eosinophil deficiency were more prone to developing insulin resistance [14]. Accumulation of AT eosinophils is mediated by IL-5, secreted predominantly by resident ILC2 cells [15]. The studies summarized above demonstrate that eosinophils can protect against metabolic defects associated with obesity-induced inflammation.
In the current report, we show that CCR2 deficiency leads to an increased eosinophil number in AT, with no differences in bone marrow, blood, or spleen. An increased AT eosinophil number positively correlated with AT Il5 expression, suggesting that local eosinophil recruitment, proliferation, and/or decreased apoptosis may account for the increased numbers in CCR2−/− mice. Similar to previous reports [14], increased AT eosinophils occurred concomitantly with M2-like macrophage activation and increased AT expression of type 2 cytokines Il4, Il5, and Il13. The tissue localization of macrophages in AT is known to be an indicator of their function, with cells located in CLSs showing greater inflammatory potential [17]. While eosinophils are confined to interstitial spaces in AT of obese WT mice, we found that eosinophils localize to CLSs and interstitial spaces in AT of obese CCR2−/− mice. To our knowledge, this is the first report associating CCR2 deficiency with increases in AT eosinophils, implicating that this receptor plays an important role in eosinophil migration to—or cell turnover in—AT. Furthermore, we found that CCR2−/− bone marrow cultures differentiate in vitro with higher expression of genes critical to the eosinophil lineage compared with WT bone marrow. This study provides a unique setting in which local AT eosinophil accumulation can be studied and contrasted to that of systemically elevated eosinophils observed in the IL-5 transgenic mouse model [14]. In addition, this mouse model provides a platform for identifying novel mechanisms of AT eosinophil and type 2 immunity regulation by CCR2.
MATERIALS AND METHODS
Animals
Male WT and CCR2−/− mice on a C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and maintained in the VUMC Animal Facility. At 8 weeks of age, mice were fed a chow diet or HFD with 60% kcal from fat (Research Diets, New Brunswick, NJ, USA) ad libitum for 6–8 weeks and had free access to water. Given the primary goal of inducing dietary obesity, we used a diet with a high-caloric composition derived from fat (60% from total kcal); however, other micronutrients were not matched between diets. All animal procedures were carried out with prior approval from VUMC’s Institutional Animal Care and Usage Committee.
Tissue collection and SVF separation
Mice were euthanized by isofluorane overdose, followed by cervical dislocation. Perfusions were performed via the left ventricle with ∼10 ml PBS (Life Technologies, Grand Island, NY, USA). Tissues were collected, weighed, and snap frozen in liquid nitrogen. When appropriate, the SVF of AT was separated from the adipocytes. SVF preparations and flow cytometry were performed as described [18]. In brief, separation involved mincing AT in 0.5% BSA (Sigma-Aldrich, St. Louis, MO, USA) in PBS, digesting in a 2 mg/ml collagenase II solution (Sigma-Aldrich) for 25 min at 37°C with agitation, filtering cells through a 100 μm filter, centrifuging for 10 min at 1000 relative centrifugal field at 8°C to separate floating adipocytes, and removing RBCs by incubating in ACK lysis buffer (K·D Medical, Columbia, MD, USA) for 2 min on ice. Blood was collected via the retroorbital venous plexus by use of heparinized collection tubes.
Peritoneal immune cell collection
Peritoneal immune cells were collected by flushing the peritoneal cavity with 10 ml DMEM. Cells were washed 2× and then used for flow cytometry as described below.
Flow cytometry and FACS
Cells of the isolated SVF, peritoneal cavity, blood, spleen, liver, or bone marrow-derived eosinophils were incubated in Fc Block (BD Biosciences, San Diego, CA, USA) for 5 min on ice. Cells were subsequently stained for 30 min at 4°C with fluorophore-conjugated antibodies: F4/80-APC (eBioscience, San Diego, CA, USA), CD11b-FITC/APC-Cy7 (eBioscience), and SiglecF-PE (BD Biosciences). Immediately before flow cytometric analysis, samples were incubated with 1 μg/ml DAPI as a viability dye. Cells were analyzed on a 5-laser LSRII flow cytometer (BD Biosciences) or FACSAria III cell sorter (BD Biosciences) and data analyzed by use of FlowJo software. For flow cytometry and FACS, cells were first gated on all DAPI-negative live cells and then gated for specific lineage markers [18]. The lineage-specific gating was performed as follows: macrophages (F4/80hi, CD11bhi, SiglecF−) and eosinophils (F4/80lo, CD11blo, SiglecF+).
RNA isolation, cDNA synthesis, and real-time RT-PCR
RNA was isolated by the guanidine isothiocyanate-phenol-chloroform method by use of TRIzol (BD Biosciences), according to the manufacturer’s protocol. Potentially contaminating DNA was digested by use of DNase1 (Life Technologies). RNA was reverse transcribed into cDNA by use of iScript RT (Bio-Rad Laboratories, Hercules, CA, USA). Relative gene expression between samples was assessed by use of the carboxyfluorescein-conjugated TaqMan Gene Expression Assay (Life Technologies), normalized to GAPDH, and statistically analyzed by the Pfaffl method [19].
Cell and tissue imaging
Immunohistochemistry
Intact AT pieces of ∼3 mm3 in size were fixed in 1% paraformaldehyde for 1 hour with agitation. Samples were washed in PBS and blocked in goat serum at a 1:5 dilution in PBS for 1 hour at room temperature with agitation. Rat anti-mouse SiglecF-PE (BD Biosciences) and anti-mouse F4/80-APC (eBioscience) were applied at a 1:50 dilution in goat serum overnight at 4°C. Samples were washed and then stained with 1 μg/ml DAPI (BD Biosciences) for 3 minutes at room temperature with agitation. Samples were mounted on No. 1.0 coverglass bottom-culture dishes in 90% buffered glycerol solution for imaging on an Olympus FV-1000 confocal inverted microscope. Four to 5 images were captured from multiple stained pieces of AT for each mouse. There were 4 mice/group for a total of ∼15–20 images/experimental group. Cell number was quantified from each image by use of ImageJ software and averaged for a representative count. Multiple confocal slices, spanning ∼50 μm,were reconstructed into a 3D rendering by use of Icy software (Bio Image Analysis, Paris, France) for greater clarity than is achieved with Z-stack analysis alone.
H&E stain
Cytospins were performed with 8000–40,000 cells spun for 5 minutes at 600–1000 rpm, stained with Hemacolor (LABSCO, Louisville, KY, USA).
Fast Green/Neutral Red stain
FACS cells were cytospun for 5 minutes at 600 rpm. Slides were fixed for 10 minutes in ice-cold 100% methanol for 10 minutes, immersed in Fast Green FCF (Sigma-Aldrich) for 24 hours at room temperature, and immersed in Neutral Red (Sigma-Aldrich) for 48–72 hours at room temperature.
Major basic protein detection by HRP
FACS cells were cytospun for 5 minutes at 600 rpm. Slides were fixed for 10 minutes in ice-cold 100% methanol and incubated in 3% H2O2 for 15 minutes at room temperature to remove endogenous peroxidase activity; nonspecific binding was blocked with 5% normal goat serum for 1 hour at room temperature, incubated with primary rat anti-mouse MBP antibody (kindly provided by the laboratories of Drs. Nancy and Jamie Lee, Mayo Clinic, Scottsdale, AZ, USA) overnight at 4°C, and incubated in secondary HRP goat anti-rat antibody (Santa Cruz Biotechnology, Dallas, TX, USA) for 1 hour at room temperature; and 3,3′-diaminobenzidine reaction was carried out according to the manufacturer’s instructions (Life Technologies) and counterstained with Hemacolor Nuclear Stain #3 (LABSCO). All slides for imaging were allowed to dry briefly, mounted with permount, and examined on an Axiophot widefield light microscope (Zeiss, Thornwood, NY, USA). The only modification to images was removal of background discoloration, neutralizing to a white background for even comparison.
Bone marrow-derived eosinophil differentiation
Bone marrow was extracted from femurs and tibias of lean WT and CCR2−/− mice and was differentiated as adapted from Dyer et al. [20]. In brief, RBCs were removed by suspension in ACK lysing buffer. Remaining cells were cultured for 4 days at 37°C at 1 × 106 cells/ml in RPMI, supplemented with 20% FBS, 100 IU/ml penicillin, 10 μg/ml streptomycin, 2 mM glutamine, 25 mM HEPES, 1× nonessential amino acids, 1 mM sodium pyruvate, and 50 μM 2-ME. During days 0–4, 100 ng/ml SCF and 100 ng/ml FLT3 ligand (PeproTech, Rocky Hill, NJ, USA) were added. During days 5–12, SCF and FLT3 were replaced with IL-5 (R&D Systems, Minneapolis, MN, USA). Fresh medium was applied on days 4, 8, and 10. Cell aliquots were collected for RNA isolation on days 0, 4, 8, 10, and 12. Cytospins were performed on days 4 and 10. Eosinophil differentiation was confirmed on day 10 by flow cytometry. Eosinophils were identified as F4/80lo, CD11blo, and SiglecF+.
Statistics
GraphPad Prism 5.0 software was used for all statistical analyses. Data were analyzed by use of a Student’s t-test or a 1-way ANOVA, followed by a post hoc Student’s t-test if the ANOVA was significant. A 2-way ANOVA was used to compare measurements with 2 different variables. Outliers were excluded from the data for each individual parameter if outside the range of the mean ± 2 sd. P ≤ 0.05 was considered significant.
RESULTS
CCR2 deficiency leads to an increased percent and total number of eosinophils in AT
To determine the role of CCR2 signaling on AT immune cell composition, WT and CCR2−/− mice were placed on a chow diet or HFD for 6–8 weeks to induce obesity. At the start of the study, there were no differences between the genotypes in body weight (data not shown). All mice fed a HFD became obese, but there were no genotype effects on body weight or metabolic phenotype, as we have reported previously [8]. To elaborate, we previously found no difference in glucose tolerance between WT and CCR2−/− mice at 6 and 12 weeks of HFD, yet there was a modest improvement in these parameters in CCR2−/− mice after an extended 20 weeks of HFD feeding [8]. There were no differences in insulin tolerance, weight gain, percent adiposity, or eAT weight between genotypes at 6, 12, or 20 weeks on HFD.
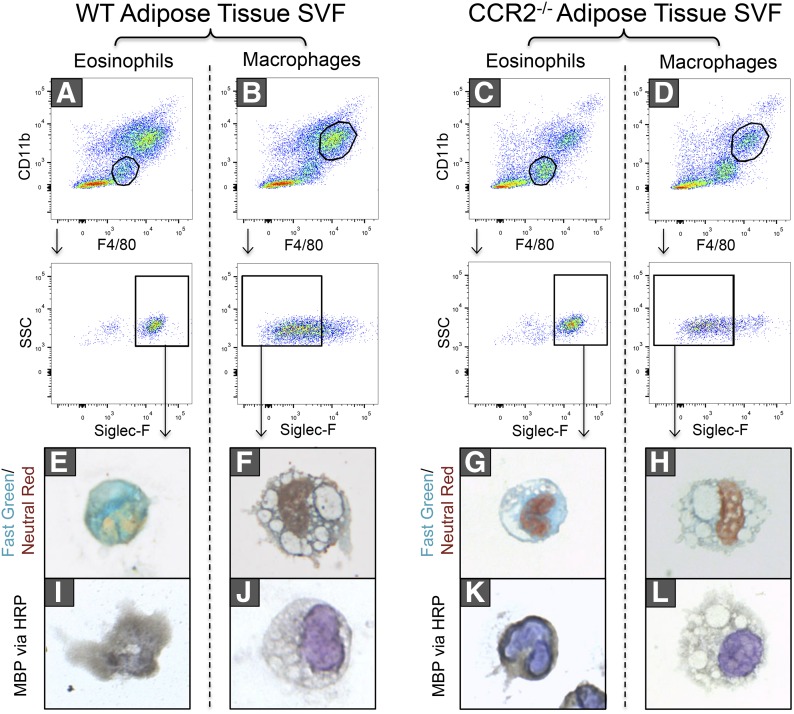
Immune cells in eAT from WT (Fig. 1A and B) and CCR2−/− (Fig. 1C and D) mice was analyzed by flow cytometry. Based on previous work by our group [8] and others [14, 15], eosinophils were identified as CD11blo, F4/80lo, SiglecF+ cells (Fig. 1A and C). Identification of eosinophils was further confirmed by the presence of characteristic green granules with a Fast Green/Neutral Red stain (Fig. 1E and G) and by staining of MBP via HRP (Fig. 1I and K), both of which were absent in the macrophage population identified as CD11bhi, F4/80hi, and SiglecF− cells (Fig. 1B, D, F, H, J, and L). In addition, cytospins of sorted CD11blo, F4/80lo, SiglecF+ cells demonstrated multilobular (Fig. 1E, G, and K) or doughnut-shaped nuclei (Fig. 1I), characteristic of eosinophils. This is in striking contrast to sorted macrophages that exhibited round nuclei with higher cytoplasmic volume (Fig. 1F, H, J, and L).
Figure 1. Identification of eosinophils by flow cytometry, Fast Green/Neutral Red, and major basic protein.
eAT from lean WT and CCR2−/− mice was collected, the SVF isolated, and cells sorted by FACS. FACS (A and C) eosinophils (CD11blo, F4/80lo, SiglecF+) and (B and D) macrophages (CD11bhi, F4/80hi, SiglecF−) from the SVF of AT of WT (left) and CCR2−/− (right) mice. SSC, Side-scatter. (E–H) Fast Green/Neutral Red staining and (I–L) MBP detection by HRP were performed on eosinophils (E, I, G, and K) and macrophages (F, J, H, and L). Images are representative of n = 2–3 mice/group. The only modification to images was removal of background discoloration and neutralizing to a white background for even comparison.
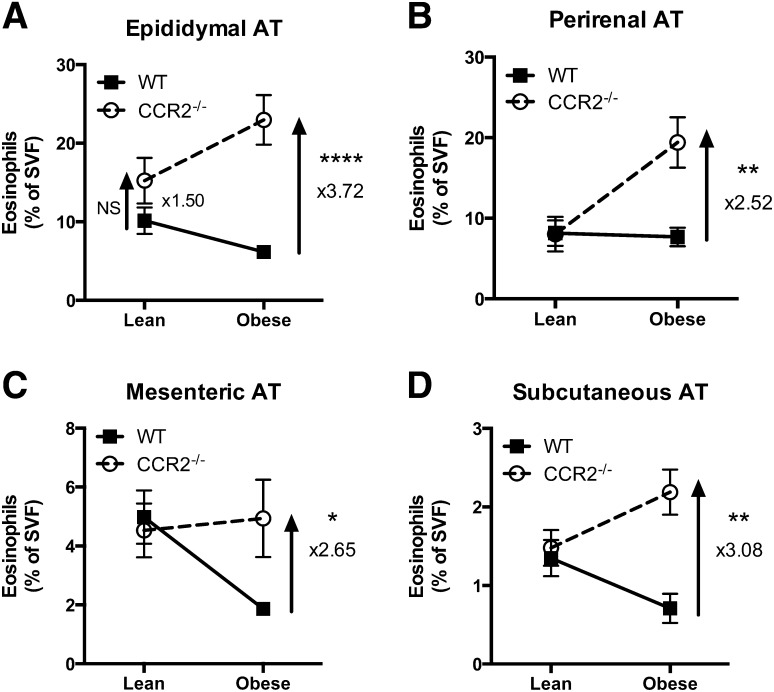
With the validated gating strategy described above, we next evaluated the relative difference in percent AT eosinophils from WT and CCR2−/− mice when in the lean versus obese state. Replicating published data [14], the percent eosinophils of live stromal vascular cells decreased in eAT from WT mice with obesity (Fig. 2A). In contrast, we show that eAT eosinophil percentages were 3.72-fold higher in obese CCR2−/− mice compared with obese WT mice (Fig. 2A; P < 0.0001). A similar increase in eosinophils of obese CCR2−/− compared with WT mice was observed in other AT depots analyzed: pAT (Fig. 2B; P < 0.005), mesenteric (Fig. 2C; P < 0.05), and subcutaneous (Fig. 2D; P < 0.005). Although the percentage of eosinophils in other fat pads was lower than eAT, the fold change between genotypes was 2- to 4-fold higher in all white fat pads of obese CCR2−/− mice examined.
Figure 2. HFD-induced obesity in CCR2−/− mice leads to increased eosinophils in white AT.
White AT from lean and obese WT and CCR2−/− mice was collected, the SVF isolated, and the percentage of eosinophils quantified by flow cytometry. (A) eAT, (B) pAT, (C) mesenteric AT, and (D) subcutaneous AT. Data are presented as means ± sem representing the difference in percent eosinophils between lean and obese for each genotype; n = 5–10 mice/group. *P < 0.05, difference between genotypes of mice on the same diet; **P < 0.005, difference between genotypes of mice on the same diet; ****P < 0.0001, difference between genotypes of mice on the same diet.
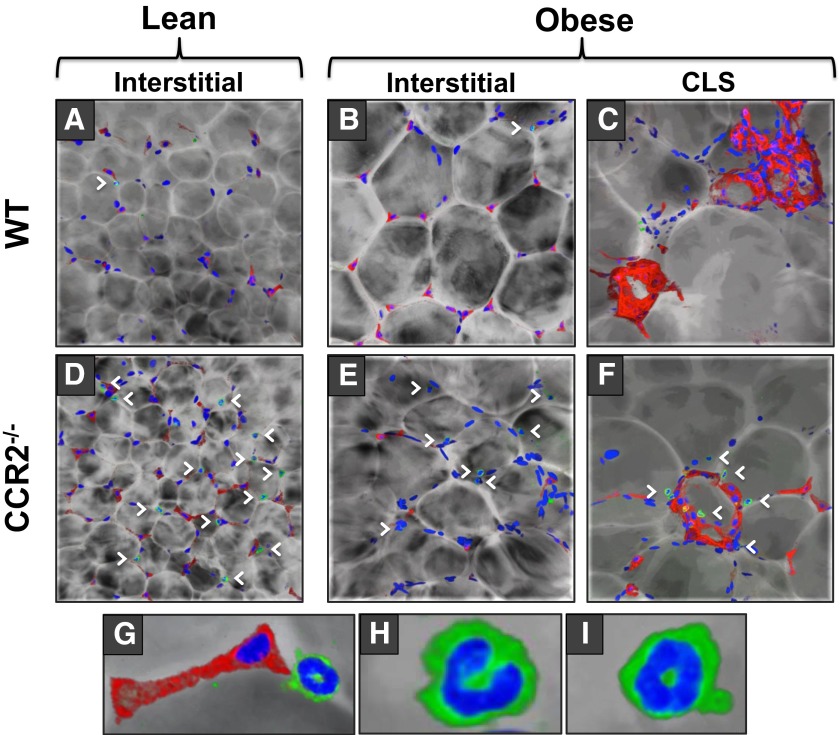
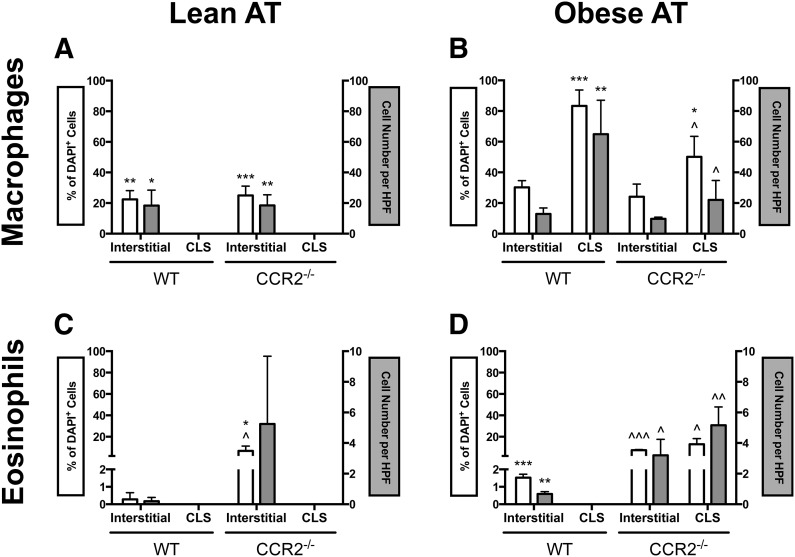
Although the following results detail AT macrophage and eosinophil composition, the results should be interpreted with the understanding that AT is composed of a variety of other cell types, including but not limited to the adipocytes themselves, preadipocytes, mesenchymal stem cells, endothelial progenitor cells, T cells, and B cells, as well as the vascular and neuronal network. Furthermore, AT is often discussed in terms of interstitially spaced regions and CLSs, as a result of the inflammatory variation of immune cells in such regions. It has been established that macrophages in CLSs possess a proinflammatory phenotype [17]; however, the localization of eosinophils to CLSs has not been reported. To examine AT macrophage and eosinophil localization in obese CCR2−/− mice and obese WT mice, we used confocal immunofluorescence microscopy to construct a 3D rendering of images spanning ∼50 μm. The 3D constructs allowed us to distinguish macrophages by F4/80 staining and eosinophils by SiglecF staining and by visualization of the multilobular and doughnut-shaped nuclei, which are not discernible in a traditional Z-stack analysis. In lean mice of both genotypes, eAT macrophages and eosinophils localized interstitially, although CCR2−/− mice had a significantly higher percentage of interstitial eosinophils than WT (Figs. 3A and D and 4A and C). During obesity, a surge of macrophages accumulated in eAT of WT mice and macrophages was >2.5-fold more likely to localize to CLSs than interstitial spaces (Figs. 3B and C and 4B) as has been reported by numerous groups [11, 12, 17, 21]. This surge of CLS-macrophage accumulation during obesity was blunted in eAT of CCR2−/− mice (Figs. 3F and 4B). CLSs were frequently found in both obese genotypes. However, in contrast to obese WT mice, in which eosinophils were confined to interstitial spaces (Figs. 3B and C and 4D), eosinophils in obese CCR2−/− mice were often found in CLSs (Figs. 3E and F and 4D). This is the first time eosinophils have been shown to localize to CLS in any mouse model. It is interesting to observe that these relatively subtle fluxes in eosinophil number correlate with more drastic alterations in macrophage content (see scale bars of Fig. 4B and D). An example of an F4/80+ macrophage and SiglecF+ eosinophil in close proximity in eAT is shown in Fig. 3G. Magnified images of eAT eosinophils with the typical multilobular and doughnut-shaped nuclei are shown in Fig. 3H and I.
Figure 3. Localization of eosinophils and macrophages to interstitial spaces or CLSs in eAT.
eAT was collected from lean and obese WT and CCR2−/− mice. AT was stained with SiglecF (green) for eosinophils, F4/80 (red) for macrophages, and DAPI (blue) for nuclei and imaged by confocal immunofluorescence microscopy, visualized as a computer-generated 3D rendering. Interstitially spaced macrophages and eosinophils from lean (A) WT and (D) CCR2−/− mice and from obese (B) WT and (E) CCR2−/− mice. CLS-localized macrophages and eosinophils from obese (C) WT and (F) CCR2−/− mice. (G) Magnified image of juxtaposed macrophage and eosinophil for comparison. (H and I) High magnification images of CCR2−/− AT eosinophils exhibiting prototypical multilobular or doughnut-shaped nuclei. Arrowheads demonstrate some of the eosinophils visible in each image. Images are representative of 3 images/mouse, with n = 4 mice/group.
Figure 4. Quantification of macrophages and eosinophils in CLS or interstitially spaced regions of eAT.
Immune cell localization from images in Fig. 3 was quantified by percent of DAPI+ cells (white bars) and absolute cell number per high-power field (HPF; gray bars). (A) Macrophages in eAT of lean mice. (B) Macrophages in eAT of obese mice.; (C) Eosinophils in eAT of lean mice. (D) Eosinophils in eAT of obese mice. Data are shown as the means ± sem, with n = 3–4 mice/group. *P < 0.05, difference between locations within the same genotype; **P < 0.005, difference between locations within the same genotype; ***P < 0.0005, difference between locations within the same genotype; ^P < 0.05, difference between genotypes within the same location; ^^P < 0.005, difference between genotypes within the same location; ^^^P < 0.0001, difference between genotypes within the same location.
As the total eosinophil percentage was also relatively high in pAT (Fig. 2B), we performed confocal immunofluorescence to determine the localization of eosinophils in this fat pad (Supplemental Figs. 1 and 2). Macrophages were selectively found in interstitial spaces of pAT from lean WT mice but were equally distributed between interstitial spaces and CLSs within pAT of lean CCR2−/− mice. Similar to eAT, eosinophils were detected more frequently in pAT of CCR2−/− mice compared with WT. Likewise, pAT eosinophils of CCR2−/− mice localized to interstitial spaces and CLSs in a similar pattern to eAT eosinophils in lean and obese mice.
In addition to AT, eosinophils accumulate in the peritoneal cavity of CCR2−/− mice
The number of eosinophils in bone marrow, blood, spleen, and liver was quantified. Whereas eosinophils in livers of lean WT mice were higher than all other groups, no differences in other tissues were detected (Supplemental Fig. 3A–D). In contrast, quantification of nonelicited peritoneal cavity cells revealed a >5-fold increase in peritoneal eosinophils in CCR2−/− mice compared with WT mice (Supplemental Fig. 3E; P < 0.05).
Bone marrow-derived CCR2−/− eosinophils display increased expression of key eosinophil genes during differentiation in vitro but present the same numerical yield as WT
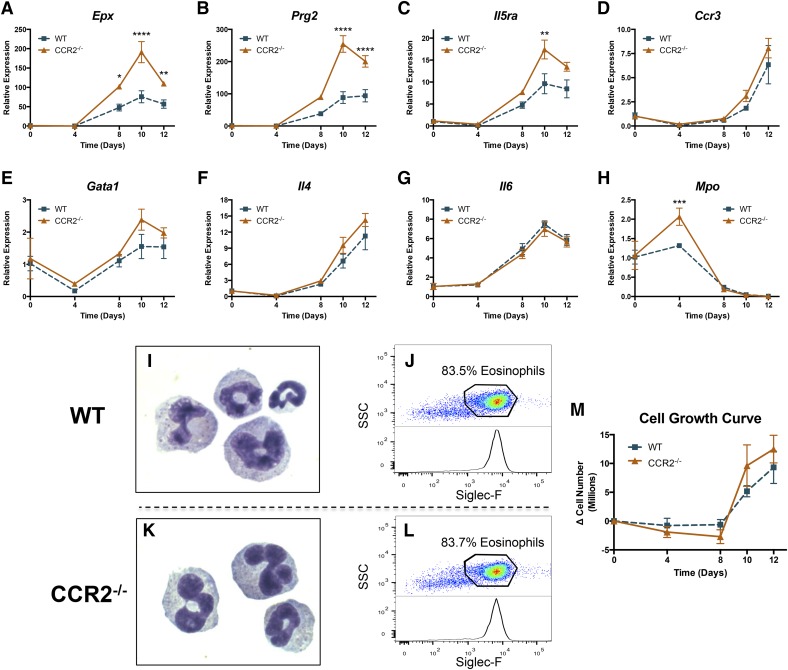
To determine a potential mechanism for AT eosinophil accumulation in CCR2−/− mice, we tested whether bone marrow cells from CCR2−/− mice have an altered potential to differentiate into eosinophils. Bone marrow cells were isolated from the femurs and tibias of lean WT and CCR2−/− mice and differentiated into eosinophils, as described previously [20] and detailed in Materials and Methods. Upon differentiation with IL-5 over a time course of 0, 4, 8, 10, and 12 days, we observed an increase (up to 300-fold) in many eosinophil-associated genes. These included: 1) eosinophil granule proteins: EPO and MBP; 2) eosinophil receptors: IL-5Rα and chemokine C-C motif receptor 3; 3) eosinophil transcription factor: GATA1; and 4) eosinophil cytokines: IL-4 and IL-6 (gene names: Epx, Prg2, Il5ra, Ccr3, Gata1, Il4, and Il6, respectively). These data indicate that the bone marrow cells had differentiated into eosinophils in both genotypes (Fig. 5A–G). Interestingly, Epx, Prg2, and IL5ra were significantly elevated in CCR2−/− bone marrow-derived eosinophils when compared with WT eosinophils; however, other eosinophil-associated genes, i.e., Ccr3, Gata1, Il4, and Il6 were not differentially expressed between genotypes. Mpo, a neutrophil-specific marker, was reduced in both genotypes, confirming the purity and specificity of the differentiation process (Fig. 5H). To confirm further the purity of eosinophil cultures, cells were cytospun after 10 days of differentiation and analyzed by Hemacolor staining. Figure 5I and K show the characteristic multilobular nuclei and granularity of differentiated eosinophils in WT and CCR2−/− cultures. At day 10 of differentiation, ∼84% of cells were eosinophils in WT and CCR2−/− cultures (Fig. 5J and L). Likewise, the growth curves of WT and CCR2−/− cultures were statistically indistinguishable from each other (Fig. 5M). These data show that although CCR2 deficiency alters expression of some genes in bone marrow-derived eosinophils, it does not alter the numerical yield. Thus, eosinophil differentiation is unlikely to account directly for the observed increase in eosinophil number in AT of CCR2−/− mice.
Figure 5. Bone marrow-derived CCR2−/− eosinophils display increased expression of key eosinophil genes during differentiation in vitro.
Bone marrow cells were collected from lean WT and CCR2−/− mice and differentiated into eosinophils in vitro, according to Materials and Methods. In brief, cells were cultured with SCF and FLT3 ligand for 4 days and subsequently cultured with IL-5 for 8 days. Expression of eosinophil-specific genes (A) Epx, (B) Prg2, (C) Il5ra, (D) Ccr3, (E) Gata1; eosinophil-secreted cytokines (F) Il4 and (G) Il6; and noneosinophil specific gene (H) Mpo throughout differentiation. Cytospin Hemacolor images of bone marrow-derived eosinophils at 10 days of differentiation from (I) WT and (K) CCR2−/− mice. Percent purity of eosinophils from (J) WT and (L) CCR2−/− at 10 days of differentiation. (M) Total cell growth rate throughout differentiation expressed as change in cell number over time. Data are normalized to day 0 for each genotype and are presented as means ± sem, with n = 2 for day 0, and n = 4 for days 4–12. *P < 0.05, difference between genotypes; **P < 0.01, difference between genotypes; ***P < 0.0005, difference between genotypes; ****P < 0.0001, difference between genotypes.
Increased eosinophils in AT correlate with increased expression of local Il5
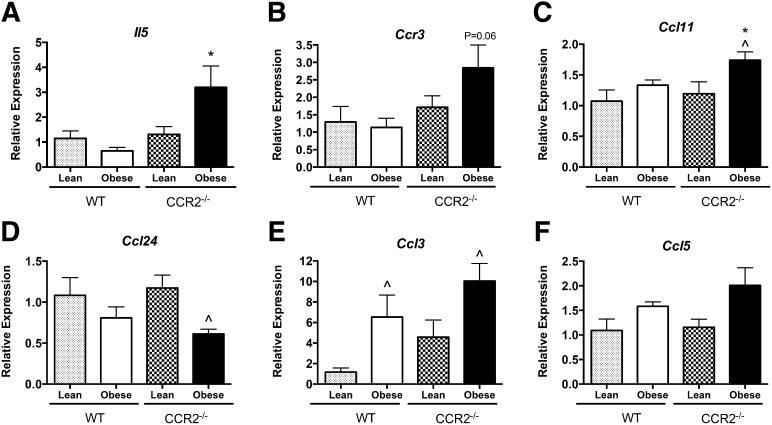
We next looked for AT-specific mechanisms to explain the local increase in eosinophil number, namely, the expression of the eosinophil growth factor, IL-5, as well as eosinophil chemokines CCL11, CCL24, CCL3, and CCL5. IL-5 is strongly linked with eosinophil function, as it regulates cellular differentiation, proliferation/apoptosis, activation, and accumulation [22, 23] and was recently shown to contribute to eosinophil accumulation in AT [15]. We found that eAT of obese CCR2−/− mice had significantly higher Il5 expression than that of WT counterparts (Fig. 6A), strongly correlating with the high number of eosinophils in CCR2−/− eAT (Fig. 2).
Figure 6. Eosinophil-chemoattractant expression in AT.
Total RNA was isolated from eAT of lean and obese WT and CCR2−/− mice and used for real-time RT-PCR analyses. Relative expression of (A) Il5, (B) Ccr3, (C) Ccl11, (D) Ccl24, (E) Ccl3, and (F) Ccl5. Data are normalized to lean WT control and presented as means ± sem, with n = 5–7 mice/group. *P < 0.05, difference between genotypes of mice on the same diet; ^P < 0.05, difference between diets in mice of the same genotype.
Tissue expression of the chemokine receptor CCR3 is associated with eosinophil recruitment [24]. Ccr3 expression trended toward an increase in eAT of CCR2−/− mice during obesity, positively correlating with increased eosinophils in the tissue (Fig. 6B; P = 0.06). A subset of chemokines, known as eotaxins, binds CCR3 to promote eosinophil chemotaxis. There are 2 known eotaxins in mice: eotaxin 1 (CCL11) and eotaxin 2 (CCL24), both of which have been shown to be important in eosinophil chemotaxis under different inflammatory conditions (reviewed in refs. [24, 25]). Expression of Ccl11 was up-regulated in obese CCR2−/− mice compared with obese WT mice (Fig. 6C; P < 0.05), as well as compared with their own lean controls (P < 0.05). The expression of Ccl24 was decreased in obese CCR2−/− mice (P < 0.05) compared with their respective lean controls but was not different between genotypes (Fig. 6D). The chemokines CCL3 and CCL5 also promote eosinophil chemotaxis via binding to CCR1 [24]. Obesity significantly increased the expression of Ccl3 (Fig. 6E) in both genotypes compared with lean controls, but there was no difference between genotypes. Expression of Ccl5 was increased modestly by obesity in both genotypes (Fig. 6F).
We analyzed all chemokine data with respect to eosinophil number and found that expression of Il5 in eAT had a strong positive correlation (r2 = 0.9210, P < 0.05) to the mean number of eAT eosinophils in the 4 groups of mice. This is in contrast to the absence of correlation of Ccl11 (r2 = 0.4582, P = 0.32), Ccl24 (r2 = 0.1432, P = 0.62), Ccl3 (r2 = 0.2899, P = 0.46), and Ccl5 (r2 = 0.2573, P = 0.49) expression with eAT eosinophil number. These data implicate IL-5 as the putative local modulator of AT-specific eosinophil cell turnover (i.e., recruitment, proliferation, apoptosis).
CCR2−/− mice manifest increased M2-like macrophages and type 2 cytokine expression in eAT
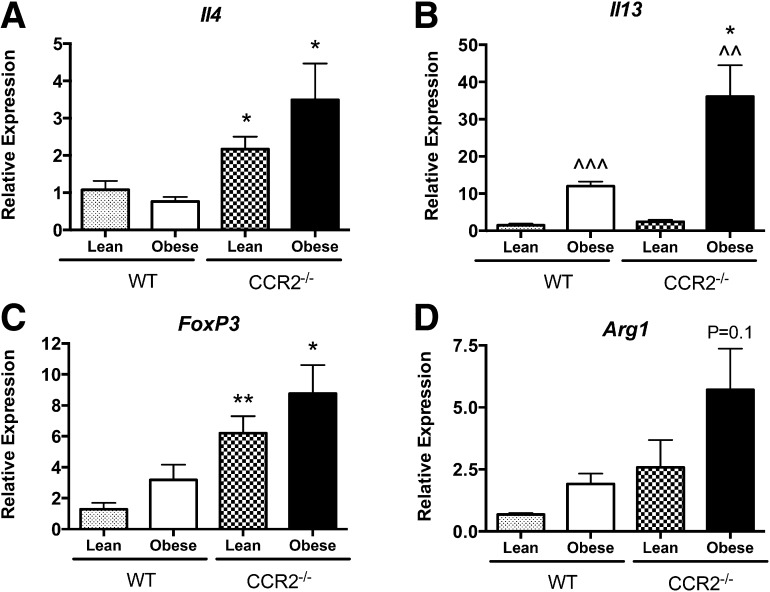
To investigate the inflammatory state of immune cells in AT, expression of M2 macrophage-polarizing cytokines, Il4 and Il13, was first assessed in RNA isolated from whole eAT. Expression of eAT Il4 was increased significantly in lean (P < 0.05) and obese (P < 0.05) CCR2−/− mice compared with their WT counterparts (Fig. 7A). Likewise, although eAT Il13 expression was increased in both genotypes during obesity (P < 0.01), CCR2−/− eAT had significantly higher Il13 mRNA levels (P < 0.05; Fig. 7B). We determined that eAT Il4 was expressed mainly in the SVF fraction of obese WT mice (data not shown), indicating that adipocytes were not the source of IL-4. Interestingly, eAT Il13 expression was higher in adipocytes compared with the SVF of obese WT mice (data not shown). In addition to eosinophils, Tregs have been found to play an important role in sustaining an anti-inflammatory environment in AT [26]. Foxp3 is a transcription factor critical for the differentiation and function of Tregs, and it is exclusively expressed by these cells [27, 28]. Expression of Foxp3 in eAT was significantly higher in lean (P < 0.01) and obese (P < 0.05) CCR2−/− mice compared with respective WT controls (Fig. 7C).
Figure 7. AT eosinophil accumulation is associated with type 2 cytokine expression.
Total RNA was isolated from eAT of lean and obese WT and CCR2−/− mice and used for real-time RT-PCR analysis. Relative expression of (A) Il4, (B) Il13, (C) Foxp3, and (D) Arg1. Data are presented as means ± sem, with n = 5–7 mice/group. *P < 0.05, difference between genotypes of mice on the same diet; **P < 0.01, difference between genotypes of mice on the same diet; ^^P < 0.01, difference between diets in mice of the same genotype; ^^^P < 0.001, difference between diets in mice of the same genotype.
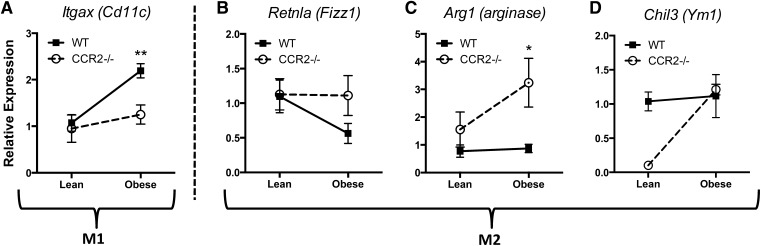
We showed previously an increase in Arg1 expression in AT of CCR2−/− mice [8]. In the current study, we were able to recapitulate increased Arg1 expression in total eAT (Fig. 7D), indicating a predominant M2-like macrophage polarization in the CCR2−/− mice. To determine macrophage polarization specifically, eAT macrophages from WT and CCR2−/− mice were isolated by FACS, and expression of various M1 and M2 markers was assessed. The gene for CD11c (Itgax), the classical M1-like protein identified in AT [17], was increased in eAT macrophages from obese WT mice as expected. However, Itgax was maintained at lower levels in macrophages from eAT of obese CCR2−/− mice (Fig. 8A). With regards to M2 markers, the gene for Fizz1 protein, Retnla, was reduced in eAT macrophages from obese WT mice, but levels of this M2 marker were preserved in eAT macrophages from obese CCR2−/− mice (Fig. 8B). Furthermore, whereas M2 genes for arginase and Ym1, (Arg1 and Chil3, respectively) were unchanged in eAT macrophages during obesity in WT mice, they were up-regulated in eAT macrophages of CCR2−/− mice (Fig. 8C and D), albeit with the caveat that Chil3 started at a lower value in CCR2−/− eAT macrophages compared with WT. In line with the field’s understanding that in vivo macrophages are rarely, entirely polarized to M1 or M2, we found that some genes were not changed by obesity in either genotype (Clec10a, Nos2, and Tnfα; data not shown). Nevertheless, these changes demonstrated a general pattern of alternative activation of macrophages in eAT of CCR2−/− mice.
Figure 8. CCR2 deficiency counteracts typical AT M1 macrophage polarization associated with obesity.
F4/80hi;CD11bhi;SiglecF− macrophages were sorted from the eAT SVF of lean and obese WT and CCR2−/− mice and used for real-time RT-PCR analysis. Relative expression of (A) Itgax (CD11c), (B) Retnla (Fizz1), (C) Arg1 (arginase), and (D) Chil3 (Ym1). Data are normalized to lean WT control and presented as means ± sem, with n = 4–5 mice/group. *P < 0.05, difference between genotypes of mice on the same diet; **P < 0.01, difference between genotypes of mice on the same diet.
DISCUSSION
In addition to defense against foreign pathogens, immune cells are involved in the normal physiology of metabolic organs and in the pathogenesis of metabolic disorders. In AT, several cells have been identified as promoters of insulin resistance, among them “M1”-polarized macrophages [11], B cells [12], CD4+ TH1 cells, and CD8+ T cells [11, 29], whereas other immune cells have been identified as insulin sensitizers, as is the case for M2-polarized macrophages [17], CD4+ TH2 cells, Tregs [26], and eosinophils [14–16]. Recent studies by Wu et al. [14] demonstrated that eosinophils sustain alternative activation of AT macrophages and contribute to improved glucose tolerance and insulin sensitivity in mice. These findings demonstrated that the inflammatory, insulin-resistant AT in obese mice has a reduction in eosinophils, suggesting that eosinophils help maintain AT homeostasis. Additionally, IL-5 transgenic mice, with global elevations in eosinophils, are protected from HFD-induced obesity and insulin resistance. In subsequent work, Molofsky et al. [15] demonstrated that ILC2 cells are responsible for producing IL-5, which recruits and sustains eosinophils in AT. Such findings encouraged further study by Hams et al. [16], who showed that ILC2 and NKT cells influence glucose homeostasis and body weight in HFD-fed mice by inducing and maintaining eosinophils and M2-like macrophages in visceral AT. Furthermore, AT eosinophils produce IL-4, and ILC2 cells produce IL-13 [14, 15]. Thus, in AT, eosinophils and ILC2 cells are thought to coordinate a homeostatic type 2 immune environment that maintains an insulin-sensitive state.
There is a short list of published work addressing the role of eosinophils in AT inflammation thus far; however, it is interesting to consider and compare the metabolic phenotypes seen in their models (metabolic improvements [14]) with that of our previous report (no metabolic improvements [8]). There are 2 primary differences between the studies: the systemic tissue distribution of eosinophils and localization of eosinophils to specific regions within AT. First, the studies by Wu et al. [14] used IL-5-overexpressing mice, which have systemic elevations in eosinophils [15]. Whereas one of the major metabolic phenotypes seen in their model was in the AT and attributed to AT eosinophils, a role for eosinophils in other tissues cannot be ruled out. It is also important to note that the IL-5 transgenic mice had reduced body weight compared with controls, which could account for the improved insulin sensitivity. In the current study, we demonstrate that the CCR2−/− mouse is a model of peritoneal and AT-specific eosinophil accumulation, as increased eosinophils were not detected in the bone marrow, blood, or other tissues examined. Of note, not all known sites of eosinophil accumulation were assessed, including the GI tract, lung, and thymus. Therefore, systemic versus AT-specific eosinophil accumulation may be a cause of the different metabolic phenotypes in the 2 studies.
Second, differential localization of eosinophils within the AT could be an important indication of their function. Our data showed that while in WT mice, eosinophils were only seen in interstitial spaces, in CCR2−/− mice, eosinophils localized interstitially and to CLSs. In fact, in obese CCR2−/− mice, ∼13% of the CLS cell milieu were eosinophils. It is established that AT macrophages in CLSs are M1 like, proinflammatory, and induce insulin resistance [17, 30], whereas AT macrophages in interstitial spaces are M2 like, anti-inflammatory, and protect from insulin resistance [17, 30]. It would be intriguing for future studies to determine whether AT eosinophil function also varies depending on localization within the tissue. It is important to note that the novel and surprising finding of eosinophils in CLSs of CCR2−/− mice correlated with a reduction in the absolute number and percent of macrophages in CLS. This reduction in CLS macrophages could be a result of the absence of CCL2-mediated recruitment in CCR2−/− mice. An alternative explanation would be that CLS eosinophils reduce accumulation of macrophages. Also unknown is whether interstitially spaced eosinophils produce basal levels of mediators beneficial to AT homeostasis, whereas CLS eosinophils may release some of their more potent inflammatory agents, i.e., MBP, EPO, TNF-α, and others. Findings from our current study, integrated with recently published work, now suggest that AT eosinophil number alone is not sufficient to determine the effect on insulin resistance. Context-dependent eosinophil function will need to be examined more critically in future studies, in acknowledgment of the newly forming dogma that eosinophils are multifunctional cells that can impart destructive or restorative effects [31–33]. In addition, this comparison elicits further investigation to separate a potential therapeutic effect of increased levels of IL-5 and/or eosinophils outside of the AT on metabolism and glucose tolerance.
Our data point to at least 2 mechanisms for altered eosinophil content in AT of CCR2−/− mice. First, we demonstrate that genes, such as Epx, Prg2, and Il5ra, were increased during the differentiation process in bone marrow cells lacking intrinsic CCR2 expression, although numerical yield of eosinophils did not differ. Eosinophils express CCL2 and eotaxin, both of which can interact with the CCR2 receptor [25, 34, 35]. Thus, a lack of the receptor in CCR2−/− bone marrow progenitors may inhibit autoregulation of differentiation, accounting for the greater expression of certain eosinophil genes. Second, we found a strong positive correlation between expression of Il5 in AT and levels of eosinophils in both genotypes. ILC2s were recently shown to produce Il5 in AT that then modulated the accumulation of eosinophils [15]. If the same mechanism is at play, then our data suggest that CCR2 deficiency increases production of IL-5 by ILC2s and/or the numbers of these cells, leading to increased recruitment of eosinophils. This hypothesis remains to be tested but could provide interesting insights into the biology of the newly discovered ILC2s.
In addition to AT, a role for resident eosinophils in homeostasis has been reported in a variety of tissues. Eosinophils have been shown to regulate tissue damage in the lung [36] and liver [37], epidermal wound healing [38], the onset and duration of estrus in the uterus [39], mammary gland development [40], and GI tract remodeling [41]. Each of these processes involves angiogenesis, fibrin/collagen deposition, and/or cell turnover, all of which can be regulated specifically by eosinophils and are also key processes in AT homeostasis. These environments also possess a unique milieu of local stimuli that likely generate specific eosinophil phenotypes. This notion is supported by the LIAR hypothesis that suggests "eosinophils are actually regulators of Local Immunity And/or Remodeling/Repair in both health and disease…(and)...accumulation occurs as part of a strategy(ies) to maintain tissue homeostasis" [32]. It is plausible that resident eosinophils assist in maintaining AT function based on local environmental signals, which ultimately affects the state of insulin resistance locally and systemically.
Our data show that AT eosinophil accumulation in CCR2−/− mice occurs concomitantly with elevated expression of typical type 2 cytokines, Il4, Il5, and Il13, and macrophage M2-like polarization. Our results regarding AT macrophage polarization in CCR2−/− mice are similar to previous reports. For instance, studies using CCR2 inhibitors have shown decreased M1 and increased M2 polarization of AT macrophages [42]. In contrast, Weisberg et al. [9] also demonstrated increased inflammatory genes but did not report whether there were changes in M2 markers. Ito et al. [43] also reported an increase in expression of Itgax and Tlr4 but did not see any changes in the M2 markers mannose receptor C type 1 or CD163. Thus, there are multiple bodies of work reporting altered macrophage polarization associated with the modulation of CCR2. However, the mechanism for this polarization has yet to be defined. We are the first to demonstrate an increased AT eosinophil number in CCR2−/− mice, and thus, we suggest that eosinophils may account for the previously unexplained mechanism of macrophage polarization in CCR2−/− mice. Future studies may now test whether the maintained M2-like phenotype of AT macrophages in CCR2−/− mice is a result of the increased eosinophils or of the systemic absence of CCR2 signaling.
Several studies have demonstrated that CCR2−/− mice have a pronounced type 2 polarization [7, 13]; however, to date, the function of CCR2 in this context has not been fully delineated. Furthermore, to our knowledge, there is no previous evidence that has linked CCR2 deficiency to eosinophilia or to any hypereosinophilic diseases. Thus, because of the advanced stages in the development of several CCR2 antagonists as drugs against atherosclerosis, arthritis, and autoimmune disease [44], it is worthwhile to consider eosinophilic hyperplasia and tissue accumulation as a likely side effect of these therapies that could be beneficial or detrimental depending on the context in which the drugs are administered. Eosinophilia has several clinical implications, including the response against helminthic parasite infections [45], in which an overactive type 2 response would promote resistance toward infection. In fact, CCR2−/− mice were recently found to be resistant to infection by the helminthic parasite Trichuris muris [46], suggesting a therapeutic potential for the inhibition of CCR2 in fighting parasitic infections. Conversely, CCR2 deficiency was shown to promote infection in the lung by the dimorphic fungus Histoplasma capsulatum as a result of increased IL-4 levels and an increased type 2 response [13]. Thus, depending on the pathogen and/or the clinical condition of the affected patient, CCR2 inhibition may improve or worsen the infection. It is also important to note that a hypereosinophilic state has been shown to promote or arrest several tumors and cancers [47, 48]. Clearly, altered eosinophil content can impart a plurality of effects for a diverse range of diseases, which demands further study to understand the nuances of eosinophil function.
In summary, in this study, we demonstrate that CCR2 deficiency leads to increased eosinophil number, alternative macrophage activation, and type 2 cytokine expression in white AT. CCR2 and its ligand CCL2 have been studied heavily in the context of AT inflammation during obesity, leading to conflicting results and conclusions. Several studies have shown CCR2 deficiency or antagonism to have a protective effect on AT inflammation and insulin resistance [8, 9, 17, 30, 42, 43, 49] but have focused on a decreased number of recruited macrophages to AT as the explanation. Additionally, many of these studies have shown M2 macrophage polarization as a result of CCR2 deficiency or inhibition, without a corresponding mechanism for this observation. The current study provides a link between the newly discovered role of eosinophils in sustaining alternative macrophage activation [14, 15] and the enigmatic consequences of CCR2 deficiency/inhibition on AT macrophages and inflammation. Additionally, we now present a model of localized increases in AT eosinophils that could be used in future studies to distinguish the effects of AT-specific eosinophil accumulation versus systemically elevated eosinophils on metabolism.
AUTHORSHIP
W.R.B. and D.A.G. contributed the most substantial amount of work toward the study’s conception, design, and performance. E.K.A.-B., A.J.K., and A.H.H. also contributed to the study’s conception, design, and performance. W.R.B., D.A.G., E.K.A.-B., A.J.K., and A.H.H. each assisted in drafting the article for submission and have approved the final version.
ACKNOWLEDGMENTS
This project was supported by a Veterans Health Administration Merit Award (5I01BX002195) and by an American Heart Association Established Investigator Award (12EIA8270000; to A.H.H.). D.A.G. was supported by an individual National Research Service Award (DK091128), W.R.B. was supported by the Molecular Endocrinology Training Grant (DK07563), and A.J.K. was supported by a postdoctoral fellowship from the ADA. E.K.A.-B. was supported by a predoctoral American Heart Association (AHA) fellowship (12PRE11910047). A.H.H. was also supported by U.S. National Institutes of Health National Heart, Lung, and Blood Institute Grant HL089466 and an ADA Career Development Award (1-07-CD-10). Flow cytometry experiments were performed in the VUMC Flow Cytometry Shared Resource, and confocal imaging experiments were performed in the VUMC Cell Imaging Shared Resource, both with scholarship provided by the Vanderbilt Digestive Disease Research Center (DK058404). The authors thank the members of their laboratory for their insightful comments on this project.
Glossary
- 3D
3-dimensional
- ACK
ammonium-chloride-potassium
- ADA
American Diabetes Association
- APC
allophycocyanin
- ARG1
arginase 1
- AT
adipose tissue
- CCR2−/−
chemokine receptor 2 global-deficient mouse
- Chil3
chitinase 3-like protein 3 gene
- CLS
crown-like structure
- eAT
epididymal adipose tissue
- EPO
eosinophil peroxidase
- Epx
eosinophil peroxidase gene
- Fizz1
found in inflammatory zone 1
- FOXP3
forkhead box P3
- GATA1
GATA-binding protein 1
- GI
gastrointestinal
- HFD
high-fat diet
- ILC2
innate lymphoid type 2 cell
- ITGAX
integrin α X
- LY6C
lymphocyte antigen 6 complex
- locus C1, M1
classically activated macrophage/inflammatory macrophage
- M2
alternatively activated macrophage/anti-inflammatory macrophage
- MBP
major basic protein
- MPO
myeloperoxidase
- pAT
perirenal adipose tissue
- Prg2
major basic protein gene
- SCF
stem cell factor
- SVF
stromal vascular fraction
- Treg
regulatory T cell
- VUMC
Vanderbilt University Medical Center
- WT
wild-type
- YM1
chitinase 3-like protein 3
Footnotes
SEE CORRESPONDING EDITORIAL ON PAGE 451
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Boring L., Gosling J., Monteclaro F. S., Lusis A. J., Tsou C. L., Charo I. F. (1996) Molecular cloning and functional expression of murine JE (monocyte chemoattractant protein 1) and murine macrophage inflammatory protein 1alpha receptors: evidence for two closely linked C-C chemokine receptors on chromosome 9. J. Biol. Chem. 271, 7551–7558. [DOI] [PubMed] [Google Scholar]
- 2.Kurihara T., Bravo R. (1996) Cloning and functional expression of mCCR2, a murine receptor for the C-C chemokines JE and FIC. J. Biol. Chem. 271, 11603–11607. [DOI] [PubMed] [Google Scholar]
- 3.Charo I. F., Ransohoff R. M. (2006) The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621. [DOI] [PubMed] [Google Scholar]
- 4.Loetscher P., Seitz M., Baggiolini M., Moser B. (1996) Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J. Exp. Med. 184, 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuziel W. A., Morgan S. J., Dawson T. C., Griffin S., Smithies O., Ley K., Maeda N. (1997) Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. USA 94, 12053–12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsou C. L., Peters W., Si Y., Slaymaker S., Aslanian A. M., Weisberg S. P., Mack M., Charo I. F. (2007) Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Invest. 117, 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boring L., Gosling J., Chensue S. W., Kunkel S. L., Farese R. V. Jr., Broxmeyer H. E., Charo I. F. (1997) Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Invest. 100, 2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez D. A., Kennedy A., Orr J. S., Anderson E. K., Webb C. D., Gerrald W. K., Hasty A. H. (2011) Aberrant accumulation of undifferentiated myeloid cells in the adipose tissue of CCR2-deficient mice delays improvements in insulin sensitivity. Diabetes 60, 2820–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weisberg S. P., Hunter D., Huber R., Lemieux J., Slaymaker S., Vaddi K., Charo I., Leibel R. L., Ferrante A. W. Jr (2006) CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 116, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shu C. J., Benoist C., Mathis D. (2012) The immune system’s involvement in obesity-driven type 2 diabetes. Semin. Immunol. 24, 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W. Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., Sole J., Nichols A., Ross J. S., Tartaglia L. A., Chen H. (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szymczak W. A., Deepe G. S. Jr (2009) The CCL7-CCL2-CCR2 axis regulates IL-4 production in lungs and fungal immunity. J. Immunol. 183, 1964–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu D., Molofsky A. B., Liang H. E., Ricardo-Gonzalez R. R., Jouihan H. A., Bando J. K., Chawla A., Locksley R. M. (2011) Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molofsky A. B., Nussbaum J. C., Liang H. E., Van Dyken S. J., Cheng L. E., Mohapatra A., Chawla A., Locksley R. M. (2013) Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 210, 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hams E., Locksley R. M., McKenzie A. N., Fallon P. G. (2013) Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J. Immunol. 191, 5349–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lumeng C. N., Bodzin J. L., Saltiel A. R. (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orr J. S., Kennedy A. J., Hasty A. H. (2013) Isolation of adipose tissue immune cells. J. Vis. Exp. e50707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyer K. D., Moser J. M., Czapiga M., Siegel S. J., Percopo C. M., Rosenberg H. F. (2008) Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J. Immunol. 181, 4004–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E., Wang S., Fortier M., Greenberg A. S., Obin M. S. (2005) Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355. [DOI] [PubMed] [Google Scholar]
- 22.Broughton S. E., Dhagat U., Hercus T. R., Nero T. L., Grimbaldeston M. A., Bonder C. S., Lopez A. F., Parker M. W. (2012) The GM-CSF/IL-3/IL-5 cytokine receptor family: from ligand recognition to initiation of signaling. Immunol. Rev. 250, 277–302. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi Y., Suda T., Ohta S., Tominaga K., Miura Y., Kasahara T. (1991) Analysis of the survival of mature human eosinophils: interleukin-5 prevents apoptosis in mature human eosinophils. Blood 78, 2542–2547. [PubMed] [Google Scholar]
- 24.Sabroe I., Hartnell A., Jopling L. A., Bel S., Ponath P. D., Pease J. E., Collins P. D., Williams T. J. (1999) Differential regulation of eosinophil chemokine signaling via CCR3 and non-CCR3 pathways. J. Immunol. 162, 2946–2955. [PubMed] [Google Scholar]
- 25.Rothenberg M. E., Hogan S. P. (2006) The eosinophil. Annu. Rev. Immunol. 24, 147–174. [DOI] [PubMed] [Google Scholar]
- 26.Feuerer M., Herrero L., Cipolletta D., Naaz A., Wong J., Nayer A., Lee J., Goldfine A. B., Benoist C., Shoelson S., Mathis D. (2009) Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benoist C., Mathis D. (2012) Treg cells, life history, and diversity. Cold Spring Harb. Perspect. Biol. 4, a007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cipolletta D., Feuerer M., Li A., Kamei N., Lee J., Shoelson S. E., Benoist C., Mathis D. (2012) PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 486, 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M., Otsu M., Hara K., Ueki K., Sugiura S., Yoshimura K., Kadowaki T., Nagai R. (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920. [DOI] [PubMed] [Google Scholar]
- 30.Lumeng C. N., DelProposto J. B., Westcott D. J., Saltiel A. R. (2008) Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57, 3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg H. F., Dyer K. D., Foster P. S. (2013) Eosinophils: changing perspectives in health and disease. Nat. Rev. Immunol. 13, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J. J., Jacobsen E. A., McGarry M. P., Schleimer R. P., Lee N. A. (2010) Eosinophils in health and disease: the LIAR hypothesis. Clin. Exp. Allergy 40, 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobsen E. A., Helmers R. A., Lee J. J., Lee N. A. (2012) The expanding role(s) of eosinophils in health and disease. Blood 120, 3882–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogilvie P., Bardi G., Clark-Lewis I., Baggiolini M., Uguccioni M. (2001) Eotaxin is a natural antagonist for CCR2 and an agonist for CCR5. Blood 97, 1920–1924. [DOI] [PubMed] [Google Scholar]
- 35.Izumi S., Hirai K., Miyamasu M., Takahashi Y., Misaki Y., Takaishi T., Morita Y., Matsushima K., Ida N., Nakamura H., Kasahara T., Ito K. (1997) Expression and regulation of monocyte chemoattractant protein-1 by human eosinophils. Eur. J. Immunol. 27, 816–824. [DOI] [PubMed] [Google Scholar]
- 36.Hogan S. P., Koskinen A., Foster P. S. (1997) Interleukin-5 and eosinophils induce airway damage and bronchial hyperreactivity during allergic airway inflammation in BALB/c mice. Immunol. Cell Biol. 75, 284–288. [DOI] [PubMed] [Google Scholar]
- 37.Proctor W. R., Chakraborty M., Chea L. S., Morrison J. C., Berkson J. D., Semple K., Bourdi M., Pohl L. R. (2013) Eosinophils mediate the pathogenesis of halothane-induced liver injury in mice. Hepatology 57, 2026–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leitch V. D., Strudwick X. L., Matthaei K. I., Dent L. A., Cowin A. J. (2009) IL-5-overexpressing mice exhibit eosinophilia and altered wound healing through mechanisms involving prolonged inflammation. Immunol. Cell Biol. 87, 131–140. [DOI] [PubMed] [Google Scholar]
- 39.Robertson S. A., Mau V. J., Young I. G., Matthaei K. I. (2000) Uterine eosinophils and reproductive performance in interleukin 5-deficient mice. J. Reprod. Fertil. 120, 423–432. [DOI] [PubMed] [Google Scholar]
- 40.Gouon-Evans V., Lin E. Y., Pollard J. W. (2002) Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 4, 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jordan H. E., Speidel C. C. (1923) Blood cell formation and distribution in relation to the mechanism of thyroid-accelerated metamorphosis in the larval frog. J. Exp. Med. 38, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamura Y., Sugimoto M., Murayama T., Minami M., Nishikaze Y., Ariyasu H., Akamizu T., Kita T., Yokode M., Arai H. (2010) C-C chemokine receptor 2 inhibitor improves diet-induced development of insulin resistance and hepatic steatosis in mice. J. Atheroscler. Thromb. 17, 219–228. [DOI] [PubMed] [Google Scholar]
- 43.Ito A., Suganami T., Yamauchi A., Degawa-Yamauchi M., Tanaka M., Kouyama R., Kobayashi Y., Nitta N., Yasuda K., Hirata Y., Kuziel W. A., Takeya M., Kanegasaki S., Kamei Y., Ogawa Y. (2008) Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. J. Biol. Chem. 283, 35715–35723. [DOI] [PubMed] [Google Scholar]
- 44.Struthers M., Pasternak A. (2010) CCR2 antagonists. Curr. Top. Med. Chem. 10, 1278–1298. [DOI] [PubMed] [Google Scholar]
- 45.Shin M. H., Lee Y. A., Min D. Y. (2009) Eosinophil-mediated tissue inflammatory responses in helminth infection. Korean J. Parasitol. 47(Suppl), S125–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mullaly S. C., Oudhoff M. J., Min P. H., Burrows K., Antignano F., Rattray D. G., Chenery A., McNagny K. M., Ziltener H. J., Zaph C. (2013) Requirement for core 2 O-glycans for optimal resistance to helminth infection. PLoS ONE 8, e60124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gatault S., Legrand F., Delbeke M., Loiseau S., Capron M. (2012) Involvement of eosinophils in the anti-tumor response. Cancer Immunol. Immunother. 61, 1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Podjasek J. C., Butterfield J. H. (2013) Mortality in hypereosinophilic syndrome: 19 years of experience at Mayo Clinic with a review of the literature. Leuk. Res. 37, 392–395. [DOI] [PubMed] [Google Scholar]
- 49.Kang Y. S., Lee M. H., Song H. K., Ko G. J., Kwon O. S., Lim T. K., Kim S. H., Han S. Y., Han K. H., Lee J. E., Han J. Y., Kim H. K., Cha D. R. (2010) CCR2 antagonism improves insulin resistance, lipid metabolism, and diabetic nephropathy in type 2 diabetic mice. Kidney Int. 78, 883–894. [DOI] [PubMed] [Google Scholar]