Review on the mechanisms by which STIM and ORAI proteins regulate neutrophil SOCE as it relates to calcium signaling in neutrophil activation.
Keywords: sensors, channels, NADPH oxidase, neutrophil activation
Abstract
Calcium signals in neutrophils are initiated by a variety of cell-surface receptors, including formyl peptide and other GPCRs, FcRs, and integrins. The predominant pathway by which calcium enters immune cells is termed SOCE, whereby plasma membrane CRAC channels allow influx of extracellular calcium into the cytoplasm when intracellular ER stores are depleted. The identification of 2 key families of SOCE regulators, STIM calcium “sensors” and ORAI calcium channels, has allowed for genetic manipulation of SOCE pathways and provided valuable insight into the molecular mechanism of calcium signaling in immune cells, including neutrophils. This review focuses on our current knowledge of the molecules involved in neutrophil SOCE and how study of these molecules has further informed our understanding of the role of calcium signaling in neutrophil activation.
Introduction
Calcium is an evolutionarily conserved signaling molecule that transduces signals critical for a diverse array of biologic processes, including fertilization, pancreatic enzyme release, and muscle contraction. SOCE is considered the main mechanism of calcium entry into immune cells, including neutrophils [1, 2]. In this process, the calcium-filling levels within the ER “stores” regulate the influx of calcium from the extracellular space. Whereas calcium and SOCE have been known for some time to be important for neutrophil activation, the molecular regulation of calcium entry into neutrophils has remained somewhat of an enigma for many years. In the past 7–8 yr, the identification of the calcium-sensing STIM proteins and ORAI calcium channel proteins has allowed rapid progress toward understanding the molecular regulation of SOCE in immune cells. Whereas studies in neutrophils have significantly lagged behind those in other lineages, recent work has begun to ask how these molecules contribute to SOCE in neutrophils.
SOCE IN IMMUNE CELLS
Active transport of calcium tightly controls intracellular calcium concentrations so that influx of calcium from outside of the cell can initiate intracellular signals, which lead to functional responses. In resting cells, the majority of intracellular calcium is located within the ER, which is often referred to as the ER “store.” Cytosolic calcium levels are maintained at relatively low levels by several calcium pumps, including plasma membrane and SERCA that pump calcium into the extracellular and ER spaces, respectively [3]. Multiple mechanisms allow calcium entry into the cytoplasm. Receptor-operated calcium channels permit ion flux when gated directly by ligand. Voltage-gated calcium channels predominate in excitable cells, such as neurons and myocytes. However, in nonexcitable cells, including immune cells, SOCE is the primary mechanism of calcium entry in response to cell stimulation [3]. Engagement of cell-surface receptors initiates a signaling cascade leading to phosphorylation of phospholipase family members. Phospholipases convert the membrane lipid phosphatidylinositol 4,5 bisphosphate into DAG and IP3, which binds to its receptor on the ER and releases calcium from the ER store. The efflux of calcium from the ER is followed by a second, prolonged calcium influx across the plasma membrane. This sustained calcium entry activates numerous calcium-dependent signaling molecules and transcription factors that modulate critical immune effector functions, including degranulation, cytokine production, cellular polarization, and proliferation [3].
Patch-clamp experiments in mast cells and T cells demonstrated that ER store depletion triggers calcium entry through plasma membrane channels known as CRAC channels [4, 5]. Multiple studies described in detail the electrophysical characteristics of the CRAC channel—highly calcium selective, low conductance [6]—however, the identity of this molecule and its gating mechanism have only been elucidated recently. In 2006, ORAI1 was identified as the pore subunit of the CRAC channel in studies that use RNA interference screening in Drosophila and genetic linkage analysis in a family with severe immunodeficiency characterized by absent SOCE and CRAC channel currents [7, 8]. Based on work mostly in lymphocytes and mast cells, ORAI1 is considered to be the dominant SOCE channel in immune cells. Two homologs, ORAI2 and ORAI3, have similar channel properties; however, their physiologic role remains to be determined [9, 10]. Nearly simultaneously, STIM1 was identified as an ER calcium sensor that opens the CRAC channel via direct physical interaction [11, 12]. STIM1 and its homolog STIM2 are structurally similar, comprising a transmembrane segment, a cytosolic portion, and an ER-luminal portion, which contains an EF hand that binds calcium at low affinity. When ER calcium stores are depleted, the calcium ions dissociate from STIM, leading to conformational change in the protein and redistribution into puncta at sites of ER-plasma membrane apposition (also termed membrane contact sites or cortical ER). STIM proteins then physically bind to ORAI channels, leading to their oligomerization in the membrane and opening of the CRAC channel [3, 13].
The importance of SOCE in the immune system is underlined by the severe immunodeficiency generated in humans with mutations in STIM1 or ORAI1 [8, 14, 15]. These children develop repeated viral, bacterial, and fungal infections along with signs of autoimmunity and lymphoproliferation. Nonimmune manifestations include ectodermal dysplasia and skeletal myopathy [14]. Investigation into the mechanism underlying this immune phenotype has focused primarily on the function of the adaptive immune system. T cell development appears grossly unaffected with STIM1 or ORAI1 mutations; however, activation and proliferation of peripheral T cells are markedly impaired [8, 15]. Mice deficient in STIM1 or ORAI1 die in the late embryonic or early perinatal period, likely secondary to myopathy and respiratory failure. However, lineage-specific deletion of STIM1 or studies from fetal liver chimeras demonstrate severely impaired SOCE in lymphocytes, mast cells, and macrophages, similar to the defects seen in human cells [16–19]. STIM1 appears to play a dominant role in SOCE, following receptor engagement in immune cells, whereas the absence of STIM2 in T cells only partially impairs SOCE [19]. However, sustained NFAT translocation and cytokine production are severely disrupted in the absence of STIM2. Mice with T cell-specific deficiency in STIM1 and STIM2 develop a lymphoproliferative disorder as a result of dysfunction of regulatory T cells, demonstrating the physiologic importance of both STIM molecules [19]. Similar findings have been found in B lymphocytes—deficiency of STIM1 and STIM2 leads to a significant block in cytokine production following engagement of the B cell antigen receptor [18]. Interestingly, ORAI1 deficiency in murine lymphocytes or mast cells results in a much milder defect in SOCE compared with human cells [9, 10]. Naïve T cells demonstrate only a slight defect in SOCE; however, in vitro-differentiated T cells almost completely lack SOCE, suggesting that ORAI2 or ORAI3 may comprise the CRAC channel in naïve murine but not human cells. Notably, some children with mutations in ORAI1 or STIM1 develop overwhelming bacterial sepsis, suggesting that neutrophil dysfunction may contribute to this immunodeficiency [13, 14].
The cloning of STIM and ORAI proteins has resulted in significant advances in our understanding of the molecular mechanism of SOCE and the role of calcium signaling in the immune system. Calcium signals in neutrophils are initiated by a variety of cell-surface receptors, including formyl peptide and other GPCRs, FcRs, and integrins [1]. Whereas a role for calcium and SOCE in neutrophil activation has been described for some time, studies have relied predominantly on inhibitors of SOCE or by manipulation of extracellular calcium concentrations, techniques that are limited in specificity. Study of STIM and ORAI molecules in neutrophils offers an opportunity to use genetic approaches to identify the molecular machinery required for SOCE in neutrophils, define the requirement for calcium signaling for key neutrophil functions, and determine how disrupted calcium signaling impacts neutrophil function in vivo.
SENSING THE NEUTROPHIL CALCIUM STORES
Before the identification of STIM1, several hypotheses were put forth to explain the mechanism linking store depletion to extracellular calcium influx, including “conformational coupling” of the ER channel with the plasma membrane channel, a diffusible calcium-influx factor, and vesicle fusion [20–22]. Indeed, some of these hypotheses were supported by work done in neutrophils, such as studies by Itagaki and Hauser [23], who demonstrated a role for sphingosine-1-phosphate as the diffusible mediator. However, work over the past 6 yr suggests that STIM1 serves as the link between ER calcium depletion and CRAC channel opening in neutrophils as well. Initial studies by Tschirhart and colleagues [24] demonstrated that in neutrophil-like HL-60 cells, siRNA knockdown of STIM1 impairs calcium influx in response to fMLF or the SERCA pump inhibitor thapsigargin. The disruption of STIM1-mediated SOCE in this system significantly impairs ROS production downstream of both receptors. Interestingly, STIM2 knockdown had no effect on SOCE or oxidative burst; however, STIM2 expression in these experiments was still 50% of wild-type, and so, a partial contribution of STIM2 to neutrophil SOCE cannot be excluded. A subsequent study from this group extended these findings to show that STIM1 is required for FcγR-induced SOCE and phagosomal ROS production as well [25]. Together, these studies suggest a functional role for SOCE in activation of the NADPH oxidase. Zou and colleagues [26] recently confirmed the requirement for STIM1 in fMLF-induced calcium flux and demonstrated a role for STIM1-mediated SOCE in cell polarization in response to fMLF, also in HL-60 cells. Activation of Akt, Src, and Rac2, following fMLF stimulation, is also impaired following siRNA knockdown of STIM1 in HL-60 cells. Additionally, it is reported that STIM1 knockdown affects the response of HL-60 cells to hypoxic stimuli [27].
There is limited data in primary cells. To study STIM1 function in primary murine neutrophils, Nunes et al. [28] bred Stim1-floxed mice to LysM-cre animals and found that the absence of STIM1 did not affect neutrophil development, even though mature cells showed a 90% reduction in STIM1 expression. The lack of STIM1 caused an ∼50% reduction in FcγR-mediated phagocytosis of IgG-opsonized RBCs. The authors correlated the reduced phagocytosis with a loss of a localized calcium efflux from the phagosomal compartment into the cytosol, suggesting that STIM1 in the ER couples to ORAI1 channel proteins to mediate calcium fluxes needed for efficient phagocytosis. Recent work from our laboratory corroborates this finding and also demonstrates that STIM1 is required for SOCE downstream of multiple receptor families (β2-integrins, GPCRs, FcγRs, dectin-1) or with passive store-emptying (thapsigargin) [29]. The disruption of SOCE has modest effects on degranulation; however, neutrophil adhesion and migration are intact in vitro (Transwell migration) and in vivo (migration to MIP2 in the footpad and neutrophil accumulation during infection and sterile inflammation). This result is surprising, given the studies in HL-60 cells that demonstrate impaired polarization (in Zigmond chambers), as polarization is an integral step in directional migration [26]. Perhaps this incongruity is a result of differences in experimental conditions or divergent signaling mechanisms in human neutrophils compared with murine cells or cell lines. Resolution of this discrepancy will require further study.
Interestingly, the disruption of STIM1-mediated calcium entry in neutrophils markedly impairs superoxide release following engagement of multiple receptors, confirming the importance of SOCE for activation of the NADPH oxidase in primary cells. We focused on the potential that calcium-sensitive PKC isoforms might be involved, based primarily on the observation that treatment of STIM1-deficient neutrophils with phorbol esters (PMA) rescued their defect in NADPH oxidase activation. Although calcium-sensitive PKCs have been implicated as the kinases involved in phosphorylation of p47phox and p40phox subunits of the oxidase, other enzymes may be involved [30]. Indeed, STIM1-deficient neutrophils fail to fully activate PKCα and -β, as determined by reduced membrane translocation of these enzymes, following integrin or fMLF stimulation. As a result, phosphorylation of p40phox and p47phox is reduced dramatically in STIM1-deficient neutrophils, explaining the impaired ROS production in these cells. Interestingly, in HL-60 cells, Steinckwich et al. [25] suggested that SOCE is coupled to phagosomal NADPH oxidase through calcium binding to the S100A8-A9 proteins. This finding is also supported by a study by Schenten et al. [31], who demonstrated S100A8-A9 translocation to the plasma membrane following fMLF-induced activation of the oxidase. This process was calcium store dependent and sphingosine kinase 1 dependent but PKC independent, suggesting that likely multiple calcium-dependent pathways are involved in activation of the NADPH oxidase. In vivo, hematopoietic loss of STIM1 results in dramatic protection from oxidative tissue injury in neutrophil-dependent hepatic ischemia-reperfusion injury. Interestingly, bacterial clearance in Staphylococcus aureus pneumonia is markedly impaired, supporting the notion that neutrophil dysfunction likely contributes to the immunodeficiency in patients with STIM1 and/or ORAI1 mutations. Collectively, these studies point to a dominant role for STIM1 in neutrophil SOCE.
A ROLE FOR STIM2?
Whereas SOCE and neutrophil activation are disrupted in the absence of STIM1, some residual function remains, suggesting that other proteins may also contribute to the gating mechanism of SOCE in neutrophils. While STIM1 and STIM2 can promote SOCE in overexpression systems, the physiologic importance of STIM2 differs between cell lineages [32]. Indeed, in dendritic cells, STIM2 may be the primary calcium-sensing molecule used [33]. Additionally, some receptors may differentially activate STIM1 and STIM2 within the same cell type. For example, in mast cells, STIM2 is critical for FcεR-triggered SOCE but is dispensable for calcium entry downstream of the leukotriene receptor [34]. The STIM2 EF hand has a lower affinity for calcium and therefore, lower activation threshold; thus, some STIM2 resides in puncta, even at basal levels of ER calcium, and has been shown to regulate basal calcium levels in cell lines [35]. It has also been proposed that STIM2 is preferentially used for SOCE caused by mild store depletion (low-concentration agonists) [36]. Per data from the ImmGen database, neutrophils express abundant levels of STIM2, relatively more compared with other immune cells, suggesting that perhaps this protein may partially compensate to support some neutrophil SOCE in the absence of STIM1. Alternatively, STIM1 and STIM2 may play complementary roles downstream of various neutrophil receptors. Further studies that use STIM2 and STIM1/STIM2 doubly deficient neutrophils will be required to determine the role of STIM2 in regulation of calcium levels in resting and activated neutrophils.
THE NEUTROPHIL CRAC CHANNEL
As described above, ORAI1 is considered to be the dominant SOCE channel in immune cells, particularly human cells [9, 10]. Work that has accumulated since the identification of ORAI1 suggests that this channel also plays a role in neutrophils as well. In HL-60 cells, siRNA knockdown of ORAI1 decreases SOCE and ROS production in response to stimulation through GPCRs or FcγRs, albeit only by 20–50%, even with ORAI1 protein levels reduced by >80% [25, 37, 38]. A series of papers from the Simon lab [38] nicely demonstrated that neutrophil arrest under shear flow requires ORAI1-mediated calcium flux. Subsequent studies further show that shear stress induces colocalization of LFA-1, Kindlin-3, and ORAI1 and that these initial steps are required for high-affinity LFA-1-induced calcium flux that orients the polymerization of actin along a uropod-pseudopod axis to establish cell polarity and migratory direction [39]. These studies were primarily in HL-60 cells; however, similar findings were obtained by use of Orai1+/− murine neutrophils.
It is interesting that ORAI1 is required for neutrophil migrational guidance, whereas STIM1 is dispensable for in vitro and in vivo migration in murine neutrophils. Potentially, STIM2 may be coupling to ORAI1 to mediate its function in LFA-1-induced calcium flux. It is also possible that the experimental system of shear flow in microfluidic chambers is a more sensitive assay for detecting alterations in neutrophil movement or that adhesive interactions other than high-affinity integrin ligation are sufficient to guide neutrophil recruitment in vivo, at least in the infection or hepatic ischemia/reperfusion models. Additionally, the measured calcium flux in these studies is the summation of 2 stimuli (integrin plus, e.g., GPCR or thapsigargin), so it is difficult to compare these results directly with other studies. Regardless, it is clear that SOCE is only partially disrupted in the absence or reduction of ORAI1. This conclusion is also supported by data presented at The Neutrophil Symposium [unpublished results], demonstrating that in primary murine neutrophils derived from mice with neutrophil-specific Orai1 deletion, there is only a minor defect in SOCE over a range of calcium concentrations and with multiple agonists. These ORAI1-deficient cells also show completely normal ROS production. Collectively, these results are markedly different from Stim1−/− neutrophils and suggest that ORAI1 is not the only calcium channel mediating SOCE in neutrophils.
Additional candidates for the neutrophil CRAC channel include the 2 other ORAI family members, ORAI2 and ORAI3, which have structural and channel properties similar to ORAI1. To date, their function remains largely unknown, although ORAI2 knockdown in HL-60 cells does decrease SOCE by 20–30% [25, 40]. Interestingly, in murine neutrophils, the expression of ORAI2 is at least 3-fold higher than ORAI1, as judged by quantitative PCR. Before the identification of ORAI1, considerable attention was focused on the TRP family channels, although the electrophysical properties of these channels do not match those of the CRAC channel. Indeed, reports in some lineages, including mast cells and neutrophils, suggest that STIM proteins may also gate TRPC channels, either alone or in heteromers with ORAI1 [37, 41, 42]. Neutrophils have been reported to express multiple TRPC, TRPV, and TRPM family members [43]. Interestingly, repression of TRPC1 and TRPC6 by use of siRNA in HL-60 cells decreases SOCE to a similar degree as ORAI1 [37]. Additionally, Trpc6−/− murine neutrophils have modestly decreased calcium flux in response to fMLF, but it is not clear whether this is a true SOCE-mediated response [44]. At the RNA level, TRPC6 is very weakly expressed relative to the ORAI channel proteins ([unpublished observation] and ref. [37]). Future studies are required examining knockdown or knockout of single and multiple ORAI and TRPC family members to determine the relative contribution of these molecules to neutrophil SOCE.
CONCLUDING REMARKS
In summary, we are beginning to understand the molecular machinery required for calcium entry into neutrophils through genetic manipulation of STIM and ORAI (summarized in Fig. 1). Clearly, STIM1 plays a primary role in neutrophil SOCE; however, whether STIM2 contributes to calcium-sensing in all or some contexts remains to be seen. The role of ORAI1 is less clear, and the available data suggest that likely multiple channels permit calcium entry, at least in murine neutrophils. Further studies of ORAI2, ORAI3, and perhaps TRPC channels will be required to delineate fully the channels operating in neutrophils SOCE. Given the differences in the expression pattern and function of calcium channels and sensors in human versus murine cells and primary cells versus cell lines, it will be critical to confirm these results in primary human neutrophils to assess whether these molecules are viable therapeutic targets. Furthermore, clearly, receptors, even within the same receptor family, initiate distinct signaling pathways, and their reliance on the various STIM and ORAI family members may differ. Thus, much work remains to be done.
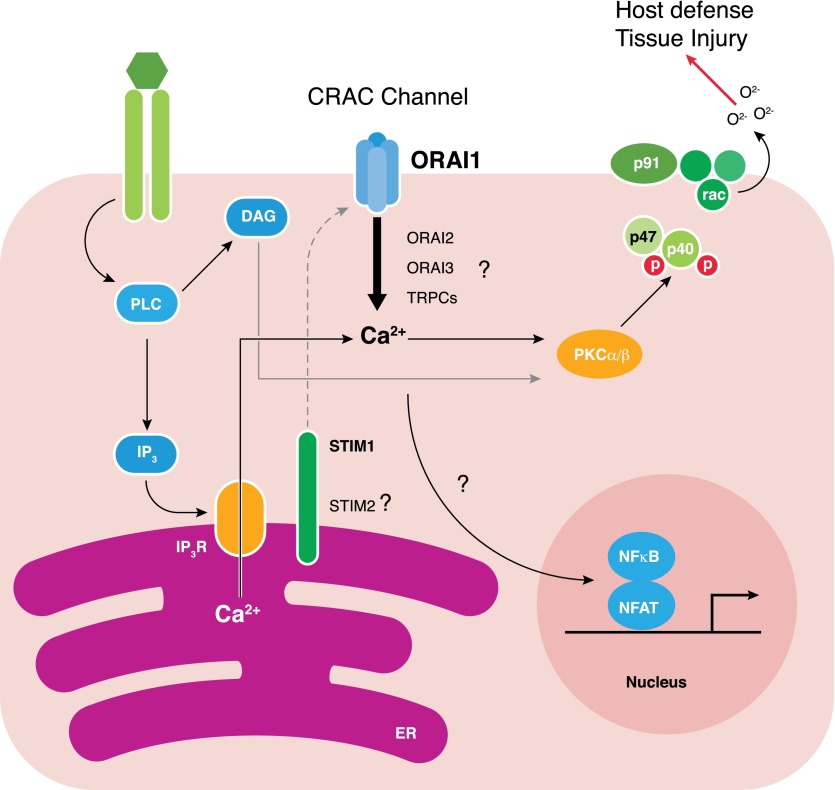
Figure 1. Schematic pathway of SOCE in neutrophils, leading to activation of the NAPDH oxidase.
Solid lines indicate known pathways; dotted lines indicate pathways requiring further investigation. Following engagement of surface receptors (shown as green ovals), activation of phospholipase C (PLC) leads to production of IP3 and DAG. IP3 activates the IP3R in the ER, leading to calcium release from ER stores into the cytoplasm. The decrease in calcium concentration in the ER activates calcium sensors STIM1 and potentially STIM2. One or the other (or both; hence, the dotted-line connection) of these sensors couples to surface calcium channels to induce entry of calcium from the extracellular space. The relative contribution of ORAI1, ORAI2, ORAI3, or TRPC channels to calcium entry remains to be investigated. Nevertheless, sustained elevation in intracellular calcium works with DAG to activate PKC isoforms (shown is PKCα and PKCβ; however, PKCδ is also known to be involved [45]), which phosphorylates NADPH subunits and allows assembly of the NADPH oxidase in the plasma/phagosomal membrane for superoxide production. Extracellular calcium influx is also likely coupled to transcriptional changes in neutrophils (shown by the dotted line), probably through calcineurin, as in other immune cells.
In addition to understanding the molecular process of SOCE, studies of STIM1- and ORAI1-deficient cells have illuminated our knowledge of how SOCE participates in neutrophil activation. SOCE is clearly required for activation of the NADPH oxidase and for efficient phagocytosis and degranulation. However, much remains to be learned. The role of STIM, ORAI, and SOCE in cell polarization and migration appears more complex, and further studies are required to understand this process fully. What other neutrophil functions require SOCE? Is SOCE required for NETosis, cytokine production, or cell survival? Extracellular calcium influx is likely coupled to transcriptional changes in neutrophils, probably through calcineurin, as in other immune cells. Indeed, neutrophils derived from calcineurin-deficient mice manifest impaired up-regulation of IL-10, cyclooxygenase 2, and early growth response 1/2, known transcriptional targets of calcineurin, following stimulation of dectin-1, which translates into increased sensitivity to fungal infection in vivo [46]. Similar experiments remain to be done in STIM1- or ORAI-deficient neutrophils. SOCE is not a stereotyped on-off switch; neutrophil activation through different receptors initiates calcium flux of markedly variable amplitude, morphology, and duration, suggesting that more complex regulation of SOCE machinery likely occurs. Once the sensor-channel machinery has been defined in neutrophils, we can then begin to ask how this complex is modulated at the molecular level (e.g., septin, SOCE-associated regulatory factor, Junctate, etc.). These will be important questions to address in the future. Given the central role of SOCE in neutrophil activation, the understanding of the molecular machinery involved in this process may identify therapeutic targets to manipulate neutrophil activation in inflammatory disease.
AUTHORSHIP
R.A.C. and C.A.L. wrote and revised the manuscript.
ACKNOWLEDGMENTS
This work was supported by grants to C.A.L. from the National Institute of Allergy and Infectious Diseases U.S. National Institutes of Health (RO1 AI065495 and RO1 AI068150). R.A.C. is a fellow of the Pediatric Scientist Development Program, supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K12-HD000850). The authors thank Clare Abram for careful review of the manuscript and Yongmei Hu for assistance with studies of STIM1- and ORAI1-deficient mice.
Glossary
- CRAC
calcium release-activated calcium (channel)
- DAG
diacylglycerol
- EF hand
helix-loop-helix structural domain
- ER
endoplasmic reticulum
- GPCR
G-protein-coupled receptor
- IP3
inositol 1,4,5 trisphosphate
- ORAI
calcium release-activated calcium channel protein
- PKC
protein kinase C
- Rac2
Ras-related C3 botulinum toxin substrate 2
- ROS
reactive oxygen species
- SERCA
sarco-endoplasmic reticulum calcium ATPase
- siRNA
small interfering RNA
- SOCE
store-operated calcium entry
- STIM
stromal interaction molecule
- TRP
transient receptor potential
DISCLOSURES
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Davies E. V., Hallett M. B. (1998) Cytosolic Ca2+ signalling in inflammatory neutrophils: implications for rheumatoid arthritis (Review). Int. J. Mol. Med. 1, 485–490. [DOI] [PubMed] [Google Scholar]
- 2.Itagaki K., Kannan K. B., Livingston D. H., Deitch E. A., Fekete Z., Hauser C. J. (2002) Store-operated calcium entry in human neutrophils reflects multiple contributions from independently regulated pathways. J. Immunol. 168, 4063–4069. [DOI] [PubMed] [Google Scholar]
- 3.Hogan P. G., Lewis R. S., Rao A. (2010) Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 28, 491–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoth M., Penner R. (1992) Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355, 353–356. [DOI] [PubMed] [Google Scholar]
- 5.Zweifach A., Lewis R. S. (1993) Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. USA 90, 6295–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parekh A. B., Putney J. W. Jr. (2005) Store-operated calcium channels. Physiol. Rev. 85, 757–810. [DOI] [PubMed] [Google Scholar]
- 7.Prakriya M., Feske S., Gwack Y., Srikanth S., Rao A., Hogan P. G. (2006) Orai1 is an essential pore subunit of the CRAC channel. Nature 443, 230–233. [DOI] [PubMed] [Google Scholar]
- 8.Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S. H., Tanasa B., Hogan P. G., Lewis R. S., Daly M., Rao A. (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185. [DOI] [PubMed] [Google Scholar]
- 9.Gwack Y., Srikanth S., Oh-Hora M., Hogan P. G., Lamperti E. D., Yamashita M., Gelinas C., Neems D. S., Sasaki Y., Feske S., Prakriya M., Rajewsky K., Rao A. (2008) Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol. Cell. Biol. 28, 5209–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vig M., DeHaven W. I., Bird G. S., Billingsley J. M., Wang H., Rao P. E., Hutchings A. B., Jouvin M. H., Putney J. W., Kinet J. P. (2008) Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat. Immunol. 9, 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S. L., Yu Y., Roos J., Kozak J. A., Deerinck T. J., Ellisman M. H., Stauderman K. A., Cahalan M. D. (2005) STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., Veliçelebi G., Stauderman K. A. (2005) STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 169, 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feske S. (2007) Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 7, 690–702. [DOI] [PubMed] [Google Scholar]
- 14.Feske S. (2011) Immunodeficiency due to defects in store-operated calcium entry. Ann. N. Y. Acad. Sci. 1238, 74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picard C., McCarl C. A., Papolos A., Khalil S., Lüthy K., Hivroz C., LeDeist F., Rieux-Laucat F., Rechavi G., Rao A., Fischer A., Feske S. (2009) STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N. Engl. J. Med. 360, 1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baba Y., Kurosaki T. (2008) [Regulation of store-operated calcium entry by STIM1]. Seikagaku 80, 1123–1128. [PubMed] [Google Scholar]
- 17.Braun A., Gessner J. E., Varga-Szabo D., Syed S. N., Konrad S., Stegner D., Vögtle T., Schmidt R. E., Nieswandt B. (2009) STIM1 is essential for Fcgamma receptor activation and autoimmune inflammation. Blood 113, 1097–1104. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto M., Fujii Y., Baba A., Hikida M., Kurosaki T., Baba Y. (2011) The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity 34, 703–714. [DOI] [PubMed] [Google Scholar]
- 19.Oh-Hora M., Yamashita M., Hogan P. G., Sharma S., Lamperti E., Chung W., Prakriya M., Feske S., Rao A. (2008) Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat. Immunol. 9, 432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson R. L., van Rossum D. B., Gill D. L. (1999) Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell 98, 487–499. [DOI] [PubMed] [Google Scholar]
- 21.Yao Y., Ferrer-Montiel A. V., Montal M., Tsien R. Y. (1999) Activation of store-operated Ca2+ current in Xenopus oocytes requires SNAP-25 but not a diffusible messenger. Cell 98, 475–485. [DOI] [PubMed] [Google Scholar]
- 22.Randriamampita C., Tsien R. Y. (1993) Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature 364, 809–814. [DOI] [PubMed] [Google Scholar]
- 23.Itagaki K., Hauser C. J. (2003) Sphingosine 1-phosphate, a diffusible calcium influx factor mediating store-operated calcium entry. J. Biol. Chem. 278, 27540–27547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bréchard S., Plançon S., Melchior C., Tschirhart E. J. (2009) STIM1 but not STIM2 is an essential regulator of Ca2+ influx-mediated NADPH oxidase activity in neutrophil-like HL-60 cells. Biochem. Pharmacol. 78, 504–513. [DOI] [PubMed] [Google Scholar]
- 25.Steinckwich N., Schenten V., Melchior C., Brechard S., Tschirhart E. J. (2011) An essential role of STIM1, Orai1, and S100A8-A9 proteins for Ca2+ signaling and FcgammaR-mediated phagosomal oxidative activity. J. Immunol. 186, 2182–2191. [DOI] [PubMed] [Google Scholar]
- 26.Zou W., Meng X., Cai C., Zou M., Tang S., Chu X., Wang X., Zou F. (2012) Store-operated Ca2+ entry (SOCE) plays a role in the polarization of neutrophil-like HL-60 cells by regulating the activation of Akt, Src, and Rho family GTPases. Cell. Physiol. Biochem. 30, 221–237. [DOI] [PubMed] [Google Scholar]
- 27.Ma S., Cai C., Ma Y., Bai Z., Meng X., Yang X., Zou F., Ge R. (2014) Store-operated Ca(2)(+) entry mediated regulation of polarization in differentiated human neutrophil-like HL-60 cells under hypoxia. Mol. Med. Rep. 9, 819–824. [DOI] [PubMed] [Google Scholar]
- 28.Nunes P., Cornut D., Bochet V., Hasler U., Oh-Hora M., Waldburger J. M., Demaurex N. (2012) STIM1 juxtaposes ER to phagosomes, generating Ca(2)(+) hotspots that boost phagocytosis. Curr. Biol. 22, 1990–1997. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H., Clemens R. A., Liu F., Hu Y., Baba Y., Theodore P., Kurosaki T., Lowell C. A. (2014) STIM1 calcium sensor is required for activation of the phagocyte oxidase during inflammation and host defense. Blood 123, 2238–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Benna J., Dang P. M., Gougerot-Pocidalo M. A., Marie J. C., Braut-Boucher F. (2009) p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp. Mol. Med. 41, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schenten V., Melchior C., Steinckwich N., Tschirhart E. J., Bréchard S. (2011) Sphingosine kinases regulate NOX2 activity via p38 MAPK-dependent translocation of S100A8/A9. J. Leukoc. Biol. 89, 587–596. [DOI] [PubMed] [Google Scholar]
- 32.Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E. Jr., Meyer T. (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 15, 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bandyopadhyay B. C., Pingle S. C., Ahern G. P. (2011) Store-operated Ca²+ signaling in dendritic cells occurs independently of STIM1. J. Leukoc. Biol. 89, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kar P., Bakowski D., Di Capite J., Nelson C., Parekh A. B. (2012) Different agonists recruit different stromal interaction molecule proteins to support cytoplasmic Ca2+ oscillations and gene expression. Proc. Natl. Acad. Sci. USA 109, 6969–6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandman O., Liou J., Park W. S., Meyer T. (2007) STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 131, 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thiel M., Lis A., Penner R. (2013) STIM2 drives Ca2+ oscillations through store-operated Ca2+ entry caused by mild store depletion. J. Physiol. 591, 1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bréchard S., Melchior C., Plançon S., Schenten V., Tschirhart E. J. (2008) Store-operated Ca2+ channels formed by TRPC1, TRPC6 and Orai1 and non-store-operated channels formed by TRPC3 are involved in the regulation of NADPH oxidase in HL-60 granulocytes. Cell Calcium 44, 492–506. [DOI] [PubMed] [Google Scholar]
- 38.Schaff U. Y., Dixit N., Procyk E., Yamayoshi I., Tse T., Simon S. I. (2010) Orai1 regulates intracellular calcium, arrest, and shape polarization during neutrophil recruitment in shear flow. Blood 115, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dixit N., Yamayoshi I., Nazarian A., Simon S. I. (2011) Migrational guidance of neutrophils is mechanotransduced via high-affinity LFA-1 and calcium flux. J. Immunol. 187, 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feske S., Skolnik E. Y., Prakriya M. (2012) Ion channels and transporters in lymphocyte function and immunity. Nat. Rev. Immunol. 12, 532–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki R., Liu X., Olivera A., Aguiniga L., Yamashita Y., Blank U., Ambudkar I., Rivera J. (2010) Loss of TRPC1-mediated Ca2+ influx contributes to impaired degranulation in Fyn-deficient mouse bone marrow-derived mast cells. J. Leukoc. Biol. 88, 863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan J. P., Kim M. S., Zeng W., Shin D. M., Huang G., Worley P. F., Muallem S. (2009) TRPC channels as STIM1-regulated SOCs. Channels (Austin) 3, 221–225. [DOI] [PubMed] [Google Scholar]
- 43.Heiner I., Eisfeld J., Halaszovich C. R., Wehage E., Jüngling E., Zitt C., Lückhoff A. (2003) Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem. J. 371, 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Damann N., Owsianik G., Li S., Poll C., Nilius B. (2009) The calcium-conducting ion channel transient receptor potential canonical 6 is involved in macrophage inflammatory protein-2-induced migration of mouse neutrophils. Acta Physiol. (Oxf.) 195, 3–11. [DOI] [PubMed] [Google Scholar]
- 45.Cheng N., He R., Tian J., Dinauer M. C., Ye R. D. (2007) A critical role of protein kinase Cδ activation loop phosphorylation in formyl-methionyl-leucyl-phenylalanine-induced phosphorylation of p47phox and rapid activation of nicotinamide adenine dinucleotide phosphate oxidase. J. Immunol. 179, 7720–7728. [DOI] [PubMed] [Google Scholar]
- 46.Greenblatt M. B., Aliprantis A., Hu B., Glimcher L. H. (2010) Calcineurin regulates innate antifungal immunity in neutrophils. J. Exp. Med. 207, 923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]