Accumulation of IM and increased monocyte turnover during SIV infection is associated with chronic pulmonary inflammation and tissue damage during disease progression.
Keywords: HIV/AIDS, pathogenesis, lung
Abstract
We recently reported that increasing blood monocyte turnover that was associated with tissue macrophage death better predicts terminal disease progression in adult SIV-infected macaques than does declining CD4+ T cell levels. To understand better mechanisms of pathogenesis, this study relates severity of lung-tissue damage to the ratio, distribution, and inflammatory responses of lung macrophage subsets during SIV infection in rhesus macaques exhibiting varying rates of monocyte turnover. In vivo BrdU incorporation was used to evaluate kinetics of monocyte/tissue macrophage turnover. Tissue damage was scored microscopically from H&E-stained lung-tissue sections, and cytokine expression was examined via immunohistochemistry and confocal microscopy. Increased monocyte turnover in SIV-infected rhesus macaques significantly correlated with severity of lung-tissue damage, as exhibited by perivasculitis, vasculitis, interstitial pneumonia, alveolar histiocytosis, foamy macrophages, multinucleated giant cells, fibrin, and edema in the alveoli. In addition, the higher monocyte turnover correlated with declining AI ratio, increased accumulation of IM in the perivascular region of the lung, and higher expression of IL-6 in the IM of the lung tissue exposed to a LPS, calcium ionophore, and tumor promoter combination stimulation ex vivo. Accumulation of IM associated with increasing monocyte turnover during SIV infection appears to contribute to chronic pulmonary inflammation and tissue damage during disease progression to AIDS.
Introduction
HIV is readily detected in the respiratory tract, and pulmonary disease is reported in >75% of autopsies performed on HIV-infected patients [1]. Infectious and noninfectious factors appear to contribute to pulmonary disease in persons living with HIV. Infectious contributions to lung disease during AIDS progression include the virus itself, as well as bacterial pneumonia and pulmonary opportunistic infections that impact morbidity and mortality, especially in the absence of antiretroviral therapy [2]. Noninfectious factors observed in HIV-infected individuals may include COPD, interstitial lung disease, pulmonary arterial hypertension [2], pulmonary fibrosis [2], and neoplasms [3].
Macrophages are abundant in the respiratory tract [4], and our recent study in rhesus macaques showed that AMs and IMs together comprise ∼70% of immune-response cells in the lung in steady state [5]. In addition, macrophages, such as CD4+ T cells, are targeted for infection by HIV and SIV binding to surface coreceptors, such as CCR5 and CCR3 [6]. Decreased expression of mannose receptors (CD206) on AM in HIV-1-infected patients was reported to correlate with defects in adherence and phagocytosis of Pneumocystis jiroveci (Pneumocystis carinii) [7]. AM from AIDS patients also exhibited dysregulation in secretion of the proinflammatory cytokine, IL-12, after challenge with Salmonella spp. in vitro [8]. These findings suggest that macrophage dysfunction contributes to increased susceptibility to opportunistic infections in AIDS patients.
We reported previously that increased turnover of blood monocytes induced by macrophage death in lymph nodes correlates better with rapid disease progression to AIDS in SIV-infected rhesus macaques than does declining CD4+ T cell levels [9]. Additional recent data showed that shorter-lived IMs, in contrast to longer-lived AMs, are replenished continuously by monocytes in the lung of rhesus macaques [5]. BAL specimens, however, primarily contain AM and not IM, so thus, may not fully represent innate immune responses to infections, such as to HIV in humans. The purpose of this study was to characterize lung macrophages and pulmonary pathogenesis in relation to monocyte turnover rate as a biomarker of disease progression by use of the rhesus macaque SIV-infection model of AIDS.
MATERIALS AND METHODS
Animals and virus inoculations
A total of 40 adult male Indian rhesus macaques (Macaca mulatta), between 3.3 and 22 yr of age, were used in various component of this study and housed at the Tulane National Primate Research Center (in Covington, LA, USA). Six of the monkeys served as uninfected controls. The remaining animals were inoculated i.v. or intravaginally with virus strains SIVmac251, SIVmac239, SIVmac239∆GY, or SIVmac239∆Nef. All animal procedures were performed according to the “NIH Guide for the Care and Use of Laboratory Animals” (National Research Council, National Academic Press, Washington, DC, USA, 1996) and were approved by the Tulane University Institution Animal Care and Use Committee.
Tissue specimen collection and measurement of cell turnover
The thymidine analogs, BrdU (Sigma-Aldrich, St. Louis, MO, USA) and EdU (Molecular Biology, Carlsbad, CA, USA), were prepared at 30 mg/ml and 25 mg/ml in PBS (pH 7.2, Ca/Mg-free; Mediatech, Manassas, VA, USA), respectively, and sterilized by passage through 0.22 μm filters. BrdU (60 mg/kg) or EdU (50 mg/kg) was injected i.v., 24 or 48 h before necropsy. At necropsy, lung tissue was obtained for flow cytometry analyses, in vitro stimulation of inflammatory cytokines, and (immuno)histochemistry for confocal imaging.
Isolation of macrophages and lymphocytes from lung tissue
Single-cell suspensions were prepared from lung tissue by use of an enzymatic digestion method, as described previously [5]. In brief, 0.5 mm-thick lung-tissue sections were removed of bronchi and resuspended in 30 ml lamina propria lymphocyte isolation medium comprising RPMI 1640 (Cellgro, Manassas, VA, USA), supplemented with 5% FBS (Cat. No. 26140-079; Gibco, Grand Island, NY, USA), 100 IU/ml penicillin/streptomycin (EMD Millipore, Billerica, MA, USA), 2 mM l-glutamine (Cellgro), 25 mM HEPES (Molecular Biology), 200 U/ml type IV collagenase (Cat. No. 4189; Worthington Biochemical, Lakewood, NJ, USA), and 0.05 mg/ml DNAase I (Cat. No. 10104159001; Roche Applied Science, Indianapolis, IN, USA) [5]. The tissue digest suspension was then incubated at 37°C for 30 min, followed by pipetting and incubation for an additional 10 min at 37°C. After discontinuous density centrifugation over 24% and 50% Percoll (Cat. No. 17-0891-01; GE Healthcare, Boston, MA, USA) at 2000 rpm for 20 min (Allegra X-12R; Beckman Coulter, Brea, CA, USA), the cells were recovered from the 24–50% Percoll interface, washed with 2% PBS-FBS (PBS containing 2% FBS), and stored in liquid nitrogen until further analyses.
Histopathological evaluation of pulmonary damage
Lung tissues collected during necropsy were processed for routine histopathology by fixation in 10% buffered formalin, embedded in paraffin, sectioned, and stained with H&E on an automated Leica AutoStainer XL (Leica Biosystems, Buffalo Grove, IL, USA). Pulmonary tissue sections from 6 uninfected controls and 17 SIV-infected rhesus macaques were coded and evaluated for histologic scoring of lesions. Multiple tissue sections from different lung lobes from each animal were examined for lesions without knowing the source of the tissues (Supplemental Table 1A), and the levels of pulmonary lesion severity were scored as 0 (normal), 1 (minimal), 2 (mild), 3 (moderate), and 4 (severe; Supplemental Table 1B), developed from a system described by Baskin et al. [10]. The lesion scores were then used to compare disease and severity of lesions in relation to monocyte turnover rate of the uninfected and SIV-infected rhesus macaques by use of Spearman’s correlation analysis.
Flow cytometry
For antibody staining, 200 μl whole blood or 106 cells from lung tissue was prepared and stained, as described previously [5]. The BD PharMingen BrdU Flow Kit (Cat. 559619; BD Biosciences, San Jose, CA, USA) and Click-iT EdU Pacific Blue Flow Cytometry Assay kit (Cat. C-10418; Invitrogen, Carlsbad, CA, USA) were used to detect BrdU and EdU incorporation, respectively, in conjunction with surface-marker antibody staining, according to the manufacturer’s protocols. A 3-laser FACSAria (BD Biosciences) was used to detect the surface markers or intracellular BrdU/EdU incorporation on the multicolor-stained cells. Antibodies used in these analyses are shown in Supplemental Table 2A. Results were analyzed by use of FlowJo software (Version 9.6.2; TreeStar, Ashland, OR, USA).
Ex vivo stimulation of cytokine expression in lung tissue
Lung-tissue sections were obtained during necropsy and stimulated ex vivo for cytokine expression, as described previously by Ramesh et al. [11]. Tissue sections of ∼1 cm3 each were placed in PBS (pH 7.2; Gibco), rinsed, and sliced into 2 mm-thick sections by use of a TM1000 tissue slicer (ASI Instruments, Warren, MI, USA). The tissue sections were then incubated at 37°C for 4 h in RPMI 1640 containing 10% FBS and stimulated with a macrophage-stimulation cocktail containing PHA (Cat. 10576-015; Life Technologies, Carlsbad, CA, USA) at 5 ng/ml, PMA (Cat. P8139; Sigma-Aldrich) at 10 ng/ml, calcium ionophore A23187 (Cat. C9275; Sigma-Aldrich) at 25 ng/ml, and LPS (Cat. L3755; Sigma-Aldrich) at 1 ng/ml. In addition, BFA (Cat. B-7450; Life Technologies) at 10 μg/ml medium was included to block cytokine secretion from the cells. After stimulation, the tissues were fixed in 2% paraformaldehyde, cryopreserved in 30% sucrose in PBS, and snap frozen in cryomolds (25 mm × 20 mm × 5 mm) containing Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA, USA). Controls included untreated tissues incubated in medium and BFA only.
Antibody staining and confocal microscopy imaging
Lung-tissue sections were incubated with antibodies listed in Supplemental Table 2B. Imaging was performed with a Leica TCS SP2 confocal microscope, equipped with 3 lasers (Leica Microsystems, Buffalo Grove, IL, USA) at 400×, 630×, or 1260× magnification. Adobe Photoshop software (Version 7.0; Adobe Systems, San Jose, CA, USA) was used to process and assemble the images, and Image-Pro Plus software (Version 6.0; Media Cybernetics, Rockville, MD, USA) was used to measure fluorescence intensity. Quantification of AM and IM was performed by manually counting 20 fields of each slide at 200× final magnification.
Statistical analysis
Comparisons between mean values were analyzed by Student's t-test, and Spearman’s test was used for correlation analysis. Data were analyzed and graphed by use of GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA), and P < 0.05 was considered statistically significant.
RESULTS
Monocyte turnover correlates with severity of pulmonary lesions in SIV-infected monkeys progressing to AIDS
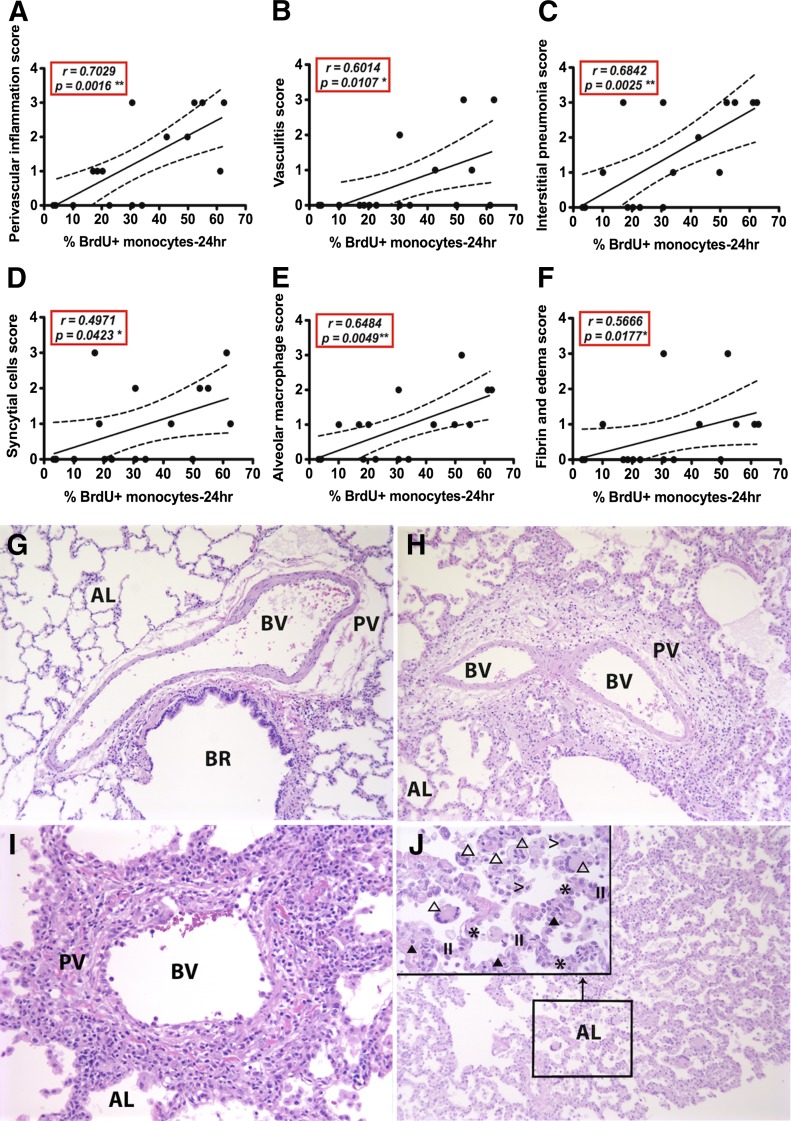
Lung tissues from 17 SIV-infected rhesus macaques (Supplemental Table 1A) were scored histologically for lesions, as described previously [10]. Pulmonary lesions that significantly correlated with blood monocyte turnover rates in the SIV-infected macaques (Fig. 1) included perivascular inflammation (Fig. 1A; P = 0.0016), vasculitis (Fig. 1B; P = 0.0107), interstitial pneumonia (Fig. 1C; P = 0.0025), syncytial cells (Fig. 1D; P = 0.0423), AM accumulation (Fig. 1E; P = 0.0049), and fibrin and edema (Fig. 1F; P = 0.0177). Histologically, the tunica adventitia of small- to medium-sized blood vessels displayed multifocal sites of edema and infiltrates of macrophages, lymphocytes, and plasma cells (perivasculitis; Fig. 1H–J) in SIV-infected animals expressing high blood monocyte turnover. Some blood vessel walls were expanded by edema, fibrin, and infiltration of lymphocytes, plasma cells, neutrophil, and macrophages. The intimas were lined by hypertrophic endothelial cells and with increased interendothelial separations (vasculitis; Fig. 1I). The alveolar septa exhibited diffuse edema, fibrin deposition, and infiltration of macrophages, lymphocytes, plasma cells, and rare neutrophils with type II pneumocyte hyperplasia (interstitial pneumonia; Fig. 1H). Moreover, the alveoli were filled with foamy macrophages, multinucleate giant cells, fibrin, and edema (Fig. 1J), and the presence and severity of these lesions correlated with monocyte turnover rate. Bronchus-associated lymphoid tissue swelling, hemorrhage, and chronic pleuritic, however, did not correlate in severity with the turnover of blood monocytes (Supplemental Fig. 1). Normal lung tissue from uninfected animals exhibited a thin alveolar septum lined by a single layer of flattened epithelial cells with macrophages rarely observed in the alveoli (Fig. 1G).
Figure 1. Severity of pulmonary lesions correlates with increasing monocyte turnover in SIV-infected monkeys progressing to AIDS.
Pulmonary lesions in SIV-infected monkeys were evaluated histologically by use of a scoring system modified from Baskin et al. [10] as follows: 0 (normal), 1 (minimal), 2 (mild), 3 (moderate), and 4 (severe). Spearman correlation analysis was used to relate severity of pulmonary lesions with blood monocyte turnover rate. The 9 categories of pulmonary lesions described by Baskin et al. [10] were evaluated, and numbers of animals affected are shown in Supplemental Table 1B. The types of pulmonary lesions that correlated significantly with increasing blood monocyte turnover during SIV infection in rhesus macaques included; pulmonary perivasculitis (A; r = 0.7029, P = 0.0016), vasculitis (B; r = 0.6014, P = 0.0107), interstitial pneumonia (C; r = 0.6842, P = 0.0025), syncytial cell formation (D; r = 0.4971, P = 0.0423), AM accumulation (E; r = 0.6484, P = 0.0049), fibrin and edema (F; r = 0.5666, P = 0.0177). (G–J) Normal lung (G), moderate perivasculitis (H; score = 3), moderate vasculitis (I; score = 3), moderate interstitial pneumonia (J; score = 3), mild multinucleate giant cell pneumonia and alveolar histiocytosis (J inset; score = 2). BR, Bronchioles; BV, blood vessel; AL, alveoli; PV, perivascular interstitium; II, hyperplastic type II pneumocytes; >, AMs; *, alveolar septum; open triangle, multinucleated giant cells; closed triangle, mononuclear cells. Original magnification, 100×; J, inset, 400×.
The AI ratio in lung inversely correlates with increasing blood monocyte turnover during SIV infection
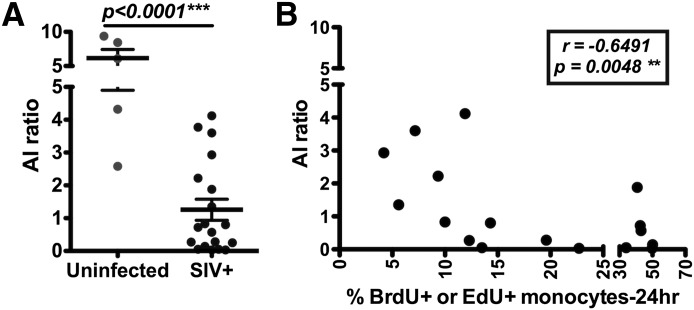
As tissue macrophages are believed to derive, in part, from circulating monocytes, we examined the relationship between monocyte-turnover rates with total numbers of lung macrophages and ratios of macrophage subpopulations in rhesus macaques during different stages of SIV infection. Single-cell suspensions from lung tissues of SIV-infected and -uninfected rhesus macaques were stained with antibodies (Supplemental Table 2A, II and III) and analyzed by flow cytometry by use of a 3-laser FACSAria. In uninfected rhesus macaques, the mean AI ratio was 6.158 ± 1.259, which was significantly higher than a mean of 1.169 ± 0.3204 in SIV-infected animals (P < 0.0001; Fig. 2A). Moreover, the AI ratio in SIV-infected monkeys inversely correlated with increased blood monocyte turnover (r = −0.6491; P = 0.0048; Fig. 2B).
Figure 2. The AI ratio in the lung inversely correlates with monocyte turnover in SIV-infected monkeys.
Student’s t-test was used to compare lung tissues from uninfected (n = 5) and SIV-infected (n = 19) rhesus macaques (A) and demonstrated a significant decreased AI ratio in SIV-infected animals. Blood monocyte turnover rates were measured by detecting BrdU or EdU incorporation and plotted against the AI ratios in lung tissues from the corresponding SIV-infected monkeys. A signification correlation was measured by Spearman analysis (B; n = 19) between increasing monocyte turnover and declining AI ratio.
Accumulation of IM in lungs of SIV-infected monkeys during progression to AIDS
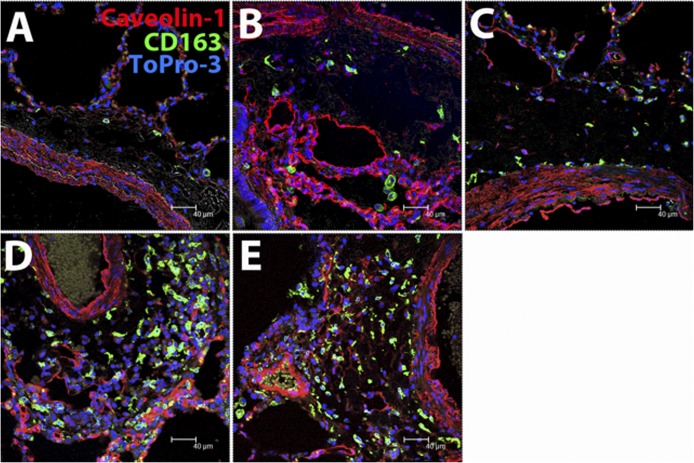
Mononuclear cells or macrophages accumulate in the lung of rhesus macaques with SHIV-associated pneumonia [12] and in HIV/AIDS patients [13]. The decrease in the AI ratio could result from a decreased number of AM, increased number of IM, or both, so we applied immunofluorescence staining for CD163 (macrophages), caveolin-1 (endothelium), and Topro-3 (nucleus) and confocal microscopy to assess the distribution, numbers, and AI ratios in lung-tissue sections. Infiltrating monocytes located in the perivascular regions were primarily CD163+ IM (Fig. 3). IM accumulation in the perivascular and subpleural areas of the interstitium (Fig. 3A–E) correlated with increased monocyte turnover rates, whereas the number of AM remained static (Table 1). Thus, increased numbers of IM accumulating in the lung rather than changes in AM levels accounted for the declining AI ratio observed in SIV-infected monkeys exhibiting high blood monocyte turnover.
Figure 3. Accumulation of IM in the lung interstitium correlates with monocyte turnover in SIV-infected monkeys.
Lung-tissue sections from 2 uninfected rhesus macaques and 7 SIV-infected monkeys were stained for the macrophage scavenger receptor CD163 (green), endothelium caveolin-1 (red), and nuclear ToPro-3 (blue) and examined by confocal microscopy at 400× original magnification. The blood monocyte turnover rates of the animals from which the lung tissues were examined were 1.61% (A), 22.7% (B), 31% (C), 40.5% (D), and 61.5% (E). Note the increasing numbers of CD163+ IM in the peribronchovascular region of lung tissue from monkeys exhibiting increasing blood monocyte turnover rates. A Leica TCS SP2 confocal microscope equipped with 3 lasers (Leica Microsystems) was used to capture the images.
TABLE 1.
Numbers of AM and IM in lung tissue of SIV-infected and -uninfected monkeysa
| Group | Animal ID | Total AM | Total IM | AI ratio | Mean ± sem (AI ratio) |
|---|---|---|---|---|---|
| Uninfected | GI53 | 525 | 171 | 3.07 | 4.17 ± 0.566 |
| EC61 | 1163 | 261 | 4.47 | ||
| GI84 | 526 | 106 | 4.96 | ||
| SIV+/low monocyte turnover | GL96 | 706 | 575 | 1.23 | 1.51 ± 0.633 |
| GH64 | 849 | 410 | 2.07 | ||
| GN24 | 282 | 232 | 1.22 | ||
| SIV+/high monocyte turnover | N/Db | N/D | N/D | N/D | N/D |
The total numbers of AM and IM were counted in 20 fields/lung-tissue section at a final magnification of 200×.
N/D, Not determined.
IL-6 expression in IM of lung following ex vivo stimulation positively correlates with monocyte turnover rates in SIV-infected monkeys
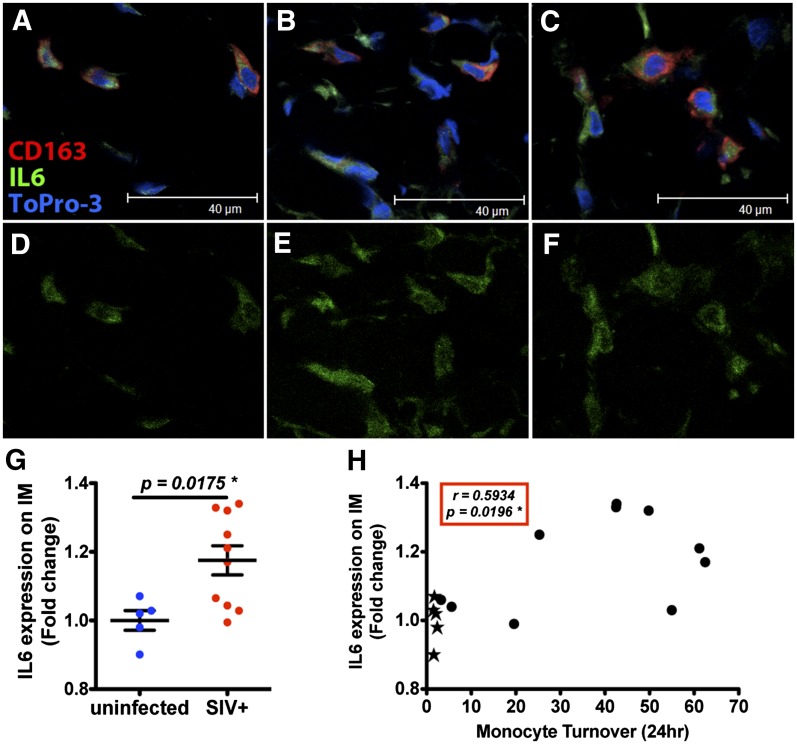
To determine whether accumulation of IM is associated with pulmonary tissue damage in AIDS, intracellular macrophage IL-6 expression was monitored by immunofluorescence staining and confocal microscopy imaging. Lung-tissue sections were stimulated ex vivo with a cell-stimulation cocktail comprised of LPS, PHA, calcium ionophore, and PMA. The mean fluorescence intensities of IL-6 in the lung AM and IM were quantified by use of Image-Pro Plus software (Version 6.0; Media Cybernetics). Confocal images showed stronger IL-6 expression in the ex vivo-stimulated lung IM in SIV-infected animals exhibiting higher blood monocyte turnover (Fig. 4C and F) than in uninfected monkeys (Fig. 4A and D) or SIV-infected monkeys with lower blood monocyte turnover (Fig. 4B and E). By t-test, induced IL-6 expression ex vivo was significantly higher in lung tissues from SIV-infected animals than uninfected animals (P = 0.0175; Fig. 4G) and correlated significantly with monocyte turnover (r = 0.5934; P = 0.0196; Fig. 4H), suggesting that functional changes in IM may contribute to chronic lung inflammation and tissue damage. We did not observe IL-6 expression by AM in lung tissues obtained from uninfected and SIV-infected macaques that were stimulated ex vivo (data not shown).
Figure 4. IL-6 expression in IM following ex vivo stimulation of lung tissue correlates with monocyte turnover rates in SIV-infected and -uninfected monkeys.
Lung-tissue sections were incubated with a cell-stimulation mixture containing LPS, PHA, PMA, and calcium ionophore for 4 h. Tissues were stained for IL-6 (green), macrophage CD163 marker (red), and nuclear ToPro-3 (blue), and images were captured with a Leica TCS SP2 confocal microscope (Leica Microsystems) at 1260× original magnification. Representative images are shown from an uninfected monkey (A and D; n = 5) and from SIV-infected monkeys with lower (≤30%) blood monocyte turnover (B and E; n = 4) and higher (>30%) blood monocyte turnover (C and F), demonstrating relatively higher IL-6 production in IM of SIV-infected monkeys. Student’s t-test was used to compare mean intensity levels of IL-6-staining cells in the ex vivo-stimulated tissues via Image-Pro Plus (Version 6.0; Media Cybernetics), and significantly higher IL-6 expression was observed from tissues of the SIV-infected macaques (G). Furthermore, there was a significant correlation between IL-6 expression and monocyte turnover rates in corresponding to lung tissues of uninfected (closed stars) and SIV-infected (closed circles) macaques by Spearman analysis (H).
Increased expression of IL-10 (anti-inflammatory cytokine) by AM has been considered critical for maintaining tolerance to ubiquitous endotoxin [14], so we also examined intracellular macrophage IL-10 expression in response to ex vivo stimulation of lung tissues from SIV-infected and -uninfected rhesus macaques. Contrary to IL-6 expression, IL-10 was expressed exclusively in AM and not IM. There appeared to be a decreased IL-10 expression after ex vivo stimulation from lung AM of SIV-infected monkeys exhibiting low blood monocyte turnover (Supplemental Fig. 2B and E) and SIV-infected monkeys with high blood monocyte turnover (Supplemental Fig. 2C and F) compared with those of uninfected monkeys (Supplemental Fig. 2A and D), but this was not quite statistically, significantly different by t-test (P = 0.075; Supplemental Fig. 2G). In addition, the expression of IL-10 did not correlate with the rate of blood monocyte turnover (P = 0.1110; Supplemental Fig. 2H).
DISCUSSION
HIV and SIV commonly infect activated CD4+ T cells, but as lentiviruses, they also infect macrophages [15, 16]. In addition, we observed that turnover of blood monocytes and death rate of tissue macrophages increase during disease progression to AIDS in SIV-infected monkeys [9]. To examine further the contribution of monocytes/macrophages to the pathogenesis of SIV infection, we focused on lung as a representative site of tissue macrophages and demonstrated that increased monocyte turnover rates correlated with development of pulmonary lesions, such as perivascular inflammation, vasculitis, interstitial pneumonia, syncytial cell formation, increased AM in the alveoli, precipitation of fibrin, and edema. Accumulation of IM in the lung parenchyma and an increased pulmonary macrophage inflammatory response in lung-tissue sections ex vivo were also associated with increased turnover of blood monocytes.
During steady-state homeostasis, macrophages predominantly function by regulating cytokine expression to maintain a quiescent environment in the lung and thereby, prevent excessive inflammation in response to environmental microbial agents and allergens during gas exchange between the lung epithelial cells and blood vessels [17, 18]. Conversely, excessive expression of proinflammatory cytokines, such as IL-6 from newly recruited IM, epithelial cells, and T cells, contributes to pathogenesis during various lung diseases commonly seen in patients with AIDS, as well as COPD, idiopathic pulmonary fibrosis, and pulmonary hypertension [19, 20]. Agustí and colleagues [21] reported that IL-6 levels in the BAL fluid was an independent predictor of mortality in immune-compromised patients. In the present study, we observed higher proinflammatory IL-6 cytokine expression by IM in lung tissue from SIV-infected monkeys in response to an endotoxin-containing stimulation cocktail ex vivo that may contribute to pulmonary pathogenesis in situ. However, we cannot rule out the possibility that IL-6, in conjunction with other inflammatory cytokines produced by cells other than macrophages, could promote lung inflammation.
In response to endotoxin-containing stimulation ex vivo, AM, in lung tissue from SIV-infected monkeys, did not express higher production of the anti-inflammatory IL-10 cytokine to maintain tolerance in the lung [14] compared with AM in lung tissue from healthy, uninfected animals. This inability to regulate inflammation in the lung could also contribute to chronic inflammation and pathogenesis in lung tissue during SIV infection. McClure et al. [22] and Soeiro Ade et al. [23] reported that chronic inflammation promotes damage to the lung-tissue architecture and leads to follicular hyperplasia and alveolar edema. Chronic inflammation may also facilitate the colonization of opportunistic pathogens [24, 25], precipitation of lung content, and oxidative stress [26, 27] that further accelerate pathogenesis of lung disease in AIDS.
In summary, these results strongly support a role for monocyte/macrophages in pulmonary pathogenesis of SIV-infected rhesus macaques. Increased monocyte turnover that is predictive of disease progression also correlated with severity of pulmonary lesions, increased numbers of IM in the lung, and relative decline in the AI ratio. Whereas ex vivo stimulation with an endotoxin-containing cocktail induced increasing IL-6 expression in AM and IM of lung biopsy tissues from SIV-infected monkeys, IL-10 expression by AM actually declined or remained the same after SIV infection. These results overall demonstrate differences among macrophage populations that contribute to pulmonary pathogenesis by use of the rhesus macaque SIV-infection model of AIDS and provide insights for further development of novel intervention strategies that target macrophages to ameliorate pulmonary diseases as a result of HIV infection.
AUTHORSHIP
Y.C. participated in the study design, experimental research, data analysis, and manuscript preparation. C.S. participated in the research design and flow cytometry data analyses. C.C.M. participated in SIV in situ hybridization, in vitro tissue stimulation, and immunostaining studies. X.A. participated in the confocal imaging and interpretation of results. D.X.L. provided histopathology of lung tissues. E.S.D. participated in data interpretation and preparation of the manuscript. A.A.L. and W.-K.K. kindly provided lung samples, CD4+ T cell data, and plasma viral load data from some of the SIV-infected monkeys in the study. M.J.K. participated in the study design, data interpretation, and manuscript preparation.
Acknowledgments
This study was supported by grants from the U.S. National Institutes of Health (AI097059, AI087302, AI091501, and AI110163 to M.J.K. and OD011104 to the Tulane National Primate Research Center) and a grant from Virginia’s Commonwealth Health Research Board (#11-09 to W.-K.K.). The authors thank Toni P. Penny, Edith Walker, Ashley N. Leach, Erin M. Haupt, Julie Bruhn, and Calvin Lanclos in the Division of Immunology and Dr. Jason P. Dufour in the Division of Veterinary Medicine at the Tulane National Primate Research Center (TNPRC) for technical assistance.
Glossary
- AI ratio
alveolar macrophage:interstitial macrophage ratio
- AM
alveolar macrophage
- BAL
bronchoalveolar lavage
- BFA
brefeldin A
- COPD
chronic obstructive pulmonary disorder
- EdU
5-ethynyl-2′-deoxyuridine
- IM
interstitial macrophage
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.Afessa B., Green W., Chiao J., Frederick W. (1998) Pulmonary complications of HIV infection: autopsy findings. Chest 113, 1225–1229. [DOI] [PubMed] [Google Scholar]
- 2.Crothers K., Huang L., Goulet J. L., Goetz M. B., Brown S. T., Rodriguez-Barradas M. C., Oursler K. K., Rimland D., Gibert C. L., Butt A. A., Justice A. C. (2011) HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am. J. Respir. Crit. Care Med. 183, 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirk G. D., Merlo C., O'Driscoll P., Mehta S. H., Galai N., Vlahov D., Samet J., Engels E. A. (2007) HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin. Infect. Dis. 45, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boorsma C. E., Draijer C., Melgert B. N. (2013) Macrophage heterogeneity in respiratory diseases. Mediators Inflamm. 2013, 769214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai Y., Sugimoto C., Arainga M., Alvarez X., Didier E. S., Kuroda M. J. (2014) In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: implications for understanding lung disease in humans. J. Immunol. 192, 2821–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He J., Chen Y., Farzan M., Choe H., Ohagen A., Gartner S., Busciglio J., Yang X., Hofmann W., Newman W., Mackay C. R., Sodroski J., Gabuzda D. (1997) CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature 385, 645–649. [DOI] [PubMed] [Google Scholar]
- 7.Koziel H., Eichbaum Q., Kruskal B. A., Pinkston P., Rogers R. A., Armstrong M. Y., Richards F. F., Rose R. M., Ezekowitz R. A. (1998) Reduced binding and phagocytosis of Pneumocystis carinii by alveolar macrophages from persons infected with HIV-1 correlates with mannose receptor downregulation. J. Clin. Invest. 102, 1332–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon M. A., Gordon S. B., Musaya L., Zijlstra E. E., Molyneux M. E., Read R. C. (2007) Primary macrophages from HIV-infected adults show dysregulated cytokine responses to Salmonella, but normal internalization and killing. AIDS 21, 2399–2408. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa A., Liu H., Ling B., Borda J. T., Alvarez X., Sugimoto C., Vinet-Oliphant H., Kim W. K., Williams K. C., Ribeiro R. M., Lackner A. A., Veazey R. S., Kuroda M. J. (2009) The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood 114, 2917–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baskin G. B., Murphey-Corb M., Martin L. N., Soike K. F., Hu F. S., Kuebler D. (1991) Lentivirus-induced pulmonary lesions in rhesus monkeys (Macaca mulatta) infected with simian immunodeficiency virus. Vet. Pathol. 28, 506–513. [DOI] [PubMed] [Google Scholar]
- 11.Ramesh G., Alvarez X., Borda J. T., Aye P. P., Lackner A. A., Sestak K. (2005) Visualizing cytokine-secreting cells in situ in the rhesus macaque model of chronic gut inflammation. Clin. Diagn. Lab. Immunol. 12, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhillon N. K., Sui Y., Pinson D., Li S., Dhillon S., Tawfik O., Callen S., Nemon O., Narayan O., Buch S. (2007) Upregulation of expression of platelet-derived growth factor and its receptor in pneumonia associated with SHIV-infected macaques. AIDS 21, 307–316. [DOI] [PubMed] [Google Scholar]
- 13.Li H., Singh S., Gorantla S., Potula R., Persidsky Y., Poluektova L., Kanmogne G. D. (2014) Dysregulation of claudin-5 in HIV-induced interstitial pneumonitis and lung vascular injury. Protective role of peroxisome proliferator-activated receptor-γ . Am. J. Respir. Crit. Care Med. 190, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chanteux H., Guisset A. C., Pilette C., Sibille Y. (2007) LPS induces IL-10 production by human alveolar macrophages via MAPKinases- and Sp1-dependent mechanisms. Respir. Res. 8, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freed E. O., Martin M. A. (1994) HIV-1 infection of non-dividing cells. Nature 369, 107–108. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal A., McAllery S., Turville S. G. (2013) Revising the role of myeloid cells in HIV pathogenesis. Curr. HIV/AIDS Rep. 10, 3–11. [DOI] [PubMed] [Google Scholar]
- 17.Wright J. R. (2005) Immunoregulatory functions of surfactant proteins. Nat. Rev. Immunol. 5, 58–68. [DOI] [PubMed] [Google Scholar]
- 18.Holt P. G., Strickland D. H., Wikström M. E., Jahnsen F. L. (2008) Regulation of immunological homeostasis in the respiratory tract. Nat. Rev. Immunol. 8, 142–152. [DOI] [PubMed] [Google Scholar]
- 19.Rincon M., Irvin C. G. (2012) Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 8, 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedroza M., Schneider D. J., Karmouty-Quintana H., Coote J., Shaw S., Corrigan R., Molina J. G., Alcorn J. L., Galas D., Gelinas R., Blackburn M. R. (2011) Interleukin-6 contributes to inflammation and remodeling in a model of adenosine mediated lung injury. PLoS ONE 6, e22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agustí C., Rañó A., Rovira M., Filella X., Benito N., Moreno A., Torres A. (2004) Inflammatory response associated with pulmonary complications in non-HIV immunocompromised patients. Thorax 59, 1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClure H. M., Anderson D. C., Fultz P. N., Ansari A. A., Lockwood E., Brodie A. (1989) Spectrum of disease in macaque monkeys chronically infected with SIV/SMM. Vet. Immunol. Immunopathol. 21, 13–24. [DOI] [PubMed] [Google Scholar]
- 23.Soeiro Ade. M., Hovnanian A. L., Parra E. R., Canzian M., Capelozzi V. L. (2008) Post-mortem histological pulmonary analysis in patients with HIV/AIDS. Clinics (Sao Paulo) 63, 497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris A., Kingsley L. A., Groner G., Lebedeva I. P., Beard C. B., Norris K. A. (2004) Prevalence and clinical predictors of Pneumocystis colonization among HIV-infected men. AIDS 18, 793–798. [DOI] [PubMed] [Google Scholar]
- 25.Deok-jong Yoo S., Worodria W., Davis J. L., Cattamanchi A., den Boon S., Kyeyune R., Kisembo H., Huang L. (2010) The prevalence and clinical course of HIV-associated pulmonary cryptococcosis in Uganda. J. Acquir. Immune Defic. Syndr. 54, 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrache I., Diab K., Knox K. S., Twigg H. L. III, Stephens R. S., Flores S., Tuder R. M. (2008) HIV associated pulmonary emphysema: a review of the literature and inquiry into its mechanism. Thorax 63, 463–469. [DOI] [PubMed] [Google Scholar]
- 27.Peterhans E. (1997) Reactive oxygen species and nitric oxide in viral diseases. Biol. Trace Elem. Res. 56, 107–116. [DOI] [PubMed] [Google Scholar]