Abstract
Purpose
The association between prediagnostic inter-leukin-6 (IL-6) concentrations and risk of colorectal cancer was evaluated in a nested case–control study and a meta-analysis of prospective studies.
Methods
Colorectal cancer cases (n = 173) and matched controls (n = 345) were identified between 1989 and 2000 among participants in the CLUE II cohort of Washington Country, Maryland. Matched odds ratios and the corresponding 95 % confidence intervals (CIs) were estimated using conditional logistic regression models.
Results
Participants in the highest third of plasma IL-6 concentration had a 2.48 times higher risk of colon cancer compared to participants in the bottom third (95 % CI 1.26–4.87; p-trend 0.02) after multivariate adjustment. This association did not differ according to the stage of disease, age, sex, or other potential modifying variables and remained statistically significant after adjustment for C-reactive protein concentrations. No statistically significant association was observed for rectal cancer risk. The meta-analysis of six prospective studies yielded an increased but borderline statistically significant risk of colon cancer per 1 U increase in naturally logarithm-transformed IL-6 (summary RR 1.22; 95 % CI 1.00–1.49; I2 46 %). An inverse association was noted for rectal cancer (RR 0.69; 95 % CI 0.54–0.88; I2 0 %), but there was evidence for small-study effects (p 0.02).
Conclusion
Our findings provide support for a modest positive association between IL-6 concentrations and colon cancer risk. More work is needed to determine whether IL-6 is a valid marker of colorectal inflammation and whether such inflammation contributes to colon and rectal cancer risk.
Keywords: Inflammation, Colorectal cancer, Cohort study, Meta-analysis
Introduction
Interleukin-6 (IL-6) is a multifunctional cytokine produced by a variety of hematopoietic and nonhematopoietic cells [1]. It exerts a pro-inflammatory role either by acting on a transmembrane type 1 cytokine receptor or by binding to a soluble IL-6 receptor (sIL6R). IL-6 up-regulates several acute-phase proteins such as C-reactive protein (CRP), fibrinogen, α1-antitrypsin, and serum amyloid A [1, 2]. There is ample mechanistic evidence, suggesting an involvement of IL-6 in colorectal cancer (CRC) development. In vivo experiments on wild-type mice have demonstrated that IL-6 is significantly augmented at the colonic tumor microenvironment, and the growth of colon tumors has been shown to be suppressed when the mice were treated with antibodies against IL6R [3]. However, results from prospective epidemiological studies regarding the association of circulating IL-6 concentrations and risk of subsequent colorectal cancer development have generally not observed strong relationships [4–8]. We have previously shown that CRP concentrations were associated with a twofold higher risk of colorectal cancer in the CLUE II cohort [9]. This report further examines the potential association between inflammation and colorectal cancer by testing an upstream to CRP biomarker, IL-6, and includes a meta-analysis of prospective epidemiological studies to summarize the evidence and study its heterogeneity.
Materials and methods
Study population
Colorectal cancer cases and controls were identified among members of the prospective CLUE II cohort. The CLUE II cohort enrolled participants from May through October 1989 primarily in Washington County, Maryland, and neighboring counties or states. The aim of the cohort was to investigate potential lifestyle and serologic risk factors for cancer and heart disease. The cohort consisted of 32,894 individuals, 25,076 of whom were residents of Washington County. For this analysis, the study participants were restricted to 22,887 adult (≥18 years old) Washington County residents, of whom 59 % were female and 98 % were white reflecting the demographics of the county at the time. All participants provided a blood sample and completed a brief medical and lifestyle exposure history questionnaire at baseline. Self-administered questionnaires were mailed to participants in 1996, 1998, and 2000 to collect and/or update information on family history of cancer, medical conditions, medication use, and lifestyle exposures. Loss to follow-up was <5 % among cohort members who were over 45 years old at baseline, which is the age range of the majority of the cases and the pool of cohort members eligible to be selected as age-matched controls. All participants provided informed consent, and the Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health approved the study.
Selection of colorectal cancer cases and controls
Participants were eligible to be selected as a case or a control if they were cancer free (except possibly for nonmelanoma skin cancer or cervix in situ) in 1989 or earlier. A total of 173 colorectal cancer cases were identified from 1990 through mid-2000 via linkage with the Washington County Cancer Registry and, since 1992, with the Maryland State Cancer Registry. The Maryland Cancer Registry is certified by the North American Association of Central Cancer Registries as being more than 95 % complete. Compared with the Maryland Cancer Registry, the Washington County Cancer Registry captured 98 % of the colorectal cancer cases diagnosed in Washington County residents in 1998. Ninety-eight percent of the cases were pathologically confirmed. A total of 124 cases had colon cancer, 48 cases had rectal cancer (one case with missing tumor location), 68 cases had stage I or II disease, and 55 cases had stage III or IV disease (50 cases were missing stage information). Ninety-nine percent and 80 % of the colon and rectal cancer tumors were adenocarcinomas, respectively. For each case, up to two controls (345 controls in total because one case was matched to only one control) matched on age (±1 year), sex, race, date of blood draw (±2 weeks), and time since the last meal (0–1, 2–3, 4–5, 6–7, ≥8 h) were selected if they were known to be alive at the time that the case was diagnosed.
IL-6 assessment
IL-6 concentrations were measured in the archived plasma specimens collected in 1989 by an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) in the laboratory of Dr. Rifai at Children’s Hospital, Boston, MA. A total of 24 quality control samples (≅5 % of the total sample) aliquoted from pooled plasma were arranged in triplets among the cases and the two controls, and each triplet was run adjacently. The laboratory was blind to the case–control and quality control sample status. The mean intra-pair coefficient of variation was 4.3 %. C-reactive protein (CRP) concentrations were also available, and details of its measurement and quality control statistics are provided elsewhere [9].
Covariate assessment
Self-reported current weight and height, weight at age 21, schooling years, and history of smoking were collected at baseline in 1989. Body mass index (BMI) was calculated at baseline and at age 21 as weight in kilograms divided by height in meters squared. Participants were asked whether they had used any medications in the 48 h prior to blood draw. Any medication that contained aspirin or nonaspirin nonsteroidal anti-inflammatory drugs was coded as nons-teroidal anti-inflammatory drugs (NSAIDs). Use of anti-diabetic and cholesterol-lowering medication was also recorded. Women were asked whether they ever used oral contraceptives or hormone replacement therapy. Daily intake of alcohol (g/day) and red meat (g/day) was estimated from the food frequency questionnaire at baseline, which was an abbreviated version of the Block food frequency questionnaire comprised of 60 food items [10], and was returned by 94 % of colorectal cancer cases and selected controls. Follow-up questionnaires in 1996, 1998, and 2000 ascertained whether participants had a first-degree family history of colorectal cancer and a personal history of inflammatory bowel disease.
Statistical analysis
The distributions of baseline characteristics were compared between cases and controls using conditional logistic regression models, and across thirds of the IL-6 distribution among controls using linear and logistic regression models adjusted for age at recruitment and sex. Matched odds ratios (ORs) and the corresponding 95 % confidence intervals (CIs) were estimated for the association of circulating IL-6 concentrations and risk of colorectal cancer using conditional logistic regression. IL-6 was modeled using continuous, after natural logarithm transformation, and categorical terms (thirds and fourths of the IL-6 distribution among controls) after inspecting nonparametric plots of IL-6 concentrations on colorectal cancer risk, where the pattern of risk changed approximately linearly with higher IL-6 concentrations in the logarithmic scale. The inferences were very similar using each of the latter IL-6 modeling approaches; thus, the main results were presented using thirds and continuous variables (per 1 U change in the natural logarithm-transformed values). Linear trends were tested by entering into the model the ordinal IL-6 concentration variable with values corresponding to the thirds of the distribution among controls. Models were adjusted for known or suspected risk factors for colorectal cancer. The ORs accounting only for the matching variables were first estimated, and then models were adjusted for education (years of schooling), BMI at baseline (<25, ≥25–<30, ≥30 kg/m2), smoking status (never, former, current), NSAIDs or aspirin use (yes, no), family history of colorectal cancer (yes, no), use of diabetes treatment (yes, no), and ever use of female hormones (yes, no). None of the above confounders had any missing data. Analyses that included further adjustments for BMI at age 21, inflammatory bowel disease, cholesterol-lowering medications, and daily intakes of red meat and alcohol gave very similar results and are not presented here. Furthermore, we conducted analyses according to tumor location (colon vs. rectum) and stage at diagnosis (I–II vs. III–IV), and heterogeneity according to these tumor characteristics was measured with the Cochran’s Q test.
Stratified analyses were conducted according to age at recruitment (cut at the median: <66 vs. ≥66 years), sex, BMI at recruitment (<25, ≥25 kg/m2), use of aspirin or NSAIDs, and ever cigarette smoking, because these variables are known to influence IL-6 concentrations and/or colorectal cancer risk. Tests for interaction were carried out by using the relevant exposure variables, indicator variables for the potentially modifying factors, and product terms of the two variables. The statistical significance of the interaction terms was evaluated by the Wald test. For these analyses, to preserve power, we broke the matched sets and performed unconditional logistic regression adjusting for the matching variables. Sensitivity analyses were performed after excluding colorectal cancer cases that developed within 2 or 5 years from the baseline blood draw to exclude the possibility that IL-6 concentrations were affected by extant cancers. To shed light on whether the association between IL-6 concentrations and colorectal cancer is independent of CRP, we further adjusted all models for CRP concentrations and performed interaction (cut at the median: <0.2 vs. ≥0.2 mg/dL) and joint analyses for thirds of IL-6 and CRP. All p values (p) were two-sided, and all analyses were performed using STATA version 12 (College Station, TX).
Meta-analysis
A comprehensive search on PubMed was conducted from 1966 through April 2014 to identify prospective epidemi-ological studies investigating the association between pre-diagnostic concentrations of circulating IL-6 and the subsequent development of colorectal cancer. The following algorithm was used: “(c-reactive protein OR inter-leukin* OR tumor necrosis factor) AND (colorectal OR colon or rectal or large bowel) AND (cancer OR carcinoma OR neoplasia OR tumor OR adenoma OR neoplasm).” A manual review of references from eligible studies was also performed. No language limitations were applied. Articles were excluded if (a) the research was not conducted in humans or was not original, (b) they were prognostic or diagnostic studies, (c) colorectal cancer was not the outcome of interest, and (d) circulating IL-6 was not the exposure of interest. The literature search, the review of the eligible studies and the data extraction was conducted independently by two investigators (CK and KKT), and discrepancies were resolved by consensus.
Maximally adjusted relative risk (RR) estimates and 95 % CIs for the association of the natural logarithm (ln) of IL-6 (per 1 U change in ln pg/mL) with colorectal cancer were obtained from the original publications, and additional risk estimates (especially by bowel location) were provided by several study authors [4, 5, 7, 8]. Specifically, Chan et al. provided updated data with more cases and controls compared to the numbers in the published paper. After weighting the study specific ln RR estimates by the inverse of their variance, we calculated summary estimates and its 95 % CIs using random effects models. Alternative analyses were based on fixed effects models. The meta-analysis was conducted for colorectal cancer risk and separately for colon and rectal cancer. Between-study heterogeneity was assessed with the I2 statistic and the p value from the Cochran’s Q test, and small-study effects bias was evaluated using funnel plots and the Egger’s regression asymmetry test. The methodological quality of the included studies was assessed independently by AK and KKT using the Newcastle–Ottawa scale, which accords a maximum of nine points to each study, with five or less points indicating a high risk of bias [11].
Results
IL-6 concentrations and colorectal cancer in CLUE II
Table 1 compares the cases and controls with respect to several lifestyle, medical, and dietary factors after taking into account the matched design using conditional logistic regression models. Compared to the controls, colorectal cancer cases were more likely to have a positive family history of colorectal cancer (p 0.06), were less likely to have used aspirin or NSAIDs (p 0.02), were more likely to have used diabetes medications (p 0.03), and had higher concentrations of plasma IL-6 (median of 2.3 pg/mL in cases and 1.9 pg/mL in controls; p 0.03) and CRP (p 0.01).
Table 1.
Baseline characteristics of colorectal cases and matched controls in the CLUE II cohort of Washington County, Maryland, 1989
| Characteristics | Cases (n = 173) | Controls (n = 345) | p valuea |
|---|---|---|---|
| Age (years), mean (SD) | 63.5 (11.3) | 63.3 (11.4) | Matched |
| Men [n (%)] | 77 (44.5) | 154 (44.6) | Matched |
| White [n (%)] | 168 (97.1) | 337 (97.7) | Matched |
| First-degree family history of CRC [n (%)] | 17 (9.8) | 18 (5.2) | 0.06 |
| Years of school completed, mean (SD) | 11.5 (3.0) | 11.9 (3.2) | 0.16 |
| BMI at recruitment (kg/m2), mean (SD) | 26.5 (4.4) | 25.9 (3.9) | 0.17 |
| BMI at age 21 (kg/m2), mean (SD) | 21.9 (4.4) | 21.4 (2.9) | 0.11 |
| Cigarette smoking status [n (%)] | |||
| Current | 21 (12.1) | 42 (12.2) | 0.44 |
| Former | 68 (39.3) | 119 (34.5) | |
| Never | 84 (48.6) | 184 (53.3) | |
| Use (in the past 48 h) of NSAIDs [n (%)] | 36 (20.8) | 105 (30.4) | 0.02 |
| Ever use of female hormones [n (%)]b | 21 (21.9) | 57 (29.8) | 0.13 |
| Use of diabetes medications [n (%)] | 14 (8.1) | 12 (3.5) | 0.03 |
| Use of cholesterol-lowering medications [n (%)] | 17 (9.9) | 20 (5.8) | 0.08 |
| History of inflammatory bowel disease [n (%)] | 2 (1.2) | 3 (0.9) | 0.75 |
| Daily intake (g), mean (SD)c | |||
| Red meat | 72.8 (58.9) | 69.8 (73.1) | 0.47 |
| Alcohol | 3.4 (9.6) | 5.6 (15.6) | 0.19 |
| Plasma interleukin-6 (pg/mL), median (IQR) | 2.3 (1.5–3.6) | 1.9 (1.3–3.0) | 0.03 |
| Plasma C-reactive protein (mg/dL), median (IQR) | 0.24 (0.13–0.55) | 0.19 (0.09–0.37) | 0.01 |
SD standard deviation, CRC colorectal cancer, BMI body mass index, NSAID nonsteroidal anti-inflammatory drug, IQR inter-quartile range
Conditional logistic regression models accounting for the matched design. Highly skewed characteristics were transformed using the natural logarithm and were summarized using the median and IQR
Use of oral contraceptives or hormone replacement therapy based on 96 female cases and 191 matched controls
Based on the participants who completed the food frequency questionnaire (151 cases and 335 matched controls)
Table 2 shows baseline characteristics by concentration of IL-6 among controls after adjusting for age at recruitment and sex. Participants with higher IL-6 concentrations were on average older (p 0.05), heavier (p < 0.01), and more likely to be men (p 0.03). No statistically significant differences were observed for other covariates.
Table 2.
Age- and sex-adjusted baseline characteristics according to thirds of circulating interleukin-6 among controls in the CLUE II cohort of Washington County, Maryland, 1989
| Characteristics | Thirds of interleukin-6 (pg/mL) |
p valuea | ||
|---|---|---|---|---|
| Lowest (≤1.40) | Second (1.41–2.56) | Third (≥2.57) | ||
| Age (years), mean | 61.5 | 63.9 | 64.4 | 0.05 |
| Men (%) | 36.5 | 47.0 | 50.6 | 0.03 |
| First-degree family history of CRC (%) | 3.2 | 8.0 | 4.4 | 0.67 |
| Years of school completed, mean | 12.3 | 11.8 | 11.7 | 0.15 |
| BMI at recruitment (kg/m2), mean | 24.2 | 26.1 | 27.5 | <0.01 |
| BMI at age 21 (kg/m2), mean | 21.4 | 21.2 | 21.7 | 0.42 |
| Ever cigarette smokers (%) | 45.2 | 44.4 | 49.5 | 0.54 |
| Use (in the past 48 h) of NSAIDs (%) | 30.0 | 32.8 | 27.3 | 0.65 |
| Ever use of female hormones (%)b | 24.3 | 30.1 | 21.3 | 0.76 |
| Use of diabetes medications (%) | 3.1 | 4.0 | 1.1 | 0.27 |
| Use of cholesterol-lowering medications (%) | 5.6 | 4.9 | 5.6 | 0.99 |
| Red meat (g/day), meanc | 67.4 | 68.6 | 73.3 | 0.54 |
| Alcohol (g/day), meanc | 6.3 | 4.3 | 6.2 | 0.96 |
CRC colorectal cancer, BMI body mass index, NSAID nonsteroidal anti-inflammatory drug
Linear or logistic regression models adjusted for age at recruitment and gender
Use of oral contraceptives or hormone replacement therapy based on 191 female controls
Based on the participants who completed the food frequency questionnaire (335 controls)
Participants above the highest third of plasma IL-6 concentrations had an approximately twofold higher risk (OR 2.09; 95 % CI 1.26–3.46; p-trend < 0.01) of col-orectal cancer compared with participants in the lowest third after taking into account the matching variables (Table 3). Further adjustment for education, smoking, BMI, aspirin/NSAIDs, diabetes medications, family history of colorectal cancer, and female hormones reduced the magnitude and statistical significance of the association (3rd vs. 1st third: OR 1.76; 95 % CI 1.01–3.06; p-trend 0.07). The fully adjusted OR of colorectal cancer per 1 U increase in the natural logarithm of IL-6 concentrations was 1.21 (95 % CI 0.94–1.56). Table 3 also shows the association of IL-6 with colorectal cancer by tumor location. Compared to participants in the lowest third of IL-6, participants in the highest third had a 2.48 times higher risk of colon cancer (OR 2.48; 95 % CI 1.26–4.87; p-trend 0.02), but no statistically significant association was observed for rectal cancer risk. When only adenocarcinoma tumors were considered among rectal cancer cases, the association remained identical (data not shown).
Table 3.
Odds ratios (OR) and 95 % confidence intervals (CI) of colorectal cancer according to thirds of the distribution of circulating interleukin-6 in the CLUE II cohort of Washington County, Maryland, 1989
| Thirds of interleukin-6 (pg/mL) |
p value for trenda | |||
|---|---|---|---|---|
| Lowest (≤1.40) | Second (1.41–2.56) | Third (≥2.57) | ||
| Colorectal cancer | ||||
| Cases/controls (n) | 34/117 | 72/113 | 67/115 | |
| OR (95 % CI)b | 1.00 (ref) | 2.17 (1.35–3.51) | 2.09 (1.26–3.46) | <0.01 |
| OR (95 % CI)c | 1.00 (ref) | 1.87 (1.13–3.11) | 1.76 (1.01–3.06) | 0.07 |
| Colon cancer | ||||
| Cases/controls (n) | 19/81 | 52/81 | 53/85 | |
| OR (95 % CI)b | 1.00 (ref) | 2.74 (1.50–5.01) | 2.80 (1.50–5.25) | <0.01 |
| OR (95 % CI)c | 1.00 (ref) | 2.55 (1.35–4.81) | 2.48 (1.26–4.87) | 0.02 |
| Rectal cancer | ||||
| Cases/controls (n) | 15/36 | 19/32 | 14/28 | |
| OR (95 % CI)b | 1.00 (ref) | 1.42 (0.62–3.24) | 1.18 (0.47–2.99) | 0.65 |
| OR (95 % CI)c | 1.00 (ref) | 0.90 (0.33–2.42) | 0.77 (0.25–2.40) | 0.65 |
Interleukin-6 was entered into the model as a single ordinal variable with values corresponding to the thirds of its distribution among controls
From a conditional logistic regression model with the interleukin-6 concentration entered as a series of indicator variables corresponding to thirds of its distribution among the controls. Cases and controls were matched on age, sex, race, date of blood draw, and time since the last meal
As inb but further adjusted for years of education, cigarette smoking status, body mass index at baseline, use of aspirin or nonsteroidal anti-inflammatory drugs, use of diabetes medications, family history of colorectal cancer, and use of oral contraceptives or hormone replacement therapy in women
Table 4 shows the risk of colorectal and colon cancer according to a 1 U increase in the natural logarithm of IL-6 concentrations in subgroup and sensitivity analyses. The OR of colon cancer per 1 U increase in the natural logarithm of IL-6 concentrations was 1.50 (95 % CI 1.12–2.01), and it was statistically significantly different from the association observed for rectal cancer (OR 0.77; 95 % CI 0.51–1.16; p-heterogeneity 0.01). However, the associations did not differ by colorectal cancer stage or according to age at recruitment, sex, BMI, use of aspirin/ NSAIDs, and smoking status. The association of IL-6 concentrations with risk of colon cancer was slightly attenuated, but remained statistically significant after excluding cases occurring within 2 or 5 years of the baseline blood draw or after adjusting for circulating CRP concentrations (Table 4). When we tested whether IL-6 and CRP concentrations might act synergistically to further increase the risk of colon cancer, no statistically significant multiplicative interaction was observed.
Table 4.
Risk of colorectal cancer according to a 1-unit increase in the natural logarithm of interleukin-6 concentration in subgroups in the CLUE II cohort of Washington County, Maryland, 1989
| Cases/controls | Odds ratio (95 % confidence interval)a |
p value for interaction/ heterogeneity |
|
|---|---|---|---|
| All colorectal cancer | 173/345 | 1.21 (0.94–1.56) | |
| Adjustment for CRP | 172/343 | 1.17 (0.88–1.55) | |
| Stage I or II CRC | 68/345 | 1.19 (0.84–1.67) | |
| Stage III or IV CRC | 55/345 | 1.32 (0.87–2.01) | 0.71b |
| Age < 66 years | 83/167 | 1.05 (0.73–1.52) | |
| Age ≥ 66 years | 90/178 | 1.43 (0.99–2.08) | 0.45 |
| Men | 96/191 | 0.91 (0.63–1.32) | |
| Women | 77/154 | 1.61 (1.11–2.35) | 0.11 |
| BMI < 25 kg/m2 | 69/152 | 1.70 (1.10–2.63) | |
| BMI ≥ 25 kg/m2 | 104/193 | 1.05 (0.76–1.44) | 0.23 |
| Users of aspirin/NSAIDs | 36/105 | 1.01 (0.54–1.90) | |
| Nonusers of aspirin/NSAIDs | 137/240 | 1.26 (0.94–1.68) | 0.64 |
| Never smokers | 84/184 | 1.35 (0.95–1.93) | |
| Ever smokers | 89/161 | 1.08 (0.73–1.60) | 0.40 |
| CRP < 0.2 mg/dL | 72/176 | 0.89 (0.57–1.38) | |
| CRP ≥ 0.2 mg/dL | 100/167 | 1.33 (0.95–1.87) | 0.13 |
| Joint third analysis IL-6/CRP | |||
| Third 1/third 1 | 17/65 | 1.00 (ref) | |
| Third 1/third 2 | 10/37 | 0.96 (0.39–2.40) | |
| Third 1/third 3 | 6/13 | 1.92 (0.61–6.07) | |
| Third 2/third 1 | 18/31 | 2.07 (0.91–4.72) | |
| Third 2/third 2 | 27/46 | 1.97 (0.94–4.14) | |
| Third 2/third 3 | 27/36 | 2.69 (1.22–5.92) | |
| Third 3/third 1 | 4/18 | 0.80 (0.23–2.75) | |
| Third 3/third 2 | 23/32 | 2.60 (1.17–5.77) | |
| Third 3/third 3 | 40/65 | 2.03 (0.97–4.28) | 0.45 |
| Exclude cases occurring within 2 years of baseline blood draw | 135/345 | 1.12 (0.85–1.48) | |
| Exclude cases occurring within 5 years of baseline blood draw | 82/345 | 1.09 (0.78–1.52) | |
| All colon cancer | 124/345 | 1.50 (1.12–2.01) | 0.01b |
| Adjustment for CRP | 123/343 | 1.40 (1.01–1.94) | |
| Stage I or II CRC | 48/345 | 1.53 (1.02–2.29) | |
| Stage III or IV CRC | 46/345 | 1.40 (0.89–2.19) | 0.77 |
| Age < 66 years | 59/167 | 1.60 (1.02–2.52) | |
| Age ≥ 66 years | 65/178 | 1.39 (0.92–2.10) | 0.17 |
| Men | 66/191 | 1.16 (0.75–1.81) | |
| Women | 58/154 | 1.88 (1.23–2.87) | 0.19 |
| BMI < 25 kg/m2 | 49/152 | 1.89 (1.16–3.09) | |
| BMI ≥ 25 kg/m2 | 75/193 | 1.28 (0.88–1.86) | 0.26 |
| Users of aspirin/NSAIDs | 28/105 | 1.15 (0.59–2.26) | |
| Nonusers of aspirin/NSAIDs | 96/240 | 1.57 (1.12–2.20) | 0.42 |
| Never smokers | 58/184 | 2.06 (1.34–3.18) | |
| Ever smokers | 66/161 | 1.17 (0.76–1.80) | 0.11 |
| CRP < 0.2 mg/dL | 46/176 | 1.26 (0.72–2.21) | |
| CRP ≥ 0.2 mg/dL | 77/167 | 1.50 (1.03–2.19) | 0.53 |
| Joint third analysis IL-6/CRP | |||
| Third 1/third 1 | 8/65 | 1.00 (ref) | |
| Third 1/third 2 | 5/37 | 1.04 (0.30–3.58) | |
| Third 1/third 3 | 5/13 | 3.75 (1.00–14.0) | |
| Third 2/third 1 | 9/31 | 2.24 (0.76–6.60) | |
| Third 2/third 2 | 21/46 | 3.41 (1.35–8.62) | |
| Third 2/third 3 | 22/36 | 5.09 (1.93–13.4) | |
| Third 3/third 1 | 4/18 | 1.83 (0.48–7.02) | |
| Third 3/third 2 | 17/32 | 4.08 (1.53–10.9) | |
| Third 3/third 3 | 32/65 | 3.70 (1.46–9.33) | 0.12 |
| Exclude cases occurring within 2 years of baseline blood draw | 98/345 | 1.44 (1.06–1.98) | |
| Exclude cases occurring within 5 years of baseline blood draw | 61/345 | 1.46 (1.01–2.13) |
From a logistic regression model according to a 1-U increase in the natural logarithm of interleukin-6 concentration adjusted for the matching variables (age, sex, race, date of blood draw, and time since the last meal), and further adjusted for years of education, cigarette smoking status, body mass index at baseline, use of aspirin or nonsteroidal anti-inflammatory drugs, use of diabetes medications, family history of colorectal cancer, and use of oral contraceptives or hormone replacement therapy in women
p values for heterogeneity using the Cochran’s Q test comparing risk estimates by stage (I/II vs. III/IV) and anatomical location (colon vs. rectal) of colorectal cancer. The remaining p values are for evaluating interactions in subgroup analyses by age, sex, BMI, aspirin/NSAID use, smoking, and CRP and are calculated using the Wald test
Meta-analysis of IL-6 concentrations and colorectal cancer
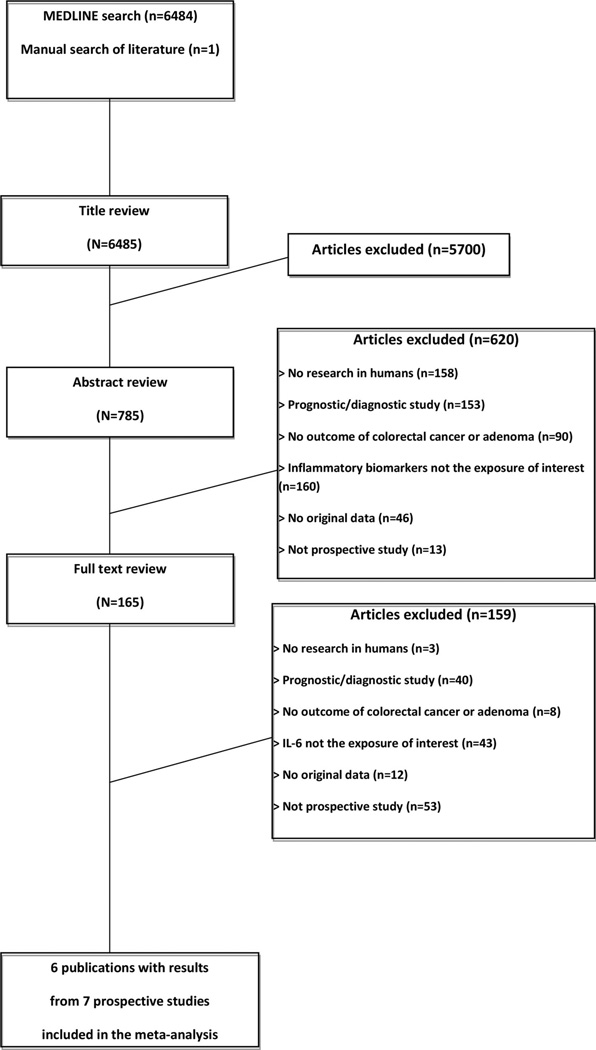
Of the 6,484 articles initially identified in PubMed, six publications with results from seven prospective studies were selected for the meta-analysis including the current study in the CLUE II cohort (Fig. 1). Five studies were conducted in the USA and two in the UK. The number of colorectal cancer cases ranged from 30 to 413, and all seven studies summed to 1,308 cases and 8,420 controls (Supplemental Table 1). Three studies had a cohort design, another three were nested case–control studies, and one used a case-cohort design. The quality of the included studies was good with a median Newcastle–Ottawa quality score of 7 (Supplemental Tables 1 and Supplemental Tables 2). Circulating IL-6 concentrations were not statistically significantly associated with risk of colorectal cancer (Fig. 2; seven studies; per 1 U change in ln IL-6; random effects summary RR 1.10; 95 % CI 0.94–1.28). There was small between-study heterogeneity (I2 34 %; p 0.17), and no evidence of small-study effects was detected by inspecting the funnel plot or using the Egger’s regression asymmetry test (p 0.77).
Fig. 1.
Flow diagram of the study selection process
Fig. 2.
Forrest plot of the seven studies included in the meta-analysis of pre-diagnostic IL-6 concentrations and risk of colorectal, colon and rectal cancer
A total of six studies provided results according to colorectal cancer location, which summed to 919 and 271 colon and rectal cancer cases, respectively. The summary association per 1 U increase in ln IL-6 for colon cancer risk was positive and borderline statistically significant using the random effects method (Fig. 2; RR 1.22; 95 % CI 1.00–1.49) with some evidence of between-study heterogeneity (I2 46 %; p 0.10). The association was nominally statistically significant with the fixed effects method (RR 1.22; 95 % CI 1.06–1.40). However, for rectal cancer risk, the association was statistically significantly inverse (RR 0.69; 95 % CI 0.54–0.88) with no evidence of heterogeneity (I2 0 %; p 0.81). The summary associations for colon and rectal cancer were statistically significantly different (p < 0.001). No evidence of small-study effects was detected in the colon cancer analysis (p 0.81), but smaller studies tended to give larger estimates of effect size compared to larger studies for the rectal cancer analysis (p 0.02).
Discussion
Prediagnostic concentrations of circulating IL-6 were associated with the subsequent development of colon cancer in the prospective CLUE II cohort. This association did not differ according to the stage of disease, age, sex, CRP concentrations, or other potential modifying variables and remained statistically significant after adjustment for CRP concentrations and after excluding cases that occurred within 2 or 5 years of follow-up, thus reducing the likelihood that the results could reflect the presence of sub-clinical disease at the time of blood collection. No association was observed between IL-6 and rectal cancer risk. The meta-analysis showed that higher IL-6 concentrations were associated with a borderline statistically significant higher risk of colon cancer, but with a significant lower risk of rectal cancer.
The overall literature evidence for an association between IL-6 concentrations and risk of colorectal cancer is sparse, as only six prospective studies exist [4–8]. II’yasova et al. [6] found that serum IL-6 concentrations were not statistically significantly associated with risk of colorectal cancer in the Health, Aging, and Body Composition Cohort. Similarly, no association was observed in two British prospective studies published in 2009 [4], but the latter three studies included small numbers of colorectal cancer cases (n = 30–40). Chan et al. [8] used 279 cases from the Nurses’ Health Study, but also did not observe a statistically significant association, and another large publication (413 cases) from the Women’s Health Initiative observational cohort yielded a similar not significant finding [7]. Only, a recent paper from the Health Professionals Follow-up Study observed a borderline significant increased risk of colorectal cancer with higher IL-6 concentrations, but this association did not remain significant when the first 2 years of follow-up was excluded [5]. In the current paper from the CLUE II cohort, we also did not observe a statistically significant association between IL-6 concentrations and risk of colorectal cancer. When we synthesized the results of the latter seven studies in a meta-analysis, we observed a nonsignificant summary RR of 1.10 (95 % CI 0.94–1.28) per 1 U change in ln IL-6. Very few reports have studied the association between IL-6 concentrations and risk of colorectal adenoma and observed inconsistent findings [12, 13].
However, the association between IL-6 concentrations and colon cancer risk was statistically significant in the CLUE II cohort. Only one other study reported results by tumor location in the original publication [5], but several study authors sent us site-specific risk estimates after communicating with them. In four studies, the association of IL-6 concentrations and colon cancer risk was not statistically significant [4, 7, 8]. However, the Health Professionals Follow-up Study (HPFS) observed a statistically significant increased risk of colon cancer with higher IL-6 concentrations [5]. When we synthesized the results of the six studies in a meta-analysis, we observed a borderline statistically significant summary RR of 1.22 (95 % CI 1.00–1.49) per 1 U change in ln IL-6 concentrations without substantial between-study heterogeneity. It is important to note that the two cohorts (CLUE II and HPFS) that observed significant increased risks of IL-6 and colon cancer risk also reported that those estimates were attenuated (the results in CLUE II remained significant) after excluding cases occurring within 2 or 5 years of the baseline blood draw. Future large prospective studies are warranted to confirm whether IL-6 concentrations are associated with colon cancer risk.
The association between IL-6 concentrations and rectal cancer risk was not statistically significant in the CLUE II cohort, but the point estimate was below unity, and this finding was significantly different from the association observed for colon cancer risk. Five studies sent us results for rectal cancer risk, and they were all quite homogeneous with estimates below unity without reaching statistical significance [4, 5, 7, 8]. When we synthesized the results of the six studies, the summary association was statistically significantly inverse (RR 0.69; 95 % CI 0.54–0.88). Findings suggesting possible inverse relationships between IL-6 concentrations and cancer risk are intriguing, but have no easy explanations. Increased IL-6 concentrations may reflect both the level of inflammatory status and an attempt by the host to suppress tumor formation or growth. Another explanation may involve the biologic differences of the colon and the rectum, but when we excluded rare histologies (e.g., squamous cell carcinomas) from the rectal cancer analysis, the results remained identical. The inverse finding could be also due to chance or bias, because it was based on only 271 rectal cancer cases and we found evidence of small-study effects in the meta-analysis, where the smaller studies observed stronger inverse estimates, which offer a hint for possible publication and other selective reporting biases, but they may also reflect genuine heterogeneity, chance, or other reasons for differences between small and large studies [14].
There is ample evidence suggesting an association between inflammation and colorectal neoplasia. Animal models and mechanistic studies implicate inflammation as a risk factor for colorectal cancer [15–17]. In addition, patients with inflammatory bowel disease are at increased risk of developing colorectal cancer [18], and regular aspirin or NSAID use reduces colorectal cancer risk or adenoma recurrence [19–22]. However, epidemiological studies of inflammatory biomarkers, like CRP and TNF-a, have observed mixed results [23–29]. A meta-analysis that our team conducted in 2008 found that increased circulating CRP concentrations were weakly associated with an increased risk of colorectal cancer [30]. More recently, two prospective studies reported an increased risk of colorectal cancer with higher CRP concentrations [31, 32], whereas four other prospective studies reported no significant association [4, 5, 8, 33]. An updated meta-analysis published in 2014 reported that CRP concentrations were weakly associated with an increased risk of colorectal and colon cancer, but not with rectal cancer risk [34]. When we investigated whether increased CRP is marking colonic inflammation, we found no such association in a colono-scopy-based study, which implies that CRP may be marking systemic factors that influence colorectal cancer risk or that the observational associations between CRP and colorectal cancer may be afflicted by biases. Further evidence from some Mendelian randomization studies has reported that the association between CRP concentration and risk of colorectal cancer may not be causal (free of biases) after using CRP genotypes as instruments of the blood concentrations [35, 36], although this evidence was not entirely consistent in other Mendelian randomization studies [37, 38]. An empirical evaluation of the cancer epidemiology literature that our team conducted in 2012 identified a statistically significant excess of positive studies, suggesting potentially the presence of selective reporting biases, among articles that investigated the association between biomarkers of inflammation and cancer risk [39]. More studies and individual-level data pooled analysis thereof are warranted to elucidate the potential association of plasma IL-6 with colonic inflammation and colorectal cancer risk.
Several factors should be considered in the interpretation of our findings. The strengths of our study include its prospective design and highly complete follow-up rate, that IL-6 was measured prediagnostically, and that almost all colorectal cancer cases were pathologically confirmed. Our study also has potential limitations. A single measurement of IL-6 does not necessarily represent an average concentration over time for this biomarker, and this might have caused regression dilution bias. However, IL-6 concentrations have been shown to be generally stable over time [40]. Moreover, plasma IL-6 might not be well correlated with tissue-specific inflammation that is most relevant for colorectal carcinogenesis. In addition, it is always possible that inadequate control for potential confounders may influence findings of epidemiological studies, but we have measured and adjusted for many potential confounders in the current study.
In summary, our findings provide support for a modest positive association between circulating IL-6 concentration and colon cancer risk. More work is needed to determine whether IL-6 is a valid marker of colorectal inflammation and whether and how such inflammation contributes to colon and rectal cancer risk.
Supplementary Material
Acknowledgments
The authors thank Despoina Capothanassi for her statistical programming support, Dr. Thomas P Erlinger for his support of research on inflammation biomarkers and colorectal cancer risk in CLUE, and the authors of the original studies (Drs. Katriina Heikkila, Andrew Chan, Gloria Ho, and Mingyang Song), who kindly and promptly replied to our requests for additional information. Cancer incidence data were provided by the Maryland Cancer Registry, Center for Cancer Prevention and Control, Department of Health and Mental Hygiene, 201 W. Preston Street, Room 400, Baltimore, MD 21201, http://phpa.dhmh.maryland.gov/cancer, 410-767-4055. We acknowledge the State of Maryland, the Maryland Cigarette Restitution Fund, the Washington County Cancer Registry, and the National Program of Cancer Registries of the Centers for Disease Control and Prevention (CDC) for the funds that helped support the availability of the cancer registry data. The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the Department of Health and Mental Hygiene.
Funding This research was funded by the American Institute for Cancer Research and the Maryland Cigarette Restitution Fund at Johns Hopkins, and the National Cancer Institute (P30 CA006973 to W.G. Nelson).
Footnotes
Author Contributions JH-B, KJH, and EAP were involved in formulating the hypothesis and the design of the study protocol. The study hypothesis arose before inspection of the data. All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. GB and NR measured the IL-6 concentrations. AK, CK, and KKT carried out the search of the literature, data abstraction, data management, and statistical analysis. DSL verified several inputs of the final database of the meta-analysis. AK, CK, EAP, and KKT wrote the first draft, and all authors contributed to further drafts and approved the final version submitted.
Compliance with ethical standards
Competing interests All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that: [1] No authors have support from any company for the submitted work; [2] no authors have relationships with any company that might have an interest in the submitted work; [3] the authors’ spouses, partners, or children do not have any financial relationships that may be relevant to the submitted work; and [4] all authors have no nonfinancial interests that may be relevant to the submitted work.
Ethical approval All participants provided informed consent, and the Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health approved the study. The evidence synthesis study did not require an ethics approval.
Electronic supplementary material The online version of this article (doi:10.1007/s10552-015-0641-1) contains supplementary material, which is available to authorized users.
References
- 1.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 2.Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 2002;4(Suppl 3):S233–S242. doi: 10.1186/ar565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker C, Fantini MC, Wirtz S, et al. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4:217–220. [PubMed] [Google Scholar]
- 4.Heikkila K, Harris R, Lowe G, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20:15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- 5.Song M, Wu K, Ogino S, Fuchs CS, Giovannucci EL, Chan AT. A prospective study of plasma inflammatory markers and risk of colorectal cancer in men. Br J Cancer. 2013;108:1891–1898. doi: 10.1038/bjc.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Il’yasova D, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 7.Ho GY, Wang T, Gunter MJ, et al. Adipokines linking obesity with colorectal cancer risk in postmenopausal women. Cancer Res. 2012;72:3029–3037. doi: 10.1158/0008-5472.CAN-11-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan AT, Ogino S, Giovannucci EL, Fuchs CS. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gas-troenterology. 2011;140:799–808. doi: 10.1053/j.gastro.2010.11.041. (quiz e11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291:585–590. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- 10.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 11.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 12.Comstock SS, Hortos K, Kovan B, McCaskey S, Pathak DR, Fenton JI. Adipokines and obesity are associated with colorectal polyps in adult males: a cross-sectional study. PLoS One. 2014;9:e85939. doi: 10.1371/journal.pone.0085939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki Y, Takeda H, Sato T, et al. Serum interleukin-6, insulin, and HOMA-IR in male individuals with colorectal adenoma. Clin Cancer Res. 2012;18:392–399. doi: 10.1158/1078-0432.CCR-11-0896. [DOI] [PubMed] [Google Scholar]
- 14.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 15.Corpet DE, Pierre F. Point: from animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 16.Corpet DE, Tache S. Most effective colon cancer chemopreventive agents in rats: a systematic review of aberrant crypt foci and tumor data, ranked by potency. Nutr Cancer. 2002;43:1–21. doi: 10.1207/S15327914NC431_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 18.Itzkowitz SH. Cancer prevention in patients with inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1133–1144. doi: 10.1016/s0889-8553(02)00047-x. [DOI] [PubMed] [Google Scholar]
- 19.Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 20.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 21.Benamouzig R, Deyra J, Martin A, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125:328–336. doi: 10.1016/s0016-5085(03)00887-4. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg L, Palmer JR, Zauber AG, Warshauer ME, Stolley PD, Shapiro S. A hypothesis: nonsteroidal anti-inflammatory drugs reduce the incidence of large-bowel cancer. J Natl Cancer Inst. 1991;83:355–358. doi: 10.1093/jnci/83.5.355. [DOI] [PubMed] [Google Scholar]
- 23.Danesh J, Lewington S, Thompson SG, et al. Plasma fib-rinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 24.Gunter MJ, Canzian F, Landi S, Chanock SJ, Sinha R, Rothman N. Inflammation-related gene polymorphisms and colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1126–1131. doi: 10.1158/1055-9965.EPI-06-0042. [DOI] [PubMed] [Google Scholar]
- 25.Ko WF, Helzlsouer KJ, Comstock GW. Serum albumin, bilirubin, and uric acid and the anatomic site-specific incidence of colon cancer. J Natl Cancer Inst. 1994;86:1874–1875. doi: 10.1093/jnci/86.24.1874. [DOI] [PubMed] [Google Scholar]
- 26.Landi S, Moreno V, Gioia-Patricola L, et al. Association of common polymorphisms in inflammatory genes interleukin (IL)6, IL8, tumor necrosis factor alpha, NFKB1, and peroxisome pro-liferator-activated receptor gamma with colorectal cancer. Cancer Res. 2003;63:3560–3566. [PubMed] [Google Scholar]
- 27.Lee YJ, Lee HR, Nam CM, Hwang UK, Jee SH. White blood cell count and the risk of colon cancer. Yonsei Med J. 2006;47:646–656. doi: 10.3349/ymj.2006.47.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theodoropoulos G, Papaconstantinou I, Felekouras E, et al. Relation between common polymorphisms in genes related to inflammatory response and colorectal cancer. World J Gas-troenterol. 2006;12:5037–5043. doi: 10.3748/wjg.v12.i31.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsilidis KK, Erlinger TP, Rifai N, et al. C-reactive protein and colorectal adenoma in the CLUE II cohort. Cancer Causes Control. 2008;19:559–567. doi: 10.1007/s10552-008-9117-x. [DOI] [PubMed] [Google Scholar]
- 30.Tsilidis KK, Branchini C, Guallar E, Helzlsouer KJ, Erlinger TP, Platz EA. C-reactive protein and colorectal cancer risk: a systematic review of prospective studies. Int J Cancer. 2008;123:1133–1140. doi: 10.1002/ijc.23606. [DOI] [PubMed] [Google Scholar]
- 31.Aleksandrova K, Jenab M, Boeing H, et al. Circulating C-reactive protein concentrations and risks of colon and rectal cancer: a nested case-control study within the European Prospective Investigation into Cancer and Nutrition. Am J Epidemiol. 2010;172:407–418. doi: 10.1093/aje/kwq135. [DOI] [PubMed] [Google Scholar]
- 32.Prizment AE, Anderson KE, Visvanathan K, Folsom AR. Association of inflammatory markers with colorectal cancer incidence in the atherosclerosis risk in communities study. Cancer Epidemiol Biomarkers Prev. 2011;20:297–307. doi: 10.1158/1055-9965.EPI-10-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-re-active protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27:2217–2224. doi: 10.1200/JCO.2008.19.8440. [DOI] [PubMed] [Google Scholar]
- 34.Zhou B, Shu B, Yang J, Liu J, Xi T, Xing Y. C-reactive protein, interleukin-6 and the risk of colorectal cancer: a meta-analysis. Cancer Causes Control. 2014;25:1397–1405. doi: 10.1007/s10552-014-0445-8. [DOI] [PubMed] [Google Scholar]
- 35.Allin KH, Nordestgaard BG, Zacho J, Tybjaerg-Hansen A, Bojesen SE. C-reactive protein and the risk of cancer: a mendelian randomization study. J Natl Cancer Inst. 2010;102:202–206. doi: 10.1093/jnci/djp459. [DOI] [PubMed] [Google Scholar]
- 36.Heikkila K, Silander K, Salomaa V, et al. C-reactive protein-associated genetic variants and cancer risk: findings from FINRISK 1992, FINRISK 1997 and Health 2000 studies. Eur J Cancer. 2011;47:404–412. doi: 10.1016/j.ejca.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 37.Prizment AE, Folsom AR, Dreyfus J, et al. Plasma C-re-active protein, genetic risk score, and risk of common cancers in the Atherosclerosis Risk in Communities study. Cancer Causes Control. 2013;24:2077–2087. doi: 10.1007/s10552-013-0285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nimptsch K, Aleksandrova K, Boeing H, et al. Association of CRP genetic variants with blood concentrations of C-reactive protein and colorectal cancer risk. Int J Cancer. 2015;136:1181–1192. doi: 10.1002/ijc.29086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsilidis KK, Papatheodorou SI, Evangelou E, Ioannidis JP. Evaluation of excess statistical significance in meta-analyses of 98 biomarker associations with cancer risk. J Natl Cancer Inst. 2012;104:1867–1878. doi: 10.1093/jnci/djs437. [DOI] [PubMed] [Google Scholar]
- 40.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Rimm EB. Leisure-time physical activity and reduced plasma levels of obesity-related inflammatory markers. Obes Res. 2003;11:1055–1064. doi: 10.1038/oby.2003.145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.