Abstract
The vitamin E family includes both tocopherols and tocotrienols, where α-tocopherol (αTOC) is the most bioavailable form. Clinical trials testing the therapeutic efficacy of high-dose αTOC against stroke have largely failed or reported negative outcomes when a “more is better” approach to supplementation (>400 IU/d) was used. This work addresses mechanisms by which supraphysiologic αTOC may contribute to stroke-induced brain injury. Ischemic stroke injury and the neuroinflammatory response were studied in tocopherol transfer protein-deficient mice maintained on a diet containing αTOC vitamin E at the equivalent human dose of 1680 IU/d. Ischemic stroke-induced brain injury was exacerbated in the presence of supraphysiologic brain αTOC levels. At 48 h after stroke, S100B and RAGE expression was increased in stroke-affected cortex of mice with elevated brain αTOC levels. Such increases were concomitant with aggravated microglial activation and neuroinflammatory signaling. A poststroke increase in markers of oxidative injury and neurodegeneration in the presence of elevated brain αTOC establish that at supraphysiologic levels, αTOC potentiates neuroinflammatory responses to acute ischemic stroke. Exacerbation of microglial activation by excessive αTOC likely depends on its unique cell signaling regulatory properties independent of antioxidant function. Against the background of clinical failure for high-dose αTOC, outcomes of this work identify risk for exacerbating stroke-induced brain injury as a result of supplementing diet with excessive levels of αTOC.—Khanna, S., Heigel,M., Weist, J., Gnyawali, S., Teplitsky, S., Roy, S., Sen, C. K., Rink, C. Excessive α-tocopherol exacerbates microglial activation and brain injury caused by acute ischemic stroke.
Keywords: inflammation, microglia, antioxidant, vitamin
The dietary reference intakes for α-tocopherol (αTOC) vitamin E were last revised by the Food and Nutrition Board at the Institute of Medicine in the year 2000 (1). At that time, the recommended dietary allowance and upper limit for αTOC vitamin E were set at 22.35 and 1000 IU/d, respectively, for men and women >14 y of age. In consideration of these recommendations and given the broad range between the αTOC recommended daily allowance and upper limit, many commercially available αTOC supplements on shelves today are sold in doses that meet or exceed 4 times the recommended daily allowance. As reported in 2005, a review of the National Health and Nutrition Examination Survey revealed that ∼11% of US adults consume ≥400 IU/d of αTOC from such supplements (2), which is the equivalent of ≥17.9 times the recommended daily allowance for αTOC consumption.
As dietary supplement use in the United States continues to grow (3), large clinical trials testing supraphysiologic αTOC supplementation against a range of diseases, from cancer to heart disease, have largely failed or reported negative outcomes. A 2005 meta-analysis of clinical trials testing high-dose αTOC (≥400 IU/d) reported significantly increased risk for all-cause mortality in 9 of 11 clinical trials reviewed (4). Patients randomized to high-dose αTOC (n = 3520, 400 IU/d) in the extended Heart Outcomes Prevention Evaluation trial had greater risk of heart failure and associated hospitalization compared with placebo controls (5). This outcome was further corroborated by the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto (GISSI)-Prevenzione trial that reported increased risk of developing congestive heart failure in postinfarction patients without congestive heart failureat baseline when they were randomized to receive high-dose αTOC (n = 4202) (6). In the context of stroke, meta-analysis of 13 randomized controlled trials to test αTOC supplementation in the prevention of stroke (N = 166,282) found no significant benefit for healthy subjects (relative risk, 0.92; 95% confidence interval, 0.83–1.03) or those at high risk (relative risk, 1.05; 95% confidence interval, 0.98–1.12) to develop stroke (7, 8).
The clinical significance of αTOC supplementation on not only prevention, but also stroke outcome remains to be elucidated. Although the antioxidant properties of αTOC at physiologic levels served as the basis for testing αTOC against a wide array of diseases including stroke (5), pathologic outcomes associated with supraphysiologic αTOC supplementation have yet to be characterized in disease models. The current work sought to test the original hypothesis that tocopherol transfer protein knockout (TTP KO) mice with lower brain αTOC levels (9) would show an exacerbated tissue injury outcome following acute ischemic stroke. Despite multiple independent studies conducted in our laboratory, the observation consistently rejected the stated hypothesis and led to the current work testing the mechanistic underpinnings of why stroke-induced tissue injury is potentiated in the αTOC-rich brain of background matched wild-type (WT) controls.
MATERIALS AND METHODS
Animal and diet protocol
All experiments were approved by the Institutional Animal Care and Use Committee of The Ohio State University. Standard rodent chow (Teklad Rodent Diet #7912; Harlan Laboratories, Indianapolis, IN, USA), as used in Ohio State University animal vivaria, is enriched with αTOC at a concentration of 150 IU/kg. For a 25 g C57BL/6J mouse that consumes ∼4 g of chow per day (10), this translates to 0.6 IU/d (0.15 IU/g × 4 g/d), or 24 IU/d/kg body weight (0.6 IU/d ÷ 0.025 kg body weight). For a 70 kg human, this is the estimated equivalent of 1680 IU/d of αTOC vitamin E. TTP KO mice on a C57BL/6J background as used by our laboratory in previously published work (9) originated from the Chugai Research Institute for Medical Science, Japan. All offspring were born of TTP heterozygous (HT) parents and were genotyped at weaning (21 d). TTP WT, HT, and KO mice were provided ad libitum access to water and chow from weaning until euthanasia. Outcomes were separately validated in background-matched C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) maintained on standard chow (Teklad Rodent Diet #7912) or vitamin E-deficient chow (Teklad diet #88163). Five-week-old male C57BL/6J mice (n = 11) were provided ad libitum access to water and chow for 10 wk, after which they were subjected to experimental stroke.
Mouse stroke model
Transient (90 min) focal cerebral ischemia was induced in 10-wk-old male TTP WT, HT, and KO mice or C57BL/6J mice by the intraluminal suture method of middle cerebral artery occlusion (MCAO) as previously described (11–13). Laser Doppler flowmetry (DRT4; Moor Instruments, Wilmington, DE, USA) was used to confirm successful MCAO (70 ± 10% drop in middle cerebral artery territory cerebral blood flow).
MRI and infarct volume determination
T2-weighted imaging was performed on stroke-affected mice using an 11.7 T (500 MHz) MR system comprised of a vertical bore magnet (Bruker Biospin, Ettlingen, Germany) as described previously (11, 13). For stroke-volume calculations, raw MRI images were converted to digital imaging and communications in medicine format and read into ImageJ software (National Institutes of Health, Bethesda, MD, USA). After matched contrast enhancement of images in ImageJ, digital planimetry was performed by a blinded observer to delineate the infarct area in 1 mm coronal brain slices encompassing the entire neocortex. Infarct areas from brain slices were summed, multiplied by slice thickness, and corrected for edema-induced swelling (14) to determine infarct volume. Three mice (2 KO and 1 HT) were excluded from MRI data analyses and downstream processing on the basis of evidence of intracerebral hemorrhage.
Vitamin E extraction and analysis
Brain vitamin E levels were determined from mice of MRI studies using an HPLC coulometric electrode array detector (Coularray Detector model 5600 with 12 channels; ESA Inc., Chelmsford, MA, USA). The CoulArray detector uses multiple channels set at specific redox potentials. Data were collected using channels set at 600, 700, and 800 mV. The samples were snap-frozen and stored in liquid nitrogen until HPLC assay. Sample preparation, composition of the mobile phase, and specification of the column were used as previously described (15).
Immunohistochemistry
Immunohistochemical determination of protein expression in stroke-affected and contralateral control brain tissue of TTP WT, HT, and KO mice was performed as described previously (11, 12, 16). At 48 h after MCAO and reperfusion, serial 1 mm thick coronal slices including contralateral and stroke-affected S1 cortices were embedded in Optimal Cutting Temperature compound (OCT; Sakura, Flemingweg, The Netherlands) and frozen or formalin fixed for 1 wk and embedded in paraffin. Blocks were sectioned (10 μm OCT, 6 μm paraffin) and mounted onto charged slides. Sections were blocked in 10% normal serum, followed by incubation with antibodies against S100B (1:200; Epitomics, Burlingame, CA, USA), RAGE (1:200; AbD Serotec, Raleigh, NC, USA), F4/80 (1:250; AbD Serotec), IL-1B (1:100; Abcam, Cambridge, MA, USA), p67 phox (1:200; Millipore, Billerica, MA, USA), NeuN (1:100; Millipore), 8OHdG (1:100; Cosmo Bio Ltd, Tokyo, Japan), 4HNE (1:100; Cosmo Bio Ltd), and MPO (1:100; HyCult Biotech). Signal was visualized by reaction with fluorescent secondary antibodies for OCT-embedded sections (Life Technologies, Grand Island, NY, USA) or biotinylated secondary to 3,3′-diaminobenzidine chromagen (Vector Laboratories, Burlingame, CA, USA) for paraffin-embedded sections. Images were captured using an Axiovert 200M microscope (Zeiss, Göttingen, Germany) and expression quantified as percent area in stroke-affected and contralateral control S1 cortex using the AutoMeasure plug-in within Axiovert software (v4.8; Zeiss) as described previously (11, 12, 16).
Fluoro-Jade neurodegeneration stain
Brain sections of 48 h poststroke TTP WT, HT, and KO mice were stained with 0.0001% Fluoro-Jade C (Millipore) (17). Coronal slices of primary somatosensory (S1) cortex were analyzed by fluorescence microscopy (Axiovert 200M; Zeiss), and images were captured using Axiovert v4.8 software (Zeiss).
Detection of superoxide anion
Histologic detection of the superoxide anion was performed using dihydroethidium (DHE) (18). In brief, frozen 10 μm thick coronal sections of TTP WT, HT, and KO mice were prepared from 48 h poststroke mouse brain. Samples were washed with diethylpyrocarbonate water to remove OCT and incubated with DHE (0.25 μmol/L; Invitrogen, Carlsbad, CA, USA) by covering the section with DHE and a coverslip followed by incubation at 37°C in a humidified, 5% CO2 atmosphere for 30 min in dark. Sections from each treatment group were examined by fluorescence microscopy. Images were captured by microscope (Axiovert 200M), and quantification of fluorescent intensity of image was achieved by software Axiovert v4.8 software (Zeiss).
Statistical analyses
Data are reported as mean ± sd. Comparisons between means were tested by 1-way ANOVA with Tukey’s post hoc test (Figs. 1–5) or Student t test (Figs. 6 and 7). P < 0.05 was considered statistically significant.
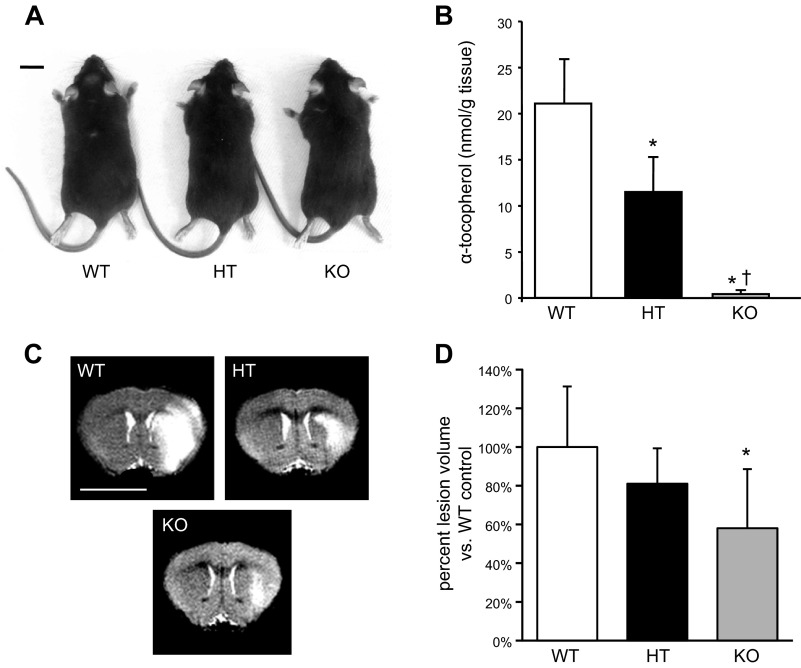
Figure 1.
Supraphysiologic αTOC levels in brain exacerbates ischemic stroke-induced lesion. A) Representative phenotype of 10-wk-old TTP-deficient mouse (KO) compared with WT and HT siblings; bar = 1 cm. B) Brain αTOC levels of TTP WT (n = 7), HT (n = 12), and KO (n = 5) mice (*P < 0.01 KO vs. WT, †P < 0.05 KO vs. HT). C) Representative T2-weighted MRI slice of WT, HT, and KO mice at 48 h after MCAO; bar = 5 mm. D) TTP KO mice had significantly smaller stroke-induced lesion volume at 48 h compared with WT controls. Data are mean ± sd percent lesion volume vs. WT (*P < 0.01 vs. WT).
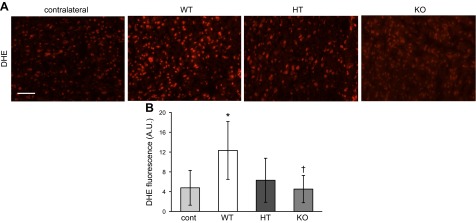
Figure 5.
Superoxide levels in stroke-affected tissue are increased by excessive brain αTOC. A) Representative photomicrographs of DHE staining in contralateral (control) and stroke-affected S1 cortex of TTP WT, HT, and KO mice; scale bar = 50 μm. B) Percent area of DHE fluorescence was used to quantify superoxide levels. Data are mean ± sd, n = 3. *P < 0.05 vs contralateral; †P < 0.05 KO vs. WT.
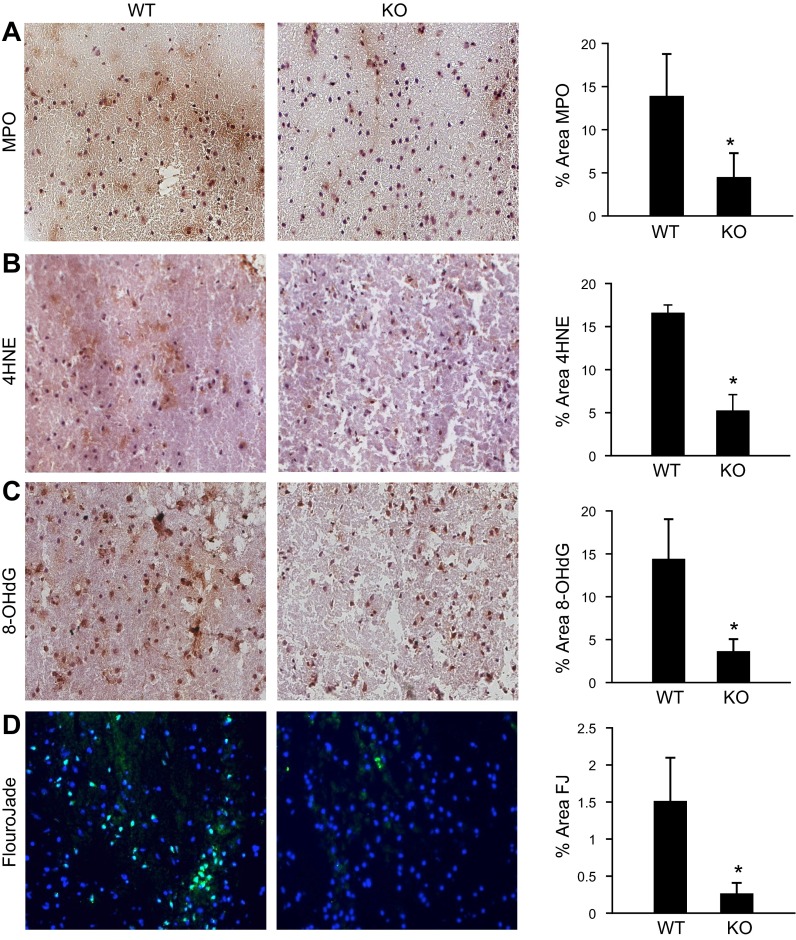
Figure 6.
Supraphysiologic brain αTOC increases poststroke myeloperoxidase expression and exacerbates oxidative stress and neurodegeneration. Representative micrographs and quantification (% area) of (A) myeloperoxidase expression, (B) markers of lipid (4HNE), and (C) DNA (8-OHdG) oxidation, and (D) neurodegeneration (FluoroJade) from stroke-affected S1 cortex off TTP WT and KO mice. Data are mean ± sd, n = 3. *P < 0.05 KO vs. WT; scale bar = 50 μm.
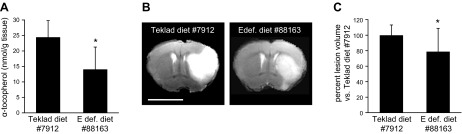
Figure 7.
Ischemic stroke-induced lesion is attenuated in C57BL/6J mice maintained on αTOC-deficient diet. A) Brain αTOC levels of C57BL/6J mice after 10 wk of unrestricted access to standard chow (Teklad diet #7912) or vitamin E–deficient chow (Teklad diet #88163). B) Representative T2-weighted MRI slice of C57BL/6J mouse maintained on Teklad diet #7912 or vitamin E–deficient diet #88163 at 48 h after MCAO; bar = 5 mm. C) C57BL/6J mice maintained on vitamin E–deficient diet #88163 had significantly smaller stroke-induced lesion volume compared with mice maintained on Teklad diet #7912. Data are mean ± sd, n = 11; *P < 0.05.
RESULTS
Excessive brain αTOC exacerbates stroke-induced lesion volume
TTP WT, HT, and KO mice were born of HT breeder pairs and genotyped at The Ohio State University Wexner Medical Center. Parents and offspring were provided access ad libitum to a fixed formula: irradiated Teklad 7912 rodent diet containing 150 IU αTOC/kg diet. This diet is the standard rodent chow given to mice in vivaria at The Ohio State University unless specialized diets are requested by investigators. At 10 wk of age, no difference in phenotype (Fig. 1A) or mean weight (WT = 27.7 ± 2.0 g, HT = 28.1 ± 3.6 g, and KO = 27.6 ± 2.5 g) were observed across genotype groups. As expected, αTOC levels in brain tissue were significantly lower in HT (11.48 ± 3.79 nmol/g tissue) and KO mice (0.43 ± 0.29 nmol/g tissue) compared with WT controls (21.07 ± 4.78 nmol/g tissue; Fig. 1B). These values are consistent with previously published results in TTP KO mice (9). At 48 h after MCAO reperfusion, stroke-induced lesion volume was determined by T2-weighted 11.7 T MRI (Fig. 1C). Strikingly, stroke lesion volume was significantly reduced in TTP KO (65.2 ± 30.5%) and HT (91.0 ± 18.2%) littermates compared with that in WT controls (Fig. 1D).
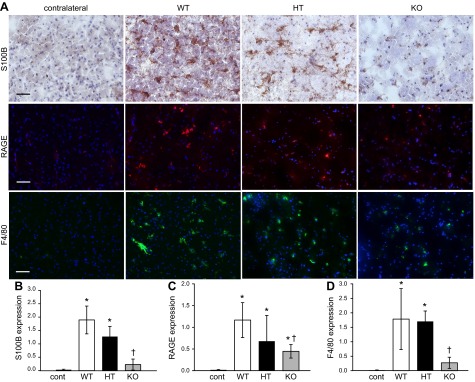
Poststroke S100B/RAGE expression and microglial activation (F4/80 expression) are increased in presence of elevated brain αTOC
To determine the effect of brain αTOC concentration on poststroke glial activation, TTP WT, HT, and KO S1 cortex was immunostained for S100B, RAGE, and F4/80 protein expression. Compared with contralateral control S1 cortex, 48 h postreperfusion S100B expression in stroke-affected S1 cortex of TTP WT, HT, and KO mice was significantly higher (Fig. 2A, B). However, S100B expression in stroke-affected S1 cortex of TTP KO mice was significantly less than that of WT and HT littermates (12.1% of WT and 18.1% of HT; Fig. 2B). Expression of RAGE, a receptor for S100B, was similarly induced across WT, HT, and KO genotype groups in stroke-affected S1 cortex (Fig. 2A, C). Compared with TTP WT mice, RAGE expression in KO mice was significantly lower in stroke-affected S1 cortex (38.5% of WT; Fig. 2C). F4/80 immunostained microglia in WT mice appeared more activated, typified by enlarged cell bodies and ramifying branches (19), compared with αTOC-depleted TTP KO mice (Fig. 2A). Quantification of F4/80 immunoreactivity in stroke-affected S1 cortex revealed significantly lower abundance in TTP KO vs. WT mice (Fig. 2D).
Figure 2.
Poststroke microglial activation is sensitive to brain αTOC levels. A) Representative micrographs depicting S100B, RAGE, and F4/80 immunostaining in contralateral control S1 cortex of WT (n = 3) and stroke-affected S1 cortex of TTP WT (n = 4), HT (n = 3), and KO (n = 3) mice. Bar = 50 μm. Expression of S100B (B), RAGE (C), and F4/80 (D) quantified as percent area. Data are mean ± sd. *P < 0.05 vs contralateral; †P < 0.05 KO vs. WT; scale bar = 50 μm.
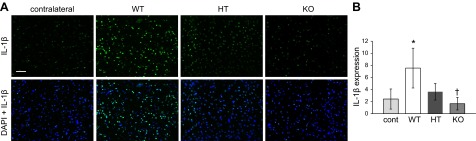
Poststroke inflammatory response is aggravated in presence of elevated brain αTOC
Given the worsening of stroke-induced lesion volume and increased microglial activation in response to increased brain αTOC, we tested whether the expression of known proinflammatory mediators were also affected by increased brain αTOC concentration. IL-1β is a proinflammatory cytokine expressed by activated microglia in the stroke-affected brain (20). Significantly higher IL-1β expression was observed in stroke-affected S1 cortex of TTP WT mice compared with contralateral control S1 cortex (Fig. 3). TTP KO mice had significantly lower IL-1β compared with WT littermates, and no significant difference in IL-1β expression was observed between the contralateral S1 cortex and the stroke-affected S1 cortex in KO mice (Fig. 3).
Figure 3.
Supraphysiologic levels of αTOC increase IL-1β expression in stroke-affected brain. Contralateral control and stroke-affected S1 cortex were immunostained to determine IL-1β expression at 48 h after MCAO reperfusion in TTP WT, HT, and KO mice. A) Representative micrographs of IL-1β staining without and with DAPI counterstain; scale bar = 50 μm. B) IL-1β expression in contralateral and stroke-affected S1 cortex quantified as percent area. Data are mean ± sd, n = 3. *P < 0.05 vs. contralateral; †P < 0.05 KO vs. WT.
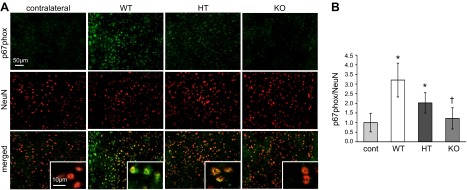
NADPH oxidase contributes to poststroke oxidative stress and inflammation (21, 22) and is the major source of NMDA-induced superoxide formation in neurons following cerebral ischemia (23). Between 24 and 72 h after stroke, expression of the NADPH oxidase subunit p67phox was increased in the neuronal cytoplasmic space (24). At 48 h after stroke, p67phox expression was significantly higher in stroke-affected neurons of TTP WT and HT mice compared with contralateral control tissue (Fig. 4). In contrast, no difference in p67phox expression was observed in the stroke-affected S1 cortex and contralateral control tissue of TTP KO mice. Furthermore, in TTP KO mice, p67phox expression was significantly lower than that of WT littermates in stroke-affected neurons (Fig. 4B). Expression of p67phox in the mouse brain did not colocalize with glial fibrillary acidic protein (Supplemental Fig. I). To evaluate the functional significance of brain αTOC levels on increased neuronal p67phox expression, superoxide generation was measured by DHE fluorescent staining. In line with p67phox expression data, DHE fluorescence was significantly higher in stroke-affected S1 cortex of TTP WT mice compared with contralateral control tissue (Fig. 5). Likewise, DHE levels in stroke-affected TTP KO brain were significantly lower than that of WTs, and no difference was observed between the contralateral control and stroke-affected TTP KO brain (Fig. 5).
Figure 4.
Elevated brain αTOC increases neuronal p67phox expression in stroke-affected tissue. At 48 h after MCAO reperfusion, contralateral control and stroke-affected brain tissue of TTP WT, HT, and KO mice was coimmunostained for p67phox and the neuronal marker NeuN. A) Representative micrographs of p67phox, NeuN, and colocalized p67phox + NeuN staining. B) p67phox expression in contralateral and stroke-affected S1 cortex were quantified (percent area p67phox/percent area NeuN). Data are mean ± sd, n = 3. *P < 0.05 vs contralateral; †P < 0.05 KO vs. WT.
Poststroke oxidative stress and neurodegeneration are exacerbated in presence of elevated brain αTOC
Unlike peripheral macrophages, resident microglia in the brain express myeloperoxidase when activated that contribute to oxidative stress and neurodegeneration (25). In stroke-affected brain tissue of TTP KO mice, myeloperoxidase abundance was significantly lower compared with WT mice (Fig. 6A). To assess oxidative stress, markers of lipid (4-HNE) and DNA (8-OHdG) oxidation were detected and quantified in the TTP WT and KO stroke-affected brain. Lipid (Fig. 6B) and DNA (Fig. 6C) oxidation was 3 times lower in the stroke-affected S1 cortex of TTP KO mice compared with the WT counterparts. Fluoro-Jade C, a selective stain for degenerating neurons, revealed significantly lower levels of neurodegeneration in stroke-affected tissue of TTP KO mice compared with WT controls (Fig. 6D).
Dietary lowering of supraphysiologic αTOC levels attenuates stroke-induced injury
To determine whether the observed effect of supraphysiologic αTOC on stroke outcome was unique to TTP transgenic mice, we used additional studies using C57BL/6J background mice. After 10 wk on a vitamin E–deficient chow (Teklad diet #88163), brain αTOC levels in C57BL/6J mice were reduced by ∼43% compared with standard chow (Teklad diet #7912) controls (Fig. 7A). At 48 h after MCAO, stroke-induced lesion volume was 21.2% smaller in mice maintained on a vitamin E-deficient diet compared with controls (Fig. 7B, C). This reduction in brain αTOC level and stroke-induced lesions was comparable to the difference observed between TTP WT and HT mice (Fig. 1).
DISCUSSION
Our observations reported during the last decade on the potent protective properties of the α-tocotrienol form of vitamin E led to our original hypothesis that TTP KO mice would show larger stroke-induced brain injury, which would then serve as a model to test the efficacy of α-tocotrienol against stroke. The αTOC form of natural vitamin E is well characterized for its antioxidant properties, such that it has been described as the body’s primary chain-breaking defense against oxidative attack of lipid membranes (26). Given the susceptibility of lipid-rich brain tissue to oxidative injury following ischemic stroke (27, 28), the therapeutic potential for dietary αTOC has been tested in preclinical studies with limited success. Using a model of permanent focal cerebral ischemia, van der Worp et al. reported a >50% reduction in stroke-induced infarct volume in rats maintained up to 16 wk on diet containing 93.4 IU/kg αTOC acetate compared with vitamin E–stripped diet controls (29). In contrast, a supraphysiologic dose of dietary αTOC (∼65.6 IU/kg body weight) was reported by Miyamoto et al. to activate microglia and significantly increase GFAP expression in brain tissue of spontaneously hypertensive rats (30). Furthermore, hippocampal neurons (CA1 pyramidal), astrocytes, and microglia of excessive αTOC-supplemented spontaneously hypertensive rats demonstrated increased numbers of lysosome-related structures and aggregates of oxidized lipid granules (lipofuscin) compared with controls (30). In TTP KO mice, we observed decreased lipid oxidation and superoxide detection in the stroke-affected brain. Of note, in the resting brain of TTP KO mice, lower levels of lipid oxidation and attenuated superoxide production have also been reported. Cuddihy et al. (31) observed lower levels of F4 neuroprostanes, a specific marker of lipid oxidation in the brain, as well as decreased cortical superoxide production (measured by DHE) in the TTP KO mouse brain compared with controls when maintained on standard rodent chow containing 81.6 IU/kg vitamin E.
In the current work, excessive dietary αTOC increased microglial activation, exacerbated oxidative stress, and worsened ischemic stroke-induced lesion volume. Taken together, these outcomes suggest that at excessive levels, αTOC may potentiate the neuroinflammatory response to acute ischemic stroke. A growing body of clinical and preclinical research also supports proinflammatory responses to supraphysiologic αTOC. Healthy human volunteers supplemented with 596 IU/d αTOC for 4 wk had significantly increased levels of lysophosphatidycholine in plasma, suggesting high-dose αTOC potentiates a general proinflammatory response by causing displacement and metabolism of membrane phospholipids (32). Of note, increased lysophosphatidycholine levels have also been reported in the stroke-affected brain tissue of rat; this is the result of pathologic lipid metabolism induced by proinflammatory cytokine signaling and executed by phospholipase A2 (33). More than just a general marker of inflammation, lysophosphatidycholines themselves are proinflammatory mediators known for stimulating rapid processing and secretion of mature IL-1β in activated brain microglia (34). In the current work, we note that supraphysiologic αTOC supplementation (TTP WT) is associated with increased microglial activation and higher expression of proinflammatory cytokine IL-1β in stroke-affected tissue compared with physiologic αTOC supplemented controls (TTP KO). The significance of dietary αTOC on proinflammatory cytokine expression has also been described using the TTP KO mouse model for the study of allergic asthma sensitization. Proinflammatory IL-5, TNF-α, and intercellular adhesion molecule 1 expression were all attenuated in lung tissue of TTP KO mice after allergen stimulation compared with TTP WT (35).
Research efforts since the early 1990s have identified unique biological activities of vitamin E isomers that are independent of antioxidant function (36, 37). For example, αTOC is known to inhibit or potentiate arachidonic acid release and metabolism depending on cell type (38). γ-Tocopherol directly binds protein kinase C-α and potentiates activation, whereas αTOC inhibits protein kinase C-α in human microvascular endothelial cells (39, 40). Finally, in the context of neuroprotection, α-tocotrienol potently protects neural cells from glutamate-induced neurotoxicity at nanomolar concentration, whereas αTOC does not (41, 42). In that light, we are led to hypothesize that the exacerbation of microglial activation by excessive αTOC depends on its unique cell signaling regulatory properties independent of antioxidant function.
CONCLUSIONS
This study provides the first evidence addressing the pathologic consequences of excessive αTOC vitamin E supplementation in the context of acute ischemic stroke. High-dose αTOC exacerbated stroke injury, increased microglial activation, and worsened oxidative stress compared with physiologic controls. Taken together with growing evidence of increased all-cause mortality (4) and without the significant benefit for stroke prevention (7, 8), this work highlights the need to exercise caution in recommending high levels of αTOC supplementation for a patient population at high risk for stroke.
Supplementary Material
Acknowledgments
This work was supported by the U.S. National Institutes of Health (NIH) Grant NS42617 (to C.K.S.), and in part by NIH Grants NS085272 (to C.R. and S.K.) and 12SDG11780023 (to C.R.).
Glossary
- αTOC
α-tocopherol
- DHE
dihydroethidium
- HT
heterozygous
- KO
knockout
- MCAO
middle cerebral artery occlusion
- OCT
Optimal Cutting Temperature compound
- TTP
tocopherol transfer protein
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.U.S. Institute of Medicine Panel on Dietary Antioxidants and Related Compounds. (2000) Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids: A Report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine, National Academy Press, Washington, DC [Google Scholar]
- 2.Ford E. S., Ajani U. A., Mokdad A. H. (2005) Brief communication: The prevalence of high intake of vitamin E from the use of supplements among U.S. adults. Ann. Intern. Med. 143, 116–120 [DOI] [PubMed] [Google Scholar]
- 3.Bailey R. L., Gahche J. J., Lentino C. V., Dwyer J. T., Engel J. S., Thomas P. R., Betz J. M., Sempos C. T., Picciano M. F. (2011) Dietary supplement use in the United States, 2003-2006. J. Nutr. 141, 261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller E. R. III, Pastor-Barriuso R., Dalal D., Riemersma R. A., Appel L. J., Guallar E. (2005) Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 142, 37–46 [DOI] [PubMed] [Google Scholar]
- 5.Lonn E., Bosch J., Yusuf S., Sheridan P., Pogue J., Arnold J. M., Ross C., Arnold A., Sleight P., Probstfield J., Dagenais G. R.; HOPE and HOPE-TOO Trial Investigators (2005) Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 293, 1338–1347 [DOI] [PubMed] [Google Scholar]
- 6.Marchioli R., Levantesi G., Macchia A., Marfisi R. M., Nicolosi G. L., Tavazzi L., Tognoni G., Valagussa F.; GISSI-Prevenzione Investigators (2006) Vitamin E increases the risk of developing heart failure after myocardial infarction: Results from the GISSI-Prevenzione trial. J. Cardiovasc. Med. (Hagerstown) 7, 347–350 [DOI] [PubMed] [Google Scholar]
- 7.Leppälä J. M., Virtamo J., Fogelholm R., Albanes D., Taylor P. R., Heinonen O. P. (2000) Vitamin E and beta carotene supplementation in high risk for stroke: a subgroup analysis of the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Arch. Neurol. 57, 1503–1509 [DOI] [PubMed] [Google Scholar]
- 8.Leppälä J. M., Virtamo J., Fogelholm R., Huttunen J. K., Albanes D., Taylor P. R., Heinonen O. P. (2000) Controlled trial of alpha-tocopherol and beta-carotene supplements on stroke incidence and mortality in male smokers. Arterioscler. Thromb. Vasc. Biol. 20, 230–235 [DOI] [PubMed] [Google Scholar]
- 9.Khanna S., Patel V., Rink C., Roy S., Sen C. K. (2005) Delivery of orally supplemented alpha-tocotrienol to vital organs of rats and tocopherol-transport protein deficient mice. Free Radic. Biol. Med. 39, 1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachmanov A. A., Reed D. R., Beauchamp G. K., Tordoff M. G. (2002) Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 32, 435–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna S., Rink C., Ghoorkhanian R., Gnyawali S., Heigel M., Wijesinghe D. S., Chalfant C. E., Chan Y. C., Banerjee J., Huang Y., Roy S., Sen C. K. (2013) Loss of miR-29b following acute ischemic stroke contributes to neural cell death and infarct size. J. Cerebral Blood Flow Metab. 33, 1197–1206 [DOI] [PMC free article] [PubMed]
- 12.Khanna S., Roy S., Slivka A., Craft T. K., Chaki S., Rink C., Notestine M. A., DeVries A. C., Parinandi N. L., Sen C. K. (2005) Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke 36, 2258–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rink C., Gnyawali S., Peterson L., Khanna S. (2011) Oxygen-inducible glutamate oxaloacetate transaminase as protective switch transforming neurotoxic glutamate to metabolic fuel during acute ischemic stroke. Antioxid. Redox Signal. 14, 1777–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loubinoux I., Volk A., Borredon J., Guirimand S., Tiffon B., Seylaz J., Meric P. (1997) Spreading of vasogenic edema and cytotoxic edema assessed by quantitative diffusion and T2 magnetic resonance imaging. Stroke 28, 419–426 [DOI] [PubMed]
- 15.Patel V., Rink C., Gordillo G. M., Khanna S., Gnyawali U., Roy S., Shneker B., Ganesh K., Phillips G., More J. L., Sarkar A., Kirkptrick R., Elkhammas E. A., Klatte E., Miller M., Firstenberg M. S., Chiocca E. A., Nesaretnam K., Sen C. K. (2012) Oral tocotrienols are transported to human tissues and delay the progression of the model for end-stage liver disease score in patients. J. Nutr. 142, 513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rink C., Roy S., Khan M., Ananth P., Kuppusamy P., Sen C. K., Khanna S. (2010) Oxygen-sensitive outcomes and gene expression in acute ischemic stroke. J. Cerebral Blood Flow Metab. 30, 1275–1287 [DOI] [PMC free article] [PubMed]
- 17.Rink C., Christoforidis G., Abduljalil A., Kontzialis M., Bergdall V., Roy S., Khanna S., Slivka A., Knopp M., Sen C. K. (2008) Minimally invasive neuroradiologic model of preclinical transient middle cerebral artery occlusion in canines. Proc. Natl. Acad. Sci. USA 105, 14100–14105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polikandriotis J. A., Rupnow H. L., Elms S. C., Clempus R. E., Campbell D. J., Sutliff R. L., Brown L. A., Guidot D. M., Hart C. M. (2006) Chronic ethanol ingestion increases superoxide production and NADPH oxidase expression in the lung. Am. J. Respir. Cell Mol. Biol. 34, 314–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ke Z. J., Gibson G. E. (2004) Selective response of various brain cell types during neurodegeneration induced by mild impairment of oxidative metabolism. Neurochem. Int. 45, 361–369 [DOI] [PubMed] [Google Scholar]
- 20.Davies C. A., Loddick S. A., Toulmond S., Stroemer R. P., Hunt J., Rothwell N. J. (1999) The progression and topographic distribution of interleukin-1beta expression after permanent middle cerebral artery occlusion in the rat. J. Cerebral Blood Flow Metab. 19, 87–98 [DOI] [PubMed]
- 21.Chen H., Song Y. S., Chan P. H. (2009) Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J. Cereb. Blood Flow Metab. 29, 1262–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walder C. E., Green S. P., Darbonne W. C., Mathias J., Rae J., Dinauer M. C., Curnutte J. T., Thomas G. R. (1997) Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke 28, 2252–2258 [DOI] [PubMed] [Google Scholar]
- 23.Brennan A. M., Suh S. W., Won S. J., Narasimhan P., Kauppinen T. M., Lee H., Edling Y., Chan P. H., Swanson R. A. (2009) NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat. Neurosci. 12, 857–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshioka H., Niizuma K., Katsu M., Okami N., Sakata H., Kim G. S., Narasimhan P., Chan P. H. (2011) NADPH oxidase mediates striatal neuronal injury after transient global cerebral ischemia. J. Cereb. Blood Flow Metab. 31, 868–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefkowitz D. L., Lefkowitz S. S. (2008) Microglia and myeloperoxidase: a deadly partnership in neurodegenerative disease. Free Radic. Biol. Med. 45, 726–731 [DOI] [PubMed] [Google Scholar]
- 26.Packer L. (1991) Protective role of vitamin E in biological systems. Am. J. Clin. Nutr. 53(4, Suppl)1050S–1055S [DOI] [PubMed] [Google Scholar]
- 27.Chan P. H. (1996) Role of oxidants in ischemic brain damage. Stroke 27, 1124–1129 [DOI] [PubMed] [Google Scholar]
- 28.Rink C., Khanna S. (2011) Significance of brain tissue oxygenation and the arachidonic acid cascade in stroke. Antioxid. Redox Signal. 14, 1889–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Worp H. B., Bär P. R., Kappelle L. J., de Wildt D. J. (1998) Dietary vitamin E levels affect outcome of permanent focal cerebral ischemia in rats. Stroke 29, 1002–1005, discussion 1005–1006 [DOI] [PubMed] [Google Scholar]
- 30.Miyamoto K., Shiozaki M., Shibata M., Koike M., Uchiyama Y., Gotow T. (2009) Very-high-dose alpha-tocopherol supplementation increases blood pressure and causes possible adverse central nervous system effects in stroke-prone spontaneously hypertensive rats. J. Neurosci. Res. 87, 556–566 [DOI] [PubMed] [Google Scholar]
- 31.Cuddihy S. L., Ali S. S., Musiek E. S., Lucero J., Kopp S. J., Morrow J. D., Dugan L. L. (2008) Prolonged alpha-tocopherol deficiency decreases oxidative stress and unmasks alpha-tocopherol-dependent regulation of mitochondrial function in the brain. J. Biol. Chem. 283, 6915–6924 [DOI] [PubMed] [Google Scholar]
- 32.Wong M., Lodge J. K. (2012) A metabolomic investigation of the effects of vitamin E supplementation in humans. Nutr. Metab. (Lond) 9, 110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H. Y., Liu C. B., Wu H. W., Kuo J. S. (2010) Direct profiling of phospholipids and lysophospholipids in rat brain sections after ischemic stroke. Rapid Commun. Mass Spectrom. 24, 2057–2064 [DOI] [PubMed] [Google Scholar]
- 34.Stock C., Schilling T., Schwab A., Eder C. (2006) Lysophosphatidylcholine stimulates IL-1beta release from microglia via a P2X7 receptor-independent mechanism. J. Immunol. 177, 8560–8568 [DOI] [PubMed] [Google Scholar]
- 35.Lim Y., Vasu V. T., Valacchi G., Leonard S., Aung H. H., Schock B. C., Kenyon N. J., Li C. S., Traber M. G., Cross C. E. (2008) Severe vitamin E deficiency modulates airway allergic inflammatory responses in the murine asthma model. Free Radic. Res. 42, 387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sen C. K., Khanna S., Roy S. (2006) Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 78, 2088–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Traber M. G., Packer L. (1995) Vitamin E: beyond antioxidant function. Am. J. Clin. Nutr. 62(6, Suppl)1501S–1509S [DOI] [PubMed] [Google Scholar]
- 38.Tran K., Chan A. C. (1990) R,R,R-alpha-tocopherol potentiates prostacyclin release in human endothelial cells. Evidence for structural specificity of the tocopherol molecule. Biochim. Biophys. Acta 1043, 189–197 [DOI] [PubMed] [Google Scholar]
- 39.Abdala-Valencia H., Berdnikovs S., Cook-Mills J. M. (2012) Vitamin E isoforms differentially regulate intercellular adhesion molecule-1 activation of PKCα in human microvascular endothelial cells. PLoS ONE 7, e41054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCary C. A., Yoon Y., Panagabko C., Cho W., Atkinson J., Cook-Mills J. M. (2012) Vitamin E isoforms directly bind PKCα and differentially regulate activation of PKCα. Biochem. J. 441, 189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osakada F., Hashino A., Kume T., Katsuki H., Kaneko S., Akaike A. (2004) Alpha-tocotrienol provides the most potent neuroprotection among vitamin E analogs on cultured striatal neurons. Neuropharmacology 47, 904–915 [DOI] [PubMed] [Google Scholar]
- 42.Sen C. K., Khanna S., Roy S., Packer L. (2000) Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells. J. Biol. Chem. 275, 13049–13055 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.