ABSTRACT
Objectives:
To describe the incidence, pattern, and outcome of priapism in homozygous sickle cell (SS) disease.
Methods:
Regular review, for periods up to 40 years, was done of all 162 males with SS disease detected during the screening of 100 000 consecutive non-operative deliveries at the main government maternity hospital in Kingston, Jamaica, between June 1973 and December 1981.
Results:
Priapism occurred in 52 (32.7%) patients overall, the incidence rising steeply in late adolescence to 32% by age 20 years and a cumulative incidence of nearly 60% of patients by age 40 years. Many cases were elicited only on direct questioning because of embarrassment and the lack of realization that priapism complicates SS disease. Initial events were recurrent stuttering episodes in 39 patients, a single short-term event in six patients and a major attack (more than six hours) in seven patients. Erectile function was preserved in almost all patients with simple stuttering or single events. Major attacks (> 6 hours) occurred in 17 patients, preceded by stuttering episodes in nine, by a single event in one, and occurring de novo in seven. In these, erectile function was unknown in five, deemed satisfactory in five (sometimes improving over three years), weak in three and impotence persisted in four (two with major attacks three and six months previously).
Conclusions:
A history of stuttering priapism should be routinely enquired and prophylactic measures used if attacks exceed once weekly. Major events generally result in short-term impotence, but the late recovery of erectile function cautions against the early insertion of penile prostheses.
Keywords: Cohort study, impotence, natural history, priapism, sickle cell disease
RESUMEN
Objetivos:
Describir la incidencia, patrones y evolución clínica del priapismo en la enfermedad de células falciformes (ECF) homocigótica.
Métodos:
Se realizó una revisión sistemática por períodos hasta de 40 años, de un total de 162 varones con la enfermedad ECF detectada durante la tamización de 100 000 partos consecutivos no-quirúrgicos en el hospital de maternidad del gobierno principal de Kingston, Jamaica, entre junio de 1973 y diciembre de 1981.
Resultados:
En general se presentó priapismo en 52 pacientes (32.7%), produciéndose un aumento abrupto de la incidencia a 32% en la adolescencia tardía a los 20 años de edad, y una incidencia acumulada de casi el 60% en los pacientes a la edad de 40 años. Muchos casos fueron conocidos solamente gracias a un interrogatorio directo, debido a lo embarazoso del asunto y la falta de conciencia de que el priapismo complica la ECF. Los eventos iniciales fueron: episodios recurrentes de priapismo en 39 pacientes, un solo evento a corto plazo en seis pacientes, y un ataque mayor (de más de seis horas) en siete pacientes. La función eréctil fue preservada en casi todos los pacientes con simple recurrencia o eventos únicos. Los ataques mayores (> 6 horas) ocurrieron en 17 pacientes, precedidos por episodios recurrentes en nueve, por un solo evento en uno, y ocurriendo de novo en siete. En estos, la función eréctil fue desconocida en cinco, considerada satisfactoria en cinco (a veces con mejoría en tres años), débiles en tres, en tanto que la impotencia persistió en cuatro (dos con ataques mayores en los tres y seis meses anteriores).
Conclusiones:
Las historias de priapismo deben ser sistemáticamente investigadas, y deben ponerse en práctica medidas profilácticas si se producen ataques más de una vez por semana. Los eventos mayores generalmente causan impotencia a corto plazo, pero la recuperación tardía de la función eréctil advierte que hay que tener cautela en cuanto a una temprana inserción de una prótesis peniana.
INTRODUCTION
Priapism, an involuntary and usually painful penile erection, is a major problem in patients with homozygous sickle cell (SS) disease, and is most common in adolescence and early adult life. There are two principal patterns, stuttering episodes and major attacks. Stuttering episodes are predominantly nocturnal, last three to four hours, are relieved by simple physical procedures such as cold showers or exercise, and patients maintain normal erectile function. Major attacks last more than six hours and sometimes for several days, are often excruciatingly painful, and tend to be followed by permanent damage to the vascular erectile system and impotence. Stuttering episodes are inconvenient since they interfere with sleep and lead to daytime sleepiness but, more seriously, may be a prodrome for major attacks. These events are under-reported partly because of embarrassment but also because patients do not realize that they are caused by sickle cell disease. It is therefore good practice in the management of sickle cell disease to question patients directly about these events, and routine questionnaires found these events in 40% of post-pubertal males attending sickle cell clinics (1, 2). A large bibliography has accumulated on priapism in sickle cell disease but most are case reports or small series focussing on risk factors and treatment of priapism. Almost all of this experience has been derived from symptomatically acquired patients with many inherent biases. In a representative sample of patients followed from birth, there is little information on the prevalence of stuttering or major attacks, the relationships between these events and their final outcome in terms of erectile function. This information has now been provided in the Jamaican Cohort Study where, following newborn diagnosis, patients have been systematically reviewed for the last 33-40 years.
SUBJECTS AND METHODS
The patients participated in the Jamaican Cohort Study which followed all cases of homozygous sickle cell (SS) disease detected during the screening of 100 000 consecutive deliveries at the main government maternity hospital, Victoria Jubilee, between June 25, 1973 and December 28, 1981 (3). A total of 315 subjects with SS disease were identified, of whom 311 (162 males) were located and recruited to the study. All subjects were scheduled for review at regular intervals (monthly to six months, alternate months to one year, three monthly to five years, six monthly thereafter) and efforts were made to locate defaulting patients, although some losses were inevitable (58 deaths, 36 emigrated, 1 default). The presented data were derived from examination of prospectively recorded data in dedicated dockets in the Sickle Cell Clinic at The University of the West Indies, Kingston, Jamaica, and interviews conducted with 65 patients (61 in Jamaica, and by phone with four emigrated patients in the United Kingdom or United States of America) during the study period (August 2012–August 2013).
Diagnostic criteria
The diagnosis of SS disease was based on a major haemoglobin band in the position of HbS and the absence of HbA on both alkali and acid haemoglobin electrophoresis, HbA2 levels below 4.5% and supported by the presence of HbS in all tested parents. Priapism was defined as an involuntary painful erection but focussed interviews revealed events sometimes as short as several minutes, so for the present study, stuttering priapism was arbitrarily defined as 30 minutes or longer.
Statistical methods
Time from birth to first occurrence of priapism is presented using a Kaplan-Meier curve and three alternative priapism end-points: any priapism, stuttering priapism, major priapism. A formal logrank test for ‘time to priapism’ differences between the three subgroups is not presented as stuttering or major priapism are subsets of the full priapism group and nine of 17 patients with major priapism had preceding stuttering priapism.
RESULTS
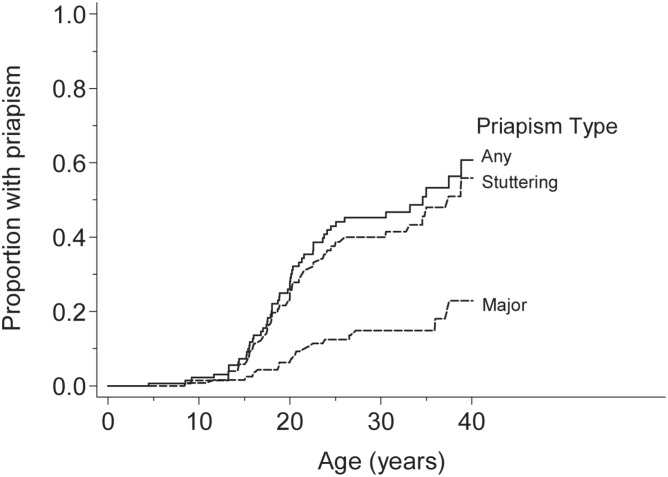
Priapism was reported in 52 patients (Table 1) or 32.7% of male patients followed from birth. Survival curve analysis showed that events were rare before age 10 years, rose steeply in late adolescence to affect 32% by 20 years, and a cumulative incidence of nearly 60% of patients by the age of 40 years (Fig. 1). Initial presentation was recurrent stuttering episodes in 39 patients, a single episode in six patients, and a major attack in seven patients.
Table 1. Summary of patients reporting priapism ranked by age of onset.
| # | Age (yrs) | Initial episode | Subsequent course | Last seen | Children | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | Duration | Period | Major | Post major | Erections | status | age | before | after | |||
| 1 | 8.5 | Major (3d) | no attacks before/since | 0 | – | Unknown | Died | 13.2 | ? | ? | ||
| 2 | 11.6 | Major (6d) | no attacks before/since | 0 | – | Good | Live | 34.4 | 0 | 1 | ||
| 3 | 13.3 | Stuttering | 2–5/w | 1–3h | 10m | 0 | – | Good | Emig. | 19.1 | ? | ? |
| 4 | 13.3 | Stuttering | 2–3/m | 3–4h | 3y | 16.0y–6d | – | Good | Died | 26.5 | 0 | 1 |
| 5 | 13.3 | Stuttering | 1/w | 1–2h | 7y | 20.5y–2d | Impotent 3y | Weak | Live | 31.2 | 2 | 1 |
| 6 | 13.9 | Stuttering | 1–2/m | – | 3y | 0 | – | Unknown | Died | 25.2 | 0 | 0 |
| 7 | 14.4 | Stuttering | ? | ? | ? | 0 | – | Unknown | Emig. | 14.6 | ? | ? |
| 8 | 15.2 | Major (4d) | no attacks before/since | 0 | – | Weak | Emig. | 38.8 | 0 | 0 | ||
| 9 | 15.2 | Stuttering | 3–4/w | 1–6h | continue | 0 | – | Good | Live | 37.8 | 0 | 0 |
| 10 | 15.3 | Stuttering | 2/m | 1–2h | 2y | 0 | – | Good | Live | 35.9 | 0 | 0 |
| 11 | 15.5 | Stuttering | ? | ? | 1y | 16.4y | Impotent | Nil | Live | 33.4 | 0 | 0 |
| 12 | 15.6 | Stuttering | 1–2/m | 3h | 4m | 0 | – | Good | Died | 24.5 | 0 | 0 |
| 13 | 15.8 | Stuttering | 2–3/w | 2–3h | 6y | 27.2y–5d | Impotent | Nil | Live | 39.8 | 0 | 0 |
| 14 | 15.9 | Stuttering | 1/m | 1h | 5y | 0 | – | Good | Died | 34.7 | 0 | 0 |
| 15 | 16.0 | Stuttering | 1/y | 1h | continue | 0 | – | Good | Live | 32.7 | 0 | 2 |
| 16 | 16.5 | Stuttering | 4–5/w | 2–3h | 5y | 0 | – | Good | Live | 33.9 | 0 | 1 |
| 17 | 16.6 | Stuttering | 1–2/w | 5h | continue | 0 | – | Good | Live | 35.6 | 0 | 3 |
| 18 | 16.8 | Stuttering | 1–2/m | 2–3h | continue | 0 | – | Good | Live | 28.8 | 0 | 0 |
| 19 | 17.0 | Stuttering | 1/m | 0.5h | continue | 0 | – | Good | Live | 31.3 | 0 | 0 |
| 20 | 17.5 | Stuttering | 1–2/m | 0.5–1h | continue but les | 0 | – | Good | Live | 34.1 | 0 | 0 |
| 21 | 17.8 | Stuttering | 2–4/m | 0.5–1h | continue | 0 | – | Good | Live | 34.7 | 0 | 2 |
| 22 | 17.8 | Stuttering | 1–2/m | 2–3h | 2y | 0 | – | Good | Live | 36.7 | 0 | 1 |
| 23 | 17.9 | Single | – | 2h | – | 0 | – | Good | Emig. | 26.7 | 0 | 1 |
| 24 | 17.9 | Stuttering | 1–2/m | 3–5h | 4y until | 21.8y | ? | Unknown | Emig. | 21.8 | ? | ? |
| 25 | 18.2 | Stuttering | 2–3/y | – | 5y | 0 | – | Good | Live | 31.1 | 0 | 0 |
| 26 | 18.4 | Single | – | – | – | 18.8y–14h | Stuttering 4y | Unknown | Died | 22.6 | 0 | 0 |
| 27 | 18.8 | Major (2d) | details unknown | 0 | – | Unknown | Live | 38.2 | 0 | 0 | ||
| 28 | 19.7 | Stuttering | 5–6/y | 30m | continue but les | 0 | – | Good | Live | 36.3 | 0 | 0 |
| 29 | 19.8 | Stuttering | 2–3/w | 1–2h | 8y | 0 | – | Good | Emig. | 29.1 | 0 | 0 |
| 30 | 20.0 | Stuttering | 4/y | ? | 5y | 0 | – | Good | Live | 31.7 | 0 | 1 |
| 31 | 20.0 | Major (4d) | ? | 0 | ? | Unknown | Dead | 25.2 | 0 | 0 | ||
| 32 | 20.0 | Stuttering | 4-5/w | 3–4h | 8m | 20.7y–2d | Impotent 8m | Good | Live | 37.0 | 0 | 3 |
| 33 | 20.3 | Stuttering | 2-3/m | 1h | 3y | 0 | – | Good | Live | 34.8 | 0 | 0 |
| 34 | 20.7 | Stuttering | 2/w | 1-2h | 10y | 0 | – | Good | Emig. | 34.8 | 0 | 0 |
| 35 | 21.3 | Stuttering | ? | 0.5h | 3y | 0 | – | Good | Live | 37.2 | 0 | 0 |
| 36 | 21.5 | Stuttering | 2/w | 0.5-1h | continue but les | 0 | – | Good | Live | 34.5 | 0 | 1 |
| 37 | 21.9 | Stuttering | 1/w | 1–2h | 5y | 0 | – | Good | Live | 39.0 | 0 | 1 |
| 38 | 22.0 | Single | – | 5h | – | 0 | – | Good | Live | 32.2 | 0 | 1 |
| 39 | 22.5 | Major (2d) | – | – | – | stuttering 4m | Weak | Died | 30.3 | 0 | 0 | |
| 40 | 22.6 | Stuttering | 2/w | 1–2h | 2m | 0 | – | Unknown | Emig. | 26.7 | 0 | 0 |
| 41 | 23.8 | Stuttering | 1/w | 2–3h | 1m | 23.9y–2d | Impotent 3m | Good | Live | 36.8 | 0 | 0 |
| 42 | 24.1 | Stuttering | 1–2/m | ? | 13y | 37.5y–2d | Impotent 6m | Nil | Live | 37.9 | 0 | 0 |
| 43 | 24.1 | Stuttering | 2–3/y | 5h | continue | 0 | – | Good | Live | 25.4 | 0 | 0 |
| 44 | 25.0 | Stuttering | 1/y | 0.5–1h | 8y | 0 | – | Good | Live | 34.9 | 0 | 0 |
| 45 | 26.0 | Single | – | ?h | – | 0 | – | Good | Live | 32.7 | 2 | 1 |
| 46 | 26.5 | Major (1d) | – | – | – | 27.6, 28.8y–3d | – | Good | Live | 31.0 | 0 | 0 |
| 47 | 31.9 | Stuttering | 2–3/w | 1–2h | 6y | 36.0y-2d | Impotent 3m | Nil | Live | 36.2 | 0 | 0 |
| 48 | 33.2 | Stuttering | 1/m | 0.5h | continue | 0 | – | Good | Live | 35.2 | 1 | 0 |
| 49 | 34.6 | Single | – | 1h | – | 0 | – | Good | Live | 36.0 | 0 | 0 |
| 50 | 35.0 | Stuttering | variable | 2h | continue | 0 | – | Good | Live | 37.0 | 0 | 0 |
| 51 | 37.5 | Single | – | 3h | – | 0 | – | Good | Live | 37.9 | 0 | 0 |
| 52 | 38.8 | Stuttering | 2 attacks | 1h | – | 0 | – | Good | Live | 39.1 | 0 | 0 |
Fig. 1. Survival curve analysis of time to first episode of priapism.
Stuttering episodes
Of the 39 with recurrent stuttering episodes, 30 ran an uncomplicated course but nine proceeded to have major attacks after a median interval of four years (range 0.1-13.4 years). Of the six patients reporting single events, five resolved without recurrence but one developed a major attack four months later which lasted 14 hours and was followed by stuttering episodes (case 26).
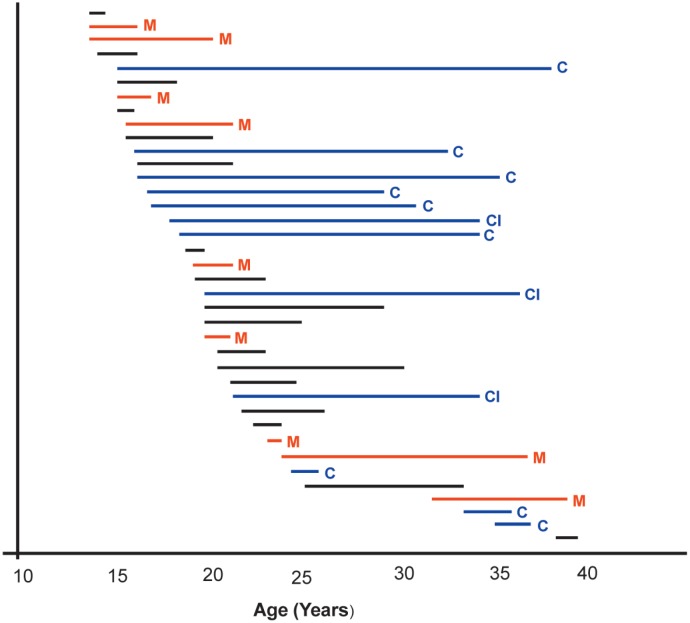
The frequency of stuttering episodes varied from four to five per week to one per year and the duration of individual events from 0.5-6 hours. Overall duration of priapism was available in 38 of 39 patients with stuttering priapism. Of these, priapism ceased in 16 subjects after a median period of 4.0 years (range 0.3-10 years) 4-17 years earlier but continued in 12 patients over intervals of 1-22.6 years, although they were becoming less frequent in three patients (Fig. 2). There were no recognized precipitating factors, and relieving factors were cold or iced water in 11 (showers, bath, or iced water splashed on the penis or over the back), exercise in five, and ganja in two.
Fig. 2. Duration of priapism history in 38 patients with stuttering episodes and adequate data.
C: priapism continues at time of interview
CI: priapism continues but reducing in frequency
M: Major attack
Major attacks
These occurred in 17 patients: seven as initial events, nine followed stuttering episodes, and one was preceded by a single short-term event. Duration of major attacks did not differ between those presenting as initial events and those preceded by stuttering priapism. Assessing the risk of a major attack in relation to the frequency of stuttering episodes, major attacks were not seen in six patients reporting 1-6 events/year, but occurred in 2/11 (18%) with 1-2 events/month, 1/3 (33%) with 2-3 events/month, 2/7 (29%) with events weekly and in 3/7 (43%) with ≥ 2 events weekly. In individual patients, major attacks may follow increasing frequency of stuttering episodes (case 47). Recurrence of major attacks was rare, although one patient had three attacks (case 46).
Subsequent erectile function
Of the 30 patients with stuttering episodes not progressing to major attacks, erectile function was satisfactory in 27 and unknown in three (one dead, two emigrated) [Fig. 2]. Of the nine patients in whom stuttering episodes progressed to major attacks, erections were deemed satisfactory in three (two had impotent periods of three and eight months), weak in one (impotence persisted for three years but appeared to be gradually resolving), impotence continued in four (major attacks having occurred 0.3, 0.5, 9 and 17 years earlier), and the outcome was unknown in one. In the seven patients reporting initial major attacks, the outcome was unknown in three (one with severe psychiatric disorder, two dead), erections were deemed good in two and weak in two. In the six patients with initial single events, five had satisfactory erections and outcome was unknown in one who developed a major attack and died four years later. Data on conception of children after the onset of priapism were available in 48/52, of whom 33 had no children, 11 had one child, two had two children and two had three children. Of the seven patients with initial major attacks, one had a single child, five had none and outcome was unknown in one; of the nine major attacks preceded by stuttering events, outcome was unknown in one, six had no children, two had single children and one had three children. In the patient with a major event following a single stuttering episode, there were no children.
Therapeutic interventions
For prophylaxis of stuttering episodes, diethylstilboestrol, previously shown to be effective in a controlled trial (4), was used in nine patients, six to control initial stuttering episodes (cases 9, 13, 24, 29, 32, 36), two patients with post-major stuttering episodes (cases 26, 39) and in one, it was used, probably inappropriately, in a major attack at an unusually early age (case 1). Of the 17 patients with major attacks, seven patients (cases 5, 13, 26, 31, 42, 46, 47) had surgical drainage, involving aspiration, irrigation, occasionally dorsal slits or spongioso-cavernosal shunts, and 10 received conservative therapy or refused surgery. With the exception of case 13 in whom surgery was complicated by infection, there was no obvious relationship between intervention and long-term outcome.
CASE REPORTS
Case 11: Long-term impotence: Stuttering priapism from age 15.5 years was followed by a major attack at 16.4 years associated with a paraphimosis which was surgically corrected. He remains impotent 17 years later although a drug-induced psychosis complicates assessment of his history.
Case 13: Long-term impotence: Stuttering priapism from age 15.8 years increased in frequency over eight months, resolved spontaneously for three years, and recurred at age 22 years with episodes controlled by diethylstilboestrol. There were no further episodes until a major attack for five days at age 27.2 years with admission for shunting surgery which became infected and was followed by total impotence persisting 13 years later.
Case 26: Stuttering events post major attack: A single event of stuttering priapism at age 18.4 years was followed four months later by a major attack lasting 14 hours when he was admitted and received surgical drainage. Seven months later, stuttering priapism developed with intermittent attacks controlled by diethylstilboestrol until his death from acute chest syndrome at age 22 years.
Case 46: Multiple major attacks: Admitted at age 26.5 years for a major attack lasting one day, priapism was treated conservatively and resolved until admission for a second major attack at age 27.6 years (details unknown as hospital docket untraceable). At age 28.8 years, there was a third admission for a major attack lasting three days which was treated by aspiration, irrigation, dorsal slits and, finally, spongioso-cavernosal shunt. He was discharged after 13 days with apparent resolution and when interviewed three years later, claimed to have erections for satisfactory intercourse.
Case 47: Increasing stuttering events preceding major attack: His wife stated that from 2008, he would get attacks once per week relieved by exercise or cold showers but these gradually increased in frequency to three to four per week until a major attack requiring hospital admission occurred in May 2013. Since then he has been impotent.
DISCUSSION
Priapism in homozygous sickle cell (SS) disease is a common complication underestimated partly because of embarrassment but also because patients do not realize that it is caused by the disease. Clinical management of patients should therefore include direct enquiries prefaced by the acknowledgement that this is a common and potentially serious condition. In the present study, direct questioning revealed several previously un-reported events resulting in an overall prevalence of 32.7% of male patients and a cumulative incidence of 60% by the age of 40 years.
Stuttering priapism, uncomplicated by major events, tended to resolve completely with normal erectile function consistent with the 94% subsequent potency rate recorded elsewhere (5). Major events had a less favourable and less predictable outcome, although of the 17 affected patients, subsequent erectile function was known in 12 (adequate in five, poor in three, impotent in four). In some patients with impotence initially, erectile function recovered gradually; three patients regained erectile function after impotent periods of three months and eight months and one reported improving erections up to three years later. Of the four patients with impotence, two had had major attacks only three and six months earlier and the impotence might resolve but two remained impotent 12 and 17 years later, one in whom surgery was complicated by infection and another in whom a psychiatric disorder complicated assessment. Late recovery of erectile function cautions against the early introduction of penile pros-theses despite the fact that such surgery is technically easier in patients before fibrosis of the corpora cavernosa occurs. The relatively poor outcome of major attacks indicates that these should be avoided if at all possible, although with the exception of the small trial on diethylstilboestrol (4), there is currently little convincing evidence of the effectiveness of the many proposed prophylactic therapies (6). Stuttering episodes greater than once weekly may predict major attacks, similar to the observation of Emond et al (1) who noted that 28% of patients with stuttering events proceeded to a major attack. These patients should receive prophylaxis to control stuttering episodes, although this would not be possible in the seven presenting with an initial major attack. Observations on children born to patients after attacks of priapism required cautious interpretation as these were sometimes limited by the short duration of follow-up before death or emigration and although most offspring carried the HbS gene, paternity was not confirmed by DNA.
These observations on the natural history of priapism have been made possible because of the unique resource available in the Jamaican Cohort Study which has allowed serial observations of patients from birth to the age of 40 years. Although priapism is much less frequent in patients with the Asian haplotype of SS disease (7, 8), the disease in Jamaica is predominantly of the Benin haplotype and these observations may guide the management of the disease in areas associated with African haplotypes of the disease. Most medical practitioners would agree that the management of priapism is unsatisfactory despite the large number of suggested approaches and therapies, few of which have been subjected to properly controlled trials (9). It is believed that the current data on the natural history of this complication will provide the basis against which to judge such therapies in the future.
ACKNOWLEDGEMENTS
Much of these data were collected during the tenure of the Medical Research Council Laboratories, The University of the West Indies, Kingston, Jamaica, from 1973 to 1999. The authors are grateful to Dr Jennifer Knight-Madden, currently Director of the Sickle Cell Unit for access to data collected after this period. This work was supported in part by the National Health Fund of Jamaica and the Alcoa Foundation. These organizations supported the infrastructure of the study but did not participate in its writing or execution.
REFERENCES
- 1.Emond A, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med. 1980;140:1434–1437. [PubMed] [Google Scholar]
- 2.Fowler JE, Koshy M, Strub M, Chinn SK. Priapism associated with sickle cell hemoglobinopathies: prevalence, natural history and sequelae. J Urol. 1991;145:65–68. doi: 10.1016/s0022-5347(17)38248-4. [DOI] [PubMed] [Google Scholar]
- 3.Serjeant GR, Serjeant BE, Forbes M, Hayes RJ, Higgs DR, Lehmann H. Haemoglobin gene frequencies in the Jamaican population: a study of 100,000 newborns. Br J Haematol. 1986;64:253–262. doi: 10.1111/j.1365-2141.1986.tb04117.x. [DOI] [PubMed] [Google Scholar]
- 4.Serjeant GR, DeCeulaer K, Maude GH. Stilboestrol and stuttering priapism in homozygous sickle cell disease. Lancet. 1985;2:1274–1276. doi: 10.1016/s0140-6736(85)91555-7. [DOI] [PubMed] [Google Scholar]
- 5.Hamre MR, Harmon EP, Kirkpatrick DV, Stern MJ, Humbert JR. Priapism as a complication of sickle cell disease. J Urol. 1991;145:1–5. doi: 10.1016/s0022-5347(17)38229-0. [DOI] [PubMed] [Google Scholar]
- 6.Kato GJ. Priapism in sickle-cell disease: a hematologist's perspective. J Sex Med. 2012;9:70–78. doi: 10.1111/j.1743-6109.2011.02287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kar BC, Satapathy RK, Kulozik AE, Kulozik M, Sirr S, Serjeant BE, et al. Sickle cell disease in Orissa State, India. Lancet. 1986;2:1198–1201. doi: 10.1016/s0140-6736(86)92205-1. [DOI] [PubMed] [Google Scholar]
- 8.Padmos MA, Roberts GT, Sackey K, Kulozik A, Bail S, Morris JS, et al. Two different forms of homozygous sickle cell disease occur in Saudi Arabia. Br J Haematol. 1991;79:93–98. doi: 10.1111/j.1365-2141.1991.tb08013.x. [DOI] [PubMed] [Google Scholar]
- 9.Chinegwundoh F, Anie KA. Treatments for priapism in boys and men with sickle cell disease. Cochrane Database Syst Rev. 2004;18 doi: 10.1002/14651858.CD004198.pub2. CD004198. [DOI] [PubMed] [Google Scholar]