Abstract
Sour taste is detected by taste receptor cells that respond to acids through yet poorly understood mechanisms. The cells that detect sour express the protein PKD2L1, which is not the sour receptor but nonetheless serves as a useful marker for sour cells. By use of mice in which the PKD2L1 promoter drives expression of yellow fluorescent protein, we previously reported that sour taste cells from circumvallate papillae in the posterior tongue express a proton current. To establish a correlation between this current and sour transduction, we examined its distribution by patch-clamp recording. We find that the current is present in PKD2L1-expressing taste cells from mouse circumvallate, foliate, and fungiform papillae but not in a variety of other cells, including spinal cord neurons that express PKD2L1. We describe biophysical properties of the current, including pH-dependent Zn2+ inhibition, lack of voltage-dependent gating, and activation at modest pH values (6.5) that elicit action potentials in isolated cells. Consistent with a channel that is constitutively open, the cytosol of sour taste cells is acidified. These data define a functional signature for the taste cell proton current and indicate that its expression is mostly restricted to the subset of taste cells that detect sour.—Bushman, J. D., Ye, W., Liman, E. R. A proton current associated with sour taste: distribution and functional properties.
Keywords: PKD2L1, ion channel, pH, acid, sensory transduction
Sensory receptors expressed by taste receptor cells of the tongue and palate epithelium respond to ingested compounds and transduce their chemical properties into a biologic response to predict nutritive quality and to distinguish between edible and inedible compounds. Receptors that mediate sweet, bitter, umami, and salty taste in mammals have mostly been discovered, whereas the receptors for sour remain unknown (1, 2). Sour contributes to the rejection of unripe or spoiled foods and is elicited by acids found in these foods. Genes expressed in taste cells that encode for proteins sensitive to pH changes have been reported, including several selectively expressed in sour responsive taste cells (3–7), but evidence that any of these contributes significantly to sour taste is still lacking (8). Most recently, it was proposed that PKD2L1, a protein that is a member of the transient receptor potential family of ion channels, was a component of a sour receptor (9). Indeed, the expression of Pkd2l1 in the tongue is restricted to type III taste cells that detect sour (9–11). Pkd2l1 gene expression has also been detected in a subset of spinal neurons whose cell bodies lie close to the central canal and which respond to acidic pH with action potentials (10). Despite the association of the expression of Pkd2l1 with sensitivity to acids, knockout of PKD2L1 in mice does not eliminate neural responses to sour stimuli, indicating that PKD2L1 is not a required component of the sour receptor (12, 13). Nonetheless, PKD2L1 expression serves as a useful marker of sour-responsive taste cells.
In search of the ionic mechanism of sour taste transduction, we recently identified an inward current that was evoked in response to extracellular acidification in PKD2L1-expressing taste cells (14). The magnitude and reversal potential of this current were unchanged when the concentration of extracellular Na+ and Ca2+ or the concentration of intracellular Cl− were varied, leading us to conclude that it was carried by protons. This current reached several tens of picoamperes in response to acidic solutions (e.g., pH 5), and similar to many proton conducting proteins (15), it was sensitive to block by extracellular Zn2+. A similar current was not observed in taste cells that express TRPM5, which marks the population of bitter, sweet, and umami responsive cells (16, 17). Based on the observation that action potentials (APs) could be elicited in response to apical delivery of protons in the absence of other permeant cations and that this response was blocked by Zn2+, we proposed that the proton current mediates sour taste transduction (14). Consistent with a major contribution of this current to sour taste, Zn2+ blocks the gustatory nerve response to strong acids (18).
To further characterize this novel current, we now investigated its distribution in a variety of cells types and have measured some of its biophysical characteristics. Our data support the view that the proton current is specifically associated with sour taste receptor cells in the tongue, where it may participate in sour transduction. It is noteworthy that it is absent from a variety of other cells types, including the spinal neurons that express PKD2L1. We have also conducted the first in depth analysis of the biophysical properties of this current. Our results show that the current is blocked by Zn2+ in a pH-dependent manner, is open over a wide range of extracellular pH values and is not gated by voltage. Consequently, as expected for an open proton conductance, the intracellular pH (pHi) of sour taste cells is acidified compared with cells that mediate other taste sensations. These observations extend our previous characterization of this ionic current (14) and provide clues in the search for its molecular identity.
MATERIALS AND METHODS
Animals
Mouse care, euthanasia, and tissue collection procedures were followed as described previously (14), with the approval from the University of Southern California Institutional Animal Care and Use Committee. Transgenic mouse strains in which yellow fluorescent protein (YFP) is driven by the PKD2L1 promoter and green fluorescent protein (GFP) is driven by the TRPM5 promoter were described previously (14, 19, 20). The 2 strains were mated to generate a double reporter transgenic line, and genotype was determined as described previously (14).
Cell culture
Cell lines were grown in culture for ≥1 wk after thawing before use in patch-clamp experiments. Culture media for each cell type were as follows: DMEM for HEK 293, Min6, and STC-1; DMEM/F12 1:1 for CHO-K1; Eagle’s minimal essential medium for Neuro2A; and SF-900 II SFM for SF-9; all media contained 10% fetal bovine serum and 0.05% gentamicin. Cells were lifted and isolated using 0.05% trypsin in PBS and passaged or plated for recording. All cell culture reagents were from Life Technologies (Grand Island, NY, USA), with the exception of PBS (Invitrogen, Grand Island, NY, USA) and Eagle’s minimal essential medium (Sigma-Aldrich, St. Louis, MO, USA).
Solutions
Solutions for electrophysiology were exchanged using a piezo-driven stepper motor system (SF-77B; Warner Instruments, Hamden, CT, USA) to move a linear array of square capillary tubes. Tyrode’s solution contained (mM) 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] (HEPES) and 10 d-glucose, pH 7.4. Sodium solution was (mM) 150 NaCl, 10 HEPES, and 2 CaCl2, pH 7.4. In testing the distribution of proton currents, NaCl was replaced with 150 tetraethylammonium chloride (TEACl) or 160 N-methyl-d-glucamine (NMDG), with pH adjusted with TEAOH or HCl, respectively. For solutions of pH 6.7 to pH 4.5, 2-N-morpholino-ethane sulfonic acid (MES) replaced HEPES, and pH was adjusted with HCl. Unless otherwise stated, the pipette solution contained (mM) 120 CsAsp, 8 NaCl, 7 CsCl, 10 HEPES, 2 MgATP, 0.3 Tris-GTP, 2.4 CaCl2, and 5 EGTA (100 nM free Ca2+), pH 7.4. For the experiments shown in Figs. 6, 7C; 8A, B; and 9C–G, the extracellular solution contained (mM) 130 TEA-methane-sulfonic acid (MA), 20 TEACl, 10 HEPES, and 2 CaCl2, pH 7.4, and the pipette solution contained (mM) 115 TEA-Asp, 20 TEACl, 10 HEPES, 2 MgATP, 0.3 Tris-GTP, 2.4 CaCl2, and 5 EGTA (100 nM free Ca2+), pH 7.4. In Fig. 6F, 10 HEPES was replaced by 10 MES, and the pH was adjusted to 6.1; correspondingly, CaCl2 was reduced to 8 μM to maintain the same nanomolar free Ca2+ at this pH. For experiments showing Zn2+ block, Zn2+ was 1 mM, unless stated otherwise.
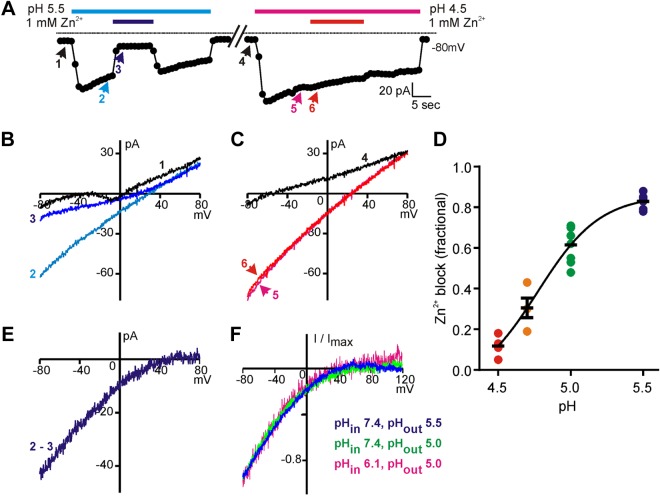
Figure 6.
Zn2+ blocks the proton current in a pH-dependent manner. A) Representative example of the block by 1 mM Zn2+ of current evoked in a PKD2L1 taste cell in response to pH 5.5 and pH 4.5 in TEA-MA–based solution. Note that Zn2+ blocks a larger percentage of the acid-evoked current in pH 5.5 than in pH 4.5. B, C) The I-V relationships measured at the indicated time points from the trace in A. D) The fraction of the acid-evoked currents blocked by 1 mM Zn2+ at each indicated pH; data from all cells is plotted, along with the means ± sem. The data were fit with a dose-response curve with a Hill coefficient of 2.0 and IC50 of pH 4.8. E) The I-V relationship of the Zn2+-blocked component of the proton current in B, obtained by subtracting the current recorded in the presence of Zn2+ from the current recorded in its absence. F) The averaged normalized I-V relationships of the Zn2+-sensitive currents recorded at the pHout and pHin indicated (n = 2–7).
Figure 7.
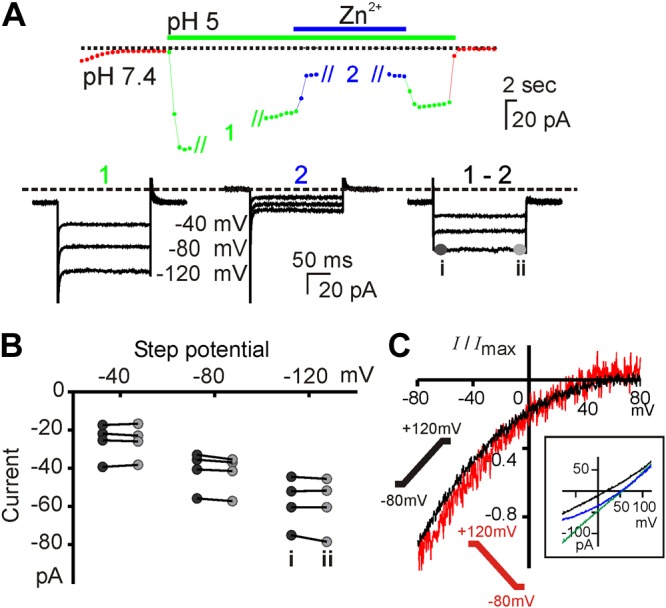
Lack of voltage dependence to the activation of the taste cell proton conductance. A) The current (Vm = −80 mV) in a PKD2L1 taste cell evoked in response to pH 5 extracellular solution (green and blue symbols; TEA-based solution). During stimulus applications 1 and 2, the recording protocol was changed to 200 ms voltage steps from a holding potential of 0 mV to voltages shown. Subtraction of 1 – 2 gives the Zn2+-sensitive component of the proton current. B) Summary data of the current amplitude measured at the beginning (i) and end (ii) of each voltage step from 4 cells. Comparison of i and ii for each voltage was not significant using multiple 2-tailed Student's t tests with Sidak-Bonferroni correction. C) The Zn2+-sensitive current evoked by pH 5 solution measured in response to a depolarizing ramp (−80 to +120 mV; 1 V/s) from a holding potential of −80 mV (black) or hyperpolarizing voltage ramp from a holding potential of 0 mV (red) from experiments as in Fig. 6. Average of normalized traces from 4 experiments. Inset) I-V relationship of 1 cell under the reversal ramp in pH 7.4 (black), pH 5 (green), and pH 5 with 1 mM Zn2+ (blue). Note that there was no difference in rectification under the 2 conditions.
Figure 8.
Proton channels in sour taste cells are open near neutral pH. A) Current evoked in a PKD2L1-YFP taste cell to extracellular acidification (TEA-MA based) from a solution at pH 7.4. The current elicited at pH 6.5 is completely blocked by 1 mM Zn2+. B) Current amplitude plotted against H+ concentration (n = 4 cells). The relationship is linear at low concentrations of protons, but saturates beyond pH 6.0. The red curve shows the fit to a one site binding curve with a Kd of 1.9 μM. C) Time series of cytosolic pH (pHi) for PKD2L1 and TRPM5 taste cells loaded with BCECF-AM in Tyrode’s from which resting values of pHi were measured. High-K+ + 20 μM nigericin (pH 6.0 to pH 8.0 in 0.5 pH unit increments) was used to generate a linear calibration curve from which fluorescent intensities were converted to pHi. D) Scatterplot of resting pHi in Tyrode’s pH 7.4 determined as in C. ***P < 0.001, 1-tailed Student's t test.
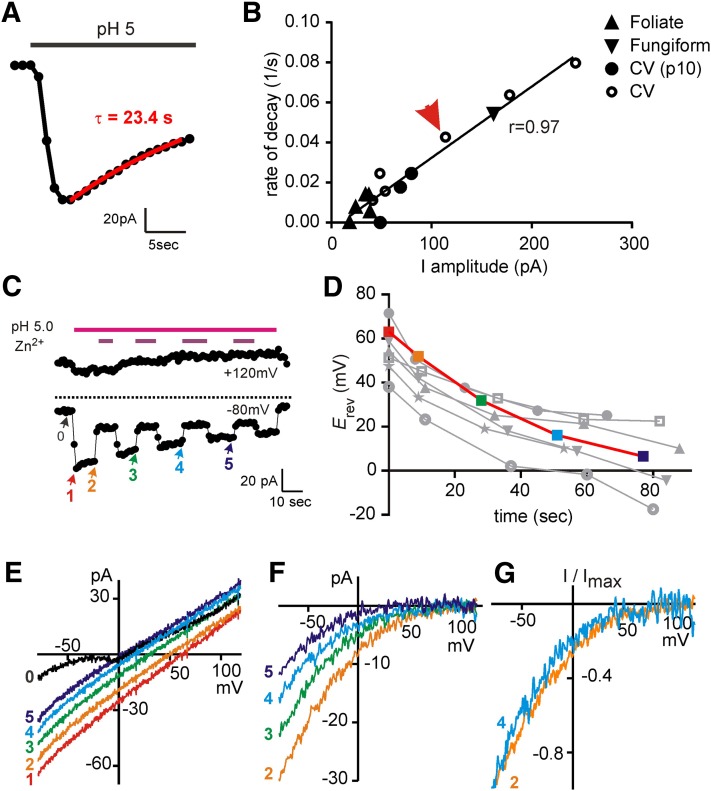
Figure 9.
Time-dependent decay of the proton currents. A) Representative data showing the activation and decay of the proton current in the presence of a steady stimulus. The decay was fit with a monoexponential function with a time constant of 23.4 s. B) Plot of the rate of current decay as a function of current amplitude. Each point represents data from a different cell isolated from the taste field indicated (same cells as in Fig. 2). There is a linear relationship with the correlation coefficient shown. Arrow denotes a representative cell from A. C) The current magnitude as a function of time for a representative cell (TEA-MA–based solution). Zn2+ was added every 20 s to allow leak subtraction. D) The reversal potential of the current elicited as in C with no leak subtraction (n = 7; each symbol represents data from a different cell, with the cell shown in C highlighted in color). E) The unsubtracted I-V relationships from the experiment in C at the time points indicated. Note that Erev is shifted leftward as plotted in D. F) The I-V relationships of the Zn2+-sensitive component of the current at the time points indicated in C. G) The Zn2+-sensitive component of the current at 2 time points, normalized to the current at −80 mV. Note that the rectification properties do not change as a function of time.
Taste cell isolation
Taste cells were isolated from adult mice (ages 6–10 wk) essentially as described previously (14). In brief, Tyrode’s solution containing 1 mg/ml elastase (Worthington, Lakewood Township, NJ, USA), 2.5 mg/ml dispase II (Roche, Indianapolis, IN, USA), and 1 mg/ml trypsin inhibitor (Sigma-Aldrich) was injected between the epithelium and the muscle of the isolated tongue, which was incubated for 20 min in Tyrode’s solution bubbled with 95% O2/5% CO2. The epithelium was then peeled off from the tongue. For circumvallate papillae, a small piece of epithelium containing the papillae was further incubated in the enzyme mixture for 20 min at room temperature. The reaction was stopped by washing the tissue in Ca2+-free saline. For foliate and fungiform papillae, the epithelium was pinned upside down in a chamber containing Ca2+-free solution, and the taste buds were suctioned out with a fire-polished wide-bore capillary. For both preparations, single cells were isolated by trituration in Tyrode’s solution with fire-polished Pasteur pipettes and were used within 6 h.
Spinal cord tissue isolation and cell dissociation
Animals at ages postnatal days 3–7 were euthanized, and the vertebral column was removed. The spinal cord was isolated either by flushing out the spinal canal into ice-cold PBS using a 20–25 gauge needle, or the vertebral column was cut open in ice-cold PBS with a microdissection scissors and the cord was lifted out by forceps. The cord was chopped into 300–500 μm slices in the transverse plane along the longitudinal axis from approximately the upper thoracic to lower lumbar segments with a McIlwain tissue chopper (Mickle Lab Engineering Company, Goose Green, Guildford, Surrey, United Kingdom). The tissue surrounding the central canal was then dissected either with Vannas microdissection scissors (Roboz Surgical, Gaithersburg, MD, USA) or using a “punch-out” approach with a fire-polished glass Pasteur pipette. Combined dissected tissue was incubated in an enzyme cocktail containing 1 mg/ml elastase (Worthington), 3 mg/ml dispase II (Roche), and 1 mg/ml trypsin (Sigma-Aldrich) in DMEM/F12 medium for 25–40 min. This enzyme cocktail was tested on taste tissue, and the proton current was observed in PKD2L1-positive cells (data not shown). Tissue was centrifuged for 5 min at 1500 g and then resuspended in DMEM/F12 containing 10% fetal bovine serum and 1 μg/ml DNAse (Thermo Fisher Scientific, Pittsburgh, PA, USA), and mechanically dissociated using a fire-polished glass Pasteur pipette. After dissociation, the cell suspension was filtered with a 40 μm nylon cell strainer (BD Falcon, Sparks, MD, USA), centrifuged for 3 min at 1500 g and 2 min at 2500 g, resuspended in Tyrode’s solution, and plated on coverslips. YFP+ cells containing an apical process with a bulbous ending were used within 5 h for patch-clamp recording.
Electrophysiology
Methods were similar to those described previously (14). In brief, isolated cell currents were recorded with loose patch or whole-cell voltage-clamp configurations. Loose patch data were collected gap-free using an applied voltage of 0 mV. For whole cell recording, the membrane potential was held at −80 mV, and voltage ramps from −80 to +80 or +120 mV (1 V/s) were applied every second, unless stated otherwise. Borosilicate glass micropipettes (VWR, South Plainfield, NJ, USA) were fire-polished using a MF-830 microforge (Narishige, Los Angeles, CA, USA). Data were collected with an Axopatch 200B amplifier using pClamp software suite (Axon Instruments, Sunnyvale, CA, USA), and current amplitudes were graphed with Origin 6 (OriginLab, Northampton, MA, USA).
Immunohistochemistry
The methods for taste tissue staining were as described previously (14). For spinal cord tissue collection, the cord was isolated from a P4 mouse by flushing it out of the vertebral column and then chopped into slices as described above. Slices were fixed at room temperature in 4% paraformaldehyde for 30 min and then overnight in 30% sucrose. Slices were cryosectioned at 35 μm in the sagittal plane and collected on coverslips. Sections were permeabilized with 0.1% Triton X-100 in PBS (Sigma-Aldrich) and blocked with normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Sections were incubated with chicken anti-GFP antibody diluted 1:1000 (Aves Labs, Tigard, OR, USA). Secondary antibody was goat anti-chicken conjugated to Alexa-488 diluted 1:1000 (Invitrogen). A 10 μm Z-stack of spinal cord images at ×40 magnification was collected on a Fluoview FV-1000 laser-scanning confocal microscope system and compressed with ImageJ (National Institutes of Health, Bethesda, MD, USA).
RT-PCR from pooled single-cell and tissue cDNAs
Methods for cell collection, reverse transcription, tailing, and amplification were previously described (14). Multiple YFP+ isolated spinal cord neurons were transferred into a PCR tube for RNA processing. RNA from isolated lingual epithelium tissue from the circumvallate (CV) was reverse transcribed with random hexamer primers and treated with TURBO DNase (Invitrogen) to remove genomic DNA. Gene-specific oligonucleotides were designed to anneal to all currently annotated RefSeq gene variants and validated by PCR from cDNA libraries of single PKD2L1 taste cells collected from CV (data not shown). Detection of expression for the following genes was assessed using the indicated oligomers: TRPM5 (NM_020277) with ATGGAGAAACGGAGGAGGG (forward) and TTTCAGGTGTCAGAGGGTGG (reverse); PKD2L1 (NM_181422) with CTGAAGATGCTGGAGAGGAAAGG (forward) and GTAAGTGCCCAGGAAGAGAGG (reverse); PKD1L3 variant 1 (NM_001039700) with CTCGTCTGTTACTATGCCTTCATCC (forward) and CCAGTGTTGTCAGCGTGTTCC (reverse); CAR4 (NM_007607) with CTAGCAAGGAGGACTCGAAGG (forward) and GAGCCATTGTAACGGAAGTAGG (reverse); SNAP25 variant 1 (NM_011428) with TGTCACCTTCTTTCTGTCAACC (forward) and CATCCATTTCACATAGTTTCAGCC (reverse); and GAD1 (NM_008077) with GCCATTGTCTATCTTTGGGCAGG (forward) and TGTGGGACTGGTCAGTGTATCGG (reverse).
pHi measurements
Taste cells were dissociated as described previously (14) from mice containing both TRPM5-expressing GFP+ cells and PKD2L1-expressing YFP+ cells and plated onto a solution chamber coverslip. Cells were washed repeatedly, and images of fluorescent cells were collected before dye-loading to identify TRPM5-GFP and PKD2L1-YFP cells, respectively. GFP+ cells were distinguished from YFP+ cells using a 2′,7′-Bis-(2-Carboxyethyl)-5-(and-6)-Carboxyfluorescein filter set (71015; Chroma Technology, Bellows Falls, VT, USA), taking advantage of the difference between the relative excitation of GFP and YFP at 440 and 495 nm. Cells were subsequently loaded with 2 μM BCECF-AM (Molecular Probes, Grand Island, NY, USA) in Tyrode’s solution, washed repeatedly, and incubated 15 min before start of imaging, per the manufacturer’s recommendations. Cells were alternately excited every 15 s with light of 495 and 440 nm using a Lambda 10-3 optical filter changer (Sutter, Novato, CA, USA), and emission at 530 nm was captured with a Hamamatsu digital CCD camera attached to an Olympus IX71 microscope. At the end of the experiment, the indicator was calibrated with high K+ solution containing (mM) 140 KCl, 5 NaCl, 10 HEPES or 10 MES, 2 CaCl2, 1 MgCl2, 10 glucose, and 0.02 nigericin, with pH adjusted with HCl or KOH (Fig. 8C). BCECF intensity was converted to pHi, using calibration curves constructed by plotting the emission ratio (I490/I440) as a function of the pHi in individual cells assuming that in the presence of the calibration solutions, pHi = pHo. The resting pHi of each cell was determined by linear interpolation (21). Note that because the fluorescent proteins are excited at the wavelengths used and emit at 530 nm, we subtracted the emission in response to excitation at 490 and 440 nm measured before loading with BCECF from the data used for calibration or measuring pHi.
Statistics and analysis
Bar and summary scatterplots were prepared in Prism (GraphPad Softwasre, La Jolla, CA, USA). Significance was determined with a Student's t test unless indicated otherwise.
RESULTS
PKD2L1 taste cells from different parts of the tongue respond to acids with membrane depolarization and action potentials
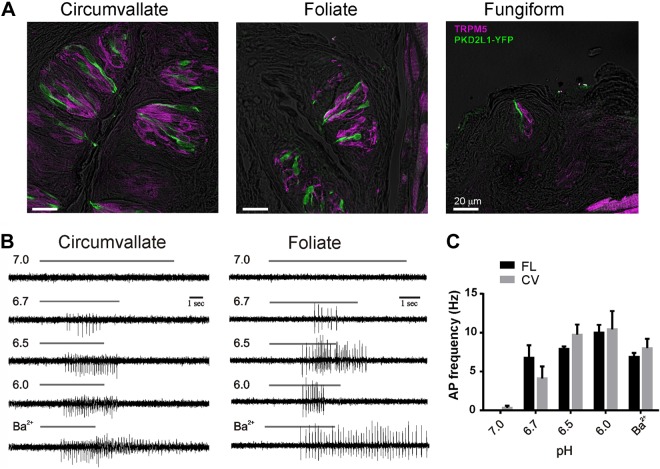
Taste cells that express PKD2L1 and detect sour are located on the palate epithelium and on 3 distinct zones of the tongue: in fungiform (FU) papillae at the front of the tongue, foliate (FL) papillae on the sides of the tongue, and CV papillae at the back of the tongue (Fig. 1A) (9–11). Previously, we have shown that sour cells from the CV, identified by expression of YFP using a PKD2L1-YFP mouse, respond to acids with trains of action potentials (14). To determine whether PKD2L1-expressing taste cells from other parts of the tongue respond similarly to acids and to quantify their sensitivity, we recorded APs in cell-attached mode from cells isolated from CV and FL papillae. In these experiments, extracellular solutions contained (mM) 150 NaCl and 2 CaCl2, buffered with 10 HEPES or 10 MES, and the pH was adjusted to values from 6.0 to 7.4 with HCl. As seen in Fig. 1B, APs could be elicited to a surprising mild acidification of the extracellular solution to pH 6.7, and PKD2L1-expressing cells from CV and FL papillae had similar sensitivity to extracellular acidification. Response in both cells types saturated in frequency by pH 6.5, and further acidification somewhat diminished the amplitude of the APs, most likely because Na+ currents were partly inactivated. The sparse density of PKD2L1-expressing cells in the FU and palate epithelium precluded similar analysis. Together these data reveal an unexpectedly high sensitivity of PKD2L1-expressing taste cells to extracellular acidification.
Figure 1.
PKD2L1 cells from different parts of the tongue fire action potentials in response to acids. A) Confocal image showing the distribution of PKD2L1 cells identified by expression of YFP under the PKD2L1 promoter in each of the major taste fields on the tongue. YFP (green) and TRPM5 (purple) were detected with protein-specific antibodies, and fluorescent images were overlaid onto a differential interference contrast image of the same field (gray). Note that there is no overlap between the 2 populations. B) Action potentials evoked by solutions of varying pH recorded from dissociated PKD2L1 taste cells from the 2 taste fields indicated using the loose patch recording method. Barium (2 mM) was used as a positive control. Each vertical series is from the same cell except for pH 6.7 in foliate was from a different cell than other pHs. C) Summary data of AP frequency in response to acid stimuli; 4 cells were tested for each condition. No significant differences were found between cells from the CV or FL papillae (2-tailed Student's t test).
A proton current is present in all PKD2L1-expressing taste cells
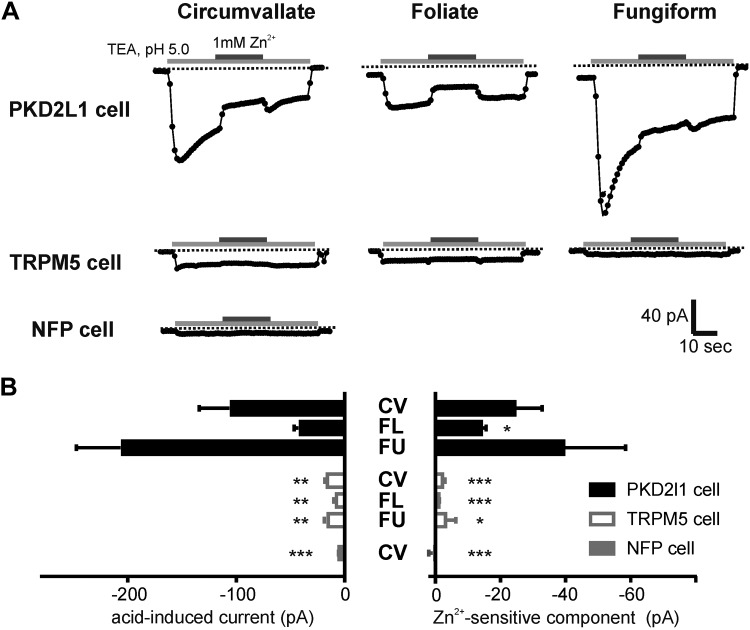
We previously reported the presence of an inward current carried by protons in PKD2L1-expressing cells of the CV (14). To determine whether a similar current is found in PKD2L1-expressing cells from other parts of the tongue, we measured responses to acids in whole-cell voltage clamp. In these experiments, the proton current was elicited by first exchanging a bath solution containing NaCl, pH 7.4, for one containing TEA-Cl, pH 7.4, to eliminate contributions from acid-gated Na+ currents and TEA-sensitive K+ currents. Rapid exchange of the extracellular solution from TEA-Cl, pH 7.4, to TEA-Cl, pH 5.0, then was used to measure the proton current. Under these conditions, a large inward current was observed in PKD2L1-expressing taste cells from all 3 taste fields of adult animals (Fig. 2A). The magnitude of the acid-evoked current varied between cells, from 18 to 243 pA, with a trend toward smaller currents in taste cells from FL papillae and larger currents in taste cells from FU papillae, but these trends did not reach statistical significance (Fig. 2B). We also recorded from 2 cells from the CV papillae of P10 animals and measured proton currents of similar magnitudes to those found in adult cells (data not shown). We previously showed that Zn2+ blocks the proton current in taste cells isolated from the CV (14). Currents recorded from cells in each of the other zones also displayed sensitivity to block by 1 mM Zn2+, although the fractional block by Zn2+ current varied somewhat from cell to cell (Fig. 2A, B). Finally, as a control, we measured responses to the same stimulus protocol using GFP-labeled taste cells isolated from TRPM5-GFP mice and nonfluorescent cells from double transgenic PKD2L1-YFP/TRPM5-GFP mice. Currents recorded in these cells in response to acids were significantly smaller than currents measured from YFP+ cells and did not display Zn2+ sensitivity (Fig. 2A, B). Thus, we can conclude that the proton current is found in PKD2L1-expressing cells from all parts of the tongue but not in TRPM5-expressing or other taste cells.
Figure 2.
A Zn2+-sensitive inward proton current is found in PKD2L1 cells from each of the taste fields on the tongue. A) Time course of the response to pH 5 solution in whole-cell recording mode from different taste cell types identified either by YFP expressed under the PKD2L1 promoter or GFP expression under the TRPM5 promoter or by the absence of either YFP or GFP (NFP) in a double transgenic animal. Cells were bathed in TEA-based solution at pH 7.4 before the stimulus. The voltage was held at −80 mV and ramped from −80 to +80 mV (1 V/s). Symbols show the current at −80 mV. B) Summary data of peak current and Zn2+-sensitive component. There was no significant difference in the magnitude of the currents in PKD2L1 cells from each taste field (2-tailed Student t test). The Zn2+-sensitive component of the current was smaller in FL taste cells (P < 0.05; 2-tailed Student's t test). Significance for GFP+ and NFP+ cells is by comparison with PKD2L1-expressing cells in the same taste field. *P < 0.05, **P < 0.01, ***P < 0.001 (1-tailed Student's t test).
Expression of the proton current may be unique to PKD2L1 taste cells
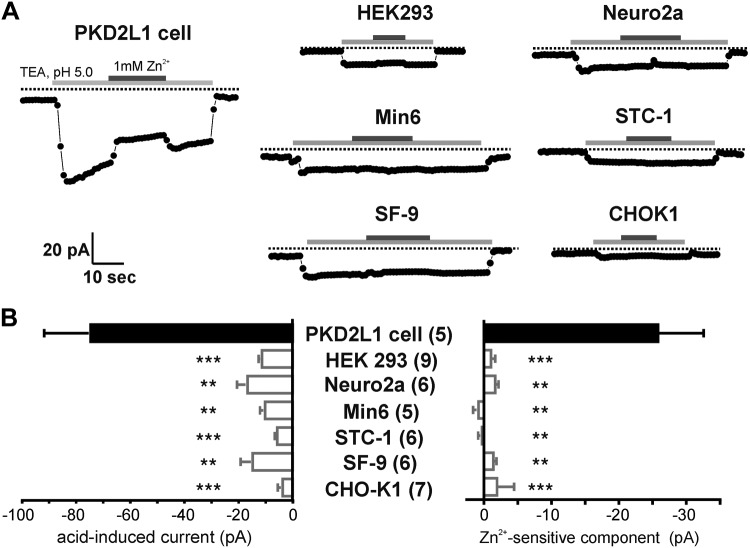
The presence of a proton current in PKD2L1-expressing taste cells could reflect a true enrichment of the current in these cells or its depletion in TRPM5-expressing and other taste cells. Thus, to examine whether the proton current is specifically expressed by PKD2L1-expressing taste cells, we measured responses to acidic pH in a variety of other cell types. We tested 6 cell lines that were chosen either because they are commonly used in laboratories (HEK 293, SF-9, and CHO-K1) or because they have been reported to have features in common with or to be derived from sensory neurons that could be acid sensitive (Neuro2a, Min6, and STC-1). In nearly all cells from all of the cell lines, a small current was evoked in response to exchange of the TEACl solution from pH 7.4 to 5.0 (Fig. 3A, B); this small current may reflect proton entry into the cell, or Cl− efflux, and we did not investigate it further. Addition of Zn2+ allowed us to distinguish this small nonspecific current from the Zn2+-sensitive proton current identified in taste cells. Notably, we observed no appreciable Zn2+ block of the current in any of the cell lines tested. These results suggest that a Zn2+-sensitive proton current is a distinct feature of PKD2L1-expressing taste cells and may be restricted to these cells.
Figure 3.
Absence of a proton current in other cell types. A) Response to acidic solutions measured in whole-cell recording from the cell types indicated. Cells were bathed in TEA-based solution at pH 7.4 before the stimulus. The voltage was held at −80 mV and ramped from −80 to +80 mV (1 V/s). Current at −80 mV is plotted. B) Summary data of peak current and zinc-sensitive component. Numbers in parentheses are number of cells recorded. **P < 0.01, ***P < 0.001 compared with PKD2L1 cells using 1-tailed Student's t test.
Spinal neurons that express PKD2L1 do not have a proton current
Pkd2l1 expression was previously reported in a subset of neurons whose cell bodies are located deep within the spinal cord (10). These cells fire APs in response to extracellular acidification, suggesting the intriguing possibility that they possess similar machinery to detect acids as do taste cells (10). To test this possibility, we isolated spinal neurons from PKD2L1-YFP mice. In these mice, PKD2L1-expressing neurons in sagittal sections of mouse spinal cord can be visually identified by fluorescent emission from the YFP reporter or by immuno-fluorescent staining with an anti-GFP antibody (Fig. 4A). Fluorescent neurons possessed a process with a bulbous-like terminal extended toward the position of the central canal, as reported previously (10). We confirmed that these cells express Pkd2l1 by RT-PCR. As shown in Fig. 4B, YFP+ spinal neurons expressed Pkd2l1, Snap25, and Gad1, all markers for sour taste cells (22), but not Trpm5, which is expressed by bitter/sweet and umami responsive taste cells. It is interesting that we could not detect expression of Pkd1l3 or Car4, both of which are specifically expressed by sour cells (9, 23, 24).
Figure 4.
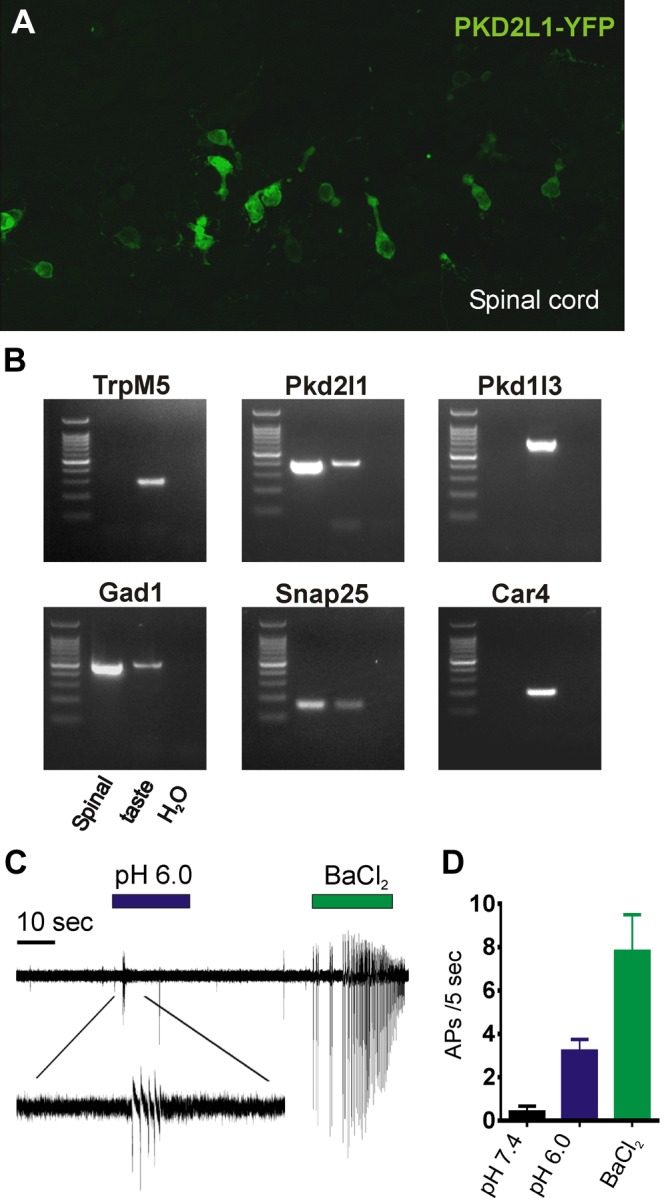
PKD2L1-expressing spinal neurons fire APs to acid. A) Confocal image of the central canal of the spinal cord from a postnatal day 4 PKD2L1-YFP mouse labeled with anti-GFP antibody. B) PCR-based expression analysis of indicated genes in samples from YFP+ spinal cord neurons, taste cDNA, or water control. Note that spinal neurons express Pkd2l1 but not Pkd1l3 or Car4. C) Loose patch recordings of acutely dissociated spinal cord neurons. Solutions are Tyrode’s at the indicated pH or with 2 mM BaCl2. D) Average data from experiments as in B (n = 4). APs were counted for the first 5 s both before the onset of the stimulus (pH 7.4) and after the onset of the stimulus (pH 6.0 or 2 mM BaCl2).
To test whether PKD2L1-expressing spinal cord neurons are sensitive to acid, we first recorded APs from YFP+ neurons using the loose patch configuration. Cells were bathed in a Na+ HEPES-buffered solution, pH 7.4, and rapidly switched to a solution buffered at pH 6.0. In all cells that were quiescent at rest (4 of 4), a burst of APs was triggered when extracellular pH was decreased (Fig. 4C). These cells also fired APs to a Ba2+ (2 mM) control solution. These data confirm that PKD2L1-expressing spinal neurons are able to sense changes in extracellular acidity (10) and show that they express some, but not all, molecular markers for sour taste cells.
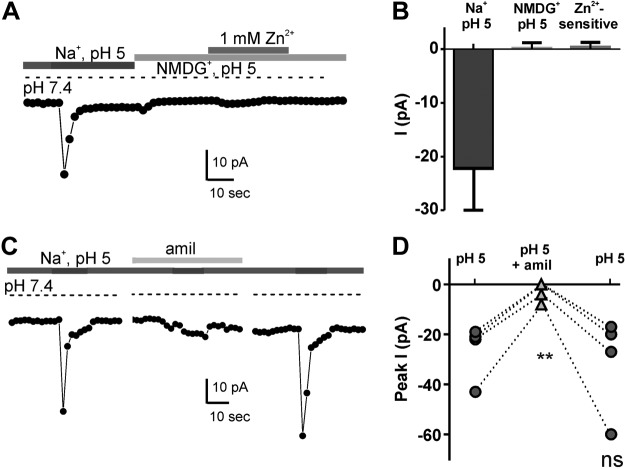
To determine whether a proton current is present in PKD2L1-expressing spinal cord neurons cells and drives AP firing, we recorded whole-cell currents from isolated YFP+ spinal neurons using a protocol we previously used to measure proton currents in taste cells (14). In this protocol, a neutral Na+-containing solution is exchanged for one that is buffered to pH 5 to evoke an acid-induced current. The permeation properties of the evoked current are then assessed by replacement of extracellular Na+ for NMDG+, which is not permeable through most cation channels. In taste cells, a large sustained current is observed in response to the Na+-containing pH 5.0 solution, and this current is unchanged in magnitude or reversal potential on substitution of NMDG+ for Na+, an initial indication that the current was carried by protons (14). The response observed in spinal neurons, shown in Fig. 5A, was entirely different. In response to the Na+-containing pH 5 solution, a transient current was elicited that decayed toward baseline, leaving only a small sustained inward current. Substituting NMDG+ for Na+ resulted in the elimination of all inward currents, and 1 mM Zn2+ had no additional effect (Fig. 5A, B). Thus, spinal neurons show no evidence of sustained inward proton current, as observed in taste cells.
Figure 5.
Absence of an inward proton current in PKD2L1-expressing spinal cord neurons. A) Whole-cell recording of acid-evoked currents in spinal neurons. Voltage was held at −80 mV and ramped from −80 to +80 mV (1 V/s). Note that the response to pH solution rapidly decays and that no acid-evoked current is observed in the absence of Na+. B) Average data from experiments as in A. Amplitudes measured relative to baseline at pH 7.4 for response to pH 5 with Na or NMDG and relative to NMDG pH 5 for Zn2+-sensitive. n = 4 cells. C) Acid-evoked current in a spinal neuron in the presence and absence of amiloride (amil). D) Data from experiments as in C. Dotted lines connect data points from same cells. **P < 0.01. ns, not significant, paired 2-tailed Student's t test.
The rapid activation and decay kinetics of the acid-evoked current in spinal neurons were reminiscent of currents carried by acid-sensing ion channels (ASICs). ASICs are highly Na+ selective and are activated by extracellular acidification. They are widely expressed in neurons throughout the CNS and peripheral nervous system (25). ASIC currents can be identified in native tissue by their sensitivity to block by the diuretic amiloride, which does not block proton current in sour taste cells (14). Thus, to test the possibility that the acid-evoked current in spinal neurons was mediated by ASICs, we measured currents in response to extracellular acidification in the presence and absence of amiloride (100 μM). We found that the transient current activated at pH 5.0 in PKD2L1-expressing spinal neurons was blocked on average by ∼80% when exposed to amiloride (Fig. 5C, D), consistent with the presence of ASICs. Thus, the response of spinal neurons to acids is not mediated by a proton current and can instead be attributed to the activation of ASICs. The function of PKD2L1 in spinal neurons remains unclear.
Biophysical properties of the proton current: Zn2+ block is pH sensitive
Our data indicate that taste cells express a novel proton current not found in other cells. To gain insight into how this current contributes to the sensory response of the cell and to provide a functional signature with which to identify the channel, we examined biophysical characteristics of the current.
A challenge in studying the proton current in native cells is that it is undoubtedly contaminated by other endogenous currents. To minimize the contributions of these currents, we used a pipette solution in which TEA was the major cation, and we replaced Cl− in the extracellular solution by methane sulfonic acid. To eliminate the contribution of leak or remaining ionic current, we subtracted the current remaining after addition of 1 mM Zn2+ to obtain the Zn2+-sensitive component of the current as shown in Fig. 6A, B, E. To gain insight into the gating and permeation of the underlying channels, this experiment was repeated using extracellular solutions that varied in pH from 4.5 to 5.5. Strikingly, the Zn2+ block of the current was highly sensitive to the extracellular pH, with 61% block at pH 5.0, whereas only 12% of the current was blocked at pH 4.5 (Fig. 6D). The relief of Zn2+ block by extracellular protons was fit with a Hill equation, with a coefficient of 2.0, indicating that binding of more than one proton is necessary to displace Zn2+.
The zn2+-sensitive component of the proton current is inwardly rectifying
In previous studies, we found that the current-voltage (I-V) relation of the current measured with ramp depolarizations was essentially linear (14). However, in these experiments, we did not have a method to eliminate background or leak currents to study the proton current in isolation. As shown in Fig. 6E, the Zn2+-sensitive component of the proton current shows strong inward rectification. Similar rectification was observed regardless of the pH of the extracellular solution or of the pH gradient (Fig. 6F), but we did not observe clear reversal of the current. The rectification was similar, regardless of whether the current was evoked by a voltage ramp that was ascending or descending (Fig. 7C), suggesting that the rectification is not caused by voltage-dependent gating. Note that some of the observed rectification could be caused by a possible voltage dependence of the Zn2+ block; however, the fact that similar rectification was observed regardless of the extracellular pH/degree of Zn2+ block argues against a major contribution for this mechanism.
The proton current is not gated by voltage
To more directly determine whether the proton current is sensitive to voltage, we used a protocol similar to that used to study voltage-dependent ion channels. In particular, because the Zn2+-sensitive component of the current is inwardly rectifying, we used a holding potential of 0 mV and looked for a voltage-dependent relaxation of the current when the voltage was stepped to more negative potentials. Currents were evoked by bath acidification (pH 5), and the Zn2+-sensitive component of the current was measured. Our results (Fig. 7A, B) show that over 200 ms, the current remained stable and did not increase or decrease at membrane potentials ranging from −40 to −120 mV. These data indicate that the taste cell proton current does not undergo voltage gating on the timescale of hundreds of milliseconds or faster. These data also argue against voltage-dependent block by Zn2+.
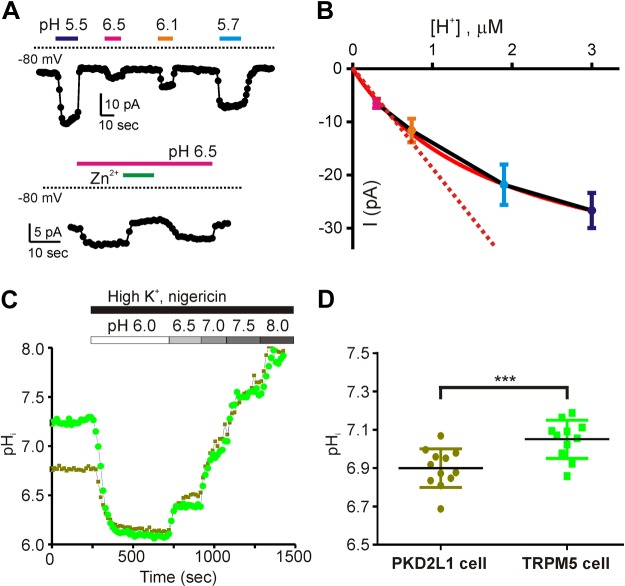
The proton current is open over a wide range of extracellular pH
An important but difficult question to answer is whether the proton current is gated by extracellular protons, as well as permeating them. Previously, we found that the magnitude of the proton current increased as the pH of the extracellular solution was lowered (14), an observation that could be attributed to either the increase in the concentration of permeant ions, the opening of channels by low pHo, or a combination of the two. To distinguish between these possibilities, we looked in more detail at the relationship between the concentration of protons and the magnitude of the current. A simple prediction is that if the channel is gated by protons then the magnitude of the current should increase sharply when a threshold pH is obtained. Thus, we plotted the magnitude of the proton current as a function of proton concentration. Note that for these experiments, we could not use the Zn2+-sensitive component of the current, because of the pH dependence of the Zn2+ block, and instead measured the current amplitude with respect to the resting current before acid application (Fig. 8A, B). Under these conditions, we observed a linear increase in the magnitude of the current over a pH range of pH 6.5 to 6.1, with saturation of the current amplitude at more acidic pH (Fig. 8B). These data are consistent with the hypothesis that the proton channels are not gated by acidic pH. Moreover, the absolute magnitude of the proton current measured at pH 6.5 (−6.5 ± 0.9 pA) appears to be sufficient to drive the APs observed in intact isolated cells.
pH imaging supports the view that the proton current is open at rest
The previous experiments suggest that the proton current is not gated either by pH or by voltage. If this is the case, even in the absence of extracellular acidification there should be a constant influx of protons propelled by the negative membrane potential of the cell. This predicts that the cytosol of sour taste cells should be more acidified under resting conditions compared with that of cells that lack the proton current. To test this possibility, we loaded acutely dissociated taste cells with the pH-sensitive ratiometric dye BCECF-AM (Fig. 8C, D and Materials and Methods) and measured the fluorescent emission. Conversion of the emission data (ratios) to pH values using nigericin calibration showed that PKD2L1 taste cells had an average resting pHi of 6.90 ± 0.03, which is significantly more acidified than the pHi of TRPM5 cells, which was 7.05 ± 0.03 (Fig. 8D).
The proton current under goes a time-dependent decay
In measuring the proton current in a large number of cells, we noted that the kinetics of the currents varied considerably from cell to cell, with some cells showing currents that were relatively stable over tens of seconds, whereas others decayed rapidly in amplitude during the stimulus. The decay of the current could reflect one of several different processes such as intrinsic stimulus-dependent inactivation of the current or a change in pHi, which could have multiple downstream effects (15, 26). Consistent with the latter possibility, inspection of individual traces showed that currents that were larger tended to decay faster (Fig. 2A). To more rigorously validate this observation, we measured the rate of decay of the proton current and plotted this as a function of the peak magnitude of the current for a large number of cells, taken from each taste region. As seen in Fig. 9A, B, the currents decayed with a rate that was correlated to the magnitude of the current (r = 0.97). One mechanism by proton entry can affect the magnitude of the proton current is through an elevation of intracellular concentrations that changes the driving force for ion entry. We therefore attempted to determine whether the reversal potential of the currents changed during a prolonged stimulus (Fig. 9C–E). Indeed, the unsubtracted current showed a time-dependent shift in Erev that coincided with the change in the current magnitude (Fig. 9C, E). However, because these currents were contaminated with leak currents, the shift in Erev could be caused by a change in the relative contribution of the proton current to the total current rather than a change in driving force for proton entry. Indeed, when we measured the Zn2+-sensitive component of the current, we found that the proton current decayed in magnitude during the acid application, without any evidence for a change in the rectification (Fig. 9F, G). In no case could we observe a reversal of the Zn2+-sensitive component of the current. Together these data suggest that decay of the proton current reflects a process of ion accumulation, leading to a reduction in the magnitude of the proton current. Because the currents do not reverse, it was not possible to determine whether there was a concomitant change in the driving force for ion entry that might also play a role in the decay of the currents.
DISCUSSION
The detection of acids by sour taste cells is still poorly understood at both the cellular and molecular levels. Previously we identified a proton current in sour-responsive cells from the circumvallate papillae and proposed that this current mediates transduction of sour taste (14). We now show that the proton current is a common feature of sour-responsive cells on the tongue and that it is absent from a large number of other cells types and cell lines not involved in sour taste. These data establish a specific association of the proton current with sour-responsive cells, lending additional support to the contention that the proton current mediates sour transduction (14). Interestingly, we did not detect the proton current in spinal neurons that express PKD2L1 and respond to acids (10), indicating that spinal neurons and taste cells have evolved independent machinery for sensing acids. We also provide evidence that the underlying proton-selective conductance is open under resting conditions and that it is insensitive to pH or voltage over physiologic ranges. Our biophysical characterization of the proton current of sour taste cells has implications for understanding how sour taste is transduced and may prove useful in the identification of the underlying proteins.
Central canal spinal neurons
PKD2L1 is expressed in a subset of spinal neurons that line the central canal (10) and that have long been thought to constitute a unique population of sensory neurons. These cells are characterized by the presence of a ciliated process that contacts the central canal and therefore have been named spinal cerebrospinal fluid-contacting neurons (CSF-cNs) (27). Consistent with a chemosensory function, Huang et al. (10) showed that the PKD2L1-expressing spinal neurons respond to acids. Moreover, this raised the intriguing possibility that CSF-cNs and taste cells share common mechanisms for detecting acids. Using YFP+ spinal neurons from the PKD2L1-YFP mouse, we confirmed that CSF-cNs respond to acids with trains of APs, much like YFP+ taste cells. However, in contrast to taste cells, spinal CSF-cNs do not have any appreciable proton current. Instead, the currents evoked by acidic pH in spinal CSF-cNs displayed characteristics of ASICs, including rapid inactivation and sensitivity to amiloride, as previously described in recordings from subependymal CSF-cNs (28). Conversely, we never observed ASIC currents in sour taste cells. Thus, we can conclude that, although PKD2L1-expressing spinal neurons are acid sensitive, they do not share molecular machinery for acid-sensing with taste cells (10).
Function of PKD2L1 in sour taste
Although PKD2L1 serves as a useful marker for sour cells, its role in taste sensation is still unknown. PKD2L1 coassembles with PKD1L3 and initial studies reported that the heteromeric channel was activated by exposure to acids, as expected for a sour receptor (9). However, in a subsequent report, the heteromeric channels were shown to be, instead, activated by removal of acids (29), an observation at odds with the expected excitatory response to acids of taste cells. More recently, careful biophysical experiments have shown that PKD2L1, also known as TRPP3, can form homomeric channels; these channels are activated by high voltages, giving rise to large tail currents measured on repolarization (30). Homomeric PKD2L1 channels are sensitive to temperature (31), and osmotic pressure (30) and show a bimodal sensitivity to pH (32). PKD2L1 has also been proposed to mediate calcium entry into the primary cilium, where it regulates developmental patterning (33). Our data add to the growing evidence that PKD2L1 and PKD1L3 do not form a sour receptor, either alone or in combination. Previously, we showed that the proton current of sour taste cells is not changed in magnitude in a Pkd1l3 knockout mouse (14). Our observation that the proton current is not diminished in cells from the fungiform papillae that express only PKD2L1 and not PKD1L3 further confirms that PKD1L3 is not a component of the proton channel. In addition, our observation that spinal neurons that express PKD2L1 alone do not display a proton current now provides evidence that PKD2L1 is not sufficient to mediate proton entry. Thus, the function of PKD2L1 in either taste cells or spinal neurons remains mysterious.
Comparison with other proton channels
The first proton currents were measured in snail neurons (34), although their existence had been postulated a decade earlier by Woody Hastings to explain bioluminescence in dinoflagellates (35). The first evidence for a proton channel in mammalian cells came from the observation that the respiratory burst of neutrophils, which involves the generation of superoxide by NADPH oxidase, was accompanied by an outward proton current. The current was characterized extensively over the next 2 decades, mainly by Decoursey et al. (15), who showed that it was selectively permeable to protons, blocked by Zn2+, and voltage activated in such a way that it carried only outward currents. The gene encoding this proton channel, Hv1, was cloned in 2006 (36, 37). The underlying structure of the protein bears strong similarity to the portion of voltage-gated K+ channels that contain the voltage sensor domain, suggesting a common origin for the voltage dependence.
The proton current we have described in this paper and previously (14) is clearly very different from Hv1. Hv1 is outwardly rectifying, reflecting a voltage dependence to the gating (15, 36, 37) of the channels, whereas the proton current we describe appears to be inwardly rectifying. Moreover, we find no evidence for voltage-dependent gating of the taste current, which shows similar rectification regardless of whether it is evoked by a positive or negative going voltage ramp and shows no time-dependent relaxation during voltage steps of 200 ms duration.
Another remarkable feature of Hv1 is its extreme ion selectivity; indeed, it has been claimed that Hv1 is not just selective for H+ but specific for H+ (38). Although the mechanism for ion conduction is still under active investigation, selectivity may arise as a consequence of the fact that H+ ions can be conducted by “hopping” along a series of aligned hydrogen bonds or through a “water wire” and do not need to move through an open pore like other ions (38–41). We previously have shown that the acid-evoked conductance in PKD2L1-expressing taste cells is not sensitive changes in the extracellular concentration of Na+ or Ca2+ or changes in the intracellular concentration of Cl−, arguing that none of these ions contributes significantly to the inward current (14). Instead, our published data (14) and the data presented here support the view that the inward current is carried by protons. Whether it is specific for protons like Hv1 cannot yet be determined as the outward rectification of the Zn2+ -sensitive component of the current precludes measurement of its reversal potential.
Hv1 and the current we describe have in common, along with many other proton transfer pathways (15), a sensitivity to extracellular Zn2+. We now show that the Zn2+ block of the taste current is relieved by acidic pH, as is the case for Hv1 and other Zn2+-sensitive ion channels (15, 42). The block we observe is relatively low affinity; at pH 5.0, 1 mM Zn2+ blocks only ∼50% of the current. By comparison, Hv1 currents measured at pH 6.5 are ∼50% inhibited by 2 μM Zn2+ (36). However, the strong pH dependence of the block predicts that the taste cell current will also show micromolar sensitivity to Zn2+ block when measured at a more basic pH. Within the Hv1 protein, Zn2+ is coordinated by 2 histidine residues, as well as aspartic acid and glutamic acid residues, which can explain the relief of block by protons (43, 44).
Functionally, the proton current of sour cells bears more similarity to the current carried by viral proton channels (15). One such protein, the M2 protein of influenza, conducts protons into the virion interior as an early step in the infection process. The M2 channel is highly selective for protons, is not gated by voltage, and shows evidence of pH regulation (26, 45). These processes are accomplished by a surprisingly small protein of just 96 amino acids, which assembles as a tetramer (46). The proton current we describe here differs from that mediated by the M2 channel, in that it displays no evidence of regulation by pH. In particular, the current magnitude over a range of pH 6.5 to 6.1 scales essentially linearly, with evidence for saturation at pH 6.0 and lower. Moreover, pH imaging shows that sour taste cells are acidified compared with other taste cells, even when the extracellular solution is at a neutral pH, supporting the view that the proton channel is not gated. Overall, the relative functional simplicity of the proton current we describe suggests that it may be encoded by a small protein, like the viral channels.
Implications of biophysical features for taste transduction
The unique biophysical characteristics of the proton channel of sour taste cells have interesting implications for the transduction of sour taste. First, the observation that the current is not gated by voltage or pH means that protons will enter through the channel without the requirement that they reach a threshold concentration. Indeed, we found that taste cells fire APs in response to a relatively modest acidification of the extracellular solution (to pH 6.7). At a similar pH (6.5), we can detect robust Zn2+-sensitive proton currents of ∼6.5 pA. In these cells, which have a relatively high input resistant of ∼3 GOhm, a current of 6.5 pA is expected to depolarize the cells by ∼20 mV, which may be sufficient to bring the membrane potential to the threshold for firing APs. It is also possible that the influx of protons is amplified by other ion channels, sensitive to changes in pHi (2). It is worth noting that in nerve recordings and psychophysical experiments (47), sour responses are generally not evoked unless the pH of the stimulus is <4.0. The difference may be attributed to the large buffering capacity of saliva, which is expected to considerably dampen fluctuations in extracellular pH, especially in the vicinity of the taste pore (48). An open proton channel would be a useful element to detect these small changes in extracellular pH.
CONCLUSIONS
Together, our data provide a detailed characterization of a novel proton conductance that is restricted to PKD2L1-expressing taste cells. Our observation that the current is tightly associated with taste cells that detect sour provides strong support that this current mediates sour transduction. Its biophysical characteristics make it well suited to detect small changes in pH in the vicinity of the taste cell.
Acknowledgments
The authors thank Eric Mulhall for expert technical assistance and Radhika Palkar and David McKemy for assistance with isolating spinal neurons and for providing neuro2a cells. This work was supported by U.S. National Institutes of Health, National Institute on Deafness and Other Communication Disorders Grants R01DC013741 (to E.R.L.) and F32DC013971 (to J.D.B.).
Glossary
- AP
action potential
- ASIC
acid-sensing ion channel
- CSF-cNs
cerebrospinal fluid-contacting neurons
- CV
circumvallate
- FL
foliate
- FU
fungiform
- GFP
green fluorescent protein
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- I-V
current-voltage
- MA
methane-sulphonic acid
- MES
2-N-morpholino-ethane sulfonic acid
- NFP
no fluorescent protein
- NMDG
N-methyl-d-glucamine
- pHi
intracellular pH
- TEA
tetraethylammonium
- YFP
yellow fluorescent protein
REFERENCES
- 1.Yarmolinsky D. A., Zuker C. S., Ryba N. J. (2009) Common sense about taste: from mammals to insects. Cell 139, 234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liman E. R., Zhang Y. V., Montell C. (2014) Peripheral coding of taste. Neuron 81, 984–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbertson T. A., Roper S. D., Kinnamon S. C. (1993) Proton currents through amiloride-sensitive Na+ channels in isolated hamster taste cells: enhancement by vasopressin and cAMP. Neuron 10, 931–942 [DOI] [PubMed] [Google Scholar]
- 4.Ugawa S., Minami Y., Guo W., Saishin Y., Takatsuji K., Yamamoto T., Tohyama M., Shimada S. (1998) Receptor that leaves a sour taste in the mouth. Nature 395, 555–556 [DOI] [PubMed] [Google Scholar]
- 5.Stevens D. R., Seifert R., Bufe B., Müller F., Kremmer E., Gauss R., Meyerhof W., Kaupp U. B., Lindemann B. (2001) Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli. Nature 413, 631–635 [DOI] [PubMed] [Google Scholar]
- 6.Liu L., Simon S. A. (2001) Acidic stimuli activates two distinct pathways in taste receptor cells from rat fungiform papillae. Brain Res. 923, 58–70 [DOI] [PubMed] [Google Scholar]
- 7.Lin W., Ogura T., Kinnamon S. C. (2002) Acid-activated cation currents in rat vallate taste receptor cells. J. Neurophysiol. 88, 133–141 [DOI] [PubMed] [Google Scholar]
- 8.Richter T. A., Dvoryanchikov G. A., Roper S. D., Chaudhari N. (2004) Acid-sensing ion channel-2 is not necessary for sour taste in mice. J. Neurosci. 24, 4088–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishimaru Y., Inada H., Kubota M., Zhuang H., Tominaga M., Matsunami H. (2006) Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. USA 103, 12569–12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang A. L., Chen X., Hoon M. A., Chandrashekar J., Guo W., Tränkner D., Ryba N. J., Zuker C. S. (2006) The cells and logic for mammalian sour taste detection. Nature 442, 934–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kataoka S., Yang R., Ishimaru Y., Matsunami H., Sévigny J., Kinnamon J. C., Finger T. E. (2008) The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem. Senses 33, 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson T. M., Lopezjimenez N. D., Tessarollo L., Inoue M., Bachmanov A. A., Sullivan S. L. (2010) Taste function in mice with a targeted mutation of the pkd1l3 gene. Chem. Senses 35, 565–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horio N., Yoshida R., Yasumatsu K., Yanagawa Y., Ishimaru Y., Matsunami H., Ninomiya Y. (2011) Sour taste responses in mice lacking PKD channels. PLoS ONE 6, e20007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang R. B., Waters H., Liman E. R. (2010) A proton current drives action potentials in genetically identified sour taste cells. Proc. Natl. Acad. Sci. USA 107, 22320–22325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decoursey T. E. (2003) Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83, 475–579 [DOI] [PubMed] [Google Scholar]
- 16.Pérez C. A., Huang L., Rong M., Kozak J. A., Preuss A. K., Zhang H., Max M., Margolskee R. F. (2002) A transient receptor potential channel expressed in taste receptor cells. Nat. Neurosci. 5, 1169–1176 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., Hoon M. A., Chandrashekar J., Mueller K. L., Cook B., Wu D., Zuker C. S., Ryba N. J. (2003) Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112, 293–301 [DOI] [PubMed] [Google Scholar]
- 18.DeSimone J. A., Phan T. H., Heck G. L., Ren Z., Coleman J., Mummalaneni S., Melone P., Lyall V. (2011) Involvement of NADPH-dependent and cAMP-PKA sensitive H+ channels in the chorda tympani nerve responses to strong acids. Chem. Senses 36, 389–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clapp T. R., Medler K. F., Damak S., Margolskee R. F., Kinnamon S. C. (2006) Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 4, 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z., Zhao Z., Margolskee R., Liman E. (2007) The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J. Neurosci. 27, 5777–5786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyall V., Feldman G. M., Heck G. L., DeSimone J. A. (1997) Effects of extracellular pH, PCO2, and HCO3- on intracellular pH in isolated rat taste buds. Am. J. Physiol. 273, C1008–C1019 [DOI] [PubMed] [Google Scholar]
- 22.DeFazio R. A., Dvoryanchikov G., Maruyama Y., Kim J. W., Pereira E., Roper S. D., Chaudhari N. (2006) Separate populations of receptor cells and presynaptic cells in mouse taste buds. J. Neurosci. 26, 3971–3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LopezJimenez N. D., Cavenagh M. M., Sainz E., Cruz-Ithier M. A., Battey J. F., Sullivan S. L. (2006) Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J. Neurochem. 98, 68–77 [DOI] [PubMed] [Google Scholar]
- 24.Chandrashekar J., Yarmolinsky D., von Buchholtz L., Oka Y., Sly W., Ryba N. J., Zuker C. S. (2009) The taste of carbonation. Science 326, 443–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waldmann R. (2001) Proton-gated cation channels—neuronal acid sensors in the central and peripheral nervous system. Adv. Exp. Med. Biol. 502, 293–304 [DOI] [PubMed] [Google Scholar]
- 26.Mould J. A., Drury J. E., Frings S. M., Kaupp U. B., Pekosz A., Lamb R. A., Pinto L. H. (2000) Permeation and activation of the M2 ion channel of influenza A virus. J. Biol. Chem. 275, 31038–31050 [DOI] [PubMed] [Google Scholar]
- 27.Djenoune, L., Khabou, H., Joubert, F., Quan, F. B., Nunes Figueiredo, S., Bodineau, L., Del Bene, F., Burckle, C., Tostivint, H., Wyart, C. Investigation of spinal cerebrospinal fluid-contacting neurons expressing PKD2L1: evidence for a conserved system from fish to primates. Front. Neuroanat. 8, 26 [DOI] [PMC free article] [PubMed]
- 28.Orts-Del’immagine A., Wanaverbecq N., Tardivel C., Tillement V., Dallaporta M., Trouslard J. (2012) Properties of subependymal cerebrospinal fluid contacting neurones in the dorsal vagal complex of the mouse brainstem. J. Physiol. 590, 3719–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inada H., Kawabata F., Ishimaru Y., Fushiki T., Matsunami H., Tominaga M. (2008) Off-response property of an acid-activated cation channel complex PKD1L3-PKD2L1. EMBO Rep. 9, 690–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu T., Janssens A., Voets T., Nilius B. (2009) Regulation of the murine TRPP3 channel by voltage, pH, and changes in cell volume. Pflugers Arch. 457, 795–807 [DOI] [PubMed] [Google Scholar]
- 31.Higuchi T., Shimizu T., Fujii T., Nilius B., Sakai H. (2014) Gating modulation by heat of the polycystin transient receptor potential channel PKD2L1 (TRPP3). Pflugers Arch. 466, 1933–1940 [DOI] [PubMed] [Google Scholar]
- 32.Shimizu T., Higuchi T., Fujii T., Nilius B., Sakai H. (2011) Bimodal effect of alkalization on the polycystin transient receptor potential channel, PKD2L1. Pflugers Arch. 461, 507–513 [DOI] [PubMed] [Google Scholar]
- 33.DeCaen P. G., Delling M., Vien T. N., Clapham D. E. (2013) Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504, 315–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas R. C., Meech R. W. (1982) Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature 299, 826–828 [DOI] [PubMed] [Google Scholar]
- 35.Fogel M., Hastings J. W. (1972) Bioluminescence: mechanism and mode of control of scintillon activity. Proc. Natl. Acad. Sci. USA 69, 690–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramsey I. S., Moran M. M., Chong J. A., Clapham D. E. (2006) A voltage-gated proton-selective channel lacking the pore domain. Nature 440, 1213–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasaki M., Takagi M., Okamura Y. (2006) A voltage sensor-domain protein is a voltage-gated proton channel. Science 312, 589–592 [DOI] [PubMed] [Google Scholar]
- 38.DeCoursey T. E., Hosler J. (2014) Philosophy of voltage-gated proton channels. J. R. Soc. Interface 11, 20130799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramsey I. S., Mokrab Y., Carvacho I., Sands Z. A., Sansom M. S., Clapham D. E. (2010) An aqueous H+ permeation pathway in the voltage-gated proton channel Hv1. Nat. Struct. Mol. Biol. 17, 869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musset B., Smith S. M., Rajan S., Morgan D., Cherny V. V., Decoursey T. E. (2011) Aspartate 112 is the selectivity filter of the human voltage-gated proton channel. Nature 480, 273–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan D., Musset B., Kulleperuma K., Smith S. M., Rajan S., Cherny V. V., Pomès R., DeCoursey T. E. (2013) Peregrination of the selectivity filter delineates the pore of the human voltage-gated proton channel hHV1. J. Gen. Physiol. 142, 625–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cherny V. V., DeCoursey T. E. (1999) pH-dependent inhibition of voltage-gated H(+) currents in rat alveolar epithelial cells by Zn(2+) and other divalent cations. J. Gen. Physiol. 114, 819–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Musset B., Smith S. M., Rajan S., Cherny V. V., Sujai S., Morgan D., DeCoursey T. E. (2010) Zinc inhibition of monomeric and dimeric proton channels suggests cooperative gating. J. Physiol. 588, 1435–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeshita K., Sakata S., Yamashita E., Fujiwara Y., Kawanabe A., Kurokawa T., Okochi Y., Matsuda M., Narita H., Okamura Y., Nakagawa A. (2014) X-ray crystal structure of voltage-gated proton channel. Nat. Struct. Mol. Biol. 21, 352–357 [DOI] [PubMed] [Google Scholar]
- 45.Pinto L. H., Holsinger L. J., Lamb R. A. (1992) Influenza virus M2 protein has ion channel activity. Cell 69, 517–528 [DOI] [PubMed] [Google Scholar]
- 46.Schnell J. R., Chou J. J. (2008) Structure and mechanism of the M2 proton channel of influenza A virus. Nature 451, 591–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roper S. D. (2007) Signal transduction and information processing in mammalian taste buds. Pflugers Arch. 454, 759–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuo R. (2000) Role of saliva in the maintenance of taste sensitivity. Crit. Rev. Oral Biol. Med. 11, 216–229 [DOI] [PubMed] [Google Scholar]