Abstract
OBJECTIVE
To estimate time trends of actual provider use of human papillomavirus (HPV) testing in cervical cancer screening by using laboratory and administrative data from the Johns Hopkins Hospital Division of Cytopathology in Baltimore, Maryland.
METHODS
In this ecologic trend study, we analyzed 178,510 Pap specimen records and 12,221 HPV tests among 85,048 patients from 2001 to 2007. Monthly frequencies and proportions of HPV reflex testing and HPV cotesting with Pap (stratified by patient ages 30 and older and 18–29 years) were calculated. Time trends of monthly HPV testing proportions were analyzed using joinpoint regression methods.
RESULTS
From April 2002, when the American Society for Colposcopy and Cervical Pathology added HPV reflex testing to its guidelines, to December 2007, the monthly the proportion of reflex testing was 95.8%. From February 2004, when the society added HPV cotesting with Pap among women aged 30 years or older to its guidelines, to December 2007, the overall proportion HPV cotesting with Pap among patients aged 30 years or older was 7.8% (compared with 4.9% among patients 18–29 years [P<.01]). The highest proportion of HPV cotesting among women aged 30 years or older, 15%, was observed in September 2006, and the trend later plateaued around 13%. The monthly proportions of HPV reflex testing and cotesting with Pap changed significantly over time.
CONCLUSION
These data reveal that a small percentage of women aged 30 years or older received HPV cotesting with Pap, thus identifying a significant opportunity for providers to improve patient care in cervical cancer prevention.
The high-risk human papillomavirus (HPV) test is approved by the U.S. Food and Drug Administration (FDA) for follow-up of a specific abnormal Pap diagnosis, atypical squamous cells of undetermined significance (ASC-US) (ie, reflex testing) and for HPV cotesting with Pap among women 30 years or older (ie, cotesting) during routine cervical cancer screening.1 The importance and value of HPV cotesting is reflected by its inclusion in screening guidelines of the American Society for Colposcopy and Cervical Pathology (ASCCP), the American Cancer Society, and other organizations.2-5
Although HPV testing plays an increasingly important role in cervical cancer prevention, little information about actual provider use of the HPV test is documented in the scientific literature. The few studies to consider this topic6 are based primarily on provider self-report and case vignettes, rather than on actual provider practice data. Provider surveys suggest there is non–evidence-based use of HPV testing,7-10 and marketing and media coverage of the HPV test and HPV vaccine has been prevalent in the public and medical communities.11,12 Rapid changes in prevention, such as FDA approval of the HPV vaccine,13 in addition to media and advertising attention, may exacerbate confusion over screening recommendations.9,14
The purpose of this ecologic study was to estimate time trends of high-risk HPV testing for two FDA-approved uses: 1) HPV reflex testing of ASC-US Pap results and 2) HPV cotesting with Pap among women 30 years or older during cervical cancer screening. We used 7 years of Pap specimen data from the Johns Hopkins Hospital Division of Cytopathology in Baltimore, Maryland.
MATERIALS AND METHODS
Data originated from the Pathology Data System, an in-house, comprehensive laboratory information system administratively maintained by the Johns Hopkins Hospital Department of Pathology. The Pathology Data System contains individual specimen record information and associated test results, diagnoses, and provider and patient information for all specimens submitted by inpatient units, outpatient clinics, and outside accounts to the Department of Pathology for processing and evaluation. In this ecologic trend study, we used a subset of the Pathology Data System data that included all Pap specimens processed by the Johns Hopkins Hospital Division of Cytopathology and HPV tests processed by the hospital’s Division of Microbiology. A statistical programmer de-identified the Pathology Data System data to create a limited data set for the analysis. Before commencement of this research, the Johns Hopkins Bloomberg School of Public Health Institutional Review Board determined the study to be exempt human subjects research and, thus, the requirement to obtain informed consent was waived.
The limited data set contained all Pap specimens processed by the Johns Hopkins Hospital Division of Cytopathology from January 1, 2001, to December 31, 2007, (N=195,981). Variables included new unique specimen identifier, new unique patient identifier, date the Pap specimen was taken, Pap diagnosis, clinic ordering the Pap, patient age, patient sex, patient home ZIP code, patient race, patient insurance type, and HPV result, if such a test was completed. The continuous variable of patient age was categorized according to decade. Categorical variables of patient race, insurance, and home ZIP code were collapsed according to their distribution and relevance to the study design and setting. SQL programming in Access 2007 was used to extract pertinent information (ie, type of Pap [conventional versus liquid-based], Pap result, possible HPV test and result) from the “free text” Pap diagnosis variable. Pap diagnoses were categorized as “negative for intraepithelial lesion or malignancy (normal),” “atypical squamous cells of undetermined significance (ASC-US),” “low-grade squamous intraepithelial lesion (LSIL),” “high-grade squamous intraepithelial lesion (HSIL),” “atypical glandular cells of undetermined significance (AGUS),” “carcinoma (cancer),” “specimen unsatisfactory/discarded,” and “cannot be classified.” Pap results that indicated two possible diagnoses in a single record were classified according to the more severe diagnosis. At the time of this study, the digene HPV test (QIAGEN, formally Digene Corporation), which detects the DNA of 13 oncogenic HPV types in a cervical specimen,1 was the only FDA-approved high-risk HPV test in the United States. HPV test results were categorized as “negative,” “positive,” and “equivocal.” The Johns Hopkins Hospital Division of Microbiology defined negative HPV results as less than 0.85 relative light units, equivocal HPV results as 0.85 to 3.0 relative light units, and positive HPV results as more than 3.0 relative light units.
For the present analysis, we excluded records missing data on patient age, patient sex, type of Pap test, or final diagnosis (n=182). Because our focus was routine cervical cancer screening among adults, we excluded records from patients younger than 18 years old (n=7,130). We also excluded specimens collected before January 1, 2001 (n=113) and from clinics that contributed Pap specimens on only 1 day in the 7-year study period (n=82). We excluded specimens that were unsatisfactory or discarded or both, and those with diagnoses that could not be classified based on information in the “free text” Pap diagnosis variable (n=1,823). Finally, because our aim was to estimate trends of HPV testing in routine cervical cancer screening, we excluded specimens from clinics that focus on non-adult screening (eg, adolescent clinics; n=1,412), referral clinics (eg. colposcopy, oncology; n=3,258), and human immunodeficiency virus (HIV) clinics (n=3,471). The final analytic data set contained 178,510 Pap specimen records (91.1% of total data) among 85,048 unique patients in 216 clinics in the Baltimore, Maryland, metropolitan area. The unique clinics originated from inpatient units of multiple Johns Hopkins hospitals, outpatient facilities affiliated with Johns Hopkins, and outpatient facilities not affiliated with Johns Hopkins, including several federally qualified health centers. The 216 clinics were heterogeneous according to setting, size of patient populations, number of providers, and so on.
Using Pap specimen as the unit, we calculated the monthly frequencies of liquid-based Pap, conventional Pap, and HPV testing. Frequencies of the two FDA-approved uses of the HPV test were our primary outcomes: HPV reflex testing and HPV cotesting with Pap among women 30 years or older. We limited our analysis of HPV testing to liquid-based Pap specimen records because the general use of the cocollection of conventional Pap and digene specimen transport medium was unknown.
HPV reflex testing was defined as use of the testing for a Pap specimen diagnosed as ASC-US regardless of the patient’s age. HPV cotesting with Pap was defined as use of the testing for a Pap specimen with a non–ASC-US diagnosis (ie, negative for intraepithelial lesion or malignancy [normal], LSIL, HSIL, or carcinoma [cancer]) for a patient aged 30 years or older. HPV cotesting with Pap among patients aged younger than 30 years is not an FDA-approved use of the HPV test. We excluded Pap diagnoses of atypical glandular cells of undetermined significance because the Johns Hopkins Hospital Division of Cytopathology occasionally ordered HPV testing for these diagnoses regardless of provider instructions.
We calculated the overall proportion of HPV reflex testing by dividing the total number of HPV tests conducted on liquid-based Pap specimens with an ASC-US diagnosis by the total number of liquid-based Pap specimens with an ASC-US diagnosis from April 2002, when the ASCCP added HPV reflex testing to its screening guidelines,15 to December 2007. For HPV cotesting with Pap, we calculated the overall proportion by dividing the total number of HPV tests conducted on liquid-based Pap specimens with a non–ASC-US diagnosis (ie, normal, LSIL, HSIL, cancer, and not atypical glandular cells of undetermined significance) by the total number of liquid-based Pap specimens with a non–ASC-US diagnosis. This calculation focused on the time between February 2004, when the ASCCP added HPV cotesting with Pap for women 30 years or older to its guidelines,3 and December 2007. Because HPV cotesting with Pap is suggested for women 30 years or older, we calculated the proportions of cotesting with Pap in strata of patient age 18–29 years and 30 years or older. We estimated the proportions of reflex testing and cotesting with Pap in categories defined by the covariates and tested for differences using Pearson χ2. Results were considered statistically significant if P≤.05.
To conduct quantitative ecologic time trend analyses, we employed joinpoint regression modeling, which identifies statistically significant changes (eg, “joinpoints”) in linear slope trends over time.16 Using the Joinpoint Regression Program 3.4.3, we fit the simplest joinpoint model to the HPV monthly proportions data. Briefly, the software begins with 0 joinpoints (ie, a straight line) and statistically tests whether adding more joinpoints (up to a total of 4) will more appropriately fit the data. Monte Carlo Permutation methods are used for tests of significance. We specified a log-linear model assuming a Poisson distribution to allow for calculating monthly percent change of regression line segments between joinpoints. The monthly percent change is calculated by fitting a linear regression to the natural log of the monthly HPV testing proportions with calendar month as the regressor. The coefficient from this regression is the slope (m) of the change in HPV testing proportions over calendar month, and the monthly percent change is calculated as: monthly percent change=100×(em−1).17 Monthly percent change estimates were reported with 95% confidence intervals. Unless otherwise noted, all data management and analysis were conducted using Stata/SE Version 11.
RESULTS
The analytic data set included 178,510 Pap specimen records; 133,938 (75.0%) were liquid-based Pap tests and 44,572 (25.0%) were conventional Pap tests, and 12,221 HPV tests were processed. The specimen records originated from 85,048 unique women. The number of Paps per unique woman ranged from 1 to 17 with a median of 1 and a mean of 2.1 (standard deviation 1.6). Sixty-eight and six-tenths percent of the study population was age 30 years or older. The study population was predominately white or black (42.6% and 36.2%, respectively), and the majority of patients (53.7%) had Baltimore City home ZIP codes. There was a wide range of insurance types (Table 1).
Table 1. Study Population Characteristics (n=85,048).
| Characteristic | n (%) |
|---|---|
| Age category (y) | |
| 18–20 | 7,228 (8.5) |
| 21–29 | 19,476 (22.9) |
| 30–39 | 18,064 (21.2) |
| 40–49 | 15,811 (18.6) |
| 50–59 | 11,669 (13.7) |
| 60–69 | 7,059 (8.3) |
| 70 or older | 5,741 (6.8) |
| Race | |
| White | 36,234 (42.6) |
| Black | 30,746 (36.2) |
| Asian | 2,109 (2.5) |
| Hispanic | 1,417 (1.7) |
| Other | 6,043 (7.1) |
| Missing | 8,499 (10.0) |
| Insurance type | |
| Preferred provider organization | 19,650 (23.1) |
| Blue Shield | 10,803 (12.7) |
| Medicaid | 10,031 (11.8) |
| Medicare | 8,200 (9.6) |
| Military | 5,999 (7.1) |
| Health maintenance organization | 4,116 (4.8) |
| Other | 2,068 (2.4) |
| Self-pay | 172 (0.2) |
| Type unknown | 9,596 (11.3) |
| Missing | 14,413 (17.0) |
| Home ZIP code | |
| Baltimore City | 45,632 (53.7) |
| Other Maryland | 19,417 (22.8) |
| Non-Maryland | 3,676 (4.3) |
| Missing | 16,323 (19.2) |
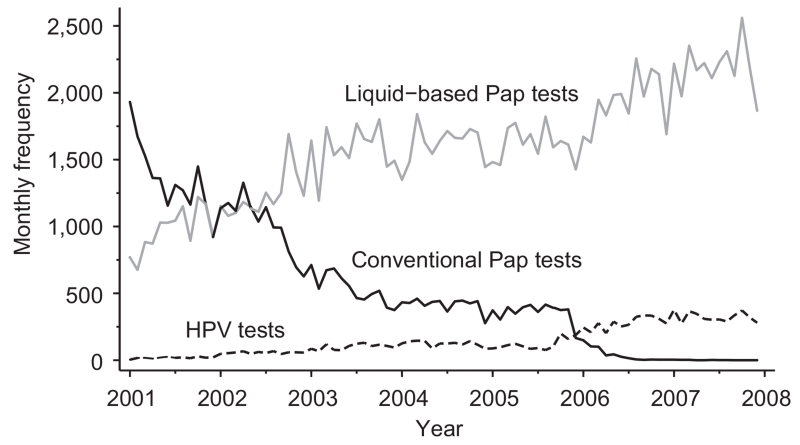
From April 2002, when the ASCCP added HPV reflex testing to its screening guidelines,15 to December 2007, the proportion of reflex testing was 95.8% (n=5,370). From February 2004, when the ASCCP added HPV cotesting with Pap for women 30 years or older to its guidelines,3 to December 2007, the proportion of cotesting with Pap among patients aged 30 years or older was 7.8% (n=4,588). In the same timeframe, the proportion of non–evidenced-based HPV cotesting with Pap among patients 18–29 years was 4.9% (n=1,158; P<.01). Cotesting with Pap differed by patient age category (P<.01). We graphed the monthly frequencies of HPV testing, conventional Pap testing, and liquid-based Pap testing. Liquid-based Pap testing increased over the 7-year study period from 769 to 1,866 tests per month. Conventional testing decreased from 1,931 to 0 tests per month. The monthly frequency of HPV testing gradually increased from 4 to 280 tests per month with a notable rise in mid-2005 (Fig. 1).
Fig. 1.
Monthly frequencies of human papillomavirus (HPV) testing, conventional Pap testing, and liquid-based Pap testing among Pap specimens processed by the Johns Hopkins Hospital Division of Cytopathology, 2001–2007.
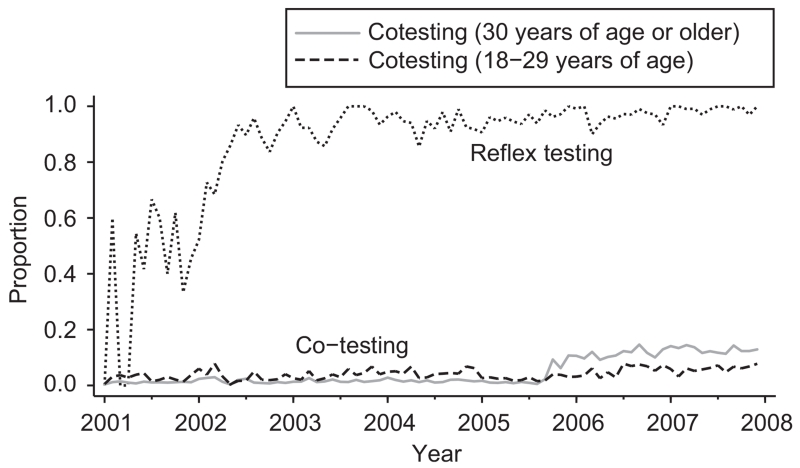
We next plotted the monthly proportions of HPV reflex testing and HPV cotesting with Pap (stratified by patient ages 18–29 years and 30 years or older). The proportion of reflex testing increased during the study time period from 0% to nearly 100% (Fig. 2). The variation in reflex testing before 2003 may be related to the changing frequencies of conventional and liquid-based Pap tests, as HPV reflex testing was generally performed only on liquid-based Pap specimens through the Johns Hopkins Hospital Division of Cytopathology. For HPV cotesting with Pap, testing was slightly more frequent among patients aged 18–29 years, which was non–evidence-based use, than among patients 30 years or older from 2001 to mid-2005. In mid- to late-2005, the proportion of cotesting among patients 30 years or older notably increased nearly 5-fold from to 2.2% to 10.7%. The highest proportion, 15%, was observed in September 2006, and the trend later plateaued around 13% (Fig. 2).
Fig. 2.
Monthly proportions of human papillomavirus (HPV) reflex testing and cotesting with Pap for Pap specimens processed by the Johns Hopkins Hospital Division of Cytopathology, 2001–2007.
To more quantitatively estimate trends of HPV testing over time, we fit joinpoint regression models to the monthly proportion data. We identified points of statistically significant change in temporal linear trends and we calculated monthly percent change for HPV reflex testing and for HPV cotesting with Pap stratified by age. For reflex testing, three trends of monthly percent change were identified: −1.1% for January 2001 to December 2001, 13.2% for December 2001 to May 2002, and 0.1% for May 2002 to December 2007 (Table 2). The monthly percent change estimate between December 2001 and May 2002, 13.2% (95% confidence interval 1.4%–26.5%), reflected a significant change in trend as the estimate was statistically significant from 0 (ie, statistically significant from 0 monthly percent change).
Table 2. Type of Human Papillomavirus Testing and Associated Time Trends, Including Monthly Percent Change Estimates.
| Time Trend 1 |
Time Trend 2 |
Time Trend 3 |
Time Trend 4 |
|||||
|---|---|---|---|---|---|---|---|---|
| HPV Testing | Period | MPC | Period | MPC | Period | MPC | Period | MPC |
| Reflex | Jan 2001– Dec 2001 |
−1.1 (−4.5–2.5) | Dec 2001– May 2002 |
13.2* (1.4–26.5) | May 2002– Dec 2007 |
0.1 (0.0–0.2) | N/A | N/A |
| Cotesting with Pap, 30 y or older |
Jan 2001– Feb 2002 |
6.6* (0.8–12.7) | Feb 2002– July 2005 |
−0.8 (−1.8–0.1) | July 2005– Nov 2005 |
66.9* (7.1–159.9) | Nov 2005– Dec 2007 |
1.0* (0.3–1.7) |
| Cotesting with Pap, 18–29 y |
Jan 2001– Dec 2004 |
1.0* (0.2–1.8) | Dec 2004– May 2005 |
−18.1 (−44.2–20.4) | May 2005– July 2006 |
9.0* (2.5–15.9) | July 2006– Dec 2007 |
0.2 (−2.4–2.9) |
HPV, human papillomavirus; MPC, monthly percent change; N/A, not applicable.
Data are % (95% confidence interval).
Monthly percent change is statistically significant from 0.
For evidence-based HPV cotesting with Pap among patients 30 years or older, four trends of monthly percent change were identified: 6.6% for January 2001 to February 2002, −0.8% for February 2002 to July 2005, 66.9% for July 2005 to November 2005, and 1.0% for November 2005 to December 2007. Three of these trends were statistically significant. For non–evidence-based HPV cotesting with Pap among patients younger than 30 years, there were also four trends of monthly percent change: 1.0% for January 2001 to December 2004, −18.1% for December 2004 to May 2005, 9.0% for May 2005 to July 2006, and 0.2% for July 2006 to December 2007. Two of these trends were statistically significant (Table 2).
DISCUSSION
Using laboratory and administrative data, we estimated actual provider use of the HPV test for two FDA-approved indications: 1) HPV reflex testing for ASC-US Pap results and 2) HPV cotesting with Pap among women 30 years or older in the Baltimore metropolitan area. HPV reflex testing of ASC-US Pap results is considered the standard of care in cervical cancer prevention in the United States, and the proportion of reflex testing increased to nearly 100% during the study period. A survey of 679 cytology laboratories in the United States found that 89.8% of laboratories offered HPV testing for reflex of ASC-US Pap test results.18
Our findings reveal that a small proportion (ie, 15% at most) of women 30 years or older received HPV testing with their Pap test for cervical cancer screening, despite HPV cotesting with Pap for this patient population being recommended since 2004 by three prominent professional organizations (reviewed in Wright et al3) and reimbursed by many insurance companies.19 This represents a significant gap in prevention practices and patient care. Although HPV cotesting with Pap is available through the Johns Hopkins Hospital Division of Cytopathology, Moriarty et al reported that only 24.5% of a sample of cytology laboratories offered non–reflex HPV testing for screening for women 30 years or older, and 59.3% offered non–reflex HPV testing for any reason.18 These data also reveal women aged 18–29 years received cotesting with Pap screening, even though the test is not FDA-approved for cotesting use among women younger than 30 years.1 Analogous relationships of “off-label” use have been observed in surveys of providers8,9 and cytology laboratories.18
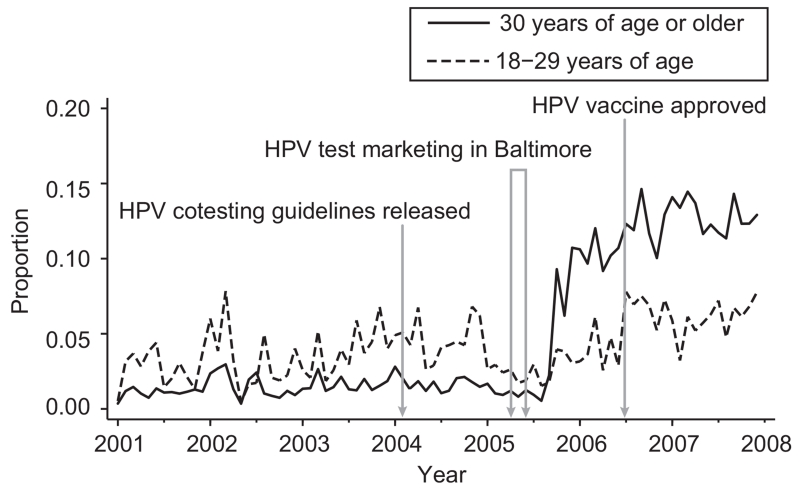
We considered the study’s findings in the context of developments in cervical cancer prevention (Fig. 3): ASCCP guidelines began to include HPV cotesting for women 30 years or older (February 2004), consumer marketing of the test in the Baltimore, Maryland (March–May 2005) and Washington, DC (April–June 2006) metropolitan areas, and FDA approval of the first HPV vaccine (June 2006). It is intriguing that the increase in 2005 takes place shortly after the HPV test marketing campaign in the Baltimore metropolitan area, although we can in no way establish causality. Patient request for the HPV test and provider misuse of the test may be related to marketing aimed at providers and patients9 and to media coverage of the HPV vaccine and cervical cancer prevention.14 It would be elucidating to characterize the relative effect of updated guidelines compared with consumer and provider marketing.
Fig. 3.
Monthly proportions of human papillomavirus (HPV) cotesting with Pap (stratified by patient age) for Pap specimens processed by the Johns Hopkins Hospital Division of Cytopathology, shown with key dates in cervical cancer prevention in the Baltimore, Maryland, metropolitan area, 2001–2007.
A large strength of this study was its unique data source, which contained data on actual provider practices (compared with self-report practices). A similar provider practice analysis was recently reported using Florida Medicaid administrative claims data.10 This study also showed an increase in usage of HPV DNA tests from 0.6% in July 2001 to 9.0% in June 2009. Other strengths of this work include its contiguous 7-year study period and that all data are from one cytology laboratory. The 178,510 Pap specimens analyzed were from 85,048 unique women in more than 200 unique clinics. In contrast to the Florida Medicaid study, there was a large amount of heterogeneity among the patients by age, race, and insurance type. Further, we were able to document time trends of HPV testing stratified by reflex and cotesting indication.
The analysis is not without limitations. The Pathology Data System is not a gold standard of provider practice data and we lacked information such as patient test request, screening history, and time when insurance companies began reimbursement for HPV cotesting. We also captured data only from Pap specimens sent to the Johns Hopkins Hospital Division of Cytopathology for evaluation. If a patient’s insurance company required the specimen be sent to Quest Diagnostics or LabCorp, we had no opportunity to capture the Pap or HPV test results or both. As such, we had no way to quantify the direction or magnitude of this selection bias, nor could we evaluate whether screening practices varied by cytology laboratory or insurance company. Whereas it is helpful to consider the role of extrinsic factors, such as marketing, on HPV test use, we cannot assume causality in time trends. Given the academic setting of Johns Hopkins and that HPV cotesting is not available in all cytology laboratories,18 the findings of this study may not be generalizable to other organizations and settings.
This study identifies a significant opportunity for improving preventive care and allows providers to compare their own practices with those reported here. The findings of this study suggest there may be barriers to adoption of HPV cotesting with Pap among women 30 years or older. More research is needed to fully characterize use of HPV testing, including system-, provider-, and patient-level factors that may affect screening practices and preferences, such as provider sex6,9 and patient race or ethnicity.20
Acknowledgments
Supported by public health research dissertation grant 1R36CK000103-01 from the Centers for Disease Control and Prevention.
The authors thank Dr. Elizabeth Platz, Dr. Angelo De Marzo, Fran Burroughs, Dee Kelly, and Jim Morgan for assistance in developing this research.
Footnotes
Presented at the American Public Health Association 137th Annual Meeting, November 7–11, 2009, Philadelphia, Pennsylvania.
The contents of this manuscript are solely the responsibility of the first author and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
Financial Disclosure
Patti E. Gravitt is a member of the Women’s Health Scientific Advisory Board for QIAGEN Corporation. The other authors did not report any potential conflicts of interest.
REFERENCES
- 1.U.S. Food and Drug Administration FDA approves expanded use of HPV test. 2003 Mar 31; Available at: http://www.fda.gov/ohrms/dockets/dockets/07p0210/07p-0210-ccp0001-01-FDA-News-vol3.pdf. Retrieved February 9, 2011.
- 2.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 3.Wright TC, Jr, Schiffman M, Solomon D, Cox JT, Garcia F, Goldie S, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304–9. doi: 10.1097/01.AOG.0000109426.82624.f8. [DOI] [PubMed] [Google Scholar]
- 4.Human papillomavirus. American College of Obstetricians and Gynecologists Obstet Gynecol. 2005;105:905–18. doi: 10.1097/00006250-200504000-00056. ACOG Practice Bulletin No. 61. [DOI] [PubMed] [Google Scholar]
- 5.Saslow D, Runowicz CD, Solomon D, Moscicki AB, Smith RA, Eyre HJ, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. J Low Genit Tract Dis. 2003;7:67–86. doi: 10.1097/00128360-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 6.McCree DH, Leichliter JS, Hogben M, St. Lawrence JS. National survey by specialty of U.S. physicians’ HPV screening practices. Prev Med. 2003;36:159–63. doi: 10.1016/s0091-7435(02)00021-x. [DOI] [PubMed] [Google Scholar]
- 7.Berkowitz Z, Saraiya M, Benard V, Yabroff KR. Common abnormal results of pap and human papillomavirus cotesting: what physicians are recommending for management. Obstet Gynecol. 2010;116:1332–40. doi: 10.1097/AOG.0b013e3181fae4ca. [DOI] [PubMed] [Google Scholar]
- 8.Saraiya M, Irwin KL, Carlin L, Chen X, Jain N, Benard V, et al. Cervical cancer screening and management practices among providers in the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) Cancer. 2007;110:1024–32. doi: 10.1002/cncr.22875. [DOI] [PubMed] [Google Scholar]
- 9.Irwin K, Montano D, Kasprzyk D, Carlin L, Freeman C, Barnes R, et al. Cervical cancer screening, abnormal cytology management, and counseling practices in the United States. Obstet Gynecol. 2006;108:397–409. doi: 10.1097/01.AOG.0000230258.07737.fa. [DOI] [PubMed] [Google Scholar]
- 10.Price RA. Association between physician specialty and uptake of new medical technologies: HPV tests in Florida Medicaid. J Gen Intern Med. 2010;25:1178–85. doi: 10.1007/s11606-010-1415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calloway C, Jorgensen CM, Saraiya M, Tsui J. A content analysis of news coverage of the HPV vaccine by U.S. newspapers, January 2002-June 2005. J Womens Health (Larchmt) 2006;15:803–9. doi: 10.1089/jwh.2006.15.803. [DOI] [PubMed] [Google Scholar]
- 12.Anhang R, Stryker JE, Wright TC, Jr, Goldie SJ. News media coverage of human papillomavirus. Cancer. 2004;100:308–14. doi: 10.1002/cncr.20006. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration FDA licenses new vaccine for prevention of cervical cancer and other diseases in females caused by human papillomavirus. 2006 Jun 8; Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108666.htm. Retrieved February 9, 2011.
- 14.Anhang R, Goodman A, Goldie SJ. HPV communication: review of existing research and recommendations for patient education. CA Cancer J Clin. 2004;54:248–59. doi: 10.3322/canjclin.54.5.248. [DOI] [PubMed] [Google Scholar]
- 15.Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. ASCCP-Sponsored Consensus Conference. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120–9. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute Joinpoint regression program. 2011 Feb 9; Available at: http://surveillance.cancer.gov/joinpoint. Retrieved.
- 18.Moriarty AT, Schwartz MR, Eversole G, Means M, Clayton A, Souers R, et al. Human papillomavirus testing and reporting rates: practices of participants in the College of American Pathologists Interlaboratory Comparison Program in Gynecologic Cytology in 2006. Arch Pathol Lab Med. 2008;132:1290–4. doi: 10.5858/2008-132-1290-HPTARR. [DOI] [PubMed] [Google Scholar]
- 19.QIAGEN Health insurance coverage for the digene HPV test. 2011 Feb 9; Available at: http://www.thehpvtest.com/getting-the-test/insurance-coverage. Retrieved.
- 20.Huang AJ, Perez-Stable EJ, Kim SE, Wong ST, Kaplan CP, Walsh JM, et al. Preferences for human papillomavirus testing with routine cervical cancer screening in diverse older women. J Gen Intern Med. 2008;23:1324–9. doi: 10.1007/s11606-008-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]