Abstract
Excessive activation of the complement system is detrimental in acute inflammatory disorders. In this study, we analyzed the role of complement-derived anaphylatoxins in the pathogenesis of experimental acute lung injury/acute respiratory distress syndrome (ALI/ARDS) in C57BL/6J mice. Intratracheal administration of recombinant mouse complement component (C5a) caused alveolar inflammation with abundant recruitment of Ly6-G+CD11b+ leukocytes to the alveolar spaces and severe alveolar-capillary barrier dysfunction (C5a-ALI; EC50[C5a] = 20 ng/g body weight). Equimolar concentrations of C3a or desarginated C5a (C5adesArg) did not induce alveolar inflammation. The severity of C5a-ALI was aggravated in C5-deficient mice. Depletion of Ly6-G+ cells and use of C5aR1−/− bone marrow chimeras suggested an essential role of C5aR1+ hematopoietic cells in C5a-ALI. Blockade of PI3K/Akt and MEK1/2 kinase pathways completely abrogated lung injury. The mechanistic description is that C5a altered the alveolar cytokine milieu and caused significant release of CC-chemokines. Mice with genetic deficiency of CC-chemokine receptor (CCR) type 5, the common receptor of chemokine (C-C motif) ligand (CCL) 3, CCL4, and CCL5, displayed reduced lung damage. Moreover, treatment with a CCR5 antagonist, maraviroc, was protective against C5a-ALI. In summary, our results suggest that the detrimental effects of C5a in this model are partly mediated through CCR5 activation downstream of C5aR1, which may be evaluated for potential therapeutic exploitation in ALI/ARDS.—Russkamp, N. F., Ruemmler, R., Roewe, J., Moore, B. B., Ward, P. A., Bosmann, M. Experimental design of complement component 5a-induced acute lung injury (C5a-ALI): a role of CC-chemokine receptor type 5 during immune activation by anaphylatoxin.
Keywords: ARDS, polymorphonuclear neutrophils, C5aR1, maraviroc, cytokines
Acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) are major health care problems, with ∼200,000 cases annually in the United States and an overall mortality rate of 20–40% (1, 2). ALI/ARDS is characterized by the acute onset of dyspnea, severe hypoxemia, and bilateral radiographic lung infiltrations. These symptoms are caused by excessive alveolar inflammation and accompanying pulmonary edema (1, 3). No specific treatment is yet available, which points up the need for a better understanding of the underlying molecular pathogenesis to eventually improve the prognosis of patients who have ALI/ARDS. Small-animal models may contribute substantially to this quest.
The exudative phase of ARDS is characterized by an intra-alveolar accumulation of inflammatory cells, the development of protein-rich edema, hemorrhage, and the formation of alveolar hyaline membranes (1). Substantial evidence implies a role for pattern recognition receptors, cytokine release, neutrophil extracellular traps, complement activation, production of reactive oxygen species in this process (4, 5). Under homeostatic conditions, the fluid lining of airways and alveoli contains substantial amounts of locally produced complement components that provide protection against inhaled pathogens (6). Although patients with hereditary complement deficiencies may display severe and recurring respiratory tract infections (7), excessive activation of the complement cascade can be extremely detrimental in the lungs (8). A particularly deleterious role has been associated with alveolar generation of activated complement component 5 (C5)a (4, 8).
Cleavage of C5 into C5a and C5b occurs in all traditional complement activation pathways. In addition, plasma/leukocyte-derived serine proteases exhibit C5-cleaving activity and can initiate a so-called extrinsic pathway of complement activation (9, 10). Whereas C5b acts as anchor for assembly of the membrane attack complex for direct pathogen lysis, C5a exhibits high biologic effects on host cells. C5a (11 kDa, 74–77 amino acids, depending on species) has a short plasma half-life as it is degraded to desarginated C5a (C5adesArg) by carboxypeptidase N within minutes (11, 12). C5a-induced cellular responses are mediated by 2 receptors: a G-protein–coupled receptor termed complement component 5a receptor 1 [C5aR1; cluster of differentiation (CD)88] and the largely homologous C5aR2 (C5L2, GPR77), which lacks an intracellular adaptor molecule (13, 14). Ligation of C5aR1 promotes chemotaxis and intracellular activation of the PI3K and MAPK pathways (15), but the role of C5aR2 in inflammation is still a matter of dispute (16).
There is compelling evidence that complement activation contributes to the pathogenesis of ARDS via local generation of C5a. Bronchoalveolar lavage fluid (BALF) from patients with ARDS contains substantial amounts of C5a (17, 18). Experimental activation of the complement system in rodents after intravenous administration of cobra venom factor leads to an ARDS-like condition in a C5a-dependent manner (19). Similar symptoms have been observed when purified C5a was administered intratracheally in several mammalian species (20–23). Interruption of endogenous C5a signaling protects rodents and primates in diverse models of ALI/ARDS (9, 24–26). The mechanistic explanation is that C5a facilitates the transmigration of polymorphonuclear neutrophils (PMNs) from the pulmonary circulation to alveolar walls and spaces (e.g., through up-regulation of adhesion molecules such as intracellular adhesion molecule-1 and P-selectin) (27, 28). Moreover, C5a is a potent inducer of the pulmonary cytokine response (26, 29).
Despite a body of evidence indicating the deleterious effects of complement activation in ALI/ARDS, a suitable experimental model for direct examination of the role of C5a and C5a-receptors has not yet been characterized in adequate detail. Consequently, the understanding of molecular mechanisms that are initiated by C5a in lungs is still insufficient. In this study, we used a model of C5a-induced ALI in mice to assess the adverse effects of alveolar generation of C5a and to identify interactions that may contribute to this process. We found that activation of CC-chemokine receptor (CCR) 5 downstream of C5aR1 determines the severity of C5a-induced ALI, which may be important in the quest for therapeutic strategies for ALI/ARDS.
MATERIALS AND METHODS
Animals
All experiments were conducted in accordance with the animal protection act of Germany, the State Investigation Office of Rhineland-Palatinate, the U.S. National Institutes of Health Guidelines, and the University Committee on Use and Care of Animals of the University of Michigan.
The following mouse strains (10- to 12-wk-old, 25 g males) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA): C57BL/6J, CCR5−/− (B6.129P2-Ccr5tm1Kuz/J), C5-deficient (B10.D2-Hc0 H2d H2-T18c/oSnJ mice), C5-sufficient (B10.D2-Hc1 H2d H2-T18c/nSnJ), and TLR4−/− (B6.B10ScN-Tlr4lps-del/JthJ). C5aR1−/− mice (backcrossed for >10 generations on C57BL/6J) were bred and housed at the University of Michigan.
ALI
Mice were anesthetized with ketamine and xylazine before surgical exposure of the trachea (22). For induction of ALI, mice received the following substances in 40 µl phosphate buffered saline (PBS) as an intratracheal instillation: recombinant mouse C5a or C3a (rmC5a or rmC3a; R&D Systems, Minneapolis, MN, USA), C5adesArg/C5a (Hycult, Plymouth Meeting, PA, USA), LPS (Escherichia coli, O111:B4, Sigma-Aldrich, St. Louis, MO, USA), or anti-BSA IgG (MP Biomedicals, Santa Ana, CA, USA), together with 0.5 mg i.v. bovine serum albumin (BSA; Sigma-Aldrich).
BALF analysis
At the end of the experiments, the lungs were lavaged with 1 ml sterile PBS. The albumin concentration was assessed by ELISA (Bethyl Laboratories, Montgomery, TX, USA). BALF cellularity was counted with a hemocytometer after lysis of erythrocytes. Multiple cytokines/chemokines were quantified with the BioPlex Pro assay (Bio-Rad, Hercules, CA, USA) (30).
Bone marrow transplantation
Bone marrow chimeric mice were generated as described elsewhere (31). In short, syngeneic recipient mice were irradiated with 13 Gy delivered in 2 fractions separated by 3 h (X-ray source). Whole bone marrow cells (5 × 106) from donor mice were infused intravenously into the recipients. Sufficient engraftment was confirmed by flow cytometry of circulating leukocytes 6 wk after transplantation and before experimental use.
High-resolution magnetic resonance imaging
Acquisition of high-resolution magnetic resonance imaging (HR-MRI) scans was performed as described by us earlier (22). Briefly, magnetic resonance images were acquired in a 7.0T MR Scanner (Agilent, Palo Alto, CA, USA) within 10 min after euthanization of the individual mice, to avoid motion artifacts caused by ventilation. A fast spin-echo sequence was used to generate axial proton density/T1-weighted images. Fifteen contiguous slices (slice thickness, 1 mm) were obtained with a total scan time of ∼4 min per mouse.
Immunoprecipitation and Western blot analysis
For detection of C5a, the lungs were homogenized and lysed in RIPA buffer containing protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA). Total lung proteins (1 mg) or BALF (500 μl) was immunoprecipitated with a customized rabbit anti-C5a affinity-purified antiserum (immunogenic sequence: CTIANKIRKESPHKPVQLGR; Lampire Biological Laboratories, Everett, PA, USA) and protein A agarose beads. Proteins were separated by electrophoresis (15% SDS-polyacrylamide gel), and blotted onto nitrocellulose membranes that were treated with the anti-C5a antiserum, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (GE Healthcare Life Sciences, Marlborough, MA, USA) and chemiluminescent substrate (Denville Scientific, South Plainfield, NJ, USA).
Microscopy
For bright-field microcopy, paraformaldehyde (4%) was used for fixation of lungs, and 3 μm paraffin-embedded sections were stained with hematoxylin and eosin (H&E) (32).
Transmission electron micrographs were acquired with a CM100 electron microscope (Philips, Leuven, The Netherlands) on 70 nm lung sections, which were prepared according to a published method (22).
For fluorescent microscopy, lungs were snap frozen in Tissue-Tek optimal cutting temperature compound (Sakura Finetek, Torrance, CA, USA), cryosectioned, and fixed in 4% formaldehyde (Thermo Fisher Scientific, Waltham, MA, USA). After blocking (10% normal mouse serum, 7% BSA) the sections were treated with rat IgG2b isotype control or rat anti-Ly6G antibody (clone RB6-8C5; eBioscience, San Diego, CA, USA) followed by 1 h with donkey anti-rat AF594 (Life Technologies-Invitrogen, Carlsbad, CA, USA) and mounted with Prolong Gold (Invitrogen). A BX-51 microscope (×40/0.9, ×60/1.4 oil, and ×100/1.4 oil), with a DP-70 camera and DP Controller Software (all from Olympus, Center Valley, PA, USA), was used for image acquisition.
PMNs were harvested by peritoneal lavage after intraperitoneal injection of 2× 1 ml casein solution (9% w/v; Sigma-Aldrich), at −15 and −3 h before lavage, and cultured in RPMI 1640 medium (25 mM HEPES, 100 U/ml penicillin-streptomycin, and 0.1% BSA) at 37°C and 5% CO2.
Flow cytometry
Cells were stained with the following anti-mouse antibodies and matched isotype controls according to standard protocols (15): Ly-6G-eFluor450 and Ly-6G-APC (clone RB6-8C5; eBioscience), p-Akt(threonine308)-PE (clone J1-223.371; BD Biosciences, Franklin Lakes, NJ, USA), p-MEK1/2(serine218/serine222)-AF647 (clone O24-836; BD Biosciences), p-ERK1(threonine203/tyrosine205)/ERK2(threonine183/tyrosine185)-AF488 (clone 20A; BD Biosciences), C5aR1-PE (clone 20/70; BioLegend, San Diego, CA, USA), CCR5-PE (clone HM-CCR5; BioLegend), CD11b-eFluor450 (clone M1/70; eBioscience), and CD45-FITC (clone 30-F11; eBioscience). In some experiments, counting beads were added to flow cytometry samples to calculate the number of cells. Data were collected on a FACSCanto II (BD Biosciences) and analyzed with FlowJo 7.6.4 (Tree Star, Ashland, OR, USA) or WinList (Verity Software House, Topsham, ME, USA) software.
Reagents
Circulating PMNs were depleted by using 200 µg/mouse of anti-mouse-Ly-6G IgG (clone RB6-8C5; eBioscience) at day –1 before ALI experiments and were used, together with isotype IgG2b (clone eB149/10H5, eBioscience), as the control. Wortmannin [1 µg/g body weight (BW); Invivogen, San Diego, CA, USA] and SL-327 (100 µg/g BW; Tocris Bioscience, Bristol, United Kingdom) were injected intraperitoneally. Maraviroc (10 µg/g BW, Tocris Bioscience) was given as an oral gavage 30 min before induction of ALI, whereas control animals received PBS vehicle (<0.1% DMSO final concentration). The doses of these drugs were deduced from previous reports (33–35).
Statistical analysis
Data were analyzed and visualized with Prism, version 6.05 (GraphPad, San Diego, CA, USA). Data are expressed as means ± sem. Group differences were tested for significance by the nonparametric Wilcoxon-Mann-Whitney test or unpaired Student’s t test. In vitro experiments were repeated a minimum of 3 times. The number of mice used for in vivo studies was ≥5/group for most experiments. We considered differences significant at P < 0.05.
RESULTS
Endogenous C5a is generated in murine models of ALI
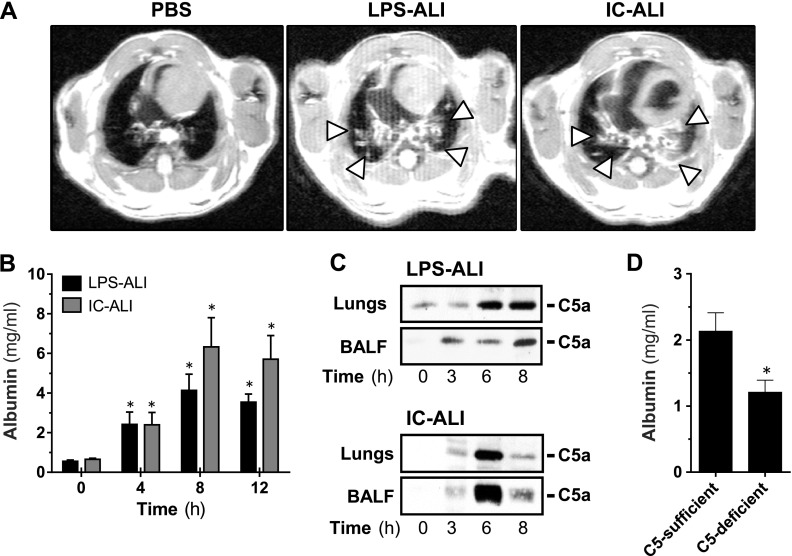
Initially, we investigated the role of endogenous C5a in 2 established experimental models of ALI. The development of bilateral pulmonary infiltrates is a major criterion for the clinical diagnosis of ARDS in humans, and HR-MRI revealed substantial infiltrates in C57BL/6J mice that were subjected to LPS-ALI or IgG immune-complex–induced ALI (IC-ALI) but not in sham-surgery control animals (Fig. 1A, arrowheads). Albumin concentrations in BALFs served as surrogate endpoints of ALI as they were used to assess the degree of damage to the alveolar-capillary barrier integrity. In LPS-ALI and IC-ALI, the peak of injury to the barrier occurred at 8 h (Fig. 1B), the time point used for all subsequent experiments. Lung injury was preceded by the generation of the anaphylatoxin C5a in BALF and lung homogenate in both ALI models (Fig. 1C). The pivotal role of endogenous C5a was confirmed, because mice that were genetically deficient in C5 were partially protected from development of LPS-ALI (Fig. 1D).
Figure 1.
Endogenous C5a mediates ALI. A) Chest scans acquired by HR-MRI of C57BL/6J mice 8 h after induction of LPS-ALI or IC-ALI or sham surgery (PBS). Arrowheads: areas of pulmonary infiltration. B) Disruption of the alveolar-capillary barrier integrity as measured by ELISA of alveolar albumin concentrations in BALF sampled 4, 8, or 12 h after induction of LPS-ALI or IC-ALI. C) Western blot analysis for C5a in BALF and whole-lung lysate that were obtained at different time points after LPS-ALI or IC-ALI. D) Alveolar albumin concentrations in C5-deficient mice vs. C5-sufficient mice 8 h after induction of LPS-ALI. All experiments were performed in C57BL/6J mice. *P < 0.05.
Airway administration of rmC5a results in recruitment of leukocytes and disruption of alveolar-capillary barrier function
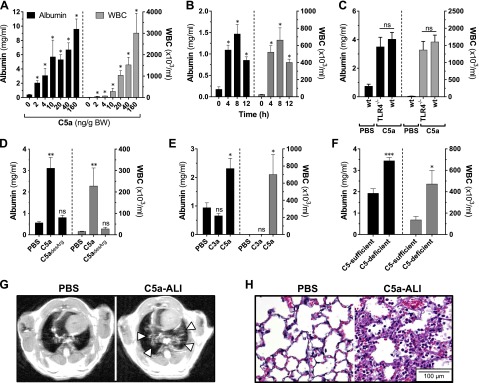
To investigate the pulmonary effects of C5a, we used a model of C5a-ALI in C57BL/6J mice (22). Instillation of rmC5a [2–160 ng/g BW, intratracheally (i.t.)] resulted in a dysfunction of the alveolar-capillary barrier as well as in voluminous recruitment of white blood cells (WBCs) to the BALF in a dose-dependent manner (Fig. 2A). For reliable induction of ALI, we used intratracheal administration of 20 ng rmC5a per gram BW in subsequent experiments. A time-course experiment revealed that maximum lung damage and influx of WBCs occurred 8 h after administration of rmC5a, although a significant increase in these hallmark parameters was detectable 4 h earlier (Fig. 2B). For subsequent experiments, samples were collected at the 8 h time point. We excluded relevant contamination with endotoxin in the E. coli–derived rmC5a by the observation that it was equally potent in TLR4−/− mice (Fig. 2C). Because C5a is inactivated immediately by the plasma enzyme carboxypeptidase N in physiologic conditions, the effects of its degradation product, C5adesArg, were also studied. Although C5adesArg was implicated in the development of ALI decades ago (20), pulmonary challenge with rmC5adesArg (in equimolar doses, as compared to doses of rmC5a) did not elicit a detectable inflammatory response (Fig. 2D). Furthermore, airway administration of rmC3a did not reproduce the ALI phenotype that was observed when equimolar concentrations of rmC5a were used (Fig. 2E).
Figure 2.
Airway administration of rmC5a causes ALI (rmC5a, rmC3a and rmC5adesArg dose, 20 ng/g BW, i.t., unless otherwise specified; negative control, equal dose of PBS). A) Alveolar albumin concentrations and WBC counts 8 h after intratracheal administration of different doses of rmC5a in C57BL/6J mice. B) Time course of C5a-ALI, as assessed by alveolar albumin concentrations and WBC counts. C) C57BL/6J (wt) mice and TLR4−/− mice received rmC5a, and alveolar albumin and WBCs were assessed 8 h later. Sham surgery (PBS) was the negative control. Alveolar albumin concentrations and WBC counts 8 h after instillation of (D) rmC5adesArg vs. rmC5a or control PBS; (E) rmC3a vs. rmC5a or control PBS; or (F) rmC5a in C5-sufficient vs. C5-deficient mice. G) Chest scans (HR-MRI) 8 h after administration of rmC5a or PBS. Arrowheads: areas of pulmonary infiltration. H) Representative micrographs of lung sections 8 h after administration of rmC5a or PBS. All experiments were performed in C57BL/6J mice unless otherwise indicated. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant when compared with PBS. H&E staining. Magnification, ×400.
It has been reported that activated alveolar macrophages and PMNs release serine proteases that cleave local C5 into C5a (an extrinsic protease pathway of complement activation) (9, 10). To test whether intratracheal C5a administration would initiate a proinflammatory autoamplification loop through cleavage of more C5 by phagocyte-derived proteases, we induced C5a-ALI in C5-deficient mice. A hypothetical amplification loop was expected to cause less lung injury in the mice. However, the opposite observation was made: C5-deficient mice displayed intensified disruption of the alveolar-capillary barrier and a higher influx of phagocytes (WBCs) in response to C5a (Fig. 2F). No differences were noted in the expression levels of C5aR1 on phagocytes from C5-deficient mice compared with levels in C5-sufficient mice (data not shown).
To further characterize C5a-ALI, we assessed lung damage by magnetic resonance tomography and microscopy studies. HR-MRI showed central bilateral lung areas of high signal intensity consistent with pulmonary consolidation in C5a-challenged animals but not in sham-treated controls (Fig. 2G, arrowheads). Histopathology sections showed characteristic features of ALI in lungs after administration of rmC5a, such as abundant presence of inflammatory cells, alveolar fibrin depositions, and intra-alveolar hemorrhage (Fig. 2H). In conclusion, these data suggest that C5a, rather than C3a or C5adesArg, is a potent mediator of the acute inflammatory response during ALI.
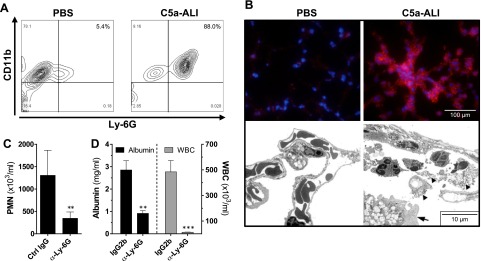
Development of C5a-ALI is dependent on Ly-6G+CD11b+ PMNs
C5a is a potent chemoattractant stimulus for PMNs. As a result, more than 85% of the WBCs that were recruited by C5a to the alveolar cavity were Ly-6G+CD11b+ PMNs (Fig. 3A). In the sham-surgery (PBS) mice only 5% of BALF cells were PMNs, whereas most of the nucleated cells in the sham group were CD11b+Ly-G6− alveolar macrophages. Fluorescence microscopy showed abundant expression of the PMN-specific surface marker Ly-6G in C5a-injured lung parenchyma (Fig. 3B, top). Moreover, transmigration of PMNs into the alveolar spaces was detected by transmission electron microscopy. The presence of fibrin depositions (arrowheads) and damaged type II alveolar epithelial cells (arrow) was also observed (Fig. 3B, bottom). To further investigate the role of PMNs in C5a-ALI, we induced experimental neutropenia by administration of a depleting antibody directed against the Ly-6G epitope on PMNs. Efficient depletion of circulating PMNs in peripheral blood was confirmed by automated cell counts (Fig. 3C). PMN-depleted C57BL/6J mice showed reduced BALF cellularity and significant protection from C5a-induced alveolar-capillary barrier dysfunction, as evaluated by alveolar albumin concentrations (Fig. 3D).
Figure 3.
C5a-induced ALI is dependent on Ly-6G+CD11b+ PMNs (C5a dose: 20 ng/g BW, i.t; negative control: equal dose of PBS). A) CD11b and Ly-6G surface expression on BALF cells was assessed by flow cytometry. Mice were subjected to C5a-ALI for 8 h. B) Top: Ly-6G expression in lungs of C57BL/6J mice that had been subjected to C5a-ALI or PBS sham surgery. Bottom: transmission electron micrographs of lungs 8 h after administration of rmC5a or PBS. Arrowheads: fibrin depositions; arrow: a damaged type II alveolar epithelial cell. Magnification, ×2600. C) Experimental neutropenia was induced by injection of a depleting antibody against Ly-6G (α-Ly-6G). Control mice received isotype IgG (Ctrl IgG). To assess neutropenia, automated complete blood counts were acquired from EDTA blood. D) Neutropenic mice and Ctrl IgG were subjected to C5a-ALI. Alveolar albumin concentrations and WBCs were detected 8 h after instillation of rmC5a. All images are of C57BL/6J mice. **P < 0.01, ***P < 0.001.
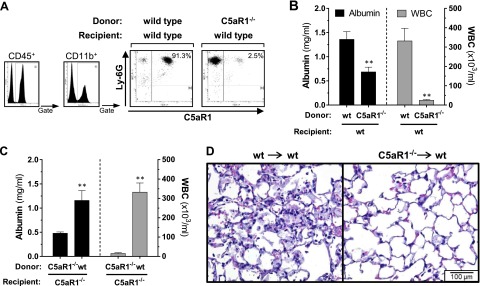
Expression of C5aR1 on hematopoietic cells is essential in the pathogenesis of C5a-ALI
The C5a receptor, C5aR1, has been shown to be expressed by hematopoietic cells as well as by a range of nonimmune cells in the alveolar microenvironment, including endothelial cells, fibroblasts, and alveolar epithelial cells (36, 37). However, in a novel C5aR1-reporter mouse strain, no substantial expression of C5aR1 was observed in alveolar epithelial cells in steady-state conditions (38). To evaluate the contribution of structural nonimmune lung cells in C5a-ALI, C5aR1−/−-deficient bone marrow was transplanted into irradiated C57BL/6J wild-type (wt) recipient mice. An irradiation and transplantation scheme was applied that also has been shown to deplete recipient alveolar macrophages efficiently (31). Sufficient engraftment of the transplanted bone marrow was demonstrated by the absence of circulating CD45+Ly-6G+CD11b+C5aR1+ cells in recipient mice 6 wk after transplantation (Fig. 4A). In the control group, bone marrow from C57BL/6J mice was transferred into irradiated syngeneic wt mice. Chimeric mice with selective absence of C5aR1 on hematopoietic cells were greatly protected from alveolar-capillary barrier dysfunction and recruitment of WBCs during C5a-ALI (Fig. 4B). Conversely, hallmark features of C5a-ALI were restored in C5aR1−/− recipient mice that had received bone marrow from the wt mice (Fig. 4C). Histopathology studies showed profound reduction of pulmonary inflammation in lung sections from wt mice that had received C5aR1−/− bone marrow (Fig. 4D). These data suggest that the detrimental effects induced by C5a are mediated by C5aR1+ hematopoietic cells (most likely PMNs) and that the effects of C5a ligation with C5aR1 on structural cells are of limited relevance during acute lung inflammation.
Figure 4.
Expression of the C5aR1 receptor on hematopoietic cells is essential during C5a-ALI (rmC5a dose: 20 ng/g BW, i.t.). A) Lethally irradiated C57BL/6J (wt) mice received C5aR1−/− bone marrow (C5aR1−/−/wt) or wt bone marrow [(wt/wt), control group] 6 wk earlier. To confirm sufficient engraftment, C5aR1 expression was assessed in peripheral blood leukocytes by flow cytometry. B) BALF albumin concentrations and WBC counts 8 h after intratracheal administration of rmC5a in bone marrow chimeric mice (wt/wt and C5aR1−/−/wt). C) Irradiated C5aR1−/− mice received either wt bone marrow (wt/C5aR1−/−) or C5aR1−/− bone marrow (C5aR1−/−/C5aR1−/−, control group). Albumin concentrations and WBC counts 8 h after instillation of rmC5a are presented. D) Representative lung sections 8 h after intratracheal administration of rmC5a in C5aR1−/−/wt and wt/wt bone marrow chimeric mice. **P < 0.01. H&E staining. Magnification, ×400.
Blockade of PI3K and MEK1/2 signaling in vivo abrogates C5a-ALI
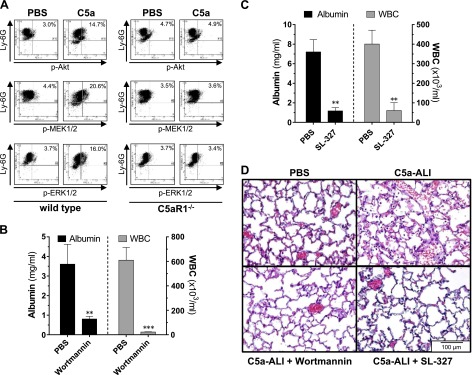
C5aR1 is a G-protein–coupled receptor that mediates intracellular phosphorylation of PI3K/Akt and MEK/ERK signaling proteins in leukocytes (15, 39). In cell cultures of PMNs (C57BL/6J), stimulation with C5a for 20 min (as compared to PBS-treated controls) resulted in increased frequencies of phospho-Akt+Ly-6G+ (3.0 vs. 14.7%), phospho-MEK1/2+Ly-6G+ (4.4 vs. 20.6%), and phospho-ERK1/2+Ly-6G+ (3.7 vs. 16.0%) cells (Fig. 5A, left). This activation was completely abrogated in PMNs derived from C5aR1−/− mice (Fig. 5A, right). To investigate the relevance of these signaling cascades in C5a-ALI, we tested specific protein kinase inhibitors . Systemic administration of the PI3K inhibitor wortmannin completely prevented the development of alveolar-capillary barrier dysfunction and PMN recruitment during C5a-ALI (Fig. 5B). Similar effects were observed after treatment with the cell-permeable MEK1/2 inhibitor SL-327 (Fig. 5C). Lungs of mice treated with either wortmannin or SL-327 before C5a-ALI showed less alveolar accumulation of PMNs, intra-alveolar hemorrhage, and fibrin deposits (Fig. 5D). These data indicate that concomitant activation of the PI3K/Akt and the MEK1/2 pathways were necessary for the development of C5a-induced lung inflammation. Whether the ERK1/2 pathway is essential, which seems likely, remains to be determined.
Figure 5.
C5aR1-mediated activation of the PI3K/Akt and MEK/ERK signaling pathways is essential in the development of C5a-ALI. A) Casein-elicited peritoneal PMNs were isolated from C57BL/6J (wt) mice or C5aR1−/− mice and stimulated with rmC5a (1 μg/ml) or PBS (negative control) for 20 min. Cells were stained for Ly-6G and phospho-Akt(Thr308), phospho-MEK1/2(Ser218/222), or phospho-ERK1(Thr203/Tyr205)/ERK2(Thr183/Tyr185) and analyzed by flow cytometry. Data are representative of 3 independent experiments. B) Alveolar albumin concentrations and WBC counts 8 h after administration of rmC5a (20 ng/g BW, i.t.) and treatment with the phosphoinositide 3-kinase inhibitor wortmannin (1 µg/g BW, i.p.) or PBS vehicle in C57BL/6J mice. C) Alveolar albumin concentrations and WBC counts 8 h after administration of rmC5a (20 ng/g BW, i.t.) and treatment with the MEK1/2-inhibitor SL-327 (100 µg/g BW, i.p.) or PBS vehicle in C57BL/6J mice. D) Representative lung sections of mice subjected to C5a-ALI (C5a: 20 ng/g BW, i.t. 8 h), treated with wortmannin, SL-327, or vehicle [concentrations as in (B, C)]. **P < 0.01, ***P < 0.001. H&E staining. Magnification, ×400.
C5a modulates the alveolar cytokine milieu and induces the release of ligands to CCR5
The activation of PI3K and MEK/ERK pathways is known to modulate cytokine responses, and we therefore sought to investigate the presence of mediators in the alveolar compartment during C5a-ALI. BALF recovered from C57BL/6J mice that had been subjected to C5a-ALI contained significantly different concentrations of several mediators, as compared to that from control mice (Table 1). Elevated concentrations were observed for IL-1β, IL-6, IL-12(p40), CCL3, CCL4, CCL5, CCL11, TNFα, granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF), whereas other mediators were not affected (Table 1).
TABLE 1.
Mediator concentrations in bronchoalveolar lavage fluids during C5a-ALI
| Analyte | Sham | C5a-ALI | P |
|---|---|---|---|
| Chemokines | |||
| CCL2 | 46.1 ± 10.6 | 83.3 ± 11.1 | 0.0511 |
| CCL3 | 21.9 ± 0.2 | 42.8 ± 6.6 | 0.006* |
| CCL4 | 9.2 ± 1.9 | 115.1 ± 25.7 | 0.0063* |
| CCL5 | 11.5 ± 1.9 | 298.5 ± 67.6 | 0.0054* |
| CCL11 | 71.8 ± 9.6 | 191.4 ± 5.0 | <0.0001* |
| CXCL1 | 221 ± 39.6 | 99 ± 9.1 | 0.0239* |
| Cytokines | |||
| IL-1α | 9.6 ± 1.1 | 9.6 ± 2.1 | 0.9903 |
| IL-1β | 49.7 ± 13.6 | 94.4 ± 9.2 | 0.0341* |
| IL-2 | 9.1 ± 2.6 | 11.4 ± 2.5 | 0.5603 |
| IL-6 | 47.5 ± 51.5 | 474.3 ± 92.4 | 0.0039* |
| IL-9 | 62.07 ± 8.7 | 77.1 ± 5.4 | 0.193 |
| IL-10 | 12.6 ± 0.8 | 15.8 ± 1.1 | 0.0553 |
| IL-12(p40) | 27.2 ± 3.9 | 53.5 ± 5.2 | 0.0068* |
| IL-12(p70) | 31.8 ± 1.3 | 30.0 ± 1.6 | 0.4281 |
| IL-13 | 32.9 ± 2.9 | 56.0 ± 10.3 | 0.0734 |
| IFN-γ | 19.5 ± 1.3 | 20.0 ± 2.7 | 0.8649 |
| TNF-α | 109.7 ± 12.4 | 1198 ± 292.5 | 0.0099* |
| G-CSF | 345.7 ± 60.5 | 1361 ± 287 | 0.0134* |
| GM-CSF | 18.5 ± 0.9 | 25.9 ± 2.7 | 0.0397* |
Data are mean ± sem concentrations (pg/ml). C5a-ALI was induced by instillation of rmC5a (40 ng/g BW, i.t.), control animals received an equivalent volume of PBS intratracheally, and BALF was recovered 8 h later. No relevant quantities of IL-3, -4, -5, and -17 were detected in the BALF (<10 pg/ml).
Significant differences by unpaired Student’s t test.
In conclusion, the alveolar cytokine milieu was selectively tilted toward a proinflammatory condition by C5a, which may indicate that indirect effects and amplification loops involving cytokines contribute to C5a-induced lung damage. In an interesting finding, C5a caused a release of the CC-chemokines, CCL3 [macrophage inflammatory protein (MIP)-1α], CCL4 (MIP-1β), and CCL5 (RANTES), which bind to a common receptor: CCR5. This chemokine release pattern suggests that engagement of CCR5 is a participant in the harmful outcomes in C5a-ALI.
Genetic deficiency or pharmacological inhibition of CCR5 protects against C5a-ALI
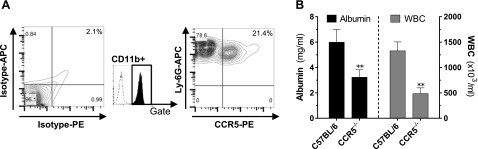
To study the role of CCR5 as a potential downstream effector mechanism during C5a-ALI, we first evaluated the expression of CCR5 on Ly-6G+CD11b+ PMNs (Fig. 6A). Next, we subjected CCR5−/− mice to C5a-ALI alongside C57BL/6J wt mice, which served as controls. CCR5−/− mice were significantly protected from the adverse events of C5a-ALI. Disruption of the alveolar-capillary barrier was reduced by ∼50% and the recruitment of leukocytes to the alveolar compartment was reduced by ∼65% (Fig. 6B).
Figure 6.
CCR5 mediates adverse events during C5a-ALI. A) Casein-elicited peritoneal PMNs were isolated, and Ly-6G+CD11b+ cells were analyzed for surface expression of CCR5 by flow cytometry. Isotype antibody was used as the control. Data are representative of 3 independent experiments. B) BALF albumin concentrations and WBC counts 8 h after administration of rmC5a (20 ng/g BW, i.t.) in C57BL/6J mice or CCR5−/− mice. *P < 0.05, **P < 0.01.
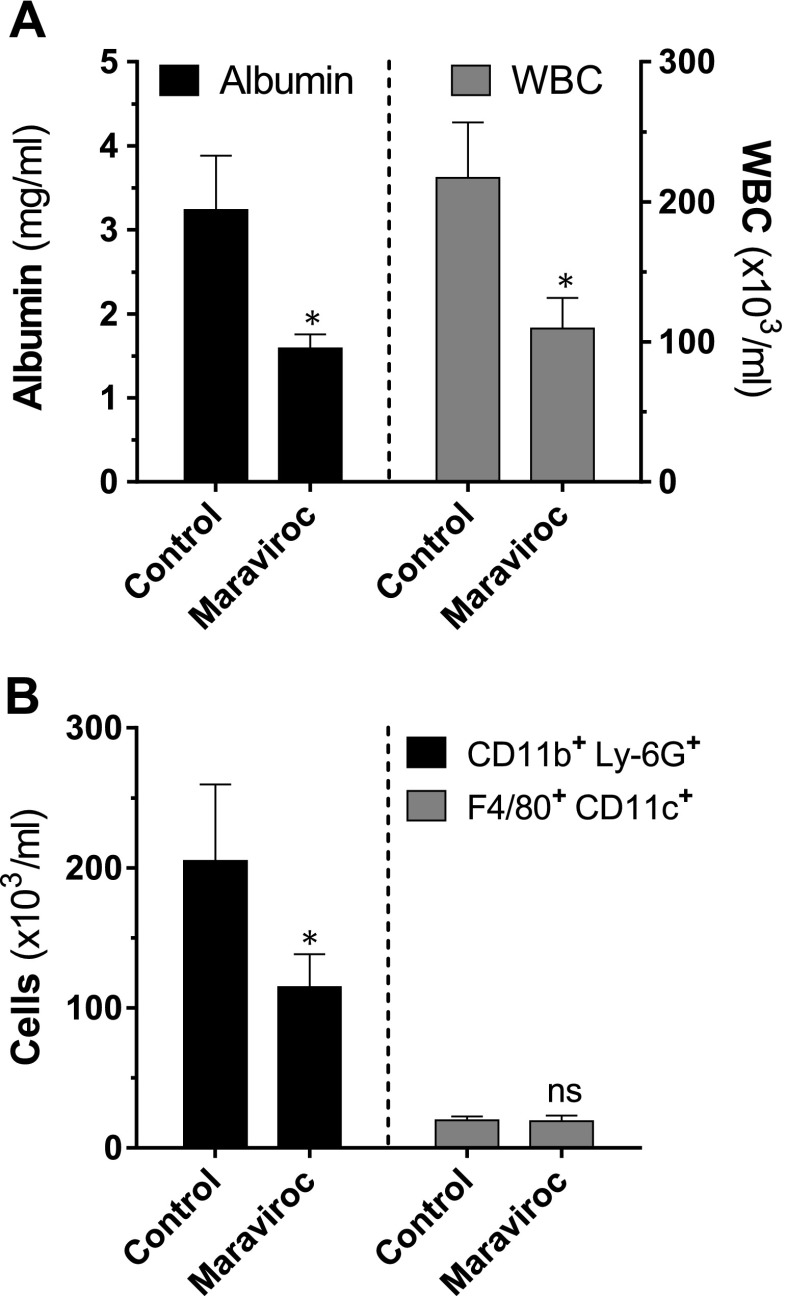
CCR5 is a coreceptor for the internalization of HIV, and CCR5 inhibitors constitute an important pillar in the treatment of HIV-positive patients. To determine whether pharmacological blockade of CCR5 would have beneficial effects during ALI, we used the antiretroviral drug and CCR5 receptor antagonist, maraviroc, in C5a-ALI. C57BL/6J mice received either maraviroc or vehicle by oral administration (gavage) briefly, before intratracheal instillation of rmC5a. C57BL/6J mice treated with maraviroc exhibited significantly attenuated BALF cellularity and alveolar-capillary barrier dysfunction during C5a-ALI (Fig. 7A). Reduction of BALF cells was caused by the diminished recruitment of Ly-6G+CD11b+ PMNs, whereas the number of F4/80+CD11c+ alveolar macrophages remained unaffected (Fig. 7B).
Figure 7.
Pharmacologic inhibition of CCR5 reduces the severity of C5a-induced ALI. A) BALF albumin concentrations and WBC counts 8 h after administration of rmC5a (20 ng/g BW, i.t.) in C57BL/6J mice treated with the CCR5 antagonist maraviroc (10 µg/g BW, orally, 30 min before C5a) or control vehicle. B) BALF cells were stained for surface expression of Ly-6G, CD11b, CD11c, and F4/80 from the same experiment. Total cell counts were obtained by flow cytometry with counting beads. *P < 0.05, **P < 0.01; ns, not significant.
In conclusion, these data identify CCR5 as a novel factor in the propagation of C5a-induced tissue damage in lungs. Antagonism of CCR5 by use of drugs such as maraviroc may have beneficial effects during ALI/ARDS.
DISCUSSION
In this study, we characterized an experimental model of C5a-ALI in mice. Our observation that CCR5 determined the severity of C5a-induced lung damage may be important in the quest for therapeutic strategies for ALI/ARDS. Furthermore, we found the PI3K/Akt and MEK1/2-ERK1/2 signaling pathways to be necessary during pulmonary recruitment of Ly-6G+CD11b+C5aR1+ hematopoietic cells. These data confirm the relevance of PI3K/Akt and MEK1/2-ERK1/2 activation downstream of C5aR1 in leukocytes in vivo (15, 39).
Intratracheal administration of rmC5a in mice rapidly caused dose-dependent changes that were pathognomonic for ALI/ARDS. Similar observations were made decades ago when C5a was purified from activated serum of pigs and humans and used to induce alveolar inflammation in guinea pigs and rabbits (20, 21, 40). However, these early reports should be interpreted carefully, because contamination with minute amounts of costimulatory molecules such as LPS cannot be excluded with certainty. A careful characterization of C5a-ALI appeared to be desirable, seeing that we and others have reported recently on the feasibility of rmC5a-mediated induction of lung inflammation (22, 23). In contrast to the earlier reports, neither C5adesArg nor C3a induced lung inflammation in our hands, when compared to equimolar concentrations of C5a. However, C5adesArg was effective in modulating the release of G-CSF in macrophage cultures (41).
Because of the discrepancy between the short half-life of C5a and its relatively long-lasting in vivo effects, a contribution of a C5a-induced autoamplification loop was considered. It has been reported that activated PMNs and alveolar macrophages produce serine proteases that are capable of local activation of complement (10, 42). Therefore, we investigated whether exogenously administered C5a induces further generation of endogenous C5a through cleavage of C5 by proteases from activated phagocytes. This hypothetical autoamplification loop should be interrupted in C5-deficient mice. However, C5-deficient mice displayed substantially increased phagocyte recruitment and a higher severity of C5a-ALI, than did C5-sufficient control mice. Because the expression levels of C5aR1 were similar in recruited phagocytes of C5-deficient and C5-sufficient mice, an explanation for the greater sensitivity of C5-deficient mice in C5a-ALI remains to be determined.
Our observations question the long-standing paradigm that C5a requires costimulatory signals for its major biologic activities. For example, we found rmC5a to be equally active in TLR4−/− mice for induction of lung inflammation, arguing against the need for an endotoxin as a costimulus with C5a in vivo.
Expression and activation of C5aR1 has been demonstrated in structural cells of the lungs, such as the alveolar epithelial cells (36, 37). However, the relevance of C5aR1 signaling in nonhematopoietic cells in vivo is not entirely clear. Data from novel C5aR1 reporter mice failed to demonstrate substantial expression of C5aR1 on nonmyeloid lung cells in steady-state conditions (38). To study the role of hematopoietic cells in C5a-ALI, we created 2 complementary groups of bone marrow chimeric mice on a C57BL/6J background. It is important to note that alveolar macrophages are tissue-resident immune cells of monocytic origin that develop early in life and proliferate self-sufficiently in the alveoli (43, 44). To exclude a confounding effect of this population, an irradiation and bone marrow transplantation scheme was chosen that efficiently depletes recipient alveolar macrophages, along with circulating and bone marrow–based hematopoietic cells. Indeed, our transplantation scheme resulted in a population of alveolar macrophages that was ∼88% donor derived (31). In the first group of chimeric mice, C5aR1 deficiency was restricted to cells of the hematopoietic lineage (donor/recipient: C5aR1−/−/wt). These animals were largely protected from C5a-induced lung damage. Conversely, reconstitution of C5aR1-deficient mice with C5aR1+ bone marrow from wt mice (donor/recipient: wt/C5aR1−/−) was sufficient to restore the detrimental effects of C5a in the lungs. These results support the idea that ligation of C5a to C5aR1 is critical in hematopoietic cells. Accordingly, direct effects of C5a on structural lung cells in the absence of C5aR1+ hematopoietic cells may be less important in C5a-ALI.
A mechanistic explanation for the development of the alveolar-capillary barrier dysfunction during C5a-ALI was recently presented, suggesting that local release of TNFα from PMNs in response to C5a causes a widening of endothelial gap junctions (45). Release of TNFα during IC-ALI requires the generation of C5a (26). In addition, the release of various other chemokines and cytokines is influenced by C5a and depends on activation of the PI3K/Akt and MEK1/2-ERK1/2 signaling pathways (26, 29, 39, 41, 46). Pulmonary deposition of C5a caused substantial release of the CC-chemokines CCL3, -4, and -5 into the alveoli. An essential role of these chemokines has been identified in other complement-dependent models of ALI (29, 47). CCL3, -4, and -5 mediate cell activation and chemotaxis through ligation with CCR5, which we found to be expressed on PMNs. CCR5−/− mice displayed markedly attenuated lung damage during C5a-ALI. Likewise, the allosteric CCR5 inhibitor maraviroc significantly reduced alveolar-capillary barrier dysfunction and PMN recruitment. These findings strongly suggest that the adverse effects of pulmonary generation of C5a are partly mediated by induction of CC-chemokines and subsequent activation of CCR5. Our data are in line with the finding that CCR5 contributes to endotoxin-induced lung inflammation (33). To what degree a formation of C5aR1/CCR5 heterodimers contributes to the inflammatory response elicited by C5a is not clear (48). Maraviroc is an FDA-approved drug with a favorable safety profile for antiretroviral treatment of HIV. This profile would facilitate clinical studies for testing maraviroc in patients at risk of ALI/ARDS.
C5-deficient mice are less protected than are C5aR1- and C5aR2-deficient mice in endotoxin-induced lung damage (LPS-ALI) (22, 49). It is possible that C5aR1 and -2 play additional roles in the acute inflammatory response to endotoxin (e.g., via crosstalk between C5aR1 and TLR4 or via a proposed heterodimerization of C5aR1 with CCR5 (48). It is in this context that we present C5a-ALI as an alternative experimental model for investigating the complement-dependent mechanisms of ALI that have been established in prior studies (22, 26, 50, 51).
In summary, pulmonary challenge with recombinant C5a reproduced many of the detrimental events that are characteristic of ALI/ARDS. Our data suggest that the detrimental pulmonary effects of C5a are predominantly mediated by recruited Ly-6G+CD11b+C5aR1+ PMNs in conjunction with a specific signature of C5a-induced mediators, which also includes the CC-chemokines, CCL3, -4, and -5. The investigation of C5a-ALI in mice may prove to be valuable for a better understanding of the pathophysiology of human ARDS. C5a-ALI, for instance, may be preferable over endotoxin-dependent models of ALI, especially given the high degree of difference in endotoxin sensitivity between species, which can result in confusing associations of inflammatory responses in humans vs. rodents (52). Beyond this, insights regarding the effects of complement activation in acute inflammation may be transferred from this model to other inflammatory diseases. Last, with the uncovered role of CCR5 downstream of the C5aR1-induced inflammatory response, we present a proinflammatory amplification loop with potential therapeutic relevance in ARDS.
Acknowledgments
The authors thank Amanda Welton for technical assistance with HR-MRI acquisition and Lisa Kubacki for secretarial assistance. This work was supported by Deutsche Forschungsgemeinschaft Grant BO3482/3-1, Federal Ministry of Education and Research Grant BMBF-01EO1003, funding from the Mainz Research Funding Program (MAIFOR) of the University Medical Center Mainz, and a Marie Curie Career Integration Grant of the European Union (Project 334486) (all to M.B.); the Boehringer Ingelheim Fonds (to N.F.R. and R.R.); and U.S. National Institutes of Health, National Institute of General Medical Sciences Grants GM-29507 and GM-61656 (to P.A.W.). The authors are responsible for the contents of this publication and declare no conflicts of interest. Parts of the data of this paper were used by N.F.R. and R.R. for their M.D. theses (Doctor of Medicine degree).
Glossary
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- BALF
bronchoalveolar lavage fluid
- BSA
bovine serum albumin
- BW
body weight
- C
complement component
- C5adesArg
desarginated complement component C5a
- C5aR1
complement component 5a receptor 1
- CCL3
chemokine (C-C motif) ligand 3 (MIP-1α)
- CCL4
chemokine (C-C motif) ligand 4 (MIP-1β)
- CCL5
chemokine (C-C motif) ligand 5 (RANTES)
- CCR5
CC-chemokine receptor type 5
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- H&E
hematoxylin and eosin
- HR-MRI
high-resolution magnetic resonance imaging
- i.t.
intratracheal
- IC-ALI
immune complex-induced acute lung injury
- LPS-ALI
lipopolysaccharide (endotoxin)-induced acute lung injury
- MIP-1α
macrophage inflammatory protein 1α (CCL3)
- MIP-1β
macrophage inflammatory protein 1β (CCL4)
- PBS
phosphate buffered saline
- PMN
polymorphonuclear neutrophil
- rmC5a
recombinant mouse complement component 5a
- WBC
white blood cell
- wt
wild-type
REFERENCES
- 1.Matthay M. A., Ware L. B., Zimmerman G. A. (2012) The acute respiratory distress syndrome. J. Clin. Invest. 122, 2731–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubenfeld G. D., Caldwell E., Peabody E., Weaver J., Martin D. P., Neff M., Stern E. J., Hudson L. D. (2005) Incidence and outcomes of acute lung injury. N. Engl. J. Med. 353, 1685–1693 [DOI] [PubMed] [Google Scholar]
- 3.Price L. C., McAuley D. F., Marino P. S., Finney S. J., Griffiths M. J., Wort S. J. (2012) Pathophysiology of pulmonary hypertension in acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 302, L803–L815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosmann M., Ward P. A. (2014) Protein-based therapies for acute lung injury: targeting neutrophil extracellular traps. Expert Opin. Ther. Targets 18, 703–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricklin D., Lambris J. D. (2013) Complement in immune and inflammatory disorders: pathophysiological mechanisms. J. Immunol. 190, 3831–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strunk R. C., Eidlen D. M., Mason R. J. (1988) Pulmonary alveolar type II epithelial cells synthesize and secrete proteins of the classical and alternative complement pathways. J. Clin. Invest. 81, 1419–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skattum L., van Deuren M., van der Poll T., Truedsson L. (2011) Complement deficiency states and associated infections. Mol. Immunol. 48, 1643–1655 [DOI] [PubMed] [Google Scholar]
- 8.Bosmann M., Ward P. A. (2012) Role of C3, C5 and anaphylatoxin receptors in acute lung injury and in sepsis. Adv. Exp. Med. Biol. 946, 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber-Lang M., Sarma J. V., Zetoune F. S., Rittirsch D., Neff T. A., McGuire S. R., Lambris J. D., Warner R. L., Flierl M. A., Hoesel L. M., Gebhard F., Younger J. G., Drouin S. M., Wetsel R. A., Ward P. A. (2006) Generation of C5a in the absence of C3: a new complement activation pathway. Nat. Med. 12, 682–687 [DOI] [PubMed] [Google Scholar]
- 10.Huber-Lang M., Younkin E. M., Sarma J. V., Riedemann N., McGuire S. R., Lu K. T., Kunkel R., Younger J. G., Zetoune F. S., Ward P. A. (2002) Generation of C5a by phagocytic cells. Am. J. Pathol. 161, 1849–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller-Ortiz S. L., Wang D., Morales J. E., Li L., Chang J. Y., Wetsel R. A. (2009) Targeted disruption of the gene encoding the murine small subunit of carboxypeptidase N (CPN1) causes susceptibility to C5a anaphylatoxin-mediated shock. J. Immunol. 182, 6533–6539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webster R. O., Larsen G. L., Henson P. M. (1982) In vivo clearance and tissue distribution of C5a and C5a des arginine complement fragments in rabbits. J. Clin. Invest. 70, 1177–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerard N. P., Gerard C. (1991) The chemotactic receptor for human C5a anaphylatoxin. Nature 349, 614–617 [DOI] [PubMed] [Google Scholar]
- 14.Okinaga S., Slattery D., Humbles A., Zsengeller Z., Morteau O., Kinrade M. B., Brodbeck R. M., Krause J. E., Choe H. R., Gerard N. P., Gerard C. (2003) C5L2, a nonsignaling C5A binding protein. Biochemistry 42, 9406–9415 [DOI] [PubMed] [Google Scholar]
- 15.Bosmann M., Sarma J. V., Atefi G., Zetoune F. S., Ward P. A. (2012) Evidence for anti-inflammatory effects of C5a on the innate IL-17A/IL-23 axis. FASEB J. 26, 1640–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R., Coulthard L. G., Wu M. C., Taylor S. M., Woodruff T. M. (2013) C5L2: a controversial receptor of complement anaphylatoxin, C5a. FASEB J. 27, 855–864 [DOI] [PubMed] [Google Scholar]
- 17.Robbins R. A., Russ W. D., Rasmussen J. K., Clayton M. M. (1987) Activation of the complement system in the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 135, 651–658 [DOI] [PubMed] [Google Scholar]
- 18.Kambas K., Markiewski M. M., Pneumatikos I. A., Rafail S. S., Theodorou V., Konstantonis D., Kourtzelis I., Doumas M. N., Magotti P., Deangelis R. A., Lambris J. D., Ritis K. D. (2008) C5a and TNF-alpha up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome. J. Immunol. 180, 7368–7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Till G. O., Johnson K. J., Kunkel R., Ward P. A. (1982) Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J. Clin. Invest. 69, 1126–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen G. L., McCarthy K., Webster R. O., Henson J., Henson P. M. (1980) A differential effect of C5a and C5a des Arg in the induction of pulmonary inflammation. Am. J. Pathol. 100, 179–192 [PMC free article] [PubMed] [Google Scholar]
- 21.Stimler N. P., Hugli T. E., Bloor C. M. (1980) Pulmonary injury induced by C3a and C5a anaphylatoxins. Am. J. Pathol. 100, 327–348 [PMC free article] [PubMed] [Google Scholar]
- 22.Bosmann M., Grailer J. J., Ruemmler R., Russkamp N. F., Zetoune F. S., Sarma J. V., Standiford T. J., and Ward P. A. (2013) Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 27, 5010–5021 [DOI] [PMC free article] [PubMed]
- 23.Trujillo G., Habiel D. M., Ge L., Ramadass M., Cooke N. E., Kew R. R. (2013) Neutrophil recruitment to the lung in both C5a- and CXCL1-induced alveolitis is impaired in vitamin D-binding protein-deficient mice. J. Immunol. 191, 848–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heller T., Hennecke M., Baumann U., Gessner J. E., zu Vilsendorf A. M., Baensch M., Boulay F., Kola A., Klos A., Bautsch W., Köhl J. (1999) Selection of a C5a receptor antagonist from phage libraries attenuating the inflammatory response in immune complex disease and ischemia/reperfusion injury. J. Immunol. 163, 985–994 [PubMed] [Google Scholar]
- 25.Stevens J. H., O’Hanley P., Shapiro J. M., Mihm F. G., Satoh P. S., Collins J. A., Raffin T. A. (1986) Effects of anti-C5a antibodies on the adult respiratory distress syndrome in septic primates. J. Clin. Invest. 77, 1812–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulligan M. S., Schmid E., Beck-Schimmer B., Till G. O., Friedl H. P., Brauer R. B., Hugli T. E., Miyasaka M., Warner R. L., Johnson K. J., Ward P. A. (1996) Requirement and role of C5a in acute lung inflammatory injury in rats. J. Clin. Invest. 98, 503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doerschuk C. M., Quinlan W. M., Doyle N. A., Bullard D. C., Vestweber D., Jones M. L., Takei F., Ward P. A., Beaudet A. L. (1996) The role of P-selectin and ICAM-1 in acute lung injury as determined using blocking antibodies and mutant mice. J. Immunol. 157, 4609–4614 [PubMed] [Google Scholar]
- 28.Mulligan M. S., Schmid E., Till G. O., Hugli T. E., Friedl H. P., Roth R. A., Ward P. A. (1997) C5a-dependent up-regulation in vivo of lung vascular P-selectin. J. Immunol. 158, 1857–1861 [PubMed] [Google Scholar]
- 29.Czermak B. J., Sarma V., Bless N. M., Schmal H., Friedl H. P., Ward P. A. (1999) In vitro and in vivo dependency of chemokine generation on C5a and TNF-alpha. J. Immunol. 162, 2321–2325 [PubMed] [Google Scholar]
- 30.Bosmann M., Russkamp N. F., Patel V. R., Zetoune F. S., Sarma J. V., Ward P. A. (2011) The outcome of polymicrobial sepsis is independent of T and B cells. Shock 36, 396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hubbard L. L., Ballinger M. N., Wilke C. A., Moore B. B. (2008) Comparison of conditioning regimens for alveolar macrophage reconstitution and innate immune function post bone marrow transplant. Exp. Lung Res. 34, 263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosmann M., Grailer J. J., Zhu K., Matthay M. A., Sarma J. V., Zetoune F. S., and Ward P. A. (2012) Anti-inflammatory effects of beta2 adrenergic receptor agonists in experimental acute lung injury. FASEB J. 26, 2137–2144 [DOI] [PMC free article] [PubMed]
- 33.Grommes J., Drechsler M., Soehnlein O. (2014) CCR5 and FPR1 mediate neutrophil recruitment in endotoxin-induced lung injury. J. Innate Immun. 6, 111–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkins C. M., Selcher J. C., Petraitis J. J., Trzaskos J. M., Sweatt J. D. (1998) The MAPK cascade is required for mammalian associative learning. Nat. Neurosci. 1, 602–609 [DOI] [PubMed] [Google Scholar]
- 35.Bosmann M., Haggadone M. D., Hemmila M. R., Zetoune F. S., Sarma J. V., Ward P. A. (2012) Complement activation product C5a is a selective suppressor of TLR4-induced, but not TLR3-induced, production of IL-27(p28) from macrophages. J. Immunol. 188, 5086–5093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wetsel R. A. (1995) Expression of the complement C5a anaphylatoxin receptor (C5aR) on non-myeloid cells. Immunol. Lett. 44, 183–187 [DOI] [PubMed] [Google Scholar]
- 37.Riedemann N. C., Guo R. F., Sarma V. J., Laudes I. J., Huber-Lang M., Warner R. L., Albrecht E. A., Speyer C. L., Ward P. A. (2002) Expression and function of the C5a receptor in rat alveolar epithelial cells. J. Immunol. 168, 1919–1925 [DOI] [PubMed] [Google Scholar]
- 38.Karsten C. M., Laumonnier Y., Eurich B., Ender F., Bröker K., Roy S., Czabanska A., Vollbrandt T., Figge J., Köhl J. (2015) Monitoring and cell-specific deletion of C5aR1 using a novel floxed GFP-C5aR1 reporter knock-in mouse. J. Immunol. 194, 1841–1855 [DOI] [PubMed] [Google Scholar]
- 39.Bosmann M., Patel V. R., Russkamp N. F., Pache F., Zetoune F. S., Sarma J. V., Ward P. A. (2011) MyD88-dependent production of IL-17F is modulated by the anaphylatoxin C5a via the Akt signaling pathway. FASEB J. 25, 4222–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henson P. M., McCarthy K., Larsen G. L., Webster R. O., Giclas P. C., Dreisin R. B., King T. E., Shaw J. O. (1979) Complement fragments, alveolar macrophages, and alveolitis. Am. J. Pathol. 97, 93–110 [PMC free article] [PubMed] [Google Scholar]
- 41.Bosmann M., Haggadone M. D., Zetoune F. S., Sarma J. V., Ward P. A. (2013) The interaction between C5a and both C5aR and C5L2 receptors is required for production of G-CSF during acute inflammation. Eur. J. Immunol. 43, 1907–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amara U., Rittirsch D., Flierl M., Bruckner U., Klos A., Gebhard F., Lambris J. D., Huber-Lang M. (2008) Interaction between the coagulation and complement system. Adv. Exp. Med. Biol. 632, 71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guilliams M., De Kleer I., Henri S., Post S., Vanhoutte L., De Prijck S., Deswarte K., Malissen B., Hammad H., Lambrecht B. N. (2013) Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 210, 1977–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarling J. D., Lin H. S., Hsu S. (1987) Self-renewal of pulmonary alveolar macrophages: evidence from radiation chimera studies. J. Leukoc. Biol. 42, 443–446 [PubMed] [Google Scholar]
- 45.Finsterbusch M., Voisin M. B., Beyrau M., Williams T. J., Nourshargh S. (2014) Neutrophils recruited by chemoattractants in vivo induce microvascular plasma protein leakage through secretion of TNF. J. Exp. Med. 211, 1307–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grailer J. J., Bosmann M., Ward P. A. (2012) Regulatory effects of C5a on IL-17A, IL-17F, and IL-23. Front. Immunol. 3, 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bless N. M., Huber-Lang M., Guo R. F., Warner R. L., Schmal H., Czermak B. J., Shanley T. P., Crouch L. D., Lentsch A. B., Sarma V., Mulligan M. S., Friedl H. P., Ward P. A. (2000) Role of CC chemokines (macrophage inflammatory protein-1 beta, monocyte chemoattractant protein-1, RANTES) in acute lung injury in rats. J. Immunol. 164, 2650–2659 [DOI] [PubMed] [Google Scholar]
- 48.Hüttenrauch F., Pollok-Kopp B., Oppermann M. (2005) G protein-coupled receptor kinases promote phosphorylation and beta-arrestin-mediated internalization of CCR5 homo- and hetero-oligomers. J. Biol. Chem. 280, 37503–37515 [DOI] [PubMed] [Google Scholar]
- 49.Rittirsch D., Flierl M. A., Day D. E., Nadeau B. A., McGuire S. R., Hoesel L. M., Ipaktchi K., Zetoune F. S., Sarma J. V., Leng L., Huber-Lang M. S., Neff T. A., Bucala R., Ward P. A. (2008) Acute lung injury induced by lipopolysaccharide is independent of complement activation. J. Immunol. 180, 7664–7672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo R. F., Ward P. A. (2005) Role of C5a in inflammatory responses. Annu. Rev. Immunol. 23, 821–852 [DOI] [PubMed] [Google Scholar]
- 51.Flierl M. A., Perl M., Rittirsch D., Bartl C., Schreiber H., Fleig V., Schlaf G., Liener U., Brueckner U. B., Gebhard F., Huber-Lang M. S. (2008) The role of C5a in the innate immune response after experimental blunt chest trauma. Shock 29, 25–31 [DOI] [PubMed] [Google Scholar]
- 52.Seok J., Warren H. S., Cuenca A. G., Mindrinos M. N., Baker H. V., Xu W., Richards D. R., McDonald-Smith G. P., Gao H., Hennessy L., Finnerty C. C., Lopez C. M., Honari S., Moore E. E., Minei J. P., Cuschieri J., Bankey P. E., Johnson J. L., Sperry J., Nathens A. B., Billiar T. R., West M. A., Jeschke M. G., Klein M. B., Gamelli R. L., Gibran N. S., Brownstein B. H., Miller-Graziano C., Calvano S. E., Mason P. H., Cobb J. P., Rahme L. G., Lowry S. F., Maier R. V., Moldawer L. L., Herndon D. N., Davis R. W., Xiao W., Tompkins R. G. Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 110, 3507–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]