Abstract
PURPOSE
Diabetes mellitus (DM) is characterized by high blood sugar levels over a prolonged period. Long term complications include but not limited heart disease, stroke, kidney failure, and ocular damage. An estimated 382 million people are diagnosed with Type 2 DM accounting for 90% of the cases. Common corneal dysfunctions associated with DM result in impaired vision due to decreased wound healing, corneal edema, and altered epithelial basement membrane. Lipids play a fundamental role in tissue metabolism and disease states. We attempt to determine the role of sphingolipids (SPL) in human Type I and Type II diabetic corneas.
MATERIALS AND METHODS
Cadaver corneas from healthy (non-diabetic/no ocular trauma), Type I (T1DM), and Type II diabetic (T2DM) donors were obtained and processed for lipidomics using LC-MS/MS.
RESULTS
Our data show significant differences in the SPL composition between control, T1DM and T2DM corneas. Both T1DM and T2DM showed a 10-fold downregulation of sphingomyelin (SM), 5-fold up regulation of Ceramides (Cer) and 2-fold upregulation of monohexosylceramides (MHC). Differences were also seen in total amounts of SPL where Cer was increased by approximately 3 fold in both T1DM and T2DM where SM decreased by 50% in both T1DM and T2DM when compared to healthy controls. No differences were seen in MHC amounts.
CONCLUSIONS
Overall, our data indicate major differences in SPL distribution in human diabetic corneas. Information on the sphingolipids role in cornea, corneal cell physiology, and diseases are very limited which highlights the importance of these findings.
Keywords: Diabetes, Cornea, Lipidomics, Sphingolipids
Introduction
Diabetes mellitus (DM) or better known as simply diabetes is a group of metabolic diseases in which high blood sugar levels are maintained over a prolonged period. Long term complications include but not limited heart disease, stroke, kidney failure, and ocular damage (1, 2). Common corneal dysfunctions associated with diabetes result in impaired vision due to decreased wound healing, corneal edema, and an altered epithelial basement membrane. There are two main types of diabetes: Type 1 (T1DM) and Type 2 (T2DM). In 2013, an estimated 382 million people were diagnosed with diabetes with type 2 accounting for 90% of the cases. Approximately 70% of them suffer from some kind of corneal complications collectively and commonly known as diabetic keratopathy (3–6).
The diabetic cornea suffers from cellular dysfunction and dysfunctional wound healing/repair mechanisms (7–10). Clinically, we have no preventive measure for T1DM diabetes, while T2DM can be managed by means of physical exercise, control weight, and healthy diet (11–14). Even then, the effect on the cornea will depend on the severity of the disease and the stage at which it was diagnosed. Diabetes is a chronic disease and corneal impairments are almost inevitable. Once the eye has been exposed to hyperglycemia long-term, the basement membrane has accumulated enough toxic end products that lead to cell death, opacity, and eventually vision impairment (4, 15–18).
Scientists have concentrated, for years, on animal studies and have developed a variety of animal models both for T1DM (19–31) and T2DM diabetes (32–39). However, there is a significant lack of reproducible paradigms of human diabetic complications and rather disappointing results of studies aimed to prevent T1DM diabetes based on treatments developed successfully in rodents. Understanding the phenotype and characteristics of the human diabetic cornea it is crucial for the development of new therapeutics.
Our study shows a novel approach for the exploration of the human diabetic cornea. Using targeted lipidomics technology, we were able to identify alterations of specific subspecies of sphingolipids. Sphingolipids are a class of bioactive lipids that have been implicated both in physiological and pathological wound-healing responses. Evidence is accumulating on the role of sphingolipids in regulating the development of tissue fibrosis in numerous organ systems, including the lungs, skin, liver, heart, and eye (40–44). Our data show significant differences in total composition as well as specific sphingolipid subspecies between the diabetic cornea and healthy cornea. Future studies will aim to dissect the mechanisms and pathways involved, which would lead to new therapeutic paradigms.
Materials and Methods
Ethics
The study met the tenets of the Declaration of Helsinki. Samples were obtained from the National Development and Research Institute (NDRI) and the Oklahoma Lions Eye Bank. All samples were anonymized before analysis. Permission from the Institutional Review Board has been obtained.
Inclusion Criteria
In order for a diabetic donor to be included, they must not only have had Type I or II diabetes, but also be free from other ocular pathology or general diseases. Healthy Individuals with no history of ocular trauma or disease were included as control groups. Samples were collected from age-matched control, T1DM, and T2DM donors. No significant differences were found between them.
Tissue processing and Targeted Lipidomics
Corneal samples were collected and incubated in Optisol upto 48h post-mortem. We analyzed minimum three corneas from three different donors per group (n=3; Control, T1DM, and T2DM). The corneal samples were washed with PBS and scrapped thoroughly in order to remove both the epithelium and endothelium layer. Furthermore they were cut into small 5–7 pieces of about 3×3mm size each and transferred to eppendorf tubes. Thereafter they were stored at −80°C and further sent for Lipidomics analysis. 5–7 pieces from each donor/per group were processed. Corneas with low endothelial cell counts were excluded. The extracted lipids were analyzed using targeted LC MS/MS methods using Shimadzu Nexera UPLC and a hybrid triple quadrupole linear ion trap (AB SCIEX 6500). Each sample was investigated for changes in sphingolipids as previously described (45–47) using a targeted lipidomics approach as previously described (45–47). Briefly, UPLC ESI MS/MS was utilized with a focus on sphingolipids. One way ANOVA analysis was used to identify statistical significance in the observed results.
Statistical analysis
Data analysis for the sample was performed by one -way ANOVA using Graph Pad Prism 6 software. Where P value (P<0.05) was considered to be statistically significant. Average values were calculated and plotted with standard error of the mean (SEM).
Results
Composition of sphingolipids
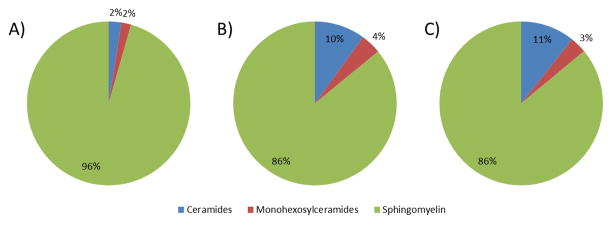
We determined the composition of major sphingolipids in corneas from healthy, T1DM, and T2DM donors (Figure 1). In healthy corneas, 96% of sphingolipids were sphingomyelins (SM), whereas ceramides (Cer) and monohexosylceramides (glycosyl+galactosyl ceramides; MHC) accounted for 2% each. However, the composition was significantly different (p<0.05) in both T1DM and T2DM when compared to healthy corneas. T1DM was composed of 86% SM, 10% Cer, and 4% MGC indicating 5-fold upregulation in Cer and 2-fold upregulation in MGC whereas T2DM accounted for 86% SM, 11% Cer, and 3% MGC. Interestingly, the composition of T1DM and T2DM was very similar and no significant differences were shown.
Figure 1.
Composition of major sphingolipids in human cadaver corneas from A) Healthy, B) T1DM, and C) T2DM donors. Healthy corneas showed 96% of sphingolipids belongs to SM, whereas Cer and MHC accounted for 2% each. T1DM was composed of 86% SM, 10% Cer, and 4% MHC whereas T2DM accounted for 86% SM, 11% Cer, and 3% MHC. At least three different samples from three different donors were used (n>=3).
Identification of major species present
Our samples were quantified, using LC-MS/MS to identify the major species present in different classes of sphingolipids. Table 1, 2, and 3 shows all major species of Cer, MHC, and SM respectively.
Table 1.
Ceramide major species in healthy corneas and diabetics (n>=3 per group).
Major Cer species were identified and quantified in human cadaver corneas from Healthy, T1DM, and T2DM donors. In healthy donors, C16:0 species were the highest followed by C24:0, C24:1, and C22:0. In T1DM, C16:0 species were also the highest followed by C18:0, C24:0, and C22:0. In T2DM, C16:0 was highest, C18:0 was second highest, followed by C24:0, and C24:1. At least three different samples from three different donors were used (n>=3).
| Ceramides Species | Healthy (pmol/mg tissue) | T1DM (pmol/mg tissue) | T2DM (pmol/mg tissue) |
|---|---|---|---|
| C14:0 | 0.08±0.01 | 0.12±0.02 | 0.13±0.02 |
| C16:0 | 1.45±0.23 | 3.76±0.49 | 3.06±0.36 |
| C18:1 | 0.11±0.03 | 0.65±0.14 | 1.10±0.26 |
| C18:0 | 0.59±0.15 | 2.17±0.33 | 2.95±0.42 |
| C20:0 | 0.38±0.10 | 0.89±0.11 | 1.16±0.20 |
| C22:0 | 0.72±0.14 | 1.10±0.16 | 1.28±0.24 |
| C24:1 | 0.88±0.14 | 0.86±0.14 | 1.49±0.29 |
| C24:0 | 0.95±0.14 | 1.36±0.18 | 1.58±0.24 |
| C26:1 | 0.11±0.03 | 0.05±0.01 | 0.08±0.02 |
| C26:0 | 0.01±0.00 | 0.14±0.02 | 0.10±0.02 |
Table 2.
Monohexosylceramides major species in healthy corneas and diabetics (n>=3 per group).
Major MHC species were identified and quantified in human cadaver corneas from Healthy, T1DM, and T2DM donors. In healthy donors, C24:1 species were the highest followed by C24:0, C22:0, and C16:0. In T1DM, C24:1 species were also the highest followed by C16:0, C24:0, and C22:0. In T2DM, C24:1 was highest, C16:0 was second highest, followed by C24:0, and C22:0. At least three different samples from three different donors were used (n>=3).
| Monohexosylceramides Species | Healthy (pmol/mg tissue) | T1DM (pmol/mg tissue) | T2DM (pmol/mg tissue) |
|---|---|---|---|
| C14:0 | 0.10±0.02 | 0.10±0.01 | 0.09±0.01 |
| C16:0 | 0.65±0.19 | 0.92±0.11 | 0.81±0.10 |
| C18:1 | 0.03±0.01 | 0.12±0.02 | 0.11±0.01 |
| C18:0 | 0.43±0.09 | 0.43±0.06 | 0.40±0.05 |
| C20:0 | 0.30±0.11 | 0.36±0.04 | 0.31±0.04 |
| C22:0 | 0.78±0.14 | 0.60±0.07 | 0.51±0.06 |
| C24:1 | 0.88±0.15 | 1.07±0.13 | 0.92±0.11 |
| C24:0 | 0.80±0.12 | 0.87±0.10 | 0.75±0.09 |
| C26:1 | 0.04±0.01 | 0.00±0.00 | 0.00±0.00 |
| C26:0 | 0.03±0.01 | 0.01±0.00 | 0.01±0.00 |
Table 3.
Sphingomyelin major species in healthy corneas and diabetics (n>=3 per group).
Major SM species were identified and quantified in human cadaver corneas from Healthy, T1DM, and T2DM donors. In healthy donors, C16:0 species were the highest followed by C24:1, C18:0, and C24:0. In T1DM, C16:0 species were also the highest followed by C18:0, C24:1, and C24:0. In T2DM, C16:0 was highest, C18:0 was second highest, followed by C24:1, and C24:0. At least three different samples from three different donors were used (n>=3).
| Sphingomyelin Species | Healthy (pmol/mg tissue) | T1DM (pmol/mg tissue) | T2DM (pmol/mg tissue) |
|---|---|---|---|
| C14:0 | 8.93±2.31 | 2.28±0.37 | 1.48±0.23 |
| C16:0 | 68.92±7.93 | 46.73±6.00 | 48.47±8.62 |
| C18:1 | 6.20±1.49 | 2.38±0.38 | 1.85±0.29 |
| C18:0 | 28.30±5.81 | 14.90±2.13 | 19.38±2.74 |
| C20:0 | 9.76±1.89 | 4.57±0.41 | 5.42±0.70 |
| C22:0 | 19.45±3.50 | 6.71±0.72 | 7.28±0.97 |
| C24:1 | 36.96±7.85 | 10.12±1.17 | 11.07±1.56 |
| C24:0 | 21.70±3.51 | 7.32±0.59 | 8.61±1.08 |
| C26:1 | 0.76±0.21 | 0.10±0.02 | 0.12±0.02 |
| C26:0 | 0.30±0.15 | 0.01±0.00 | 0.01±0.00 |
In healthy corneas C16:0 Cer accounts for the highest levels of the ceramide subspecies, followed by C24:0, C24:1, and C22:0 (Table 1). In T1DM, Cer species, C16:0 were also the highest followed by C18:0, C24:0, and C22:0 (Table 1). In T2DM Cer species, C16:0 was highest, C18:0 was second highest, followed by C24:0, and C24:1(Table 1).
Major MHC species were identified and quantified as summarized in Table 2. In healthy donors, C24:1 species were the highest followed by C24:0, C22:0, and C16:0. In T1DM, C24:1 species were also the highest followed by C16:0, C24:0, and C22:0. In T2DM, C24:1 was highest, C16:0 was second highest, followed by C24:0, and C22:0 (Table 2).
SM species were also quantified (Table 3). In healthy donors, C16:0 species were the highest followed by C24:1, C18:0, and C24:0. In T1DM, C16:0 species were also the highest followed by C18:0, C24:1, and C24:0. In T2DM, C16:0 was highest, C18:0 was second highest, followed by C24:1, and C24:0 (Table 3). The data indicate that in all major classes (Cer, MHC, and SM) of sphingolipids, the first/highest subspecies were identical between healthy, T1DM, and T2DM samples. The differences were noted on the second, third, and fourth highest subspecies.
Total amounts of sphingolipids
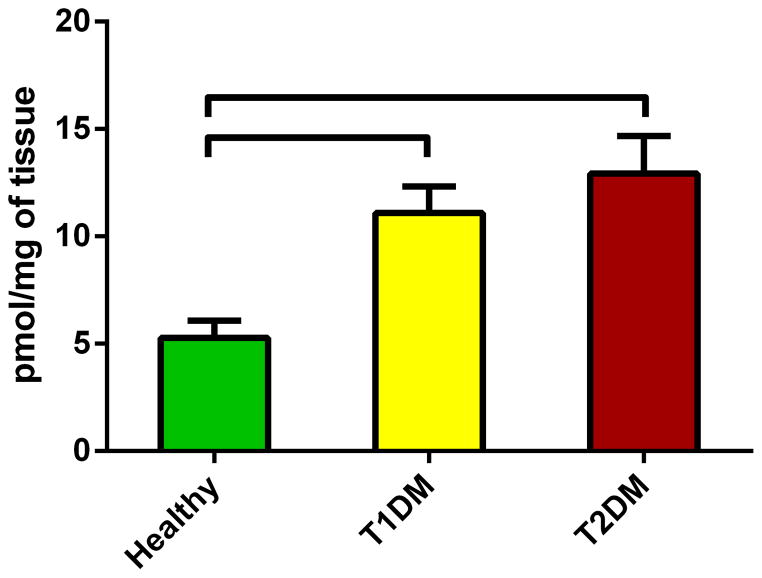
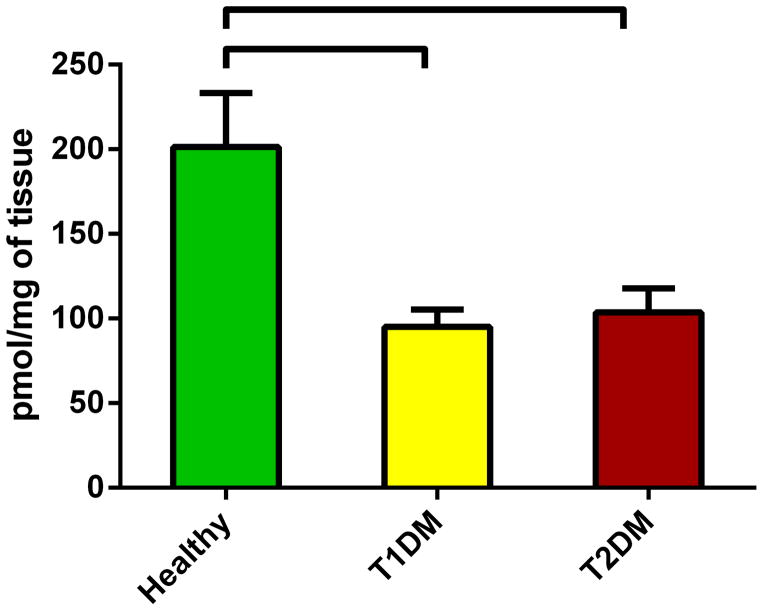
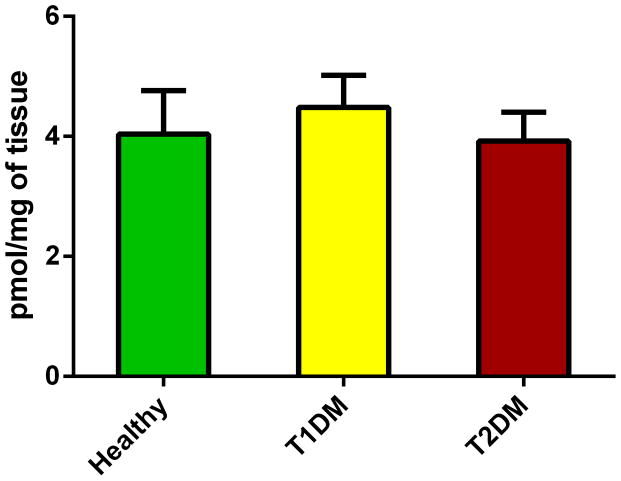
Information on sphingolipids in cornea and corneal cell physiology and disease is very limited. We quantified the total levels of sphingolipids and revealed significant differences between healthy and diabetic corneas. Figure 2 shows the total levels of Cer in all three conditions. Cer was significantly up regulated in both T1DM (Figure 2; p<0.05) and T2DM (Figure 2; p<0.05) in comparison to the healthy samples. Figure 3 shows the total SM levels, which unlike Cer, were significantly down regulated in both T1DM (Figure 3; p<0.05) and T2DM (Figure 3; p<0.05). Figure 4 shows total levels of MHC, however no significant differences were observed.
Figure 2.
Total levels of Ceramides in human cadaver corneas from Healthy, T1DM, and T2DM donors. Cer were significantly up regulated in both T1DM (p<0.05) and T2DM (p<0.05) when compared to healthy corneas. No difference was seen between T1DM and T2DM. At least three different samples from three different donors were used (n>=3).
Figure 3.
Total levels of Spingomyelins in human cadaver corneas from Healthy, T1DM, and T2DM donors. SM were significantly down regulated in both T1DM (p<0.05) and T2DM (p<0.05) when compared to healthy corneas. No difference was seen between T1DM and T2DM. No difference was seen between T1DM and T2DM. At least three different samples from three different donors were used (n>=3).
Figure 4.
Total levels of monohexosylceramides in human cadaver corneas from Healthy, T1DM, and T2DM donors. No difference was seen between Healthy and T1DM or T2DM. At least three different samples from three different donors were used (n>=3).
Discussion
Diabetes is a major public health problem. T1DM and T2DM diabetes were described and identified in 400–500 by Sushruta and Charaka (48) who they associated T1DM with youth and T2DM with being overweight (48). Today we know that T1DM is characterized by loss of the insulin-producing beta cells of the islets of Langerhans in the pancreas (49, 50). T2DM, on the other hand, is known for insulin resistance and it is the more common of the two (2, 51, 52). With regards to ophthalmic complications as a result of diabetes, more profound effects are seen in cornea and retina (53). Studies on animal models of diabetes are responsible for most of our knowledge about the pathogenesis and mechanisms of diabetes. Unfortunately, most of the treatments developed on rodents, for the treatment of diabetic individuals, have failed when tested in humans. In fact, of the 382 million people diagnosed with diabetes worldwide approximately 70% of them suffer from some kind of corneal complications collectively and commonly known as diabetic keratopathy (53, 54).
The diabetic cornea suffers from cellular dysfunction and dysfunctional wound healing/repair mechanisms. There have been an extensive range of studies looking at specific dysfunctions of the cornea. Schultz and co-authors (54) found corneal epithelial lesions in more than 65% of the population tested. Two years later (55) the same group reported diminished corneal peripheral sensation suggesting some kind of neuropathy. This has now been confirmed by multiple studies (54, 56–62) and is widely acceptable that these patients suffer from reduced corneal sensitivity and generalized neuropathy.
In addition, diabetic patients found to have abnormal adhesions of the corneal epithelium to the underlying basement membrane (15) leading to prolonged and recurrent defects. To make things even more complicated, Gekka et al (63) and Goebbels et al (64) showed improper function and weakening of the epithelial barrier in diabetic patients leading to higher risks of corneal infections and stromal fibrosis. Corneal thickness increase has also been reported (65–68) and linked to diabetes as well as endothelial dysfunction (67). Clearly, there are a lot of defects in the human diabetic cornea that may lead to severe vision impairments.
In this study, we used targeted lipidomics technology to evaluate the changes of sphingolipids and their major subspecies in the human diabetic cornea. We chose human cadaver samples, and we excluded donors with any kind of ocular history. This process ensured that our data are only regulated by the diabetic effect. Understanding the characteristics of the human diabetic cornea it is crucial for the development of new therapeutics. While sphingolipids are only a class of lipids, we found significant differences between healthy and diabetic samples. Total Cer were significantly up regulated in both T1DM and T2DM while total SM were down regulated in the same groups. Cer and SM are connected in terms of function and are known to play a significant role in cell signaling pathways.(69) The degradation of SM can produce ceramide which is involved in the apoptotic signaling pathway. In fact, SM has been found to be crucial in cell apoptosis by hydrolyzing into ceramide(70). SM can determine not only when a cell dies but how(70). Our data showing down regulation of SM at the diabetic samples might be an indicator of apoptotic resident cells. In support of this, are our data on Cer. One of the most studied roles of Cer pertains to its function as a proapoptotic molecule. Cer accumulation has been found following treatment of cells with a number of apoptotic agents including ionizing radiation (71, 72), UV light (73), TNF-alpha (74), and chemotherapeutic agents. The increase we see with the diabetic samples suggests accumulation of Cer and therefore apoptotic behavior. While our data seem convincing the role of ceramide in apoptosis and the mechanism by which this lipid regulates apoptosis remains elusive (75). The most crucial limitation of this study is the fact that we do not have an accurate number of corneal stromal cells for each sample/cornea, prior to lipidomics analysis. It is possible that the relative composition of lipids is affected by the cell numbers present in each sample/tissue.
Many laboratories haves studied the regulation of ceramide biosynthesis (76–84); however, little is known about the role of sphingolipids in cornea and even less is known about their role in the diabetic cornea. This is a novel approach in order to determine the effects of sphingolipids and determine any consequences they might present. Further studies of the regulation of sphingolipids might also be helpful in diagnosis, treatment and prevention of cornea diabetic defects.
Conclusions
We have shown here the importance of sphingolipids in human diabetic corneas. Clearly further studies are necessary in order to unravel the mechanism by which these lipids are involved in corneal diabetes. To the author’s knowledge, this is the first report of sphingolipids quantification in human diabetic corneas.
Acknowledgments
Funding
This work was supported by research grants from the Veteran’s Administration (VA Merit Review I BX001792 (CEC) and a Research Career Scientist Award 13F-RCS-002 (CEC)); from the National Institutes of Health via HL125353 (CEC), CA154314 (C.E.C), EY020886 (D.K), EY023568 (D.K), and NH1C06-RR17393 (to Virginia Commonwealth University for renovation); from unrestricted grant from Research to Prevent Blindness. Services and products in support of the research project were generated by the VCU Massey Cancer Center Shared supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
Supported, in part, by an unrestricted grant from Research to Prevent Blindness, (New York, NY USA).
Footnotes
Declaration of interests
The authors declare that there is no conflict of interest.
The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–43. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardner DG, Shoback D. Greenspan’s basic & clinical endocrinology. 9. New York: McGraw-Hill Medical; 2011. [Google Scholar]
- 3.Skarbez K, Priestley Y, Hoepf M, Koevary SB. Comprehensive Review of the Effects of Diabetes on Ocular Health. Expert Rev Ophthalmol. 2010;5(4):557–77. doi: 10.1586/eop.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herse PR. A review of manifestations of diabetes mellitus in the anterior eye and cornea. Am J Optom Physiol Opt. 1988;65(3):224–30. doi: 10.1097/00006324-198803000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Owen CG, Newsom RS, Rudnicka AR, Ellis TJ, Woodward EG. Vascular response of the bulbar conjunctiva to diabetes and elevated blood pressure. Ophthalmology. 2005;112(10):1801–8. doi: 10.1016/j.ophtha.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Schultz RO, Matsuda M, Yee RW, Edelhauser HF, Schultz KJ. Corneal endothelial changes in type I and type II diabetes mellitus. Am J Ophthalmol. 1984;98(4):401–10. doi: 10.1016/0002-9394(84)90120-x. [DOI] [PubMed] [Google Scholar]
- 7.Mishima S. The effects of the denervation and the stimulation of the sympathetic and the trigeminal nerve on the mitotic rate of the corneal epithelium in the rabbit. Jpn J Ophthalmol. 1957;1:65–73. [Google Scholar]
- 8.Alper MG. The anesthetic eye: an investigation of changes in the anterior ocular segment of the monkey caused by interrupting the trigeminal nerve at various levels along its course. Trans Am Ophthalmol Soc. 1975;73:323–65. [PMC free article] [PubMed] [Google Scholar]
- 9.Araki K, Ohashi Y, Kinoshita S, Hayashi K, Kuwayama Y, Tano Y. Epithelial wound healing in the denervated cornea. Curr Eye Res. 1994;13(3):203–11. doi: 10.3109/02713689408995778. [DOI] [PubMed] [Google Scholar]
- 10.Baker KS, Anderson SC, Romanowski EG, Thoft RA, SundarRaj N. Trigeminal ganglion neurons affect corneal epithelial phenotype. Influence on type VII collagen expression in vitro. IOVS. 1993;34(1):137–44. [PubMed] [Google Scholar]
- 11.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298(22):2654–64. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 12.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The effect of intensive diabetes therapy on the development and progression of neuropathy. The Diabetes Control and Complications Trial Research Group. Ann Intern Med. 1995;122(8):561–8. doi: 10.7326/0003-4819-122-8-199504150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Cavanagh PR. Therapeutic footwear for people with diabetes. Diabetes Metab Research Rev. 2004;20(Suppl 1):S51–5. doi: 10.1002/dmrr.435. [DOI] [PubMed] [Google Scholar]
- 15.Foulks GN, Thoft RA, Perry HD, Tolentino FI. Factors related to corneal epithelial complications after closed vitrectomy in diabetics. Arch Ophthalmol. 1979;97(6):1076–8. doi: 10.1001/archopht.1979.01020010530002. [DOI] [PubMed] [Google Scholar]
- 16.Chung H, Tolentino FI, Cajita VN, Acosta J, Refojo MF. Reevaluation of corneal complications after closed vitrectomy. Arch Ophthalmol. 1988;106(7):916–9. doi: 10.1001/archopht.1988.01060140062025. [DOI] [PubMed] [Google Scholar]
- 17.Saini JS, Khandalavla B. Corneal epithelial fragility in diabetes mellitus. Can J Ophthalmol. 1995;30(3):142–6. [PubMed] [Google Scholar]
- 18.Hatchell DL, Magolan JJ, Jr, Besson MJ, Goldman AI, Pederson HJ, Schultz KJ. Damage to the epithelial basement membrane in the corneas of diabetic rabbits. Arch Ophthalmol. 1983;101(3):469–71. doi: 10.1001/archopht.1983.01040010469029. [DOI] [PubMed] [Google Scholar]
- 19.Dahlquist G. The aetiology of type 1 diabetes: an epidemiological perspective. Acta paediatr. 1998;87:5–10. doi: 10.1111/j.1651-2227.1998.tb01244.x. [DOI] [PubMed] [Google Scholar]
- 20.Bono VH. Review of Mechanism of Action Studies of Nitrosoureas. Cancer Treat Rep. 1976;60(6):699–702. [PubMed] [Google Scholar]
- 21.Wang ZY, Gleichmann H. GLUT2 in pancreatic islets - Crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Diabetes. 1998;47(1):50–6. doi: 10.2337/diab.47.1.50. [DOI] [PubMed] [Google Scholar]
- 22.Zahner D, Malaisse WJ. Kinetic behaviour of liver glucokinase in diabetes. I. Alteration in streptozotocin-diabetic rats. J Diabetes Res. 1990;14(3):101–8. [PubMed] [Google Scholar]
- 23.Bolzan AD, Bianchi MS. Genotoxicity of streptozotocin. Mutat Res. 2002;512(2–3):121–34. doi: 10.1016/s1383-5742(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Chen M, Fialkow LB, Ellett JD, Wu R, Nadler JL. The novel anti-inflammatory compound, lisofylline, prevents diabetes in multiple low-dose streptozotocin-treated mice. Pancreas. 2003;26(4):e99–104. doi: 10.1097/00006676-200305000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Mensah-Brown EP, Stosic Grujicic S, Maksimovic D, Jasima A, Shahin A, Lukic ML. Downregulation of apoptosis in the target tissue prevents low-dose streptozotocin-induced autoimmune diabetes. Mol Immunol. 2002;38(12–13):941–6. doi: 10.1016/s0161-5890(02)00021-4. [DOI] [PubMed] [Google Scholar]
- 26.Muller A, Schott-Ohly P, Dohle C, Gleichmann H. Differential regulation of Th1-type and Th2-type cytokine profiles in pancreatic islets of C57BL/6 and BALB/c mice by multiple low doses of streptozotocin. Immunobiology. 2002;205(1):35–50. doi: 10.1078/0171-2985-00109. [DOI] [PubMed] [Google Scholar]
- 27.Holstad M, Sandler S. A transcriptional inhibitor of TNF-alpha prevents diabetes induced by multiple low-dose streptozotocin injections in mice. J Autoimmun. 2001;16(4):441–7. doi: 10.1006/jaut.2001.0506. [DOI] [PubMed] [Google Scholar]
- 28.Zuccollo A, Navarro M, Frontera M, Cueva F, Carattino M, Catanzaro OL. The involvement of kallikrein-kinin system in diabetes type I (insulitis) Immunopharmacology. 1999;45(1–3):69–74. doi: 10.1016/s0162-3109(99)00149-6. [DOI] [PubMed] [Google Scholar]
- 29.Herold KG, Lenschow DJ, Bluestone JA. CD28/B7 regulation of autoimmune diabetes. Immunol Res. 1997;16(1):71–84. doi: 10.1007/BF02786324. [DOI] [PubMed] [Google Scholar]
- 30.Reddy S, Wu D, Elliott RB. Low-Dose Streptozotocin Causes Diabetes in Severe Combined Immunodeficient (Scid) Mice without Immune Cell Infiltration of the Pancreatic-Islets. Autoimmunity. 1995;20(2):83–92. doi: 10.3109/08916939509001931. [DOI] [PubMed] [Google Scholar]
- 31.Arata M, Fabiano de Bruno L, Goncalvez Volpini WM, Quintans JC, D’Alessandro VG, Braun M, et al. Beta-cell function in mice injected with mononuclear splenocytes from multiple-dose streptozotocin diabetic mice. Proc Soc Exp Biol Med. 1994;206(1):76–82. doi: 10.3181/00379727-206-43725. [DOI] [PubMed] [Google Scholar]
- 32.Stride A, Hattersley AT. Different genes, different diabetes: lessons from maturity-onset diabetes of the young. Ann Med. 2002;34(3):207–16. [PubMed] [Google Scholar]
- 33.Krook A, O’Rahilly S. Mutant insulin receptors in syndromes of insulin resistance. Baillieres Best Pract Res Clin Endocrinol Metab. 1996;10(1):97–122. doi: 10.1016/s0950-351x(96)80330-2. [DOI] [PubMed] [Google Scholar]
- 34.Maassen JA, LMTH, Van Essen E, Heine RJ, Nijpels G, Jahangir Tafrechi RS, et al. Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes. 2004;53(Suppl 1):S103–9. doi: 10.2337/diabetes.53.2007.s103. [DOI] [PubMed] [Google Scholar]
- 35.Leibel RL, Chung WK, Chua SC., Jr The molecular genetics of rodent single gene obesities. The JBC. 1997;272(51):31937–40. doi: 10.1074/jbc.272.51.31937. [DOI] [PubMed] [Google Scholar]
- 36.Chagnon YC, Bouchard C. Genetics of obesity: advances from rodent studies. TIG. 1996;12(11):441–4. doi: 10.1016/0168-9525(96)30103-0. [DOI] [PubMed] [Google Scholar]
- 37.Zhang YY, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional Cloning of the Mouse Obese Gene and Its Human Homolog. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 38.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–5. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 39.Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, et al. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13(1):18–9. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 40.Bu S, Asano Y, Bujor A, Highland K, Hant F, Trojanowska M. Dihydrosphingosine 1-phosphate has a potent antifibrotic effect in scleroderma fibroblasts via normalization of phosphatase and tensin homolog levels. Arthritis Rheum. 2010;62(7):2117–26. doi: 10.1002/art.27463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Jiang X, Yang L, Liu X, Yue S, Li L. Involvement of sphingosine 1-phosphate (SIP)/S1P3 signaling in cholestasis-induced liver fibrosis. Am J Pathol. 2009;175(4):1464–72. doi: 10.2353/ajpath.2009.090037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shea BS, Brooks SF, Fontaine BA, Chun J, Luster AD, Tager AM. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am J Respir Cell Mol Biol. 2010;43(6):662–73. doi: 10.1165/rcmb.2009-0345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swaney JS, Moreno KM, Gentile AM, Sabbadini RA, Stoller GL. Sphingosine-1-phosphate (S1P) is a novel fibrotic mediator in the eye. Exp Eye Res. 2008;87(4):367–75. doi: 10.1016/j.exer.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Takuwa N, Ohkura S, Takashima S, Ohtani K, Okamoto Y, Tanaka T, et al. S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic mice involves reactive oxygen species. Cardiovasc Res. 2010;85(3):484–93. doi: 10.1093/cvr/cvp312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wijesinghe DS, Allegood JC, Gentile LB, Fox TE, Kester M, Chalfant CE. Use of high performance liquid chromatography-electrospray ionization-tandem mass spectrometry for the analysis of ceramide-1-phosphate levels. J Lipid Res. 2010;51(3):641–51. doi: 10.1194/jlr.D000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wijesinghe DS, Brentnall M, Mietla JA, Hoeferlin LA, Diegelmann RF, Boise LH, et al. Ceramide kinase is required for a normal eicosanoid response and the subsequent orderly migration of fibroblasts. J Lipid Res. 2014;55(7):1298–309. doi: 10.1194/jlr.M048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mietla JA, Wijesinghe DS, Hoeferlin LA, Shultz MD, Natarajan R, Fowler AA, et al. Characterization of eicosanoid synthesis in a genetic ablation model of ceramide kinase. J Lipid Res. 2013;54(7):1834–47. doi: 10.1194/jlr.M035683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poretsky L. Principles of diabetes mellitus. 2. New York: Springer; 2009. [Google Scholar]
- 49.Lambert P, Bingley PJ. What is Type 1 Diabetes? Medicine. 2002;30:1–5. [Google Scholar]
- 50.Rother KI. Focus on research: Diabetes treatment - Bridging the divide. New Engl J Med. 2007;356(15):1499–501. doi: 10.1056/NEJMp078030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shoback . In: Greenspan’s basic & clinical endocrinology. 9. David GG, Dolores, editors. New York: McGraw-Hill Medical; 2011. [Google Scholar]
- 52.Riserus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48(1):44–51. doi: 10.1016/j.plipres.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lutty GA. Effects of diabetes on the eye. IOVS. 2013;54(14):ORSF81–7. doi: 10.1167/iovs.13-12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH. Diabetic keratopathy. Trans Am Ophthalmol Soc. 1981;79:180–99. [PMC free article] [PubMed] [Google Scholar]
- 55.Schultz RO, Peters MA, Sobocinski K, Nassif K, Schultz KJ. Diabetic keratopathy as a manifestation of peripheral neuropathy. Am J Ophthalmol. 1983;96(3):368–71. doi: 10.1016/s0002-9394(14)77829-8. [DOI] [PubMed] [Google Scholar]
- 56.Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001;108(3):586–92. doi: 10.1016/s0161-6420(00)00599-6. [DOI] [PubMed] [Google Scholar]
- 57.Yoon KC, Im SK, Seo MS. Changes of tear film and ocular surface in diabetes mellitus. Korean J Ophthalmol: KJO. 2004;18(2):168–74. doi: 10.3341/kjo.2004.18.2.168. [DOI] [PubMed] [Google Scholar]
- 58.Goebbels M. Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol. 2000;84(1):19–21. doi: 10.1136/bjo.84.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saito J, Enoki M, Hara M, Morishige N, Chikama T, Nishida T. Correlation of corneal sensation, but not of basal or reflex tear secretion, with the stage of diabetic retinopathy. Cornea. 2003;22(1):15–8. doi: 10.1097/00003226-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 60.Inoue K, Kato S, Ohara C, Numaga J, Amano S, Oshika T. Ocular and systemic factors relevant to diabetic keratoepitheliopathy. Cornea. 2001;20(8):798–801. doi: 10.1097/00003226-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Schwartz DE. Corneal sensitivity in diabetics. Arch Ophthalmol. 1974;91(3):174–8. doi: 10.1001/archopht.1974.03900060182003. [DOI] [PubMed] [Google Scholar]
- 62.Rosenberg ME, Tervo TM, Immonen IJ, Muller LJ, Gronhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. IOVS. 2000;41(10):2915–21. [PubMed] [Google Scholar]
- 63.Gekka M, Miyata K, Nagai Y, Nemoto S, Sameshima T, Tanabe T, et al. Corneal epithelial barrier function in diabetic patients. Cornea. 2004;23(1):35–7. doi: 10.1097/00003226-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Gobbels M, Spitznas M, Oldendoerp J. Impairment of corneal epithelial barrier function in diabetics. Graefe’s Arch Clin Exp Ophthalmol. 1989;227(2):142–4. doi: 10.1007/BF02169787. [DOI] [PubMed] [Google Scholar]
- 65.Busted N, Olsen T, Schmitz O. Clinical Observations on the Corneal Thickness and the Corneal Endothelium in Diabetes-Mellitus. Brit J Ophthalmol. 1981;65(10):687–90. doi: 10.1136/bjo.65.10.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su DH, Wong TY, Wong WL, Saw SM, Tan DT, Shen SY, et al. Diabetes, hyperglycemia, and central corneal thickness: the Singapore Malay Eye Study. Ophthalmology. 2008;115(6):964–8. e1. doi: 10.1016/j.ophtha.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 67.Saini JS, Mittal S. In vivo assessment of corneal endothelial function in diabetes mellitus. Arch Ophthalmol. 1996;114(6):649–53. doi: 10.1001/archopht.1996.01100130641001. [DOI] [PubMed] [Google Scholar]
- 68.Lee JS, Oum BS, Choi HY, Lee JE, Cho BM. Differences in corneal thickness and corneal endothelium related to duration in diabetes. Eye. 2006;20(3):315–8. doi: 10.1038/sj.eye.6701868. [DOI] [PubMed] [Google Scholar]
- 69.Kolesnick R. Signal-Transduction through the Sphingomyelin Pathway. Mol Chem Neuropathol. 1994;21(2–3):287–97. doi: 10.1007/BF02815356. [DOI] [PubMed] [Google Scholar]
- 70.Green DR. Apoptosis and sphingomyelin hydrolysis. The flip side. J Cell Biol. 2000;150(1):F5–7. doi: 10.1083/jcb.150.1.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haimovitz-Friedman A, Kan CC, Ehleiter D, Persaud RS, McLoughlin M, Fuks Z, et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994;180(2):525–35. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dbaibo GS, Pushkareva MY, Rachid RA, Alter N, Smyth MJ, Obeid LM, et al. p53-dependent ceramide response to genotoxic stress. J Clin Invest. 1998;102(2):329–39. doi: 10.1172/JCI1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rotolo JA, Zhang J, Donepudi M, Lee H, Fuks Z, Kolesnick R. Caspase-dependent and -independent activation of acid sphingomyelinase signaling. J Biol Chem. 2005;280(28):26425–34. doi: 10.1074/jbc.M414569200. [DOI] [PubMed] [Google Scholar]
- 74.Dbaibo GS, El-Assaad W, Krikorian A, Liu B, Diab K, Idriss NZ, et al. Ceramide generation by two distinct pathways in tumor necrosis factor alpha-induced cell death. FEBS letters. 2001;503(1):7–12. doi: 10.1016/s0014-5793(01)02625-4. [DOI] [PubMed] [Google Scholar]
- 75.Taha TA, Mullen TD, Obeid LM. A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim Biophys Acta. 2006;1758(12):2027–36. doi: 10.1016/j.bbamem.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanada K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim Biophys Acta. 2003;1632(1–3):16–30. doi: 10.1016/s1388-1981(03)00059-3. [DOI] [PubMed] [Google Scholar]
- 77.Kihara A, Igarashi Y. FVT-1 is a mammalian 3-ketodihydrosphingosine reductase with an active site that faces the cytosolic side of the endoplasmic reticulum membrane. J Biol Chem. 2004;279(47):49243–50. doi: 10.1074/jbc.M405915200. [DOI] [PubMed] [Google Scholar]
- 78.Michel C, vanEchtenDeckert G. Conversion of dihydroceramide to ceramide occurs at the cytosolic face of the endoplasmic reticulum. FEBS letters. 1997;416(2):153–5. doi: 10.1016/s0014-5793(97)01187-3. [DOI] [PubMed] [Google Scholar]
- 79.van Helvoort A, van’t Hof W, Ritsema T, Sandra A, van Meer G. Conversion of diacylglycerol to phosphatidylcholine on the basolateral surface of epithelial (Madin-Darby canine kidney) cells. Evidence for the reverse action of a sphingomyelin synthase. J Biol Chem. 1994;269(3):1763–9. [PubMed] [Google Scholar]
- 80.Futerman AH, Stieger B, Hubbard AL, Pagano RE. Sphingomyelin Synthesis in Rat-Liver Occurs Predominantly at the Cis and Medial Cisternae of the Golgi-Apparatus. J Biol Chem. 1990;265(15):8650–7. [PubMed] [Google Scholar]
- 81.Perry RJ, Ridgway ND. Molecular mechanisms and regulation of ceramide transport. Biochim Biophys Acta. 2005;1734(3):220–34. doi: 10.1016/j.bbalip.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 82.Sprong H, Kruithof B, Leijendekker R, Slot JW, van Meer G, van der Sluijs P. UDP-galactose:ceramide galactosyltransferase is a class I integral membrane protein of the endoplasmic reticulum. J Biol Chem. 1998;273(40):25880–8. doi: 10.1074/jbc.273.40.25880. [DOI] [PubMed] [Google Scholar]
- 83.van Meer G, Holthuis JC. Sphingolipid transport in eukaryotic cells. Biochim Biophys Acta. 2000;1486(1):145–70. doi: 10.1016/s1388-1981(00)00054-8. [DOI] [PubMed] [Google Scholar]
- 84.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. 2004;82(1):27–44. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]