Abstract
Each of the steps of respiratory system development relies on intricate interactions and coordinated development of the lung epithelium and mesenchyme. In the past, more attention has been paid to the epithelium than the mesenchyme. The mesenchyme is a source of specification and morphogenetic signals as well as a host of surprisingly complex cell lineages that are critical for normal lung development and function. This review highlights recent research focusing on the mesenchyme that has revealed genetic and epigenetic mechanisms of its development in the context of other cell layers during respiratory lineage specification, branching morphogenesis, epithelial differentiation, lineage distinction, vascular development, and alveolar maturation.
Introduction
Development of the respiratory system proceeds through a well-described series of steps beginning with division of the anterior common foregut tube into the respiratory endoderm ventrally and the esophagus dorsally. The respiratory tract then undergoes extensive branching to form the proximal conducting airways, followed by distal septation generating the gas exchange units, or alveoli, of the mature lung. These processes are coupled with coordinated differentiation of the airway and distal lung epithelium leading to a regionally specific pattern of cell types. Formation of a functional lung also requires simultaneous development of both the pulmonary vascular system (central systemic circulation) and bronchial vascular system (local lung circulation). The genetic and epigenetic regulation, as well as the specialized intra-, inter-, and extracellular mechanisms responsible for proper development of the respiratory system continue to be elucidated. Each of the steps in lung development is reliant upon inductive cues and reciprocal interactions between the pulmonary epithelium and the surrounding mesenchyme. Loss of or abnormalities in this critical interaction can lead to severe anatomical and functional defects in the airway and alveoli. Many of the phenotypes associated with such abnormalities result in lethality or severe morbidity in humans and are being investigated in biochemical, cellular, tissue culture, organ explant, and animal models. Despite its importance in directing airway and alveoli development, many aspects of the activities and regulatory mechanisms of the lung mesenchyme are not well understood, a deficit recognized at a recent workshop hosted by the National Heart, Lung, and Blood Institute [1]. In this review, we will discuss recent (primarily within the past 2–3 years) advances in respiratory development, focusing on the role of the lung mesenchyme (Fig. 1). For more comprehensive discussions of lung development, please see recently published reviews including [2], [3], [4], [5], [6], and [7].
Figure 1.
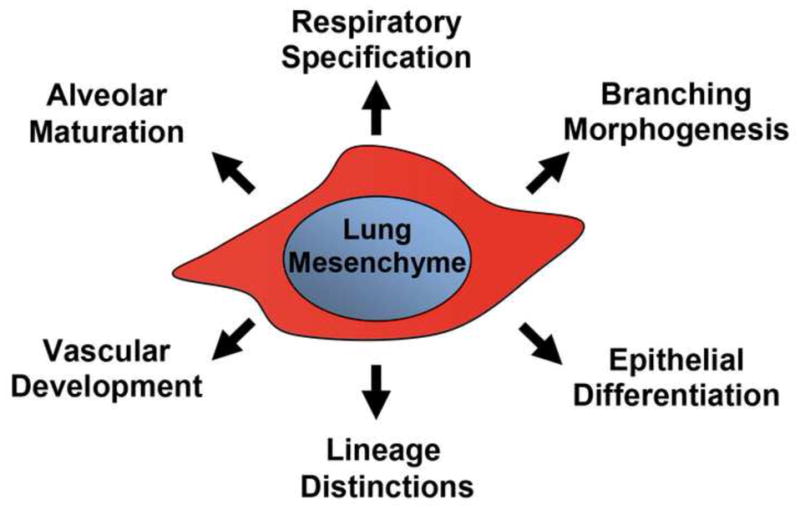
The lung mesenchyme holds a central position in the formation of a functional lung.
A diagram delineating the various topics covered in this review.
The mesenchyme provides critical signals for respiratory lineage specification
Specification of the respiratory system takes place in the ventral anterior foregut endoderm, as indicated by the expression of Nkx2-1 (also named Ttf1) beginning at embryonic day (E) 8.25 in mice [8], [9], and [10]. Collective work on respiratory lineage specification implicates the surrounding ventral mesenchyme as a critical source of signals, including FGF, WNT, BMP, RA, and TGFβ, that direct endodermal expression of Nkx2-1 in a temporal and spatial context dependent fashion [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], and [20]. Understanding the specification mechanisms initiated within the lung mesenchyme as well as identifying the specific mediators of mesenchyme-epithelium interactions has been a focus of recent research.
As an example of these signals, combined mesenchymal expression of Wnt2 and Wnt2b has been shown to be required for respiratory lineage specification and the expression of Nkx2-1 [12]. However, the upstream factors that control the expression of these signals in the mesenchyme are less clear. In Xenopus, it was shown that morpholino knockdown of Osr1 and Osr2, a pair of transcription factor genes, led to loss of Wnt2b expression in the mesenchyme [21*]. In mice, it was shown that genetic inactivation of Tbx5 prior to respiratory specification led to reduced Wnt2 and loss of Wnt2b expression in the mesenchyme and unilateral loss of Nkx2-1 expression in the prospective pulmonary epithelium [22*]. These data suggest that Osr1/2 and Tbx5 are required in the lung mesenchyme for normal Wnt2 and Wnt2b expression and subsequent specification of the respiratory foregut epithelium.
While evidence suggests that specification signals from the mesenchyme can control Nkx2-1 expression via transcription factors [15], a recent study suggests that they may also act through an epigenetic mechanism [23*]. It was shown that NANCI, a long non-coding RNA, is expressed in the ventral foregut and acts as a positive regulator of Nkx2-1 expression [23*]. Furthermore, it was found that NANCI is regulated itself by mesenchymal WNT signaling [23*].
Despite these advances, several open questions remain. For example, as each of the signals essential for respiratory specification is also active in other tissues, how they function to only specify the respiratory fate in a regionally constrained manner in the anterior ventral foregut is not understood. In addition, evidence demonstrates that the same signals, for example WNT, can function as either a promoter or inhibitor of the respiratory fate when acting at different time windows of development [12], [13], and [14]. How these different effects are mediated is not understood. Furthermore, the mechanisms by which fate specification is translated into morphogenesis, i.e. budding of the respiratory primordia from the anterior foregut tube, have not been determined.
The mesenchyme provides critical signals that drive epithelial branching morphogenesis
Following specification and physical separation of the respiratory lineage precursors from the esophagus within the anterior foregut, future conducting airways and alveolar regions are laid down according to a proximal-distal blueprint, through largely stereotypical branching events directed by cues from the adjacent mesenchyme. A cardinal mesenchymal signal that drives branching is FGF10 [24] and [25]. Its restricted expression in the distal mesenchyme at sites of future branch destination led to the proposal that the local source of FGF10 acts as a chemoattractive cue for directing nascent branches. However, a recent study showed that lungs with ubiquitous over expression of Fgf10 still formed discrete branches although the pattern is grossly abnormal especially at later developmental stages [26]. These findings together suggest that, in addition to proper regulation of Fgf10 expression, other constraints, such as heparin sulfate-based modification of signaling activity, may contribute to the construction of a stereotypical branching pattern [27*].
WNT signaling in the mesenchyme is a well-established driver of branching morphogenesis, however the specific WNT ligands and regulatory partners had not been clearly defined. Miller and colleagues showed that mesenchymal Wnt2 and epithelial Wnt7b cooperatively control branching morphogenesis, proximal-distal patterning, and development of distal lung progenitors [28]. To identify upstream activators and regulatory partners of WNT signaling in the mesenchyme, Miller et al conducted an in vitro screen that uncovered important interactions with homeobox transcription factors ESX1, MSX1/2, Nkx5-2 and the sumoylation factor PIAS4 [29]. For example, MSX1/2 were found to enhance canonical WNT signaling in a WNT ligand dependent manner specific to lung mesenchyme, possibly through transcriptional repression of WNT antagonists [29].
Proper development and activity of the mesenchyme requires interactions with both the neighboring epithelium and mesothelium. For example, mesothelial specific deletion of Fgf9 results in loss of Wnt2a expression in the mesenchyme and decreased airway branching [30]. This suggests that activation of mesenchymal WNT/β-catenin signaling is dependent on Fgf9 expressed in the mesothelium [30].
The lung mesenchyme is also a source of micro-RNAs (miRs) that control lung branching through an epigenetic mechanism. Carraro et al recently showed that miR-142-3p acts in the developing lung mesenchyme to regulate lung bud outgrowth and branching morphogenesis [31*]. It functions by promoting the activity of the WNT-FGF feed-forward signaling loop that maintains the lung mesenchyme in an undifferentiated state [31*].
A central question in the coordination between branch growth and patterning is the transition from the branching that forms the airways and the branching that forms the alveoli. Alanis et al recently demonstrated that the airway and alveolar regions are distinguished by two waves of developmental cues that interact to establish a critical boundary between these domains, the bronchoalveolar duct junction, BADJ [32**]. Mesenchymal WNT and FGF signaling is required for the first, or Sox9 wave, that progresses through all stages of embryonic lung development and generates both the proximal airway branches and distal alveoli [32**]. The second, or Sox2 wave, is initiated later and terminates earlier than the first wave under the control of glucocorticoid signaling that promotes the proximal conducting airway differentiation program [32**]. Treatment with a glucocorticoid, dexamethasone, led to premature termination of the Sox2 wave, setting a more proximal BADJ, whereas deletion of glucocorticoid receptors led to a more distal BADJ [32**].
The cellular mechanisms within the lung epithelium that mediate branching morphogenesis have been a major focus of recent research including changes in cell shape, apical-basal patterning, planar cell polarity, intercellular contacts, and extracellular matrix organization [33], [34], [35], [36]. Kadzik et al found that epithelial specific deletion of the WNT receptor Frizzled 2 (FZD2) results in impaired branch formation and disregulation of tube morphology [37**]. A close examination revealed that WNT signaling, possibly originating from the mesenchyme, acts through epithelial FZD2 to direct changes in epithelial cell shape and apical-basal lengthening, allowing for new branch formation in the early respiratory tree [37**].
Although it is clear that the mesenchyme is the key source of signals that drive branching, many questions remain. For example, three subroutines of branching have been identified: domain branching, planar bifurcation, and orthogonal bifurcation [38]. It is not clear how the mesenchymal signals interact differently to achieve each of these subroutines. Furthermore, while most research has focused on factors that control the initiation and maintenance of branching, what drives the termination of branching and lung growth remains elusive.
The mesenchyme provides critical signals that direct epithelial differentiation
Following branching morphogenesis, the conducting airway epithelium undergoes differentiation, influenced by signals from the neighboring mesenchyme. This influence was first demonstrated in tissue recombination experiments showing that proximal tracheal mesenchyme could induce distal lung epithelium to take on a more proximal cell fate, whereas distal lung mesenchyme could induce proximal tracheal epithelium to take on a more distal cell fate [39*]. The identities of these inductive cues from the mesenchyme remain unclear.
Further refining the influence from the mesenchyme, it was recently shown that the developing cartilage is a source of signals that promote basal cell fate in the adjacent epithelium [40*] and [41*]. Inactivation of Sox9 in the upper airway led to lack of cartilage specification which secondarily resulted in a decrease in the development of proximal airway basal cells [40*] and [41*]. It is possible that one of the signals that mediate the cartilage promotion of basal cell fate is FGF10. It was shown that Fgf10 expression is increased in the ventral mesenchyme, adjacent to the cartilage [11]. Disruption of cartilage formation led to a decrease in FGF target gene (Spry2) expression [40*] and [41*]. Over-expression of Fgf10 promotes p63-positive basal cell differentiation whereas inactivation of Fgf10 results in an almost complete absence of basal cells within the trachea [26]. This role for cartilage cells is of interest given the central role played by basal cells as a source of progenitors in the upper airway following injury [42], [43], and [44].
In the future, uncovering the mesenchymal signals that serve as instructive cues for epithelial identify should be an important direction of exploration. In addition, it is not clear if the airway smooth muscle cells that are arranged in a pattern apposing that of the cartilage cells are also a source of signals that direct epithelial differentiation. In the adult lung following injury such as that induced by naphthalene inhalation exposure, it was shown that the airway smooth muscle cells secrete FGF10, which then triggers a cascade of responses in the epithelium [26]. In the developing lung, differentiated airway smooth muscle is not a major source of FGF10. However, it remains possible that the airway smooth muscle in the developing lung may secrete other factors to control epithelial cell differentiation.
The mesenchyme receives cellular contribution from the cardiac mesoderm before differentiating into multiple lineages
In addition to its role as a source of signals for epithelial specification, branching and differentiation, the mesenchyme itself undergoes a regionally distinct differentiation program and gives rise to airway and vascular smooth muscle, endothelium, pericytes, and airway cartilage cells, among others (Fig. 2). A recent study by Peng et al identified a cardiopulmonary precursor population (CPP) with overlapping expression of Wnt2, Gli1, and Isl1 [45**]. Lineage tracing based on these markers demonstrated that CPPs give rise to airway and vascular smooth muscle, proximal vascular endothelium, and supporting pericyte cells within the lung (Fig. 2A) [45**]. Despite these recent findings, the relationship amongst the various lineages and the sequence by which they arise from the mesenchyme has not been clearly delineated.
Figure 2.
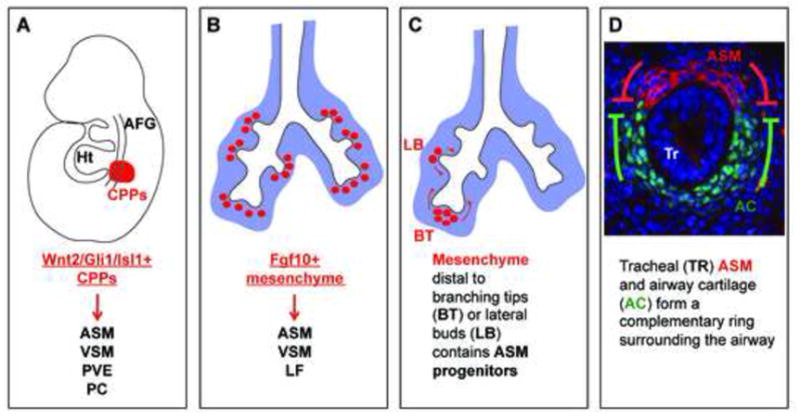
Lung mesenchyme is derived from and gives rise to multiple lineages. (A) Cardiopulmonary precursor cells (CPPs), characterized by overlapping Wnt2, Gli1, and Isl1 expression at E8.5, give rise to ASM, VSM, PVE, and PCs [45**]. (B) Fgf10 expressing cells labeled at E11.5 give rise to ASM, VSM, and LF cells [47**]. (C) ASM progenitor cells present in the mesenchyme distal to epithelial branching tips migrate proximally and take on a mature smooth muscle cell fate [48**]. (D) Tracheal ASM and cartilage have a mutually antagonistic relationship and form a complementary ring around the airway [40*] and [41*]. Abbreviations: AFG – anterior foregut, ASM – airway smooth muscle, Ht – heart, LF – lipofibroblast, PC – pericyte like (Pdgfrβ positive) cells, PVE – proximal vascular endothelium, VSM – vascular smooth muscle.
Within the lung, the airway smooth muscle precursor population has been shown to be derived from Fgf10-expressing distal lung mesenchyme that migrates proximally and takes on a differentiated smooth muscle fate [46]. Lineage tracing using an inducible cre knocked into the Fgf10 locus shows that at early branching stages, in addition to airway smooth muscle cells, the Fgf10-expressing progenitor population also gives rise to vascular smooth muscle and lipofibroblasts (Fig. 2B) [47**]. Mapping of progenitors for airway smooth muscles is further fine tuned in Kumar et al [48**]. By labeling single cells in the lung mesenchyme, it was shown that the airway smooth muscle cells are exclusively derived from mesenchyme distal to a branching tip, while mesenchymal cells that flank the epithelial stalks can only be induced to form airway smooth muscle by a lateral bud (Fig. 2C) [48**].
The subsequent differentiation of airway smooth muscle has been shown to be dependent on WNT2, which activates a smooth muscle (Myocardin, Mrtf-B) differentiation program [49]. Interestingly, WNT2 was not required for the development of adjacent proximal pulmonary vascular smooth muscle cells [49], suggesting that these two lineages may respond to different cues during their establishment.
The airway cartilage is only present in the upper airways in C-shaped half rings. In mice, at the levels of the trachea and extrapulmonary bronchi, the cartilage and smooth muscle are precisely juxtaposed and complement each other to encircle the epithelium. Recent studies showed that this juxtaposition is established early on when these opposing lineages first emerge, and is achieved primarily via mutual antagonism (Fig 2D) [40*] and [41*]. For example, loss of airway smooth muscle results in an increase in Sox9-positive cartilage precursor cell number [40*]. Conversely, loss of airway cartilage results in an increase in ACTA2 positive smooth muscle cell number [40*] and [41*]. Focusing on airway cartilage, several signaling pathways, including WNT, TGFβ, RA, SHH, and FGF have been shown to be essential for proper cartilage formation [50], [51], [18], [52], and [53].
Understanding patterning of the mesenchyme lags behind knowledge on the regionalization of the epithelium. For example, it is not clear why the cartilage is only present in the proximal part of the airway, whereas the airway smooth muscle is present in the entire airway. Furthermore, lung mesenchymal cells have been shown to act together as a niche that maintains neighboring airway precursor stem cell populations [7]. However, how this mesenchymal cell niche is generated and regulated is not fully understood and is important from a therapeutic perspective. While CPPs contribute to both the airway and vascular smooth muscle cell types [45**], it is not clear if, once CPPs enter the lung, they take on a shared “lung smooth muscle” lineage before splitting into the airway versus vascular smooth muscle fates.
The mesenchyme receives multiple signals to generate the lung vasculature
The processes of vascular development in the lung have been the source of considerable debate with both angiogenesis and vasculogenesis mechanisms being implicated. Prior work has suggested that proximal pulmonary vascular formation occurs through angiogenesis, with sprouting of vessels occurring in parallel to lung bud outgrowth while distal lung vessel formation occurs through vasculogenesis with endothelial cells deriving from mesenchymal vascular precursors [54], [55], and [56]. Additional lineage tracing experiments suggest that the pulmonary vasculature may develop purely through angiogenesis [57]. The findings that CPPs give rise to the proximal (von-Willebrand factor positive) pulmonary vascular endothelium but not distal alveolar capillary endothelium supports a dual origin of the pulmonary vascular system [45**].
Identification of the genetic pathways necessary for pulmonary vascular development has also been a recent focus. Mesenchymal specific inactivation of Sox17, although not affecting endothelial cell fate, results in abnormal dilation of the pulmonary veins and arteries as well as microvascular network simplification [58]. Pro-angiogenic miR-130a and anti-angiogenic miR-221 concurrently promote or inhibit airway branching, respectively, to give rise to a balanced system [59]. Additionally, epithelial inactivation of Wntless (Wls, also called Gpr77), a mediator of WNT ligand secretion, results in decreased distal pulmonary microvascular development associated with decreased expression of Vegfa and Ang-1, as well as their respective receptors Vegfr2 and Tie-2 [60], [61].
Despite these advances, the origin of the distal lung vasculature remains unclear. If there is indeed a dual origin of the pulmonary endothelial cells, it is not clear how they connect to each other without leakage of blood from the vascular system. Furthermore, the developmental mechanisms underlying the unique physiological properties of the pulmonary vascular network as distinct from the systemic vascular network warrant further investigation. The processes that direct the parallel development of the bronchial vascular system and the distal lymphatic vascular system are also unclear.
The mesenchyme contains key cell types that drive alveolar maturation
Following the pseudoglandular stage when the lung undergoes branching morphogenesis, the organ progresses through the canalicular, saccular, and alveologenesis stages during which the distal gas exchange units mature. Focusing on the distal lung, these steps result in a transition from columnar branching tip epithelium to thin walled airway septae required for efficient gas exchange. Accompanying the cell shape change is epithelial differentiation, generating type I and type II pneumocytes. The mechanisms of these transitions are not well understood but likely require the involvement of mesenchymal derived cell types including the myofibroblasts and lipofibroblasts.
Proper differentiation of the myofibroblast population is dependent on PDGF signaling acting via the receptor PDGFRα [62], [63], and [64]. In the Pdgfa ligand mutant, myofibroblast precursors, as marked by Pdgfrα expression, fail to spread around the primary septae [63]. As a consequence, there is little secondary septae formation, leading to simplification of the alveoli [63].
Recent work has focused on the role of HH signaling in affecting the myofibroblast population. Lin et al showed that mesenchyme specific inactivation of Suppressor of fused (Sufu), a negative regulator of HH signaling, results in increased HH activity but surprisingly reduced level of GLI1 protein, which is commonly a readout of HH activity [65*]. They further showed that GLI1 acts as a transcriptional regulator of Pdgfrα expression and its loss in the Sufu deficient mice results in a loss of myofibroblasts and subsequent impaired alveologenesis [65*].
Mice harboring a Gli1-creERT2 allele were recently used to lineage label and isolate cells responsive to HH signaling including the myofibroblast cell population [66*]. Using this technique, gene expression changes were studied in this Gli1-lineaged population during the transition from pseudoglandular, canalicular, and saccular phases of lung development [66*]. It was found that there are progressive changes in several signaling pathways including WNT, TGFβ, and PDGF pathways [66*]. For example, there is a gradual reduction of WNT signaling over this progression, characterized by a decrease in WNT activators (e.g. Ccna1) with a concurrent increase in WNT inhibitors (e.g. Wnt5a, Wif, Dkk3, and Tle1) [66*]. Furthermore, inactivation of the WNT inhibitor Apc using Gli1-creERT2 results in an expansion of Hh responsive myofibroblast precursor cells and impaired alveolar morphogenesis [66*].
To better understand the complex interactions required between the epithelium and mesenchyme during alveolar development, Greer et al recently devised an in vitro epithelium and mesenchyme co-culture system [67*]. Using this system, the authors investigated the effects of inflammation known to impair alveologenesis in infants born prematurely [67*]. This model provides the opportunity to better tease out the specific interactions between the mesenchyme and epithelium required for normal development of the alveoli, by manipulating these interactions with ease in culture.
In addition to myofibroblasts, lipofibroblasts are also required for alveologenesis, however their specific role and the genetic mechanisms that direct their development are not well understood. Injury models have demonstrated that lipofibroblasts can transdifferentiate to take on a myofibroblast fate following exposure to high (hyperoxia) or low (hypoxia) concentrations of oxygen, as well as nicotine [68], [69], [70], and [71]. PPARγ is important for lipofibroblast differentiation and its activation has been shown to reverse lipofibroblast to myofibroblast fate change [72], [73], [68], and [74]. Varisco et al demonstrated that the expression of Thy-1 increases during the initial phases of alveologenesis [75*]. This stimulates the expression of PPARγ and its heterodimer RXRα, leading to an accumulation of neutral lipid by fibroblasts. Alveologenesis is delayed in Thy-1 mutant lungs, suggesting that its effect on lipofibroblast differentiation may play a role in alveolar maturation [76].
Alveologenesis, and sometimes sacculation, are the steps of lung development that are disrupted by premature birth. Thus, delineating the genetic mechanisms that direct these phases of lung development is of clinical significance. During these phases, the individual and integrated functions of each of the mesenchymal cell population warrant investigation. It is also important to determine how the mesenchyme interacts with the epithelium as well as the infiltrating immune cells in injury settings such as under hyperoxia. Improved cell specific markers, lineage tracing tools, culture methods and 3D imaging modalities will allow for considerable advances in understanding the complex developmental process of alveolar maturation.
Summary and Future Directions
While much of the attention of lung development research has been focused on the lung epithelium, the mesenchyme is shaping up to be the new arena with an abundance of open questions. Strong lines of evidence, some of which are outlined above, unequivocally demonstrate that the lung mesenchyme is a critical source of inductive cues for the epithelium as they progress through development together. Furthermore, recent findings reveal unanticipated complexity in the lung mesenchymal populations, the intricacies of which demand attention. Innovative experimental approaches have driven considerable progress in our understanding of lung epithelial biology in the past decade. Adapting these approaches to study the lung mesenchyme will yield fruitful investigations.
Acknowledgments
We thank the members of the Sun laboratory for helpful discussions and suggestions, particularly Jamie Verheyden and Kelsey Branchfield. We also thank Elizabeth Hines for the smooth muscle and cartilage labeled trachea image included in Figure 2. DJM is supported by funding from the Department of Pediatrics, School of Medicine and Public Health, and by a Translational Research Pilot Award from the Institute for Clinical and Translational Research and the Stem Cell & Regenerative Medicine Center at the University of Wisconsin, Madison. Recent research in the Sun lab has been funded by: NHLBI-RO1HL097134, NHLBI-RO1HL113870, March of Dimes 6-FY10-339, Wisconsin Partnership Program Collaborative Research Award 2900.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morrisey EE, Cardoso WV, Lane RH, Rabinovitch M, Abman SH, Ai X, Albertine KH, Bland RD, Chapman HA, Checkley W, et al. Molecular determinants of lung development. Ann Am Thorac Soc. 2013;10:S12–16. doi: 10.1513/AnnalsATS.201207-036OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domyan ET, Sun X. Patterning and plasticity in development of the respiratory lineage. Dev Dyn. 2011;240:477–485. doi: 10.1002/dvdy.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitsett JA, Haitchi HM, Maeda Y. Intersections between pulmonary development and disease. Am J Respir Crit Care Med. 2011;184:401–406. doi: 10.1164/rccm.201103-0495PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng T, Morrisey EE. Development of the pulmonary vasculature: Current understanding and concepts for the future. Pulm Circ. 2013;3:176–178. doi: 10.4103/2045-8932.109954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hines EA, Sun X. Tissue crosstalk in lung development. J Cell Biochem. 2014;115:1469–1477. doi: 10.1002/jcb.24811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141:502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, Niklason L, Calle E, Le A, Randell SH, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- 9.Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2. 1(−/−) mouse embryos. Dev Biol. 1999;209:60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- 10.Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132:35–47. doi: 10.1242/dev.01570. [DOI] [PubMed] [Google Scholar]
- 11.Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris-Johnson KS, Domyan ET, Vezina CM, Sun X. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci U S A. 2009;106:16287–16292. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3:11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domyan ET, Ferretti E, Throckmorton K, Mishina Y, Nicolis SK, Sun X. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development. 2011;138:971–981. doi: 10.1242/dev.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–437. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Litingtung Y, Ten Dijke P, Chiang C. Aberrant Bmp signaling and notochord delamination in the pathogenesis of esophageal atresia. Dev Dyn. 2007;236:746–754. doi: 10.1002/dvdy.21075. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Gordon J, Manley NR, Litingtung Y, Chiang C. Bmp4 is required for tracheal formation: a novel mouse model for tracheal agenesis. Dev Biol. 2008;322:145–155. doi: 10.1016/j.ydbio.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen F, Desai TJ, Qian J, Niederreither K, Lu J, Cardoso WV. Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134:2969–2979. doi: 10.1242/dev.006221. [DOI] [PubMed] [Google Scholar]
- 20.Chen F, Cao Y, Qian J, Shao F, Niederreither K, Cardoso WV. A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J Clin Invest. 2010;120:2040–2048. doi: 10.1172/JCI40253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Rankin SA, Gallas AL, Neto A, Gomez-Skarmeta JL, Zorn AM. Suppression of Bmp4 signaling by the zinc-finger repressors Osr1 and Osr2 is required for Wnt/beta-catenin-mediated lung specification in Xenopus. Development. 2012;139:3010–3020. doi: 10.1242/dev.078220. Morpholino-mediated knockdown of Osr1/2 disrupts Wnt expression in the mesenchyme, and in turn affects lung specification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *22.Arora R, Metzger RJ, Papaioannou VE. Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet. 2012;8:e1002866. doi: 10.1371/journal.pgen.1002866. Genetic inactivation of Tbx5 in the mesenchyme led to reduction of Nkx2-1 expression in the prospective respiratory endoderm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Herriges MJ, Swarr DT, Morley MP, Rathi KS, Peng T, Stewart KM, Morrisey EE. Long noncoding RNAs are spatially correlated with transcription factors and regulate lung development. Genes Dev. 2014;28:1363–1379. doi: 10.1101/gad.238782.114. A lincRNA, NANCI was found to be expressed in the prospective respiratory endoderm, regulated by WNT signgaling from the mesenchyme, and in turn controls Nkx2-1 expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- 25.Abler LL, Mansour SL, Sun X. Conditional gene inactivation reveals roles for Fgf10 and Fgfr2 in establishing a normal pattern of epithelial branching in the mouse lung. Dev Dyn. 2009;238:1999–2013. doi: 10.1002/dvdy.22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volckaert T, Campbell A, Dill E, Li C, Minoo P, De Langhe S. Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Development. 2013;140:3731–3742. doi: 10.1242/dev.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27.Izvolsky KI, Zhong L, Wei L, Yu Q, Nugent MA, Cardoso WV. Heparan sulfates expressed in the distal lung are required for Fgf10 binding to the epithelium and for airway branching. Am J Physiol Lung Cell Mol Physiol. 2003;285:L838–846. doi: 10.1152/ajplung.00081.2003. Demonstrated that heparan sulfates restricts the activity of FGF10, whose local activity is essential for driving stereotypical lung branching. [DOI] [PubMed] [Google Scholar]
- 28.Miller MF, Cohen ED, Baggs JE, Lu MM, Hogenesch JB, Morrisey EE. Wnt ligands signal in a cooperative manner to promote foregut organogenesis. Proc Natl Acad Sci U S A. 2012;109:15348–15353. doi: 10.1073/pnas.1201583109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller MF, Cohen ED, Baggs JE, Hogenesch JB, Morrisey EE. High throughput genomic screen identifies multiple factors that promote cooperative Wnt signaling. PLoS One. 2013;8:e55782. doi: 10.1371/journal.pone.0055782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin Y, Wang F, Ornitz DM. Mesothelial- and epithelial-derived FGF9 have distinct functions in the regulation of lung development. Development. 2011;138:3169–3177. doi: 10.1242/dev.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *31.Carraro G, Shrestha A, Rostkovius J, Contreras A, Chao CM, El Agha E, Mackenzie B, Dilai S, Guidolin D, Taketo MM, et al. miR-142-3p balances proliferation and differentiation of mesenchymal cells during lung development. Development. 2014;141:1272–1281. doi: 10.1242/dev.105908. This microRNA was found to be present in the mesenchyme and control WNT-FGF signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **32.Alanis DM, Chang DR, Akiyama H, Krasnow MA, Chen J. Two nested developmental waves demarcate a compartment boundary in the mouse lung. Nat Commun. 2014;5:3923. doi: 10.1038/ncomms4923. Sox9 and Sox2 are found as markers of nexted waves of branching that ultimately distinguishes between airway and alveolar regions of the lung. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nechiporuk T, Klezovitch O, Nguyen L, Vasioukhin V. Dlg5 maintains apical aPKC and regulates progenitor differentiation during lung morphogenesis. Dev Biol. 2013;377:375–384. doi: 10.1016/j.ydbio.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yates LL, Schnatwinkel C, Hazelwood L, Chessum L, Paudyal A, Hilton H, Romero MR, Wilde J, Bogani D, Sanderson J, et al. Scribble is required for normal epithelial cell-cell contacts and lumen morphogenesis in the mammalian lung. Dev Biol. 2013;373:267–280. doi: 10.1016/j.ydbio.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HY, Varner VD, Nelson CM. Apical constriction initiates new bud formation during monopodial branching of the embryonic chicken lung. Development. 2013;140:3146–3155. doi: 10.1242/dev.093682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan H, Liu C, Wert SE, Xu W, Liao Y, Zheng Y, Whitsett JA. CDC42 is required for structural patterning of the lung during development. Dev Biol. 2013;374:46–57. doi: 10.1016/j.ydbio.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **37.Kadzik RS, Cohen ED, Morley MP, Stewart KM, Lu MM, Morrisey EE. Wnt ligand/Frizzled 2 receptor signaling regulates tube shape and branch-point formation in the lung through control of epithelial cell shape. Proc Natl Acad Sci U S A. 2014;111:12444–12449. doi: 10.1073/pnas.1406639111. A clear dissection of how WNT, activing via Frizzled receptor, controls branching via regulating coordinated cell shape changes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *39.Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn. 1998;212:482–494. doi: 10.1002/(SICI)1097-0177(199808)212:4<482::AID-AJA2>3.0.CO;2-D. Classical tissue recombination method was used to demonstrate the inductive effect of the respiratory mesenchyme on the epithelium. [DOI] [PubMed] [Google Scholar]
- *40.Hines EA, Jones MK, Verheyden JM, Harvey JF, Sun X. Establishment of smooth muscle and cartilage juxtaposition in the developing mouse upper airways. Proc Natl Acad Sci U S A. 2013;110:19444–19449. doi: 10.1073/pnas.1313223110. Tissue specific demonstration of the mutually antagonistic relationship between airway smooth muscle and cartilage. Additionally demonstrates role of airway cartilage in neighboring epithelial cell differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Turcatel G, Rubin N, Menke DB, Martin G, Shi W, Warburton D. Lung mesenchymal expression of Sox9 plays a critical role in tracheal development. BMC Biol. 2013;11:117. doi: 10.1186/1741-7007-11-117. Lung mesenchyme deletion of Sox9 affects both the mesenchyme, causing abnormal cartilage development, and neighboring epithelium, causing abnormal cellular differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 2004;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004;286:L643–649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- 44.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **45.Peng T, Tian Y, Boogerd CJ, Lu MM, Kadzik RS, Stewart KM, Evans SM, Morrisey EE. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature. 2013;500:589–592. doi: 10.1038/nature12358. Genetic lineage tracing was used to demonstrate a common cardiopulmonary progenitor contribute to multiple cell lineages in the lung mesenchyme. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mailleux AA, Kelly R, Veltmaat JM, De Langhe SP, Zaffran S, Thiery JP, Bellusci S. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development. 2005;132:2157–2166. doi: 10.1242/dev.01795. [DOI] [PubMed] [Google Scholar]
- **47.El Agha E, Herold S, Al Alam D, Quantius J, MacKenzie B, Carraro G, Moiseenko A, Chao CM, Minoo P, Seeger W, et al. Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development. 2014;141:296–306. doi: 10.1242/dev.099747. Genetic lineage tracing of the Fgf10-expressing cells and their progeny show that their contribution to the lung mesenchyme alters as development progresses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **48.Kumar ME, Bogard PE, Espinoza FH, Menke DB, Kingsley DM, Krasnow MA. Mesenchymal cells. Defining a mesenchymal progenitor niche at single-cell resolution. Science. 2014;346:1258810. doi: 10.1126/science.1258810. Single cell lineage tracing reveals lineage boundaries and potentials in the lung mesenchyme. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goss AM, Tian Y, Cheng L, Yang J, Zhou D, Cohen ED, Morrisey EE. Wnt2 signaling is necessary and sufficient to activate the airway smooth muscle program in the lung by regulating myocardin/Mrtf-B and Fgf10 expression. Dev Biol. 2011;356:541–552. doi: 10.1016/j.ydbio.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bell SM, Schreiner CM, Wert SE, Mucenski ML, Scott WJ, Whitsett JA. R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis. Development. 2008;135:1049–1058. doi: 10.1242/dev.013359. [DOI] [PubMed] [Google Scholar]
- 51.Vermot J, Niederreither K, Garnier JM, Chambon P, Dolle P. Decreased embryonic retinoic acid synthesis results in a DiGeorge syndrome phenotype in newborn mice. Proc Natl Acad Sci U S A. 2003;100:1763–1768. doi: 10.1073/pnas.0437920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiozzo C, De Langhe S, Carraro G, Alam DA, Nagy A, Wigfall C, Hajihosseini MK, Warburton D, Minoo P, Bellusci S. Fibroblast growth factor 10 plays a causative role in the tracheal cartilage defects in a mouse model of Apert syndrome. Pediatr Res. 2009;66:386–390. doi: 10.1203/PDR.0b013e3181b45580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller LA, Wert SE, Clark JC, Xu Y, Perl AK, Whitsett JA. Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev Dyn. 2004;231:57–71. doi: 10.1002/dvdy.20105. [DOI] [PubMed] [Google Scholar]
- 54.Schachtner SK, Wang Y, Scott Baldwin H. Qualitative and quantitative analysis of embryonic pulmonary vessel formation. Am J Respir Cell Mol Biol. 2000;22:157–165. doi: 10.1165/ajrcmb.22.2.3766. [DOI] [PubMed] [Google Scholar]
- 55.deMello DE, Sawyer D, Galvin N, Reid LM. Early fetal development of lung vasculature. Am J Respir Cell Mol Biol. 1997;16:568–581. doi: 10.1165/ajrcmb.16.5.9160839. [DOI] [PubMed] [Google Scholar]
- 56.Akeson AL, Wetzel B, Thompson FY, Brooks SK, Paradis H, Gendron RL, Greenberg JM. Embryonic vasculogenesis by endothelial precursor cells derived from lung mesenchyme. Dev Dyn. 2000;217:11–23. doi: 10.1002/(SICI)1097-0177(200001)217:1<11::AID-DVDY2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 57.Parera MC, van Dooren M, van Kempen M, de Krijger R, Grosveld F, Tibboel D, Rottier R. Distal angiogenesis: a new concept for lung vascular morphogenesis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L141–149. doi: 10.1152/ajplung.00148.2004. [DOI] [PubMed] [Google Scholar]
- 58.Lange AW, Haitchi HM, LeCras TD, Sridharan A, Xu Y, Wert SE, James J, Udell N, Thurner PJ, Whitsett JA. Sox17 is required for normal pulmonary vascular morphogenesis. Dev Biol. 2014;387:109–120. doi: 10.1016/j.ydbio.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mujahid S, Nielsen HC, Volpe MV. MiR-221 and miR-130a regulate lung airway and vascular development. PLoS One. 2013;8:e55911. doi: 10.1371/journal.pone.0055911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cornett B, Snowball J, Varisco BM, Lang R, Whitsett J, Sinner D. Wntless is required for peripheral lung differentiation and pulmonary vascular development. Dev Biol. 2013;379:38–52. doi: 10.1016/j.ydbio.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang M, Ku WY, Fu J, Offermanns S, Hsu W, Que J. Gpr177 regulates pulmonary vasculature development. Development. 2013;140:3589–3594. doi: 10.1242/dev.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bostrom H, Willetts K, Pekny M, Leveen P, Lindahl P, Hedstrand H, Pekna M, Hellstrom M, Gebre-Medhin S, Schalling M, et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–873. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- 63.Lindahl P, Karlsson L, Hellstrom M, Gebre-Medhin S, Willetts K, Heath JK, Betsholtz C. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development. 1997;124:3943–3953. doi: 10.1242/dev.124.20.3943. [DOI] [PubMed] [Google Scholar]
- 64.Sun T, Jayatilake D, Afink GB, Ataliotis P, Nister M, Richardson WD, Smith HK. A human YAC transgene rescues craniofacial and neural tube development in PDGFRalpha knockout mice and uncovers a role for PDGFRalpha in prenatal lung growth. Development. 2000;127:4519–4529. doi: 10.1242/dev.127.21.4519. [DOI] [PubMed] [Google Scholar]
- *65.Lin C, Yao E, Wang K, Nozawa Y, Shimizu H, Johnson JR, Chen JN, Krogan NJ, Chuang PT. Regulation of Sufu activity by p66beta and Mycbp provides new insight into vertebrate Hedgehog signaling. Genes Dev. 2014;28:2547–2563. doi: 10.1101/gad.249425.114. Sufu mutants reveal the key roles of HH signaling in alveologenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *66.Li C, Li M, Li S, Xing Y, Yang CY, Li A, Borok Z, De Langhe S, Minoo P. Progenitors of Secondary Crest Myofibroblasts are Developmentally Committed in Early Lung Mesoderm. Stem Cells. 2014 doi: 10.1002/stem.1911. Lineage tracing using a HH-responsive driver that labels the myofibroblasts reveal dynamic transcriptome changes across development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *67.Greer RM, Miller JD, Okoh VO, Halloran BA, Prince LS. Epithelial-mesenchymal co-culture model for studying alveolar morphogenesis. Organogenesis. 2014:e29198. doi: 10.4161/org.29198. A epithelium and mesenchyme co-culture method provides options for future studies of alveologenesis in a dish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rehan VK, Sugano S, Wang Y, Santos J, Romero S, Dasgupta C, Keane MP, Stahlman MT, Torday JS. Evidence for the presence of lipofibroblasts in human lung. Exp Lung Res. 2006;32:379–393. doi: 10.1080/01902140600880257. [DOI] [PubMed] [Google Scholar]
- 69.Rehan V, Torday J. Hyperoxia augments pulmonary lipofibroblast-to-myofibroblast transdifferentiation. Cell Biochem Biophys. 2003;38:239–250. doi: 10.1385/cbb:38:3:239. [DOI] [PubMed] [Google Scholar]
- 70.Nicola T, Ambalavanan N, Zhang W, James ML, Rehan V, Halloran B, Olave N, Bulger A, Oparil S, Chen YF. Hypoxia-induced inhibition of lung development is attenuated by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Am J Physiol Lung Cell Mol Physiol. 2011;301:L125–134. doi: 10.1152/ajplung.00074.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rehan VK, Sakurai R, Wang Y, Santos J, Huynh K, Torday JS. Reversal of nicotine-induced alveolar lipofibroblast-to-myofibroblast transdifferentiation by stimulants of parathyroid hormone-related protein signaling. Lung. 2007;185:151–159. doi: 10.1007/s00408-007-9007-0. [DOI] [PubMed] [Google Scholar]
- 72.Ferguson HE, Kulkarni A, Lehmann GM, Garcia-Bates TM, Thatcher TH, Huxlin KR, Phipps RP, Sime PJ. Electrophilic peroxisome proliferator-activated receptor-gamma ligands have potent antifibrotic effects in human lung fibroblasts. Am J Respir Cell Mol Biol. 2009;41:722–730. doi: 10.1165/rcmb.2009-0006OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGowan SE, Jackson SK, Doro MM, Olson PJ. Peroxisome proliferators alter lipid acquisition and elastin gene expression in neonatal rat lung fibroblasts. Am J Physiol. 1997;273:L1249–1257. doi: 10.1152/ajplung.1997.273.6.L1249. [DOI] [PubMed] [Google Scholar]
- 74.Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, Rehan VK. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1031–1041. doi: 10.1152/ajplung.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *75.Varisco BM, Ambalavanan N, Whitsett JA, Hagood JS. Thy-1 signals through PPARgamma to promote lipofibroblast differentiation in the developing lung. Am J Respir Cell Mol Biol. 2012;46:765–772. doi: 10.1165/rcmb.2011-0316OC. Thy-1, through its function on lipofibroblast differentiation, controls alveologenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nicola T, Hagood JS, James ML, Macewen MW, Williams TA, Hewitt MM, Schwiebert L, Bulger A, Oparil S, Chen YF, et al. Loss of Thy-1 inhibits alveolar development in the newborn mouse lung. Am J Physiol Lung Cell Mol Physiol. 2009;296:L738–750. doi: 10.1152/ajplung.90603.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


