Abstract
N-Palmitoylethanolamine or palmitoylethanolamide (PEA) is an anti-inflammatory compound that was recently shown to exert peroxisome proliferator-activated receptor-α-dependent beneficial effects on colon inflammation. The actions of PEA are terminated following hydrolysis by 2 enzymes: fatty acid amide hydrolase (FAAH), and the less-studied N-acylethanolamine-hydrolyzing acid amidase (NAAA). This study aims to investigate the effects of inhibiting the enzymes responsible for PEA hydrolysis in colon inflammation in order to propose a potential therapeutic target for inflammatory bowel diseases (IBDs). Two murine models of IBD were used to assess the effects of NAAA inhibition, FAAH inhibition, and PEA on macroscopic signs of colon inflammation, macrophage/neutrophil infiltration, and the expression of proinflammatory mediators in the colon, as well as on the colitis-related systemic inflammation. NAAA inhibition increases PEA levels in the colon and reduces colon inflammation and systemic inflammation, similarly to PEA. FAAH inhibition, however, does not increase PEA levels in the colon and does not affect the macroscopic signs of colon inflammation or immune cell infiltration. This is the first report of an anti-inflammatory effect of a systemically administered NAAA inhibitor. Because NAAA is the enzyme responsible for the control of PEA levels in the colon, we put forth this enzyme as a potential therapeutic target in chronic inflammation in general and IBD in particular.—Alhouayek, M., Bottemanne, P., Subramanian, K. V., Lambert, D. M., Makriyannis, A., Cani, P. D., and Muccioli, G. G. N-Acylethanolamine-hydrolyzing acid amidase inhibition increases colon N-palmitoylethanolamine levels and counteracts murine colitis.
Keywords: endocannabinoid, 2-AG, Crohn’s disease, PF-3845, N-acyltaurine
Inflammatory bowel diseases (IBDs) are chronic inflammatory disorders of the gastrointestinal tract. Although rarely lethal, these diseases show increased prevalence and incidence in developed countries and deeply affect patients’ quality of life (1, 2). Until the addition of biologic therapies such as anti–TNF-α antibodies, the conventional treatments for IBD included classic anti-inflammatory compounds, such as 5-aminosalicylic acid derivatives, steroids, and immunoregulatory agents, such as azathioprine and methotrexate. Although active, these treatments either induce dependence and tolerance or are accompanied by heavy side effects. Thus, these diseases are in need for new pharmacologic approaches (3, 4). Evidence suggests that interfering with bioactive lipid signaling might constitute an interesting option (5).
Along this line, N-acylethanolamines (NAEs) constitute a family of endogenous bioactive lipids implicated in the regulation of several processes, ranging from modulation of food intake to pain and inflammation (6). NAEs share the same biosynthetic and degradation pathways. They are produced on demand from membrane phospholipids by the sequential actions of an N-acyltransferase (to generate N-acylphosphatidylethanolamines or NAPEs) and an NAPE-preferring phospholipase D (NAPE-PLD). Their actions are terminated by their hydrolysis by 2 enzymes: the fatty acid amide hydrolase (FAAH), and the more recently described N-acylethanolamine-hydrolyzing acid amidase (NAAA) (7).
Despite their shared metabolic pathways, NAEs constitute a diverse set of endogenous mediators acting at different receptors and exerting a variety of biologic activities. For instance, the endocannabinoid N-arachidonoylethanolamine or anandamide (AEA) is an orexigenic, anti-inflammatory, and anxiolytic compound, whereas N-oleoylethanolamine has anorexigenic properties (6, 8, 9). N-Palmitoylethanolamine or palmitoylethanolamide (PEA) is a known anti-inflammatory compound with analgesic, neuroprotective, and antiallergic properties (10–13). Its beneficial effects have been investigated in numerous models (14). For instance, PEA administration has proven beneficial in murine models of neurodegenerative/neuroinflammatory diseases such as multiple sclerosis, Alzheimer’s disease, and Parkinson’s disease, reducing the inflammatory component and exerting a neuroprotective effect (15–17). Moreover, PEA was shown to reduce neutrophil migration (10), TNF-α production, and cyclooxygenase-2 and inducible nitric oxide synthase expression in acute inflammation (18, 19) as well as intestinal hypermotility in croton oil-induced intestinal inflammation (20). The receptors mediating the effects of PEA remain elusive, although some of its effects were shown to be peroxisome proliferator-activated receptor (PPAR)-α dependent (21).
Until recently, PEA was not investigated in the setting of IBD. In an article recently published, Esposito et al. (22) show that PEA has beneficial effects in reducing colon inflammation in dextran sodium sulfate (DSS)–induced colitis, a murine model of IBD, by a PPAR-α–dependent mechanism. Because FAAH is implicated in the regulation of PEA levels in vivo and very little is known about the effects of NAAA inhibition in physiopathologic settings, it was proposed that FAAH inhibition would potentiate the effects of PEA (22).
Here, we used inhibitors of these 2 enzymes in order to dissect their contributions to PEA levels and the control of inflammation in the colon. We confirm the beneficial effects of PEA in 2 models [the DSS-induced and the trinitrobenzene sulfonic acid (TNBS)–induced colitis models], and we further show that PEA not only reduces colon inflammation but also the colitis-related systemic inflammation. Moreover, we show that NAAA, rather than FAAH, is responsible for controlling PEA levels in the colon during inflammation. Accordingly, NAAA inhibition exerts beneficial effects in colitis, similarly to PEA, whereas FAAH inhibition has little effect. This is the first report for the implication of NAAA in the control of IBDs.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice (8–10 wk of age) were obtained from Charles River Laboratories (Brussels, Belgium), housed in standard cages, and supplied with food and drinking water ad libitum. Animals were kept in a 12 h light/dark cycle, and controlled temperature (22°C). All animal care and experimental procedures were conducted in accordance with the Belgian Law of April 6, 2010, regarding the protection of laboratory animals (agreement number LA1230314) and approved by the local ethics committee (UCL/MD/2009-010).
Induction and assessment of colitis
TNBS-induced colitis
Food (but not water) was withdrawn 24 h before administration of TNBS. Colitis was induced as previously described (23). In brief, mice (10 per group) were anesthetized by injection of a mixture of ketamine (100 mg/kg i.p.) and xylazine (10 mg/kg i.p.). TNBS (100 mg/kg, in 0.9% NaCl:ethanol, 50:50, v/v) was administered intrarectally (50 µl) into the colon using a cannula inserted 4 cm from the anus. Control mice received 50 µl of a 0.9% NaCl:ethanol (50:50, v/v) solution. To ensure a homogeneous distribution and retention of TNBS (or vehicle) within the colon, mice were held by the tail, in a vertical position, for 1 min after administration. Depending on the study, PEA (10 mg/kg), the FAAH inhibitor PF-3845 (10 mg/kg), and a combination of PEA and PF-3845, in a mixture of saline:ethanol:Tween 80 (18:1:1, v/v/v), or the vehicle alone were administered intraperitoneally 12 h before the induction of colitis and then once daily until the mice were killed. N-Arachidonoyltaurine (10 mg/kg) was administered similarly to PEA. The NAAA inhibitor AM9053 (10 mg/kg) was administered twice daily starting 2 h before colitis induction. For the therapeutic intervention study, PEA and inhibitors were administered starting 24 h after colitis induction at the same doses as the previous studies. PEA and PF-3845 dose and administration scheme were based on previous studies and the reported activity and half-life of the drugs (24). AM9053 dose administration and scheme were determined based on previous studies in the laboratory. Body weight was monitored daily, and mice were killed 3 d after TNBS administration.
DSS-induced colitis
To induce colitis, 5% DSS was added to the drinking water over a 5 d period, and this solution was replaced with normal drinking water for an additional 2 d. Mice were killed on d 7 (25, 26). PEA (10 mg/kg) or vehicle was administered intraperitoneally once daily, starting on the day of addition of DSS to the drinking water (pretreatment group, PEA I) or on d 5 (treatment group, PEA II). The d 5 time point was chosen based on previous studies indicating that, at this point, colitis is already established based on weight loss and the presence of occult blood in the feces (Supplemental Fig. 1A and B). Control mice received only normal drinking water. Body weight was monitored daily.
For both colitis models, tissues were removed at the time of killing and snap-frozen in liquid nitrogen. Before freezing, colons were examined for weight, stool consistency, gross macroscopic appearance and length, and spleen and cecal content were weighed.
Myeloperoxidase assay
The tissue-associated myeloperoxidase (MPO) assay was performed to quantitate the degree of inflammatory infiltration, which correlates with the severity of colitis (27, 28). In brief, colon fragments were snap-frozen in liquid nitrogen at the time of killing and stored for later assessment. For determination of MPO activity, tissue was placed in hexadecyltrimethylammonium bromide (HTAB) buffer [0.5% HTAB in 50 mM potassium phosphate buffer (pH 6)] on ice and gently homogenized. The homogenate was centrifuged at 2000 × g for 10 min and subsequently ultracentrifuged at 18,000 × g for 20 min at 4°C. A total of 7 µl of supernatant was then added to 96-well plates together with 200 μl of a solution of 0.167 mg/ml o-dianisidine and 500 ppm hydrogen peroxide in 50 mM potassium phosphate buffer at pH 6. Samples were analyzed in duplicate. Total protein concentration was determined using the DC protein assay (Bio-Rad, Nazareth, Belgium). MPO activity in the supernatant was measured at 460 nm and normalized for protein concentration. Results are expressed as the percentage of vehicle.
Cytokine quantification by ELISA
Levels of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) in tissues were determined by a sandwich-type ELISA technique using the Ready-Set-Go! Kit (eBioscience, Vienna, Austria) following the manufacturer’s instructions. For experiments in the colon, proteins were isolated from the same tissue samples used for real-time quantitative PCR (qPCR) using TriPure reagent (Roche, Basel, Switzerland) after RNA extraction according to the manufacturer’s instructions. Total protein concentration was determined using the DC protein assay before the ELISAs were run.
NAEs and 2-arachidonoylglycerol quantification by HPLC-mass spectrometry
Tissues were homogenized in CHCl3 (10 ml), and deuterated standards (200 pmol d4-AEA, d4-PEA, and d5-2-AG) were added. Then MeOH (5 ml) and H2O (2.5 ml) were added and the lipids extracted by vigorous mixing. The organic layer was recovered and dried under N2. The resulting lipid fraction was prepurified by solid-phase extraction over silica, and NAEs and 2-arachidonoylglycerol were eluted with ethyl acetate:acetone (1:1, v/v). The resulting lipid fraction was analyzed by HPLC-mass spectrometry (MS) using an LTQ Orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) coupled to an Accela HPLC system (Thermo Fisher Scientific). Analyte separation was achieved by using a Kinetex C-18 column (5 μm, 4.6 × 150 mm; Phenomenex, Utrecht, The Netherlands) and a C18 precolumn. Mobile phases A and B were composed of MeOH:H2O:acetic acid (75:25:0.1, v/v/v) and MeOH:acetic acid (100:0.1, v/v), respectively. The gradient (0.5 ml/min) was as follows: from 100% A to 100% B in 15 min, followed by 10 min at 100% B and subsequent re-equilibration at 100% A. MS analysis in the positive mode was performed with an atmospheric pressure chemical ionization (APCI) source. Capillary and APCI vaporizer temperatures were set at 250 and 400°C, respectively. PEA, AEA, and 2-AG were quantified by isotope dilution using their respective deuterated standard (showing identical retention times) (29, 30). The calibration curves were generated as described (31), and the data were normalized by tissue sample weight.
N-Acyltaurine quantification by HPLC-MS
Tissues were homogenized in CHCl3 (10 ml) with 17:0 N-acyltaurine (NAT) added as internal standard [synthesized following (32)]. Then MeOH (5 ml) and 1% NaCl solution (2.5 ml) were added prior to acidification of the mixture using 2 M HCl. The lipids were extracted by vigorous mixing, and then the organic layer was recovered and dried under N2. The resulting lipid fraction was prepurified by solid-phase extraction over silica. The resulting lipid fraction, containing the NATs, was analyzed by HPLC-MS using an LTQ Orbitrap mass spectrometer coupled to an Accela HPLC system. Analyte separation was achieved by using a C-18 Kinetex C-18 column (5 μm, 4.6 × 150 mm; Phenomenex) and a C18 precolumn. Mobile phases A and B were composed of MeOH:H2O:NH4OH (50:50:0.1, v/v/v) and MeOH:NH4OH (100:0.1, v/v), respectively. The gradient (0.5 ml/min) was as follows: from 100% A to 100% B in 15 min, followed by 30 min at 100% B and subsequent re-equilibration at 100% A. MS analysis in the negative mode was performed using an electrospray source. The data were normalized using the signal obtained with 17:0 NAT and by tissue sample weight.
RNA extraction and real-time qPCR
For mRNA analysis, tissues were excised at the time of sacrifice, snap frozen in liquid N2, and stored at −80°C. Total RNA was extracted using the TriPure reagent according to the manufacturer’s instructions. cDNA was synthesized using a reverse-transcription kit (Promega, Madison, WI, USA) from 1 μg total RNA. qPCR was performed with a StepOnePlus instrument and software (Applied Biosystems, Foster City, CA, USA) as described previously (23). Products were analyzed by performing a melting curve at the end of the PCR. Data are normalized to the 60S ribosomal protein L19 (RPL19) mRNA expression. Primer sequences for qPCR are listed in Table 1.
TABLE 1.
Primer sequences
| Forward primer (5′–3′) | Reverse primer (5′–3′) | |
|---|---|---|
| RPL19 | GAAGGTCAAAGGGAATGTGTTCA | CCTTGTCTGCCTTCAGCTTGT |
| TNF-α | AGCCCCCAGTCTGTATCCTT | GGTCACTGTCCCAGCATCTT |
| IL-12 | ACGGCCAGAGAAAAACTGAA | AGCTCCCTCTTGTTGTGGAA |
| IL-6 | ACAAGTCGGAGGCTTAATTACACAT | TTGCCATTGCACAACTCTTTTC |
| IL-1β | TCGCTCAGGGTCACAAGAAA | CATCAGAGGCAAGGAGGAAAAC |
| MCP-1 | GCAGTTAACGCCCCACTCA | CCCAGCCTACTCATTGGGATCA |
| CD11b | GAACATCCCATGACCTTCCA | GCTGGGGGACAGTAGAAACA |
| F4-80 | TGACAACCAGACGGCTTGTG | CAGGCGAGGAAAAGATAGTGT |
Drugs
TNBS, HTAB, and o-dianisidine were from Sigma-Aldrich (Bornem, Belgium). DSS was purchased from TdB Consultancy (Uppsala, Sweden). PEA and PF-3845 were purchased from Tocris Bioscience (R&D Systems Europe Limited, Abingdon, United Kingdom). N-Arachidonoyltaurine (from Cayman Chemicals) was purchased from Bertin Pharma (Montigny le Bretonneux, France). AM9053 was synthesized in the laboratory of A.M. AM9053 inhibits NAAA activity with a half-maximal inhibitory concentration (IC50) value of 30 nM, while having limited effect on FAAH activity (IC50 >100 µM).
Statistical analysis
Data are expressed as the mean ± SEM. Differences between groups were assessed by 1-way ANOVA followed by a Dunnett post hoc test with the colitis group as the control column or, when variances were not equal, by the Kruskal-Wallis nonparametric test followed by the Dunn’s post hoc test. Data were analyzed using GraphPad Prism version 5.0 (San Diego, CA, USA) for Windows (Microsoft, Redmond, WA, USA).
RESULTS
PEA administration ameliorates DSS-induced colitis and prevents TNBS-induced colitis
In the DSS-induced colitis model, PEA was administered to mice on the day of addition of DSS to drinking water (PEA I), or on d 5 (PEA II). Both PEA administration regimens had a beneficial effect on colitis, reducing the colon weight/length ratio, inflammation, and the disruption in intestinal motility as well as DSS-induced splenomegaly (Supplemental Fig. 2A, B, D, E), although these effects did not reach statistical significance for PEA II for some parameters. PEA administration from d 1 also reduced neutrophil infiltration, as measured by MPO activity, whereas PEA II had no effect on this parameter (Supplemental Fig. 2C). In this latter regimen, colitis is already established, immune cells have already infiltrated the mucosa when we start PEA administration, and mice are exhibiting sickness behavior. Perhaps, a 2 d administration of PEA is insufficient to induce the efflux of immune cells from the colon and reproduce all the effects of the pretreatment.
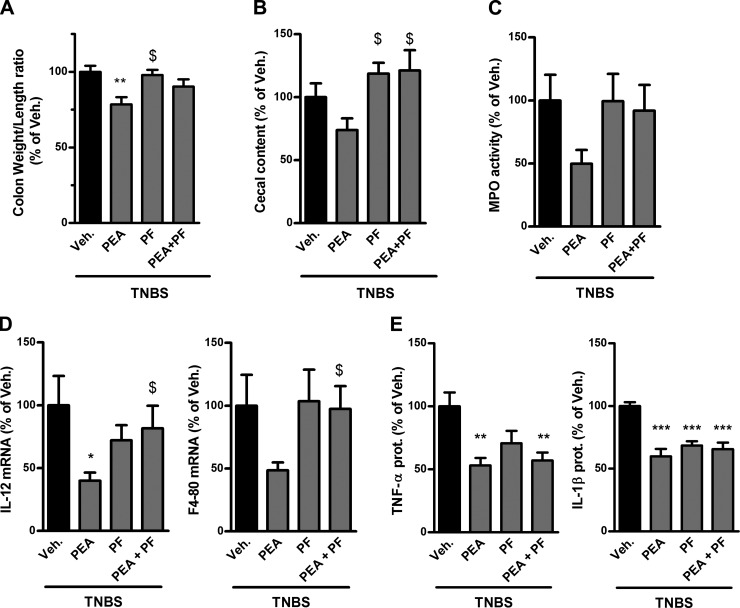
To further confirm the role of PEA in decreasing colon inflammation, PEA was also administered in the TNBS-induced colitis model. In this second model of IBD, PEA again reduced the colon weight/length ratio (Fig. 1A) and showed a nonsignificant trend to decrease cecal content weight (Fig. 1B). PEA also reduced proinflammatory cytokine mRNA expression (IL-12) and protein production (TNF-α and IL-1β) and exhibited a trend toward decreasing leukocyte infiltration as measured by MPO activity and mRNA expression of F4-80, a glycoprotein expressed on the cell surface of macrophages and thus mirroring macrophage infiltration (Fig. 1C–E).
Figure 1.
Impact of PEA administration and FAAH inhibition on colon inflammation in TNBS-induced colitis. Colitis is induced by intrarectal administration of TNBS to mice. PEA (10 mg/kg) and the FAAH inhibitor PF-3845 (PF; 10 mg/kg) were administered intraperitoneally. Mice were killed on d 3 after TNBS administration. A) Colon weight/length ratio, (B) feces weight in the cecum, (C) MPO activity, (D) IL-12 and F4-80 mRNA expression, and (E) proinflammatory cytokine production as measured by ELISA. Veh., vehicle. Results are expressed relative to the TNBS-untreated group set at 100%. Results are expressed as the mean ± SEM with n = 10 mice/group. *P < 0.05; **P < 0.005; ***P < 0.001 vs. the TNBS-untreated group; $P < 0.05 vs. the colitis group treated with PEA.
FAAH inhibition does not reproduce all the effects of PEA on TNBS-induced colitis
Having confirmed the beneficial effects of PEA administration on colitis, even when it is administered after colitis is established, we postulated that increasing endogenous PEA levels, by preventing its hydrolysis, could also exert beneficial effects in colitis. Because it is thought that FAAH is the principal enzyme that controls PEA levels in vivo, we evaluated the effects of the potent and selective FAAH inhibitor, PF-3845 (24), in TNBS-induced colitis.
Surprisingly, FAAH inhibition did not reproduce all the beneficial effects of PEA. Indeed, whereas PF-3845 administration decreased proinflammatory cytokine production (TNF-α and IL-1β) (Fig. 1E), it had no effect on the other parameters measured, such as colon weight/length ratio, leukocyte infiltration, and cecal content weight (Fig. 1A–D).
Another hypothesis was that FAAH inhibition could potentiate the effects of exogenously administered PEA by preventing its hydrolysis. However, coadministration of PEA with the FAAH inhibitor PF-3845 blocked the beneficial effects of PEA on several parameters (Fig. 1A–D). Concerning proinflammatory cytokine expression, here, the effect of coadministration of PEA and the FAAH inhibitor was similar to the effect of either compound alone (Fig. 1E).
Differential regulation of PEA and AEA levels following inflammation and FAAH inhibition
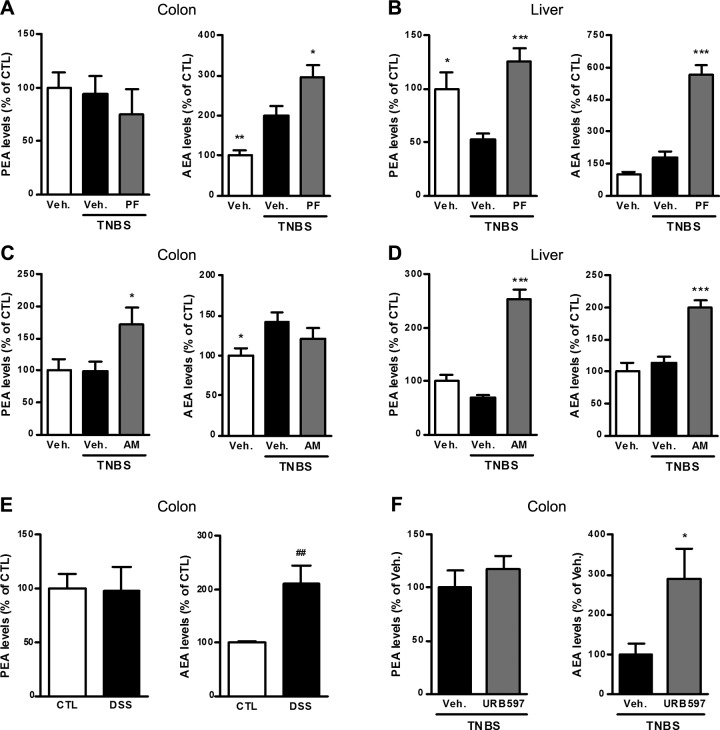
To dissect the differences between the effects of PEA administration and FAAH inhibition in the colon, we measured the levels of PEA and AEA in the colon. The first interesting observation came from comparing the colitis group to the control group: the levels of PEA are differently regulated compared to the levels of other NAEs such as AEA following colon inflammation. Indeed, PEA levels were unchanged by the colitis, whereas AEA levels were increased in the colon (Fig. 2A, C). This was also observed in the DSS-induced colitis model, where colon PEA levels were unaffected by colon inflammation, whereas AEA levels were increased (Fig. 2E). Moreover, FAAH inhibition using PF-3845 completely inhibited FAAH activity (Supplemental Fig. 3) and further increased colon AEA levels, without affecting PEA levels (Fig. 2A). This lack of effect of FAAH inhibition on PEA levels could explain why administration of the FAAH inhibitor did not reproduce the effects of PEA on colitis. It is noteworthy that when we tested another potent and well-characterized FAAH inhibitor, URB597 (33), it did not increase PEA levels in the colon of mice with TNBS-induced colitis but increased AEA levels (Fig. 2F), thus confirming the fact that FAAH does not control PEA levels in the colon.
Figure 2.
Effect of colitis, FAAH inhibition, and NAAA inhibition on PEA and AEA levels measured by HPLC-MS. PEA and AEA levels from the colon and liver of the different colitis experiments are shown. A, B) PEA and AEA levels in (A) the colon and (B) the liver of mice with TNBS-induced colitis in the presence of the FAAH inhibitor PF-3845 (PF; 10 mg/kg). C, D) PEA and AEA levels in (C) the colon and (D) the liver of mice with TNBS-induced colitis in the presence of the NAAA inhibitor AM9053 [AM; 10 mg/kg twice a day (b.i.d.)]. E) PEA and AEA levels in the colon of mice with DSS-induced colitis. F) PEA and AEA levels in the colon of mice with TNBS-induced colitis in the presence of the FAAH inhibitor URB597 (5 mg/kg b.i.d.). CTL, control. Results are expressed relative to control mice except for (F), where results are expressed compared to the TNBS-untreated group set at 100%. Results are expressed as the mean ± SEM with n = 10 mice/group for TNBS-induced colitis and n = 8 mice/group for DSS-induced colitis. *P < 0.05; ***P < 0.001 vs. the TNBS-untreated group. ##P < 0.005 for DSS-untreated mice vs. control mice.
To see if this differential regulation of NAE levels is present in other tissues than the colon, we also measured PEA and AEA levels in the liver. Interestingly, the picture is different in the liver where FAAH inhibition resulted in an increase in both the levels of PEA and AEA (Fig. 2B). However, here also, the levels of PEA and AEA were inversely affected by inflammation: PEA levels were decreased in the colitis group compared to the control group, whereas AEA levels were increased (Fig. 2B).
NAAA inhibition increases PEA levels in the colon and prevents TNBS-induced colitis
This differential regulation of PEA and AEA levels, and the lack of effect of FAAH inhibition on PEA levels in the colon, prompted us to consider the possibility that another enzyme was responsible for controlling PEA hydrolysis in the colon, which is NAAA. Here, we used one of the first systemically available NAAA inhibitors, AM9053, in order to assess its effects in TNBS-induced colitis.
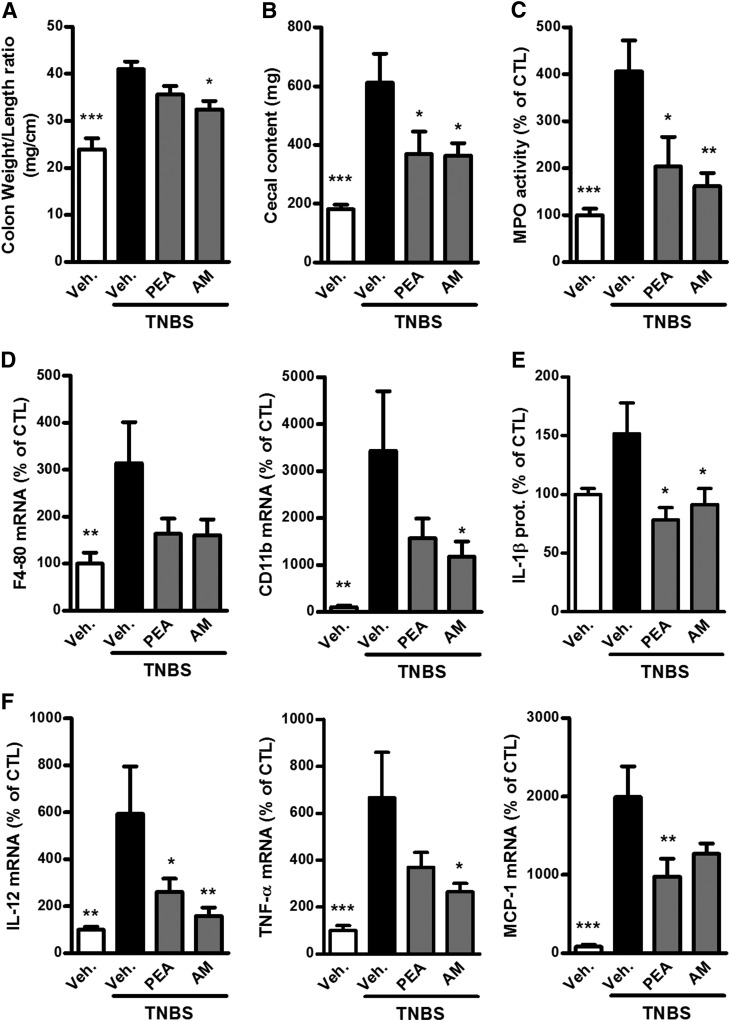
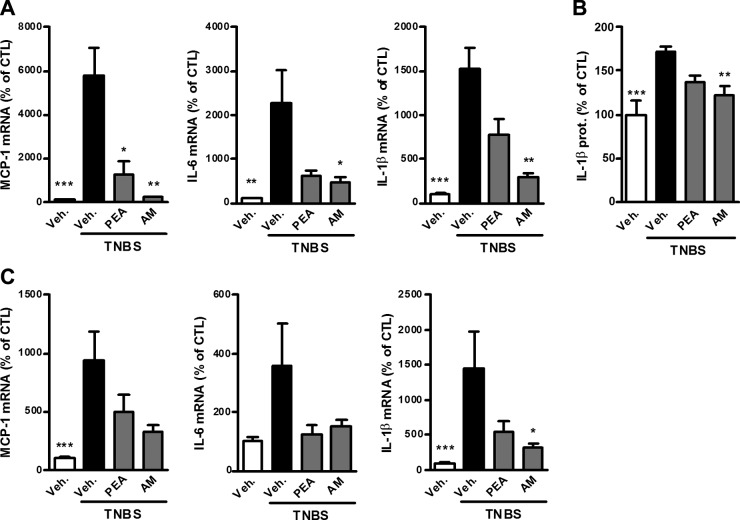
Administration of the NAAA inhibitor increased PEA, but not AEA, levels in the colon (Fig. 2C) and decreased colon inflammation in TNBS-induced colitis. Indeed, NAAA inhibition reduced the colon weight/length ratio and weight of the cecal content (Fig. 3A, B), as well as leukocyte infiltration and activation measured by MPO activity (Fig. 3C) and CD11b expression (Fig. 3D). Administration of the NAAA inhibitor also decreased the expression of proinflammatory cytokines (IL-1β, IL-12, and TNF-α) in the colon (Fig. 3E, F).
Figure 3.
Beneficial effects of PEA administration and NAAA inhibition on colon inflammation in TNBS-induced colitis. Colitis is induced by intrarectal administration of TNBS to mice. PEA (10 mg/kg) and the NAAA inhibitor AM9053 (AM; 10 mg/kg b.i.d.) were administered intraperitoneally. Mice were killed on d 3 after TNBS administration. A) Colon weight/length ratio, (B) feces weight in the cecum, (C) MPO activity, (D) mRNA expression of F4-80 and CD11b, (E) IL-1β expression measured by ELISA, and (F) mRNA expression of IL-12, TNF-α, and MCP-1. prot., protein. Results are expressed as the mean ± SEM with n = 10 mice/group. *P < 0.05, **P < 0.005, ***P < 0.001 vs. the TNBS-untreated group.
NAAA inhibition, FAAH inhibition, and PEA administration reduce the colitis-related systemic and central inflammation
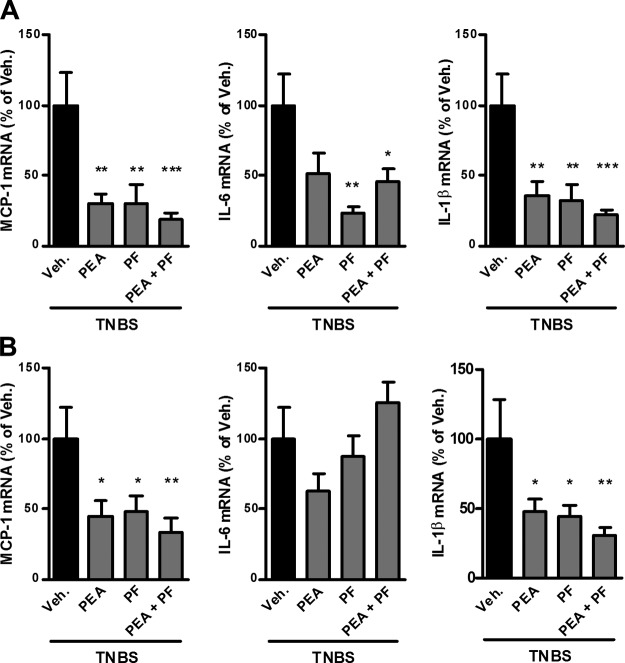
TNBS-induced colitis, as with human IBD, leads to a disruption in the integrity of the intestinal barrier due to inflammation, which results in peripheral inflammation (23). PEA administration decreased expression of IL-1β and MCP-1 in the liver and brain (Fig. 4). Moreover, NAAA inhibition, just like FAAH inhibition, increased the levels of both PEA and AEA in the liver (Fig. 2B, D) and decreased proinflammatory markers in the liver and brain, although the effect of the NAAA inhibitor was not significant in the brain, except for IL-1β (Figs. 4 and 5). Of note, whereas FAAH inhibition increased the levels of PEA and AEA in the brain, there was no effect of NAAA inhibition (Supplemental Fig. 4). This would suggest that the observed reduction in central inflammation with the NAAA inhibitor is more likely the result of the reduction of the systemic inflammation and not due to the local increase of PEA levels.
Figure 4.
Effects of PEA administration and FAAH inhibition on systemic inflammation resulting from TNBS-induced colitis. TNBS-induced colitis is accompanied by increased proinflammatory cytokine expression in the liver and brain. Effects are shown for PEA (10 mg/kg) administration and FAAH inhibition with PF-3845 (PF; 10 mg/kg) on proinflammatory cytokine expression in (A) the liver and (B) brain. Results are expressed relative to the TNBS-untreated group set at 100%. Results are expressed as the mean ± SEM with n = 10 mice/group. *P < 0.05; **P < 0.005; ***P < 0.001 vs. the TNBS-untreated group.
Figure 5.
Effects of PEA administration and NAAA inhibition on systemic inflammation resulting from TNBS-induced colitis. TNBS-induced colitis is accompanied by increased proinflammatory cytokine expression in the liver and brain. Effects are shown for PEA administration and NAAA inhibition with AM9053 (AM; 10 mg/kg b.i.d.) on (A) proinflammatory cytokine mRNA expression and (B) IL-1β protein expression in the liver and (C) proinflammatory cytokine mRNA expression in the brain. Results are expressed as the mean ± SEM with n = 10 mice/group. *P < 0.05; **P < 0.005; ***P < 0.001 vs. the TNBS-untreated group.
FAAH inhibition increases the levels of NATs
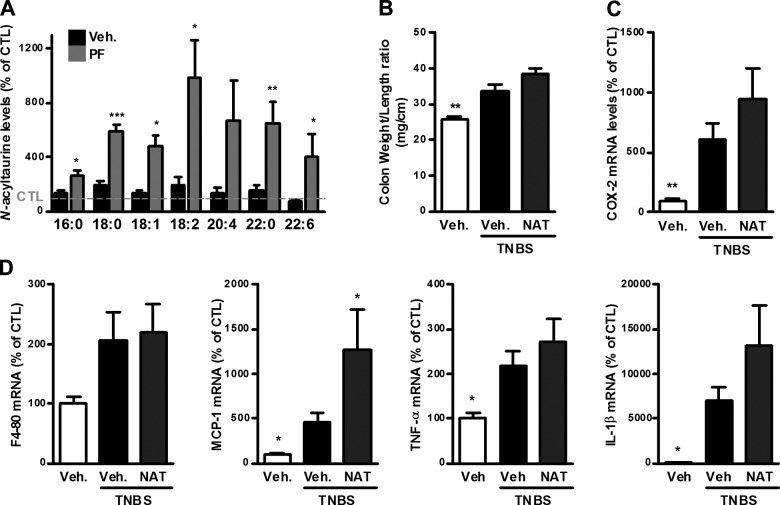
FAAH inhibition was shown in several studies to increase the levels of NATs in some tissues (34), with no reports in the digestive tract; therefore, we measured NAT levels in the ileum of mice with TNBS-induced colitis, following FAAH inhibition. The levels of several NATs were increased in the ileum of mice with TNBS-induced colitis and further increased following FAAH inhibition with PF-3845 (Fig. 6A).
Figure 6.
NATs in TNBS-induced colitis. A) NAT levels in the ileum of mice with TNBS-induced colitis in the presence of the FAAH inhibitor PF-3845 (PF; 10 mg/kg). Results are expressed relative to the control group set at 100% (dotted line). The length and degree of insaturation of the acyl chain are mentioned below their respective columns. B–D) Colitis is induced by intrarectal administration of TNBS to mice. N-Arachidonoyltaurine (NAT; 10 mg/kg) was administered intraperitoneally. Mice were killed on d 3 after TNBS administration. B) Colon weight/length ratio, (C) COX-2 mRNA expression, and (D) F4-80, MCP-1, IL-1β, and TNF-α mRNA expression in the colon. For (C) and (D), results are expressed relative to the control group set at 100%. Results are expressed as the mean ± SEM with n = 10 mice/group. *P < 0.05; **P < 0.005; ***P < 0.001 vs. the TNBS-untreated group.
Effect of N-arachidonoyltaurine on TNBS-induced colitis
We tested the effects of N-arachidonoyltaurine, which was shown to be a TRPV4 agonist in vitro (35), in TNBS-induced colitis. Administration of N-arachidonoyltaurine did not affect the TNBS-induced colon inflammation and showed a nonsignificant trend to worsen some of the parameters of TNBS-induced colitis, such as the colon weight/length ratio, as well as the expression of proinflammatory cytokines (Fig. 6B–D).
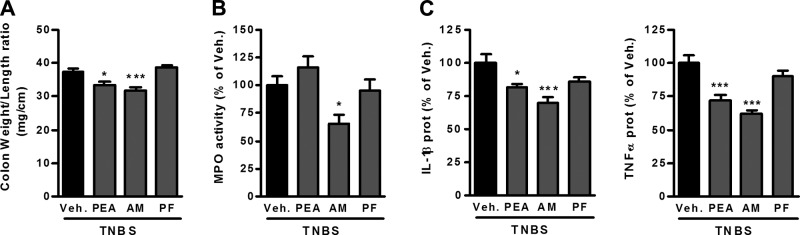
NAAA inhibition decreases colon inflammation, even when administered therapeutically
In the previous studies, we administered the enzyme inhibitors before colitis induction. We also wanted to assess their effects when administered after colitis is already induced. Therefore, PEA and inhibitors were administered to mice with TNBS-induced colitis 24 h after colitis induction. In this treatment scheme, the NAAA inhibitor showed beneficial effects similar to what was observed before, i.e., reduction of the colon weight/length ratio, MPO activity, as well as the expression of proinflammatory cytokines in the colon (Fig. 7A–C). In contrast, the FAAH inhibitor did not decrease any of the measured parameters (Fig. 7A–C).
Figure 7.
Effects of therapeutic administration of PEA and enzyme inhibitors in TNBS-induced colitis. Colitis is induced by intrarectal administration of TNBS to mice. PEA (10 mg/kg), the NAAA inhibitor AM9053 (AM; 10 mg/kg b.i.d.), and the FAAH inhibitor PF-3845 (PF; 10 mg/kg) were administered intraperitoneally, starting 24 h after colitis induction. Mice were killed on d 3 after TNBS administration. A) Colon weight/length ratio, (B) MPO activity in the colon, and (C) IL-1β and TNF-α protein expression measured by ELISA in the colon. Results are expressed as the mean ± SEM with n = 10 mice/group. *P < 0.05; ***P < 0.001 vs. the TNBS-untreated group.
DISCUSSION
Crohn’s disease and ulcerative colitis are the 2 major forms of IBD. Conventional treatments for IBD include 5-aminosalycilic acid derivatives, corticosteroids, immunomodulators, and biologics such as anti–TNF-α antibodies (3). However, because of various shortcomings of these treatments (e.g., loss of efficacy in the long run, severe side effects, and high costs), numerous studies are underway in order to find novel therapeutic targets for IBD (4). PPAR-α activation is known to have beneficial effects in colitis (36), and the effects of PEA on colon inflammation have been shown to be PPAR-α dependent (22). Moreover, the anti-inflammatory effects of 5-aminosalycilic acid derivatives and corticoids seem to be PPAR dependent (37–39); thus, increasing the endogenous levels of a PPAR-α agonist such as PEA should constitute a promising therapeutic strategy. However, the difficulty here was increasing the levels of PEA in the colon. Indeed, FAAH inhibition could be considered as the first choice to increase endogenous PEA levels due to the availability of several systemically active FAAH inhibitors and the fact that FAAH has been implicated in controlling NAE levels. However, FAAH inhibition did not increase PEA levels in the colon, prompting us to consider a more important role for NAAA in this setting. Our study puts forth the role of NAAA in controlling PEA levels in the colon and therefore provides a means for controlling colon inflammation.
In the present study, we provide further evidence to support the role of PEA as an anti-inflammatory compound, capable of alleviating inflammation in murine models of IBD. Indeed, PEA reduces the macroscopic parameters of murine colitis, namely the colon weight/length ratio and the weight of the cecal content. Moreover, PEA also reduces production of proinflammatory cytokines and the extent of immune cell infiltration.
Interestingly, PEA and AEA levels were differently regulated following colon inflammation—no variation in PEA levels was observed, whereas AEA levels were increased—thus pointing to different metabolic pathways for these 2 NAEs in the inflamed colon. This could be due to a difference in biosynthesis of PEA and AEA or to their hydrolysis by different enzymes. Concerning biosynthesis, alternative pathways have been described for AEA (7), and NAPE-PLD activity was shown to be decreased following inflammatory stimuli with a subsequent decrease of PEA levels (40). In this context, NAPE-PLD immunoreactivity in the colonic epithelium seems to be decreased in biopsies of patients with ulcerative colitis during acute flares (41). This would suggest a dysregulation in the production of NAEs during colon inflammation, with potentially reduced production. The fact that NAPE-PLD immunoreactivity is normalized following treatment (41) is another argument that increasing the levels of NAEs in general, and PEA in particular, which have anti-inflammatory properties, should be beneficial in colon inflammation.
Concerning the hydrolysis of NAEs, FAAH is thought to be the main enzyme controlling their levels. Moreover, the impact of inhibiting this enzyme in vivo has been extensively studied due to the availability of potent and selective inhibitors. However, FAAH inhibition, with 2 potent and selective inhibitors (PF-3845 and URB597), did not increase PEA levels in the colon but increased AEA levels. Accordingly, FAAH inhibition did not reproduce all the effects of PEA on colon inflammation, reducing only proinflammatory cytokine production, but not affecting colon weight/length ratio, cecal content weight, or immune cell infiltration. Perhaps this effect of FAAH inhibition on proinflammatory cytokine production is due to the slight increase in AEA levels. Conversely, FAAH inhibition in the liver increased both PEA and AEA levels and reduced the colitis-related systemic inflammation. This confirms the role of FAAH as being able to control PEA and AEA levels in the liver.
These results could seem surprising considering that a previous study with the FAAH inhibitor URB597 reported beneficial effects on TNBS-induced colitis, in a CB1/CB2-dependent manner, suggesting a role for AEA in the reduction of colon inflammation, although NAE levels were not quantified (42). Perhaps the difference with the present study is the dose of TNBS used [4 mg/mouse for (42) vs. 2 mg/mouse in this study], which could account for the difference in the effect of AEA. Moreover, the fact that the observed effects were CB1/CB2 dependent in that study (42) rules out the implication of PEA in the effects of URB597 administration because PEA is unable to bind and activate CB1 and CB2 receptors (43). Alternatively, the increase we observed in AEA levels in our studies might not be sufficient to unlock its full anti-inflammatory properties, although AEA hydrolytic activity was completely blocked in the colon of mice receiving the FAAH inhibitor (Supplemental Fig. 3); however, this remains speculative because AEA levels were not quantified in the study by Storr et al. (42).
Moreover, we show that coadministration of PEA and PF-3845 leads to blocking some effects of PEA on colon inflammation. Perhaps this could be explained by the potential increase in the levels of other FAAH substrates than NAEs, such as NATs, which have been shown to be increased following FAAH inhibition or in FAAH−/− mice (34, 35). However, this was shown in the liver, lung, kidney, and the CNS, with no reports concerning the digestive tract. Here, we show that FAAH inhibition also increases NAT levels in the digestive tract. The effects of these NATs are currently under investigation, but they have been shown to activate TRPV1 and TRPV4 receptors and could, therefore, exert effects opposite to PEA on some parameters in the colon (35). Indeed, TRPV1 activation induces colon contractions and hyperalgesia, whereas TRPV1 antagonists have been shown to decrease the severity of colitis (44–47). In addition, TRPV4 seems to be implicated in inflammation, pain, and hyperalgesia in the gastrointestinal tract (48). Accordingly, TRPV4 antagonism was shown to alleviate TNBS-induced colitis in mice (49). Therefore, it is possible that the increase in NATs following FAAH inhibition could block some of the effects of PEA, when it is administered in combination with PF-3845 (Fig. 1).
The lack of control of FAAH over PEA levels in the colon merits further investigation given the discrepant results observed in the literature and describing either pharmacologic or genetic FAAH inhibition. Indeed, whereas FAAH inhibitors and FAAH knockout mice have been tested in physiologic or pathologic conditions, the focus was mostly directed on AEA levels. However, some data with FAAH knockout mice show no differences in PEA levels in the small intestine in one study (50), whereas they are increased in the duodenum in another study (51), both in physiologic conditions. FAAH inhibition with arachidonoylserotonin (AA-5-HT) led to increased PEA levels in the stomach (52) and small intestine (50) of mice, both in physiologic conditions, and no increase in the colon of mice with preneoplastic lesions (53).
This would suggest that PEA hydrolysis in the colon could be mediated by another amidase under inflammatory conditions. NAAA could be responsible for PEA hydrolysis in some tissues or under certain conditions. Indeed, since its first characterization in macrophages, this enzyme has been considered as a PEA-preferring hydrolase (54). However, the lack of systemically active NAAA inhibitors (55) accounts for the little data available concerning the role of this enzyme in controlling PEA hydrolysis in vivo. Our data demonstrate for the first time the control of PEA levels in the colon, and in the liver (Fig. 2C, D) by NAAA. Additional data to support a role for NAAA in the inflamed colon come from studies on biopsies from patients with ulcerative colitis where immunohistochemical studies in active colitis epithelium showed an increase in NAAA expression and a decrease in NAPE-PLD expression, with infiltrating immune cells being strongly positive for NAAA (56). Thus, colon inflammation seems to impair NAE signaling, with PEA production seemingly decreased and its hydrolysis increased. Therefore, NAAA inhibition constitutes an attractive therapeutic strategy. Here, taking advantage of one of the first systemically active NAAA inhibitors, AM9053, we show that NAAA inhibition increases PEA, but not AEA, levels in the colon. Accordingly, NAAA inhibition reduces all the measured parameters of colitis, even when administered after colitis is established, as well as the related systemic inflammation.
Of course, it is important to keep in mind that these studies are done in murine models of IBD, and that, whereas these models are useful to the study of potential therapeutic targets and the identification of potential anti-inflammatory compounds, the challenge will be the later development of druggable compounds and the selection of the right disease stage or subpopulation of patients that will benefit from this potential treatment. However, because PEA has been already shown in several studies to be effective in humans (57, 58), we expect that NAAA inhibition, and the subsequent increase in PEA levels, will exert beneficial effects in inflammatory diseases.
In conclusion, we put forth the role of NAAA in controlling PEA levels in inflammatory conditions of the colon, and propose NAAA inhibitors as a potential therapeutic strategy in IBD, exerting beneficial effects through increasing PEA levels.
Supplementary Material
Acknowledgments
The authors thank Thérèse Timmerman and Owein Guillemot-Legris for their assistance. P.D.C. is a research associate from the FRS-FNRS (Fonds de la Recherche Scientifique), Belgium, and recipient of ERC Starting Grant 2013 (European Research Council, Starting Grant 336452-ENIGMO). G.G.M. is the recipient of a subsidy from the Fonds Spéciaux de Recherches (Université catholique de Louvain) and from the FRS-FNRS, Belgium (Grants CC 1.5.034.10 and FRFC 2.4555.08). A.M. is a postdoctoral researcher from the FRS-FNRS, Belgium. They are also grateful to the IBD Research Foundation for their kind financial support. The authors declare no conflicts of interest.
Glossary
- AEA
N-arachidonoylethanolamine or anandamide
- APCI
atmospheric pressure chemical ionization
- DSS
dextran sodium sulfate
- FAAH
fatty acid amide hydrolase
- HTAB
hexadecyltrimethylammonium bromide
- IBD
inflammatory bowel disease
- IC50
half-maximal inhibitory concentration
- MPO
myeloperoxidase
- MS
mass spectrometry
- NAAA
N-acylethanolamine-hydrolyzing acid amidase
- NAE
N-acylethanolamine
- NAPE
N-acylphosphatidylethanolamine
- NAPE-PLD
N-acylphosphatidylethanolamine-preferring phospholipase D
- NAT
N-acyltaurine
- PEA
N-palmitoylethanolamine or palmitoylethanolamide
- PPAR
peroxisome proliferator-activated receptor
- qPCR
quantitative PCR
- RPL19
ribosomal protein L19
- TNBS
trinitrobenzene sulfonic acid
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Khor B., Gardet A., Xavier R. J. (2011) Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strober W., Fuss I., Mannon P. (2007) The fundamental basis of inflammatory bowel disease. J. Clin. Invest. 117, 514–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mowat C., Cole A., Windsor A., Ahmad T., Arnott I., Driscoll R., Mitton S., Orchard T., Rutter M., Younge L., Lees C., Ho G. T., Satsangi J., Bloom S.; IBD Section of the British Society of Gastroenterology (2011) Guidelines for the management of inflammatory bowel disease in adults. Gut 60, 571–607 [DOI] [PubMed] [Google Scholar]
- 4.Melmed G. Y., Targan S. R. (2010) Future biologic targets for IBD: potentials and pitfalls. Nat. Rev. Gastroenterol. Hepatol. 7, 110–117 [DOI] [PubMed] [Google Scholar]
- 5.Alhouayek M., Muccioli G. G. (2012) The endocannabinoid system in inflammatory bowel diseases: from pathophysiology to therapeutic opportunity. Trends Mol. Med. 18, 615–625 [DOI] [PubMed] [Google Scholar]
- 6.Borrelli F., Izzo A. A. (2009) Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance. Best Pract. Res. Clin. Endocrinol. Metab. 23, 33–49 [DOI] [PubMed] [Google Scholar]
- 7.Muccioli G. G. (2010) Endocannabinoid biosynthesis and inactivation, from simple to complex. Drug Discov. Today 15, 474–483 [DOI] [PubMed] [Google Scholar]
- 8.Lambert D. M., Muccioli G. G. (2007) Endocannabinoids and related N-acylethanolamines in the control of appetite and energy metabolism: emergence of new molecular players. Curr. Opin. Clin. Nutr. Metab. Care 10, 735–744 [DOI] [PubMed] [Google Scholar]
- 9.Mechoulam R., Parker L. A. (2013) The endocannabinoid system and the brain. Annu. Rev. Psychol. 64, 21–47 [DOI] [PubMed] [Google Scholar]
- 10.Solorzano C., Zhu C., Battista N., Astarita G., Lodola A., Rivara S., Mor M., Russo R., Maccarrone M., Antonietti F., Duranti A., Tontini A., Cuzzocrea S., Tarzia G., Piomelli D. (2009) Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc. Natl. Acad. Sci. USA 106, 20966–20971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa B., Comelli F., Bettoni I., Colleoni M., Giagnoni G. (2008) The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB(1), TRPV1 and PPARgamma receptors and neurotrophic factors. Pain 139, 541–550 [DOI] [PubMed] [Google Scholar]
- 12.Darmani N. A., Izzo A. A., Degenhardt B., Valenti M., Scaglione G., Capasso R., Sorrentini I., Di Marzo V. (2005) Involvement of the cannabimimetic compound, N-palmitoyl-ethanolamine, in inflammatory and neuropathic conditions: review of the available pre-clinical data, and first human studies. Neuropharmacology 48, 1154–1163 [DOI] [PubMed] [Google Scholar]
- 13.Luongo L., Guida F., Boccella S., Bellini G., Gatta L., Rossi F., de Novellis V., Maione S. (2013) Palmitoylethanolamide reduces formalin-induced neuropathic-like behaviour through spinal glial/microglial phenotypical changes in mice. CNS Neurol. Disord. Drug Targets 12, 45–54 [DOI] [PubMed] [Google Scholar]
- 14.Alhouayek M., Muccioli G. G. (2014) Harnessing the anti-inflammatory potential of palmitoylethanolamide. Drug Discov. Today 19, 1632–1639 [DOI] [PubMed] [Google Scholar]
- 15.Loría F., Petrosino S., Mestre L., Spagnolo A., Correa F., Hernangómez M., Guaza C., Di Marzo V., Docagne F. (2008) Study of the regulation of the endocannabinoid system in a virus model of multiple sclerosis reveals a therapeutic effect of palmitoylethanolamide. Eur. J. Neurosci. 28, 633–641 [DOI] [PubMed] [Google Scholar]
- 16.Scuderi C., Steardo L. (2013) Neuroglial roots of neurodegenerative diseases: therapeutic potential of palmitoylethanolamide in models of Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 12, 62–69 [DOI] [PubMed] [Google Scholar]
- 17.D’Agostino G., Russo R., Avagliano C., Cristiano C., Meli R., Calignano A. (2012) Palmitoylethanolamide protects against the amyloid-β25-35-induced learning and memory impairment in mice, an experimental model of Alzheimer disease. Neuropsychopharmacology 37, 1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berdyshev E., Boichot E., Corbel M., Germain N., Lagente V. (1998) Effects of cannabinoid receptor ligands on LPS-induced pulmonary inflammation in mice. Life Sci. 63, PL125–PL129 [DOI] [PubMed] [Google Scholar]
- 19.Costa B., Conti S., Giagnoni G., Colleoni M. (2002) Therapeutic effect of the endogenous fatty acid amide, palmitoylethanolamide, in rat acute inflammation: inhibition of nitric oxide and cyclo-oxygenase systems. Br. J. Pharmacol. 137, 413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capasso R., Izzo A. A., Fezza F., Pinto A., Capasso F., Mascolo N., Di Marzo V. (2001) Inhibitory effect of palmitoylethanolamide on gastrointestinal motility in mice. Br. J. Pharmacol. 134, 945–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo Verme J., Fu J., Astarita G., La Rana G., Russo R., Calignano A., Piomelli D. (2005) The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 67, 15–19 [DOI] [PubMed] [Google Scholar]
- 22.Esposito G., Capoccia E., Turco F., Palumbo I., Lu J., Steardo A., Cuomo R., Sarnelli G., Steardo L. (2013) Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-alpha activation. Gut 63, 1300–1312 [DOI] [PubMed] [Google Scholar]
- 23.Alhouayek M., Lambert D. M., Delzenne N. M., Cani P. D., Muccioli G. G. (2011) Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J. 25, 2711–2721 [DOI] [PubMed] [Google Scholar]
- 24.Ahn K., Johnson D. S., Mileni M., Beidler D., Long J. Z., McKinney M. K., Weerapana E., Sadagopan N., Liimatta M., Smith S. E., Lazerwith S., Stiff C., Kamtekar S., Bhattacharya K., Zhang Y., Swaney S., Van B. K., Stevens R. C., Cravatt B. F. (2009) Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem. Biol. 16, 411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wirtz S., Neufert C., Weigmann B., Neurath M. F. (2007) Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2, 541–546 [DOI] [PubMed] [Google Scholar]
- 26.Kim J. J., Shajib M. S., Manocha M. M., Khan W. I. (2012) Investigating intestinal inflammation in DSS-induced model of IBD. J. Vis. Exp. (60) 3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley P. P., Christensen R. D., Rothstein G. (1982) Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood 60, 618–622 [PubMed] [Google Scholar]
- 28.Krawisz J. E., Sharon P., Stenson W. F. (1984) Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87, 1344–1350 [PubMed] [Google Scholar]
- 29.Muccioli G. G., Xu C., Odah E., Cudaback E., Cisneros J. A., Lambert D. M., López Rodríguez M. L., Bajjalieh S., Stella N. (2007) Identification of a novel endocannabinoid-hydrolyzing enzyme expressed by microglial cells. J. Neurosci. 27, 2883–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alhouayek M., Masquelier J., Cani P. D., Lambert D. M., Muccioli G. G. (2013) Implication of the anti-inflammatory bioactive lipid prostaglandin D2-glycerol ester in the control of macrophage activation and inflammation by ABHD6. Proc. Natl. Acad. Sci. USA 110, 17558–17563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muccioli G. G., Stella N. (2008) An optimized GC-MS method detects nanomolar amounts of anandamide in mouse brain. Anal. Biochem. 373, 220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saghatelian A., Trauger S. A., Want E. J., Hawkins E. G., Siuzdak G., Cravatt B. F. (2004) Assignment of endogenous substrates to enzymes by global metabolite profiling. Biochemistry 43, 14332–14339 [DOI] [PubMed] [Google Scholar]
- 33.Tarzia G., Duranti A., Gatti G., Piersanti G., Tontini A., Rivara S., Lodola A., Plazzi P. V., Mor M., Kathuria S., Piomelli D. (2006) Synthesis and structure-activity relationships of FAAH inhibitors: cyclohexylcarbamic acid biphenyl esters with chemical modulation at the proximal phenyl ring. ChemMedChem 1, 130–139 [DOI] [PubMed] [Google Scholar]
- 34.Long J. Z., LaCava M., Jin X., Cravatt B. F. (2011) An anatomical and temporal portrait of physiological substrates for fatty acid amide hydrolase. J. Lipid Res. 52, 337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saghatelian A., McKinney M. K., Bandell M., Patapoutian A., Cravatt B. F. (2006) A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochemistry 45, 9007–9015 [DOI] [PubMed] [Google Scholar]
- 36.Cuzzocrea S., Di Paola R., Mazzon E., Genovese T., Muià C., Centorrino T., Caputi A. P. (2004) Role of endogenous and exogenous ligands for the peroxisome proliferators activated receptors alpha (PPAR-alpha) in the development of inflammatory bowel disease in mice. Lab. Invest. 84, 1643–1654 [DOI] [PubMed] [Google Scholar]
- 37.Rousseaux C., Lefebvre B., Dubuquoy L., Lefebvre P., Romano O., Auwerx J., Metzger D., Wahli W., Desvergne B., Naccari G. C., Chavatte P., Farce A., Bulois P., Cortot A., Colombel J. F., Desreumaux P. (2005) Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J. Exp. Med. 201, 1205–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuzzocrea S., Bruscoli S., Mazzon E., Crisafulli C., Donato V., Di Paola R., Velardi E., Esposito E., Nocentini G., Riccardi C. (2008) Peroxisome proliferator-activated receptor-alpha contributes to the anti-inflammatory activity of glucocorticoids. Mol. Pharmacol. 73, 323–337 [DOI] [PubMed] [Google Scholar]
- 39.Riccardi L., Mazzon E., Bruscoli S., Esposito E., Crisafulli C., Di Paola R., Caminiti R., Riccardi C., Cuzzocrea S. (2009) Peroxisome proliferator-activated receptor-alpha modulates the anti-inflammatory effect of glucocorticoids in a model of inflammatory bowel disease in mice. Shock 31, 308–316 [DOI] [PubMed] [Google Scholar]
- 40.Zhu C., Solorzano C., Sahar S., Realini N., Fung E., Sassone-Corsi P., Piomelli D. (2011) Proinflammatory stimuli control N-acylphosphatidylethanolamine-specific phospholipase D expression in macrophages. Mol. Pharmacol. 79, 786–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marquéz L., Suárez J., Iglesias M., Bermudez-Silva F. J., Rodríguez de Fonseca F., Andreu M. (2009) Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS One 4, e6893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storr M. A., Keenan C. M., Emmerdinger D., Zhang H., Yüce B., Sibaev A., Massa F., Buckley N. E., Lutz B., Göke B., Brand S., Patel K. D., Sharkey K. A. (2008) Targeting endocannabinoid degradation protects against experimental colitis in mice: involvement of CB1 and CB2 receptors. J. Mol. Med. (Berl.) 86, 925–936 [DOI] [PubMed] [Google Scholar]
- 43.Lambert D. M., DiPaolo F. G., Sonveaux P., Kanyonyo M., Govaerts S. J., Hermans E., Bueb J., Delzenne N. M., Tschirhart E. J. (1999) Analogues and homologues of N-palmitoylethanolamide, a putative endogenous CB(2) cannabinoid, as potential ligands for the cannabinoid receptors. Biochim. Biophys. Acta 1440, 266–274 [DOI] [PubMed] [Google Scholar]
- 44.Kihara N., de la Fuente S. G., Fujino K., Takahashi T., Pappas T. N., Mantyh C. R. (2003) Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut 52, 713–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimball E. S., Wallace N. H., Schneider C. R., D’Andrea M. R., Hornby P. J. (2004) Vanilloid receptor 1 antagonists attenuate disease severity in dextran sulphate sodium-induced colitis in mice. Neurogastroenterol. Motil. 16, 811–818 [DOI] [PubMed] [Google Scholar]
- 46.Sugiuar T., Bielefeldt K., Gebhart G. F. (2004) TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J. Neurosci. 24, 9521–9530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szitter I., Pozsgai G., Sandor K., Elekes K., Kemeny A., Perkecz A., Szolcsanyi J., Helyes Z., Pinter E. (2010) The role of transient receptor potential vanilloid 1 (TRPV1) receptors in dextran sulfate-induced colitis in mice. J. Mol. Neurosci. 42, 80–88 [DOI] [PubMed] [Google Scholar]
- 48.Holzer P. (2011) Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol. Ther. 131, 142–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fichna J., Mokrowiecka A., Cygankiewicz A. I., Zakrzewski P. K., Małecka-Panas E., Janecka A., Krajewska W. M., Storr M. A. (2012) Transient receptor potential vanilloid 4 blockade protects against experimental colitis in mice: a new strategy for inflammatory bowel diseases treatment? Neurogastroenterol. Motil. 24, e557–e560 [DOI] [PubMed] [Google Scholar]
- 50.Capasso R., Matias I., Lutz B., Borrelli F., Capasso F., Marsicano G., Mascolo N., Petrosino S., Monory K., Valenti M., Di Marzo V., Izzo A. A. (2005) Fatty acid amide hydrolase controls mouse intestinal motility in vivo. Gastroenterology 129, 941–951 [DOI] [PubMed] [Google Scholar]
- 51.Fegley D., Gaetani S., Duranti A., Tontini A., Mor M., Tarzia G., Piomelli D. (2005) Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J. Pharmacol. Exp. Ther. 313, 352–358 [DOI] [PubMed] [Google Scholar]
- 52.Aviello G., Matias I., Capasso R., Petrosino S., Borrelli F., Orlando P., Romano B., Capasso F., Di Marzo V., Izzo A. A. (2008) Inhibitory effect of the anorexic compound oleoylethanolamide on gastric emptying in control and overweight mice. J. Mol. Med. (Berl.) 86, 413–422 [DOI] [PubMed] [Google Scholar]
- 53.Izzo A. A., Aviello G., Petrosino S., Orlando P., Marsicano G., Lutz B., Borrelli F., Capasso R., Nigam S., Capasso F., Di Marzo V.; Endocannabinoid Research Group (2008) Increased endocannabinoid levels reduce the development of precancerous lesions in the mouse colon. J. Mol. Med. (Berl.) 86, 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ueda N., Yamanaka K., Yamamoto S. (2001) Purification and characterization of an acid amidase selective for N-palmitoylethanolamine, a putative endogenous anti-inflammatory substance. J. Biol. Chem. 276, 35552–35557 [DOI] [PubMed] [Google Scholar]
- 55.Ponzano S., Bertozzi F., Mengatto L., Dionisi M., Armirotti A., Romeo E., Berteotti A., Fiorelli C., Tarozzo G., Reggiani A., Duranti A., Tarzia G., Mor M., Cavalli A., Piomelli D., Bandiera T. (2013) Synthesis and structure-activity relationship (SAR) of 2-methyl-4-oxo-3-oxetanylcarbamic acid esters, a class of potent N-acylethanolamine acid amidase (NAAA) inhibitors. J. Med. Chem. 56, 6917–6934 [DOI] [PubMed] [Google Scholar]
- 56.Suárez J., Romero-Zerbo Y., Márquez L., Rivera P., Iglesias M., Bermúdez-Silva F. J., Andreu M., Rodríguez de Fonseca F. (2012) Ulcerative colitis impairs the acylethanolamide-based anti-inflammatory system reversal by 5-aminosalicylic acid and glucocorticoids. PLoS One 7, e37729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conigliaro R., Drago V., Foster P. S., Schievano C., Di Marzo V. (2011) Use of palmitoylethanolamide in the entrapment neuropathy of the median in the wrist. Minerva Med. 102, 141–147 [PubMed] [Google Scholar]
- 58.Keppel Hesselink J. M., Kopsky D. J. (2013) Treatment of chronic regional pain syndrome type 1 with palmitoylethanolamide and topical ketamine cream: modulation of nonneuronal cells. J. Pain Res. 6, 239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.