Abstract
Vaccination with the minor capsid protein L2, notably the 17–36 neutralizing epitope, induces broadly protective antibodies, although the neutralizing titers attained in serum are substantially lower than for the licensed L1 VLP vaccines. Here we examine the impact of other less reactogenic adjuvants upon the induction of durable neutralizing serum antibody responses and protective immunity after vaccination with HPV16 and HPV31 L2 amino acids 17–36 inserted at positions 587 and 453 of VP3, respectively, for surface display on Adeno-Associated Virus 2-like particles [AAVLP (HPV16/31L2)]. Mice were vaccinated three times subcutaneously with AAVLP (HPV16/31L2) at two week intervals at several doses either alone or formulated with alum, alum and MPL, RIBI adjuvant or Cervarix. The use of adjuvant with AAVLP (HPV16/31L2) was necessary in mice for the induction of L2-specific neutralizing antibody and protection against vaginal challenge with HPV16. While use of alum was sufficient to elicit durable protection (>3 months after the final immunization), antibody titers were increased by addition of MPL and RIBI adjuvants. To determine the breadth of immunity, rabbits were immunized three times with AAVLP (HPV16/31L2) either alone, formulated with alum ± MPL, or RIBI adjuvants, and after serum collection, the animals were concurrently challenged with HPV16/31/35/39/45/58/59 quasivirions or cottontail rabbit papillomavirus (CRPV) at 6 or 12 months post-immunization. Strong protection against all HPV types was observed at both 6 and 12 months post-immunization, including robust protection in rabbits receiving the vaccine without adjuvant. In summary, vaccination with AAVLP presenting HPV L2 17–36 epitopes at two sites on their surface induced cross-neutralizing serum antibody, immunity against HPV16 in the genital tract, and long-term protection against skin challenge with the 7 most common oncogenic HPV types when using a clinically relevant adjuvant.
Keywords: Human papillomavirus, HPV16, HPV31, L2, Neutralizing antibody, Vaccine, VLP, AAV2, Adeno-associated virus, Display, Challenge, Adjuvant
1. Introduction
Cervical cancer is the third most common cancer of women worldwide, but is completely preventable. Persistent high-risk human papillomavirus (hrHPV) infection is necessary, but alone is not sufficient, for the development of cervical cancer [1,2]. Fifteen of the >120 known HPV genotypes are considered high risk [3–6], but HPV16 alone causes half of all cases of cervical cancer, and predominates in other HPV-associated anogenital cancers and those of the oropharynx [7–13]. The hrHPV cause ~5% of all cancer deaths globally but the greatest burden is among women who currently are not reached by effective cervical cancer screening programs, such that >85% of cervical cancers occur in low resource settings in the developing world [14–16]. Thus, inexpensive and broadly protective hrHPV vaccines are needed, especially in unscreened populations.
The licensed prophylactic HPV vaccines, Cervarix™ and Gardasil™ quadri- and nona-valent, are based upon virus-like particles ( VLPs) assembled from the major capsid protein L1. All contain L1 VLP derived from HPV16 and HPV18, but Gardasil 9 includes L1 VLP of 5 other hrHPV. Gardasil4 and 9 both contain L1 VLPs of HPV6 and HPV11 as these two types cause ~90% of all genital warts, a benign sexually transmitted disease. Vaccination with L1 VLPs alone induces high titers of type-restricted neutralizing antibodies [15,17–19], although all licensed vaccines utilize an aluminum-based adjuvant and Cervarix also includes a second adjuvant, the TLR4 agonist monophosphoryl lipid A (MPL). Cervarix and Gardasil4 are only licensed for protection against two hrHPV, which together cause ~70% of cervical cancer. However Gardasil9 is licensed for protection against the 7 most common hrHPV found in cervical cancer (HPV16, HPV18, HPV31, HPV33, HPV45, HPV52 and HPV58) as well as HPV6 and HPV11 [20], but its cost and therefore applicability in low resource settings is currently unknown.
An alternative approach to extend the coverage to more HPV genotypes is vaccination with a single immunogen that presents a conserved protective epitope, such as residues 17–36 within the minor capsid protein, L2 [21,22]. However, L2 is antigenically subdominant to L1 in the virus capsid and even alone is weakly immunogenic as compared to L1 VLP [23,24]. Therefore, here we present the L2 epitopes on the surface of a heterologous VLP backbone derived from VP3 of Adeno-associated virus 2. AAV2 belongs to the Parvoviridea. AAV2 is a small non enveloped virus containing an icosahedreal capsid of about 25 nm in diameter. The capsid is composed of three viral capsid proteins all encoded by the same open reading frame: VP1, VP2 and VP3. They form together the 60 subunits of the AAV2 capsid in a 1:1:8 ratio [62]. VP3 and VP2 are N-terminal truncated variants of VP1. Two main insertion sites were detected for the incorporation of peptides exposed on the capsid surface. These are insertions after amino acid position 587 and 453 [63,64]. Here, virus-like particles were formed using only the VP3 capsid protein (VP3), to produce a non-infectious protein scaffold lacking specific packaging of viral DNA. Due to the highly structured and repetitive presentation of epitopes on the capsid (60 times), combined with the intrinsic immunogenicity of AAV [65], potent B-cell responses can be expected. Nieto et al., previously showed that the simultaneous insertion of L2 epitopes comprising amino acids 17–36 of HPV16 and HPV31 in AAV2 VP3 residues 587 and 453, respectively, did not compromise assembly into Adeno-associated virus-like particles AAVLP (HPV16/31L2). Furthermore, vaccination of mice with AAVLP (HPV16/31L2) formulated in the adjuvant Montanide induced significant titers of broadly neutralizing serum antibodies [25,26]. Here we examine whether formulation of AAVLP (HPV16/31L2) with clinically relevant adjuvants can confer durable protection against experimental hrHPV challenge in the genital tract and skin of mice and rabbits, respectively. Further we examined the longevity of the protection responses up to 4 or 12 months in mice and rabbits, respectively.
2. Materials and methods
2.1. Production and purification of AAVLP (HPV16/31 L2) particles
The AAVLP (HPV16/31L2) were prepared and purified as described in Nieto et al. [26]:
Briefly the AAV VP2 sequence was cloned into the pCI plasmid (Promega). The VP2 start codon acg was substituted by gag, resulting in translation of only VP3. Nucleotide sequence of L2 residues 17–36 of HPV16 was cloned behind arginine residue 587 and nucleotide sequence of L2 residues 17–36 of HPV31 was cloned behind glycine residue 453. The amino acid sequence of the resulting AAV VP3 protein is shown in Supplementary Fig. S1. Plasmid was transfected via calcium phosphate precipitation into 293T cells. Particles were purified from cell lysate by several chromatographic steps as previously described [25,26].
The final buffer after purification was 50 mM HEPES, 200 mM NaCl and 2.5 mM MgCl2. This buffer was also used for dilution to adapt volume and concentration for immunization experiments.
Capsid titer was determined using a commercially available AAV2 titration ELISA kit (Progen, Heidelberg, Germany) according to the manufacturer’s manual.
Determination of Endotoxin content of purified material was performed by BSL Bioservice via turbidimetric kinetic LAL test (BSL Bioservice, Planegg, Germany).
2.2. Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Studies in mice were performed with the prior approval of the Johns Hopkins University Institutional Animal Care and Use Committee (IACUC). Rabbit studies were performed at Penn State University (PSU) College of Medicine following review and approval by the PSU IACUC.
2.3. Generation of HPV pseudovirus (PsV) and quasivirus (QV)
Plasmid double expression vectors for codon-optimized L1 and L2 genes were transfected with either a luciferase or alkaline phosphatase (SEAP) reporter gene plasmid into 293TT cells using TransIT-2020 transfection reagent (Mirus) [27,28]. Three days after transfection, cell pellets were collected and rinsed with Dulbecco’s modified Eagle medium (DMEM) and Dulbecco’s phosphate-buffered saline (DPBS). The pellets were washed with DPBS-Mg (DPBS supplemented with 9.5 mM MgCl2) and then transferred into siliconized tubes. Cells were lysed in DPBS-Mg supplemented with 0.25% of Brij 58 and 0.1% benzonase and incubated at 37 °C for 24 h. After this maturation step, the lysate was brought to 850 mM NaCl. Lysates were clarified by centrifugation at 10,000 × g for 10 min, loaded onto an Optiprep step gradient (27, 33, and 39%), and pseudovirions purified by centrifugation at 40,000 rpm in an SW40 rotor for 16 h at 16 °C. After centrifugation, 0.5 mL fractions were collected from the top of the gradient and tested for infectivity. Fractions producing the highest reporter gene expression were pooled, aliquoted and stored at −80 °C.
HPV Quasivirions were produced as described for PsV production but with the following modifications. L1 and L2 plasmids were transfected along with a linearized Cottontail rabbit papillomavirus genome containing the SV40 origin of replication (CRPV/SV40) into 293TT cells using Lipofectamine 2000 as previously described [29]. Quasivirions were clarified in Optiprep gradients at 50,000 rpm in an SW55T1 rotor for 3.5 h at 16 °C. The 24 h maturation step did not include benzonase which was subsequently added for an additional 1 h incubation at 37 °C prior to Optiprep gradient purification. Quasivirions were analyzed in vitro for infectious fractions by quantification of E1–E4 transcripts by QRT-PCR analysis in RK13 cells as described previously [30,31].
2.4. Enzyme-linked immunosorbent assays of L2 peptide
Immobilon plates (Nunc, Rochester, NY) were coated overnight at 4 °C with 100 ng/well of HPV16 L2 17–36 peptide. The plates were then blocked with 1% BSA in PBS for 1 h at room temperature and then the wells were drained and incubated with 2-fold dilution series of mouse sera for 1 h at room temperature. Following a wash step with PBS/0.01% (vol/vol) Tween-20, peroxidase-labeled rabbit anti-mouse IgG (Amersham) diluted 1:5000 in 1% BSA/PBS was added for 1 h. The plates were then washed again and developed with 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic) acid solution (Roche Applied Science, Indianapolis, IN) for 10 minutes. The absorbance was measured at 405 nm (A 405) in a Benchmark Plus plate reader (Bio-Rad, Hercules, CA). Pre-immune sera and mouse monoclonal antibody RG-1 were used as controls.
2.5. In vitro neutralization assays
Serum samples were serially diluted 2-fold in culture medium, mixed with an equal volume of HPV pseudovirions containing SEAP reporter plasmid, and incubated at 37 °C for 2 h. These samples were each added to 293TT cell cultures (3 × 104 cells/well). After incubation for 72 h at 37 °C in 5% CO2/95% air, 40 μL of cell-free supernatant was collected from each well, mixed with 20 μL of 0.05% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, heated at 65 °C for 30 min, and then cooled on ice. For development, 200 μL/well of p-nitrophenyl phosphate (pNPP) substrate (2 M diethanolamine with 1 mg/mL of pNPP) was added to each well at ambient temperature, and measured with an automatic enzyme-linked immunosorbent assay (ELISA) plate reader (Bio-Rad) when the optical density at 405 nm (OD405) in the wells incubated with only PsV (ODpsv) was >1. The highest dilution of serum that resulted in 50% or more reduction in ODpsv was defined as the endpoint neutralization titer and expressed as its reciprocal value.
2.6. Immunization of mice
As depicted in Supplemental Fig. S2, groups (n = 10) of female BALB/c mice, 4–6 weeks age (NCI Frederick) were vaccinated sub-cutaneously three times at two week intervals with low (0.6 μg, equivalent to 1011 particles which harbors an estimated 5.8 × 1012 L2 epitopes), or medium (6 μg) dose of AAVLP (HPV16/31L2) particles either without adjuvant or formulated with alum alone (Alhydrogel, Brenntag Biosector, Cat no.: 21645-51-2) (10 μL/mouse), or alum + MPL (Sigma L6895, from Salmonella enterica serotype minnesota Re 595; 5 μg/mouse, 5 μL of 1 μg/μL stock in DMSO), or RIBI adjuvant (Sigma S6322) containing 0.05 mg monophosphoryl lipid A (detoxified endotoxin) from S. Minnesota (MPL) and 0.05 mg synthetic trehalose dicorynomycolate (TDM) in 2% oil (squalene)-Tween 80-water. 100 μL/mouse)). Cervarix was used as a positive control (1/10th of human dose of Cervarix (comprising 2 μg of HPV16 L1 VLP and 2 μg of HPV18 L1 VLP adsorbed on to aluminum hydroxide (50 μg Al3+ as Al(OH)3) and 5 μg of MPL)). AAVLP (HPV16/31L2) and adjuvant were mixed and the volume made up to 200 μL with dilution buffer for each mouse and incubated overnight on a gentle end-over-end rotation at 4 °C. Serum samples were obtained by tail vein bleeds two weeks and 3.5 months after the final immunization.
2.7. Vaginal challenge of mice with HPV16 pseudovirus
Five days before challenge, female BALB/c mice were injected s.c. with 3 mg of medroxyprogesterone (Depo-Provera; Pfizer) to synchronize their estrus cycle. For challenge, the mice were anesthetized by isoflurane inhalation to effect. Half of the inoculum, which comprised 20 μL HPV16 PsV pre-mixed with 20 μL of 3% carboxymethyl cellulose (CMC), was delivered into the vaginal vault. This was followed by insertion and gentle rotation (15×) of a cytobrush, and upon its removal the remainder of the inoculum was deposited. Three days after challenge, the mice were again anesthetized and 20 μL of luciferin (7.8 mg/mL) was deposited in the vaginal vault. Luciferase signals were acquired for 10 min with a Xenogen IVIS 100 imager, and analysis was performed with Living Image 2.5 software [32,33].
2.8. Immunization and challenge of rabbits with HPV/CRPV quasivirions
As depicted in Supplemental Fig. S3, groups of 5 rabbits (Robinson, Inc) were vaccinated i.m. in the quadriceps three times at two week intervals with either Cervarix as a positive control (1/10th of human dose of Cervarix comprising 2 μg of HPV16 L1 VLP and 2 μg of HPV18 L1 VLP adsorbed on to aluminum hydroxide (50 μg Al3+ as Al(OH)3) and 5 μg of MPL) (Group A); AAVLP in saline without L2 inserts and without adjuvant (Group B); AAVLP (HPV16/31L2) in saline without adjuvant (Group C); AAVLP (HPV16/31L2) in RIBI adjuvant (Group D); AAVLP (HPV16/31L2) adsorbed on to aluminum hydroxide (50 μg Al3+ as Al(OH)3) and 5 μg of MPL) (Group E); and AAVLP (HPV16/31L2) admixed with 1/20th dose of Cervarix (Group F). The dose of AAVLP per immunization was 20 μg in each group. Each rabbit received 1 mL of vaccine distributed at two sites. Groups B and E contained 10 rabbits each. Six months after the final booster immunization, 5 rabbits in each group were challenged at two sites per construct with HPV quasivirions for HPV types 16, 18, 31, 35, 39, 45, 58, 59, and wildtype CRPV virions as previously described [29,34]. The doses of the HPV-QV that were used were sufficient to ensure an infection as pretested on a limited number of naïve rabbits. The remaining 10 rabbits in Groups B and E were challenged similarly to the 6 month challenge group but at 12 months after the final booster immunization. The dose of HPV QV challenge for the 12 month challenge group was the same and from the same production lot as that used for the 6 month challenge groups. The 12 month challenge doses were stored at −80 °C until needed.
2.9. Statistical analysis
Exploratory statistical analyses are performed to analyze the observed titer data. Log transformations were used to achieve normality in residuals for titer data. One-way ANOVA and pair wise multiple comparisons with Bonferroni adjustment were performed using 6 months post immunization.
3. Results
3.1. Immunization of mice with AAVLP (HPV16/31L2) and protection against cervicovaginal HPV PsV challenge
In a previous study, Nieto et al., [26] vaccinated BALB/c mice three times at two week intervals with either a low dose (LD = 0.6 μg), or high dose (HD = 30 μg) of AAVLP (HPV16/31L2), either formulated in the adjuvant Montanide ISA51 or without adjuvant, and collected serum 2 weeks later. Only the mice vaccinated with AAVLP (HPV16/31L2) that was formulated in adjuvant developed neutralizing antibodies. In addition, there was no significant difference between the LD and HD groups in the neutralizing antibody titer elicited against HPV16, HPV18, HPV45, HPV52 or HPV58, but the response to HPV31 was significantly higher in the mice receiving the higher dose. To gain a better understanding of the dose response and the impact of various adjuvants, we repeated the study using a low dose (LD = 0.6 μg), or medium dose (MD = 6 μg) of AAVLP (HPV16/31L2), or as a control 6 μg of wild type AAVLP. Furthermore, since Montanide ISA51 is not appropriate for prophylactic vaccination of patients, here we also examined whether other more clinically relevant adjuvants were sufficient to elicit neutralizing antibodies when formulated with AAVLP (HPV16/31L2). Thus 0.6 μg and 6 μg of AAVLP (HPV16/31L2) and 6 μg of AAVLP were formulated with either no adjuvant, alum alone, alum + MPL or RIBI adjuvant and used to vaccinate groups of mice (n = 10) three times at 2 week intervals. In addition, groups of mice were vaccinated with 0.1× human dose of Cervarix alone, or as a mixture with 6 μg of AAVLP (HPV16/31L2) to determine if there was any interference in the response to each HPV capsid antigen when admixed. A schema showing the experimental design is provided as Supplementary Fig. S2.
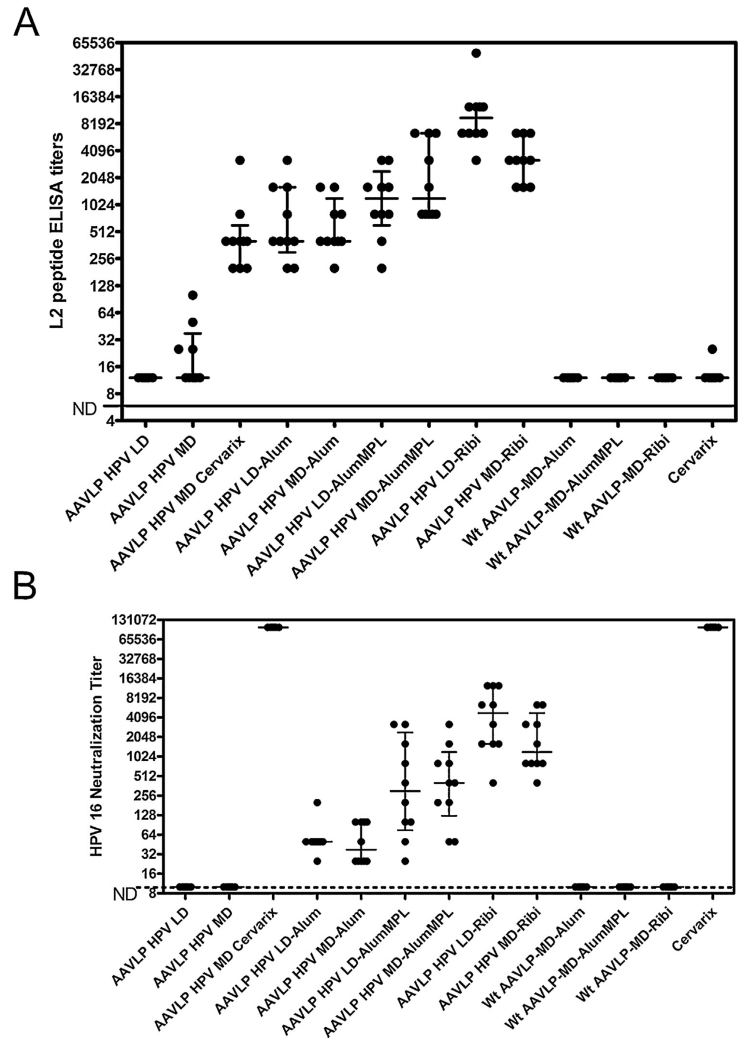
To assess the immune response, a serum sample was collected from each group (n = 5 mice) two weeks after the third immunization, both HPV16 L2 peptide and VP3 ELISA (data not shown) and in vitro neutralization assays were performed (Fig. 1A and B). As expected, there was no detectable L2 peptide-specific antibody responses in mice vaccinated with wild type AAV or Cervarix alone (Fig. 1A). Likewise, no response was detected in mice vaccinated with the low dose of AAVLP (HPV16/31L2) without adjuvant, although some L2-specific and VP3-specific response was measurable at 1:25 dilution in the sera of mice vaccinated with the medium dose of AAVLP (HPV16/31L2) without adjuvant. All mice vaccinated with low dose AAVLP (HPV16/31L2) in the presence of an adjuvant developed robust antibody responses to both L2 and VP3. For each adjuvant, the response was not significantly different between the LD and MD when measuring either the L2-peptide specific response or neutralizing antibody titer, except for the L2-peptide specific response (p < 0.05) in the AAVLP (HPV16/31L2) doses tested with RIBI adjuvant. The mean L2 peptide-specific serum antibody responses (Fig. 1A) and in vitro neutralizing antibody titers (Fig. 1B) were highest in the mice vaccinated with AAVLP (HPV16/31L2) in the presence of the RIBI adjuvant, intermediate for the alum + MPL formulation and lowest when just alum was used. At either dose level the use of alum enhances HPV16 neutralizing antibody titers elicited by AAVLP (HPV16/31L2) in the absence of an adjuvant (p < 0.001 at the low dose and p < 0.01 at the medium dose). Likewise the use of alum + MPL with AAVLP (HPV16/31L2) improved the HPV16 neutralizing antibody titers over alum (p < 0.001 at both doses) after vaccination. Finally, the use of Ribi adjuvant with AAVLP (HPV16/31L2) produced higher HPV16 neutralizing antibody titers after vaccination than alum + MPL (p < 0.001 at the low dose and p < 0.01 at the medium dose). These trends were similar for the L2 peptide response data.
Fig. 1. HPV16 L2-specific serum antibody responses in AAVLP (HPV16/31L2)-immunized mice.
Groups of mice were immunized with a variety of different antigen and formulated antigen preparations as described in the methods section. Sera from mice 2 weeks post vaccination were tested for reactivity to HPV16 L2 peptides by ELISA (A) and for neutralization of HPV16 PsV (B). ELISA and neutralization activities were calculated from titration curves representing 2-fold serial dilutions of sera tested in duplicate assay wells. From these curves, the half-maximal ELISA and neutralization values for each mouse (●) were plotted for each vaccine group. Each group contained 10 mice per antigen preparation. Median and interquartile range values for each group are represented as horizontal bars.
When a medium dose of AAVLP (HPV16/31L2) was mixed with Cervarix, then the alum + MPL in Cervarix acted as adjuvant for the L2-specific response to AAVLP (HPV16/31L2). The L2-specific serum antibody response to 6 μg of AAVLP (HPV16/31L2) formulated in alum + MPL was slightly, but significantly (p < 0.001), higher than the same dose of AAVLP (HPV16/31L2) mixed with Cervarix. It is not clear if this reflects weak antigenic competition or subtle differences in the source or formulation of the alum and/or MPL. The neutralizing antibody response to vaccination with Cervarix alone was very strong, and identical in sera of mice vaccinated with AAVLP (HPV16/31L2) formulated with Cervarix suggesting that mixing does not compromise the L1-specific neutralizing antibody response. The neutralizing antibody titers elicited by vaccination with AAVLP (HPV16/31L2) in adjuvant were 103-fold lower; although this conventional neutralization assay is known to be insensitive for L2-specific neutralizing antibodies [35] but its use facilitates comparison to other similar studies.
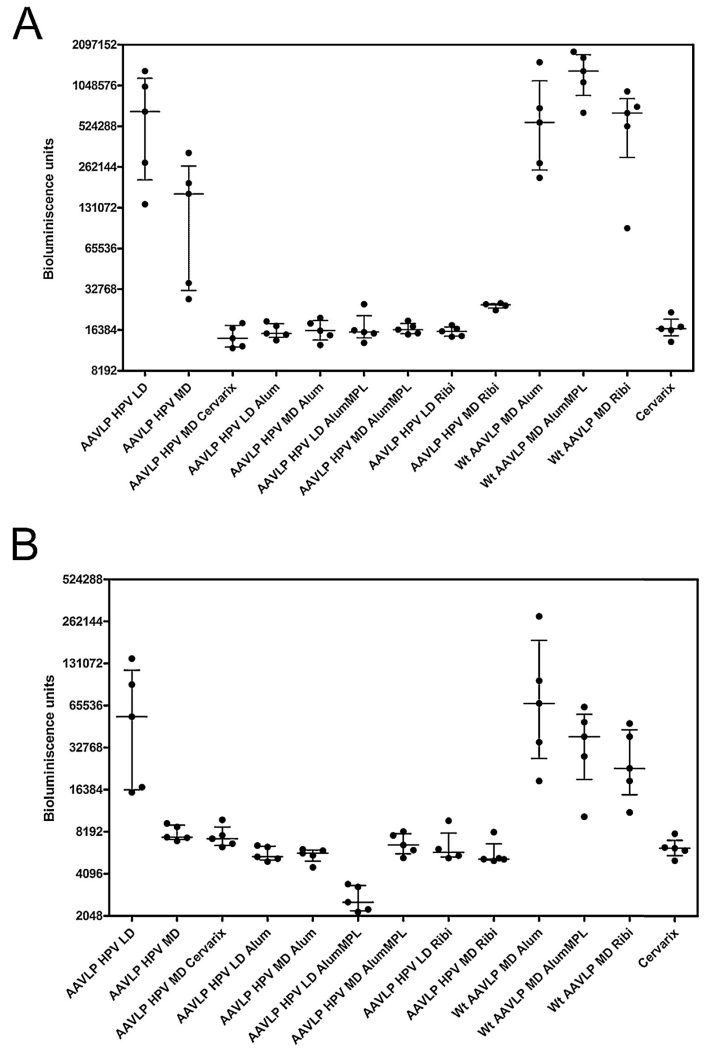
To determine whether these low titer L2-specific serum neutralizing antibody responses were sufficient to confer protection, the mice (5 mice/group) were challenged intra-vaginally with HPV16 pseudovirions (Fig. 2). All of the mice vaccinated with AAVLP (HPV16/31L2) formulated in adjuvant were completely protected against HPV16 challenge as for Cervarix. Without the use of adjuvant, partial protection was seen at the medium dose of AAVLP (HPV16/31L2), and the infection was similar between the mice vaccinated with LD AAVLP (HPV16/31L2) and wild type AAVLP, suggesting no protection. The protection observed was consistent with the L2-specific peptide responses and suggest that even weak responses are protective.
Fig. 2. Vaccine-induced Protection against HPV16 PsV challenge in the cervicovaginal mouse challenge model.
Mice that were vaccinated with the various antigen and antigen-adjuvanted preparations described in Fig. 1 were divided into two groups and challenged either 2 weeks (A) or 19 weeks (B) after the final booster vaccination (see Supplementary Fig. S2). The readout for infection was recorded as bioluminescence signals as described in Section 2 and plotted for individual mice (●) in each group. Median and interquartile range values for each group are represented as horizontal bars.
To assess the durability of the immune response and stability of protection, the remaining 5 mice in each group were bled at 3 months post vaccination and then subjected to intravaginal challenge with HPV16 pseudovirions (Fig. 2B), although at 0.1× of the challenge dose in Fig. 2A. The protective response was maintained at 19 weeks post immunization. The protection at 19 weeks post final immunization seen in the mice administered with the medium dose of AAVLP (HPV16/31L2) appeared to be stronger than seen at 2 weeks post-immunization. This latter observation is likely a consequence of a 10-fold lower challenge dose at 19 weeks post vaccination as there was no increase in neutralizing antibody titer over the first 3 months post-vaccination with the different formulations (Table 1).
Table 1.
Breadth of cross-neutralization. In vitro neutralizing antibody titers (IVNT) in sera at 0.5 and 3 months post vaccination of mice with Cervarix (0.1× human dose) as a positive control, or wild type AAVLP (wt) or AAVLP (HPV16/31 L2) at either a low dose (0.6 μg) or medium dose (6 μg) in no adjuvant, or alum, alum + MPL or Ribi adjuvant or mixed with Cervarix (*which contains alum + MPL). N.D. < 1:50.
| Vaccination groups |
HPV16 IVNT | HPV45 IVNT | HPV58 IVNT | HPV18 IVNT | HPV31 IVNT | ||
|---|---|---|---|---|---|---|---|
| Antigen | Dose | Adjuvant | 2 wks 3 mths |
2 wks 3 mths |
2 wks 3 mths |
2 wks 3 mths |
2 wks 3 mths |
| AAVLP (HPV) | Low | N.D. | N.D. | N.D. | N.D. | N.D. | |
| N.D. | N.D. | N.D. | N.D. | N.D. | |||
| AAVLP (HPV) | Medium | N.D. | N.D. | N.D. | N.D. | N.D. | |
| N.D. | N.D. | N.D. | N.D. | N.D. | |||
| AAVLP (HPV)+ Cervarix | Medium + 0.1× | Alum + MPL* | 409,600 | 100 | N.D. | >12,800 | N.D. |
| 102,400 | 100 | N.D. | >12,800 | N.D. | |||
| AAVLP (HPV) | Low | Alum | 100 | N.D. | 50 | N.D. | N.D. |
| 100 | N.D. | 50 | N.D. | N.D. | |||
| AAVLP (HPV) | Medium | Alum | 100 | N.D. | N.D. | N.D. | N.D. |
| 100 | N.D. | N.D. | N.D. | N.D. | |||
| AAVLP (HPV) | Low | Alum + MPL | 100 | N.D. | N.D. | N.D. | N.D. |
| 100 | N.D. | N.D. | N.D. | N.D. | |||
| AAVLP (HPV) | Medium | Alum + MPL | 200 | N.D. | 50 | 100 | N.D. |
| 200 | N.D. | 100 | 50 | N.D. | |||
| AAVLP (HPV) | Low | Ribi | 400 | 50 | 200 | 400 | 100 |
| 200 | 50 | 400 | 200 | 200 | |||
| AAVLP (HPV) | Medium | Ribi | 400 | 50 | 200 | 400 | 100 |
| 200 | N.D. | 100 | 100 | 50 | |||
| AAVLP (wt) | Medium | Alum | N.D. | N.D. | N.D. | N.D. | N.D. |
| N.D. | N.D. | N.D. | N.D. | N.D. | |||
| AAVLP (wt) | Medium | Alum + MPL | N.D. | N.D. | N.D. | N.D. | N.D. |
| N.D. | N.D. | N.D. | N.D. | N.D. | |||
| AAVLP (wt) | Medium | Ribi | N.D. | N.D. | N.D. | N.D. | N.D. |
| N.D. | N.D. | N.D. | N.D. | N.D. | |||
| Cervarix | 0.1× | Alum + MPL* | 409,600 | 400 | N.D. | >12,800 | N.D. |
| 204,800 | N.D. | N.D. | >12,800 | N.D. | |||
3.2. Serum antibody responses in vaccinated rabbits
A complementary study of the immunogenicity of AAVLP (HPV16/31L2) was also performed in rabbits. As summarized in Supplementary Fig. S3, rabbits were immunized three times with 0.1× human dose of Cervarix (n = 5), AAVLP with no insert (n = 10), or AAVLP (HPV16/31L2) without adjuvant (n = 5), AAVLP (HPV16/31L2) in RIBI adjuvant (n = 5), AAVLP (HPV16/31L2) in alum + MPL (n = 10), or a mixture comprising AAVLP (HPV16/31L2) and 0.1× human dose of Cervarix (n = 5). The dose of AAVLP per immunization was 20 μg in each group. Serum samples were collected from rabbits at various time points after vaccination and tested for anti-peptide activity and neutralizing activity. The anti-peptide antibody titers showed typical decays in titer over time (Supplementary Fig. S4a); titers declined from a peak of 1:3000 at 3 days after the final booster immunization, to ~1:100 at 3 months and thereafter the L2 titers were stable until end of the 12 month observation period.
Similar high L2 peptide titers were observed for other hrHPV genotypes commonly found in cervical cancer (Supplementary Fig. S4b). In vitro neutralization titers of animals in Group E were also determined and showed similar reductions in titers at 12 months (Table 2). Although the anti-peptide antibody titer decayed rapidly over the 12 month period, we did note that the neutralization titer decayed less rapidly, and remained high over the first 6 months.
Table 2.
HPV 16 Neutralizing titer time course. The serum HPV16 neutralizing titer was tested for group E (AAVLP (HPV16/31L2) + Alum + MPL) rabbits at 3 days, 3 months and 12 months post vaccination.
| Rabbit no. | 3 day titer | 3 month titer | 12 month titer |
|---|---|---|---|
| 4115 | 800 | 930 | – |
| 4116 | 400 | 600 | – |
| 4117 | 300 | 980 | – |
| 4134 | 300 | 2000 | – |
| 4135 | 930 | 5000 | – |
| 4118 | 5000 | 5000 | >1000 |
| 4119 | 980 | 990 | – |
| 4136 | 950 | 7000 | 330 |
| 4137 | 2000 | 2000 | 25 |
| 4138 | 850 | 970 | 30 |
3.3. Immunization of rabbits with AAVLP (HPV16/31L2) and protection against challenge with hrHPV quaziviruses (QV)
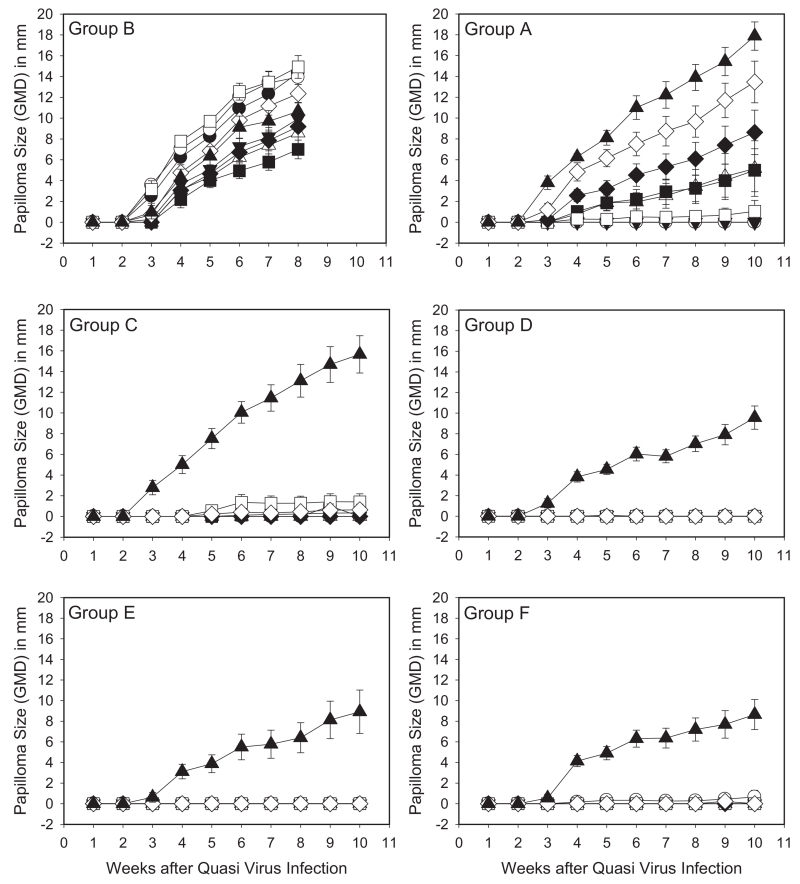
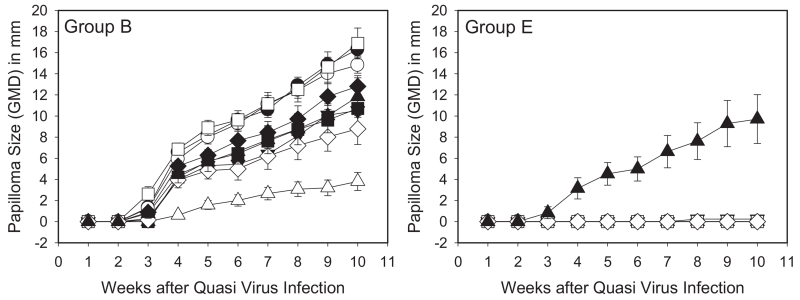
Since analysis of the in vitro neutralizing antibody responses elicited by vaccination with AAVLP (HPV16/31L2) in adjuvant suggested the possibility of broad cross-protection (Table 1), we then sought to understand the breadth of the protective response against the hrHPV most commonly detected in cervical cancer. The murine challenge model requires a separate group of mice to test protection against each type and does not have a disease endpoint. However, in the rabbit model, individual animals can be challenged concurrently with numerous hrHPV types carrying the CRPV genome which produces cutaneous warts as the endpoint. Groups of rabbits were set up to test various vaccine groups, serum was collected at various times after vaccination for ELISA and in vitro neutralization studies (Supplementary Fig. S5), and the rabbits were challenged at 6 months and 12 months post vaccination with a range of hrHPV types (Supplementary Fig. S3). Rabbits vaccinated with Cervarix showed strong protection against HPV16, 18, and 31 QV types, with strong but incomplete protective responses to HPV 45, and weak to no protection against HPV35, 39, 58, 59 (consistent with the breadth of protection observed clinically) as well as no activity against CRPV (Fig. 3, Group A). In contrast, there were strong protective responses to all HPV QV types in rabbits receiving the AAVLP (HPV16/31L2) vaccine, including the group that included AAVLP (HPV16/31L2) without adjuvant (Group C). Those groups receiving the adjuvanted AAVLP (HPV16/31L2) vaccine also showed some reduction in growth for the distantly-related native CRPV challenge (Groups D, E and F). The final group of rabbits receiving admixtures of AAVLP (HPV16/31L2) vaccine and Cervarix (Group F) showed very strong protection against the hrHPV QV types similarly to the adjuvanted AAVLP (HPV16/31L2) vaccinated rabbits. When additional rabbits in groups B (control) and E were challenged 12 months after vaccination (Fig. 4), the strong cross-protection against the listed hrHPV QV types persisted, demonstrating significant durability of the AAVLP (HPV16/31L2) vaccine. Representative rabbits at 10-weeks post-challenge are shown in Supplementary Fig. S6.
Fig. 3. Rabbit papilloma growth curves for each of the HPV/CRPV quasivirus challenge groups 6 months post immunization.
Groups of rabbits were vaccinated with various AAVLP (HPV16/31L2), cervarix and control AAVLP vaccines as described in Section 2 (and Supplementary Fig. S3). Each vaccine group contained 5 rabbits and each rabbit was challenged at duplicate skin sites for each HPV QV preparation. Mean ± SEM of geometric mean diameter (GMD) measurements of HPV QV and native CRPV-induced rabbit papillomas vaccinated with Cervarix (A), AAVLP without adjuvant (B), AAVLP (HPV16/31L2) without adjuvant (C), AAVLP (HPV16/31L2) in RIBI adjuvant (D), AAVLP (HPV16/31L2) in Alum + MPL (E) and AAVLP (HPV16/31L2) + Cervarix (F). Papillomas were induced at 18 sites on each rabbit with 5 μL of HPV16/CRPV (●), HPV18/CRPV (○), HPV31/CRPV (▼), HPV35/CRPV (△), HPV39/CRPV (■), HPV45/CRPV (□), HPV58/CRPV (♦), HPV59/CRPV (◇) and wild-type CRPV (▲).
Fig. 4. Papilloma growth curves at 12 months post immunization with AAVLP (HPV16/31L2) vaccine.
Two groups of rabbits were vaccinated with either AAVLP without peptide insert (A) or AAVLP (HPV16/31L2) vaccine in Alum + MPL as described in Section 2 (and Supplementary Fig. S3). Each vaccine group contained 5 rabbits and each rabbit was challenged at duplicate skin sites for each HPV QV preparation. Mean + SEM of geometric mean diameter (GMD) measurements of HPV QV and native CRPV-induced rabbit papillomas vaccinated with AAVLP without adjuvant (A), and AAVLP (HPV16/31L2) in Alum + MPL (B). Papillomas were induced at 18 sites on each rabbit with 5 μL of HPV16/CRPV (●), HPV18/CRPV (○), HPV31/CRPV (▼), HPV35/CRPV (△), HPV39/CRPV (■), HPV45/CRPV (□), HPV58/CRPV (♦), HPV59/CRPV (◇) and wild-type CRPV (▲).
4. Discussion
Recent studies have indicated that L2-based vaccines develop broadly cross-neutralizing antibodies to multiple HPV types [24,36]. These findings contrast with the highly type-specific responses that develop in response to L1 VLP-based HPV vaccines [37–39]. The potency of L1-associated epitopes stems from the multi-epitope nature of the L1 epitopes when presented on a VLP [40,41]. Corresponding enhancements to the antigenicity of L2 epitopes have been achieved using VLP-based platforms with L2-epitope insertions into papillomavirus VLPs [42–45], bacteriophage VLPs [46,47], tobacco mosaic virus VLPs [48] and adeno-associated virus VLP [26]. These latter modifications are needed because of the sub-dominance of L2 within native HPV particles and the reduced immunity of L2 antigens presented separately [21,49,50]. Additional strategies to enhance the antigenicity of L2 have included L2 sequences fused to TLR5 activators [33], IgG1 Fc [51] and thioredoxin [52]. In some of these studies, the antigenicity of the L2 sequences remained low [53,54] and required potent and non-clinically relevant adjuvants to induce strong protection. The purpose of the current studies was three-fold: (i) to measure the durability of the immune response to neutralizing L2 epitopes presented on an AAV-VLP-based platform; (ii) to assess the impact upon immunogenicity of various clinically relevant adjuvants as well as admixture of AAVLP (HPV16/31L2) with an adjuvanted L1 VLP vaccine; and (iii) to test the breadth and duration of protection in vivo in individuals against multiple hrHPV types using preclinical mouse and rabbit models.
In the current study, AAVLP (HPV16/31L2) immunogens provided excellent and long-lasting protective immunity to various hrHPV types in vivo. The mechanism by which this protection was achieved was likely via neutralizing antibody as indicated by passive immunization being sufficient to mediate protection [26]. Although passive transfer studies do not rule out a complementary role for cellular immunity after active vaccination, in the mouse challenge model no L2 protein is expressed (only luciferase) after infection, suggesting it could therefore not be targeted by L2 17–36 specific cellular immune responses. Likewise in the rabbit model, since all quasivirions deliver the CRPV genome, only CRPV L2 would be expressed. Since the vaccine protects against all hrHPV quasivirions, but minimally to CRPV itself (Fig. 3, Groups D, E and F), this again argues against a role for cell mediated immunity after AAVLP (HPV16/31L2) vaccination in the rabbit model. We have noted in previous studies that even broader cross-protection (e.g. to CRPV) can be achieved using HPV16 L2 protein fragments from aa 11 to 200 [56]. It is possible therefore that the poor response to wild-type CRPV challenge in the current study is due to the diminished size of the L2 region (aa 17–36) and reduced cross-reactivity to CRPV L2 in this short peptide. In this latter situation therefore, only a weak cross-neutralizing activity can be achieved, and the response is also dependent upon effective adjuvants (Fig. 3, Groups D, E and F).
The licensed L1 VLP vaccines have demonstrated durable protection over a decade, but with the comparatively lower immunogenicity of L2-based vaccines there is a concern about the durability of protection. Strong protection was also observed 6 months after challenge in the rabbit cutaneous model even when no adjuvants were used with the AAVLP (HPV16/31L2) vaccine (Fig. 4 and Supplementary Fig. S6). There was also weak protection in the mouse cervicovaginal challenge model with the non-adjuvanted vaccine despite the apparent absence of significant anti-L2 antibody in the in vitro neutralization assay (Fig. 1B). The lack of sensitivity of the in vitro neutralization assays to detect serum antibody responses associated with in vivo protection have been observed previously in both the mouse and rabbit models [55,56] and our data also suggest that the in vitro neutralization assays are less sensitive than the in vivo protection models [35,57]. Indeed, recent modifications to the in vitro assay [35,58;59] have confirmed that L2 neutralizing activity was not adequately detected in the standard PsV neutralization assays despite this assay being very sensitive to the neutralizing activity of L1-based neutralizing antibodies [58].
When using 0.1× of a human dose, we noted in the rabbit cutaneous challenge model that Cervarix strongly protected against vaccine-matched (16 and 18) and closely-related HPV types (31 and 45), but failed to cross-protect against 58, 35, 59 when the challenge was delayed by 6 months. Clinical studies have also indicated that there are similar rates of cross-protection against vaccine-related types [15]. However, this cross-protection is of unclear duration and not sufficient to protect against all hrHPV. These finding have driven efforts to increase the valency of the HPV vaccine (e.g. the recently licensed Gardasil-9 nonavalent L1 VLP vaccine), and the development of broadly protective vaccines based upon a monovalent L2-based immunogen [36].
An important feature of the current study is the demonstration of durable protection following the administration of AAVLP (HPV16/31L2) vaccine in clinically relevant adjuvants, and the stabilization of neutralizing antibody titers. In addition, strong protection at 6-months post vaccination was achieved without adjuvants in the rabbit challenge model. These observations suggest the value of providing the L2 epitopes as multimeric structures on VLP-based platforms and that such vectors enhance the antigenicity of L2 epitopes for long-term protection against a broad range of HPV types. However, it is important that the L2 epitope is appropriately displayed to improve the immunogenicity and maintain broad cross-protection [59]. The breadth of cross-protection can be further enhanced by including L2 epitopes from more than one HPV type. Here the HPV31 L2 epitope is paired with HPV16 because the sequence within HPV31 L2 17–36 is somewhat divergent from the other hrHPV, and this type has proven more difficult to neutralize [26,60,61].
Peptide ELISAs for rabbits immunized with AAVLP (HPV16/31L2) were examined over 12 months post vaccination and declined as predicted. Interestingly, the neutralizing titers appeared to decay less rapidly suggesting that the anti-peptide ELISA was not a good correlate for neutralizing capacity. One possible explanations for these differences include the likelihood that the anti-peptide antibodies contained a mixture of neutralizing and non-neutralizing antibodies, and that the former had a longer half-life in the sera. We noted that strong protection was observed in these rabbits at 12 months post-vaccination suggesting longevity of the neutralizing antibody.
In general, we found that the mouse and rabbit HPV challenge models showed good agreement including both cross-protection to various HPV types and durability of the antibody response. We did note however, that the rabbit model demonstrated strong protection following immunization with AAVLP (HPV16/31L2) without adjuvants, whereas the response in mice with AAVLP (HPV16/31L2) was weak. In these experiments, the rabbits received a higher dose of AAVLP (HPV16/31L2) vaccine (20 μg) than the mice (6 μg) and the reduced vaccine dose may have produced less neutralizing antibody than the rabbit vaccine dose. An alternative explanation for these differences is that the challenges doses for the mice and rabbits had different titers of infectious particles. The study overall demonstrated the strength of the combined animal model approach in which the mouse model was better able to assess different vaccine doses and adjuvant preparations, and the rabbit model was better able to assess cross-protection to multiple HPV types on the same animal.
Finally it was demonstrated in both animal models that the combination of Cervarix and AAVLP (HPV16/31L2) was able to broaden the protection against different HPV serotypes as immunization with the different antigens did not compromise immune responses and protection to either.
In conclusion, we present preclinical data addressing the in vivo efficacy of AAVLP (HPV16/31L2) for the long-term protection against a broad range of HPV types using clinically relevant adjuvants. These studies suggest the potential of such recombinant VLP-based vaccines as a simple single immunogen-based second-generation HPV vaccine that induces broad prophylactic immunity against the most common hrHPV. Confirmation of their immunogenicity in patients awaits clinical trials. One question remains as to the direct application of AAVLP-based vaccines in patient populations in which pre-existing serum antibody to AAV has been noted [65–67]. Intriguingly, however, it was previously shown that a pre-existing immune response directed against the AAVLP carrier protein boosts rather than attenuates the specific antibody response against the inserted epitopes [26]. This is an important finding as approximately 80% of the human population is sero-positive for several AAV serotypes [65–67]. Collectively, these data suggest that AAVLPs are promising carriers to generate long lasting immune response against otherwise weakly immunogenic peptides through capsid display.
Supplementary Material
Acknowledgements
We thank members of the PSU College of Medicine veterinary staff for daily care of rabbits used in these studies.
Funding statement
The study was funded by grants from Medigene AG to NDC and RBSR, and Public Health Service (grants.nih.gov) grants P50 CA098252 and CA118790 to RBSR.
Footnotes
Declaration of competing interests
We have read the journal’s policy and the authors of this manuscript have the following competing interests: The study was funded by grants from Medigene AG to Neil Christensen and Richard Roden. Richard Roden is an inventor of L2-related patents (US application 20090047301 Papillomavirus L2 N-Terminal Peptides for the Induction of Broadly Cross-Neutralizing Antibodies and US application 20130177585 PAPILLOMAVIRUS L2 N-TERMINAL PEPTIDES FOR THE INDUCTION OF BROADLY CROSS-NEUTRALIZING ANTIBODIES) licensed to Shantha Biotechnics Ltd., GlaxoSmithKline, PaxVax, Inc. and Acambis, Inc. Richard Roden and TC Wu are founders of Papivax LLC and scientific advisors to Papivax Biotech Inc. The terms of these arrangements are managed by Johns Hopkins University in accordance with its conflict of interest policies. Kerstin Pino Tossi is an inventor of the AAV related patent application WO 2008/145400 “Mutated structural protein of a parvovirus” and related patent applications. Kerstin Pino Tossi, and Margit Weghofer are employees of Medigene AG. This does not alter our adherence to all the journal’s policies on sharing data and materials.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2015.09.005.
References
- [1].Van DK, Bernard HU, Chen Z, de Villiers EM, Zur Hausen H, Burk RD. Papillomaviruses: evolution Linnaean taxonomy and current nomenclature. Trends Microbiol. 2011;19(2):49–50. doi: 10.1016/j.tim.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zur Hausen H. Human papillomavirus & cervical cancer. Indian J Med Res. 2009;130(3):209. [PubMed] [Google Scholar]
- [3].Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88(1):63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Munoz N, Bosch FX, Castellsague X, et al. Against which human papillomavirus types shall we vaccinate and screen?, The international perspective. Int J Cancer. 2004 Aug;111(2):278–85. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- [5].Munoz N, Bosch FX, de SS, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003 Feb;348(6):518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- [6].Joura EA, Ault KA, Bosch FX, et al. Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1997–2008. doi: 10.1158/1055-9965.EPI-14-0410. [DOI] [PubMed] [Google Scholar]
- [7].Bosch FX, Broker TR, Forman D, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013 Dec;31(Suppl 7):H1–31. doi: 10.1016/j.vaccine.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Combes JD, Chen AA, Franceschi S. Prevalence of human papillomavirus in cancer of the oropharynx by gender. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2954–8. doi: 10.1158/1055-9965.EPI-14-0580. [DOI] [PubMed] [Google Scholar]
- [9].Bernard HU, Burk RD, Chen Z, Van DK, Zur HH, de Villiers EM. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010 May;401(1):70–9. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Alemany L, Saunier M, Tinoco L, et al. Large contribution of human papillomavirus in vaginal neoplastic lesions: a worldwide study in 597 samples. Eur J Cancer. 2014 Nov;50(16):2846–54. doi: 10.1016/j.ejca.2014.07.018. [DOI] [PubMed] [Google Scholar]
- [11].Alemany L, Saunier M, Alvarado-Cabrero I, et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer. 2015;136(1):98–107. doi: 10.1002/ijc.28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hauck F, Oliveira-Silva M, Dreyer JH, et al. Prevalence of HPV infection in head and neck carcinomas shows geographical variability: a comparative study from Brazil and Germany. Virchows Arch. 2015 Mar;:98–107. doi: 10.1007/s00428-015-1761-4. [DOI] [PubMed] [Google Scholar]
- [13].Stephen JK, Worsham MJ. Human papilloma virus (HPV) modulation of the HNSCC epigenome. Methods Mol Biol. 2015;1238:369–79. doi: 10.1007/978-1-4939-1804-1_20. [DOI] [PubMed] [Google Scholar]
- [14].Van d V, Boily MC, Drolet M, et al. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model-based analysis. J Natl Cancer Inst. 2012 Nov;104(22):1712–23. doi: 10.1093/jnci/djs395. [DOI] [PubMed] [Google Scholar]
- [15].Malagon T, Drolet M, Boily MC, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(10):781–9. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- [16].Wang JW, Roden RB. Virus-like particles for the prevention of human papillomavirus-associated malignancies. Expert Rev Vaccines. 2013;12(2):129–41. doi: 10.1586/erv.12.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002 Nov;347(21):1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- [18].Luna J, Plata M, Gonzalez M, et al. Long-term follow-up observation of the safety, immunogenicity, and effectiveness of Gardasil in adult women. PLoS ONE. 2013;8(12):e83431. doi: 10.1371/journal.pone.0083431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Einstein MH, Takacs P, Chatterjee A, et al. Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: end-of-study analysis of a Phase III randomized trial. Hum Vaccin Immunother. 2014;10(12):3435–45. doi: 10.4161/hv.36121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015 Feb;372(8):711–23. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- [21].Alphs HH, Gambhira R, Karanam B, et al. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc Natl Acad Sci USA. 2008 Apr;105(15):5850–5. doi: 10.1073/pnas.0800868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gambhira R, Karanam B, Jagu S, et al. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol. 2007 Dec;81(24):13927–31. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Roden RB, Yutzy WH, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000 May;270(2):254–7. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- [24].Kanda T, Kondo K. Development of an HPV vaccine for a broad spectrum of high-risk types. Hum Vaccin. 2009;5(1):43–5. doi: 10.4161/hv.5.1.6554. [DOI] [PubMed] [Google Scholar]
- [25].Nieto K, Stahl-Hennig C, Leuchs B, Muller M, Gissmann L, Kleinschmidt JA. Intranasal vaccination with AAV5 and 9 vectors against human papillomavirus type 16 in rhesus macaques. Hum Gene Ther. 2012;23(7):733–41. doi: 10.1089/hum.2011.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nieto K, Weghofer M, Sehr P, et al. Development of AAVLP (HPV16/31L2) particles as broadly protective HPV vaccine candidate. PLoS ONE. 2012;7(6):e39741. doi: 10.1371/journal.pone.0039741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004 Jan;78(2):751–7. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med. 2005;119:445–62. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- [29].Mejia AF, Culp TD, Cladel NM, et al. Preclinical model to test human papillomavirus virus (HPV) capsid vaccines in vivo using infectious HPV/cottontail rabbit papillomavirus chimeric papillomavirus particles. J Virol. 2006 Dec;80(24):12393–7. doi: 10.1128/JVI.01583-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Culp TD, Christensen ND. Quantitative RT-PCR assay for HPV infection in cultured cells. J Virol Methods. 2003 Aug;111(2):135–44. doi: 10.1016/s0166-0934(03)00170-8. [DOI] [PubMed] [Google Scholar]
- [31].Culp TD, Cladel NM, Balogh KK, Budgeon LR, Mejia AF, Christensen ND. Papillomavirus particles assembled in 293TT cells are infectious in vivo. J Virol. 2006 Nov;80(22):11381–4. doi: 10.1128/JVI.01328-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Roberts JN, Buck CB, Thompson CD, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13(7):857–61. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- [33].Kalnin K, Tibbitts T, Yan Y, et al. Low doses of flagellin-L2 multimer vaccines protect against challenge with diverse papillomavirus genotypes. Vaccine. 2014 Jun;32(28):3540–7. doi: 10.1016/j.vaccine.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cladel NM, Hu J, Balogh K, Mejia A, Christensen ND. Wounding prior to challenge substantially improves infectivity of cottontail rabbit papillomavirus and allows for standardization of infection. J Virol Methods. 2008 Mar;148(1–2):34–9. doi: 10.1016/j.jviromet.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang JW, Jagu S, Kwak K, et al. Preparation and properties of a papillomavirus infectious intermediate and its utility for neutralization studies. Virology. 2014 Jan;449:304–16. doi: 10.1016/j.virol.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang JW, Hung CF, Huh WK, Trimble CL, Roden RB. Immunoprevention of human papillomavirus-associated malignancies. Cancer Prev Res (Phila) 2015;8(2):95–104. doi: 10.1158/1940-6207.CAPR-14-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Roden RB, Hubbert NL, Kirnbauer R, Christensen ND, Lowy DR, Schiller JT. Assessment of the serological relatedness of genital human papillomaviruses by hemagglutination inhibition. J Virol. 1996;70(5):3298–301. doi: 10.1128/jvi.70.5.3298-3301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Giroglou T, Sapp M, Lane C, et al. Immunological analyses of human papillomavirus capsids. Vaccine. 2001 Feb;19(13–14):1783–93. doi: 10.1016/s0264-410x(00)00370-4. [DOI] [PubMed] [Google Scholar]
- [39].Sehr P, Rubio I, Seitz H, et al. High-throughput pseudovirion-based neutralization assay for analysis of natural and vaccine-induced antibodies against human papillomaviruses. PLoS ONE. 2013;8(10):e75677. doi: 10.1371/journal.pone.0075677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chackerian B, Lenz P, Lowy DR, Schiller JT. Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. J Immunol. 2002 Dec;169(11):6120–6. doi: 10.4049/jimmunol.169.11.6120. [DOI] [PubMed] [Google Scholar]
- [41].Wang JW, Roden RB. L2, the minor capsid protein of papillomavirus. Virology. 2013 Oct;445(1–2):175–86. doi: 10.1016/j.virol.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schellenbacher C, Kwak K, Fink D, et al. Efficacy of RG1-VLP vaccination against infections with genital and cutaneous human papillomaviruses. J Invest Dermatol. 2013;133(12):2706–13. doi: 10.1038/jid.2013.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Slupetzky K, Gambhira R, Culp TD, et al. A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11. Vaccine. 2007 Mar;25(11):2001–10. doi: 10.1016/j.vaccine.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kondo K, Ochi H, Matsumoto T, Yoshikawa H, Kanda T. Modification of human papillomavirus-like particle vaccine by insertion of the cross-reactive L2-epitopes. J Med Virol. 2008;80(5):841–6. doi: 10.1002/jmv.21124. [DOI] [PubMed] [Google Scholar]
- [45].Varsani A, Williamson AL, de VD, Becker I, Christensen ND, Rybicki EP. Chimeric human papillomavirus type 16 (HPV-16) L1 particles presenting the common neutralizing epitope for the L2 minor capsid protein of HPV-6 and HPV-16. J Virol. 2003 Aug;77(15):8386–93. doi: 10.1128/JVI.77.15.8386-8393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tumban E, Peabody J, Peabody DS, Chackerian B. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS ONE. 2011;6(8):e23310. doi: 10.1371/journal.pone.0023310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tyler M, Tumban E, Dziduszko A, Ozbun MA, Peabody DS, Chackerian B. Immunization with a consensus epitope from human papillomavirus L2 induces antibodies that are broadly neutralizing. Vaccine. 2014 Jul;32(34):4267–74. doi: 10.1016/j.vaccine.2014.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Palmer KE, Benko A, Doucette SA, et al. Protection of rabbits against cutaneous papillomavirus infection using recombinant tobacco mosaic virus containing L2 capsid epitopes. Vaccine. 2006 Jun;24(26):5516–25. doi: 10.1016/j.vaccine.2006.04.058. [DOI] [PubMed] [Google Scholar]
- [49].Jagu S, Karanam B, Gambhira R, et al. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J Natl Cancer Inst. 2009 Jun;101(11):782–92. doi: 10.1093/jnci/djp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jagu S, Kwak K, Karanam B, et al. Optimization of multimeric human papillomavirus L2 vaccines. PLoS ONE. 2013;8(1):e55538. doi: 10.1371/journal.pone.0055538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chen X, Liu H, Zhang T, et al. A vaccine of L2 epitope repeats fused with a modified IgG1 Fc induced cross-neutralizing antibodies and protective immunity against divergent human papillomavirus types. PLoS ONE. 2014;9(5):e95448. doi: 10.1371/journal.pone.0095448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rubio I, Bolchi A, Moretto N, et al. Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20–38) peptide displayed on bacterial thioredoxin. Vaccine. 2009 Mar;27(13):1949–56. doi: 10.1016/j.vaccine.2009.01.102. [DOI] [PubMed] [Google Scholar]
- [53].Karanam B, Gambhira R, Peng S, et al. Vaccination with HPV16 L2E6E7 fusion protein in GPI-0100 adjuvant elicits protective humoral and cell-mediated immunity. Vaccine. 2009 Feb;27(7):1040–9. doi: 10.1016/j.vaccine.2008.11.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Karanam B, Jagu S, Huh WK, Roden RB. Developing vaccines against minor capsid antigen L2 to prevent papillomavirus infection. Immunol Cell Biol. 2009 May;87(4):287–99. doi: 10.1038/icb.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang JW, Jagu S, Wang C, et al. Measurement of neutralizing serum antibodies of patients vaccinated with human papillomavirus L1 or L2-based immunogens using furin-cleaved HPV Pseudovirions. PLoS ONE. 2014;9(7):e101576. doi: 10.1371/journal.pone.0101576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gambhira R, Jagu S, Karanam B, et al. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J Virol. 2007 Nov;81(21):11585–92. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Longet S, Schiller JT, Bobst M, Jichlinski P, Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol. 2011 Dec;85(24):13253–9. doi: 10.1128/JVI.06093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Day PM, Pang YY, Kines RC, Thompson CD, Lowy DR, Schiller JT. A human papillomavirus (HPV) in vitro neutralization assay that recapitulates the in vitro process of infection provides a sensitive measure of HPV L2 infection-inhibiting antibodies. Clin Vaccine Immunol. 2012;19(7):1075–82. doi: 10.1128/CVI.00139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tumban E, Peabody J, Tyler M, Peabody DS, Chackerian B. VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papillomavirus. PLoS ONE. 2012;7(11):e49751. doi: 10.1371/journal.pone.0049751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Rubio I, Seitz H, Canali E, et al. The N-terminal region of the human papillomavirus L2 protein contains overlapping binding sites for neutralizing, cross-neutralizing and non-neutralizing antibodies. Virology. 2011 Jan;409(2):348–59. doi: 10.1016/j.virol.2010.10.017. [DOI] [PubMed] [Google Scholar]
- [61].Seitz H, Schmitt M, Bohmer G, Kopp-Schneider A, Muller M. Natural variants in the major neutralizing epitope of human papillomavirus minor capsid protein L2. Int J Cancer. 2013 Feb;132(3):E139–48. doi: 10.1002/ijc.27831. [DOI] [PubMed] [Google Scholar]
- [62].Kronenberg S, Kleinschmidt JA, Bottcher B. Electron cryo-microscopy and image reconstruction of adeno-associated virus type 2 empty capsids. EMBO Rep. 2002;2:997–1002. doi: 10.1093/embo-reports/kve234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Girod A, Ried M, Wobus C, Lahm H, Leike K, Kleinschmidt J, et al. Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2. Nat Med. 1999;5:1438. doi: 10.1038/71021. [DOI] [PubMed] [Google Scholar]
- [64].Boucas J, Lux K, Huber A, Schievenbusch S, von Freyend MJ, Perabo L, et al. Engineering adeno-associated virus serotype 2-based targeting vectors using a new insertion site-position 453-and single point mutations. J Gene Med. 2009;11:1103–13. doi: 10.1002/jgm.1392. [DOI] [PubMed] [Google Scholar]
- [65].Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21:704–12. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- [66].Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–90. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Calcedo R, Morizono H, Wang L, McCarter R, He J, Jones D, et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin Vaccine Immunol. 2011;18:1586–8. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.