Abstract
Purpose:
To screen for keratoconus and potential associated risk factors in a tertiary student population sample.
Population and Methods:
This cross sectional study included 1234 students attending An-Najah National University (Nablus, West Bank, Palestine), that were randomly selected from a total of 20,000 university students. 634 (51.3%) student participants responded by completing a self-administered questionnaire and were assessed by means of corneal topography. Following initial evaluation, participants were referred for Pentacam evaluation if they demonstrated either a mean keratometry of more than 45 diopters, corneal astigmatism of more than 2 diopters and/or if asymmetric topographic patterns were present. Pentacam images were analyzed by an experienced ophthalmologist based on a number of indices and the participants were classified as normal, keratoconus suspects, and keratoconus patients.
Results:
A total of 620 participants (mean age, 20.1±1.6 years) were included in this study, 379 (61.1%) were females and 241 (38.9%) were males. Nine subjects were diagnosed with keratoconus, demonstrating a prevalence of 1.5%. 52 (8.4%) participants showed at least one abnormal pentacam index, and were considered as KC suspects.
Conclusion:
Keratoconus is a prevalent disease among the tertiary Palestinian student population. This may be related to a combination of genetic and environmental factors. The results of this study signal the need for public health outreach and intervention for keratoconus.
Keywords: Keratoconus, pentacam, prevalence, risk factors, topography.
INTRODUCTION
Keratoconus (KC) is the most common ectatic disorder of the cornea [1], and a major cause of ocular morbidity with significant social and economic impact as the disease affects younger generations [1]. It is an ectatic debilitating corneal disorder characterized by a progressive corneal thinning that results in corneal protrusion, irregular astigmatism, and decreased visual performance [2, 3].
The onset of KC is generally observed during puberty, with a variable amount of progression, which may last until the third or fourth decade of life, when progression usually halts [4, 5]. This progression is manifested by variable significant loss of visual acuity (VA) which cannot be corrected by spectacles nor contact lenses in advanced cases [3, 6]. Recent studies found that the onset of KC is most common in the second and third decades of life. Although it has been reported to develop in later ages [6].
Subclinical or forme fruste KC is usually an asymptomatic manifestation of KC, which can prove difficult to diagnose in early stages based on slit-lamp examination alone [4, 7]. The most sensitive and accurate screening and diagnostic method for subclinical KC is computer-assisted videokeratography (topography), which allows for the early detection of KC, before clinical findings and patient symptoms become evident [8, 9]. Early diagnosis of subclinical KC is essential, as early management may delay or avoid the need for corneal transplantation, and help in preserving functional visual acuity. This cross sectional study will measure the prevalence of KC among tertiary students, and assess its associated risk factors.
METHODS
Subject Population
A quantitative cross sectional study was conducted to determine the prevalence of KC among all students enrolled in An-Najah National University (ANNU), which at the time of data collection were about 20,000 students. The study was conducted between September 2014 and December 2014. One thousand two hundred and thirty four participants (N=1234) were recruited to participate in the study using proportionate random sampling technique. A 50% increase in sample size was utilized to compensate for the expected non-response rate among this population, as reported by a similar study conducted in Jerusalem [10]. Students were recruited through notice on the Bulletin boards and web notifications. All registered students included in the study were Palestinian citizens, with no history of corneal pathology other than KC, traumatic corneal scars, nor history of corneal keratoplasty for reasons other than KC.
This research followed the tenets of the Declaration of Helsinki. Ethical approval to conduct this study was obtained from the Institutional Review Board (IRB) committee at ANNU. Informed consent was obtained from all the participants surveyed in the study. All consent forms entailing study objectives and significance were provided to students in Arabic language to ensure they fully understand what their participation requires.
Screening Protocol
Participants who responded to the research call underwent a self-administered questionnaire. Bilateral topography acquisition and auto-refracto-keratometry was then obtained for all participants, using the color mapping 32 Software for KR-8000PA Supra (8000PA, Topcon, Tokyo, Japan).
Any participant who demonstrated either mean keratometry readings (K) more than 45 diopters (D) [10-12], topographic pattern suspicious of KC (all irregular patterns except symmetric bowtie (SB), oval, and round patterns) and/or corneal astigmatism of more than 2D [13] was considered to have abnormal topographical indices and was then referred to An-Najah University Hospital for further evaluation by corneal tomography (pentacam). The final analysis and diagnosis of KC based on tomography results was completed by an experienced ophthalmologist (MSH) and classified according to the criteria shown in Table 1 into: Normal, if all pentacam indices were normal; KC suspect, if pentacam exhibited one or more known abnormal parameters which do not meet the criteria for KC diagnosis [14]; and KC, if a definite diagnosis was confirmed by pentacam.
Table 1.
The classification criteria for keratoconus.
Data and Statistical Analysis
The Statistical Package of Social Sciences version 16.0 (SPSS Inc., Chicago, IL, USA) was utilized for data entry and statistical analysis. Only the participants who completed the study were included in data analysis. Chi-square test was used to compare the characteristics of each classification group. A multivariate analysis was used to evaluate the associations with KC. P-values ≤ 0.05 were considered statistically significant.
RESULTS
634 participants responded to the research call (response rate= 51.3%), of which 14 were excluded as they did not meet the inclusion criteria. The age of the participants ranged between 17 and 27 years (mean 20.1 ± 1.6), and 379 (61.2%) were females and 241 (38.8%) were males. All participants included were of Palestinian origin.
Ocular and Medical Characteristics of the Participants
One third (30.2%) of the participants were using spectacles to improve their visual function. The number of participants who were contact lens wearers was 35 (5.6%), of which 33 (94.3%) were using soft contact lenses, while only 2 (5.7%) participants were using rigid gas permeable (RGP) contact lenses.
70 (11%) participants reported daily significant eye rubbing, while about 20% reported having atopy and 70 (11%) participants reported having vernal keratoconjunctivitis (VKC). A positive family history of KC was reported by 47 (7.5%) participants. Furthermore, 159 (25.6%) participants had parents who had a consanguineous marriage, of which, 115 (72.3%) parents were first cousins. None of the participants reported having any chronic diseases or genetic conditions associated with KC. A summary of the data concerning the ocular and medical characteristics of participants is shown in Table 2.
Table 2.
Ocular and medical characteristics of participants.
| Variable | Frequency | Percent (%) |
|---|---|---|
| Spectacles use | 191 | 30.3% |
|
Contact lenses
Soft Hard |
35 33 2 |
5.6% 94.3% 5.7% |
|
Eye surgery
Refractive corneal Cross linking |
12 10 2 |
1.9% 84.6% 15.4% |
| Eye rubbing | 72 | 11.6% |
| History of eye rubbing | 253 | 40.8% |
| VKC | 65 | 10.5% |
| Family history of KC | 45 | 7.30% |
|
Consanguinity First degree Second degree Third degree |
159 115 24 20 |
25.6% 72.3% 15.1% 12.6% |
| Atopy | 126 | 20.3% |
| Chronic diseases | 0 | 0.00% |
Refractive & Topographic Characteristics
Participants had a keratometry range between 36.5 to 47.75 D, with a mean of 43.24 ± 1.34 D. Thirty-seven subjects (65 eyes) had keratometry values higher than 45 (referral cutoff) in one or both eyes. Corneal astigmatism ranged between 0.00 to -5.75 D (mean 0.45 ± 0.44 D), with 17 subjects (23 eyes) having higher than 2.00 D (referral cutoff) astigmatism. Topographic patterns analyzed showed that the majority (89%) of participants had a symmetrical pattern (symmetric bowtie, oval, and round), and the remaining 11% were divided among the other abnormal patterns.
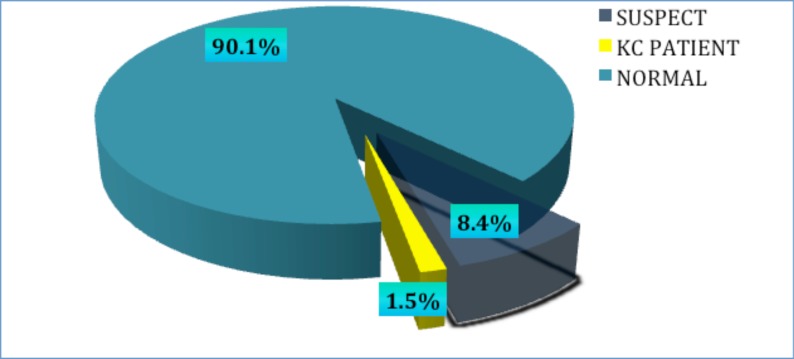
Based on the referral criteria developed, 91 subjects were referred for further evaluation using pentacam. Of those, 82 (90.11%) participants responded to the call. Definite KC was found in nine individuals, indicating a prevalence of 1.5% (Fig. 1). Eight cases presented with bilateral KC, and only one participant had evident keratoconus in only one eye. Two (22%) subjects were previously diagnosed with KC, while the other seven (78%) participants were unaware of having KC before their participation in the study.
Fig. (1).
Prevalence of keratoconus among a tertiary student population.
Of the 82 participants who underwent additional pentacam analysis, 52 (8.4%) participants were diagnosed as KC suspects, having an abnormal topographic pattern and at least one abnormal pentacam index. A definite KC diagnosis could not be established for these participants based on the criteria utilized. Only one suspect was defined as a possible case of post LASIK ectasia.
Participants diagnosed with definite KC were classified according to the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study [15], where 3 participants were found to have mild KC, 4 have moderate KC, and 2 have severe KC.
The prevalence of KC was 1.6% in females and 1.2% in males (p= 0.097). The presence of family history of KC was found to be significantly associated with a diagnosis of KC (p=0.002). Participants who had a positive family history were found to have a 13 fold increased risk of having KC, compared to those without family history. Contact lens wear was also associated with 6.8 times higher risk for having KC, but this was not statistically significant (p=0.060). Although there is an increased risk for KC with female gender, history of eye rubbing, atopy, and vernal keratoconjunctivitis, none of these associations demonstrated statistical significant difference.
DISCUSSION
In this cross sectional study, the prevalence of KC was 1.5% (1500 per 100 000). This prevalence is consistent with the results reported in other middle-eastern countries where a much higher prevalence of KC is demonstrated in comparison to other regions of the world.
The prevalence in our study was lower than the other study conducted in Lebanon (3300/100000) [16]. This may be attributed to the fact that the mean age of participants in the current study was 20.1 years, which was less than the mean age of participants in Lebanon study. Although the mean age in this study is within the period of onset of the disease, a few cases may develop later. This was clear in a study conducted in Tehran, Iran [17], to determine the prevalence of KC based on topographic maps in a population with ages between 14-81 years. The prevalence of keratoconus was 3.3%. However, the prevalence of KC was 0.8% in the 14-29-year-old age group and 7.5% in those ≥ 60 years. The study showed that prevalence of keratoconus significantly increased with age [17]. Another study conducted in Malaysia reported that 26.4% of KC patients were under 23 years of age, while 52.8% were in between 23 and 32 years of age [18].
The prevalence of 1.5% found in this study, represents only definite forms of KC that matched the diagnostic criteria utilized. Study results showed that 8.4% of participants with abnormal topography patterns were also found to have many abnormal pentacam indices, but indices values were insufficient to confirm a diagnosis of KC because they did not meet the diagnostic criteria. Many of these participants may have early form of KC and a definite diagnosis can only be confirmed with future follow-ups. In the longitudinal study conducted by Li et al. [19], a total of 2501 eyes enrolled at baseline and 1627 eyes were followed for a median of 4.1 years after being classified as normal, KC suspects, early KC, and KC based on topography, slit lamp, and retinoscopy examination. Of the originally classified eyes, 277 were considered KC suspects (identified by having asymmetric topographical pattern only), 174 KC suspects continued the follow up and 22 (12.6%) of them developed early KC (abnormal topography and retinoscopy) and 27 (15.5%) developed KC (abnormal topography, retinoscopy, and slit lamp). Thus it is important to follow-up participants to further investigate suspicious KC tomographic patterns and their risk of developing KC later in life.
It is important to mention that keratometry of > 45D is not a sensitive screening protocol for post corneal refractive surgery ectasia, as ectasia may develop with much lower K values. However, other screening protocols such as abnormal corneal topographic patterns and astigmatism > 2D may help to detect the ectasia in its early phase.
It is possible that environmental factors may have contributed to the high prevalence found in the Middle East region where the climate is characterized by dry conditions for most of the year and hot summer periods. On the other hand, in regions such as Denmark, Minnesota, Japan, and the Urals in Russia [20-23] where the weather is much colder with lower average annual temperature, lower KC prevalence has been reported. Thus it is reasonable to suspect that in the middle-east, sun exposure, which is also associated with higher incidence of atopy and eye rubbing, may play a significant role in KC development [24].
Ethnic differences may also account for the discrepancy in prevalence between the various studies as higher prevalence where mostly reported among Arabs, south- Asians (Indian, Pakistani and Bangladeshi) [25, 26], Persians [17], while lower prevalence rates have been reported among the Japanese and white Caucasians. Concerning gender differences, KC affects both genders, although it is unclear whether significant differences between males and females exist. In our study, there was slightly increased prevalence among females, although this was not statistically significant. However, the predominance of males over females has been noted in recent studies. A study conducted in India [27] reported a difference of 2.6 times higher prevalence in men compared to women. In addition, there was a significant difference between males and females in the Jerusalem study [10] (prevalence 4.91% vs 1.07%; P < 0.001), which could be due to the significant difference in the mean age between the male group (25.91 years) which was higher than that of the female group (21 years) in their sample.
Family history of KC has been found to be variable in association with KC among different studies, ranging between 6% and 23.5%. In this study, the strongest predictor to be associated with KC was family history, where 44% of KC patients had a positive family history of KC, while only 6.1% of normal participants reported to have a positive family history of KC. Having a positive family history of KC has around a 13 fold higher risk for developing KC. However, no significant association was found with parental consanguinity.
In the current study and contrary to many previous studies [28], we did not find a significant association between eye rubbing and KC. This difference could be explained on the basis that, in all the studies reporting on eye rubbing, the force of the actual rubbing is not considered, and only the frequency of rubbing is assessed, which is measured on a variety of different scales. In addition, it should be noted that other factors should be taken into account when assessing eye rubbing and its association with KC, such as the method of rubbing (knuckle or finger pads), duration, and seasonal variations.
The association between atopy and keratoconus has been reported extensively. A review of the literature reveals contradictory data in favor of and against this association. Additionally, KC has been commonly associated with VKC. In this study, 3 (33%) patients reported to have atopic conditions, which was found not to be statistically significant (p= 0.42). 22% of KC patients reported having VKC compared to 10.9% of the normal participants, although no significant association with VKC was evident. However, most of atopic patients admit that they rubbed their eyes, making it unclear whether atopy itself or the eye rubbing was the most important factor in the etiology of KC.
In this study, there was a significant correlation between contact lens use and KC. It is possible that mechanical trauma induced by eye rubbing and hard contact lens wear, acts as an external factor that enhance the progression of the disorder in genetically predisposed individuals.
Our study is limited by its design to enroll participants, a selection bias may have occurred since individuals who knew they had the disease may have refrained from participating in the study because they were under ophthalmic care, while others with visual problems (and no previous diagnosis) may have been more likely to volunteer. This could explain that in this study only 2 (22%) participants with KC already knew their diagnosis. Nevertheless, the results of the study showed that the prevalence of KC among tertiary students in Palestine is relatively high, and among the highest in the world. Seventy eight percent of the KC patients were newly diagnosed in the study, meaning that screening programs may be essential for early KC detection. Additionally, 8.4% were KC suspects, and they could have an early form of KC, and should be followed regularly using tomography. Regulated screening programs for KC for university students or younger populations, in order to detect KC in its earliest forms, could prove useful for early diagnosis and management of this corneal entity.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Gatinel D., Saad A. The challenges of the detection of subclinical keratoconus at its earliest stage. Int J Keratoco Ectatic Corneal Dis. 2012;1(1):36–43. doi: 10.5005/jp-journals-10025-1007. [DOI] [Google Scholar]
- 2.Ramos-López D., Martínez-Finkelshtein A., Castro-Luna G.M., Burguera-Gimenez N., Vega-Estrada A., Piñero D., Alió J.L. Screening subclinical keratoconus with placido-based corneal indices. Optom. Vis. Sci. 2013;90(4):335–343. doi: 10.1097/OPX.0b013e3182843f2a. [DOI] [PubMed] [Google Scholar]
- 3.Romero-Jiménez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Cont Lens Anterior Eye. 2010;8; 33(4):157–66. doi: 10.1016/j.clae.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Vazirani J., Basu S. Keratoconus: current perspectives. Clin. Ophthalmol. 2013;7:2019–2030. doi: 10.2147/OPTH.S50119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wheeler J, Hauser MA, Afshari NA, Allingham RR, Liu Y. The genetics of keratoconus: a review. J Microsc. 2012;Suppl 6:001. doi: 10.4172/2161-038X.S6-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assiri A.A., Yousuf B.I., Quantock A.J., Murphy P.J. Incidence and severity of keratoconus in Asir province, Saudi Arabia. Br. J. Ophthalmol. 2005;89(11):1403–1406. doi: 10.1136/bjo.2005.074955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson A.N. Keratoconus: time to rewrite the textbooks. Rev Ophthalmol. 2009;16:66. [Google Scholar]
- 8.Wygledowska-Promieńska D., Zawojska I. [Use of AutoTopograf TMS-3 keratoconus screening program for keratoconus detection]. Klin. Oczna. 2000;102(4):237–240. [PubMed] [Google Scholar]
- 9.Saad A., Gatinel D. Topographic and tomographic properties of forme fruste keratoconus corneas. Invest. Ophthalmol. Vis. Sci. 2010;51(11):5546–5555. doi: 10.1167/iovs.10-5369. [DOI] [PubMed] [Google Scholar]
- 10.Millodot M., Shneor E., Albou S., Atlani E., Gordon-Shaag A. Prevalence and associated factors of keratoconus in Jerusalem: a cross-sectional study. Ophthalmic Epidemiol. 2011;18(2):91–97. doi: 10.3109/09286586.2011.560747. [DOI] [PubMed] [Google Scholar]
- 11.Holladay J.T. Keratoconus detection using corneal topography. J. Refract. Surg. 2009;25(10) Suppl.:S958–S962. doi: 10.3928/1081597X-20090915-11. [DOI] [PubMed] [Google Scholar]
- 12.Kanellopoulos A.J., Asimellis G. Revisiting keratoconus diagnosis and progression classification based on evaluation of corneal asymmetry indices, derived from Scheimpflug imaging in keratoconic and suspect cases. Clin. Ophthalmol. 2013;7:1539–1548. doi: 10.2147/OPTH.S44741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serdarogullari H., Tetikoglu M., Karahan H., Altin F., Elcioglu M. Prevalence of keratoconus and subclinical keratoconus in subjects with astigmatism using pentacam derived parameters. J. Ophthalmic Vis. Res. 2013;8(3):213–219. [PMC free article] [PubMed] [Google Scholar]
- 14.Belin M.W., Kim J.T., Zloty P., Ambrosio R., Jr Simplified nomenclature for describing keratoconus. Int J Keratoco Ectatic Corneal Dis. 2012;1:31–35. doi: 10.5005/jp-journals-10025-1006. [DOI] [Google Scholar]
- 15.Zadnik K., Barr J.T., Edrington T.B., Everett D.F., Jameson M., McMahon T.T., Shin J.A., Sterling J.L., Wagner H., Gordon M.O. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Invest. Ophthalmol. Vis. Sci. 1998;39(13):2537–2546. [PubMed] [Google Scholar]
- 16.Waked N., Fayad A.M., Fadlallah A., El Rami H. Dépistage du kératocône dans une population universitaire au Liban. J. Fr. Ophtalmol. 2012;35(1):23–29. doi: 10.1016/j.jfo.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Hashemi H., Khabazkhoob M., Fotouhi A. Topographic Keratoconus is not Rare in an Iranian population: the Tehran Eye Study. Ophthalmic Epidemiol. 2013;20(6):385–391. doi: 10.3109/09286586.2013.848458. [DOI] [PubMed] [Google Scholar]
- 18.Mohd-Ali B., Abdu M., Yaw C.Y., Mohidin N. Clinical characteristics of keratoconus patients in Malaysia: a review from a cornea specialist centre. J. Optom. 2012;5(1):38–42. doi: 10.1016/j.optom.2012.01.002. [DOI] [Google Scholar]
- 19.Li X., Yang H., Rabinowitz Y.S. Keratoconus: classification scheme based on videokeratography and clinical signs. J. Cataract Refract. Surg. 2009;35(9):1597–1603. doi: 10.1016/j.jcrs.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen K., Hjortdal J., Aagaard Nohr E., Ehlers N. Incidence and prevalence of keratoconus in Denmark. Acta Ophthalmol. Scand. 2007;85(8):890–892. doi: 10.1111/j.1600-0420.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy R.H., Bourne W.M., Dyer J.A. A 48-year clinical and epidemiologic study of keratoconus. Am. J. Ophthalmol. 1986;101(3):267–273. doi: 10.1016/0002-9394(86)90817-2. [DOI] [PubMed] [Google Scholar]
- 22.Tanabe U., Fujiki K., Ogawa A., Ueda S., Kanai A. [Prevalence of keratoconus patients in Japan]. Nippon Ganka Gakkai Zasshi. 1985;89(3):407–411. [PubMed] [Google Scholar]
- 23.Gorskova E.N., Sevost’ianov E.N. [Epidemiology of keratoconus in the Urals]. Vestn. Oftalmol. 1998;114(4):38–40. [PubMed] [Google Scholar]
- 24.Cristina Kenney M., Brown D.J. The cascade hypothesis of keratoconus. Cont. Lens Anterior Eye. 2003;26(3):139–146. doi: 10.1016/S1367-0484(03)00022-5. [DOI] [PubMed] [Google Scholar]
- 25.Georgiou T., Funnell C.L., Cassels-Brown A., O’Conor R. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asians and white patients. Eye (Lond.) 2004;18(4):379–383. doi: 10.1038/sj.eye.6700652. [DOI] [PubMed] [Google Scholar]
- 26.Pearson A.R., Soneji B., Sarvananthan N., Sandford-Smith J.H. Does ethnic origin influence the incidence or severity of keratoconus? Eye (Lond.) 2000;14(Pt 4):625–628. doi: 10.1038/eye.2000.154. [DOI] [PubMed] [Google Scholar]
- 27.Jonas J.B., Nangia V., Matin A., Kulkarni M., Bhojwani K. Prevalence and associations of keratoconus in rural maharashtra in central India: the central India eye and medical study. Am. J. Ophthalmol. 2009;148(5):760–765. doi: 10.1016/j.ajo.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Weed K.H., MacEwen C.J., Giles T., Low J., McGhee C.N. The Dundee University Scottish Keratoconus study: demographics, corneal signs, associated diseases, and eye rubbing. Eye (Lond.) 2008;22(4):534–541. doi: 10.1038/sj.eye.6702692. [DOI] [PubMed] [Google Scholar]