Abstract
Cytomegalovirus (CMV) is a highly complex pathogen which, despite modern prophylactic regimens, continues to affect a high proportion of thoracic organ transplant recipients. The symptomatic manifestations of CMV infection are compounded by adverse indirect effects induced by the multiple immunomodulatory actions of CMV. These include a higher risk of acute rejection, cardiac allograft vasculopathy after heart transplantation, and potentially bronchiolitis obliterans syndrome in lung transplant recipients, with a greater propensity for opportunistic secondary infections. Prophylaxis for CMV using antiviral agents (typically oral valganciclovir or intravenous ganciclovir) is now almost universal, at least in high-risk transplants (D+/R−). Even with extended prophylactic regimens, however, challenges remain. The CMV events can still occur despite antiviral prophylaxis, including late-onset infection or recurrent disease, and patients with ganciclovir-resistant CMV infection or who are intolerant to antiviral therapy require alternative strategies. The CMV immunoglobulin (CMVIG) and antiviral agents have complementary modes of action. High-titer CMVIG preparations provide passive CMV-specific immunity but also exert complex immunomodulatory properties which augment the antiviral effect of antiviral agents and offer the potential to suppress the indirect effects of CMV infection. This supplement discusses the available data concerning the immunological and clinical effects of CMVIG after heart or lung transplantation.
Cytomegalovirus (CMV) (Figure 1) is one of the most common pathogens in humans, infecting more than 60% of the general population and as many as 100% within some geographic areas. In the immunocompetent host, it usually has a benign, asymptomatic course, but in the immunocompromised or immune-immature host—such as transplant recipients or newborns—it may develop clinically meaningful clinical syndromes. The biology of CMV lifecycle is among the most complex of the known human viruses thanks to its ability to interact with the immune system via several strategies by which it modulates and escapes host immune response.1 Indeed, fewer than 30% of CMV genes are required for virus replication and many of the others relate to regulation of the host's cellular mechanisms.2,3 Despite intensive efforts to reduce the toll of CMV infection after thoracic transplantation, it remains the most clinically relevant infectious agent in this setting, representing a major cause of morbidity and, if untreated, mortality. The intense immunosuppression required after heart or lung transplantation compared with other solid organ transplants places these recipients at particularly high risk for CMV events, compounded after lung transplantation by a high transfer of latent CMV in grafts from seropositive donors.
FIGURE 1.
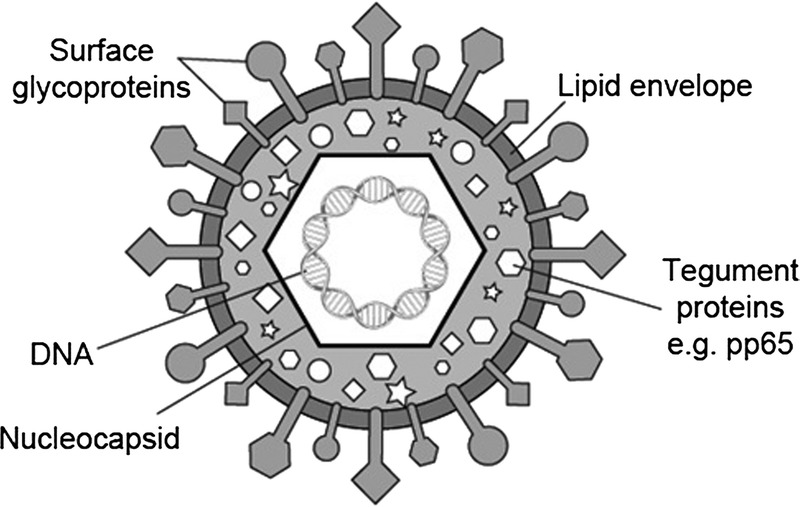
The CMV virion.
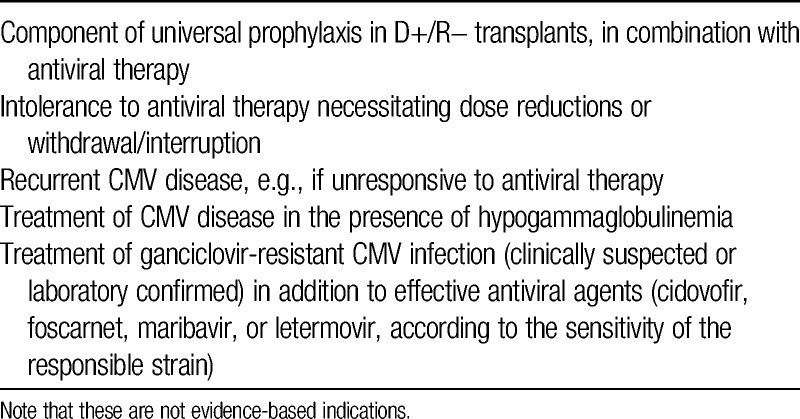
Despite the long experience with CMV immunoglobulin (CMVIG) in thoracic organ transplantation, there is still a wide variability among centers regarding its use in the prophylaxis and treatment of CMV infection or CMV disease (Table 1). Randomized trials are rare in this setting4 such that evidence-led decision-making, although desirable, is difficult. Against this background, a meeting of heart and lung transplant experts was convened in San Diego, CA, in April 2014. The purpose of the discussions was to review the available data relating to CMVIG therapy in the setting of thoracic organ transplantation and to consider the most appropriate strategies for its deployment to help reduce the impact of CMV infection on patient outcomes. The key findings and conclusions of the expert panel are reported in this supplement.
TABLE 1.
Possible settings for CMVIG administration in thoracic organ transplantation
The Burden of CMV
Estimates of CMV infection rates vary, partly due to differences in diagnostic criteria, but recent studies using modern CMV prophylaxis regimens have reported incidences of 11% to 30% in heart transplant recipients5-8 and 20% to 40% in lung transplant recipients.9-11 Encouragingly, markedly lower rates have been observed in patients treated with a mammalian target of rapamycin inhibitor5,7,12,13 or given extended antiviral prophylaxis.14
High-level CMV infection can manifest as the well-characterized CMV syndrome typified by mononucleosis-like fever. If the infection progresses to organ-invasive CMV disease, it most often affects the gastrointestinal system (colitis, ulceration), the liver (hepatitis) and, particularly in lung transplant recipients, the lungs (pneumonitis), with potentially life-threatening consequences. In addition, however, persistent low-level CMV infection can exert a number of damaging indirect effects.15 Notably, CMV infection seems to be associated with a significant increase in the risk of acute rejection after thoracic transplantation even in the era of valganciclovir prophylaxis.9 The CMV infection upregulates major histocompatibility complex antigens in the graft, likely by stimulating interferon-γ production by activated CD4+ cells, thus increasing graft immunogenicity16 and prompting rejection. The CMV infection also promotes the development of cardiac allograft vasculopathy after heart transplantation16-20 by triggering an early inflammatory response in the graft vasculature, ultimately leading to enhanced intimal thickness and a reduced lumen.16 Similarly, there is evidence that CMV infection or CMV pneumonitis increases the risk of bronchiolitis obliterans syndrome (BOS) in lung transplant recipients,21-25 again by provoking interferon-γ release by CD4+ cells26 and other immunomodulatory effects,27 although conflicting results have been reported.28-30 The CMV infection is also associated with an increased risk of opportunistic secondary infections, such as invasive fungal disease, again due to CMV-induced modulation of the host immune system.31
Issues in the Management of CMV
Host defences are severely curtailed in solid organ transplant patients receiving chronic immunosuppression, particularly in the first weeks posttransplant when the high risk of rejection necessitates potent regimens. Since the 1990s, antiviral prophylaxis for CMV using antiviral agents has become more widely used after heart32-34 or lung35,36 transplantation, particularly in high-risk D+/R− transplants.35 Evidence from kidney transplantation that extending antiviral prophylaxis to a minimum of 6 months reduces the risk of CMV disease37 has been mirrored in heart8,38 and lung39-41 transplantation. Prophylactic treatment for CMV after lung transplantation is almost entirely based on oral valganciclovir, preceded by intravenous ganciclovir at approximately half of specialist centers.35 If, alternatively, a preemptive strategy is followed, prevention of primary CMV infection or viral reactivation from latency sites requires constant immune surveillance. Preemptive therapy, although effective,42,43 seems to be less frequently used than prophylaxis; 1 international survey reported its use in only 14% of lung transplant centers.36
Despite the improvements in CMV infection rates after adoption of modern antiviral protocols, considerable challenges remain. Even using extended valganciclovir regimens within the controlled environment of a clinical trial, CMV infection occurs in at least 10% of heart6 and lung14,41 transplant patients. Furthermore, late-onset primary CMV infection after withdrawal of antiviral agents routinely occurs in a proportion of patients receiving universal prophylaxis,44,45 whereas cases of recurrent CMV disease and, in particular, CMV strains resistant to ganciclovir or valganciclovir require alternative treatment approaches, such as foscarnet.46 Patients with severe hypogammaglobulinemia, for example, induced by immunosuppressive therapies, such as mycophenolic acid, are another instance in which conventional antiviral therapy alone may not be adequate. Additionally, the dose-limiting hematological side effects of ganciclovir47 can necessitate a switch of strategy.
CMV Immunoglobulin Therapy
The highly sophisticated mechanisms by which CMV modulates cell signalling pathways to adapt the host immune system1 may support a rationale for a combination approach to prevention and treatment of CMV infection. The CMVIG and antiviral agents have complementary modes of action: CMVIG eliminates virus particles before they reach the host cell, whereas antiviral agents (ganciclovir, foscarnet and cidofovir) target viral DNA polymerase to block viral replication within the cell. The importance of an effective immunoglobulin G response to CMV infection was highlighted by the randomized VICTOR study, in which solid organ transplant patients (predominantly kidney transplant recipients) with negative CMV serostatus at the point when antiviral therapy started were significantly more likely to develop recurrent CMV disease than those who were positive for CMV IgG.48 The high titers of CMVIG in commercial preparations provide passive CMV-specific immunity in heavily immunosuppressed patients, neutralizing free viral particles by promoting opsonization and phagocytosis, and stimulating lysis by complement- and antibody-mediated responses. After the initial viremic phase, CMVIG enhances the antibody-dependent cellular cytotoxicity response to CMV infection such that its effect peaks a few weeks after first administration, when cytokine-producing CD4+ cells are produced. As discussed in the subsequent section “The immunology of posttransplant CMV infection: Potential effect of CMV immunoglobulins on distinct components of the immune response to CMV” CMVIG has complex immunomodulatory properties. In addition to augmenting the antiviral effect of antiviral agents, these may potentially suppress the indirect effects of CMV infection, such as allograft rejection, transplant vasculopathy, and BOS.
The CMVIG preparations are, essentially, concentrated doses of endogenous immunoglobulins from healthy individuals and as such, safety is not considered a major concern. Adverse events are rare and where they occur are typically mild injection site reactions. For example, in 1 series of 377 heart transplant recipients given CMVIG as sole prophylaxis, all patients received the full dose without any overt side effects,49 confirmed in a smaller early series of 15 patients.50 Postmarketing surveillance for the Cytotect preparation found only 4 events with a possible or certain relation to CMVIG administration across a total of 13 716 applications in 2286 patients during a 3-year period, comprising 1 case of headache, 1 infusion-related reaction, and 2 cases of anti-hepatitis B surface antibody positivity.51 Given this level of safety, virtually all the studies described in this article do not report safety endpoints, and little evidence of adverse events associated with CMVIG is available in the literature.
CMV-specific hyperimmunoglobulin preparations have been used to treat or prevent CMV infection after thoracic transplantation for more than 30 years.52 During that time, production methods have evolved and now incorporate sophisticated viral inactivation procedures which ensure high-quality and safety profiles and generate CMV-specific immunoglobulin.53 Before effective antiviral agents became available, CMVIG was used more widely than now to prevent CMV infection. In the United States, data suggest that CMVIG is currently given prophylactically in fewer than 10% of heart transplant recipients overall,33,34 but with higher usage in D+/R− patients (~40%).36 A recent international survey of lung transplant centers also found that 30% to 40% use CMVIG for prophylaxis in D+/R− transplants.35,36 Information on the application of CMVIG therapy for the treatment of established CMV infection or disease is more sparse, but across all types of solid organ transplants, it has been reported that approximately 10% of clinicians routinely administer CMVIG as an adjunct to antiviral treatment, mostly in D+/R− cases.36
Use of CMVIG in heart and lung transplant recipients has increased in recent years, possibly in response to growing problems with CMV management based on antiviral therapy alone, notably the emergence of ganciclovir-resistant strains. Current consensus recommendations, however, note that there are only limited data to support prophylactic use of CMVIG when appropriate antivirals are given.15,54 Guidelines from the Transplantation Society and the American Society of Transplantation do not advise CMVIG prophylaxis after kidney or liver transplantation, but include the option for CMVIG as adjunctive therapy to antiviral prophylaxis after heart or lung transplantation in high-risk D+/R− patients.15,54,55 Regarding use of CMVIG to treat CMV disease, the recommendations point out that the evidence is unclear but suggest that it could be considered in severe forms, such as pneumonitis.15,54,55
The relative scarcity of high-quality trials in this area prohibits development of robust, evidence-based guidance. The articles in this supplement discuss the available data concerning the immunological and clinical effects of CMVIG as prophylaxis or treatment for CMV infection after heart or lung transplantation.
Footnotes
All authors received an honorarium from Biotest AG to attend the meeting upon which this publication is based. Paolo Grossi is a member of a speaker's bureau and advisory board for Biotest AG. Paul Mohacsi and Zoltán Szabolcs have no other conflicts of interest to declare.
This supplement was funded by Biotest AG, Dreieich, Germany.
All authors attended a meeting to discuss the content of this supplement and review the available evidence, after which the article was developed by a freelance medical writer. All authors undertook a detailed critique of draft texts and approved the final article for submission.
REFERENCES
- 1. Roy S, Arav-Boger R. New cell-signaling pathways for controlling cytomegalovirus replication. Am J Transplant. 2014; 14: 1249– 1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mocarski ES., Jr Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 2002; 10: 332– 339. [DOI] [PubMed] [Google Scholar]
- 3. Kudchodkar SB, Yu Y, Maguire TG, et al. Human cytomegalovirus infection induces rapamycin-insensitive phosphorylation of downstream effectors of mTOR kinase. J Virol. 2004; 79: 11030– 11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonaros N, Mayer B, Schachner T, et al. CMV-hyperimmune globulin for preventing cytomegalovirus infection and disease in solid organ transplant recipients: a meta-analysis. Clin Transplant. 2008; 22: 89– 97. [DOI] [PubMed] [Google Scholar]
- 5. Eisen HJ, Kobashigawa J, Starling RC, et al. Everolimus versus mycophenolate mofetil in heart transplantation: a randomized, multicenter trial. Am J Transplant. 2013; 13: 1203– 1216. [DOI] [PubMed] [Google Scholar]
- 6. Rodríguez-Serrano M, Sánchez-Lázaro I, Almenar-Bonet L, et al. Does the calcineurin inhibitor have influence on cytomegalovirus infection in heart transplantation? Clin Transplant. 2014; 28: 88– 95. [DOI] [PubMed] [Google Scholar]
- 7. Andreassen AK, Andersson B, Gustafsson F, et al. SCHEDULE Investigators Everolimus initiation and early calcineurin inhibitor withdrawal in heart transplant recipients: a randomized trial. Am J Transplant. 2014; 14: 1828– 1838. [DOI] [PubMed] [Google Scholar]
- 8. Doesch AO, Repp J, Hofmann N, et al. Effects of oral valganciclovir prophylaxis for cytomegalovirus infection in heart transplant patients. Drug Des Devel Ther. 2012; 6: 289– 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stern M, Hirsch H, Cusini A, et al. Members of Swiss Transplant Cohort Study Cytomegalovirus serology and replication remain associated with solid organ graft rejection and graft loss in the era of prophylactic treatment. Transplantation. 2014; 98: 1013– 1018. [DOI] [PubMed] [Google Scholar]
- 10. Johansson I, Mårtensson G, Nyström U, et al. Lower incidence of CMV infection and acute rejections with valganciclovir prophylaxis in lung transplant recipients. BMC Infect Dis. 2013; 13: 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitsani D, Nguyen MH, Kwak EJ, et al. Cytomegalovirus disease among donor-positive/recipient-negative lung transplant recipients in the era of valganciclovir prophylaxis. J Heart Lung Transplant. 2010; 29: 1014– 1020. [DOI] [PubMed] [Google Scholar]
- 12. Ghassemieh B, Ahya VN, Baz MA, et al. Decreased incidence of cytomegalovirus infection with sirolimus in a post hoc randomized, multicenter study in lung transplantation. J Heart Lung Transplant. 2013; 32: 701– 706. [DOI] [PubMed] [Google Scholar]
- 13. Kobashigawa J, Ross H, Bara C, et al. Everolimus is associated with a reduced incidence of cytomegalovirus infection following de novo cardiac transplantation. Transpl Infect Dis. 2013; 15: 150– 162. [DOI] [PubMed] [Google Scholar]
- 14. Palmer SM, Limaye AP, Banks M, et al. Extended valganciclovir prophylaxis to prevent cytomegalovirus after lung transplantation: a randomized, controlled trial. Ann Intern Med. 2010; 152: 761– 769. [DOI] [PubMed] [Google Scholar]
- 15. Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ. Transplantation. 2013; 96: 333– 360. [DOI] [PubMed] [Google Scholar]
- 16. Koskinen PK, Kallio EA, Tikkanen JM, et al. Cytomegalovirus infection and cardiac allograft vasculopathy. Transpl Infect Dis. 1999; 1: 115– 126. [DOI] [PubMed] [Google Scholar]
- 17. Potena L, Grigioni F, Magnani G, et al. Prophylaxis versus preemptive anti-cytomegalovirus approach for prevention of allograft vasculopathy in heart transplant recipients. J Heart Lung Transplant. 2009; 28: 461– 467. [DOI] [PubMed] [Google Scholar]
- 18. Petrakopoulou P, Kübrich M, Pehlivanli S, et al. Cytomegalovirus infection in heart transplant recipients is associated with impaired endothelial function. Circulation. 2004; 110(11 Suppl 1): II207– II212. [DOI] [PubMed] [Google Scholar]
- 19. Weill D. Role of cytomegalovirus in cardiac allograft vasculopathy. Transpl Infect Dis. 2001; 3(Suppl 2): 44– 48. [DOI] [PubMed] [Google Scholar]
- 20. Koskinen PK, Nieminen MS, Krogerus LA, et al. Cytomegalovirus infection and accelerated cardiac allograft vasculopathy in human cardiac allografts. J Heart Lung Transplant. 1993; 12: 724– 729. [PubMed] [Google Scholar]
- 21. Paraskeva M, Bailey M, Levvey BJ, et al. Cytomegalovirus replication within the lung allograft is associated with bronchiolitis obliterans syndrome. Am J Transplant. 2011; 11: 2190– 2196. [DOI] [PubMed] [Google Scholar]
- 22. Snyder LD, Finlen-Copeland CA, Turbyfill WJ, et al. Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. Am J Respir Crit Care Med. 2010; 181: 1391– 1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duncan SR, Paradis IL, Yousem SA, et al. Sequelae of cytomegalovirus pulmonary infections in lung allograft recipients. Am Rev Respir Dis. 1992; 146: 1419– 1425. [DOI] [PubMed] [Google Scholar]
- 24. Johansson I, Mårtensson G, Andersson R. Cytomegalovirus and long-term outcome after lung transplantation in Gothenburg, Sweden. Scand J Infect Dis. 2010; 42: 129– 136. [DOI] [PubMed] [Google Scholar]
- 25. Chmiel C, Speich R, Hofer M, et al. Ganciclovir/valganciclovir prophylaxis decreases cytomegalovirus-related events and bronchiolitis obliterans syndrome after lung transplantation. Clin Infect Dis. 2008; 46: 831– 839. [DOI] [PubMed] [Google Scholar]
- 26. Waldman WJ, Knight DA, Adams PW, et al. In vitro induction of endothelial HLA class II antigen expression by CMV-activated CD4+ T cells. Transplantation. 1993; 56: 1504– 1512. [DOI] [PubMed] [Google Scholar]
- 27. Tikkanen JM, Krebs R, Bruggeman C, et al. Platelet-derived growth factor regulates cytomegalovirus infection-enhanced obliterative bronchiolitis in rat tracheal allograft. Transplantation. 2004; 77: 655– 658. [DOI] [PubMed] [Google Scholar]
- 28. Danziger-Isakov LA, Worley S, Michaels MG, et al. The risk, prevention, and outcome of cytomegalovirus after pediatric lung transplantation. Transplantation. 2009; 87: 1541– 1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Costa C, Curtoni A, Sidoti F, et al. Detection of human cytomegalovirus in transbronchial biopsies from lung transplant recipients. Arch Virol. 2013; 158: 1461– 1465. [DOI] [PubMed] [Google Scholar]
- 30. Schlischewsky E, Fuehner T, Warnecke G, et al. Clinical significance of quantitative cytomegalovirus detection in bronchoalveolar lavage fluid in lung transplant recipients. Transpl Infect Dis. 2013; 15: 60– 69. [DOI] [PubMed] [Google Scholar]
- 31. Snydman DR, Limaye AP, Potena L, et al. Update and review: state-of-the art management of cytomegalovirus infection and disease following thoracic organ transplantation. Transplant Proc. 2011; 43(Suppl 3): S1– S17. [DOI] [PubMed] [Google Scholar]
- 32. Laske A, Gallino A, Mohacsi P, et al. Prophylactic treatment with ganciclovir for cytomegalovirus infection in heart transplantation. Transplant Proc. 1991; 23(1 Pt 2): 1170– 1173. [PubMed] [Google Scholar]
- 33. Snydman DR, Kistler KD, Ulsh P, et al. Cytomegalovirus prevention and long-term recipient and graft survival in pediatric heart transplant patients. Transplantation. 2010; 90: 1432– 1438. [DOI] [PubMed] [Google Scholar]
- 34. Snydman DR, Kistler KD, Ulsh P, et al. The impact of CMV prevention on long-term recipient and graft survival in heart transplant recipients: analysis of the Scientific Registry of Transplant Recipients (SRTR) database. Clin Transplant. 2011; 25: E455– E462. [DOI] [PubMed] [Google Scholar]
- 35. Zuk DM, Humar A, Weinkauf JG, et al. An international survey of cytomegalovirus management practices in lung. Transplantation. 2010; 90: 672– 676. [DOI] [PubMed] [Google Scholar]
- 36. Le Page AK, Jager MM, Kotton CN, et al. International survey of cytomegalovirus management in solid organ transplantation after the publication of consensus guidelines. Transplantation. 2013; 95: 1455– 1456. [DOI] [PubMed] [Google Scholar]
- 37. Humar A, Limaye AP, Blumberg EA, et al. Extended valganciclovir prophylaxis in D+/R− kidney transplant recipients is associated with long-term reduction in cytomegalovirus disease: two-year results of the IMPACT study. Transplantation. 2010; 90: 1427– 1431. [DOI] [PubMed] [Google Scholar]
- 38. Gupta S, Mitchell JD, Markham DW, et al. High incidence of cytomegalovirus disease in D+/R− heart transplant recipients shortly after completion of 3 months of valganciclovir prophylaxis. J Heart Lung Transplant. 2008; 27: 536– 539. [DOI] [PubMed] [Google Scholar]
- 39. Zamora MR, Nicolls MR, Hodges TN, et al. Following universal prophylaxis with intravenous ganciclovir and cytomegalovirus immune globulin, valganciclovir is safe and effective for prevention of CMV infection following lung transplantation. Am J Transplant. 2004; 4: 1635– 1642. [DOI] [PubMed] [Google Scholar]
- 40. Jaksch P, Zweytick B, Kerschner H, et al. Cytomegalovirus prevention in high-risk lung transplant recipients: comparison of 3- vs 12-month valganciclovir therapy. J Heart Lung Transplant. 2009; 28: 670– 675. [DOI] [PubMed] [Google Scholar]
- 41. Finlen Copeland CA, Davis WA, Snyder LD, et al. Long-term efficacy and safety of 12 months of valganciclovir prophylaxis compared with 3 months after lung transplantation: a single-center, long-term follow-up analysis from a randomized, controlled cytomegalovirus prevention trial. J Heart Lung Transplant. 2011; 30: 990– 996. [DOI] [PubMed] [Google Scholar]
- 42. Vrtovec B, Thomas CD, Radovancevic R, et al. Comparison of intravenous ganciclovir and cytomegalovirus hyperimmune globulin pre-emptive treatment in cytomegalovirus-positive heart transplant recipients. J Heart Lung Transplant. 2004; 23: 461– 465. [DOI] [PubMed] [Google Scholar]
- 43. Atabani SF, Smith C, Atkinson C, et al. Cytomegalovirus replication kinetics in solid organ transplant recipients managed by preemptive therapy. Am J Transplant. 2012; 12: 2457– 2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kijpittayarit-Arthurs S, Eid AJ, Kremers WK, et al. Clinical features and outcomes of delayed-onset primary cytomegalovirus disease in cardiac transplant recipients. J Heart Lung Transplant. 2007; 26: 1019– 1024. [DOI] [PubMed] [Google Scholar]
- 45. Schoeppler KE, Lyu DM, Grazia TJ, et al. Late-onset cytomegalovirus (CMV) in lung transplant recipients: can CMV serostatus guide the duration of prophylaxis? Am J Transplant. 2013; 13: 376– 382. [DOI] [PubMed] [Google Scholar]
- 46. Zürcher S, Mooser C, Lüthi AU, et al. Sensitive and rapid detection of ganciclovir resistance by PCR based MALDI-TOF analysis. J Clin Virol. 2012; 54: 359– 363. [DOI] [PubMed] [Google Scholar]
- 47. McGavin JK, Goa KL. Ganciclovir: an update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients. Drugs. 2001; 61: 1153– 1183. [DOI] [PubMed] [Google Scholar]
- 48. Asberg A, Humar A, Jardine AG, et al. VICTOR Study Group Long-term outcomes of CMV disease treatment with valganciclovir versus IV ganciclovir in solid organ transplant recipients. Am J Transplant. 2009; 9: 1205– 1213. [DOI] [PubMed] [Google Scholar]
- 49. Kocher AA, Bonaros N, Dunkler D, et al. Long-term results of CMV hyperimmune globulin prophylaxis in 377 heart transplant recipients. J Heart Lung Transplant. 2003; 22: 250– 257. [DOI] [PubMed] [Google Scholar]
- 50. Aguado JM, Gomez-Sanchez MA, Lumbreras C, et al. Prospective randomized trial of efficacy of ganciclovir versus that of anti-cytomegalovirus (CMV) immunoglobulin to prevent CMV disease in CMV-seropositive heart transplant recipients treated with OKT3. Antimicrob Agents Chemother. 1995; 39: 1643– 1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Data on file, Biotest® AG, Dreieich, Germany. [Google Scholar]
- 52. Freeman R, Gould FK, McMaster A. Management of cytomegalovirus antibody negative patients undergoing heart transplantation. J Clin Pathol. 1990; 43: 373– 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Radosevich M, Burnouf T. Intravenous immunoglobulin G: trends in production methods, quality control and quality assurance. Vox Sang. 2010; 98: 12– 28. [DOI] [PubMed] [Google Scholar]
- 54. Humar A, Syndman D, AST Infectious Diseases Community of Practice. Cytomegalovirus in solid organ transplant recipients. Am J Transplant. 2009; 9(Suppl 4): S78– 86. [DOI] [PubMed] [Google Scholar]
- 55. Razonable RR, Humar A. Cytomegalovirus. Chapter 14. The AST Handbook of Transplant Infections. 2011. (1st ed). Blackwell Publishing Ltd; Ed. Kumar D, Humar A. [Google Scholar]


