Abstract
The growth in health professions education (HPE) and a desire on the part of nurse and medical educators to disseminate their work have raised important questions about the ethical conduct of education research. At the center of the debate is the institutional review board (IRB) and its proper role in the oversight of HPE research. This article examines the IRB process and types of reviews for education research and presents an Education Project Summary Template to use for IRB reviews.
Keywords: health professions education research, human subjects review, institutional review board, IRB, nursing education research, research: ethical considerations
Health professions education (HPE) has entered a new era. Anonymous lectures in darkened halls and unstructured apprenticeships of varying quality are disappearing. Training future health care workers is evolving into a highly structured process with carefully formatted objectives, interactive teaching strategies, and multimodal evaluations.1,2 Institutions and accrediting bodies have established thresholds of achievement and proficiency as milestones for graduating learners to independent practice.3,4 The number of questions arising regarding the quality and efficacy of educational practice has fed a growing field of HPE research. This growth in HPE and a desire on the part of nursing and medical educators to disseminate their work have raised equally important questions about the ethical conduct of such work, particularly with the complexity and conflict presented for subject-learners, external funders, and regulatory demands. At the center of the debate is the institutional review board (IRB) and its proper role in the oversight of HPE.
Background
Traditionally, most educational program design and evaluation have not been considered part of the research enterprise. Clinicians or researchers would spend time teaching, but this was considered wholly separate from their other academic pursuits. The work of Glassick5 in the late 1990s provided a taxonomy and framework for a more deliberative teaching process with key components of careful design, meaningful evaluation, peer review, and reflective critique intended to spawn opportunities for presentation and publication. With the evolution of the “scholarship of teaching,” educators began to reflect on the research-like attributes of their work as well as the implications of its design and delivery for their subjects. In 2004, an article appeared detailing the plight of a grant-funded curricular program in geriatrics.6 The article described how medical students at a university in which faculty initiated curricular change with grant funding ultimately were forced to cancel the program when students protested the fact that they were not consented prior to the innovation and that the project had not undergone IRB review.
While lacking the historical weight of the Belmont Report, this cautionary tale raised a number of important points, including (1) learners may be considered not just passive participants but actually subjects of education research and that educators need to consider their rights and identities when participating in these projects, (2) a lack of training and awareness among health professions educators about the protection of human subjects and the IRB process and a prevailing sense that IRB review is an optional process and something to be avoided if possible, and (3) the growth of external funding raising the possibility that local activities might be influenced by demands and requirements of outside agencies. These points speak to the core of the mission of the IRB in that health professions educators need to consider the ethical implications of the design and execution of their work.
Significance
HPE is recognized as a key ingredient in high-quality nursing and medical care.7 A demand exists for evidence to inform how we educate health care professionals, and educational programs have become a key tool for implementing improvement. Admittedly, the science underlying education research is often complex and requires use of a variety of study designs—both experimental and quasi-experimental—and multiple approaches for evaluation, including both qualitative and quantitative.8 The evolving sophistication and complexity of HPE research point to a need for a centralized source for review of conduct and quality of these projects. Moreover, education researchers are gathering, collating, and analyzing large amounts of data. These data need to be collected and stored in a way that ensures its integrity and protects the identity of learners. Collaboration across institutions adds an additional layer of complexity to this process.9
It is often the intent of medical and nursing education researchers to disseminate their work through presentation and publication. This intent to generalize has important implications for the use of information and protection of the identity of participants. Furthermore, HPE is moving toward measuring impact of teaching interventions on outcomes including patient care. The examination of a learner’s performance in real clinical care requires a consideration of how to handle not only the learner data, but also that of the patient as well. Finally, consideration must be given to the learner as a vulnerable subject, prone to coercion, exposure, and undue psychological stressors.
The IRB Process
Health professions educators may be best served to reframe these challenges as compelling reasons to engage the IRB as a partner in the process of ensuring sound design and the responsible and ethical conduct of educational research. This “reframing” starts with a careful examination of the principles underlying the formation and mission of the IRB in the Code of Federal Regulations and a point-by-point examination of their relevance to the education researcher.
Types of IRB Reviews
The responsibility of an IRB is to ensure that the regulatory criteria for approval of a study are met and in so doing help protect the rights and welfare of human subjects involved in research, including ensuring that risks of participating in a study are reasonable in relation to the potential benefits. Current federal regulations require that all nonexempt human subjects research be approved by an IRB before an investigator may conduct a study.
Making decisions regarding the IRB review process for research studies begin with first determining if human subjects are involved and if the investigation is truly designed to contribute to generalizable knowledge.10 The Federal Policy for the Protection of Human Subjects Research is known as the “Common Rule” and guides oversight and provides guidance for those engaged in research involving living individuals.11 Human subjects research means obtaining data through intervention or interaction with an individual or by using identifiable private information to answer a research question.10 In the case of HPE, learners may be considered “subjects” when considering their rights and protections as participants. There are 3 types of IRB reviews for new protocols: exempt, expedited, and full-committee review. A full description of these reviews is found in Table 1.
Table 1.
Institutional Review Board Review Types

The IRB Review Process for Education Research
There are specific categories of research involving human subjects that are eligible for exemption; some types of education research meet the definition for these categories. To assist education project leaders in determining what types of projects meet the exempt education research criteria, the Office for Human Research Protections in section 45 CFR 46.101(b1-6)10 includes educational research categories that could potentially be considered exempt:
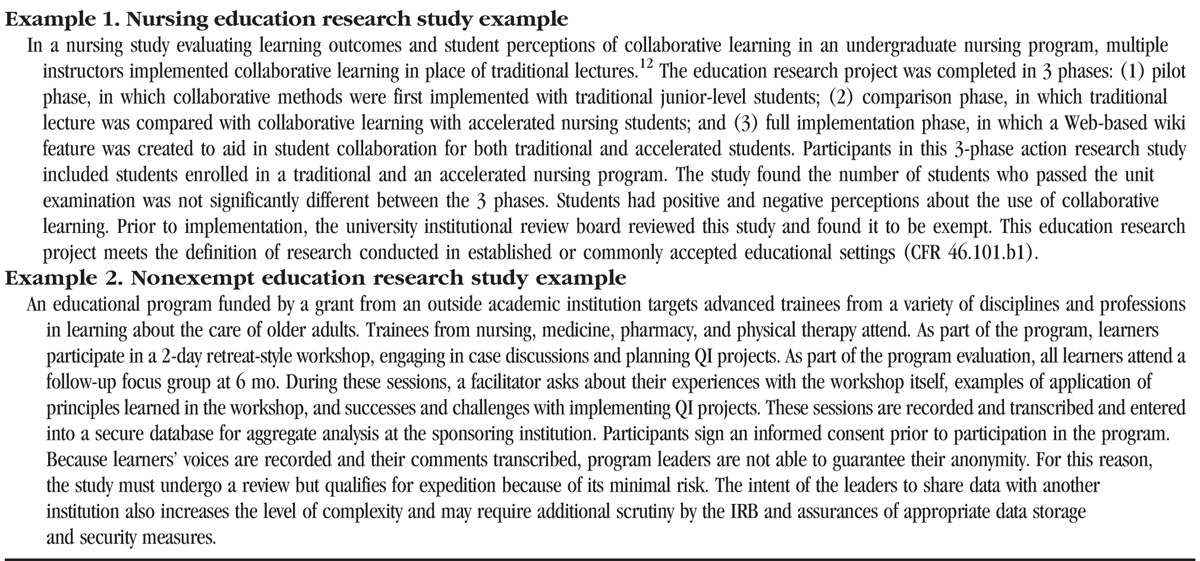
Research conducted in established or commonly accepted educational settings, involving normal educational practices, such as (i) research on regular and special education instructional strategies or (ii) research on the effectiveness of or the comparison among instructional techniques, curricula, or classroom management methods (CFR 46.101.b1). In Table 2, example 1 is an exempt education research study.
Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview, or observation of public behavior, unless (i) information obtained is recorded in such a manner that human subjects can be identified, directly or through identifiers linked to the subjects; and (ii) any disclosure of the human subjects’ responses outside the research could reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, or reputation (CFR 46.101.b2). This exemption does not apply when surveys, interviews, or observation of public behaviors include individuals younger than 18 years when the investigator is participating in the activities being observed.
Table 2.
Examples of Education Research and Types of IRB Reviews
Research involving the collection of existing data, documents, or records, if publicly available, or if information is recorded by the investigator in such a manner that subjects are not identifiable (directly or through identifiers) may also be exempt (CFR 46.101.b4).10 Careful evaluation of the above categories determines whether an education research project meets the exemption criteria or requires an expedited or full-committee IRB review. A complete description of potentially exempt research categories can be found at http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html.
When a research study does not meet the criteria for exemption, the risks to subjects participating in the project should be further evaluated. In Table 2, example 2 is of a nonexempt education research study. Federal regulations define minimal risk as the “probability and magnitude of harm or discomfort anticipated in the research is not greater in and of themselves than that ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests” (45 CFR 46.102[i]).10 Most education research studies are minimal risk studies when protection of the rights and welfare of subjects involved are carefully considered. Education research subjects must not be unduly influenced to participate.13 Protection of the identity of involved subjects must be considered. If any information is collected that can directly identify a subject or when an identifier can be linked to this individual, then it is not eligible for exemption unless it meets one of the 6 exemption categories listed in the federal regulations (45 CFR 46.101).14 Measures to protect the privacy and confidentiality of subjects should be taken and clearly described in the study.
When students are directly involved themselves, even exempt education research studies must inform the subjects about the project, and they must be provided details as to how confidentiality of the information will be protected. Subjects must also be told that participation is voluntary and that they can refuse to answer questions that they do not wish to answer. Subjects should be allowed to withdraw at any time without repercussion.
Written consent may be required if the project includes collection of recorded data via voice, video, digital, or image. Individual or group interviews or focus groups may require written consent as well. First consider whether the research is more than minimal risk of harm to subjects and if it involves no procedures for which written consent is normally required outside the research environment (45 CFR 46.117.c2). There are many situations where consent documentation can be waived, and the IRB can request the investigators to provide subjects with a written statement regarding the research.
Several examples are provided to give further guidance. Examination of nursing students’ perceptions about a specific disease such as human immunodeficiency virus (HIV) or cancer and their attitudes toward caring for a patient through interviews or focus groups requires research measures to ensure that student participation is voluntary and anonymity is protected. This type of research would not be exempt and requires informed consent. In comparison, evaluation of the effectiveness of a curriculum designed to allow students to explore their attitudes toward caring for patients with HIV or cancer may be seen as standard educational practice and would meet the criteria for exempt research if anonymity is maintained and no identifiers are collected. Even if written consent is not required, learners responding to surveys or other measures should be informed of the voluntary nature of their participation and the intent of the educator to analyze and disseminate the data.
Improving the Process
HPE researchers and their supporting institutions aspire to innovate in a way that will improve both the ability of the graduating learner and the care they provide. To achieve these goals in a responsible and ethical way, steps must be taken to bridge the gap between their work and the review process. These improvements may take several forms including (1) education and orientation programs, (2) expansion of the types of expertise and assistance available at the IRB, and (3) development of HPE-friendly review templates and processes.
Institutions should begin by introducing programs to inform both educators and IRB members about issues specific to education-related projects. Educators need to gain greater understanding of the process and definitions set forth in the CFR to more accurately prepare submissions for review. Specifically, such programs might focus on criteria for exemption versus review, risk for learners, and indications for consent. Health professions educators would also benefit from more in-depth orientation to data management and protection and, specifically, institutional resources designed to help with these issues. Finally, clear guides to the logistical aspects of submissions are essential.
The IRB itself also should participate in a review of its capabilities for reviewing educational projects. This may involve orientation for its board members on the specific issues and characteristics of education research. In addition, many have proposed that IRBs should seek to include reviewers with specific expertise in social and behavioral sciences (often lacking on medical campuses).15 At institutions with active education research communities, addition of a dedicated chair or board may be necessary.
In addition to orientation and education, the development of submission templates and targeted reviews specific for education projects may help the utility and efficiency of the process.16 At Duke University, a group of educators from our Schools of Medicine and Nursing developed a template in collaboration with leadership of the IRB to address needed improvements in the process. Our work followed the example set by the development and implementation of a template for submissions for quality improvement (QI) projects.17 Our team first met with representative chairs of the IRB to discuss the structure and content of the template as well as a plan for piloting the submission and review process. A draft version of the template was circulated among educators and IRB members for feedback and revision. The template, derived from the traditional research submission form, offered a modified organization and language to provide a better fit for education-related projects as well as clarity for reviewers (Table 3). A pilot group of submissions was routed to 2 IRB chairs involved in the development of the template. The template proved quite popular among educators and reviewers in both form and function. The form was posted on the IRB Web site, and our development team offered a series of workshops for faculty on its use.
Table 3.
Education Project Summary Template for IRB

Conclusion
It is important for health professions educators to consider the ethical implications of conducting education research. Increased understanding of the categories for exempt research and how to determine when an education research study does not meet the criteria for exemption are essential for those involved in HPE. Specifically, educators need to recognize learners as subjects and anticipate and acknowledge risks inherent in their participation in education research. In addition, educators need to seek greater knowledge and resources for data collection and management to ensure protection of subject identity and study integrity. Improvements in the review process for educational research studies will require a collaboration between educators and the IRB as outlined in this article. Development of education programs for researchers and reviewers, integration of educational expertise on the IRB, and standardization and dissemination of submission templates will all enhance the process and improve the quality of the work.
Footnotes
The authors declare no conflicts of interest.
Published ahead of print: November 25, 2015
References
- 1. Cooke M, Irby DM, O’Brien BC. Educating Physicians: A Call for Reform of Medical School and Residency. San Francisco, CA: Jossey-Bass; 2010. [Google Scholar]
- 2. Broome ME. Building the science for nursing education: vision or improbable dream. Nurs Outlook. 2009; 57(4): 177- 179. [DOI] [PubMed] [Google Scholar]
- 3. Nasca TJ, Philibert I, Brigham T, Flynn TC. The next GME accreditation system—rationale and benefits. N Engl J Med. 2012; 366(11): 1051- 1056. [DOI] [PubMed] [Google Scholar]
- 4. Pijl-Zieber EM, Barton S, Konkin J, Awosoga O, Caine V. Competence and competency-based nursing education: finding our way through the issues. Nurse Educ Today. 2014; 34(5): 676- 678. [DOI] [PubMed] [Google Scholar]
- 5. Glassick CE. Boyer’s expanded definitions of scholarship, the standards for assessing scholarship, and the elusiveness of the scholarship of teaching. Acad Med. 2000; 75(9): 877- 880. [DOI] [PubMed] [Google Scholar]
- 6. Tomokowiak JM, Gunderson AJ. To IRB or not to IRB? Acad Med. 2004; 79(7): 628- 632. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine (US) Committee on the Health Professions Education Summit; Greiner AC, Knebel E, eds. Health Professions Education: A Bridge to Quality. Washington, DC: National Academies Press (US); 2003. [PubMed] [Google Scholar]
- 8. Broome ME, Ironside PM, McNelis AM. Research in nursing education: state of the science. J Nurs Educ. 2012; 51(9): 521- 524. [DOI] [PubMed] [Google Scholar]
- 9. Oermann MH, Hallmark BF, Haus C, Kardong-Edgren SE, McColgan JK, Rogers N. Conducting multisite research studies in nursing education: brief practice of CPR skills as an exemplar. J Nurs Educ. 2012; 51(1): 23- 28. [DOI] [PubMed] [Google Scholar]
- 10.Code of Federal Regulations, Title 45, Public Welfare, Department of Health and Human Services, National Institutes of Health, Office for Protection From Research Risks, part 46, Protection of Human Subjects. Effective January 15, 2009. Available at www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm. Accessed May 9, 2015.
- 11.Federal Policy for the Protection of Human Subjects (‘Common Rule’). Available at http://www.hhs.gov/ohrp/humansubjects/commonrule/index.html. Accessed May 9, 2015.
- 12. Schoening AM, Selde MS, Goodman JT, et al. Implementing collaborative learning in prelicensure nursing curricula: student perceptions and learning outcomes. Nurse Educ. 2015; 40(4): 183- 188. [DOI] [PubMed] [Google Scholar]
- 13. Miser WF. Educational research—to IRB, or not to IRB? Fam Med. 2005; 37(3): 168- 173. [PubMed] [Google Scholar]
- 14. Sullivan GM. Education research and human subject protection: crossing the IRB quagmire. J Grad Med Educ. 2011; 3(1): 1- 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dyrbye LN, Thomas MR, Mechaber AJ, et al. Medical education research and IRB review: an analysis and comparison of the IRB review process at six institutions. Acad Med. 2007; 82(7): 654- 660. [DOI] [PubMed] [Google Scholar]
- 16. Johansson AC, Durning SJ, Gruppen LD, Olson ME, Schwartzstein RM, Higgins PA. Perspective: medical education research and the institutional review board: reexamining the process. Acad Med. 2011; 86(7): 809- 817. [DOI] [PubMed] [Google Scholar]
- 17. Demeo SD, Nagler A, Heflin MT. Development of a health professions education research specific institutional review board template. Acad Med. 2015. In press. [DOI] [PubMed] [Google Scholar]


