Abstract
OBJECTIVE
The management of postoperative hyperglycemia is controversial and generally does not take into account pre-existing diabetes. We analyzed clinical and economic outcomes associated with postoperative hyperglycemia in cardiac surgery patients, stratifying by diabetes status.
RESEARCH DESIGN AND METHODS
Multicenter cohort study in 4,316 cardiac surgery patients operated on in 2010. Glucose was measured at 6-h intervals for 48 h postoperatively. Outcomes included cost, hospital length of stay (LOS), cardiac and respiratory complications, major infections, and death. Associations between maximum glucose levels and outcomes were assessed with multivariable regression and recycled prediction analyses.
RESULTS
In patients without diabetes, increasing glucose levels were associated with a gradual worsening of outcomes. In these patients, hyperglycemia (≥180 mg/dL) was associated with an additional cost of $3,192 (95% CI 1,972 to 4,456), an additional hospital LOS of 0.8 days (0.4 to 1.3), an increase in infections of 1.6% (0.5 to 2.8), and an increase in respiratory complications of 2.6% (0.0 to 5.3). However, among patients with insulin-treated diabetes, optimal outcomes were associated with glucose levels considered to be hyperglycemic (180 to 240 mg/dL). This level of hyperglycemia was associated with cost reductions of $6,225 (−12,886 to −222), hospital LOS reductions of 1.6 days (−3.7 to 0.4), infection reductions of 4.1% (−9.1 to 0.0), and reductions in respiratory complication of 12.5% (−22.4 to −3.0). In patients with non–insulin-treated diabetes, outcomes did not differ significantly when hyperglycemia was present.
CONCLUSIONS
Glucose levels <180 mg/dL are associated with better outcomes in most patients, but worse outcomes in patients with diabetes with a history of prior insulin use. These findings support further investigation of a stratified approach to the management of patients with stress-induced postoperative hyperglycemia based on prior diabetes status.
Introduction
Glycemic abnormalities and diabetes are on the rise globally (1). According to the most recent statistics, 9.3% of the U.S. population, 29.1 million individuals, live with diabetes, and the level of glycemia in the general public (mean fasting plasma glucose) since 1980 has risen by 2.5 mg/dL per decade in women, and by 3.2 mg/dL per decade in men (1,2). Hyperglycemia is common after stressful events, such as myocardial infarction, stroke, and sepsis, or in the postoperative setting, after cardiac surgery (3). Stress-induced hyperglycemia is a transient phenomenon, distinct from the chronic glucose dysregulation brought about by diabetes (3). Studies (4–7) have shown that stress hyperglycemia after cardiac surgery, which occurs in patients both with and without diabetes, is associated with a higher risk of complications, including major infections, and increased mortality.
The management of stress hyperglycemia in patients receiving critical care is a matter of great controversy (8). The rationale of glucose control management rests on the hypothesis that the relationship between hyperglycemia and adverse outcomes is one of causation. Trials assessing the potential benefits of strict glycemic control (target range 80–110 mg/dL) (9–12) have produced conflicting results, with early studies reporting decreased mortality and morbidity, and subsequent studies showing a lack of benefits or even worse outcomes, along with an increased risk of hypoglycemia. These trials included a heterogeneous selection of patients, which may have influenced the response to short-term changes in glucose levels. Given the uncertainty about the effectiveness of different protocols targeting normoglycemia, most medical societies have endorsed a moderate approach to glucose control in perioperative and critical care settings, recommending that patients, regardless of their diabetes status, have their serum glucose levels maintained at <180 mg/dL (6,13). More recently, due the ongoing debate, the Surgical Care Improvement Project, a national program undertaken to improve outcomes in surgery whose measures are publicly reported on the Centers for Medicare and Medicaid hospital website and affect reimbursement, has suspended its recommendation on maintaining postoperative glucose levels at <180 mg/dL (14).
An increasing body of evidence shows that the association between stress hyperglycemia and adverse outcomes varies depending on the pre-existence of diabetes (3,15–17). Although diabetes is a heterogeneous disease with a broad spectrum of manifestations and symptom severity (18), most of the previous studies have analyzed the impact of stress hyperglycemia in diabetes without further stratification by prior treatment. However, prior treatment history and degree of glycemic control may be important effect modifiers (19). Consideration of these factors would permit the selection of more appropriate glucose targets for specific groups of patients, particularly in intensive care, where complications can be life threatening and costs are the highest. The purpose of this study is to assess the clinical and economic outcomes associated with postoperative hyperglycemia among patients without and with diabetes with different treatment histories who have undergone cardiac surgery.
Research Design and Methods
Study Population
Between February and October 2010, the Cardiothoracic Surgical Trials Network conducted a multicenter prospective cohort study to assess the incidence of hospital-acquired infections. All adult cardiac surgery patients (≥18 years old) without pre-existing infection on hospital admission were eligible to participate (N = 5,158) (20). Of the 10 participating centers (9 American and 1 Canadian), only patients from U.S. centers (n = 4,614) were included in order to avoid the confusion of mixing data from different health care systems with very different reimbursement methods. Billing data for these nine centers were obtained from the University HealthSystem Consortium, an alliance of U.S. academic medical centers with the goal of promoting improvements in the quality, safety, and efficiency of health care. Costs for 4,320 patients (93.6%) were available (21). The final study population included 4,316 patients in whom glucose was measured within 48 h after surgery. The study protocol was approved by the institutional review boards of each of the participating study centers.
Clinical and Economic Variables
Baseline variables prior to surgery included the following: demographics, anthropometrics, laboratory results, and comorbid conditions. Pre-hospital admission diabetes status was defined by prior therapy with oral antidiabetes medication only, a history of non–insulin-treated diabetes mellitus (NITDM), or a history of insulin-treated diabetes mellitus (ITDM). The latter group included patients treated with insulin only or a combination of insulin and oral antidiabetic medications. Hemoglobin A1c (HbA1c) was assessed preoperatively in patients with diabetes. In 83 NITDM patients (15%) and 66 ITDM patients (16%), HbA1c values were missing. Glomerular filtration rate (GFR) was estimated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration equation (22). Surgical parameters included sternotomy, hospital admission type (elective, urgent, emergent), procedure type (isolated valve, isolated coronary artery bypass graft [CABG], transplantation or ventricular assist device, CABG with valve, thoracic aortic, other), and surgery duration. The study protocol included blood glucose measurements every 6 h for 48 h after surgery. Based on previous research using maximum blood glucose level as a measure of blood glucose control (23), we used the highest value among these measurements. Hyperglycemia was defined as having at least one measurement >180 mg/dL. To convert glucose values to millimoles per liter, multiply by 0.0555.
Participating centers provided their protocol for managing glucose in the intensive care unit (ICU) after cardiac surgery. The standard protocol of each ICU was then used to approximate the actual treatment that the individual patients of that hospital received. Among the centers, three had guidelines recommending a target range for blood glucose concentration between 80 and 120 mg/mL, whereas the other six centers had a target range between 140 and 180 mg/mL. The protocols were grouped into the following two categories for the analysis: “tight” (80–120 mg/dL) and “nontight” (140–180 mg/mL).
Outcomes related to resource use included hospitalization costs and hospital length of stay (LOS). Clinical outcomes during the hospital stay were defined as death, major infection, cardiac complications, and respiratory complications. We used a composite end point including all these complications. Hospitalization costs were calculated from the billing data using Medicare cost center–specific cost-to-charge ratios. This method of approximating cost is widely used and provides reasonably accurate estimates of actual costs (24). Postoperative infections were reviewed and adjudicated by an independent committee of infectious disease experts. Major infections were classified using definitions adapted from the Centers for Disease Control and Prevention/National HealthCare Safety Network (20). Complications other than infections were identified through ICD-9 codes, and were defined as cardiac complications (code 9971) and respiratory failure (codes 5185, 51881, 51882, 51884, and 7991), with exclusion of those flagged as “present on admission.”
Literature Review
Previous studies related to the existence of possible differences between patients with and without diabetes, with respect to outcomes associated with stress hyperglycemia, were searched on PubMed using the terms “critical care,” “critically ill,” “ICU,” “hyperglycemia,” and “diabetes mellitus,” and English language and human species were used as filters. The search retrieved 653 citations, including 254 review articles. After reviewing titles and abstracts, 209 were considered relevant to the focus of this study. We finally selected 11 articles for a full-text review.
Statistical Analysis
We divided the study population in subgroups of patients with no diabetes, NITDM, and ITDM. Differences in baseline characteristics were assessed using Kruskal-Wallis one-way ANOVA for continuous variables and χ2 tests for categorical variables. The relationship between maximum glucose levels and outcomes was modeled using multivariable generalized linear regression analysis. Models included all baseline variables and interactions between a variable indicating diabetes status (no diabetes, NITDM, and ITDM) and maximum glucose levels when analyzed continuously, or hyperglycemia when analyzed dichotomously. Predictors were selected using costs as outcome and the Akaike information criterion, which calculates the log-likelihood penalized for the number of parameters included. Final regression models included the maximum glucose-diabetes treatment interaction term, age, sex, race (white vs. other), BMI, white blood cell count, GFR, hemoglobin level, ejection fraction, renal insufficiency, lung disease, congestive heart failure, prior cardiac surgery, corticosteroid use, hospital admission type (elective/urgent/emergent), procedure type, surgery duration, thoracic approach, and medical center. Within an analysis restricted to patients with diabetes, we also included HbA1c values. Missing HbA1c values were imputed by multiple imputation with a multivariable algorithm including all predictors, costs, hospital LOS, and the composite end point of complications. Nonlinear associations of continuous variables were modeled by restricted cubic spline functions with four knots (25). For estimating costs, we used a γ distribution with a log link function; for hospital LOS, we used a negative binomial distribution with a log link function; and for complications, we used logistic regression.
To demonstrate the adjusted change in each outcome with varying glucose levels, we used the recycled prediction method (26). In brief, we predicted the outcome of interest in each patient by using the regression equation and varying blood glucose concentrations (between 120 and 300 mg/dL when continuous, and >180 vs. ≤180 mg/dL when dichotomous) while keeping the other parameters fixed at their observed value. To take into account parameter uncertainty, regression models were refitted in 1,000 bootstrap data sets, and subsequently predictions were made in each bootstrap. After ordering the 1,000 bootstrap estimates, 2.5% and 97.5% quantiles were used for calculating 95% CIs.
To examine how a routine implementation of the recommended threshold <180 mg/dL would affect our different patient subgroups, we analyzed the expected outcomes above and below this threshold. Differences in the individual complications were adjusted for age, sex, and type of procedure only. In addition, we predicted outcomes for various hypothetical scenarios of postoperative glucose control. Each scenario represented the implementation of a different glucose control threshold ranging from 120 to 300 mg/dL, and assumed the perfect situation in which patients’ glucose levels were maintained below this threshold. Per scenario, patients were reassigned a maximum glucose value equal to the threshold when their actual value was higher. At lower thresholds, more patients have a glucose value above the threshold and will be reassigned to the threshold value; whereas, at higher thresholds, more patients will keep their actual glucose value. Finally, we graphically depicted the difference between the expected outcome of each scenario and the originally observed outcome. This difference will tend to zero at higher thresholds, given that more patients will keep their actual glucose levels. Parameter uncertainty was expressed as 95% CIs using 1,000 bootstraps, as described above.
All analyses were performed using R version 3.1.0 (R Foundation for Statistical Computing [http://www.r-project.org/]).
Results
Study Population
The average age in the study population was 65.5 years, and 66% were male. Among patients with diabetes, 43% were ITDM patients. These patients generally had glucose levels that were less well controlled than NITDM patients, as indicated by their HbA1c levels. Moreover, patients with ITDM had lower renal function, and a higher prevalence of congestive heart failure and chronic lung disease compared with the other patient groups (Table 1). Hyperglycemia during the first 48 h after surgery was reported in 70% of patients with diabetes and 36% of patients without diabetes. Hyperglycemia was less frequent in hospitals with a tight glucose control protocol compared with hospitals with nontight glucose control. Hypoglycemia, conversely, was more frequent in hospitals with tight glucose control, particularly in ITDM patients (Table 1). These results were confirmed in multivariable analyses, where the presence of a tight glucose control standard protocol in the hospital ICU increased the likelihood of the development of hypoglycemia in ITDM patients compared with a nontight protocol (odds ratio of hypoglycemia in ITDM patients = 2.39 [95% CI 1.64 to 3.48]).
Table 1.
Patients’ baseline characteristics
| No DM (n = 3,344) | NITDM (n = 553) | ITDM (n = 419) | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 64.7 (54.9, 74.5) | 67.6 (60.0, 75.2) | 66.0 (57.7, 72.9) | <0.0001 |
| Male | 2,209 (0.66) | 380 (0.69) | 261 (0.62) | 0.111 |
| White | 2,824 (0.84) | 421 (0.76) | 294 (0.70) | <0.0001 |
| Laboratory analyses | ||||
| Nontight glucose control* | ||||
| Maximum glucose, mg/dL | 170 (152, 194) | 201 (178, 232) | 213 (181, 249) | <0.0001 |
| Average glucose, mg/dL | 135 (126, 146) | 150 (137, 164) | 153 (140, 170) | <0.0001 |
| Hyperglycemia (≥180 mg/dL) | 820 (0.37) | 239 (0.73) | 195 (0.76) | <0.0001 |
| Hypoglycemia (<70 mg/dL) | 87 (0.04) | 11 (0.03) | 10 (0.04) | 0.885 |
| ≥2 Hyperglycemic measures, n (%) | 287 (12.9) | 144 (43.8) | 138 (53.7) | <0.0001 |
| Tight glucose control** | ||||
| Maximum glucose, mg/dL | 168 (151, 189) | 196 (170, 234) | 209 (169, 243) | <0.0001 |
| Average glucose, mg/dL | 133 (123, 144) | 147 (131, 163) | 145 (125, 169) | <0.0001 |
| Hyperglycemia (≥180 mg/dL) | 374 (0.34) | 140 (0.62) | 110 (0.68) | <0.0001 |
| Hypoglycemia (<70 mg/dL) | 44 (0.04) | 11 (0.05) | 15 (0.09) | 0.011 |
| ≥2 Hyperglycemic measures, n (%) | 125 (11.2) | 89 (39.7) | 75 (46.3) | <0.0001 |
| HbA1c | ||||
| % | NA | 6.7 (6.3, 7.4) | 7.5 (6.6, 8.6) | |
| mmol/mol | 50 (45, 57) | 58 (49, 70) | <0.0001 | |
| WBC count, cells × 103/mL | 6.8 (5.7, 8.2) | 7.3 (5.9, 8.8) | 7.5 (6.1, 9.0) | <0.0001 |
| Serum creatinine, mg/dL | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.3) | 1.1 (0.9, 1.6) | <0.0001 |
| GFR, mL/min/1.73 m2 | 76 (60, 92) | 69 (53, 88) | 63 (39, 86) | <0.0001 |
| Hemoglobin, g/dL | 13.5 (12.2, 14.6) | 12.8 (11.4, 14.0) | 12.2 (10.9, 13.6) | <0.0001 |
| Medical history and physical examination | ||||
| BMI, kg/m2 | 27.7 (24.7, 31.4) | 30.3 (26.7, 34.4) | 30.8 (27.3, 36.0) | <0.0001 |
| Ejection fraction, % | 55 (50, 60) | 55 (45, 60) | 50 (39, 59) | <0.0001 |
| Congestive heart failure | 824 (0.25) | 162 (0.29) | 173 (0.41) | <0.0001 |
| Prior cardiac surgery | 672 (0.20) | 97 (0.18) | 90 (0.21) | 0.264 |
| Cerebrovascular accident | 291 (0.09) | 71 (0.13) | 56 (0.13) | 0.0003 |
| Peripheral vascular disease | 273 (0.08) | 87 (0.16) | 81 (0.19) | <0.0001 |
| Renal insufficiency | 275 (0.08) | 86 (0.16) | 125 (0.30) | <0.0001 |
| Hypertension | 2,351 (0.70) | 499 (0.90) | 384 (0.92) | <0.0001 |
| Lung disease | 449 (0.13) | 92 (0.17) | 88 (0.21) | <0.0001 |
| Corticosteroid use | 100 (0.03) | 17 (0.03) | 18 (0.04) | 0.350 |
| Surgical parameters | ||||
| Duration, h | 4.3 (3.4, 5.4) | 4.4 (3.6, 5.4) | 4.5 (3.7, 5.6) | 0.004 |
| Sternotomy | 2,965 (0.89) | 517 (0.93) | 406 (0.97) | <0.0001 |
| Hospital admission type | <0.0001 | |||
| Elective | 2,503 (0.75) | 340 (0.61) | 241 (0.58) | |
| Urgent | 760 (0.23) | 192 (0.35) | 162 (0.39) | |
| Emergent | 81 (0.02) | 21 (0.04) | 16 (0.04) | |
| Procedure | <0.0001 | |||
| Isolated valve | 782 (0.23) | 242 (0.44) | 207 (0.49) | |
| Isolated CABG | 1,157 (0.35) | 116 (0.21) | 72 (0.17) | |
| Transplantation or VAD | 76 (0.02) | 10 (0.02) | 24 (0.06) | |
| CABG plus valve | 365 (0.11) | 84 (0.15) | 58 (0.14) | |
| Thoracic aortic | 199 (0.06) | 18 (0.03) | 4 (0.01) | |
| Other | 765 (0.23) | 83 (0.15) | 54 (0.13) |
Continuous variables are reported as the median (interquartile range), and categorical variables as n (proportion). The χ2 test was performed for comparing categorical variables, and Kruskal-Wallis one-way ANOVA was performed for comparing continuous variables. DM, diabetes mellitus; NA, not applicable; VAD, ventricular assist device implantation or explantation; WBC, white blood cell.
*The standard protocol of the ICU had a blood glucose target range within 140–180 mg/dL (n = 2,816).
**The standard protocol of the ICU had a blood glucose target range within 80–120 mg/dL (n = 1,500).
Outcomes by Diabetes Status
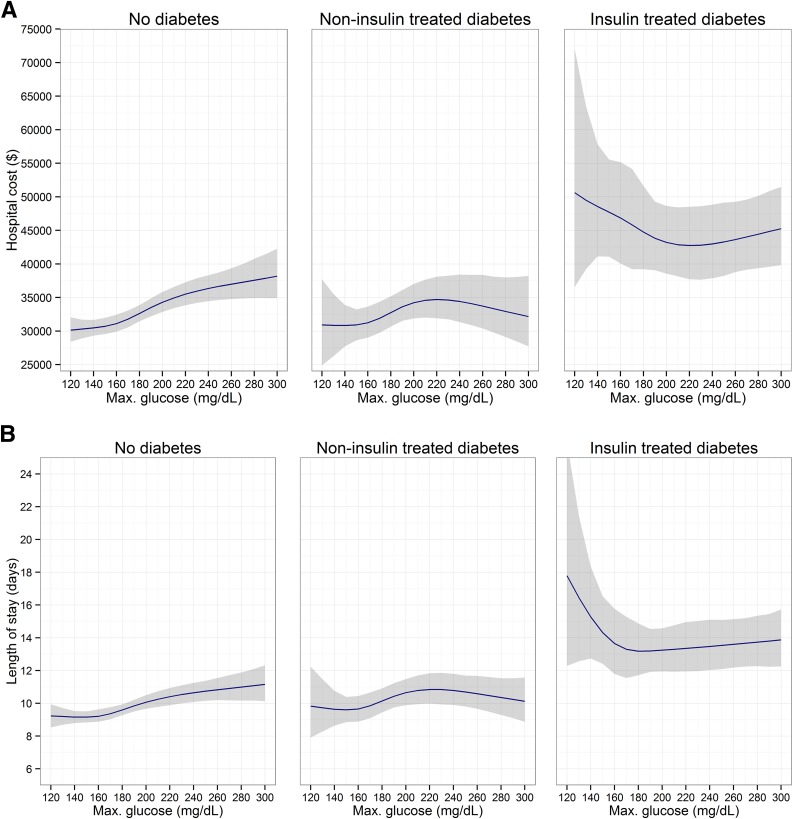
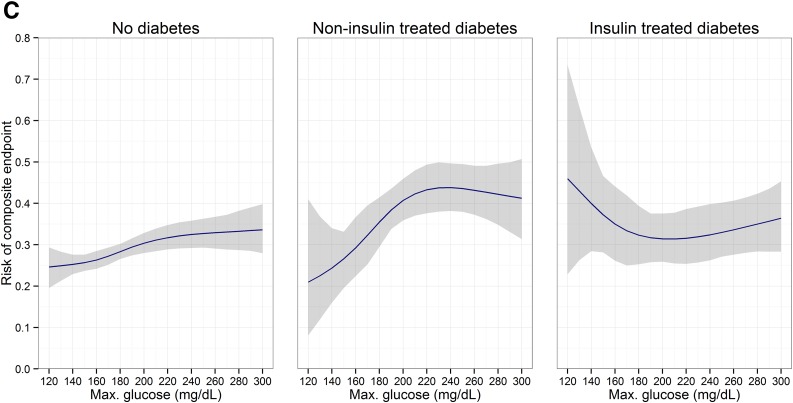
ITDM patients had, on average, higher cost and longer hospital LOS than patients with no diabetes or NITDM. There were 29 (0.9%), 5 (0.9%), and 7 (1.7%) in-hospital deaths, respectively, among patients with no diabetes, NITDM, and ITDM. Furthermore, there were remarkable differences in the relationship of increasing glucose levels with resource use and clinical outcomes among patients with no diabetes, NITDM, and ITDM (Fig. 1). In patients without diabetes, the cost of hospitalization, hospital LOS, and the risk of complications increased with increasing glucose levels. In NITDM patients, cost, hospital LOS, and risk of complications peaked at ∼220 mg/dL, and slightly decreased with further glycemic increases. In contrast, in ITDM patients, cost, hospital LOS, and the risk of complications during the hospital stay were highest at glucose concentrations <180 mg/dL, and decreased with increasing glucose values, reaching a minimum between 180 and 240 mg/dL. Persistent hyperglycemia, which was defined as two or more measurements >180 mg/mL, occurred in 12.3% of patients without diabetes and 45.9% of patients with diabetes. In ITDM patients, an increase in the number of hyperglycemic measurements was associated with reduced cost, number of complications, and hospital LOS, compared with patients with no hyperglycemia. Conversely, in patients with no diabetes and patients with NITDM, such an increase correlated with higher cost, more complications, and prolonged hospital LOS (Supplementary Fig. 1).
Figure 1.
Adjusted association between maximum glucose and outcomes. 95% CIs are shown as the shaded area. A: Total costs. B: Hospital LOS. C: Composite end point of complications. Max., maximum.
HbA1c was significantly higher in ITDM patients than in NITDM patients (7.5 vs. 6.7% [58 vs. 50 mmol/mol]) on average (Table 1). In patients with diabetes, outcomes improved with increasing HbA1c values. However, these improvements were not statistically significant in the multivariable complete case analysis. Relative changes were 0.91 (95% CI 0.80 to 1.04) for the odds of complications, 0.98 (95% CI 0.95 to 1.00) for hospital LOS, and 0.98 (95% CI 0.95 to 1.00) for total costs. Estimates based on multiple imputation were not different from those based on complete case analysis.
Among patients without diabetes, the cost of those with hyperglycemia was, on average, nearly $10,000 higher than the cost of those without hyperglycemia; whereas, among ITDM patients, the cost of those patients with hyperglycemia was on average approximately $15,000 lower (Table 2). The change in cost for patients with NITDM was more modest and not significant. After adjustment for baseline and procedure variables, the change in cost with hyperglycemia was approximately an extra $3,000 in patients without diabetes and a decrease of $6,000 in patients with ITDM. In patients with NITDM, the change in cost with hyperglycemia was positive but not significant. Adjusted changes in hospital LOS associated with hyperglycemia followed a pattern similar to that of cost, with 0.8 additional days (95% CI 0.4 to 1.3) in patients without diabetes, and a decrease of 1.6 days (95% CI −3.7 to 0.4) in ITDM patients, although the latter did not reach statistical significance.
Table 2.
Clinical and economic outcomes associated with postoperative hyperglycemia
| Outcome | No DM |
NITDM |
ITDM |
|||
|---|---|---|---|---|---|---|
| No hyperglycemia | Hyperglycemia | No hyperglycemia | Hyperglycemia | No hyperglycemia | Hyperglycemia | |
| Hospital costs (U.S. $) | ||||||
| Unadjusted mean (95% CI) | 28,987 (27,850 to 30,246) | 38,664 (36,537 to 40,856) | 31,264 (28,134 to 34,744) | 34,835 (31,845 to 38,730) | 55,004 (45,104 to 67,403) | 39,599 (35,072 to 44,371) |
| Adjusted incremental (95% CI)* | Reference | 3,192 (1,972 to 4,456) | Reference | 2,151 (−572 to 5,034) | Reference | −6,225 (−12,886 to −222) |
| Hospital LOS (days) | ||||||
| Unadjusted mean (95% CI) | 8.7 (8.4 to 9.1) | 11.3 (10.7 to 11.9) | 9.7 (8.9 to 10.5) | 10.7 (9.9 to 11.7) | 16.6 (14.0 to 19.9) | 12.4 (11.2 to 13.7) |
| Adjusted incremental (95% CI)* | Reference | 0.8 (0.4 to 1.3) | Reference | 0.6 (−0.2 to 1.5) | Reference | −1.6 (−3.7 to 0.4) |
| Composite clinical end point | ||||||
| Unadjusted mean (95% CI) | 0.262 (0.243 to 0.280) | 0.310 (0.284 to 0.338) | 0.310 (0.241 to 0.378) | 0.406 (0.358 to 0.456) | 0.430 (0.342 to 0.519) | 0.305 (0.254 to 0.365) |
| Adjusted incremental (95% CI)* | Reference | 0.038 (0.010 to 0.067) | Reference | 0.126 (0.050 to 0.204) | Reference | −0.052 (−0.140 to 0.039) |
| Major infections | ||||||
| Unadjusted mean (95% CI) | 0.019 (0.013 to 0.025) | 0.040 (0.030 to 0.051) | 0.023 (0.005 to 0.048) | 0.037 (0.019 to 0.057) | 0.061 (0.019 to 0.112) | 0.020 (0.006 to 0.036) |
| Adjusted incremental (95% CI)** | Reference | 0.016 (0.005 to 0.028) | Reference | 0.012 (−0.021 to 0.040) | Reference | −0.041 (−0.091 to 0.000) |
| Cardiac complications | ||||||
| Unadjusted mean (95% CI) | 0.150 (0.136 to 0.165) | 0.178 (0.157 to 0.199) | 0.161 (0.110 to 0.217) | 0.222 (0.181 to 0.260) | 0.193 (0.124 to 0.270) | 0.164 (0.125 to 0.211) |
| Adjusted incremental (95% CI)** | Reference | 0.016 (−0.010 to 0.042) | Reference | 0.049 (−0.018 to 0.118) | Reference | −0.037 (−0.123 to 0.042) |
| Respiratory complications | ||||||
| Unadjusted mean (95% CI) | 0.133 (0.086 to 0.111) | 0.166 (0.107 to 0.144) | 0.190 (0.133 to 0.250) | 0.232 (0.193 to 0.276) | 0.307 (0.222 to 0.395) | 0.180 (0.138 to 0.227) |
| Adjusted incremental (95% CI)** | Reference | 0.026 (0.000 to 0.053) | Reference | 0.042 (−0.034 to 0.114) | Reference | −0.125 (−0.224 to −0.030) |
DM, diabetes mellitus.
*Adjusted for age, sex, race, BMI, white blood cell count, GFR, hemoglobin, history of heart failure, renal insufficiency, ejection fraction, prior cardiac surgery, history of lung disease, corticosteroids, surgery time, sternotomy performed yes/no, surgery type, procedure, and study site.
**Adjusted for age, sex, and procedure.
In patients without diabetes, after adjustment, hyperglycemia was associated with a 1.6% (95% CI 0.5 to 2.8) increased risk of major infections, a 2.6% (95% CI 0.0 to 5.3) increased risk of respiratory complications, and a trend toward increased cardiac complications (Table 2). In NITDM patients, the risk of such complications associated with hyperglycemia increased as well, but without reaching statistical significance. Conversely, in patients with ITDM, hyperglycemia was associated with a reduced risk of adverse outcomes, particularly with respect to respiratory complications (−12.5% [95% CI −22.4 to −3.0]) and major infections (−4.1% [95% CI −9.1 to 0.0]).
Impact of Glucose Thresholds
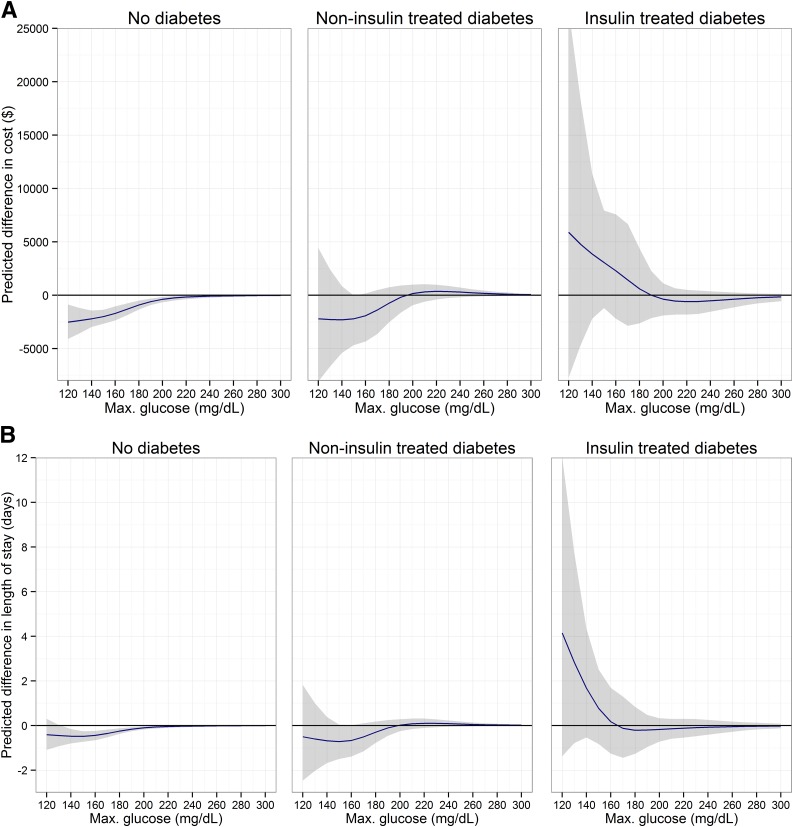
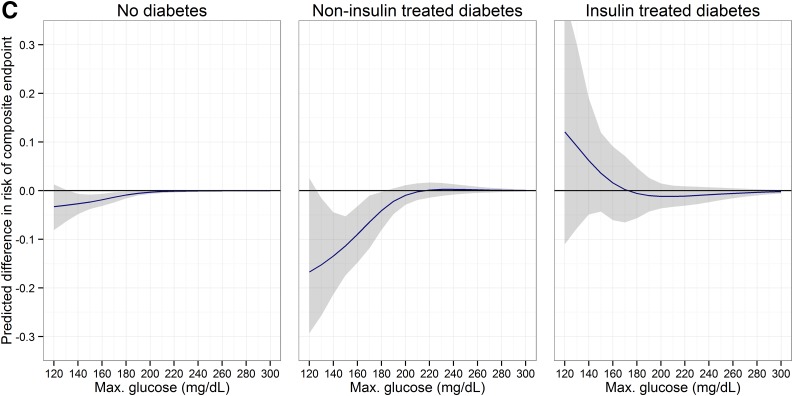
In the analysis of hypothetical scenarios of different glucose thresholds, the outcomes after cardiac surgery predicted in patients without diabetes and patients with NTIDM would improve with lowering postoperative glucose values. The expected benefits comprised an approximate $2,000 cost reduction and a 5–15% reduction in complications. In contrast, in patients with ITDM, thresholds <180 mg/dL would be harmful, although in this range outcomes were uncertain (Fig. 2).
Figure 2.
Potential impact of using different maximum glucose thresholds. 95% CIs are shown as the shaded area. A: Total costs. B: Hospital LOS. C: Composite end point of complications. Max., maximum.
Conclusions
In this study, we demonstrated a significant association of postoperative stress hyperglycemia with economic and clinical outcomes, which varies with the presence of diabetes and its treatment. In patients with diabetes treated with insulin, glucose levels below the generally recommended threshold of 180 mg/dL were associated with an increase in costs, hospital LOS, and complications. Conversely, in patients without and with diabetes not treated with insulin, adverse outcomes were decreased or unchanged. In these patient groups, the association between glucose levels and outcomes was characterized by a dose-response relationship with increasing complication rates at higher glucose levels. The results of this study suggest that targeting a glucose range below the threshold of 180 mg/dL, an approach in line with current guidelines, may be harmful in patients with diabetes treated with insulin.
There are several possible explanations for a differential response among patients with and without ITDM. First, metabolic homeostasis is altered in the cells of patients with diabetes, particularly in ITDM patients (27). The appropriate concentration of glucose for survival under stress-related conditions may be much higher in patients with ITDM to provide energy for wound repair and to recover from major physiological insults (28). The long-term exposure to higher blood glucose levels could result in the downregulation of glucose transporters limiting the influx of glucose across the cellular membrane (29).
Independent of glucose level, higher HbA1c levels decreased the likelihood of complications in patients with diabetes, as assessed with our composite end point. Although it has been shown that higher HbA1c values may decrease the risk of atrial fibrillation (30) and mortality in patients with acute hyperglycemia (31), we found only a nonsignificant trend for decreasing the risk of complications. The protective effect mentioned above and a decreased risk of hypoglycemic events could be an explanation for this phenomenon.
There is accumulating evidence that patients without a previous diagnosis of diabetes face a worse prognosis than patients with diabetes when stress hyperglycemia occurs (15,32). In a large retrospective analysis (33), the association between mortality risk and hyperglycemia in critically ill patients without diabetes was significantly stronger than in patients with diabetes. More recently, an increased risk of postoperative adverse events, such as myocardial infarction, stroke, transient ischemic attack, infectious complication, and renal insufficiency, was linked to hyperglycemia in patients without diabetes, but not in patients with diabetes (34). In contrast with our findings, we found earlier reports (6,35,36) showing that lower glucose levels in patients with diabetes were linked to lower mortality and lower infection rates. However, the mode of insulin delivery in these earlier studies may not be directly comparable to the current glucose management practice. Moreover, in these studies, previous treatment of diabetes was not considered, and results could have been driven by a majority of patients not previously being treated with an insulin regimen. Randomized trials comparing intensive insulin therapy to more moderate approaches have reported mixed results (37). Such persisting discrepancies in the effects of intensive insulin therapy likely result from the high variability existing across centers in the multiple components of glucose control (i.e., monitoring, feeding, and glucose targets) and the changes in standard practice that have occurred over time. These historic changes have modified the difference in insulin protocol between the intensive treatment and the control group in randomized clinical trials. However, in randomized controlled trials that did report a survival benefit from tight glycemic control, patients with diabetes have been the exception, demonstrating no benefit from the intervention (10,38).
Our data expand this body of evidence in two crucial aspects. By considering the type of prior diabetes treatment, we show that the association between glycemic control and outcomes is different for those patients with a history of insulin therapy compared with patients without diabetes and patients with NITDM, after adjustment for baseline clinical variables. In addition, we report the economic burden associated with stress hyperglycemia for patients with and without diabetes. However, our analysis was based on an earlier, observational study (20) whose original aim was to evaluate the incidence of hospital-acquired infections after cardiac surgery, and therefore a number of limitations need to be mentioned. First, HbA1c level was not assessed in all patients. Consequently, patients with undiagnosed diabetes may have been misclassified as not having diabetes, and for these patients diabetes control before hospital admission could not be assessed. Studies (39) have reported a 10% rate of latent diabetes in ICU patients with hyperglycemia. However, such misclassification, if present, would not affect the findings related to the group of patients with diabetes. Second, insulin usage and parenteral nutrition protocols differed among centers, and data on adherence to these protocols were not collected. Therefore, we could not include this information in our analysis. On the other hand, we adjusted for the standard insulin protocol used in the ICUs of the study centers, which reflects to some extent the differences in glucose management among different ICUs. Third, the glucose measurements data, which were only collected during the 48 h after surgery (at 6-h intervals), likely did not capture all the measurements that were performed in the ICU after the surgery. However, such a limited sampling is unlikely to have biased the results differentially for patients with no diabetes, NITDM, and ITDM. Fourth, we used ICD-9 codes to define complications other than infection, which did not allow us to evaluate the sequence of events that occurred, limiting conclusions about causal pathways. Nevertheless, the consistency in the associations of glucose across economic and clinical outcomes further supports our conclusions. Although we adjusted for a broad range of demographic characteristics and illness-related confounders, residual confounding may still have biased our findings to some extent. As is the case for observational studies, our observations only support an association between postoperative glucose levels and clinical outcomes, and not a causal relationship. Likewise, we cannot infer from our results whether previous insulin therapy has a causative role or is merely a marker of severity of illness associated with differential outcomes.
The pandemic of diabetes calls for improved management of hyperglycemia, both outside and inside the hospital. Roughly 20% of cardiac surgery patients have pre-existing diabetes, with a large proportion having more advanced disease requiring insulin therapy. More than 60% of these patients have at least one blood glucose measurement >180 mg/dL, the glycemic threshold recommended by current clinical guidelines (6,40). In the context of findings by others, our results support conducting a randomized controlled trial to evaluate a stratified approach to glucose control based on diabetes history and prior use of insulin.
In summary, our findings suggest that current recommendations, which use a single maximum glucose threshold for the control of stress hyperglycemia after cardiac surgery, may not achieve the intended benefits in all patient subgroups. Such a blanket approach could instead be harmful to patients with more advanced diabetes. Given the substantial clinical and economic benefits that may be attained, patient stratification with indicators of chronic glucose dysregulation should be investigated.
Supplementary Material
Article Information
Acknowledgments. The authors thank the Cardiothoracic Surgical Trials Network (CTSN) for patient enrollment, follow-up, data collection, and adjudication of infection events. (A listing of the members of the CTSN and their institutions is located in Supplementary Data.)
Funding and Duality of Interest. This study was funded by National Heart, Lung, and Blood Institute grant 7U01-HL-088942, the Canadian Institutes of Health Research, and the Institute for Health Technology Studies (InHealth).
G.G. reports grants from InHealth during the conduct of the study. A.E.N. reports receiving grants and nonfinancial support from the National Institutes of Health during the conduct of the study. A.M.G. reports receiving personal fees from Edwards Lifesciences, Medtronic, St. Jude Medical, On-X, Tendyne, AtriCure, and Abbott outside of the submitted work. A.C.G. and A.J.M. report receiving grants from the National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research during the conduct of the study. No other potential conflicts of interest relevant to this article were reported.
The funding sources did not participate in the study design, data collection, analysis and interpretation of the data, or the decision to submit the article for publication.
Author Contributions. G.G. and B.S.F. contributed to the conception and design of the study; performed the statistical analysis; contributed to the acquisition, analysis, and interpretation of the data; wrote the manuscript; contributed to critical revision of the manuscript for important intellectual content; and approved the final version to be published. D.A.D., W.S., K.A.H., A.R., S.W., E.B., A.E.N., D.L.W., A.G., J.N.B., A.M.G., M.L.M., J.K.-M., L.S.G., S.F.H., A.C.G., and P.T.O. contributed to the acquisition, analysis, and interpretation of the data; contributed to critical revision of the manuscript for important intellectual content; and approved the final version to be published. A.J.M. contributed to the conception and design of the study; contributed to the acquisition, analysis, and interpretation of the data; contributed to critical revision of the manuscript for important intellectual content; and approved the final version to be published. G.G. and B.S.F. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-1817/-/DC1.
References
- 1.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011;378:31–40 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2014 [article online], 2014. Available from http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed 1 July 2015
- 3.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet 2009;373:1798–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ata A, Lee J, Bestle SL, Desemone J, Stain SC. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg 2010;145:858–864 [DOI] [PubMed] [Google Scholar]
- 5.Estrada CA, Young JA, Nifong LW, Chitwood WR Jr. Outcomes and perioperative hyperglycemia in patients with or without diabetes mellitus undergoing coronary artery bypass grafting. Ann Thorac Surg 2003;75:1392–1399 [DOI] [PubMed] [Google Scholar]
- 6.Lazar HL, McDonnell M, Chipkin SR, et al. The Society of Thoracic Surgeons practice guideline series: blood glucose management during adult cardiac surgery. Ann Thorac Surg 2009;87:663–669 [DOI] [PubMed] [Google Scholar]
- 7.Breithaupt T. Postoperative glycemic control in cardiac surgery patients. Proc Bayl Univ Med Cent 2010;23:79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz MJ, Harmsen RE, Spronk PE. Clinical review: strict or loose glycemic control in critically ill patients–implementing best available evidence from randomized controlled trials. Crit Care 2010;14:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–1297 [DOI] [PubMed] [Google Scholar]
- 10.Van den Berghe G, Wilmer A, Milants I, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes 2006;55:3151–3159 [DOI] [PubMed] [Google Scholar]
- 11.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359–1367 [DOI] [PubMed] [Google Scholar]
- 12.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006;354:449–461 [DOI] [PubMed] [Google Scholar]
- 13.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 2009;15:353–369 [DOI] [PubMed] [Google Scholar]
- 14.Joint Commission Online, 2015. Available from http://www.jointcommission.org/assets/1/23/jconline_January_28_15.pdf. Accessed 6 February 2015
- 15.Egi M, Bellomo R, Stachowski E, et al. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med 2008;36:2249–2255 [DOI] [PubMed] [Google Scholar]
- 16.Krinsley JS, Egi M, Kiss A, et al. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Crit Care 2013;17:R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krinsley JS, Meyfroidt G, van den Berghe G, Egi M, Bellomo R. The impact of premorbid diabetic status on the relationship between the three domains of glycemic control and mortality in critically ill patients. Curr Opin Clin Nutr Metab Care 2012;15:151–160 [DOI] [PubMed] [Google Scholar]
- 18.Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L. The many faces of diabetes: a disease with increasing heterogeneity. Lancet 2014;383:1084–1094 [DOI] [PubMed] [Google Scholar]
- 19.Plummer MP, Bellomo R, Cousins CE, et al. Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med 2014;40:973–980 [DOI] [PubMed] [Google Scholar]
- 20.Gelijns AC, Moskowitz AJ, Acker MA, et al. Management practices and major infections after cardiac surgery. J Am Coll Cardiol 2014;64:372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greco G, Shi W, Michler RE, et al. Costs associated with health care-associated infections in cardiac surgery. J Am Coll Cardiol 2015;65:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ascione R, Rogers CA, Rajakaruna C, Angelini GD. Inadequate blood glucose control is associated with in-hospital mortality and morbidity in diabetic and nondiabetic patients undergoing cardiac surgery. Circulation 2008;118:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taira DA, Seto TB, Siegrist R, Cosgrove R, Berezin R, Cohen DJ. Comparison of analytic approaches for the economic evaluation of new technologies alongside multicenter clinical trials. Am Heart J 2003;145:452–458 [DOI] [PubMed] [Google Scholar]
- 25.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, Springer, 2001 [Google Scholar]
- 26.Fu AZ, Qiu Y, Radican L, Wells BJ. Health care and productivity costs associated with diabetic patients with macrovascular comorbid conditions. Diabetes Care 2009;32:2187–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature 2014;511:167–176 [DOI] [PubMed] [Google Scholar]
- 28.Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care Med 2013;41:E93–E94 [DOI] [PubMed] [Google Scholar]
- 29.Vanhorebeek I, Van den Berghe G. Diabetes of injury: novel insights. Endocrinol Metab Clin North Am 2006;35:859–872 [x] [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita T, Asai T, Suzuki T, Kambara A, Matsubayashi K. Preoperative hemoglobin A1c predicts atrial fibrillation after off-pump coronary bypass surgery. Eur J Cardiothorac Surg 2012;41:102–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egi M, Bellomo R, Stachowski E, et al. The interaction of chronic and acute glycemia with mortality in critically ill patients with diabetes. Crit Care Med 2011;39:105–111 [DOI] [PubMed] [Google Scholar]
- 32.Sechterberger MK, Bosman RJ, Oudemans-van Straaten HM, et al. The effect of diabetes mellitus on the association between measures of glycaemic control and ICU mortality: a retrospective cohort study. Crit Care 2013;17:R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med 2009;37:3001–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotagal M, Symons RG, Hirsch IB, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg 2015;26:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2003;125:1007–1021 [DOI] [PubMed] [Google Scholar]
- 36.Zerr KJ, Furnary AP, Grunkemeier GL, Bookin S, Kanhere V, Starr A. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg 1997;63:356–361 [DOI] [PubMed] [Google Scholar]
- 37.Mesotten D, Preiser JC, Kosiborod M. Glucose management in critically ill adults and children. Lancet Diabetes Endocrinol 2015;3:723–733 [DOI] [PubMed] [Google Scholar]
- 38.Umpierrez G, Cardona S, Pasquel F, et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG Trial. Diabetes Care 2015;38:1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cely CM, Arora P, Quartin AA, Kett DH, Schein RM. Relationship of baseline glucose homeostasis to hyperglycemia during medical critical illness. Chest 2004;126:879–887 [DOI] [PubMed] [Google Scholar]
- 40.Swanson CM, Potter DJ, Kongable GL, Cook CB. Update on inpatient glycemic control in hospitals in the United States. Endocr Pract 2011;17:853–861 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.