Abstract
OBJECTIVE
Gestational diabetes mellitus (GDM) predicts incident cardiovascular disease (CVD). However, mechanisms linking GDM to CVD beyond intervening incident diabetes are not well understood. We examined the relation of GDM with echocardiographic parameters of left ventricular (LV) structure and function, which are important predictors of future CVD risk.
RESEARCH DESIGN AND METHODS
We studied 609 women (43% black) from the Coronary Artery Risk Development in Young Adults (CARDIA) study who delivered one or more births during follow-up and had echocardiograms in 1990–1991 (mean age 28.8 years) and 2010–2011.
RESULTS
During the 20-year follow-up, 965 births were reported, with GDM developing in 64 women (10.5%). In linear regression models adjusted for sociodemographic factors, BMI, physical activity, parity, smoking, use of oral contraceptives, alcohol intake, family history of coronary heart disease, systolic blood pressure, and lipid levels, women with GDM had impaired longitudinal peak strain (−15.0 vs. −15.7%, P = 0.025), circumferential peak strain (−14.8 vs. −15.6%, P = 0.028), lateral e′ wave velocity (11.0 vs. 11.8 cm/s, P = 0.012), and septal e′ wave velocity (8.6 vs. 9.3 cm/s, P = 0.015) in 2010–2011 and a greater 20-year increase in LV mass indexed to body surface area (14.3 vs. 6.0 g/m2, P = 0.006) compared with women with non-GDM pregnancies. Further adjustment for incident type 2 diabetes after pregnancy did not attenuate these associations.
CONCLUSIONS
Pregnancy complicated by GDM is independently associated with increased LV mass and impaired LV relaxation and systolic function. Implementation of postpartum cardiovascular health interventions in women with a history of GDM may offer an additional opportunity to reduce future CVD risk.
Introduction
Gestational diabetes mellitus (GDM), defined as impaired glucose tolerance of variable severity with first recognition during pregnancy (1), complicates 9.2% of all pregnancies in the U.S. (2). Over the past 20 years, the incidence of GDM has increased by as much as 127% among all ethnic groups (3). Women with a history of GDM are at higher risk for the development of type 2 diabetes (4) and cardiovascular disease (CVD) (5–8) later in life. GDM may be considered as an early expression of metabolic syndrome that is characterized by the overexpression of proinflammatory factors (9), oxidative stress (10), and vascular dysfunction (impaired endothelium-dependent vasodilation and increased vessel stiffness) (11,12). Therefore, whereas gestational diabetes is by definition transient, its actions on the cardiovascular system may influence future CVD risk.
Pathways linking GDM to incident CVD require elucidation. It is known that a state of hyperglycemia results in impaired cardiac autonomic function, especially among women (13). Some studies (14) indicate that left ventricular (LV) dysfunction represents the earliest preclinical manifestation of diabetic cardiomyopathy that may progress to overt heart failure, whereas others (15) show elevated LV mass (LVM) and geometry predicting incident cardiovascular events. Despite this, longitudinal investigations of the relation of GDM with LV structure and function are limited. A prior study (14) among a small group of pregnant women showed mild diastolic dysfunction in women with GDM compared with those without GDM, even 8 weeks postpartum. To date, no population-based longitudinal studies have been conducted to assess the impact of GDM on physiological changes in LV structure and function among women of reproductive age.
Therefore, the aims of this study were to assess the relation of GDM to echocardiographic indices of LV structure and function among women enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) study, and to compare how these parameters change over time among women with a history of GDM with those with non-GDM pregnancies.
Research Design and Methods
Study Population
The CARDIA study is an ongoing multicenter longitudinal observational study conducted at four U.S. communities (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA) to study cardiovascular risk trends in young adults. At year 0 (1985–1986), 5,115 healthy adults were recruited from the general population to be balanced on sex, race (white or black), age (18–24 or 25–30 years), and education (≤12, >12 years). Since then, seven follow-up examinations have occurred at years 2, 5, 7, 10, 15, 20, and 25 with 72% of the surviving cohort attending the year 25 examination. Data collection and follow-up protocols were approved by the institutional review boards of each field center with all participants providing written informed consent. Details of the study design and methods are described elsewhere (16).
Sample Selection
Participants were eligible for inclusion in this analytic sample if they had one or more births during follow-up. Of the 2,787 women enrolled at baseline, 1,362 had one or more post–year 0 births; among them, 1,339 had births not preceded by diabetes. Because echocardiograms were first performed at year 5, we considered this time to be the baseline. Among these parous women, 910 had one or more births after year 5, of whom 672 underwent echocardiography at both year 5 and year 25. We further excluded women who were pregnant during echocardiogram readings (n = 42), who reported ever having a history of GDM before or at year 5 (n = 16), and who reported a physician diagnosis of valvular heart disease at baseline (n = 5), resulting in an analytic sample of 609 women (Supplementary Fig. 1). Women who did not have echocardiograms at both year 5 and year 25 were more likely to be of black race, younger, and current smokers and to have fewer years of education at baseline.
Pregnancy and GDM Status
At each CARDIA study clinic visit, women were asked whether they were currently pregnant or breastfeeding, the number and outcomes of pregnancies occurring since the previous visit (i.e., abortions, miscarriages, stillbirths, or live births), as well as delivery dates and lengths of gestation. Pregnancies ending in miscarriage or abortions, and/or those of <20 weeks of gestation were classified as pregnancy losses. Live births were defined as delivery of a live infant of ≥20 weeks of gestation that occurred between year 5 and year 25. Women were also asked about the occurrence of diabetes during pregnancy. A positive response to this question in the absence of overt diabetes before conception defined GDM. Self-reported GDM was validated among a subsample of 165 women whose glucose tolerance test results were abstracted from their prenatal records to determine whether blood glucose level during pregnancy was elevated to levels meeting GDM diagnostic criteria (1). It was found that the sensitivity for self-reported GDM was 100%, whereas the specificity was 92% (17).
Echocardiographic Methods
Echocardiography was performed in all participants at follow-up years 5 and 25 using standardized protocols across all field centers. At year 5, participants underwent echocardiography using an ACUSON cardiac ultrasound system (Siemens) with images recorded on videotape and read at the University of California, Irvine, reading center (18). At year 25, echocardiography was performed using an Artida cardiac ultrasound scanner (Toshiba Medical Systems, Otawara, Japan) by trained sonographers, with images digitized and read at the Johns Hopkins University reading center (19).
In years 5 and 25, LVM was calculated using the Devereux formula (20). Measurements of LV dimensions (internal diameter in diastole, septal and posterior wall thicknesses in diastole) were acquired from 2-dimensional (2D)-guided M-mode echocardiograms obtained from optimized parasternal short-axis views. LVM and LV end-systolic volume (LVESV) were then indexed to body surface area. Stroke volume was calculated as the difference between LVESV and LV end-diastolic volume (LVEDV), whereas LV ejection fraction (LVEF) was calculated as the ratio of stroke volume to LVEDV and converted to a percentage by multiplying by 100. LVM-to-volume ratio (LVMVR) was calculated by dividing LVM by LVEDV. Cardiac output was determined as the product of stroke volume and heart rate. Relative wall thickness (RWT) was calculated by dividing the sum of the posterior wall and interventricular septal thickness by the internal LV diameter. Finally, the E/A ratio was calculated as the ratio of mitral peak early (E) to late (A) diastolic filling velocity. Peak early diastolic mitral annular velocity (e′) was calculated from the average of the septal and lateral mitral annular velocities. The 20-year change in these echocardiographic parameters was calculated as the difference between measures undertaken at year 25 and those obtained at year 5. To determine the agreement between repeated echocardiographic parameters, a subset of year 5 echocardiographic measures were reread at the Johns Hopkins University reading center at year 25, and, generally, a good agreement, as determined by Bland-Altman plots, was found (21).
At year 25, 2D speckle-tracking echocardiography was also performed. 2D speckle-tracking imaging for myocardial strain was analyzed on a 16-segment basis for the LV midwall layer using Wall Motion 2D Tracking software (UltraExtend version 2.7; Toshiba Medical Systems, Otawara, Japan) (19). Strain was calculated as the change in segment length relative to its end-diastolic length from peak systolic values. Four-chamber longitudinal peak strain (Ell) was assessed from four-chamber views, whereas circumferential peak strain (Ecc) was measured from the parasternal short-axis view at the midventricular level (19). More negative values of strain indicate greater shortening or better function.
Covariates
Participants were asked to fast for at least 12 h prior to each clinic visit, and to avoid smoking and heavy physical activity for the preceding 2 h. Age, race, education, family history of coronary heart disease (CHD) and diabetes, use of lipid-lowering and antihypertensive medications, and use of oral contraceptives were all self-reported. Body weight was measured by trained and certified technicians to the nearest 0.2 kg using a calibrated scale with participants wearing light clothing. Height (without shoes) was measured to the nearest 0.5 cm using a vertical ruler. BMI was calculated by dividing weight in kilograms by height in meters squared. Marijuana use in the previous 30 days and smoking status were self-reported. Blood pressure was measured using a random-zero sphygmomanometer at year 5 and an Omron aneroid device at year 25, with participants seated and after 5 min of rest. The average of the second and third consecutive measurements was used for analysis. Physical activity was assessed using a modified version of the Minnesota Leisure Time Physical Activity Questionnaire, with total scores representing total moderate-to-vigorous activity expressed in exercise units. Alcoholic beverage (beer, wine, and liquor) consumption was assessed using an interviewer-administered questionnaire, with current drinkers defined as participants who consumed any alcoholic beverages in the past year, whereas those who did not were classified as nondrinkers. Plasma total cholesterol and HDL cholesterol levels were measured enzymatically by Northwest Lipid Research Clinic Laboratory. Diabetes was defined by one or more of the following: fasting plasma glucose levels ≥126 mg/dL, oral glucose tolerance test results ≥200 mg/dL, hemoglobin A1C level ≥6.5%, or self-reported use of diabetes medications (insulin or oral hypoglycemic agents).
Statistical Analysis
Descriptive statistics according to GDM status were created for all study variables at year 5, with comparisons undertaken using a t test for continuous variables and a χ2 test for categorical variables. Bivariate Pearson correlation coefficients were computed for year 5 and year 25 echocardiography parameters (LVM, LVM index, LVESV, LVESV index [LVESVI], LVEF, LVMVR, RWT, E/A ratio, cardiac output, and stroke volume). General linear regression models were used to assess the association of GDM with each echocardiography parameter (chosen a priori) at year 25 and the 20-year change from year 5 to year 25. Two models with progressive degrees of adjustments were used for all outcomes of interest. Model 1 was adjusted for the following year 5 measures: age, race, education, clinic site, BMI, physical activity, parity, cigarette smoking, oral contraceptive use, marijuana use, alcohol use, family history of CHD, systolic blood pressure, and total cholesterol level. Diabetes status is considered a mediator of the association of GDM and CVD. However, as changes in LV structure and function may precede the onset of overt diabetes among women with GDM, we conducted further analyses adjusting for incident diabetes (occurring any time after year 5 up until year 25) to assess the temporality of the association (Model 2). The observed associations were also stratified by incident GDM and incident diabetes during follow-up. To account for the influence of metabolic syndrome on the observed results, we performed sensitivity analyses, adjusting for metabolic syndrome occurring after pregnancy during follow-up. Additionally, to understand the underlying mechanism linking GDM to LV structure and function, we used a piecewise linear mixed model to assess the mean rate of change in fasting glucose levels before and after the first GDM or non-GDM pregnancy (index pregnancy). All statistical tests were two-sided and were performed at the 0.05 level of significance using SAS software, version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Description of Study Participants
Descriptive statistics for the cohort are presented in Table 1. Approximately 43% of the cohort reported black race with a mean age of 28.8 ± 3.5 years at year 5 (1990–1991). There were no statistically significant differences in sociodemographic characteristics, BMI, parity, systolic blood pressure, total cholesterol level, physical activity, smoking status, oral contraceptive use, marijuana use, and family history of CHD at either year 5 or year 25 (Table 1) among women in whom GDM developed and among those who had non-GDM pregnancies. By the end of year 25 (2010–2011), 965 live births were recorded among the analytic sample of 609 women, with GDM developing in 64 of them. Of the women who reported the development of GDM during follow-up, 58 had GDM during one pregnancy, 5 during two different pregnancies, and 1 during three different pregnancies. There were no differences between women with a history of GDM and those without GDM in either the age at first birth after baseline (31.7 vs. 32.0 years, P = 0.66), age at last birth after baseline (35.0 vs. 35.6 years, P = 0.27), or time from last birth to the year 25 examination (14.2 vs. 13.8 years, P = 0.47). There was no significant difference in fasting glucose levels at year 0 (5 years before the baseline for the current study) (Table 1) or the mean annual rate of change in fasting glucose levels before the index pregnancy for women with and without a history of GDM (0.57 vs. 0.58 mg/dL, P = 0.97). However, after the index pregnancy, women with GDM were observed to have a higher rate of change in glucose levels (1.24 vs. 0.41 mg/dL, P = 0.001) (Supplementary Fig. 2). Overall, the proportion of women in whom diabetes developed by year 25 was 8.5%. As expected, the proportion of women with a history of GDM in whom diabetes later developed was almost fivefold higher than women who had non-GDM pregnancies (29.7 vs. 6.1%, P < 0.001).
Table 1.
Characteristics at years 5 and 25 among women in whom GDM later developed or did not during follow-up
| Year 5 |
Year 25 |
|||||
|---|---|---|---|---|---|---|
| GDM (n = 64) | No GDM (n = 545) | P value | GDM (n = 64) | No GDM (n = 545) | P value | |
| Age, years | 28.5 (3.9) | 28.8 (3.5) | 0.599 | 48.6 (3.8) | 48.9 (3.6) | 0.522 |
| Race | 0.350 | 0.350 | ||||
| White | 51.6 | 58.2 | 51.6 | 58.2 | ||
| Black | 48.4 | 41.8 | 48.4 | 41.8 | ||
| High school education or less | 25.0 | 19.6 | 0.324 | 14.1 | 15.0 | 0.835 |
| Parity | 0.896 | 0.781 | ||||
| None | 57.8 | 58.7 | 0.0 | 0.0 | ||
| One | 28.1 | 24.4 | 20.3 | 24.2 | ||
| Two | 9.4 | 11.4 | 45.3 | 43.7 | ||
| Three or more | 4.7 | 5.5 | 34.4 | 32.1 | ||
| Current oral contraceptive use | 31.3 | 39.8 | 0.223 | 29.7 | 20.4 | 0.106 |
| Current cigarette use | 28.1 | 21.1 | 0.203 | 14.1 | 13.8 | 0.947 |
| Current marijuana use | 15.6 | 11.9 | 0.420 | 7.8 | 8.6 | 0.826 |
| Current alcohol use | 84.4 | 85.1 | 0.854 | 70.3 | 82.4 | 0.027 |
| Heart rate, bpm | 67.0 (9.0) | 67.7 (9.9) | 0.618 | 67.2 (10.9) | 66.5 (10.4) | 0.641 |
| Systolic blood pressure, mmHg | 103.3 (10.0) | 103.0 (9.5) | 0.802 | 114.1 (13.1) | 114.2 (14.9) | 0.966 |
| Blood pressure medication use | 1.6 | 0.4 | 0.284 | 23.4 | 18.3 | 0.325 |
| Total cholesterol, mg/dL | 176.1 (37.5) | 177.8 (30.5) | 0.731 | 191.7 (43.9) | 194.3 (34.6) | 0.650 |
| HDL cholesterol, mg/dL | 55.9 (13.3) | 59.0 (13.8) | 0.099 | 61.0 (21.5) | 64.2 (19.4) | 0.221 |
| LDL cholesterol, mg/dL | 105.3 (34.8) | 105.4 (28.6) | 0.981 | 109.4 (39.0) | 111.0 (30.4) | 0.740 |
| Triglycerides, mg/dL* | 72.3 (41.3) | 64.8 (33.7) | 0.215 | 106.3 (52.5) | 96.3 (66.7) | 0.074 |
| Lipid-lowering medication use | 0.0 | 0.0 | 14.1 | 9.2 | 0.211 | |
| Body surface area | 1.7 (0.2) | 1.7 (0.2) | 0.655 | 1.9 (0.2) | 1.9 (0.2) | 0.963 |
| BMI, kg/m2 | 26.0 (6.8) | 24.7 (5.7) | 0.171 | 30.0 (7.3) | 29.3 (7.6) | 0.471 |
| Waist circumference, cm | 77.5 (14.5) | 75.2 (11.3) | 0.227 | 90.6 (16.1) | 88.3 (15.6) | 0.270 |
| Physical activity, exercise units | 290 (242) | 318 (258) | 0.421 | 303.2 (275) | 299.8 (254) | 0.924 |
| High sensitive C-reactive protein, μg/mL† | 3.5 (4.3) | 3.5 (7.2) | 0.980 | 3.5 (4.7) | 3.1 (5.0) | 0.054 |
| Insulin, μU/mL | 8.5 (3.8) | 8.0 (3.3) | 0.250 | 13.6 (32.3) | 9.7 (7.1) | 0.341 |
| Fasting glucose, mg/dL‡ | 81.3 (9.6) | 79.0 (7.4) | 0.063 | 104.6 (34.8) | 92.7 (20.3) | 0.009 |
| 2-h oral glucose, mg/dL§ | 101.1 (21.6) | 97.5 (36.9) | 0.455 | 132.3 (49.0) | 104.2 (40.2) | 0.001 |
| Glycated hemoglobin, % (mmol/mol) | 6.0 (42) | 5.5 (37) | 0.001 | |||
| Diabetes | 0.0 | 0.0 | 29.7 | 6.1 | 0.001 | |
| Family history of diabetes | 26.6 | 14.1 | 0.016 | 60.3 | 40.4 | 0.003 |
| Family history of CHD | 12.5 | 15.2 | 0.711 | 31.7 | 31.4 | 0.960 |
Values are reported as the mean (SD) for continuous variables and % for categorical variables, unless otherwise indicated.
*The P value from logged analyses.
†Values measured at year 7 were substituted for year 5.
‡Values measured at year 0 were substituted for year 5.
§First measured at CARDIA study year 10.
With regard to LV parameters, there were no statistically significant differences in echocardiographic parameters measured at year 5 between women in whom GDM developed during follow-up and those who did not; an exception was LVMVR, which was lower in women with GDM (Supplementary Table 1).
Echocardiographic Parameters at Year 25
At year 25, women who reported a history of GDM were observed to have a higher LVM index than women who had only non-GDM pregnancies (Table 2).
Table 2.
Echocardiography measures (unadjusted) among women by GDM status at year 25 and 20-year change
| Year 25 |
20-year change |
|||||
|---|---|---|---|---|---|---|
| GDM (n = 64) | No GDM (n = 545) | P value | GDM (n = 64) | No GDM (n = 545) | P value | |
| LV structure | ||||||
| LVM (g) | 154.7 (54.0) | 143.1 (40.8) | 0.108 | 36.4 (49.3) | 18.8 (40.7) | 0.010 |
| LVM index (g/m2) | 82.6 (22.5) | 77.2 (18.9) | 0.039 | 14.5 (23.2) | 4.9 (24.7) | 0.001 |
| LVM/volume ratio | 1.6 (0.6) | 1.5 (0.4) | 0.215 | 0.5 (0.6) | 0.3 (0.5) | 0.071 |
| LVESVI (mL/m2) | 20.0 (7.2) | 19.0 (6.6) | 0.267 | −4.4 (6.1) | −4.0 (7.8) | 0.820 |
| RWT | 0.35 (0.08) | 0.34 (0.07) | 0.355 | 0.03 (0.1) | 0.01 (0.1) | 0.037 |
| LV systolic function | ||||||
| Stroke volume (mL) | 63.3 (15.8) | 60.7 (14.1) | 0.186 | −9.2 (19.8) | −8.9 (20.4) | 0.960 |
| Cardiac output (L/min) | 4.3 (1.3) | 4.0 (1.0) | 0.126 | −0.6 (1.5) | −0.7 (1.7) | 0.813 |
| LVEF (%) | 62.3 (7.6) | 61.6 (6.9) | 0.465 | −3.3 (9.6) | −1.8 (8.7) | 0.435 |
| LV diastolic function | ||||||
| E/A ratio | 1.29 (0.4) | 1.34 (0.4) | 0.389 | 1.33 (0.4) | 1.28 (0.4) | 0.300 |
Values are reported as the mean (SD), unless otherwise indicated.
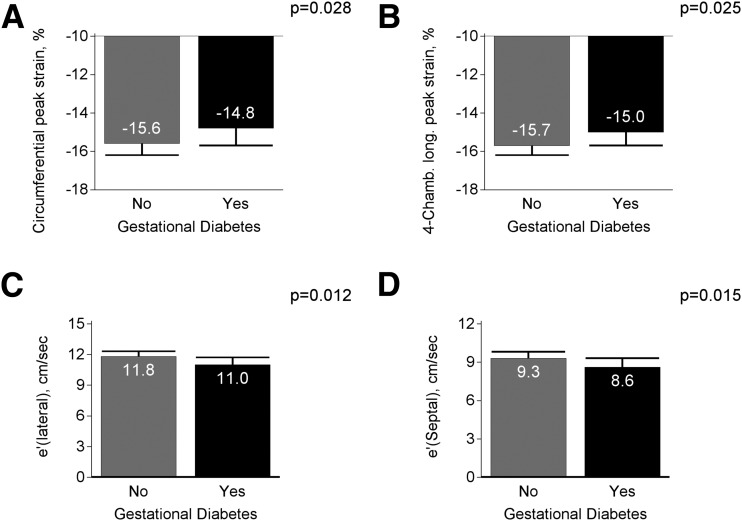
In adjusted analyses assessing the cross-sectional association of GDM with echocardiographic parameters measured only at year 25, women with GDM had impaired Ell, Ecc, and lateral and septal e′ wave velocities (Fig. 1).There were no significant differences by GDM status with regard to peak radial strain, E/e′ ratio, and left atrial volume indexed to body surface area (Supplementary Table 2). Differences between groups with regard to Ecc were found to be explained by incident diabetes after pregnancy. However, the association of GDM with Ell and mitral annular velocities persisted with further adjustment for incident diabetes after pregnancy.
Figure 1.
Echocardiography parameters measured at year 25 among women by GDM status. Adjusted for year 25 covariates (age, race, education, center, BMI, physical activity, parity, cigarette smoking, use of oral contraceptives, marijuana use, alcohol intake, family history of CHD, systolic blood pressure, and total cholesterol level).
Twenty-Year Changes in Echocardiographic Parameters
During 20 years of follow-up, there were significant changes in all echocardiographic parameters among participants, regardless of GDM status between year 5 and year 25 (P < 0.001). Measures of LV structure (with the exception of LVESVI) and LV diastolic function increased during this period, whereas indices for LV systolic function decreased (Table 2). Moreover, echocardiographic parameters measured at year 5 were found to correlate positively with parameters measured 20 years later, with correlation coefficients ranging from 0.01 to 0.4. In multivariable-adjusted models, women with GDM were observed to have significantly higher changes in LVM (14.8 g [95% CI 3.3, 26.3]) and LVM index (8.27 g/m2 [95% CI 2.4, 14.1]) (Table 3). Despite diabetes being adversely associated with parameters of LV structure and function (Supplementary Tables 3 and 4), further adjustment for incident diabetes after pregnancy or 20-year changes in weight and systolic blood pressure did not attenuate the elevated changes in LVM and LVM index (Table 3). In analyses stratified by GDM and incident diabetes status, women who had incident GDM without diabetes during follow-up had significantly elevated parameters of LV structure and function, with those women having both conditions experiencing more adverse outcomes (Supplementary Table 5).
Table 3.
Mean differences in adjusted 20-year change in echocardiographic measures of LV structure and function among women with GDM compared with women without GDM
| Model 1 |
Model 2 |
Model 3 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) |
P | SE | R2 | β (95% CI) | P | SE | R2 | β (95% CI) | P | SE | R2 | |
| LV structure | ||||||||||||
| LVM (g) | 14.79 (3.32, 26.26) | 0.012 | 5.84 | 0.112 | 14.67 (2.74, 26.59) | 0.016 | 6.07 | 0.112 | 15.53 (4.24, 26.82) | 0.007 | 5.75 | 0.196 |
| LVM index (g/m2) | 8.27 (2.40, 14.14) | 0.006 | 2.99 | 0.083 | 8.30 (2.20, 14.40) | 0.008 | 3.11 | 0.083 | 8.19 (2.31, 14.06) | 0.006 | 2.99 | 0.136 |
| LVM/volume ratio | 0.13 (−0.11, 0.37) | 0.279 | 0.12 | 0.099 | 0.12 (−0.14, 0.38) | 0.375 | 0.13 | 0.099 | 0.08 (−0.18, 0.34) | 0.531 | 0.13 | 0.128 |
| LVESVI (mL/m2) | 0.07 (−3.65, 3.78) | 0.971 | 1.89 | 0.098 | 0.14 (−3.85, 4.12) | 0.944 | 2.02 | 0.098 | −0.03 (−3.93, 3.87) | 0.987 | 1.98 | 0.087 |
| RWT | 0.018 (−0.005, 0.041) | 0.118 | 0.01 | 0.079 | 0.012 (−0.011, 0.036) | 0.311 | 0.01 | 0.079 | 0.012 (−0.011, 0.036) | 0.307 | 0.01 | 0.101 |
| LV systolic function | ||||||||||||
| Stroke volume (mL) | −0.86 (−10.43, 8.71) | 0.860 | 4.86 | 0.098 | 0.09 (−10.03, 10.21) | 0.986 | 5.14 | 0.100 | −0.15 (−10.38, 10.08) | 0.977 | 5.19 | 0.103 |
| Cardiac output (L/min) | −0.13 (−0.94, 0.69) | 0.761 | 0.41 | 0.082 | −0.17 (−1.04, 0.71) | 0.705 | 0.44 | 0.082 | −0.22 (−1.10, 0.67) | 0.629 | 0.45 | 0.087 |
| LVEF (%) | −2.92 (−7.11, 1.26) | 0.170 | 2.13 | 0.053 | −3.14 (−7.57, 1.30) | 0.165 | 2.25 | 0.053 | −2.70 (−7.10, 1.70) | 0.228 | 2.23 | 0.059 |
| LV diastolic function | ||||||||||||
| E/A ratio | −0.026 (−0.128, 0.077) | 0.624 | 0.05 | 0.157 | −0.017 (−0.122, 0.089) | 0.758 | 0.05 | 0.157 | −0.024 (−0.128, 0.081) | 0.658 | 0.05 | 0.181 |
Model 1, adjusted for year 5 covariates (age, race, education, center, BMI, physical activity, parity, cigarette smoking, oral contraceptives, marijuana, alcohol intake, family history of CHD, systolic blood pressure, and total cholesterol); Model 2, Model 1 plus diabetes during follow-up; Model 3, Model 2 plus change in weight and systolic blood pressure during follow-up.
In sensitivity analyses, we observed that accounting for metabolic syndrome accompanying pregnancy during follow-up did not significantly attenuate the associations of GDM with LV dysfunction reported above (Supplementary Table 3).
Conclusions
In this population-based prospective study of women with a history of pregnancy, GDM was independently associated with greater LVM, impaired LV relaxation, and impaired longitudinal and circumferential strain indicative of lower LV systolic function. To our knowledge, this is the first long-term longitudinal study to comprehensively assess the association of GDM with LV structure and function among women of reproductive age.
Normal pregnancy is associated with several hemodynamic and physiological alterations in the cardiovascular system (22,23). LVM, cardiac output, LVESV, LV posterior wall diameter, and end-diastolic diameters increase throughout pregnancy above prepregnancy values (22–24). The increases in volume overload coupled with alterations in heart rate, preload, and contractility results in physiological LV remodeling, as well as a decrease in LV systolic and diastolic functions (24). Among healthy women, these changes in cardiovascular functional parameters return to prepregnancy levels after delivery when vascular volumes decrease (22,25). In women with pregnancy complications, however, cardiovascular functional parameters remain altered and are thought to persist several years after childbirth, and later manifest in overt CVD (26,27). Despite this, investigations into the effect of GDM on LV structure and function are sparse.
Zakovicova et al. (27) previously reported LV dysfunction among 31 women with GDM compared with 34 healthy control subjects. However, these differences were explained by metabolic changes found in women with gestational diabetes. In a small study that examined diastolic function during pregnancy and 8 weeks postpartum in 13 women with GDM and 13 control subjects, Freire et al. (14), observed a mild degree of diastolic abnormality among women with GDM. Women with GDM had a significantly higher atrial contraction wave (A wave) and lower E/A ratio, both during the third trimester of pregnancy and 8 weeks postpartum (14). Furthermore, no statistically significant differences among groups were identified with regard to other echocardiography parameters, namely LVM, LV posterior wall thickness, LVEF, and left atrial diameter (14). Contrary to the report by Freire et al. (14), we found in the current study that GDM was positively associated with changes in LVM and LVM index during 20 years of follow-up. Potential reasons for the discrepancy in results from the current study and those from the study by Freire et al. (14) include methodological differences pertaining to study design, sampling procedure, referral bias, and inadequate statistical adjustments for important confounders such as BMI, blood pressure, and other CVD risk factors. Further, we speculate that low statistical power due to the relatively smaller sample size of previous studies or potential sample selection bias in the study by Freire et al. (14) may have led to the inability to detect significant differences in LVM between groups, as was seen in this relatively large population-based cohort with a 20-year duration.
Although previous retrospective studies (28,29) reported no association between gestational diabetes and macrovascular dysfunction, recent prospective epidemiologic evidence points to a positive association between GDM and incident CVD (5,30), with the risk elevated for women with a family history of type 2 diabetes (6). In a retrospective population-based cohort study of 435,696 women aged 20 to 49 years, Retnakaran and Shah (30) reported a 66% higher risk of incident CVD after pregnancy among women with GDM. Similarly, Shah et al. (5), in a related study using data from the same cohort, observed that the elevated CVD risk among women with GDM was attenuated when type 2 diabetes status was taken into account, suggesting that the elevated CVD risk among women with GDM may be attributable to the subsequent development of diabetes. However, other evidence (7,8) linking GDM with subclinical CVD challenges this idea, and results from our study lend support to this, because differences among groups in LVM, LV relaxation, and longitudinal strain remained statistically significant after adjusting for incident diabetes. Taken together, these findings suggest that GDM may be an independent risk factor for LV abnormalities through other mechanisms apart from those involving the development of postpartum type 2 diabetes. Both mild and severe hyperglycemia during pregnancy adversely impact the cardiovascular system and influence long-term changes in inflammatory markers, vessel stiffness, and cardiometabolic profiles of women with a history of GDM (27,30–33). At 36 weeks of pregnancy, women with GDM were observed to have higher interventricular septal, posterior wall and RWT of the left ventricle (27), which points to an acute effect of GDM on cardiac remodeling long before the onset of diabetes.
Explanations for the elevated LVM, impaired LV relaxation, and lower LV longitudinal shortening found in women with GDM beyond the incidence of diabetes are not well established. Women with GDM are known to have higher prepregnancy BMI and to gain more weight during their first trimester of pregnancy than women without hyperglycemia during pregnancy (34). This accelerated weight gain, coupled with prepregnancy obesity, may directly influence the development and progression of LV remodeling in women with GDM by increasing hemodynamic volume overload, leading to LV dilation and elevated LVM (19). Several years after the index pregnancy, cardiometabolic anomalies (32,33), increased insulin resistance (35), and elevated inflammatory marker levels (31) develop in most women with a history of GDM. The persistent hyperglycemia and elevated inflammatory marker levels impair endothelium-dependent vasodilation that damages vascular walls (36). Additionally, hyperglycemia has been associated with poor cardiac wall motion (37). In experimental studies (38), changes in glucose metabolism in rats were linked to profibrotic and prohypertrophic mechanisms that are thought to influence the early remodeling of cardiac parenchyma. Protein glycosylation caused by hyperglycemia may also lead to small increases in LV remodeling (39). It is possible that the slightly elevated levels of fasting glucose prior to GDM that were observed in the current study may have contributed to changes in LV structure and function, with the markedly elevated glucose levels associated with GDM compounding this effect.
Others have also suggested that elevated plasma insulin concentrations may have direct growth-stimulating effects on the myocardium (39), and may alter vascular compliance, increase renal sodium absorption, and elevate blood pressure (13). However, some emerging evidence disputes the role of hyperinsulinemia on LV remodeling (40). Alternatively, the atherogenic dyslipidemia, hypertension, and hemostatic abnormalities that occur during impaired glucose tolerance may increase oxidative stress, alter energy metabolism, accelerate fibrosis, and enhance the accumulation of advanced glycation end products in the myocardium, which may adversely impact LVM (39).
This study has several notable strengths, including the use of a large population-based biracial sample of women of reproductive age with or without a history of GDM during pregnancy who had extensive assessment of CVD risk factors and repeated echocardiography measurements coupled with a sufficiently long follow-up with minimal attrition during 20 years of follow-up.
On the other hand, several limitations ought to be acknowledged in interpreting our results. First, GDM was self-reported. However, it was validated and shown to be highly reliable, with a sensitivity and specificity of 100% and 92%, respectively (17). Second, diabetes status at year 5 was determined by self-reported use of diabetes medications, which could have led to some misclassification. Because women were excluded if they had births after year 5 that were preceded by diabetes (defined using fasting and/or 2-h oral glucose tests), the influence of such misclassification on our results, if present, may be minimal. Third, values for glycated hemoglobin and 2-h oral glucose tests were not available at baseline, and information on the treatment of hyperglycemia during pregnancy was not ascertained. Finally, echocardiograms were performed 20 years apart using different equipment, sonographers, and reading centers, which may affect the comparability of the indices of LV structure and function. For example, the harmonic imaging used at year 25 is known to slightly overestimate LVM measurements (21). However, a reliability study (21) conducted in this cohort showed good agreement between repeated echocardiographic measures.
In summary, this study demonstrates impaired LV relaxation, lower LV systolic function, and a significantly higher progression of LVM among women in whom GDM developed during a 20-year period. These associations, which are largely independent of incident diabetes, highlight the possibility that GDM identifies a subpopulation of women who are at higher risk of CVD during midlife. With the knowledge that LVM predicts cardiovascular events and mortality, coupled with the rising burden of GDM in the U.S., these findings are of significant clinical and public health importance. Therefore, the early detection of GDM affords clinicians an opportunity to prevent future development of cardiovascular events in a relatively young population at high risk by implementing effective pregnancy and postpartum interventions, such as risk awareness education and implementation of healthy lifestyles, as well as pharmacological interventions.
Supplementary Material
Article Information
Acknowledgments. The authors thank the other investigators, the staff, and the participants of the CARDIA study for their contributions.
Funding. The CARDIA study is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI); the Intramural Research Program of the National Institute on Aging (NIA); and an intra-agency agreement between the NIA and NHLBI (AG0005). The analyses were supported by grants K01-DK-059944 (E.P.G., Principal Investigator) and R01-DK-090047 (E.P.G., Principal Investigator) from the National Institute of Diabetes and Digestive and Kidney Diseases. D.A. was supported by NHLBI training grant T32-HL-007779.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.A. researched the data, conducted the data analysis, and wrote the manuscript. P.J.S., E.P.G., D.R.J., and J.A.L. collected the data, contributed to the writing and interpretation of results, and reviewed and edited the manuscript. S.H.K., C.C.N., I.A.E., H.K.W., D.C.G., I.A.K., and S.S.G. reviewed and revised the manuscript critically for important intellectual content. D.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-1759/-/DC1.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36(Suppl. 1):S67–S74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007-2010. Prev Chronic Dis 2014;11:E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007;30(Suppl. 2):S141–S146 [DOI] [PubMed] [Google Scholar]
- 4.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25:1862–1868 [DOI] [PubMed] [Google Scholar]
- 5.Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care 2008;31:1668–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr DB, Utzschneider KM, Hull RL, et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care 2006;29:2078–2083 [DOI] [PubMed] [Google Scholar]
- 7.Li JW, He SY, Liu P, Luo L, Zhao L, Xiao YB. Association of gestational diabetes mellitus (GDM) with subclinical atherosclerosis: a systemic review and meta-analysis. BMC Cardiovasc Disord 2014;14:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunderson EP, Chiang V, Pletcher MJ, et al. History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: the Coronary Artery Risk Development in Young Adults study. J Am Heart Assoc 2014;3:e000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson AC, Carpenter MW. Inflammatory mediators in gestational diabetes mellitus. Obstet Gynecol Clin North Am 2007;34:213–224, viii [DOI] [PubMed] [Google Scholar]
- 10.Lappas M, Hiden U, Desoye G, Froehlich J, Hauguel-de Mouzon S, Jawerbaum A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signal 2011;15:3061–3100 [DOI] [PubMed] [Google Scholar]
- 11.Carpenter MW. Gestational diabetes, pregnancy hypertension, and late vascular disease. Diabetes Care 2007;30(Suppl. 2):S246–S250 [DOI] [PubMed] [Google Scholar]
- 12.Heitritter SM, Solomon CG, Mitchell GF, Skali-Ounis N, Seely EW. Subclinical inflammation and vascular dysfunction in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab 2005;90:3983–3988 [DOI] [PubMed] [Google Scholar]
- 13.Rutter MK, Parise H, Benjamin EJ, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation 2003;107:448–454 [DOI] [PubMed] [Google Scholar]
- 14.Freire CM, Nunes MdoC, Barbosa MM, et al. Gestational diabetes: a condition of early diastolic abnormalities in young women. J Am Soc Echocardiogr 2006;19:1251–1256 [DOI] [PubMed] [Google Scholar]
- 15.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008;52:2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–1116 [DOI] [PubMed] [Google Scholar]
- 17.Gunderson EP, Lewis CE, Tsai AL, et al. A 20-year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes 2007;56:2990–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gidding SS, Xie X, Liu K, Manolio T, Flack JM, Gardin JM. Cardiac function in smokers and nonsmokers: the CARDIA study. The Coronary Artery Risk Development in Young Adults Study. J Am Coll Cardiol 1995;26:211–216 [DOI] [PubMed] [Google Scholar]
- 19.Kishi S, Armstrong AC, Gidding SS, et al. Association of obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling: the CARDIA study (Coronary Artery Risk Development in Young Adults). JACC Heart Fail 2014;2:500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57:450–458 [DOI] [PubMed] [Google Scholar]
- 21.Gidding SS, Liu K, Colangelo LA, et al. Longitudinal determinants of left ventricular mass and geometry: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Circ Cardiovasc Imaging 2013;6:769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keser N. Echocardiography in pregnant women. Anadolu Kardiyol Derg 2006;6:169–173 [PubMed] [Google Scholar]
- 23.Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation 2014;130:1003–1008 [DOI] [PubMed] [Google Scholar]
- 24.Kametas NA, McAuliffe F, Hancock J, Chambers J, Nicolaides KH. Maternal left ventricular mass and diastolic function during pregnancy. Ultrasound Obstet Gynecol 2001;18:460–466 [DOI] [PubMed] [Google Scholar]
- 25.Schannwell CM, Zimmermann T, Schneppenheim M, Plehn G, Marx R, Strauer BE. Left ventricular hypertrophy and diastolic dysfunction in healthy pregnant women. Cardiology 2002;97:73–78 [DOI] [PubMed] [Google Scholar]
- 26.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet 2001;357:2002–2006 [DOI] [PubMed] [Google Scholar]
- 27.Zakovicova E, Charvat J, Mokra D, Svab P, Kvapil M. The optimal control of blood glucose is associated with normal blood pressure 24 hours profile and prevention of the left ventricular remodeling in the patients with gestational diabetes mellitus. Neuroendocrinol Lett 2014;35:327–333 [PubMed] [Google Scholar]
- 28.Savvidou MD, Anderson JM, Kaihura C, Nicolaides KH. Maternal arterial stiffness in pregnancies complicated by gestational and type 2 diabetes mellitus. Am J Obstet Gynecol 2010;203:274.e1–7. [DOI] [PubMed] [Google Scholar]
- 29.Bulzico DA, Zajdenverg L, Cabizuca CA, de Oliveira JE, Salles GF. Assessment of arterial stiffness in women with gestational diabetes. Diabet Med 2012;29:227–231 [DOI] [PubMed] [Google Scholar]
- 30.Retnakaran R, Shah BR. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: a population-based cohort study. CMAJ 2009;181:371–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Benedetto A, Russo GT, Corrado F, et al. Inflammatory markers in women with a recent history of gestational diabetes mellitus. J Endocrinol Invest 2005;28:34–38 [DOI] [PubMed] [Google Scholar]
- 32.Lauenborg J, Mathiesen E, Hansen T, et al. The prevalence of the metabolic syndrome in a danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab 2005;90:4004–4010 [DOI] [PubMed] [Google Scholar]
- 33.Meyers-Seifer CH, Vohr BR. Lipid levels in former gestational diabetic mothers. Diabetes Care 1996;19:1351–1356 [DOI] [PubMed] [Google Scholar]
- 34.Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol 2010;115:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kousta E, Lawrence NJ, Godsland IF, et al. Insulin resistance and β-cell dysfunction in normoglycaemic European women with a history of gestational diabetes. Clin Endocrinol (Oxf) 2003;59:289–297 [DOI] [PubMed] [Google Scholar]
- 36.Anastasiou E, Lekakis JP, Alevizaki M, et al. Impaired endothelium-dependent vasodilatation in women with previous gestational diabetes. Diabetes Care 1998;21:2111–2115 [DOI] [PubMed] [Google Scholar]
- 37.Iwakura K, Ito H, Ikushima M, et al. Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J Am Coll Cardiol 2003;41:1–7 [DOI] [PubMed] [Google Scholar]
- 38.D’Souza A, Howarth FC, Yanni J, et al. Left ventricle structural remodelling in the prediabetic Goto-Kakizaki rat. Exp Physiol 2011;96:875–888 [DOI] [PubMed] [Google Scholar]
- 39.Ilercil A, Devereux RB, Roman MJ, et al. Relationship of impaired glucose tolerance to left ventricular structure and function: the Strong Heart Study. Am Heart J 2001;141:992–998 [DOI] [PubMed] [Google Scholar]
- 40.Galvan AQ, Galetta F, Natali A, et al. Insulin resistance and hyperinsulinemia: no independent relation to left ventricular mass in humans. Circulation 2000;102:2233–2238 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.