Sleep restriction is associated with insulin resistance and an increased risk for type 2 diabetes (1–3). Here, we investigated whether only 2 nights of recovery sleep, as may occur on weekends, reverses the negative effects of short-term sleep restriction on glucose homeostasis.
Nineteen healthy young lean men were studied under controlled laboratory conditions during normal sleep and sleep restriction in a randomized order, as previously reported (1,2,4). The institutional review board of The University of Chicago approved the protocol, and all participants gave written informed consent. During normal sleep, participants were allowed 8.5 h in bed (2300–0700) for 4 consecutive nights. During sleep restriction, participants were allowed 4.5 h in bed (0100–0530) for 4 consecutive nights, immediately followed by recovery sleep for 2 consecutive nights with 12 h in bed on the first night (2200–1000) and 10 h in bed on the second night (2200–0800). A frequently sampled intravenous glucose tolerance test (ivGTT) was performed at 1000 after 4 nights of normal sleep, 4 nights of sleep restriction, and 2 nights of recovery sleep to assess insulin sensitivity, acute insulin response to glucose, and disposition index (i.e., insulin sensitivity × acute insulin response to glucose). Participants received standardized meals during the 24 h prior to each ivGTT. We previously reported the effects of sleep restriction versus normal sleep on sleep stages, insulin sensitivity, and insulin response from this cohort (1,2,4). The effects of sleep condition on glucose homeostasis were assessed using a mixed-effects regression model.
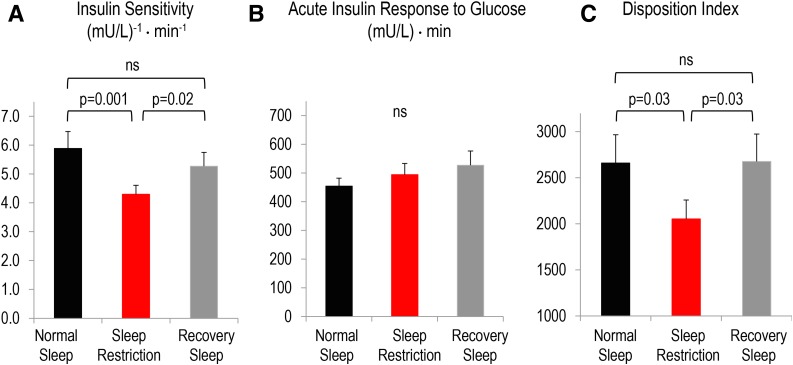
On average, participants slept 7.8 ± 0.1 h during normal sleep, 4.3 ± 0.02 h during sleep restriction, and 9.7 ± 0.2 h during sleep recovery (P < 0.001; all data are mean ± SEM). Weight measured prior to each ivGTT was similar between sleep conditions (P = 0.21). Insulin sensitivity was reduced by 23% after sleep restriction relative to normal sleep, which improved after recovery sleep (Fig. 1A). Acute insulin response to glucose did not differ between conditions (Fig. 1B). Disposition index was reduced by 16% following sleep restriction relative to normal sleep, consistent with increased diabetes risk, which reverted back to normal sleep levels after recovery sleep (Fig. 1C).
Figure 1.
Effects of normal sleep (black bars), sleep restriction (red bars), and recovery sleep (gray bars) on insulin sensitivity (A), acute insulin response to glucose (B), and disposition index (C). The disposition index is insulin sensitivity × acute insulin response and is a marker of diabetes risk. Data are mean ± SEM. Overall P values for sleep condition were P = 0.003 for insulin sensitivity, P = 0.19 for acute insulin response to glucose, and P = 0.047 for disposition index. The effects of sleep condition insulin sensitivity, acute insulin response to glucose, and disposition index were assessed using a mixed-effects linear regression model using restricted maximum likelihood with a small-sample adjustment to hypothesis tests using the Kenward and Roger method.
A common question is whether, and how quickly, an individual can recover from the adverse effects of sleep loss on glucose homeostasis. We have demonstrated that 2 nights of recovery sleep averaging nearly 10 h per night following 4 nights of sleep restriction in healthy young lean men is sufficient to improve insulin sensitivity and restore disposition index (a marker of diabetes risk) to the levels observed after normal sleep. Our findings suggest that catching up on sleep can reverse the negative metabolic effects of short-term sleep restriction. These data are clinically relevant because such sleep patterns (i.e., short-term sleep restriction on workdays and recovery sleep on weekends) are quite common in modern society (5). Future studies in real-world settings are needed to investigate whether catching up on sleep could be an effective behavioral intervention in the prevention and management of type 2 diabetes.
Article Information
Acknowledgments. The authors thank Paul Rue (Department of Medicine, The University of Chicago) for performing the insulin assays, the nursing and dietary staff of The University of Chicago General Clinical Research Center for their expert assistance, and the volunteers for participating in this study.
Funding. This work was supported by grants from the National Heart, Lung, and Blood Institute (R01-HL-075079 and R01-HL-086459), the National Institute on Aging (P01-AG11412), the Foundation for the National Institutes of Health (CTSA-UL1 TR000430), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P50-HD057796), and the National Institute of Diabetes and Digestive and Kidney Diseases (P60-DK20595). This work was also supported by a Department of Defense Air Force Civil Engineer Center award (W81XWH-07-2-0071) and Society in Science–The Branco Weiss Fellowship, administered by the ETH Zürich (to J.L.B.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.L.B. contributed to study design, data acquisition and analysis, and wrote the manuscript. K.W. contributed to data analysis and reviewed and edited the manuscript for intellectual content. J.M.K. contributed to data acquisition and analysis and reviewed and edited the manuscript for intellectual content. E.T. obtained funding, designed the study, collected and analyzed data, and wrote the manuscript. All authors gave final approval of the version to be published. E.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Broussard JL, Chapotot F, Abraham V, et al. Sleep restriction increases free fatty acids in healthy men. Diabetologia 2015;58:791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med 2012;157:549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol 2015;3:52–62 [DOI] [PubMed] [Google Scholar]
- 4.Broussard JL, Kilkus JM, Delebecque F, et al. Elevated ghrelin predicts food intake during experimental sleep restriction. Obesity (Silver Spring). 15 October 2015. [Epub ahead of print]. DOI: 10.1002/oby.21321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Sleep Foundation 2013 international bedroom poll: summary of findings [Internet], 2013. Arlington, VA, National Sleep Foundation. Available from https://sleepfoundation.org/sites/default/files/RPT495a.pdf. Accessed 7 October 2015