Abstract
OBJECTIVE
B lymphocytes play an important role in the immunopathogenesis of autoimmune diabetes. We hypothesized that the altered B-cell subset phenotype is associated with autoimmune diabetes.
RESEARCH DESIGN AND METHODS
Patients with type 1 diabetes (T1D) (n = 81), latent autoimmune diabetes in adults (LADA) (n = 82), or type 2 diabetes (T2D) (n = 95) and healthy control subjects (n = 218) with normal glucose tolerance (NGT) were recruited. We determined the percentage of circulating B-lymphocyte subsets, including CD19+CD23−CD21+ (marginal zone B [MZB]), CD19+CD23+CD21− (follicular B [FoB]), and CD19+CD5+CD1dhi (interleukin-10–producing regulatory B [B10]) cells by flow cytometry.
RESULTS
Patients with T1D or LADA had increased percentages of MZB cells and decreased percentages of FoB cells compared with healthy control subjects with NGT and patients with T2D. Moreover, patients with T1D showed the lowest frequency of B10 cells compared with patients with LADA or T2D, whereas healthy control subjects expressed the highest frequency of B10 cells. Of note, the frequency of MZB cells was negatively associated and the frequency of FoB cells was positively associated with fasting C-peptide (FCP). The frequency of B10 cells was positively correlated with FCP and negatively correlated with hemoglobin A1c.
CONCLUSIONS
The data show that patients with T1D or LADA express an altered frequency of B-cell subsets, which is associated with islet function and glycemia. These findings suggest that B lymphocytes may be involved in loss of self-tolerance and β-cell destruction and could be used as a biomarker and potential target for immunological intervention.
Introduction
Type 1 diabetes (T1D) is characterized by autoimmune destruction of pancreatic β-cells, primarily mediated by T lymphocytes, resulting in insulin deficiency and lifelong exogenous insulin dependence (1). Latent autoimmune diabetes in adults (LADA) is a subtype of autoimmune diabetes, but the progression of autoimmune β-cell destruction is slow; therefore, patients with LADA are not insulin dependent at the initial phase (at least 6 months) of disease onset. Clinical manifestation of LADA shares the features of classical T1D and type 2 diabetes (T2D) (2). T1D, LADA, and T2D present a spectrum of diabetes. They range from patients with classic childhood T1D characterized by autoimmune-mediated destruction of β-cells with the lowest C-peptide levels to age-related exacerbation of glucose tolerance with the highest BMI seen in T2D (3). Furthermore, patients with LADA have susceptibility genes for both T1D and T2D (4), suggesting that LADA lies between T1D and T2D.
Increasing evidence demonstrates a role for B cells in autoimmune diabetes. B cells infiltrate the islets of young NOD mice, the commonly used mouse model for human T1D, and play a role in the initiation of β-cell destruction by the autoimmune response (5). Studies have shown that marginal zone B (MZB) cells and follicular B (FoB) cells can effectively activate CD4+ T cells and facilitate their proliferation by serving as important antigen-presenting cells, especially as antigen-specific antigen-presenting cells (6,7). B-cell–targeted therapy has been shown to delay the fall of C-peptide levels in patients with recent-onset T1D (8). Clinical trials have identified B cells as a possible therapeutic target for the prevention and reversal of T1D. B-cell–targeted therapy also has been successfully used in treating other autoimmune diseases, including rheumatoid arthritis (9), systemic lupus erythematosus (10), and multiple sclerosis (11). Moreover, studies have shown that B cells are important in regulating both innate and adaptive immunity (12). A subset of B cells expressing CD19+CD5+CD1dhi, which have a regulatory function in infection, inflammation, and autoimmune disease, recently has been identified. These regulatory B cells can regulate T-cell responses and inhibit inflammation in an interleukin-10 (IL-10)–dependent manner (13–16). This subset of B cells, therefore, is designated as B10 cells. The maturation and expansion of B10 cells require IL-21 and CD40 signals both in vivo and ex vivo (17,18).
Considerable evidence shows that patients with autoimmune diabetes have altered frequencies of circulating immune cells, including T cells (19), dendritic cells (20), natural killer cells (21,22), and neutrophils (23). Although studies have reported no significant change in total B cells in peripheral blood of patients with T1D (21,24), little is known about B-cell subpopulations with distinct immune functions that may play a role in the diabetes spectrum of T1D, LADA, and T2D. We hypothesized that an altered phenotype of B-cell subsets is associated with autoimmune diabetes. This study investigated a change of peripheral B-cell subsets in patients with T1D, LADA, and T2D compared with healthy control subjects. The results show altered frequencies of various B-cell subsets associated with autoimmune diabetes.
Research Design and Methods
Participants
Two hundred fifty-eight patients with T1D (n = 81, mean diabetes duration 3.1 ± 3.5 years), LADA (n = 82, 6.1 ± 5.9 years), or T2D (n = 95, 3.0 ± 3.7 years) and 218 control subjects with normal glucose tolerance (NGT) (Table 1) were enrolled in the current study from The Second Xiangya Hospital of Central South University (Changsha, Hunan, China). Diabetes was diagnosed according to World Health Organization (WHO) criteria (25). Diagnosis of T1D was based on acute-onset ketosis or ketoacidosis with immediate insulin replacement therapy; at least one positive islet autoantibody (GAD antibody [GADA], insulinoma-associated protein-2 antibody [IA-2A], or zinc transporter 8 antibody [ZnT8A]); or impaired C-peptide secretion. LADA inclusion criteria were 1) any positive islet autoantibody (GADA, IA-2A, ZnT8A), 2) age ≥30 years at onset of diabetes, 3) insulin independence for at least 6 months after diagnosis, and 4) no ketosis or ketoacidosis (2,26). Diagnostic criteria of T2D were 1) typical history of hyperglycemia according to WHO criteria, 2) negative for islet autoantibodies, and 3) not requiring immediate insulin treatment. Healthy control subjects underwent a standardized 75-g oral glucose tolerance test for normoglycemia (fasting plasma glucose [FPG] <6.1 mmol/L, 2-h plasma glucose <7.8 mmol/L). Exclusion criteria for control subjects were secondary diabetes; inflammation, infectious disease, or other autoimmune disease; history of immunosuppressive medication or steroids for >7 days; pregnancy; and malignant disease. This study was approved by the ethical committee of The Second Xiangya Hospital of Central South University, and all subjects provided written informed consent for the study protocol.
Table 1.
Anthropometric and metabolic data of participants
| NGT (n = 218) | T2D (n = 95) | LADA (n = 82) | T1D (n = 81) | |
|---|---|---|---|---|
| Male (%) | 40.4 (88) | 47.4 (45) | 59.8 (49)** | 54.3 (44) |
| Age (years) | 41.37 ± 13.52 | 48.78 ± 12.58*** | 52.48 ± 14.21***‡‡‡ | 28.53 ± 16.21***††† |
| BMI (kg/m2) | 23.10 ± 3.77 | 24.20 ± 3.39* | 22.40 ± 3.15††‡‡‡ | 19.52 ± 3.17***††† |
| SBP (mmHg)¶ | 111 (105–122) | 128 (118–140)** | 118 (110–130)*‡‡‡ | 110 (99–123)† |
| DBP (mmHg)¶ | 74 (70–80) | 80 (70–90)* | 77 (71–83) | 72 (65–80)† |
| TG (mmol/L)§ | 1.05 (0.69–1.61) | 2.06 (1.21–2.93)*** | 1.22 (0.80–1.96)††† | 0.92 (0.65–1.47)††† |
| TC (mmol/L) | 4.90 ± 1.00 | 5.26 ± 1.22* | 4.83 ± 1.28† | 4.57 ± 1.32† |
| LDL-C (mmol/L) | 2.74 ± 0.87 | 3.21 ± 1.06** | 2.90 ± 1.10 | 2.70 ± 1.17 |
| HDL-C (mmol/L)¶ | 1.36 (1.14–1.58) | 1.08 (0.85–1.31)*** | 1.20 (0.94–1.50)* | 1.24 (0.91–1.59)† |
| HbA1c (%)¶ | NA | 7.10 (5.90–9.50) | 7.40 (6.20–9.73) | 8.10 (7.10–11.80)† |
| HbA1c (mmol/mol) | NA | 54 (41–80) | 57 (44–83) | 65 (54–105) |
| FPG (mmol/L)¶ | 5.09 (4.70–5.40) | 7.22 (5.85–9.46)*** | 8.00 (6.31–10.29)*** | 8.49 (5.94–12.60)*** |
| FCP (nmol/L)¶ | 0.42 (0.33–0.54) | 0.53 (0.35–0.76) | 0.31 (0.14–0.50)***†††‡‡‡ | 0.06 (0.03–0.18)***††† |
Data are % (n), mean ± SD, and median (25th–75th percentile). NA, not appropriate. DBP, diastolic blood pressure; SBP, systolic blood pressure.
¶Compared by the nonparametric Kruskal-Wallis test.
§Compared after log transformation.
*P < 0.05 compared with NGT.
**P < 0.01 compared with NGT.
***P < 0.001 compared with NGT.
†P < 0.05 compared with T2D.
††P < 0.01 compared with T2D.
†††P < 0.001 compared with T2D.
‡‡‡P < 0.001 compared with T1D.
Study physicians recorded body height and weight, waist circumference, hip circumference, and blood pressure. Fasting venous blood samples were tested for triglycerides (TGs), total cholesterol (TC), HDL cholesterol (HDL-C), LDL cholesterol (LDL-C), FPG, hemoglobin A1c (HbA1c), and fasting C-peptide (FCP).
C-Peptide and HbA1c Assays
HbA1c was measured by automated liquid chromatography (VARIANT II Hemoglobin Testing System; Bio-Rad Laboratories, Hercules, CA). FCP was measured by a chemiluminescence method (ADVIA Centaur; Siemens, Munich, Germany).
Islet Autoantibody Assays
GADA, IA-2A, and ZnT8A were measured by radioligand assays as previously described (27,28). The cutoff indices of positivity for GADA, IA-2A, and ZnT8A were 18 units/mL (WHO units), 3.3 units/mL, and 0.011 (ZnT8A index), respectively, which were determined as the 99th percentile of 405 healthy control subjects. The positive samples were tested twice for confirmation. The sensitivity of the current GADA, IA-2A, and ZnT8A assays was 78%, 74%, and 70%, respectively, and the specificity was 96.7%, 96.7%, and 98.9%, respectively, in the Islet Autoantibody Standardization Program (IASP2012).
Immunostaining and Flow Cytometry
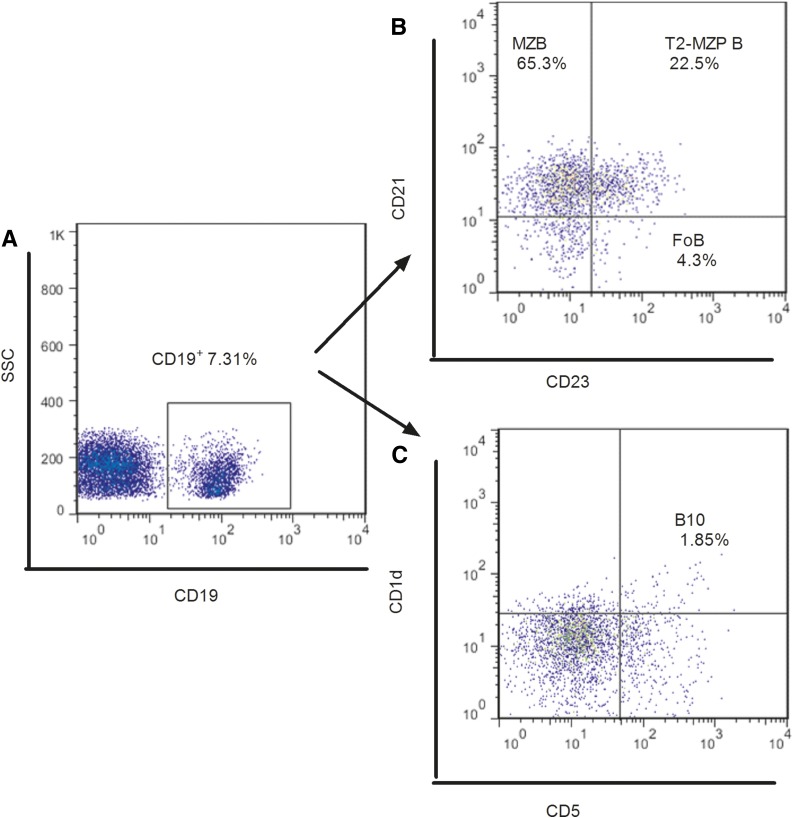
Fresh venous blood samples were drawn into sodium heparin tubes from fasting subjects and processed within 2 h. The peripheral blood mononuclear cells were isolated by standard Ficoll-Paque Plus density-gradient centrifugation and stained with various monoclonal antibodies (allophycocyanin-Cy7-CD19 [HIB19], fluorescein isothiocyanate-CD5 [UCHT2], allophycocyanin-CD1d [51.1], peridinin chlorophyll protein-Cy5.5-CD23 [EBVCS-5], and phycoerythrin-Cy7-CD21 [LT21]; BioLegend, San Diego, CA) to determine the percentage of B-cell subsets according to the manufacturer’s protocol. The stained cells were analyzed with a BD FACSCanto II system, which was calibrated daily with appropriate single fluorochrome-stained samples. Fifty thousand lymphocytes were collected in a forward scatter/side scatter (FSC-A/SSC-A) lymphocyte gate and analyzed with FlowJo version 7.6 software (Tree Star, Inc., Ashland, OR). Dead cells were excluded from the analysis on the basis of their forward- and side-light scatter properties and propidium iodide staining. Doublets were excluded by FSC-A/FSC-H. Gating strategies for all B-cell populations examined are described in Fig. 1. The following antibodies were used to identify B-cell subsets: CD19+ (pan B marker), CD19+CD23−CD21+ (MZB cells), CD19+CD23+CD21− (FoB cells), CD19+CD23+CD21+ (transitional 2-marginal zone precursor [T2-MZP] B cells) (29), and CD19+CD5+CD1dhi (B10 cells). The intra-assay and interassay coefficients of variation for the percentages of CD19+ were 3.71% and 12.15%, respectively.
Figure 1.
Flow cytometry analysis of peripheral B-cell subsets. A: The initial CD19+ gate was derived from a lymphocyte gate (defined on FSC and SSC) followed by single-cell discrimination. B: Representative dot plot showing the gating strategy for MZB, FoB, and T2-MZP B cells gated on CD19+ B cells. C: Representative dot plot showing the gating strategy for B10 cells gated on CD19+ B cells.
Statistical Analysis
Data are presented as mean ± SD or as median (25th–75th percentile). Logarithmic transformations were applied for nonnormally distributed parameters before applying these statistical tests. ANOVA was performed to compare groups after adjustment for potentially confounding variables, including age, sex, and BMI. All the significant differences between groups are the results of adjusted analyses unless stated otherwise. Associations of the frequencies of B-cell subsets with other parameters were estimated by Pearson correlations or Spearman nonparametric correlations. For the statistical analysis, we used SPSS version 19.0 (IBM Corporation, Chicago, IL) and GraphPad Prism 5 (GraphPad Software, San Diego, CA) software. Differences were considered significant at a two-tailed P < 0.05.
Results
Anthropometric and Metabolic Data
Table 1 summarizes the features of the subjects in each group. The control subjects with NGT were younger than patients with LADA and T2D but significantly older than patients with T1D. The percentage of males in the LADA group was higher than in the NGT group. The BMI of the NGT and LADA groups was lower than the T2D group but higher than the T1D group. Patients with T2D or LADA had higher systolic and diastolic blood pressures than patients with T1D and subjects with NGT. Patients with T2D had distinctive TG, TC, LDL-C, and HDL-C levels compared with the other groups. The FPG levels were similar in all the patients with diabetes but higher than in the subjects with NGT. HbA1c in patients with T1D was different from that in patients with T2D. There was a significant decrease of FCP in the LADA and T1D (lowest) groups compared with the T2D and NGT groups.
Change of B-Cell Subsets in Patients With Diabetes
We did not find any significant changes in the frequency of CD19+ B cells among the patient groups, regardless of age, sex, and BMI, compared with the NGT group (P > 0.05) (Supplementary Fig. 1).
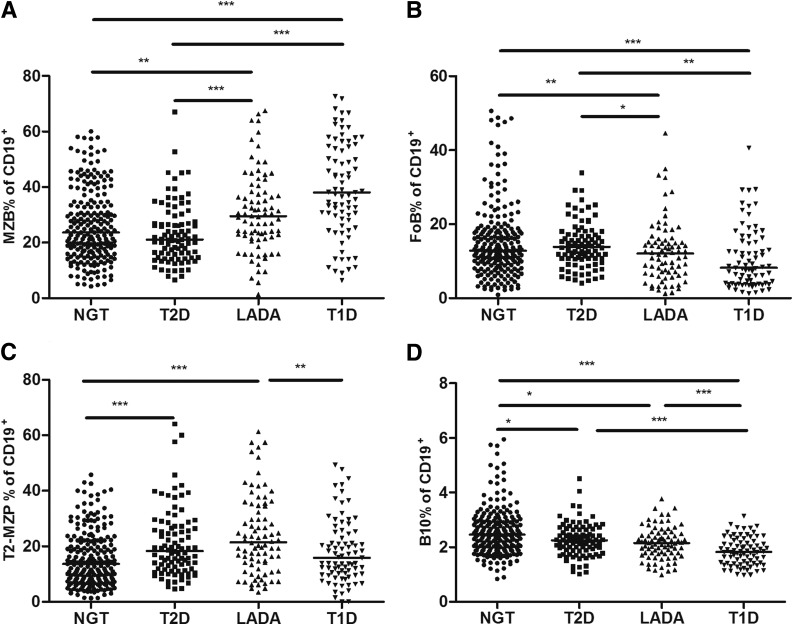
The frequencies of MZB cells in the T1D and LADA groups were significantly higher than in the NGT and T2D groups (T1D vs. NGT, T1D vs. T2D, LADA vs. T2D P < 0.001; LADA vs. NGT P < 0.01) (Fig. 2A). We found that MZB cells were more frequent in the T1D group than in the LADA group, but the differences were diminished after adjustment for age, sex, and BMI. FoB cells were less frequent in the T1D group compared with the other three groups; however, the percentages of FoB cells in the T1D and LADA groups were decreased after adjustment for age, sex, and BMI compared with the NGT and T2D groups (T1D vs. NGT, P < 0.001; T1D vs. T2D, LADA vs. NGT, P < 0.01; LADA vs. T2D, P < 0.05) (Fig. 2B). We also assessed the percentages of T2-MZP B cells, which were reported to have a regulatory function (29), and found these to be increased in the LADA group compared with the NGT and T1D groups (LADA vs. NGT P < 0.001; T1D vs. LADA P < 0.01) (Fig. 2C), whereas they were more frequent in the T2D group than in the NGT group (T2D vs. NGT P < 0.001) (Fig. 2C).
Figure 2.
The frequency of B-cell subsets in subtypes of diabetes and control subjects. The frequency of MZB (A), FoB (B), T2-MZP B (C), and B10 (D) cells gated on CD19+ B cells. P values refer to comparison of data after adjustment for age, sex, and BMI. Each point represents the percentage of B-cell subsets of an individual. Horizontal lines show medians. *P < 0.05, **P < 0.01, ***P < 0.001.
Of note, regardless of the adjustment for age, sex, and BMI, the proportions of B10 cells were significantly decreased in all the patients with diabetes compared with the control subjects with NGT, but these were lowest in the T1D group (T1D vs. NGT P < 0.001; T2D vs. NGT, LADA vs. NGT P < 0.05) (Fig. 2D). B10 cells were higher in the T2D and LADA groups than in the T1D group (P < 0.001 for both) (Fig. 2D).
After matching for age and sex, these findings remained the same for the percentages of CD19+, MZB, B10, and T2-MZP B cells after adjustment for BMI among groups (Supplementary Table 1 and Supplementary Fig. 2). However, patients with LADA but not those with T1D had decreased percentages of FoB cells compared with the control subjects with NGT and the patients with T2D.
B-Cell Subsets Are Associated With Clinical Diabetes
We analyzed whether the change of B-cell subsets had any association with the clinical manifestations and found that 1) MZB and FoB cells were correlated with age, 2) T2-MZP B cells were associated with sex, and 3) B10 and MZB cells were correlated with BMI (Table 2), regardless of the type of diabetes. Of note, FPG was positively correlated with MZB and T2-MZP B cells but negatively correlated with B10 cells. HbA1c was also negatively associated with B10 cells. However, FCP showed positive correlations with FoB and B10 cells but negative correlations with CD19+ and MZB cells. In terms of lipid profile, we found that T2-MZP B cells were positively associated with TG and LDL-C levels but negatively associated with HDL-C level.
Table 2.
Correlation between circulating B-cell subset frequencies and anthropometric and metabolic variables and islet autoantibodies
| CD19+ | B10 | MZB | FoB | T2-MZP | |
|---|---|---|---|---|---|
| Sex | −0.018 | −0.090 | 0.016 | −0.086 | 0.134** |
| Age | −0.076 | 0.005 | −0.176*** | 0.169*** | 0.040 |
| BMI | −0.081 | 0.104* | −0.109* | 0.279 | 0.041* |
| SBP¶ | −0.004 | −0.008 | −0.002 | 0.268 | 0.150** |
| DBP¶ | −0.055 | −0.049 | 0.010 | 0.500 | 0.105 |
| TC | 0.031 | 0.004 | 0.042 | 0.101 | 0.072 |
| TG§ | −0.032 | −0.053 | 0.028 | 0.154 | 0.164*** |
| LDL-C | 0.078 | −0.006 | 0.054 | 0.080 | 0.115* |
| HDL-C¶ | −0.029 | 0.051 | 0.004 | 0.455 | −0.139** |
| FPG¶ | −0.003 | −0.196*** | 0.096* | 0.097 | 0.150*** |
| HbA1c¶ | 0.147 | −0.194* | −0.067 | 0.352 | 0.036 |
| FCP¶ | −0.125* | 0.179*** | −0.118* | 0.122* | 0.029 |
| GADA¶ | 0.017 | 0.051 | −0.110 | 0.726 | 0.052 |
| IA-2A¶ | 0.082 | 0.018 | 0.022 | 0.081 | 0.182 |
| ZnT8A¶ | −0.012 | 0.195 | −0.017 | −0.123 | 0.231 |
Correlation analyses between circulating B-cell subset frequencies (after log transformation) and anthropometric and metabolic variables were performed using Pearson test. Boldface text indicates a significant correlation. DBP, diastolic blood pressure; SBP, systolic blood pressure.
¶Spearman correlations.
§Pearson correlations after log transformation.
*P < 0.05.
**P < 0.01.
***P < 0.001.
In addition to the associations in all participants, we examined the correlations between the various B-cell subsets and clinical manifestations in the different types of diabetes. In patients with T1D, the proportion of CD19+ B cells associated negatively with FCP (r = −0.248, P < 0.05), and the MZB cells exhibited a negative correlation with age (r = −0.498, P < 0.001), BMI (r = −0.364, P < 0.01), and HbA1c (r = −0.324, P < 0.01). In patients with LADA, CD19+ B cells negatively correlated with BMI (r = −0.498, P < 0.001) and FCP (r = −0.248, P < 0.05), whereas FoB cells showed a positive correlation with age (r = 0.236, P < 0.05). Finally, in patients with T2D, the percentage of T2-MZP B cells was positively associated with FCP (r = 0.364, P < 0.05). We did not find any correlation between B-cell subtypes and islet autoantibodies.
Conclusions
Increasing evidence suggests that B cells are as important as T cells in the immunopathogenesis of autoimmune diabetes. Most, if not all, diabetogenic T cells are known to be T-helper 1 subsets, but little is known about B-cell subsets in autoimmune diabetes. We investigated the phenotype and frequency of various B-cell subsets in the peripheral blood across the clinical spectrum of diabetes. The major finding of this study was a distinct alteration of B-cell subsets across the diabetes spectrum and their association with clinical features.
We found no significant difference in the frequency of CD19+ B cells, which is in accordance with the published studies (21,30); however, we did not find a negative correlation between total B cells and age, as previously reported (24,31). The discrepancy might be a result of the different patient populations studied. The current study went further than the examination of the frequency of total B cells, focusing on B-cell subsets with distinct immunological functions. Of note, we found that patients with T1D or LADA had an increased frequency of MZB cells but decreased frequency of FoB cells compared with control subjects with NGT and patients with T2D. In all groups, we found that FCP was negatively associated with MZB cells but positively associated with FoB cells. Attanavanich and Kearney (6) reported that MZB cells were more effective at activating naive CD4+ T cells than FoB cells. MZB cells in NOD mice were also shown to be important antigen-presenting cells for the activation of T cells in pancreatic-draining lymph nodes (32). Given the negative correlation between MZB cells and FCP, it is possible that MZB cells may promote β-cell destruction in patients with T1D or LADA. Decreased frequency of FoB cells in the circulation of patients with T1D and LADA suggests that some FoB cells migrated from the blood to the pancreas or the draining lymph nodes as a result of the local inflammatory response. Along with the disease progression, more immune cells are known to infiltrate to the islets of both NOD mice and patients with T1D (5,33), but the patients with T1D in the current study have a relatively long history of diabetes (3.1 years). Thus, FoB cells appear to be more important in initial rather than established pancreatic β-cell autoimmunity (34). We showed that patients with T2D and LADA more frequently had T2-MZP B cells than control subjects with NGT, which associates with parameters of blood pressure, lipidemia, and glycemia but not with HbA1c or FCP. The finding suggests that T2-MZP B cells are involved in metabolic regulation. These observations are consistent with a previous study showing that glycemia and lipidemia are associated with immune cells (35).
Another interesting finding was the decrease of B10 cell frequency in all types of diabetes, being the lowest in T1D. Whether this phenotype is mediated through a common mechanism or different ones is not clear. What is clear is that immune mechanisms are involved and that more studies, especially functional studies, are required. Although B cells are generally acknowledged for their function in humoral immune response by producing antibodies, they also have been demonstrated to play an immunoregulatory role in the prevention of autoimmune disease by producing IL-10 and downregulating T-cell responses (13,14,36). The observed B10 cell reduction could contribute to the breakdown of self-tolerance that leads to β-cell destruction in patients with T1D (24) or LADA. Antigen-activated IL-10–producing B cells may selectively inhibit autoreactive T-cell responses to islet-specific antigen to maintain self-tolerance, and hyperglycemic NOD mice and patients with T1D lack this population of regulatory B cells (37). In the clinical trial where B cells in patients with new-onset T1D were depleted, the patients showed a transient remission (8). However, blanket targeting of all B cells, as used in the clinical trial and in treating other autoimmune disorders, needs to be improved by selectively deleting pathogenic B cells while preserving regulatory B cells to promote tolerance. Our preclinical study showed that more regulatory B cells were regenerated after B-cell deletion in an animal model (38). Chronic and systemic inflammation are known to play an important role in T2D. Furthermore, studies have shown that patients with long-standing T2D could have a secondary autoimmune response toward islet β-cells (39). The reduction of B10 regulatory cells might contribute to the dysregulation of immune response in T2D. A functional study on IL-10–producing B cells and further phenotyping will be the goal of future work.
In the current study, we found altered B-cell subsets in a spectrum of diabetes. Although we currently cannot identify a specific subset that could be used for diagnostic purposes, the results strongly imply that the change of B-cell subtypes is related to the pathogenesis of autoimmune diabetes. Therefore, it is important to reconsider the approach of B-cell–targeted therapy in patients with T1D and aim to selectively remove pathogenic B cells but promote regulatory B cells.
In summary, the current data demonstrate distinct differences in the frequencies of peripheral B-cell subsets in T1D and LADA, which are associated with islet function and glycemia. We found a reduction of B10 cells in T1D to be particularly interesting. The findings support the notion that these immunological alterations are involved in loss of self-tolerance and β-cell destruction. The findings also suggest that, similar to regulatory T-cell therapy, transfusion of ex vivo expanded autologous B10 cells might open a new and effective therapeutic strategy in treating autoimmune diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank Susan Wong (Institute of Molecular and Experimental Medicine, Cardiff School of Medicine, Cardiff University, Cardiff, U.K.) for critical reading and editing of the manuscript.
Funding. This study was supported by the National Key Technologies R&D Program of China (2012BAI02B04) and grants from the National Clinical Research Center for Metabolic Diseases in China (2013BAI09B12) and the National Natural Science Foundation of China (81400817). Y.X. was supported by the Shenghua Yuying Talented Program of Central South University. L.W. was supported by the National Institutes of Health (DK-100500 and DK-092882) and the American Diabetes Association (BS222).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.D. contributed to the experiments, data analysis, discussion, and writing of the manuscript. Y.X. contributed to the study design, data research, discussion, and critical review and editing of the manuscript. T.T., Z.R., and C.C. contributed to the experiments. G.H. contributed to the islet autoantibodies assay. L.W. contributed to the discussion and editing of the manuscript. Z.Z. contributed to the study design, discussion, and editing of the manuscript. C.D. and Y.X. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 18th Annual Meeting of the Chinese Diabetes Society, Guangzhou, China, 5–8 November 2014.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-1765/-/DC1.
References
- 1.Todd JA. Etiology of type 1 diabetes. Immunity 2010;32:457–467 [DOI] [PubMed] [Google Scholar]
- 2.Zhou Z, Xiang Y, Ji L, et al.; LADA China Study Group . Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic-based cross-sectional study. Diabetes 2013;62:543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leslie RD, Kolb H, Schloot NC, et al. Diabetes classification: grey zones, sound and smoke: Action LADA 1. Diabetes Metab Res Rev 2008;24:511–519 [DOI] [PubMed] [Google Scholar]
- 4.Xiang Y, Zhou Z, Deng C, Leslie RD. Latent autoimmune diabetes in adults in Asians: similarities and differences between East and West. J Diabetes 2013;5:118–126 [DOI] [PubMed] [Google Scholar]
- 5.Diana J, Simoni Y, Furio L, et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med 2013;19:65–73 [DOI] [PubMed] [Google Scholar]
- 6.Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J Immunol 2004;172:803–811 [DOI] [PubMed] [Google Scholar]
- 7.Mariño E, Villanueva J, Walters S, Liuwantara D, Mackay F, Grey ST. CD4(+)CD25(+) T-cells control autoimmunity in the absence of B-cells. Diabetes 2009;58:1568–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pescovitz MD, Greenbaum CJ, Bundy B, et al.; Type 1 Diabetes TrialNet Anti-CD20 Study Group . B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care 2014;37:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mariette X, Rouanet S, Sibilia J, et al. Evaluation of low-dose rituximab for the retreatment of patients with active rheumatoid arthritis: a non-inferiority randomised controlled trial. Ann Rheum Dis 2014;73:1508–1514 [DOI] [PubMed] [Google Scholar]
- 10.Duxbury B, Combescure C, Chizzolini C. Rituximab in systemic lupus erythematosus: an updated systematic review and meta-analysis. Lupus 2013;22:1489–1503 [DOI] [PubMed] [Google Scholar]
- 11.He D, Guo R, Zhang F, Zhang C, Dong S, Zhou H. Rituximab for relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev 2013;12:CD009130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 2005;23:7–18 [DOI] [PubMed] [Google Scholar]
- 13.Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol 2010;125:1114–1124 [DOI] [PubMed] [Google Scholar]
- 14.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008;28:639–650 [DOI] [PubMed] [Google Scholar]
- 15.Jeong YI, Hong SH, Cho SH, Lee WJ, Lee SE. Induction of IL-10-producing CD1dhighCD5+ regulatory B cells following Babesia microti-infection. PLoS One 2012;7:e46553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol 2002;3:944–950 [DOI] [PubMed] [Google Scholar]
- 17.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol 2009;182:7459–7472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshizaki A, Miyagaki T, DiLillo DJ, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 2012;491:264–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaaba SA, Al-Harbi SA. Abnormal lymphocyte subsets in Kuwaiti patients with type-1 insulin-dependent diabetes mellitus and their first-degree relatives. Immunol Lett 1995;47:209–213 [DOI] [PubMed] [Google Scholar]
- 20.Angelini F, Del Duca E, Piccinini S, Pacciani V, Rossi P, Manca Bitti ML. Altered phenotype and function of dendritic cells in children with type 1 diabetes. Clin Exp Immunol 2005;142:341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akesson C, Uvebrant K, Oderup C, et al. Altered natural killer (NK) cell frequency and phenotype in latent autoimmune diabetes in adults (LADA) prior to insulin deficiency. Clin Exp Immunol 2010;161:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodacki M, Svoren B, Butty V, et al. Altered natural killer cells in type 1 diabetic patients. Diabetes 2007;56:177–185 [DOI] [PubMed] [Google Scholar]
- 23.Valle A, Giamporcaro GM, Scavini M, et al. Reduction of circulating neutrophils precedes and accompanies type 1 diabetes. Diabetes 2013;62:2072–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson WS, Pekalski ML, Simons HZ, et al. Multi-parametric flow cytometric and genetic investigation of the peripheral B cell compartment in human type 1 diabetes. Clin Exp Immunol 2014;177:571–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553 [DOI] [PubMed] [Google Scholar]
- 26.Hawa MI, Thivolet C, Mauricio D, et al.; Action LADA Group . Metabolic syndrome and autoimmune diabetes: Action LADA 3. Diabetes Care 2009;32:160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen JS, Hejnaes KR, Moody A, et al. Detection of GAD65 antibodies in diabetes and other autoimmune diseases using a simple radioligand assay. Diabetes 1994;43:459–467 [DOI] [PubMed] [Google Scholar]
- 28.Huang G, Xiang Y, Pan L, Li X, Luo S, Zhou Z. Zinc transporter 8 autoantibody (ZnT8A) could help differentiate latent autoimmune diabetes in adults (LADA) from phenotypic type 2 diabetes mellitus. Diabetes Metab Res Rev 2013;29:363–368 [DOI] [PubMed] [Google Scholar]
- 29.Evans JG, Chavez-Rueda KA, Eddaoudi A, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol 2007;178:7868–7878 [DOI] [PubMed] [Google Scholar]
- 30.Habib T, Funk A, Rieck M, et al. Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. J Immunol 2012;188:487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morbach H, Eichhorn EM, Liese JG, Girschick HJ. Reference values for B cell subpopulations from infancy to adulthood. Clin Exp Immunol 2010;162:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mariño E, Batten M, Groom J, et al. Marginal-zone B-cells of nonobese diabetic mice expand with diabetes onset, invade the pancreatic lymph nodes, and present autoantigen to diabetogenic T-cells. Diabetes 2008;57:395–404 [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes 2014;63:3880–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serreze DV, Chapman HD, Niens M, et al. Loss of intra-islet CD20 expression may complicate efficacy of B-cell-directed type 1 diabetes therapies. Diabetes 2011;60:2914–2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menart-Houtermans B, Rütter R, Nowotny B, et al.; German Diabetes Study Group . Leukocyte profiles differ between type 1 and type 2 diabetes and are associated with metabolic phenotypes: results from the German Diabetes Study (GDS). Diabetes Care 2014;37:2326–2333 [DOI] [PubMed] [Google Scholar]
- 36.Carter NA, Rosser EC, Mauri C. Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Res Ther 2012;14:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleffel S, Vergani A, Tezza S, et al. Interleukin-10+ regulatory B cells arise within antigen-experienced CD40+ B cells to maintain tolerance to islet autoantigens. Diabetes 2015;64:158–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang Y, Peng J, Tai N, et al. The dual effects of B cell depletion on antigen-specific T cells in BDC2.5NOD mice. J Immunol 2012;188:4747–4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donath MY, Dalmas É, Sauter NS, Böni-Schnetzler M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab 2013;17:860–872 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.