Introduction
The Best Pharmaceuticals for Children Act of 2002 mandated that the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) carries out critical reviews of the gaps in knowledge and unmet needs regarding safe and effective pharmacologic treatment of infants, children, and adolescents in a broad range of disease areas. In 2012, NICHD selected diabetes mellitus as one of the pediatric disorders for review. Dr. William V. Tamborlane was named chair, and Dr. Linda DiMeglio, vice-chair, of the Diabetes Working Group. Together with Dr. George Giacoia of NICHD, they assembled a distinguished group of medical experts in childhood diabetes, including clinicians/clinical investigators from leading academic centers and from industry and representatives from the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), to carry out this review. It is very important to note that the views expressed in this article, as well as in other reports from the Diabetes Working Group, are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the FDA or EMA or any of the organizations or pharmaceutical companies represented in our working group.
As shown in Supplementary Table 1, the large Diabetes Working Group was divided into five committees: Type 1 Diabetes (T1D): Therapeutics, Type 2 Diabetes (T2D): Therapeutics, T1D: Natural History and Biomarkers, T2D: Natural History and Biomarkers, and Diabetes Pharmacology. The consensus of the T2D Therapeutics Committee was that its efforts should address the crisis in care that clinicians face in treating this disorder in adolescents. Despite a plethora of new drug classes and new agents within each class that have been approved for use in adults with T2D, in 2015, metformin and insulin remain the only drugs that are approved by the FDA and EMA for use in patients with T2D <18 years of age. Although there are obstacles to the successful completion of phase 3 studies of new treatment modalities in a number of childhood disorders (1), the magnitude of the problem is particularly challenging in studying youth with T2D.
In this white paper, we describe the population of pediatric patients with T2D in the U.S. and Europe, address the limitations of current therapy for youth with T2D, and summarize how inclusion and exclusion criteria, study design, and investigator-related issues have made it especially challenging to complete the investigation plans for new drugs that have been submitted to the regulatory agencies to fulfill pediatric study requirements. We have also developed a proposal, described here, that may better facilitate the collection of adequate and well-controlled pediatric efficacy and safety clinical trial data to inform clinicians regarding the pediatric use of new drugs to treat T2D.
Part 1: The Challenges
Limited Treatment Options for Youth With T2D
Glucose-lowering treatments for which efficacy and safety have been evaluated in completed randomized clinical trials in children and adolescents with T2D are extremely limited. This is in stark contrast to the multiple treatment modalities that have been evaluated for efficacy and safety and approved for use in adults with essentially the same disease. Metformin and insulin remain the only antidiabetes treatments approved by the U.S. and Europe for the treatment of youth with T2D. It is noteworthy that no pivotal clinical trial of insulin has yet to be completed specifically in pediatric T2D subjects.
Metformin is quite effective in achieving near-normal hemoglobin A1c (A1C) levels (i.e., <7.0%) in most adolescents early in the course of T2D. However, results of the Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) study in youth with duration of T2D <2 years suggest that T2D has a more aggressive course in adolescents than in adults. Despite mean baseline A1C levels of 5.9%, A1C levels increased to >8.0% in more than 50% of subjects treated with metformin monotherapy in the TODAY study over an average of 11 months (2). However, assessment of medication compliance in the TODAY study was carried out with pill counts and the actual consumption of medications was unknown. The Pediatric Diabetes Consortium (PDC) established the first registry of youth with T2D in the U.S. in 2011. The clinical and demographic characteristics of the first 500 patients enrolled in the registry were very similar to other pediatric T2D cohorts, in that two-thirds were female, >90% were racial and ethnic minorities, and most patients were from economically disadvantaged families (3). Data from the PDC (Table 1) show that treatment with insulin alone or metformin plus insulin usually fails to achieve target A1C levels of <7.5% that are recommended by the International Society for Pediatric and Adolescent Diabetes (4). Difficulties in complying with complex insulin injection regimens may contribute to the elevated A1C levels in insulin-requiring pediatric T2D patients. These data also show that only 3% of the PDC T2D Registry patients were receiving off-label use of the newer antidiabetes drugs approved for use in adults with T2D.
Table 1.
Enrollment data of 500 youth with T2D in the PDC T2D Clinic Registry (3)
| Treatment | Median T2D duration (years) | Median A1C (25, 75 percentile) | % of total cohort |
|---|---|---|---|
| Drug naïve |
1.7 |
6.4 (5.7, 7.6) |
9 |
| Metformin alone |
2.0 |
6.2 (5.6, 7.3) |
37 |
| Insulin alone |
2.9 |
8.4 (6.5, 10.0) |
12 |
| Metformin plus insulin |
2.5 |
8.7 (6.6, 10.5) |
39 |
| Other | 3.8 | 8.9 (6.0, 9.2) | 3 |
Regulatory Framework
The U.S. FDA and the European EMA require pharmaceutical companies to describe, at an early stage in drug development, how they will develop new medicinal products for use in children (defined as age <18 years). Unless an exemption (waiver) is granted, the companies must agree to a research plan with each agency separately and, although the two agencies work closely together, their recommendations may occasionally differ.
Most of the pediatric research plans for each product consist of at least two separate studies:
A pharmacokinetic (PK)/pharmacodynamic (PD) study (establishing dose and tolerability)
A confirmatory efficacy and safety phase 3 study with a controlled phase of at least 12 weeks and a subsequent safety extension up to a total of 52 weeks to establish drug safety and efficacy
All of these studies include waivers for children <10 years of age based on the grounds that T2D is exceedingly rare in children <10 years of age.
A review of www.clinicaltrials.gov reveals a number of phase 3 studies that have been open for recruitment for up to 2–6 years (Table 2). In the case of saxagliptin, this includes two separate clinical trials involving the use of this drug as initial monotherapy versus metformin as well as add-on therapy in metformin-treatment failures versus placebo. In the case of sitagliptin, separate trials were undertaken to study its efficacy and safety when used in fixed-dose combinations with metformin, as well as when used alone. The requirement to carry out more than one pivotal trial for an individual drug has contributed to the problems in completing the trials of any new drugs in pediatric T2D. More recent examples of pediatric investigation plans (PIPs) for drugs in T2D proposed by industry and approved by the EMA are listed in Table 3. The proposed dates of completion of these studies stretches out to 2027 and more PIPs are in negotiation.
Table 2.
Examples of ongoing phase 3 studies in youth with T2D (as of 30 April 2015)
| Drug | Trial indentifer | Subjects (N) | Status | Duration |
|---|---|---|---|---|
| Colesevelam |
NCT01258075 |
200 |
Open |
4 years, 5 months |
| Exenatide |
NCT00658021 |
195 |
Open |
6 years, 11 months |
| Linagliptin |
NCT01342484 |
117 |
Open |
4 years |
| Liraglutide |
NCT01541215 |
172 |
Open |
2 years, 5 months |
| Saxagliptin |
NCT01434186 |
224 |
Open |
3 years, 5 months |
| Saxagliptin |
NCT01204775 |
136 |
Open |
3 years, 10 months |
| Sitagliptin |
NCT01485614 |
170 |
Open |
3 years, 2 months |
| Sitagliptin |
NCT01472367 |
90 |
Open |
3 years, 4 months |
| Sitagliptin | NCT01760447 | 90 | Open | 2 years, 2 months |
Table 3.
Examples of recent PIPs for drugs in T2D approved by the EMA
| Drug | Anticipated completion date |
|---|---|
| Taspoglutide |
March 2017 |
| Empagliflozin |
February 2019 |
| Exenatide |
July 2019 |
| Alogliptin |
May 2020 |
| Albiglutide |
April 2021 |
| Omarigliptin |
February 2022 |
| Dulaglutide |
June 2022 |
| Lixisenatide |
October 2022 |
| Sotagliflozin |
February 2024 |
| Ertugliflozin |
March 2026 |
| Glucagon receptor antagonist | July 2027 |
The reality is that most of the pivotal phase 3 studies that have been launched are finding it very difficult to enroll enough pediatric patients, despite recruitment of many centers in Europe, the U.S., and other countries around the world, underscoring the need to critically assess current study eligibility requirements and trial designs.
In 2014, it was estimated that conducting all of the individual pediatric T2D studies that companies have agreed to with the regulatory agencies will require up to 3,800 pediatric patients (5), and this number continues to increase with every new study proposal. Having been first recognized in the 1990s, pediatric T2D is an epidemic in relative rather than absolute terms, and the number of pediatric patients with T2D remains small compared with the number of patients with T1D. On the basis of the SEARCH for Diabetes in Youth (SEARCH) study data, the estimated number of T2D patients between 10 and 18 years of age in the U.S. was ≤25,000 (6) and the prevalence is much lower in Europe (7,8). Moreover, the wide geographical distribution of patients in the U.S. results in relatively small numbers of patients in any single pediatric diabetes treatment center.
As in the TODAY study (2), 85% of patients in the PDC T2D Registry were black and Hispanic adolescents from low-income families (3). In these disadvantaged families, socioeconomic factors (e.g., parents missing work for study visits, transportation issues that restrict recruitment to a limited geographical area) and cultural/historical issues (e.g., mistreatment of minority subjects in past clinical trials) are major obstacles to recruitment. In Europe, the situation is different: the prevalence of T2D in young people remains lower than in the U.S. and migrant families from Africa, India, Pakistan, and the Middle East are overrepresented, thus creating additional challenges for recruitment.
Other common reasons why potential subjects are excluded from these studies are the presence of major medical and psychiatric conditions and treatment with glucocorticoids, atypical antipsychotics, and other excluded medications. In addition, most eligible subjects are teenagers who are often difficult to recruit and retain in clinical trials and are frequently lost to follow-up with treating physicians (9).
Easing the Impact of Eligibility Restrictions
Eligibility criteria in many of the early T2D studies in pediatrics resulted in the exclusion of a substantial proportion of the relatively small pool of patients who might otherwise be available for participation in these studies. Until recently, most studies only included subjects with A1C levels ≥7.0%. As observed in the TODAY study in patients with T2D duration <2 years (10), and substantiated by the PDC T2D Registry (Table 1), this criteria eliminates almost half of T2D patients who are well controlled on metformin alone or on treatment with lifestyle modification. In some more recent trials, the A1C inclusion criteria has been lowered to ≥6.5%, which increases the available pool of patients by an additional 10%. Nevertheless, ∼35–40% of potential pediatric subjects with T2D who have A1C levels <6.5% would still be excluded. As the TODAY study showed that A1C levels rise rapidly in adolescents with T2D who are well controlled on metformin monotherapy, it might be possible to reduce the lower limit of A1C to values <6.5%.
Prior to the February 2013 EMA workshop in London, all but one of the active pivotal trials of pediatric T2D excluded patients on current treatment with insulin (11). As indicated in Table 1, this exclusion criteria eliminated ∼50% of pediatric patients with T2D. Currently, eligibility criteria in most but not all studies have been revised to allow inclusion of patients treated with insulin with or without metformin.
Obstacles to Participation in T2D Studies by Academic Pediatric Diabetes Centers in the U.S.
Most studies of T2D in pediatrics in the U.S. are carried out at academic medical centers, and there are a number of issues at these institutions that have made it difficult for investigators to participate in industry-sponsored T2D studies. Major problems include the ever-increasing time and effort required of investigators and research staff to complete the regulatory and certification processes, the lack of the administrative infrastructure to assist with regulatory approvals and negotiations of trial budgets, and the lack of clinical research infrastructure and experienced staff to carry out the studies. In T2D research in pediatrics, where local numbers of eligible participants are small, these hurdles often become insurmountable. A potential solution to this problem is the use of shared personnel, infrastructure, and other resources by investigators in different disciplines.
Most academic research institutions are accustomed to the budgeting of research grants and contracts based on the National Institutes of Health (NIH) model of committed full-time equivalent support for prespecified effort of investigators and research staff. Protected research time can be provided to investigators and personnel can be hired because the funds to support them are already committed over a specified period of time. In contrast, the predominant fee-for-service model of industry-sponsored trials provides piecemeal funding that is almost entirely dependent on the number of subjects who are enrolled in the studies. This method of funding poses a particular challenge for centers to participate in the current, short-term T2D trials that will enroll only a small number of patients.
Academic medical centers have traditionally placed limited scholarly value on their faculty’s participation in industry-sponsored clinical trials as compared with peer-reviewed, investigator-initiated studies funded by the NIH or foundation grants. Clinical investigators, themselves, have had a vastly different approach to participating in NIH-sponsored than industry-sponsored multicenter clinical trials. The NIDDK-sponsored TODAY study provides an excellent example that is particularly relevant to this discussion (12). In the TODAY study, 15 clinical centers enrolled >1,200 adolescents with T2D of <2 years’ duration and 699 subjects were randomized into the study. In contrast, industry-sponsored clinical trials in pediatric T2D have enrolled hundreds of centers but the number of subjects who have completed the studies is very small. In Europe, although the incidence and prevalence of T2D has been well documented through robust diabetes population registries, there is currently no clinical trials network that compares with the TODAY study, although there are plans to establish a network through European Network of Paediatric Research at the EMA. Thus in Europe, recruitment to T2D studies has been through industry sponsorship and limited by the overall low numbers of eligible subjects.
Results of Pediatric Endocrine Society Survey of Diabetes Treatment Centers
Two of the authors of this white paper (R.G.-K. and K.B.) developed a survey that was sent by the Pediatric Endocrine Society to its members to ascertain the barriers to participation in industry-sponsored clinical trials in adolescents with T2D. The results of the survey support the challenges that have been described above. Specifically, the most common obstacles to participation included the following:
Clinics caring for ≤50 T2D patients under the age of 18 years
Lack of interest in participating in research by patients and families
Restrictive inclusion criteria
Exclusion of subjects due to past or current use of glucose-lowering agents other than metformin
Inadequate reimbursement
Part 2: Innovative Approaches and Novel Study Designs
The Case for Extrapolation
Both the EMA and FDA have defined their concepts and necessary criteria to extrapolate the efficacy of medicinal products from adults to the pediatric population (13). In general, to permit extrapolation, the disease must be sufficiently similar in both populations. In addition, similar responses to intervention and similar exposure-response relationships in the adult and pediatric populations would have to be substantiated. Although the basic pathophysiology of insulin resistance and progressive β-cell dysfunction is similar in adolescents and adults (14,15), there appears to be a faster decline in β-cell function in youth than in adults with T2D, a suggestion that is supported by the higher-than-predicted failure rate of metformin monotherapy in the TODAY study (2). In addition, due to the hormonal changes of puberty (16), obese adolescents with T2D may be more insulin resistant than adults with T2D. The FDA and EMA have judged that there is insufficient evidence regarding the similarities between adolescents and adults with T2D to justify full extrapolation of efficacy from studies in adults to adolescents.
Partial extrapolation of efficacy can be used when uncertainty exists about the assumptions underlying full extrapolation. Partial extrapolation of efficacy can range from requiring a single adequate and well-controlled phase 3 study (as opposed to the two separate trials required in adults) to requiring only a PK/PD exposure-response study that shows similarities in exposure-response relationships between adult and pediatric patients. Safety data would also need to be collected at the recommended dose(s).
Virtually all of the recent pediatric T2D program agreements accept partial extrapolation of efficacy from adults by allowing a single phase 3 study in the pediatric population compared with the requirement for at least two separate studies. It remains to be determined whether the concept of partial extrapolation can be further extended to reduce the number of pediatric patients required in pediatric programs without compromising the adequacy of the pediatric efficacy assessment.
Studies of Drug PK and PD in Youth With T2D
It is standard procedure for one of the first clinical studies in the pediatric development of a drug to be a phase 1 pediatric study to assess drug PK based on the assumption that growth and developmental changes in factors influencing absorption, distribution, metabolism, and excretion will lead to changes in PK measures and parameters. To achieve drug exposure values, e.g., area under the drug concentration curve and peak drug concentration, in children similar to values associated with effectiveness and safety in adults, it is considered necessary to evaluate the drug PK over the entire pediatric age range in which the drug will be used. However, as almost all pediatric patients with T2D are ≥10 years of age, some of the complexities challenging clinical pharmacology studies in younger children are avoided.
In early studies, the PK of metformin (17), glimepiride (18), and pioglitazone (19) in adolescents with T2D were not different than the PK of approved doses of the drugs in adults. More recently, the doses of newer drugs such as exenatide, liraglutide, and sitagliptin that are being used in pivotal safety and efficacy trials in pediatric patients with T2D were also shown to be very similar to the doses approved for use in adults (Table 4). These data suggest that the PK properties of T2D drugs in adolescent patients are not significantly different from that in adults. Similar drug exposures in adolescents compared with adults with T2D are likely to be related to the age, pubertal development, and the marked obesity in this patient population. Whether increases in glomerular filtration rate or other physiologic factors contribute to increased rates of drug clearance in youth with T2D has not been established.
Table 4.
Recently completed pediatric T2D PK studies (ClinicalTrials.gov)
| Drug | Subjects (N) | Start date | End date | Pediatric dose in phase 3 trial | Adult dose |
|---|---|---|---|---|---|
| Exenatide |
13 |
February 2006 |
February 2007 |
5 μg b.i.d. AND 10 μg b.i.d. |
5 μg b.i.d. AND 10 μg b.i.d. |
| Liraglutide |
21 |
November 2009 |
September 2011 |
1.8 mg or maximum tolerated dose: 0.6/1.2/1.8 mg |
1.2 mg or 1.8 mg q.d. |
| Sitagliptin |
36 |
July 2008 |
February 2011 |
100 mg q.d. |
100 mg q.d. |
| Sitagliptin | 24 | July 2012 | April 2014 | Sitagliptin 100 mg q.d. AND Metformin extended release 1,000 to 2,000 mg q.d. | Sitagliptin 100 mg q.d. AND Metformin extended release 1,000 to 2,000 mg q.d. |
Depending on the sample size, performance of dedicated PK studies in this difficult-to-recruit population usually takes between 1 and 2.5 years to complete, as only about one subject per month successfully completes the study (Table 3). Due to the difficulties with performing a standard phase 1 study in this population, alternative approaches have been considered. One alternative includes the adoption of modeling and simulation to predict the pediatric exposure and dose with confirmation of exposure of the predicted dose obtained through sparse sampling for PK parameters embedded within the pivotal pediatric study to assess safety and efficacy. This approach is now being accepted by the FDA; it eliminates the need for a dedicated phase 1 pediatric study to assess PK, with significant savings to both time and cost.
New Study Designs of Pivotal Randomized Clinical Trials
Leaders at the EMA and FDA have recognized that innovative approaches to the design of regulatory studies that address feasibility and recruitment issues in pediatric T2D are needed (5). An innovative approach presented by James Wason, MRC Biostatistics Unit Hub for Trials Methodology Research, Cambridge, U.K., at the EMA Workshop in 2013 would use simultaneous multi-agent/multicompany pivotal trials carried out by independent networks of leading academic pediatric diabetes treatment centers in the U.S., Europe, Australia, and elsewhere (11).
Multi-agent clinical trials could be restricted to products from the same pharmaceutical company or involve multiple companies; similarly, they could include products within the same class or in different classes. For regulatory purposes, the study design would only test each new treatment against the control treatment, so that the safety and efficacy of each individual drug can be established. There would be no regulatory requirement to test the potential superiority (or noninferiority) of one new treatment against another new treatment.
The use of multiple experimental treatment groups versus a single, shared control group will substantially lower the total number of subjects needed to complete these studies compared with current studies that use a separate control for each experimental group. For example, a four-arm trial would reduce the number of subjects that would be required for separate trials by 33% because the number of control subjects would be cut by 67%.
Multi-agent rather than the current single-agent approach could be used in a number of different study designs that would make as many subjects as possible eligible for inclusion.
Subjects Who Are Poorly Controlled (A1C >7.0% or 6.5%) on Treatment With Metformin and Insulin Alone or in Combination
These would be trials of the safety and efficacy of add-on therapy with experimental agents versus placebo. The primary efficacy outcome would be superiority in lowering of A1C versus placebo after a specified period of time.
Subjects Who Are Well Controlled (A1C <7.0 or 6.5%) on Treatment With Metformin Alone
Trials of Experimental Agents as Monotherapy of T2D.
In these studies, subjects who are well controlled on metformin alone would be randomized to either remaining on metformin or switching to one of the experimental agents. The primary efficacy outcome would be noninferiority in the difference in A1C levels versus the metformin group after 12 months.
Trials of Early Combination Therapy in Adolescents With T2D.
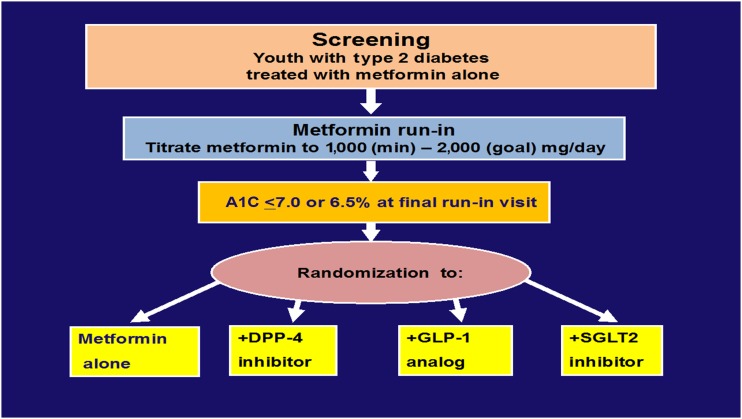
As shown in Fig. 1, in these clinical trials, subjects who are well controlled on metformin alone would be randomized to either metformin plus placebo or combination therapy of metformin plus an experimental agent. The concept of studying early combination therapy in patients with T2D who are well controlled on metformin alone rather than waiting for the failure of metformin monotherapy has already been established in youth in the TODAY study and in adults in the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE) study (20). As in the TODAY study, long-term efficacy, as defined as time to treatment failure, would require longer follow-up periods (e.g., up to 3–4 years) to complete.
Figure 1.
Example of a multi-agent study design of early combination therapies of pediatric T2D modeled after the TODAY study. DPP-4, dipeptidyl peptidase 4; GLP-1, glucagon-like peptide 1; SGLT2, sodium–glucose cotransporter 2.
From a patient perspective, multi-agent trials increase the likelihood of getting randomized to an active treatment arm rather than a control arm as compared with a single-agent trial. An advantage for pharmaceutical companies could be to benefit from each other’s know-how in terms of recruitment strategy and trial expertise. Companies would no longer have to compete against each other to recruit the few available patients and make their individual study workable but would collaborate for one joint study. It is important to note that, in Europe and the U.S., the extension of patent protection depends only on the successful completion of the agreed development plan rather than whether or not the plan leads to a pediatric indication.
Of course, multi-agent clinical trials involving multiple companies also present significant challenges, which would need to be overcome. Which agents would be included and how would the study costs be divided? Although a three-arm study involving almost 700 pediatric T2D patients was successfully completed by the TODAY Study Group, it remains to be determined whether such a large multi-arm, industry-sponsored study would be as successful.
Expanding the Pool of Eligible Subjects
Additional steps can be taken to increase the pool of subjects who are eligible for these studies. There is a strong rationale for including subjects up to 21 or even 25 years of age who had the onset of their disease prior to 18 years of age, as it has been extremely rare for such patients to be included in adult T2D pivotal trials. Moreover, as a result of the February 2013 EMA workshop on PIPs in T2D, the EMA has indicated that “young adults behave more like adolescents than adults” (11). It is also noteworthy that 21 years of age is considered the upper limit of age for pediatric studies of medical devices.
New Organizational Structures
New organizational structures are needed for the development and implementation of clinical trials for approval of new drugs for youth with T2D to replace the current system of companies competing against one another and even against themselves when they have more than one agent to study. This approach will involve close cooperation of all stakeholders, including pharmaceutical companies, investigators at pediatric diabetes research centers, and regulatory agencies. In the U.S., the NIH could also play a role as a facilitator.
Pharmaceutical industry partners could provide the financial support to develop a network of investigators and centers with recognized expertise in performing clinical studies in pediatric T2D in the U.S., the European Union, Australia, and elsewhere, as well as covering the costs of performing the studies. Within the European Network of Pediatric Research, a Diabetes and Endocrinology network is currently being established and the PDC in the U.S. has recently received additional support to increase the number of its centers to more than 35. Similar to some of the NIH-supported clinical trial groups, such as the Diabetes Research in Children Network (DirecNet), these consortia could use a hybrid approach to funding: a relatively modest amount of committed support might be provided at each site to help build the research team and this support will be supplemented by the number of subjects who are enrolled in active treatment trials. The longer study periods of the new study designs and the steady stream of new therapeutic agents that require investigation for the foreseeable future will ensure the financial stability of the centers.
Many of the regulatory and financial hurdles can be reduced by a more coordinated approach using an independent central coordinating center. The coordinating center will have the responsibility for the preparation of regulatory documents, share the collective network experience in responding to regulatory concerns, monitor the institutional review board approval, certify the sites and investigators, implement the standard research agreements across the network, and assist in the development of study budgets.
Ideally, the clinical center principal investigators and the coordinating center(s) for each study will share the responsibility for the development of study protocols in conjunction with input from the pharmaceutical industry, the FDA, and the EMA. As much as possible, guidelines from both of these agencies regarding the essential study elements would take into consideration the expert recommendations of study investigators and be harmonized between the two agencies. A steering committee composed of all the stakeholders will establish subcommittees that will be responsible for the monitoring the recruitment of subjects, implementation of study protocols, proposals for ancillary studies, preparation of publications and presentations, and evaluations of the performance of individual centers.
As each subject completes the randomized phase of each of these trials, they will be invited to continue to be followed in a T2D registry and clinic network made up of all of the clinical centers that participated in the study for the collection of additional postapproval safety and efficacy data.
Pipe Dream or Future Reality?
The authors of this white paper remain committed to the idea that appropriate studies should be done to provide the evidence needed to secure an indication for the use of these drugs in youth with T2D where appropriate rather than to advocate for off-label use of these agents in pediatrics. We have outlined a number of approaches to aid and improve the collection of pediatric T2D safety and efficacy data that could help to facilitate the approval of new, safe, and effective glucose-lowering agents for youth with T2D. We all believe that these ideas and other novel approaches can provide a real solution to the problems we currently face in providing the best possible care to youth with this difficult condition.
Supplementary Material
Article Information
Acknowledgments. The authors would like to thank Debra Stein, Diabetes Working Group Project Leader, of Circle Solutions, Inc., for her tireless work on coordinating the writing of this white paper.
Duality of Interest. W.V.T. has been a consultant for AstraZeneca, Boehringer Ingelheim, Janssen, Novo Nordisk Inc., Sanofi, and Takeda. R.S. is an employee of Merck & Co. P.H.H. is an employee of Novo Nordisk Inc. R.P. is an employee of Novartis Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-1649/-/DC1.
Member roster by committee is available in Supplementary Data online.
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the U.S. Food and Drug Administration or the European Medicines Agency or one of its committees or working parties.
References
- 1.Finkelstein JB. Pediatric drug trials facing some obstacles. J Natl Cancer Inst 2005;97:1720–1721 [DOI] [PubMed] [Google Scholar]
- 2.Zeitler P, Hirst K, Pyle L, et al.; TODAY Study Group . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregg B, Connor CG, Ruedy KJ, et al.; Pediatric Diabetes Consortium. C-peptide levels in pediatric type 2 diabetes in the Pediatric Diabetes Consortium T2D Clinic Registry. Pediatr Diabetes. 5 May 2015 [Epub ahead of print]. DOI: 10.1111/pedi.12280 [DOI] [PubMed]
- 4.Tamborlane WV, Ruedy K, Van Name M, Klingensmith GJ. Can we get it right for youth with type 2 diabetes? Diabetes Res Clin Pract 2014;106:643–644 [DOI] [PubMed] [Google Scholar]
- 5.Karres J, Pratt V, Guettier JM, et al. . Joining forces: a call for greater collaboration to study new medicines in children and adolescents with type 2 diabetes. Diabetes Care 2014;37:2665–2667 [DOI] [PubMed] [Google Scholar]
- 6.Dabelea D, Mayer-Davis EJ, Saydah S, et al.; SEARCH for Diabetes in Youth Study . Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014;311:1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feltbower RG, McKinney PA, Campbell FM, Stephenson CR, Bodansky HJ. Type 2 and other forms of diabetes in 0-30 year olds: a hospital based study in Leeds, UK. Arch Dis Child 2003;88:676–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Awa WL, Fach E, Krakow D, et al.; DPV Initiative; German BMBF Competence Networks Diabetes mellitus and Obesity . Type 2 diabetes from pediatric to geriatric age: analysis of gender and obesity among 120,183 patients from the German/Austrian DPV database. Eur J Endocrinol 2012;167:245–254 [DOI] [PubMed] [Google Scholar]
- 9.Reinehr T, Schober E, Roth CL, Wiegand S, Holl R; DPV-Wiss Study Group . Type 2 diabetes in children and adolescents in a 2-year follow-up: insufficient adherence to diabetes centers. Horm Res 2008;69:107–113 [DOI] [PubMed] [Google Scholar]
- 10.Copeland KC, Zeitler P, Geffner M, et al.; TODAY Study Group . Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab 2011;96:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Medicines Agency. Workshop on paediatric investigation plans in type-2 diabetes mellitus, 2013. Available from http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/events/2013/01/event_detail_000695.jsp&mid=WC0b01ac058004d5c3. Accessed 3 September 2014
- 12.Laffel L, Chang N, Grey M, et al.; TODAY Study Group . Metformin monotherapy in youth with recent onset type 2 diabetes: experience from the prerandomization run-in phase of the TODAY study. Pediatr Diabetes 2012;13:369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Medicines Agency. Concept paper on extrapolation of efficacy and safety in medicine development, 2012. Available from http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129285.pdf. Accessed 3 September 2014
- 14.Giannini C, Weiss R, Cali A, et al. . Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes 2012;61:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss R, Caprio S, Trombetta M, Taksali SE, Tamborlane WV, Bonadonna R. Beta-cell function across the spectrum of glucose tolerance in obese youth. Diabetes 2005;54:1735–1743 [DOI] [PubMed] [Google Scholar]
- 16.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med 1986;315:215–219 [DOI] [PubMed] [Google Scholar]
- 17.Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 2002;25:89–94 [DOI] [PubMed] [Google Scholar]
- 18.Gottschalk M, Danne T, Vlajnic A, Cara JF. Glimepiride versus metformin as monotherapy in pediatric patients with type 2 diabetes: a randomized, single-blind comparative study. Diabetes Care 2007;30:790–794 [DOI] [PubMed] [Google Scholar]
- 19.Christensen ML, Meibohm B, Capparelli EV, Velasquez-Mieyer P, Burghen GA, Tamborlane WV. Single- and multiple-dose pharmacokinetics of pioglitazone in adolescents with type 2 diabetes. J Clin Pharmacol 2005;45:1137–1144 [DOI] [PubMed] [Google Scholar]
- 20.Nathan DM, Buse JB, Kahn SE, et al.; GRADE Study Research Group . Rationale and design of the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE). Diabetes Care 2013;36:2254–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.