Abstract
Background
Cisplatin-based neoadjuvant chemotherapy (NAC) before cystectomy is the standard of care for muscle-invasive bladder cancer (MIBC), with 25–50% of patients expected to achieve a pathologic response. Validated biomarkers predictive of response are currently lacking.
Objective
To discover and validate biomarkers predictive of response to NAC for MIBC.
Design, setting, and participants
Pretreatment MIBC samples prospectively collected from patients treated in two separate clinical trials of cisplatin-based NAC provided the discovery and validation sets. DNA from pretreatment tumor tissue was sequenced for all coding exons of 287 cancer-related genes and was analyzed for base substitutions, indels, copy number alterations, and selected rearrangements in a Clinical Laboratory Improvements Amendments–certified laboratory.
Outcome measurements and statistical analysis
The mean number of variants and variant status for each gene were correlated with response. Variant data from the discovery cohort were used to create a classification tree to discriminate responders from nonresponders. The resulting decision rule was then tested in the independent validation set.
Results and limitations
Patients with a pathologic complete response had more alterations than those with residual tumor in both the discovery (p = 0.024) and validation (p = 0.018) sets. In the discovery set, alteration in one or more of the three DNA repair genes ATM, RB1, and FANCC predicted pathologic response (p < 0.001; 87% sensitivity, 100% specificity) and better overall survival (p = 0.007). This test remained predictive for pathologic response in the validation set (p = 0.033), with a trend towards better overall survival (p = 0.055). These results require further validation in additional sample sets. Conclusions: Genomic alterations in the DNA repair-associated genes ATM, RB1, and FANCC predict response and clinical benefit after cisplatin-based chemotherapy for MIBC. The results suggest that defective DNA repair renders tumors sensitive to cisplatin.
Patient summary
Chemotherapy given before bladder removal (cystectomy) improves the chance of cure for some but not all patients with muscle-invasive bladder cancer. We found a set of genetic mutations that when present in tumor tissue predict benefit from neoadjuvant chemotherapy, suggesting that testing before chemotherapy may help in selecting patients for whom this approach is recommended.
Keywords: Bladder cancer, Urothelial carcinoma, Biomarkers, Cisplatin sensitivity, Cisplatin resistance, ATM, RB1, FANCC, DNA repair, Neoadjuvant chemotherapy
1. Introduction
Muscle-invasive bladder cancer (MIBC) is characterized by a propensity to metastasize. Currently, neoadjuvant cisplatin-based chemotherapy followed by cystectomy is the standard of care for MIBC on the basis that phase 3 clinical trial data and meta-analyses show better overall survival (OS) with this approach [1,2]. Pathologic response at the time of cystectomy predicts survival [3]. Unfortunately, only approximately a third of patients achieve such a response [1,4]. Genomic profiling is an increasingly useful tool for understanding the molecular etiology of bladder cancer; however, while molecular biomarkers are currently used clinically to guide treatment selection in melanoma (BRAF), lung cancer (EGFR) and colorectal cancer (KRAS), validated genomic biomarkers predictive of response to therapy are currently lacking for bladder cancer [5–7]. We recently reported the results of a clinical trial using three cycles of neoadjuvant accelerated methotrexate, vinblastine, doxorubicin, and cisplatin (AMVAC) in patients with MIBC [4]. Using a discovery set of prospectively collected pretreatment tumor samples from patients treated in this study, we sought to identify potential genomic biomarkers of response, hypothesizing that genomic alterations could be identified that would effectively predict response to DNA-damaging chemotherapy in MIBC. A set of identically collected samples from a follow-up trial of similar design testing three cycles of neoadjuvant dose-dense gemcitabine and cisplatin (DDGC) [8] served as the validation cohort.
2. Patients and methods
2.1. Study design and patients
The discovery (AMVAC) and validation (DDGC) sets consisted of pretreatment tumor samples collected from all MIBC patients treated during two previously reported trials (NCT01031420 [4] and NCT01611662 [8], respectively) who received all three cycles of chemotherapy and for whom adequate pretreatment tissue was available. For each cohort, pretreatment formalin-fixed paraffin-embedded (FFPE) sections were obtained and sequenced as described below. Patients provided informed consent for study treatment and collection and testing of archival tissue, clinical data, demographic data, and follow-up data as part of each trial. We considered two definitions of response: pathologic complete response, defined as no remaining tumor in the specimen (pT0pN0cM0), and tumors that were downstaged to non-MIBC disease (≤pT1pN0cM0). Both have been used as endpoints in clinical trials and correlate with improved progression-free survival (PFS) and OS in our AMVAC clinical trial and others [1,4,8–10].
2.2. Genomic sequencing
Genomic DNA was extracted from 40 μg of FFPE tumor tissue using a Maxwell 16 FFPE Plus LEV DNA Purification kit (Promega, Madison, WI, USA). Samples of 50–200 ng of extracted DNA were sheared to ~100–400 bp by sonication and were then subjected to end-repair, dA-addition, and ligation of indexed Illumina (San Diego, CA, USA) sequencing adaptors [11]. Sequencing libraries were hybridization-captured using a pool of >24 000 individually synthesized 5′-biotinylated DNA oligonucleotides (Integrated DNA Technologies, Coralville, IA, USA). These baits were designed to target ~1.5 MB of the human genome, including 4557 exons of 287 cancer-related genes, 47 introns of 19 genes frequently rearranged in cancer, and 3549 polymorphisms located throughout the genome. DNA sequencing was performed using the HiSeq instrument (Illumina) with 49 × 49 paired-end reads, targeting >500× unique median sequence coverage. Sequence data analysis and quality control measures are described in the Supplementary material, along with gene expression data analysis, support vector machine training with sequence and protein structural features, and ATM protein modeling.
2.3. Statistical analysis
A classification tree was used to identify a parsimonious decision rule to discriminate responders from nonresponders. At each branch point, the relationship between gene (variant vs wild type) and responder status was assessed within the subtree using two-sided Fisher’s exact tests. The branch was then split on the gene that resulted in the lowest p value. This process was repeated until it was no longer possible to identify a gene that was significantly (p ≤0.05) associated with response in the subtree. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and two-sided 95% exact confidence intervals (CIs) were computed to characterize the operating characteristics of the resulting ATM/RB1/FANCC decision rule using the discovery and validation data sets with each definition of pathologic response (≤pT1pN0cM0 and pT0pN0cM0). We assessed the significance of the resulting decision rule in the AMVAC discovery set using a permutation-based approach by randomly reordering the responder/nonresponder status of the individuals, keeping variant information fixed. The proportion of reorderings resulting in a misclassification rate at least as small as that for the original tree was taken as an estimate of the overall p value. The number of genomic variants detected in responders (pT0) was compared to that in nonresponders by two-sample t tests separately within the AMVAC and DDGC data sets. OS and PFS were calculated using the Kaplan-Meier method.
3. Results
3.1. Discovery
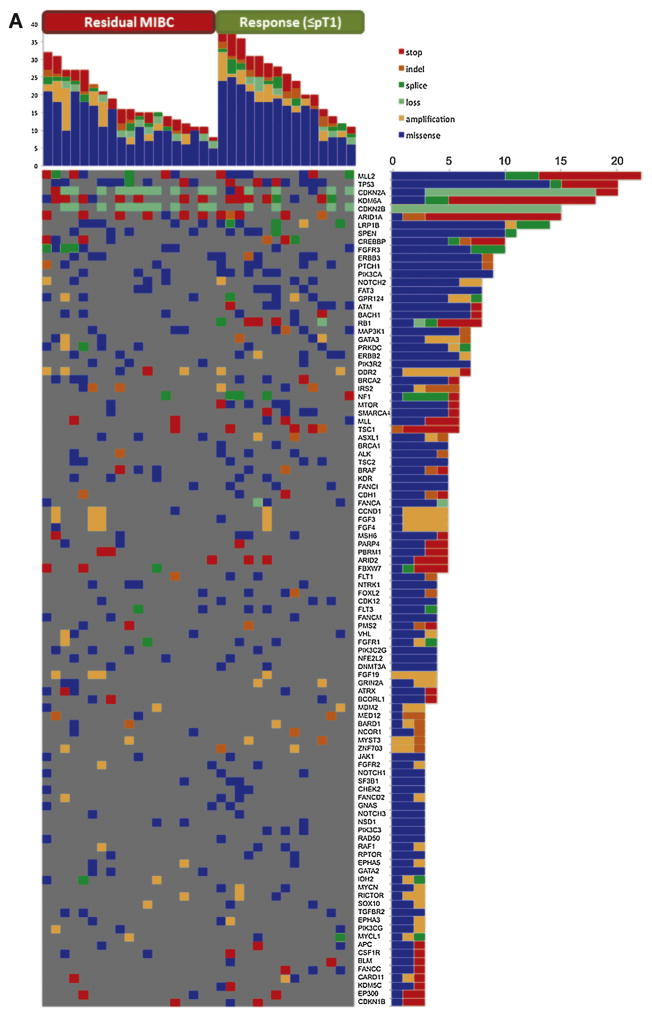
Of the 44 patients treated on the AMVAC study, 37 received all three cycles of chemotherapy. Three additional patients were excluded because of insufficient pretreatment tissue, yielding a discovery set of 34 for genomic analysis. The baseline characteristics of this cohort are described in Table 1. Within this discovery set, 728 alterations in 212 genes were detected (Fig. 1A) and correlated with pathologic response (defined as no residual MIBC, ≤pT1pN0cM0), PFS, and OS.
Table 1.
Patient characteristics and treatment outcomes for the discovery and validation sets
| Discovery (n = 34) | Validation (n = 24) | |
|---|---|---|
| Median age, yr (range) | 64 (44–83) | 68 (55–82) |
| Gender, n (%) | ||
| Male | 23 (68) | 17 (71) |
| Female | 11 (32) | 7 (29) |
| Race, n (%) | ||
| White (non-Latino) | 31 (91) | 23 (96) |
| African American | 2 (6) | 1 (4) |
| Asian | 1 (3) | 0 (0) |
| ECOG performance status, n (%) | ||
| 0 | 31 (91) | 16 (67) |
| 1 | 3 (9) | 8 (33) |
| Baseline clinical stage, n (%) | ||
| T2N0M0 | 10 (29) | 9 (38) |
| T3N0M0 | 16 (47) | 10 (42) |
| T4N0M0 | 5 (15) | 1 (4) |
| T any N1 | 3 (9) | 4 (17) |
| Pathologic response to NAC, n (%) | ||
| Complete response (T0N0M0) | 14 (41) | 9 (38) |
| Residual disease (any) | 20 (59) | 15 (63) |
| Downstaged to ≤T1N0M0, n (%) | 15 (44) | 11 (46) |
ECOG = Eastern Cooperative Oncology Group; NAC = neoadjuvant chemotherapy.
Figure 1.
Distribution of alteration in samples by alteration type and responder status for the (A) AMVAC and (B) DDGC data sets. For each panel, the top graph indicates the alteration counts per sample. Somatic mutations in all sequenced genes were taken into account. Samples were subdivided into nonresponders (left section of the graph) and responders (right section) and sorted by the total number of all alterations in descending order. For each panel, the right-hand graph provides alteration counts per gene. For each panel, the main field indicates the presence of the mutation in a given sample in a given gene. Only the most deleterious mutation in the indicated gene is shown for cases in which two or more mutations were identified in the same patient. The type of mutation is color-coded as shown by the legend. MIBC = muscle-invasive bladder cancer.
Decision tree analysis showed that 13/15 (87%) patients with a response to AMVAC had an alteration in one or more of the genes ATM, RB1, and FANCC, whereas none (0%) of the nonresponders had an alteration in any of these genes (p < 0.001). This decision rule for response has 87% specificity (95% CI 60–98%), 100% sensitivity (95% CI 82–100%), 100% PPV (95% CI 75–100%), 90% NPV (95% CI 70–99%), and 94% accuracy (95% CI 80–99%; Table 2). To determine the probability that chance alone could explain our results and to take into account the multiple genes tested, a permutation analysis of the decision rule was performed. Using the more stringent definition of response of no residual disease (pT0pN0cM0), this analysis showed that the probability that the decision rule would result in ≥97% accuracy by chance alone is 0.0001. No classification signature for nonresponse was identified.
Table 2.
Operating characteristics of the ATM/RB1/FANCC test according to the definition of pathologic response
| Set | Response definition | RSPs (n) | NRSPs (n) | SSY (%) | SPY (%) | PPV (%) | NPV (%) | p value |
|---|---|---|---|---|---|---|---|---|
| Discovery (n = 34) | pT0pN0cM0 | 14 | 20 | 93 | 100 | 100 | 95 | <0.001 |
| Discovery (n = 34) | ≤pT1pN0cM0 | 15 | 19 | 87 | 100 | 100 | 90 | <0.001 |
| Validation (n = 24) | pT0pN0cM0 | 9 | 15 | 56 | 73 | 56 | 73 | 0.22 |
| Validation (n = 24) | ≤pT1pN0cM0 | 11 | 13 | 64 | 85 | 78 | 73 | 0.033 |
RSPs = responders; NRSPs = nonresponders; SSY = sensitivity; SPY = specificity; PPV = positive predictive value; NPV = negative predictive value.
3.2. Validation
To validate our findings, we used tumor samples from MIBC patients treated on a separate, similarly designed neoadjuvant clinical trial testing a different cisplatin-based chemotherapy regimen (DDGC; NCT01611662) [4]. Of the 31 patients treated in the study, 25 completed all three cycles of chemotherapy. One additional patient was excluded because of insufficient tissue, leaving 24 pretreatment samples for analysis (Table 1). These samples were sequenced and analyzed in an identical fashion to the discovery set. Within this DDGC validation set, 434 alterations were detected among 170 genes (Fig. 1). Seven of 11 (64%) responders (≤pT1pN0cM0) had an alteration in one or more of ATM, RB1, and FANCC, compared to 2/13 (15%) of the nonresponders (p = 0.033), validating the results from our discovery set. The operating characteristics of this decision rule using the discovery and validation data sets for each definition of pathologic response (≤pT1pN0cM0 and pT0pN0cM0) are summarized in Table 2.
The ATM/RB1/FANCC decision rule was also predictive of better PFS (p = 0.0085) and OS (p = 0.007) in the AMVAC discovery set over a median follow-up period of 28.3 mo. In the DDGC validation set, for which follow-up is less mature (median 16.75 mo), 9/24 patients developed metastases and 6/24 have died. All six deaths occurred among ATM/RB1/FANCC wild-type patients, and 8/9 of the patients with disease progression had wild-type ATM/RB1/FANCC genes, indicating a nonsignificant improvement in PFS (p = 0.1018) and a trend towards improved OS (p = 0.0545; Fig. 2).
Fig. 2.
Progression-free survival (PFS) and overall survival (OS) by ATM/RB1/FANCC mutation status for the AMVAC discovery and DDGC validation sets. Alteration in any one of ATM/RB1/FANCC predicts better PFS (p = 0.0085) and OS (p = 0.007) in the AMVAC discovery set, with a trend towards significance for OS (p = 0.0545) in the DDGC validation set. wt = wild type; mut = mutation; PTs = patients.
The gene alterations grouped by responders and non-responders are depicted in Figure 1. For the AMVAC discovery set, the number of variants for each sample was correlated with response to chemotherapy according to a two-sample t test. Responders (≤pT1pN0cM0) had more alterations (mean 25.00, range 11–39) than nonresponders (mean 18.58, range 8–32; p = 0.03). Correlation between the number of alterations and response was not confirmed in the validation DDGC data set when using ≤pT1pN0cM0 as the response definition, but was significant using the more stringent cutoff of pT0pN0cM0. The mean number of alterations for the pT0pN0cM0 group was 22.67 (range 14–35) compared to 15.33 (range 7–29) among those with residual disease at cystectomy (p = 0.018; Table 3).
Table 3.
Number of alterations as a predictor of response
| Set | Response definition | RSPs (n) | NRSPs (n) | Mean alterations, n (median) {range} | p value | |
|---|---|---|---|---|---|---|
| NRSPs | RSPs | |||||
| Discovery (n = 34) | pT0pN0cM0 | 14 | 20 | 18.65 (16) {8–32} | 25.36 (27) {11–39} | 0.024 |
| Discovery (n = 34) | ≤pT1pN0cM0 | 15 | 19 | 18.58 (16) {8–32} | 25.00 (26) {11–39} | 0.030 |
| Validation (n = 24) | pT0pN0cM0 | 9 | 15 | 15.33 (13) {7–29} | 22.67 (22) {14–35} | 0.018 |
| Validation (n = 24) | ≤pT1pN0cM0 | 11 | 13 | 16.15 (15) {7–29} | 20.36 (21) {8–35} | 0.181 |
RSPs = responders; NRSPs = nonresponders.
3.3. Functional prediction modeling
Not all mutations lead to deficits in protein function. To investigate whether the ATM, RB1, and FANCC mutations observed were likely to affect protein function, we applied a prediction model to assess the potential biologic consequences of the alterations. Using a support vector machine trained with sequence and protein structural features as previously described [13], we assigned missense mutations as deleterious or neutral (Fig. 3, Supplementary Tables 1 and 2, and Supplementary Figs. 1 and 2). For RB1, experimental structures were used as the input; no solved structures exist for ATM, so we used homology modeling to generate a structure based on the related structures of PI3K and mTOR. In the AMVAC discovery set, all alterations were predicted to be deleterious except for one (K2413Q in ATM) that was predicted to be neutral. In the DDGC validation set, nine patient samples contained a total of 14 distinct alterations among ATM, RB1, and FANCC; two samples contained alterations in more than one of these three genes. Of the 14 alterations, 12 were predicted to be deleterious, disrupting protein structure and/or important protein-protein interaction interfaces. Of the remaining two, only one (D1791N in ATM) was predicted to be neutral (score 0.32; also neutral by Polyphen2, score 0.046), and occurred in a responding patient without other alterations in the other two genes. This analysis emphasized the functional impact of mutations in the gene signature, supporting our hypothesis that the functional DNA repair deficiency imparted by these alterations explains the chemosensitivity demonstrated.
Fig. 3.
ATM and RB protein domains and structures annotated with alterations. (A) RB domains and variants. The positions of RB missense variants and truncations are mapped with respect to known domains. The domains of RB are denoted along with their sequence ranges (except Rb-C, which corresponds to residues 829–872). RbN A and RbN B denote the A and B N-terminal domains. Pocket A and Pocket B denote the two pocket domains of RB. Rb-C is the C-terminal conserved motif. Truncations are marked with arrows. Red triangles denote missense mutations in responders, while green triangles denote missense mutations in nonresponders. Mutations predicted to be deleterious are marked with a black border around the triangles; those predicted to be neutral do not have a border. Mutations found in the same patient are connected with thin red lines. TCGA mutations associated with bladder cancer are denoted below the protein domain diagram with blue triangles. (B) ATM domains and variants. The positions of ATM missense variants and truncations are mapped with respect to known domains. (C) Predicted structure of ATM. The FAT (light green), PI-3/PI-4 kinase (light blue), and FATc (orange helix in the background) domains in a predicted structure of ATM are shown in ribbon representation. The wild-type residues found where missense variants were determined in this study are shown with red spheres. The magenta spheres represent a PI-3 kinase inhibitor molecule and thus mark the active site of the kinase domain.(D) Structure of RB1. RB-C domain bound to transcription factor Dp-1 and transcription factor E2F1 (Protein Data Bank entry 2AZE). The RB-C domain is shown in orange, Dp-1 in green, and E2F1 in cyan. The wild-type residue for mutation S862G is shown in red spheres; it forms a side- chain/side-chain hydrogen bond with E864 of RB, shown in orange spheres. It forms backbone hydrogen bonds with C274 of Dp-1 (not shown).
3.4. Genomic alterations and gene expression subsets
The pretreatment tumor samples from our AMVAC discovery cohort were previously analyzed and reported by Choi et al [12] defining three distinct subsets of urothelial cancer according to gene expression data: basal, p53-like, and luminal. In total, 33 of our AMVAC discovery samples had both genomic alteration data and an assigned subset. The ATM/RB1/FANCC signature did not correlate with subset assignment. Alterations predicted to be deleterious did not reliably result in the absence of gene expression. For a cutoff of p < 0.05 we found that deleterious ATR and VHL mutations occurred more commonly in the p53-like cluster, while deleterious SMARCA4 mutations occurred exclusively in the basal cluster (Supplementary Table 3). We did not identify any genes for which expression was significantly associated with ATM or RB1 mutation status; however, the expression levels of multiple genes, including the GAGE cancer testes antigens, were significantly (false discovery rate <0.05 and fold-change >2.0 or <0.5) associated with FANCC alterations. This pattern was not discernable in the two FANCC altered bladder cancer samples in the TCGA data set, although the low frequency of FANCC alterations (2/130) may be a confounder [5]. Together, these data emphasize a lack of congruence between genomic mutation and gene expression that has implications in selecting a modality for biomarkers of treatment response.
4. Discussion
Failure to repair treatment-induced DNA damage has been widely reported as a key mechanism of sensitivity to cytotoxic chemotherapy [14–16]. Cisplatin, the key component of the AMVAC and DDGC chemotherapy regimens, acts like an alkylating agent, inducing DNA damage by causing intrastrand and interstrand DNA crosslinks [17]. It has been posited that effective DNA repair is a mechanism of resistance to these regimens in bladder cancer.
ATM, RB1, and FANCC are mutated in approximately 11%, 14%, and 2% of urothelial carcinomas, respectively, and each gene is important for DNA repair [6,18,19]. It has been reported that defects in each of these genes confer sensitivity to chemotherapy in cell lines and animal models [20–26]. On the basis of known functions of the ATM, RB1, and FANCC gene products, we hypothesize that deleterious defects in these genes among chemosensitive patients in our AMVAC trial represent an Achilles heel for tumors: an inability to repair the DNA damage induced by chemotherapy. The observation that higher numbers of alterations correlate with response to chemotherapy was reported by Van Allen et al [9] and is observed in our study (Table 3), supporting the hypothesis that the higher frequency of alterations among responders than nonresponders reflects phenotypic accumulation of DNA damage due to a genotypic defect in DNA repair among responding tumors. While the majority of alterations in ATM, RB1 and FANCC were predicted to be deleterious to protein function, deleterious alterations did not correlate with expression transcripts of these genes, suggesting that the genetic alterations may lead to expression of crippled or inactive protein products.
We found tight correlation between ATM/RB1/FANCC alterations and pathologic response (p < 0.001), PFS (p = 0.0085), and OS (p = 0.007) in the AMVAC discovery set. The ability of the ATM/RB1/FANCC signature to predict response was confirmed in the DDGC validation set (p = 0.033). The majority of progression events and all deaths in the DDGC validation group were among those without ATM/RB1/FANCC alterations. However, this association between ATM/RB1/FANCC alterations and clinical benefit in terms of PFS and OS must be viewed as a trend because it did not meet criteria for significance, potentially because of the small sample size and short follow-up. As with any study investigating markers predictive of response to NAC, it is difficult to determine how the rate of pT0 after transurethral resection alone (historically 4.6% at our center) may have affected our results [27]. Other reports examining retrospectively collected samples suggest that alterations in ERCC2 or ERBB2 enhance cisplatin sensitivity, but the heterogeneity of chemotherapy regimens, the number of cycles administered, and exclusion of patients with intermediate responses represent limitations [9,28]. Further confounding cross-study comparison, Groenendijk et al [28] considered pathologically node-positive patients as intermediate or partial responders if the pathologic T stage was ≤T2, excluding this group from the correlation analysis. Pathologicallynode-positivepatients were considered nonresponders by Van Allen et al [9] and in our study, irrespective of T stage. ERCC2 was not included in the panel of genes tested and ERBB2 did not correlate with response or improve the predictive signature in our analysis. Definitive validation of these and other biomarkers will require investigation in larger independent data sets controlled for specific chemotherapy regimens, ideally with a control cohort of patients who do not receive NAC to define the signature as predictive rather than simply prognostic.
5. Conclusions
Genomic alterations in the DNA repair-associated genes ATM, RB1, and FANCC predicted response and clinical benefit after cisplatin-based chemotherapy for MIBC in our two independent prospective data sets. These results suggest that defective DNA repair renders tumors sensitive to cisplatin. Larger independent prospective data sets of homogeneously treated patients are needed to further clarify and validate these findings. We expect that our ability to understand and define the capacity of individual tumors to repair chemotherapy-induced damage will evolve, ultimately leading to improvement and refinement of a predictive signature that can be clinically applied to select appropriate treatments for patients with urothelial and other advanced cancers.
Supplementary Material
Acknowledgments
This work was presented in part at the 2014 and 2015 Annual Meetings of the American Society of Clinical Oncology and the 2015 American Society of Clinical Oncology Genitourinary Symposium.
Funding/Support and role of the sponsor: This work was supported in part by the Fox Chase Cancer Center Institutional Research Pilot Program, Fox Chase Cancer Center NCI Core Grant #P30CA00692, and Grant IRG-92-027-17 from the American Cancer Society.
The authors would like to thank the patients enrolled in our two clinical trials for consenting to donate tissue samples for this study, as well as research staff at the Fox Chase Cancer Center Extramural Research Program (B. Adaire-Halenda, G. Duncan, and C. Jerome), the Fox Chase Cancer Center Protocol Management Office (C. Cione, C. O’Sullivan, K.C. Wright, and A. Eigenbrode), and Thomas Jefferson University Hospital (D. Kilpatrick). Ilya G. Serebriiskii received partial salary support for this project from the Russian Government Program of Competitive Growth of Kazan Federal University. We dedicate this manuscript to the memory of Nancy Finnegan, supporter of bladder cancer research.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eururo.2015.07.009.
Footnotes
Author contributions: Elizabeth R. Plimack had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Plimack, Ross, Golemis.
Acquisition of data: Plimack, Dunbrack, Brennan, Andrake, Alpaugh, Dulaimi, Palma, Hoffman-Censits, Bilusic, Wong, Kutikov, Viterbo, Greenberg, Chen, Lallas, Trabulsi, Yelensky, McConkey, Miller.
Analysis and interpretation of data: Plimack, Dunbrack, Brennan, Andrake, Zhoul, Serebriiskii, Slifker, Yelensky, McConkey, Miller, Golemis, Ross.
Drafting of the manuscript: Plimack, Dunbrack, Andrake, Golemis, Ross.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Ross, Zhou, Slifker.
Obtaining funding: Plimack.
Administrative, technical, or material support: Plimack. Supervision: Plimack.
Other: None.
Financial disclosures: Elizabeth R. Plimack certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: ER Plimack and EA Ross have a patent filing related to these findings.
References
- 1.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 2.Vale CL. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–6. doi: 10.1016/j.eururo.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Sonpavde G, Goldman BH, Speights VO, et al. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009;115:4104–9. doi: 10.1002/cncr.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated meth-otrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol. 2014;32:1895–901. doi: 10.1200/JCO.2013.53.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–9. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyer G, Al-Ahmadie H, Schultz N, et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J Clin Oncol. 2013;31:3133–40. doi: 10.1200/JCO.2012.46.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plimack ER, Hoffman-Censits JH, Kutikov A, et al. Neoadjuvant dose-dense gemcitabine and cisplatin (DDGC) in patients (pts) with muscle-invasive bladder cancer (MIBC): final results of a multicenter phase II study. ASCO Meeting Abstr. 2014;32:4513. [Google Scholar]
- 9.Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014;4:1140–53. doi: 10.1158/2159-8290.CD-14-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choueiri TK, Jacobus S, Bellmunt J, et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfil-grastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol. 2014;32:1889–94. doi: 10.1200/JCO.2013.52.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnirke A, Melnikov A, Maguire J, et al. Solution hybrid selection with ultra-long oligonucleotides for massively parallel targeted sequencing. Nat Biotechnol. 2009;27:182–9. doi: 10.1038/nbt.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–65. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Q, Xu Q, Dunbrack RL., Jr Prediction of phenotypes of missense mutations in human proteins from biological assemblies. Proteins. 2013;81:199–213. doi: 10.1002/prot.24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291–5. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 15.Bouwman P, Jonkers J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat Rev Cancer. 2012;12:587–98. doi: 10.1038/nrc3342. [DOI] [PubMed] [Google Scholar]
- 16.Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–83. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 17.Kufe DW, Frei E, Holland JF. Cancer medicine. 7. London: Elsevier; 2006. [Google Scholar]
- 18.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9:759–69. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 20.Köcher S, Spies-Naumann A, Kriegs M, Dahm-Daphi J, Dornreiter I. ATM is required for the repair of topotecan-induced replication-associated double-strand breaks. Radiother Oncol. 2013;108:409–14. doi: 10.1016/j.radonc.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Samuelson AV, Lowe SW. Selective induction of p53 and chemo-sensitivity in RB-deficient cells by E1A mutants unable to bind the RB-related proteins. Proc Natl Acad Sci U S A. 1997;94:12094–9. doi: 10.1073/pnas.94.22.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knudsen KE, Booth D, Naderi S, et al. RB-dependent S-phase response to DNA damage. Mol Cell Biol. 2000;20:7751–63. doi: 10.1128/mcb.20.20.7751-7763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosco EE, Mayhew CN, Hennigan RF, Sage J, Jacks T, Knudsen ES. RB signaling prevents replication-dependent DNA double-strand breaks following genotoxic insult. Nucleic Acids Res. 2004;32:25–34. doi: 10.1093/nar/gkg919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taniguchi T, Tischkowitz M, Ameziane N, et al. Disruption of the Fanconi anemia-BRCA pathway in cisplatin-sensitive ovarian tumors. Nat Med. 2003;9:568–74. doi: 10.1038/nm852. [DOI] [PubMed] [Google Scholar]
- 25.D’Andrea AD, Grompe M. The Fanconi anaemia/BRCA pathway. Nat Rev Cancer. 2003;3:23–34. doi: 10.1038/nrc970. [DOI] [PubMed] [Google Scholar]
- 26.Chirnomas D, Taniguchi T, de la Vega M, et al. Chemosensitization to cisplatin by inhibitors of the Fanconi anemia/BRCA pathway. Mol Cancer Ther. 2006;5:952–61. doi: 10.1158/1535-7163.MCT-05-0493. [DOI] [PubMed] [Google Scholar]
- 27.Canter D, Long C, Kutikov A, et al. Clinicopathological outcomes after radical cystectomy for clinical T2 urothelial carcinoma: further evidence to support the use of neoadjuvant chemotherapy. BJU Int. 2011;107:58–62. doi: 10.1111/j.1464-410X.2010.09442.x. [DOI] [PubMed] [Google Scholar]
- 28.Groenendijk FH, de Jong J, Fransen van de Putte EE, et al. ERBB2 mutations characterize a subgroup of muscle-invasive bladder cancers with excellent response to neoadjuvant chemotherapy. Eur Urol. doi: 10.1016/j.eururo.2015.01.014. In press. http://dx.doi.org/10.1016/j.eururo.2015.01.014. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.