Abstract
Research has shown that lymphocytes of high-distress patients have reduced DNA repair relative to that of low-distress patients and healthy controls. Furthermore, deficits in repair are associated with an increased risk of cancer. Using and academic stress model, we hypothesized that students would exhibit lower levels of Nucleotide Excision Repair (NER) during a stressful exam period when compared to a lower stress period. Participants were 19 healthy graduate level students. NER was measured in lymphocytes using the unscheduled DNA synthesis (UDS) assay with slide autoradiography. Contrary to prediction, mean values for NER significantly increased during the higher stress period relative to the lower stress period controlling for background differences in repair. Furthermore, lymphocytes had significantly increased repair of endogenous damage during the higher stress period. Stress appears to directly increase DNA repair. Additionally, stress may increase DNA repair indirectly by increasing damage to DNA.
Keywords: Psychological stress, academic stress, DNA repair, DNA damage, nucleotide excision repair
Human DNA is constantly exposed to both endogenous (e.g., superoxide and hydroxyl radicals) and exogenous (e.g., ultraviolet radiation, X-rays, chemicals) genotoxic agents (Feigelson, Ross, Yu, Coetzee, Reichardt and Henderson, 1997). Unrepaired damage due to a reduction or loss of DNA repair leads to permanent somatic mutations and an accumulation of mutations within a single cell drives that cell towards malignancy and cancer (Hall and Johnson, 1996; Vogelstein and Kinzler, 1993). There are several DNA repair pathways organized according to the type of damage repaired or the mechanism of repair (Bohr, 1995; Friedberg, Walker and Siede, 1995). For example, oxidative damage is repaired by the Base Excision Repair (BER) pathway, which removes a single damaged base (Yu, Chen, Ford, Brackley and Glickman, 1999). Lesions induced by UV radiation are repaired by the Nucleotide Excision Repair (NER) pathway, which removes a long 22–30 bp patch of DNA (Yu et al., 1999). BER, NER, and Double Strand Break (DSB) repair pathways repair lesions induced by ionizing radiation. (Yu et al., 1999)
People who inherit DNA repair deficiency syndromes such as xeroderma pigmentosum (XP), Bloom’s syndrome, ataxia telengiectasia (AT), or hereditary nonpolyposis colorectal cancer (HNPCC) are at increased risk for developing cancer (Setlow, 1978; Bonn, 1998). For instance, people born with XP are prone to skin cancer because they have a mutation in an NER gene that prevents them from repairing damage caused by exposure to UV light (Bootsma et al., 1995; Bonn, 1998). People with HNPCC have a mutation in a gene encoding for mismatch repair, a system involved in correcting replication errors (Jiricny, 1994).
The connection between DNA repair and cancer is not limited to people with inherited syndromes. DNA repair deficits (NER) in blood (PBL’s) have been linked to sporadic cancers such as basal cell carcinoma (Wei, Matanoski, Fanner, Hedayati and Grossman, 1994), breast cancer (Kovacs, Stucki, Weber and Muller, 1986), lung cancer (Wei, Cheng, Hong and Spitz, 1996) and head and neck cancer (Cheng, Eicher, Guo, Hong, Spitz and Wei, 1998).
In addition, research has shown that increasing age, independent of antioxidant status, is associated with a decrease in oxidative repair and an increase in mutation in lymphocytes (Bootsma et al., 1995). Aging was also associated with a decrease in the ability to repair UV damage and an increase in DNA mutability in both cultured skin and blood cells (Barnett and King, 1995). Finally, an age-dependent decline in DNA repair has been linked with increases in accumulated mutations in lymphocytes (Moriwaki, Ray, Tarone, Kraemer and Grossman, 1996). However, little work has focused on examining modifiable factors that contribute to the normal variation in DNA repair. Understanding these factors is important for understanding the complex process of carcinogenesis.
There is some evidence that stress can affect DNA repair. In a sample of nonpsychotic psychiatric inpatients, high-distress patients had reduced repair in lymphocytes (i.e., decreased nucleoid sedimentation rates) two hours and five hours after X-irradiation relative to low-distress patients and matched controls. In addition, repair rates in the psychiatric patients were reduced 5 hours post-irradiation compared with healthy controls (Kiecolt-Glaser, Stephens, Lipetz, Speicher and Glaser, 1985). This study provided the first evidence that stress altered DNA repair capacity in humans. If stress suppresses aspects of DNA repair, there may be important consequences for several aspects of cancer prevention and control.
Stress has also been shown to inhibit the repair of carcinogen-induced DNA damage in rats (Glaser, Thorn, Tarr, Kiecolt-Glaser and D’Ambrosio, 1985). Rats were given 50 parts per million (ppm) of dimethylnitrosamine (DMN; a carcinogen) in their drinking water for 16 days and were randomly assigned to a stress or no stress condition. The stress condition consisted of four 24-hr periods of rotational stress in their home cage. After 16 days, the stressed animals had significantly less spleenic methytransferase activity (an important DNA repair enzyme) than the nonstressed animals that had the same level of carcinogen exposure. This suggests that stress may moderate the effect of genotoxic exposures via alteration of DNA repair mechanisms.
The primary objective of this research was to evaluate the relationship between stress and repair of exogenously damaged DNA. Academic examinations are commonplace stressors that have been studied extensively. They are reliably associated with alterations in erythron variables (e.g., hematocrit, hemoglobin, mean corpuscular volume) (Maes, et al., 1998), wound healing (Marucha, Kiecolt-Glaser and Favagehi, 1998), total serum protein (Van Hunsel et al., 1998), serum immunoglobulins, complement, and acute phase proteins (Maes et al., 1997), reactivation of latent Epstein-Barr Virus (Glaser, Pearl, Kiecolt-Glaser and Malarkey, 1994), and inhibition of radiation-induced apoptosis (Tomei, Kiecolt-Glaser, Kennedy and Glaser, 1990). These data suggest that an examination stress model may be a productive way of studying the effects of stress on DNA repair.
METHODS
This study was a prospective examination of perceived stress and DNA repair in 19 students during both a low stress (time 1) and higher stress period (time 2). The low stress period was scheduled within 2 days of returning from spring or summer break, and the higher stress period occurred during the week preceding final or board exams. The primary hypothesis, derived from Kiecolt-Glaser et al. (1985), was that students would exhibit lower levels of DNA repair in peripheral blood lymphocytes during the stressful exam period than during the (relatively) low stress period. This study was conducted after review and approval by the University of Pittsburgh Institutional Review Board.
Participants
Participants were healthy first and second-year students recruited from the University of Pittsburgh Medical School (n = 8), Dental School (n = 4), Law School (n = 4), and School of Pharmacy (n = 3). Exclusion criteria included smoking, personal history of cancer, and personal history of major psychiatric disorder. Subjects were paid $10 at the end of each session.
Procedure
Upon arrival at the testing site, the study and procedures were explained to the participants and informed consent was obtained. After the subjects completed background and perceived stress questionnaires, 30 ml of blood were drawn into three 10 ml green top tubes (Vacutainer Brand) by a trained phlebotomist or medical assistant. Subjects were then given written information about the time and date of their second appointment and were paid. Procedures for both the low and higher stress periods were similar.
Measures
Basic demographic information and family history of cancer were measured as background variables. In order to evaluate whether the higher stress period was perceived as more stressful, stress appraisal was measured on the Perceived Stress Scale (PSS), a 14-item self-report measure of the extent to which respondents feel their lives are unpredictable, uncontrollable, and overloaded (Cohen, 1986). The PSS demonstrates good internal consistency (α=.85) (Cohen, Kamarck and Mermelstein, 1983).
NER was measured in peripheral blood lymphocytes using the unscheduled DNA synthesis (UDS) assay with autoradiography (Cleaver and Thomas, 1981). UDS is a robust functional assay that measures the amount of [3-H]-thymidine incorporated into DNA after an exogenous dose of damaging UV-C light in vitro. This permits the quantitative measurement of overall genomic repair (both transcribed and untranscribed genes). The advantage of using a functional assay for NER is that the assay can examine the coordinated functioning of all of the gene products (approximately 30, 11 of which have been cloned) that operate in this repair pathway (Latimer, Hultner, Cleaver and Pedersen, 1996). Additionally, the autoradiographic procedure for UDS, as opposed to the scintillation counting method, allows for a clear-cut elimination through visual inspection of cells incorporating [3-H]-thymidine that are in S-phase.
Blood Preparation
Blood samples were obtained from the participants via standard phlebotomy procedures. Peripheral blood lymphocytes (PBL) were isolated by Ficoll-Hypaque Gradient Centrifugation (Coligan, Kruisbeek, Margulies, Shevach and Strober, 1995). After the final wash, pellets were resuspended in media containing RPMI (Beckman TJ-6) supplemented with 10% autologous serum and 1% pen/strep (Gibco). Autologous serum was used because it contains relevant stress hormones (e.g., cortisol) or biological response modifiers characteristic of the person from whom the sample was obtained (Larcom and Smith, 1988; Larcom, Morris and Smith, 1990). These factors would not be present in samples incubated with fetal bovine sera. Aliquots were placed into culture on chamber slides coated with a diluted form of Matrigel (Collaborative Research, Inc.). This allowed the attachment of the PBLs to the slide (Latimer et al., 1996; Latimer et al., submitted), enabling autoradiographic analysis. Three chamber slides were prepared for each subject.
Assay Procedures
After 3 days in culture with autologous serum and without passaging, the PBL’s were assayed for NER. One side of each slide was irradiated with UV-C light (254 nm at a mean fluence of 1.2J/m−2s−1×12 seconds for a total of 14J/m2) using a machine specifically built to deliver a reproducible dose of UV radiation (Steier and Cleaver, 1969). The unirradiated side of the slide served as an untreated control and reflected the rate of NER prior to exogenous damage. Immediately following irradiation, samples were cultured in labeling medium containing 10μCi ml [3H]-methyl-thymidine (80Ci/mmol) (NEN Dupont) and allowed to repair for 2 hours at 37°C. The kinetics of UV repair is such that 2 hours of incubation are enough time to repair induced 6–4 photoproducts but not pyrimidine dimers (Latimer et al., submitted). After 2 hours, the labeling medium was removed and replaced with a chasing medium containing non-radioactive thymidine (Sigma) allowing any residual radioactive thymidine to be removed from the intracellular nucleotide pools. The slides were then immersed in photographic emulsion (Kodak type NTB2), and allowed to develop for 11 to 15 days in complete darkness (Cleaver and Thomas, 1981). Tester slides with control cells (foreskin fibroblasts and MDA MB231) were used to assess the optimal time of exposure.
In addition to the peripheral blood lymphocytes, human foreskin fibroblasts were plated, irradiated, and labeled as described for the lymphocyte samples for each experiment. These cells have been documented to have the highest level of NER in mammals and have traditionally served as positive internal controls for the UDS assay (Latimer et al., 1996; Latimer et al., submitted). Normal foreskin fibroblasts have also traditionally been used as a standard in the clinical diagnosis of the classic NER disorder xeroderma pigmentosum, a recessively inherited deficiency in the repair of UV damage.
Determination of NER of exogenously damaged DNA
Following development of the slides, the nuclei were stained with Giemsa and the number of radiolabled grains over the nuclei of 88 non-S-phase cells per chamber was counted at 1000X magnification under oil-immersion. Local background counts for each microscopic field were subtracted from the grain counts of each nucleus as a correction. The average number of grains per nucleus for both the irradiated and unirradiated sides of the slide was then calculated. The final values for the mean number of grains per nucleus for each slide were calculated by subtracting the corrected unirradiated mean grains per nucleus from the corrected irradiated means.
Mean grain counts for each subject were determined by averaging the mean counts for each of the three slides. This represents the corrected average number of grains over the nuclei of at least 276 cells per subject. Results are expressed as a percentage of irradiated foreskin fibroblast repair, the positive standard of comparison for each experiment.
Normalizing the average number of grains per nucleus in lymphocytes to that of foreskin fibroblast, run in the same experiment, enables inter-assay comparisons and controls for inter-assay variation.
Determinations of NER of Endogenously Damaged DNA
Levels of repair of endogenous damage were calculated using the average number of grains per nucleus from the unirradiated side of the chamber slide. Mean grain counts for each subject were determined by averaging the mean counts for each of the three slides. Results are expressed as a percentage of irradiated foreskin fibroblast repair, the positive standard of comparison for each experiment. As previously stated, normalizing the average number of grains per nucleus in lymphocytes to that of foreskin fibroblasts, run in the same experiment, enables inter-assay comparisons and controls for inter-assay variation.
RESULTS
Demographics
Nineteen subjects completed the protocol at time 1. The mean age of the sample was 25.3 years (range = 19–35). The sample was 68% female (n = 13) and 84% of participants (n = 16) described themselves as Caucasian. Eighty-nine percent (n = 17) were single and never married. This sample consisted of students in a professional academic training program; all of them had completed at least 16 years of schooling. Sixty-three percent (n = 12) reported at least one family member with cancer. Demographic data are summarized in Table 1.
Table 1.
Sample demographic characteristics
| Variable | Mean (SD) | Frequency | % |
|---|---|---|---|
| Age in years | 25.3 (4.34) | ||
| Gender | |||
| Male | N = 6 | 31.6 | |
| Female | N = 13 | 68.4 | |
| Ethnicity | |||
| African-American | N = 1 | 5.3 | |
| Asian | N = 1 | 5.3 | |
| Caucasian | N = 16 | 84.2 | |
| Other | N = 1 | 5.3 | |
| Marital Status | |||
| Single | N = 17 | 89.5 | |
| Married | N = 1 | 5.3 | |
| Divorced | N = 1 | 5.3 | |
| Family History of Cancer | |||
| Yes | N = 12 | 63.2 | |
| No | N = 7 | 36.8 | |
Fourteen of the nineteen subjects returned to provide data for time 2. Values for the demographic variables, PSS scores, and NER at time 1 did not differ significantly between those who completed both time points and those who did not complete the second assessment. The demographic characteristics of the sample did not change. All subsequent analyses were carried out using data from only those subjects who completed both assessments.
For the manipulation check, paired t-tests were performed on the measures of perceived stress. As expected, the students reported significantly higher levels of perceived stress just prior to exams (time 2) than after returning from a semester or spring break, t (13) = −2.53, p < .05, two-tailed.
DNA Repair
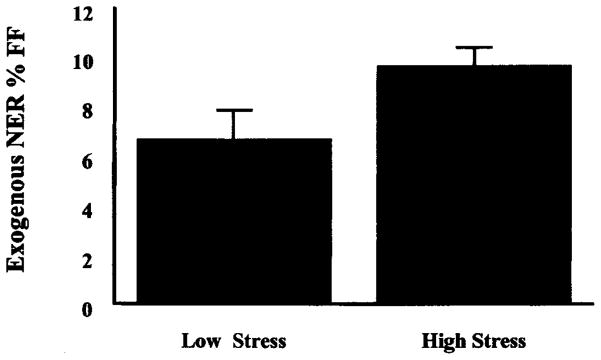
In order to test the primary hypothesis that DNA repair would be reduced during the examination period, mean values for NER were compared, again using a paired t-test. Contrary to our original hypothesis, mean values for NER during the examination period (M =9.58%, SD = .79) were significantly greater than mean values for NER during the low stress period (M =6.23%, SD = 1.31), t(13) = −2.47, p <.05 (see Figure 1). There were no differences in DNA repair at time 1 or time 2 as a function of gender or family history of cancer.
Figure 1.
Repair of exogenously damaged DNA by stress period.
Note: Mean values for NER are significantly higher during the high stress period, t(13) = −2.75, p <. 05.
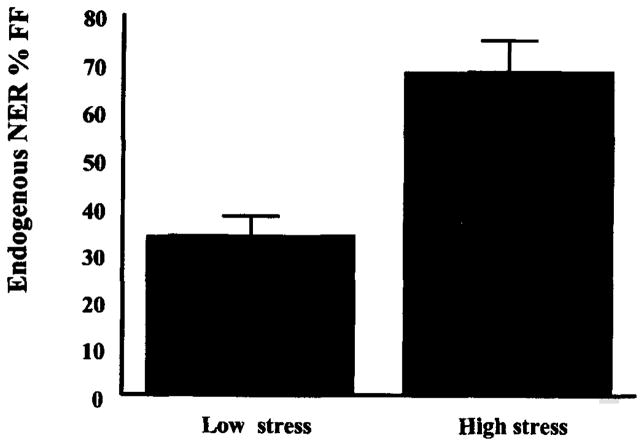
To examine the possibility that increased NER might be a function of endogenous DNA damage, secondary analyses with the NER values of the lymphocytes from the unirradiated side of the chamber slide were conducted. This allowed us to examine preirradiated levels of NER during the exam and non-exam periods. While this value is typically removed from the calculation of NER as a background control, it has previously been viewed as an index of DNA repair that may reflect pre-existing levels of DNA damage (Fischman, Pero and Kelly, 1996). The levels of NER during the exam period (M =67.78% SD = 25.19) were almost twice that for the non-exam period (M =36.63% SD = 17.74), t(13) = −4.99, p < .001, suggesting that stress may be damaging to DNA in a way that can be remediated by the NER pathway (see Figure 2).
Figure 2.
Repair of endogenously damaged DNA by stress period.
Note: Mean values for NER are significantly higher during the high stress period, t(13) = 4.99, p < .001.
DISCUSSION
This study tested the hypothesis that stress associated with academic examinations can inhibit the repair of exogenously damaged DNA. This hypothesis was examined by evaluating the NER capacity of peripheral blood lymphocytes from healthy, nonsmoking, graduate students during both a low and high stress period. Contrary to prediction, DNA repair increased significantly during the examination period and was independent of age, gender, and family history of cancer. Thus, stress may account for some of the non-age related variation in NER that has been reported previously (Setlow, 1983; Wei et al., 1994; Grossman, 1997).
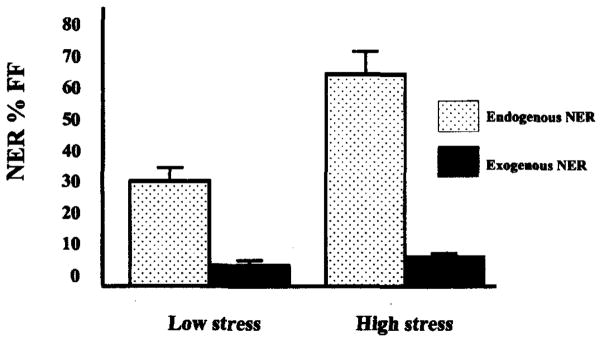
Additionally, stress may increase DNA repair indirectly by increasing damage to DNA. In other words, stress may have direct or indirect genotoxic effects that would require adaptive increases in DNA repair to keep the rate of mutation the same. We examined this possibility in secondary analyses with the NER values of the lymphocytes from the unirradiated side of the chamber slide. As noted previously, increased levels of NER prior to exogenously induced damage may reflect increases in endogenous DNA damage (Fischman, Pero and Kelly, 1996). The observed increase in unirradiated levels of NER during the high stress period lends support to the hypothesis that the higher levels of DNA repair observed during the high stress examination period were due at least in part, to greater DNA damage (see Figure 3).
Figure 3.
Repair of exogenously and endogenously damaged DNA by stress period.
Note: Mean values for NER of exogenously damaged DNA are significantly higher during the high stress period, t(13) = −2.75, p < .05.
Mean values for NER of endogenously damaged DNA are significantly higher during the high stress period, t(13) = 4.99, p < .001.
These results are different from those reported by Kiecolt-Glaser et al. (1985) and the extensive dissimilarities in both the samples and the methods of these studies make comparison somewhat problematic. However, these differences may account for some discrepancies. Severity of stress is likely to be important, and the present study measured modest, transient stress in a sample of young, healthy, nonsmoking, community-dwelling students. Kiecolt-Glaser et al. (1985) measured more severe distress in psychiatric inpatients. Another important difference concerns the timing of the putative stressor. Exam related stress could be considered an acute or episodic event of a relatively fixed duration. In contrast, mental illness severe enough to warrant hospitalization is typically more chronic in nature. The differential effects of acute and chronic stress exposure on DNA repair are unknown.
Additionally, each study examined different repair systems. The present study looked at the NER of UV damaged DNA. UV light creates very specific types of lesions in DNA (i.e. 6–4 photoproducts and pyrimidine dimers). These lesions are remediated specifically though the NER pathway. In contrast, X-ray damage induces a much wider variety and severity of lesions (e.g., single and double strand breaks, oxidative damage) requiring the involvement of many different repair systems including NER. It is not clear how the mechanisms of UV and X-ray damage repair relate to each other.
Another difference concerns preparation of the cells prior to irradiation. We cultured our cells in media supplemented with autologous serum, not fetal bovine serum (FBS). Larcom, Morris and Smith (1990) report that autologous serum always yields higher values than FBS for UDS reasoning that the serum contains biological response modifiers that could affect cellular processes related to repair. This may also partially account for our higher values and the persistence of the stress effects across three days of culture.
Finally, our study allowed only two hours for repair of the DNA before removing the radioactive label. Kiecolt-Glaser et al. (1985) measured repair at 0, 2, and 5 hours. When compared with healthy blood donors, differences were not apparent until the repair process had gone on for 5 hours, with no difference in repair observed at two hours. The many differences in stress-related and DNA repair-related variables suggest that future studies will need to pay closer attention to the various methodological details in measurement of DNA damage and repair.
Our results also differ from those of Glaser et al. (1985). This is not surprising as there are differences in the DNA repair systems of rats and humans (Cleaver, Speakman and Volpe, 1995). For example, UDS in human cells is 5–10 fold higher than in rodent cells and excises more pyrimidine dimers (Layer and Cleaver, 1997). Additionally, rodents appear to repair damage in genomic regions that are actively transcribed while humans repair both transcribed and untranscribed regions (Layer and Cleaver, 1997). Finally, Glaser et al. (1985) did not measure NER, but the expression of spleenic methytransferase activity which functions as part of the Base Excision Repair (BER) pathway.
Despite the small size of our study sample, our findings are statistically significant. The within-subjects design provides more power than a between-subjects design with a similar sample size and is unprecedented in the DNA repair literature. Additionally, small sample size (and the resultant low power) often contributes to the inability to find significant differences that truly exist. Our significant result argues against this possibility. However, we do acknowledge that our small sample size is a limitation that argues for caution when generalizing these results. A larger more representative sample would increase variance and could yield different results.
A selection bias may also exist in our volunteer sample. However, the bias seems to have favored those students that were least stressed during exams. That is, the most distressed students did not participate because they did not want their blood drawn immediately prior to their exams. This made detection of a significant difference more difficult, not less. Despite this fact, the difference in repair was still significant.
A third variable other than stress may be responsible for the observed increase in repair. Changes in diet, exercise, sleep or some other unidentified variable may influence DNA repair. The lack of a control group does not allow us to evaluate these alternative hypotheses.
We have suggested that studying the relationship between stress and DNA repair could help explain variation in DNA repair capacity and the subsequent long-term cancer risk. This in turn may offer insight into discrepancies in the literature looking at stress and cancer. Previous research examining the effects of stress on cancer has often led to inconsistent and inconclusive results (Cassileth, 1996; Fox, 1995; Hilakivi-Clarke, Rowland, Clarke and Lippman, 1994; Garssen and Goodkin, 1999; McGee, Williams and Elwood, 1996; Spiegel and Kato, 1996). Methodological problems such as recall and reporting biases, inadequate timing of stress measurements and failure to account for strong biological (e.g., stage of tumor) or behavioral (e.g., smoking history) factors, have made drawing firm conclusions impossible. More importantly, efforts to link stress with the etiology of cancer have failed to identify the intrinsic genetic mechanisms that are affected by stress and that influence neoplastic growth. Research in this area is strengthened by focusing on potential biological mechanisms such as increases in DNA damage, alterations in the amount or rate of DNA repair, inhibition of apoptosis, increases in somatic mutations, and failures of immune surveillance.
Contrary to our hypothesis, we found that NER of exogenously damaged DNA was increased during a period of high stress in young healthy students. Furthermore, we found increases in levels of NER prior to irradiation suggesting as one possibility that stress may be causing an increase in endogenous DNA damage. There is a small literature supporting the relationship between stress and DNA damage in animals. Psychological stress has been shown to damage DNA at the molecular (Adachi, Kawamura and Takemoto, 1993) and chromosomal levels in rats (Fischman, 1989; Fischman, Pero and Kelly, 1996), but there are no such studies in humans. These findings suggest that damage studies directly measuring DNA adducts or excreted DNA damage products should be a priority in research on stress and carcinogenesis.
Acknowledgments
The authors would like to thank Dr. Gregory Miller and Dr. Steven Grant for their intellectual and creative input into the preparation of this manuscript. We would also like to thank Elena Kisin for running the UDS assay, and Laurie Hall and Trish Piatti for their expert assistance in blood collection.
References
- Adachi S, Kawamura K, Takemoto K. Oxidative damage of nuclear DNA in liver of rats exposed to psychological stress. Cancer Research. 1993;53:4153–4155. [PubMed] [Google Scholar]
- Barnett YA, King CM. An investigation of antioxidant status, DNA repair capacity and mutation as a function of age in humans. Mutation Research. 1995;338:115–128. doi: 10.1016/0921-8734(95)00017-z. [DOI] [PubMed] [Google Scholar]
- Bonn D. How DNA-repair pathways may affect cancer risk. Lancet. 1998;351(9095):42. doi: 10.1016/S0140-6736(05)78079-X. [DOI] [PubMed] [Google Scholar]
- Bootsma D, Weeda G, Vermeulen W, Van Vuure H, Troelstra C, Van Der Spek P, Hoeijmakers JHJ. Nucleotide excision repair syndromes: Molecular basis and clinical symptoms. Philosophical Transactions of the Royal Society London. 1995;347:75–81. doi: 10.1098/rstb.1995.0012. [DOI] [PubMed] [Google Scholar]
- Bohr V. DNA repair fine structure and its relation to genomic instability. Carcinogenesis. 1995;16(12):2885–2892. doi: 10.1093/carcin/16.12.2885. [DOI] [PubMed] [Google Scholar]
- Cassileth BR. Stress and the development of breast cancer. Cancer. 1996;77(6):1015–1016. doi: 10.1002/(sici)1097-0142(19960315)77:6<1015::aid-cncr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Cheng L, Eicher SA, Guo Z, Hong WK, Spitz MR, Wei Q. Reduced DNA repair capacity in head and neck cancer patients. Cancer Epidemiology Biomarkers Prevention. 1998;7:465–468. [PubMed] [Google Scholar]
- Cleaver JE, Thomas GH. Measurement of unscheduled synthesis by autoradiography. In: Friedberg EC, Hanawalt PC, editors. DNA Repair: A Laboratory Manual of Research Procedures. New York: Marcel Dekker; 1981. pp. 277–287. [Google Scholar]
- Cleaver JE, Speakman JR, Volpe JP. Nucleotide excision repair: variations associated with cancer development and speciation. Cancer Surveys. 1995;25:125–142. [PubMed] [Google Scholar]
- Cohen S. Contrasting the hassles scale with the perceived stress scale: Who’s really measuring appraised stress? American Psychologist. 1986;41(6):716–718. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. New York: John Wiley and Sons, Inc; 1995. pp. 7.1.1–7.1.7. [Google Scholar]
- Feigelson HS, Ross RK, Yu MC, Coetzee GA, Reichardt JKV, Henderson BE. Genetic susceptibility to cancer form exogenous and endogenous exposures. Journal of Cell Biochemistry. 1997;255:15–22. doi: 10.1002/(sici)1097-4644(1996)25+<15::aid-jcb2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Fischman H. Sister chromatid exchanges induced by behavioral stress. Loss, Grief and Care. 1989;3(3–4):203–213. [Google Scholar]
- Fischman H, Pero RW, Kelly DD. Psychogenic stress induces chromosomal and DNA damage. International Journal of Neuroscience. 1996;84:219–227. doi: 10.3109/00207459608987267. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W. DNA Repair and Mutagenesis. Washington, DC: ASM Press; 1995. [Google Scholar]
- Fox BH. The role of psychological factors in cancer incidence and prognosis. Oncology. 1995;9(3):245–256. [PubMed] [Google Scholar]
- Garssen B, Goodkin K. On the role of immunological factors as mediators between psychosocial factors and cancer progression. Psychiatry Research. 1999;85:51–61. doi: 10.1016/s0165-1781(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Glaser R, Pearl DK, Kiecolt-Glaser JK, Malarkey WB. Plasma cortisol levels and reactivation of latent herpes virus in response to examination stress. Psychoneuroendocrinology. 1994;19(8):765–772. doi: 10.1016/0306-4530(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Glaser R, Thorn BE, Tarr KL, Kiecolt-Glaser J, D’Ambrosio SM. Effects of stress on methytransferase synthesis: An important DNA repair enzyme. Health Psychology. 1985;4(5):403–415. doi: 10.1037//0278-6133.4.5.403. [DOI] [PubMed] [Google Scholar]
- Grossman L. Epidemiology of ultraviolet-DNA repair capacity and human cancer. Environmental Health Perspectives. 1997;105(supp 4):927–930. doi: 10.1289/ehp.97105s4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M, Johnson RT. The role of DNA repair in the prevention of cancer. Molecular Aspects of Medicine. 1996;17(3):235–283. doi: 10.1016/s0098-2997(96)00001-5. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Rowland J, Clarke R, Lippman ME. Psychosocial factors in the development and progression of breast cancer. Breast Cancer Research and Treatment. 1994;29(2):141–160. doi: 10.1007/BF00665676. [DOI] [PubMed] [Google Scholar]
- Jiricny J. Colon cancer and DNA repair: Have mismatches met their match? Trends in Genetics. 1994;10(5):164–168. doi: 10.1016/0168-9525(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Stephens RE, Lipetz PD, Speicher CE, Glaser R. Distress and DNA repair in human lymphocytes. Journal of Behavioral Medicine. 1985;8(4):311–320. doi: 10.1007/BF00848366. [DOI] [PubMed] [Google Scholar]
- Kovacs E, Stucki D, Weber W, Muller Hj. Impaired DNA repair-synthesis in lymphocytes of breast cancer patients. European Journal of Cancer and Clinical Oncology. 1986;22:863–869. doi: 10.1016/0277-5379(86)90375-5. [DOI] [PubMed] [Google Scholar]
- Larcom LL, Morris TE, Smith ME. Factors which affect DNA repair in human lymphocytes. In: Sutherland BM, Woodhead AD, editors. DNA Repair in Human Tissues. New York: Plenum Press; 1990. pp. 263–276. [DOI] [PubMed] [Google Scholar]
- Larcom LL, Smith ME. Quantitative assay for evaluation immunocompetence and DNA repair capacity. Journal of the National Cancer Institute. 1988;80(14):1112–1118. doi: 10.1093/jnci/80.14.1112. [DOI] [PubMed] [Google Scholar]
- Latimer JJ, Hultner ML, Cleaver JE, Pedersen RA. Elevated DNA excision repair capacity in the extraembryonic mesoderm of the midgestation mouse embryo. Experimental Cell Research. 1996;228:19–28. doi: 10.1006/excr.1996.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer JJ, Nazir T, Dimsdale J, Kisin E, Kanbour-Shakir A, Flowers L. DNA repair capacity in normal breast and ovarian epithelium under review. [Google Scholar]
- Layer SL, Cleaver JE. Quantification of XPA gene expression levels in human and mouse cell lines by competitive RT-PCR. Mutation Research. 1997;383:9–19. doi: 10.1016/s0921-8777(96)00040-7. [DOI] [PubMed] [Google Scholar]
- Maes M, Hendriks D, Van Gastel A, Demedts P, Wauters A, Neels H, Janca A, Scharpe S. Effects of psychological stress on serum immunoglobulin, complement and acute phase protein concentrations in normal volunteers. Psychoneuroendocrinology. 1997;22(6):397–410. doi: 10.1016/s0306-4530(97)00042-5. [DOI] [PubMed] [Google Scholar]
- Maes M, Van Der Planken M, Van Gastel A, Bruyland K, Van Hunsel F, Neels H, Hendriks D, Wauters A, Demedts P, Janca A, Scharpe S. Influence of academic examination stress on hematological measurements in subjectively healthy volunteers. Psychiatry Research. 1998;80(3):201–212. doi: 10.1016/s0165-1781(98)00059-6. [DOI] [PubMed] [Google Scholar]
- Marucha PT, Kiecolt-Glaser JK, Favagehi M. Mucosal wound healing is impaired by examination stress. Psychosomatic Medicine. 1998;60(3):362–365. doi: 10.1097/00006842-199805000-00025. [DOI] [PubMed] [Google Scholar]
- McGee R, Williams S, Elwood M. Are life events related to the onset of breast cancer? Psychological Medicine. 1996;26(3):441–448. doi: 10.1017/s0033291700035522. [DOI] [PubMed] [Google Scholar]
- Moriwaki Ray, Tarone Kraemer, Grossman The effect of donor age on the processing of UV-damaged DNA by cultured human cells: Reduced DNA repair capacity and increased DNA mutability. Mutation Research. 1996;364:117–123. doi: 10.1016/0921-8777(96)00029-8. [DOI] [PubMed] [Google Scholar]
- Setlow RB. Repair deficient human disorders and cancer. Nature. 1978;271:713–717. doi: 10.1038/271713a0. [DOI] [PubMed] [Google Scholar]
- Setlow RB. Variation in DNA repair among humans. In: Harris C, Autrup H, editors. Human Carcinogenesis. New York: Academic Press; 1983. pp. 231–244. [Google Scholar]
- Spiegel D, Kato PM. Psychosocial influences on cancer incidence and progression. Harvard Review of Psychiatry. 1996;4:10–26. doi: 10.3109/10673229609030518. [DOI] [PubMed] [Google Scholar]
- Steier H, Cleaver JE. Exposure chamber for quantitative ultraviolet photobiology. Lab Practice. 1969;18:1295. [PubMed] [Google Scholar]
- Tomei LD, Kiecolt-Glaser JK, Kennedy S, Glaser R. Psychological stress and phorbol ester inhibition of radiation-induced apoptosis n human peripheral blood leukocytes. Psychiatry Research. 1990;33(1):59–71. doi: 10.1016/0165-1781(90)90149-y. [DOI] [PubMed] [Google Scholar]
- Van Hunsel F, Van Gastel A, Neels H, Wauters A, Demedts P, Bruyland K, Demeester I, Scharpe S, Janca A, Song C, Maes M. The influence of psychological stress on total serum protein and patterns obtained in serum protein electrophoresis. Psychological Medicine. 1998;28(2):301–309. doi: 10.1017/s0033291797006351. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler K. The multistep nature of cancer. Trends in Genetics. 1993;9(4):138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- Wei Q, Cheng L, Hong WK, Spitz MR. Reduced DNA repair capacity in lung cancer patients. Cancer Research. 1996;56:4103–4107. [PubMed] [Google Scholar]
- Wei Q, Matanoski GM, Farmer ER, Hedayati MA, Grossman L. DNA repair related to multiple skin cancers and drug use. Cancer Research. 1994;54:437–440. [PubMed] [Google Scholar]
- Yu Z, Chen J, Ford BN, Brackley ME, Glickman BW. Human DNA repair systems: An overview. Environmental and Molecular Mutagenesis. 1999;33:3–20. doi: 10.1002/(sici)1098-2280(1999)33:1<3::aid-em2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]