Abstract
Ferula communis L., subsp. communis, namely giant fennel, has extensively been used in traditional medicine for a wide range of ailments. Fresh plant materials, crude extracts and isolated components of F. communis showed a wide spectrum of in vitro and in vivo pharmacological properties including antidiabetic, antimicrobial, antiproliferative, and cytotoxic activities. The present paper, reviews the traditional uses, botany, phytochemistry, pharmacology and toxicology of F. communis in order to reveal its therapeutic potential and future research opportunities. A bibliographic literature search was conducted in different scientific databases and search engines including Scopus, Cochrane Library, Embase, Google Scholar, Pubmed, SciFinder, and Web of science. Phytochemical studies have led to the isolation of different compounds such as sesquiterpenes from F. communis. This plant has two different chemotypes, the poisonous and non-poisonous chemotypes. Each chemotype is endowed with various constituents and different activities. The poisonous chemotype exhibits anticoagulant and cytotoxic activities with sesquiterpene coumarins as major constituents, while the non-poisonous one exhibits estrogenic and cytotoxic effects with daucane sesquiterpene esters as the main compounds. In addition, although various pharmacological properties have been reported for F. communis, anti-microbial activities of the plant have been investigated in most studies. Studies revealed that F. communis exhibits different biological activities, and contains various bioactive compounds. Although, antibacterial and cytotoxic activities are the two main pharmacological effects of this plant, further studies should focus on the mechanisms underlying these actions, as well as on those biological activities that have been reported traditionally.
Keywords: Anticoagulant, Ferula communis L., Sesquitepene coumarins, Sesquiterpenes, Toxicity
Introduction
The genus Ferula (Apiaceae) with about 170 species, mostly occurs throughout central Asia, the Mediterranean region and Northern Africa. Ferula spp. has a long history of medicinal use and their pharmacological effects are well documented in both the human and animal studies. A large number of compounds have been identified in this genus. These include sesquiterpenes (1, 2), sesquiterpene coumarins (3-5), sesquiterpene lactones (6) and sulfur-containing compounds (7-10).
Ferula communis L. subsp. communis (Giant fennel) is a latex-containing perennial plant, 1–2.5 m high, odoriferous, with dense roots. Its cylindrical peduncle is green, striated, with slimy exudate. The branches, 8–10 cm long, are alternate (inferior) or opposite (superior). The leaves are glabrous, with a large sheath. The inflorescence is attached on the terminal part of the peduncle. The plant, despite the name, is not a type of fennel proper belonging to the other genus Foeniculum. The name of the phenolic compound ferulic acid, which can be isolated from giant fennel, is derived from the Latin name of the plant.
Two chemotypes of F. communis L. have been characterized with different biological effects. Poisonous chemotype contain mainly prenyl coumarins such as ferulenol (1) and related compounds that are responsible for ferulosis (a lethal haemorrhagic disorder which mainly affects goats, sheep, cattle, and horses) and its toxicity. The other chemotype is non-poisonous and contains daucane esters (11, 12). Genetically, these two chemotypes are different and distributed in different geographic regions (13). Non-poisonous plant is used traditionally as a hormonal plant because of its phytoestrogens content, while the poisonous one may cause different intoxication and even death (14).
In the present paper, we aimed to review the traditional uses, pharmacology, phytochemistry and toxicology of this ancient medicinal plant. All related available information on F. communis was collected via electronic search (using Pubmed, SciFinder, Scirus, Embase, Google Scholar, Scopus, Cochrane Library and Web of Science) without time limit.
Traditional uses
F. communis has traditionally been used as anti-hysteria and for the treatment of dysentery (15, 16). The rhizomes of this plant which is known as Al-kalakh in Saudi Arabia are used locally as a traditional remedy for the treatment of skin infections, while the roasted flower buds are used against fever and dysentery (17).
Traditional uses of non-poisonous F. communis as phytohormone are attributed to ferutinin (21), an aromatic ester of a daucane alcohol. It has been reported that this plant acts as a possible source of phytoestrogens of the daucane type. In Morocco, F. communis has traditionally been used as a hypoglycemic medicinal plant (18) but its use has been restricted due to its toxicity.
In traditional medicinal books such as Dioscorides, numerous beneficial applications have been reported for this plant. For example, administration of mashed fresh kernel is useful for expulsion of oral bloody humors, and for the treatment of stomachache with diarrhea. It is also recommended for the treatment of snakebite. Preparation of the crushed plants in the form of wick seal bleeding of the bleeding organ. Administration of plant seeds improves cramps, and if rubbing the combination of mashed seeds with oil on the skin, causes perspiration (19).
Phytochemistry
Out of all compounds identified in F. communis, sesquiterpene and their derivatives (11, 20, 21), sesquiterpene coumarins (22) and prenylated sesquiterpene coumarins are found abundantly (11, 23, 24).
Investigations on F. communis have shown that each of the two identified chemotypes has their own major phytochemicals: one chemotype contains sesquiterpene daucane esters, and the other one is dominated by prenylated coumarins (20, 21).
To consider the chemical differences between two chemotypes of F. communis, Arnoldi et al (13), studied daucanes and coumarins of two chemotypes using HPLC-DAD-UV, HPLC-ESI-MS and HPLC-APCI-MS analyses. The chromatogram of non-poisonous chemotype showed two main peaks attributed to ferutinin (21) and the prenylated coumarin ferulenol (1) as well as 10 minor peaks contributing to daucane esters including lapiferin (43) and jaeskeanadiol benzoate (ferutinol benzoate) (66). The chromatogram of poisonous chemotype was totally different from the non-poisonous one. Five main peaks were detected possessing a 4-hydroxy-coumarin structure. This chemotype was reported to contain the toxic coumarins ferulenol (1) and its ω-hydroxy derivatives (25). Daucane derivatives could not be detected in the ‘poisonous’ chemotype of F. communis (13).
Not only have phytochemical studies been established on various chemotypes of F. communis, but several studies also have investigated the major compounds in different plant parts. Studying the fruits of F. communis showed that prenylated coumarins were absent, whereas sesquiterpenoid esters, such as the phenylpropane laserin (79) (26) and the daucane ester of anisate (45) were present (20, 26). The fruits from the plants containing prenylated coumarins in their roots and leaves gave in fact a series of l-oxojaeskeanadiol esters. The major constituent of the fruits was a crystalline mixture of the 5-(O)-angeloyl (46) and isovaleroyl (47) esters of 1-oxojaeskeanadiol. Also, the 5(O)-anisoyl ester of 1-oxojaeskeanadiol (48), was obtained from the more polar fractions (26).
Sesquiterpene coumarins (prenylated coumarins)
Ferula is a genus rich in coumarins, particularly sesquiterpene coumarins (27-29). To date, many sesquiterpene coumarins have been identified from this genus (29). In Figure 1, the chemical structures of sesquiterpene coumarins of this species that have been reported to date, are depicted. Ferulenol (1) is the most abundant and the first coumarin isolated from this species (30). Biological activities of ferulenol have been investigated in several studies (31). In 1986, Valle et al isolated two 4-hydroxycoumarin derivatives bearing a farnesylic [ferulenol (1)] and a 12-hydroxy farnesylic residue (2) at C-3 from the latex of F. communis (20). In a separate study, these two compounds were isolated from the root sap of F. communis var. genuina with haemorrhagic activity and toxicity (25). Derivatives of prenylated coumarins ferulenol and ferprenin (3) (a pyrano coumarin) (24) with functional groups hydroxyl, acetoxyl, aldehydic carbonyl at the end of the prenyl residue, have also been isolated from the poisonous chemotype of F. communis (11).
Figure 1.
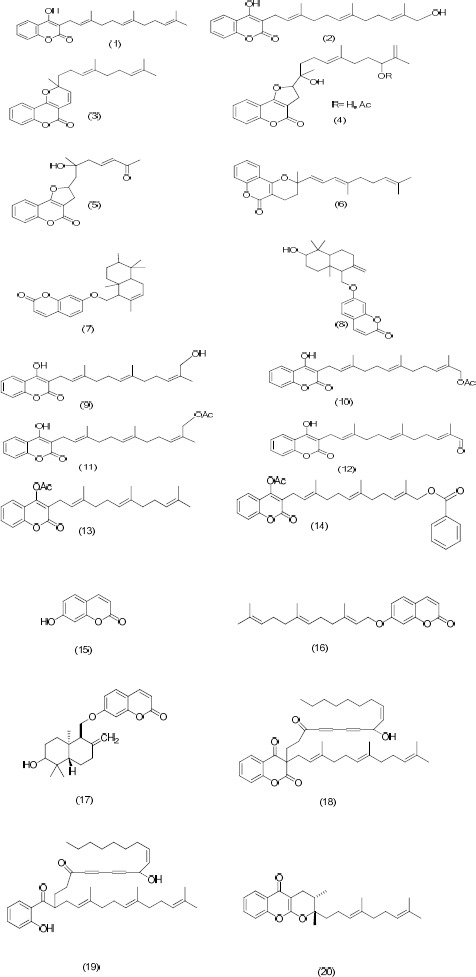
Sesquiterpene coumarins isolated from F. communis
Some other sesquiterpene coumarins reported from F. communis include samarcandin (22), two new cyclic farnesylcoumarins (4,5), isoferprenin (6) (32), 2-nor-1,2-secoferulenol (33), feselol (7) and colladonin (8) (21, 34).
Further investigation on aerial parts of F. communis grown in Saudi Arabia led to the identification of three compounds namely umbelliferone (15), umbelliprenin (16), and farnesiferol A (17) (35). The mentioned compounds have been reported from other Ferula Species (36, 37), however, the scientific name of plant used in that study needs to be confirmed by further investigation. To our knowledge, F. communis is a Mediterranean species of Ferula and there is no another document that confirms the presence of F. communis in Saudi Arabia.
Daucane sesquiterpene esters
A large number of daucane sesquiterpene esters have been isolated from F. communis with a l,5-trans-fused daucane ring system. For instance, Minski et al isolated a number of (21-37) daucane sesquiterpenes from the benzene extract of the dried roots of F. communis subsp. communis (21). Their further investigation on this plant led to the identification of the daucane ester 14-p-anisoyloxy-dauc-4,8-diene (69), fercomin (50), and fercolide (51) (daucane-γ-lactone) which is the first daucane-γ-lactone identified (21, 38). Daucane esters including compounds 22, 52-54 were also reported from this species (20). Daucane esters (55-61) from leaves and seeds of F. communis var. breuzfolia have been reported which are related to jaeschkeanadiol (67) (39). The major constituent of the fruits was a crystalline mixture of the 5-(O)-angeloyl (62) and isovaleroyl (63) esters of 1-oxojaeskeanadiol (39). In addition, 5-(O)-anisoyl ester of 1-oxojaeskeanadiol (64), was identified from more polar fractions (39). Compound 63 had previously been isolated as the oil from a plant of the Asteraceae family (17).
Investigation of F. communis rhizomes, using a bioautography-guided isolation technique, has led to the isolation and characterization of three antibacterial sesquiterpenes, namely, the daucane ester 14-(O-hydroxycinnamoyloxy)-dauc-4,8-diene (65), 2a-acetyl-6a-(benzoyl) jaeschkeanadiol (66) and 2a-acetyl-6a-(p-anisoyl)-jaeschkeanadiol (67) (40).
In a recent work, three daucane sesquiterpenes including acetoxyferutinin, oxojaeskeanadioyl anisate (48) and ferutidin (22), together with ferulenol, have also been identified from the aerial parts of F. communis (31).
Volatile constituents of F. communis
A number of studies have reported the chemical composition of Ferula essential oils (41, 42). In a study on F. communis leaf oil, forty seven components were identified. The main constituents were myrcene (53.5%) and aristolene (8.5%) (43), however, the oil contained a high content of monoterpenes. In addition, two main sesquiterpenes including, aristolene (8.5%) and (E,E)-farnesol (4.3%), were also present in the volatile oil (43). Chemical composition of essential oils from different parts of the plant showed that the content of aristolene reached 19.0% in leaf oil, while it was absent in flower oil. In contrast, the chemical composition of samples obtained from peduncle was different and dominated by sesquiterpenes. The sesquiterpenes aristolene, β –gurjunene, α- and β -selinenes were the major components of the essential oil of peduncle. It is notable that the yields of peduncle oil were at least two times lower than those of leaf and flower oils (43).
The study of the chemical composition of the oil from flower-heads using a carbon dioxide super-critical extraction (SPE) (44) showed that the main constituents in the oil were α-gurjunene, β-gurjunene, α-selinene and β-selinene. The oil obtained by SPE had a different appearance with that of obtained by a conventional hydrodistillation method. Indeed, the hydrodistilled oil due to the presence of chamazulene had a blue color while the SPE oil showed a pale yellow color (44). In the SPE oil, there is no thermal degradation of matricin and its conversion to the blue compound chamazulene.
Figure 2.
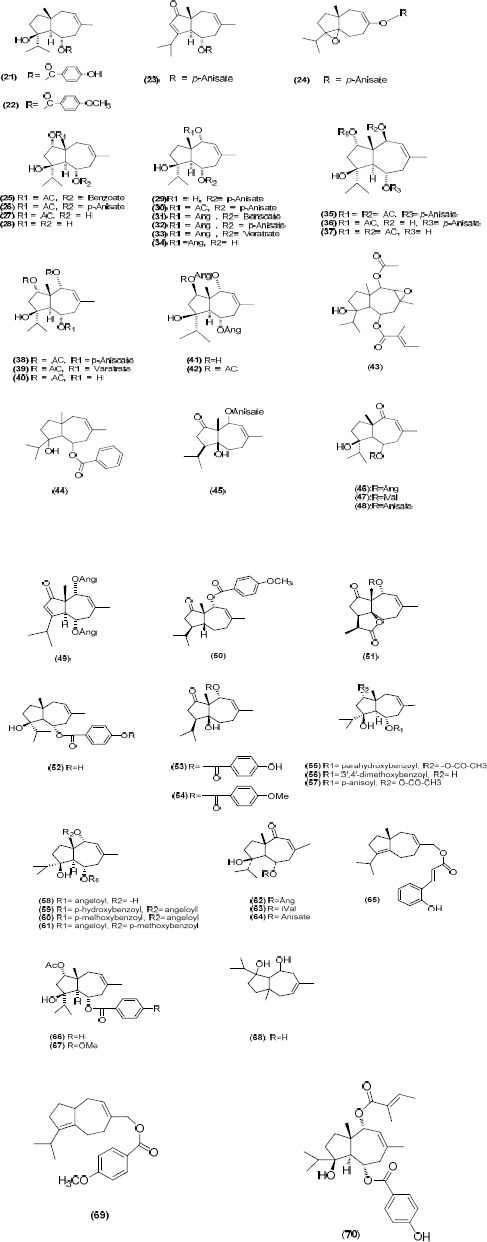
Daucane esters isolated from Ferula communis
Figure 3.
Miscellaneous compounds isolated from Ferula communis
Recently, ninety three compounds have also been identified in the total essential oil of Greek F. communis subsp. communis using a hydrodistillation method. Sesquiterpenes were the most dominant class of compounds in the oil of leaves and inflorescences, while the oil of infructescence was rich in monoterpenes, particularly α-pinene (35.2-40.6%) as the major component (45).
Miscellaneous compounds
Other compounds such as 3,4-methylenedioxy-5-hydroxy-propiophenone (71) (21), phenylpro-panoids 1-hydroxy-1-(3’-methoxy-4’,5’-methyl-enedioxy) phenylpropane (72) and 2-epihel-manticine (73) (21) and elemicin (46) were identified from F. communis (22). An unusual minor component fercoperol (74) which is a cyclic-endoperoxynero-lidol derivative has been also reported (47).
Chromones are the other class of compounds which have been isolated from F. communis. For example, an investigation on F. communis grown in turkey caused the identification of two cyclic farnesyl-chromone derivatives (75, 76) (22). The chromone sesquiterpene ferchromone (77) (40) was also obtained from the plant (21).
Acetylenes including ferulinolone [3-(1,2 dihydrofalcarinolonyl)-ferulenol] (18) and decarboxyferulinolone [2-nor-3-(1,2-dihydrofal-carinolonyl-ferulenol] (19) are the other class of metabolites identified from the roots of F. communis (48).
Biological effects
Most of in vitro studies on F. communis and its constituents have been focused on their antimicrobial properties. In 1998, Al-Yahya et al, examined anti-bacterial activity of constituents extracted from the rhizome of F. communis (40) including 14-(O-hydroxycinnamoyloxy)-dauc-4,8-diene (65), the sesquiterpene coumarin ferulenol (1), and the chromane sesquiterpene ferchromone (77). The petroleum ether extract of F. communis and its constituents showed anti-bacterial effects. Their findings revealed that the sesquiterpene 14-(O-hydroxycinnamoyloxy)-dauc-4,8-diene possessed strong activity against Staphylococcus aureus, Bacillus subtilis, Streptococcus durans and Enteracoccus faecalis comparable to those of streptomycin sulfate. On the other hand, ferulenol exhibited potent activity against four Mycobacterium strains, including M. intracellulare, M. xenopei, M. chelonei and M. smegmatis. The compound 14-(O-hydroxycinnamoyloxy)-dauc-4,8-diene exhibited significant activity against Gram-positive bacteria, while ferchromone was found to be less active (40).
Unasho et al also studied the in vitro antibacterial activities of F. communis root extract against clinical isolates of Streptococcus pyogenes and Streptococcus pneumonia (49). But the fractions of the plant were found to have weak antibacterial effects to the mentioned bacteria.
The antibacterial activities of three daucane sesquiterpenes including acetoxyferutinin, oxojaes-keanadioyl anisate (48), ferutidin (22) were examined against Botryotinia fuckeliana, Penicillium digitatum, Penicillium expansum, Monilinia laxa, Monilinia fructigena and Aspergillus spp. F. communis root extract only affected colony growth of Bortryo fuckeliana, Monilinia laxa and Monilinia fructigena and no inhibitory activity was observed on conidial germination under applied experimental concentrations. F. communis aerial part extract showed no activity on colony growth and was not tested on Conidia (31).
Fercoperol (74) which is a cyclic-endoperoxynerolidol derivative has been also reported to have amoebicidal activity. Anti-malarial effects of fercoperol are comparable with other standard anti-malarial compounds (47).
Ferulenol and its acetate exhibited antibacterial activity against Mycobacterium intracellulare, Mycobacterium xenopei, Mycobacterium chelonei and Mycobacterium smegmatis, with MIC values much lower (1.25–5.0 µg/ml) than those of the positive controls streptomycin sulfate, isonicotinic acid hydrazide (INH) and amikacin sulfate (40, 50). In addition, ferulenol showed synergistic action with INH towards the same strains of Mycobacterium.
Plant defensive effects of Ferula extracts have been examined against insect herbivores (such as Spodoptera littoralis and Myzus persicae) and the compounds responsible for these activities were identified (51) by bio-guided fractionation method. The nematicidal (Meloidogyne javanica) and phytotoxic (Lactuca sativa and Lolium perenne) activity of these extracts and compounds have been also investigated (51). Bioassay-guided fractionation led to the identification of ferulenol as the active anti-feedant compound.
Toxicological properties
Consumption of F. communis L. has been found to be associated with bleeding (ferulosis) in animals and humans (52). Studies showed that prenylated 4-hydroxycoumarins play an important role in pro-haemorrhagic effects of F. communis (11, 12, 14, 23, 53).
It has been known for a long time that the consumption of F. communis L. can cause in livestock an often lethal disease known as ferulosis. Cases of human poisoning from ingestion of F. communis have also been reported. Poisoning from F. communis causes symptoms in cattle similar to those described for the intoxication by fermented sweet clover, and it has been suggested that the plant contains antithrombic coumarin derivatives (53-55).
Biological tests established that, among compounds isolated from F. communis, only prenylated coumarins are toxic (56). Ferulenol (20) and ferprenin (24), the prenylated coumarins isolated from toxic variety of giant fennel, exhibited haemorrhagic effects in vivo. Extracts lacking these two compounds still remained toxic to experimental animals. This toxicity was assigned to more polar coumarins which were derivatives of ferulenol and ferprenin (24, 56). Other studies also confirm that only plants containing prenylated coumarins can elicit the toxic symptoms of ferulosis, whereas those containing daucane esters are not toxic (56).
In the root extract of prenylated coumarin-containing plants, based on the ratio of ferulenol to its ω-oxygenated derivatives, two patterns of constituents are detected; one in which ferulenol is the major constituent and one which consists almost exclusively of its more polar ω-oxygenated derivatives (11). But a very similar pattern of constituents is also found in leaves.
The toxic and the non-toxic varieties of F. communis differ not only in the presence of prenylated coumarins or sesquiterpene esters, but also in other classes of metabolites including volatile terpenoids and phenylpropanoids (11).
Alzweiri et al proposed acetylated ferulenol-oxy-ferulenol as a marker for fresh Ferula toxicity by using a metabolomic approach (57). Their results showed that chloroform extract of fresh F. communis had significantly different constituents from those detected in dried plants. One of the main different peaks in HPLC analysis was attributed to the mentioned compound.
Studying the mitochondrial effects of ferulenol revealed that it inhibited oxidative phosphorylation (58). Ferulenol has a dual effect on mitochondrial functions depending on concentration. At low concentrations, ferulenol inhibited ATP synthesis, while it did not have any effect on mitochondrial respiration. In contrast, at higher concentrations, ferulenol inhibited oxygen consumption. This data showed that ferulenol, and F. communis chemotype containing ferulenol, might exert their toxicity via other mechanisms including mitochondrial dysfunction (58).
Monti et al investigated the mechanisms by which F. communis caused severe bleeding and characterized anti-coagulant properties of prenylated coumarin ferulenol (59). Ferulenol did not exert its anti-coagulant activity directly. This compound exhibited hepatocyte cytotoxicity, and at non-cytotoxic concentrations (<100 nM), could reduce factor X biosynthesis. Structure activity studies showed that the presence of the prenyl residue in ferulenol derivatives is essential for ferulenol activity (59). Animals treated with ferulenol indicated a sharp decrease in activity for several coagulation factors (60).
Cytotoxic and antiproliferative activity
Beacause of the importance of cytotoxic effects of F. communis, we reviewed this activity and its related mechanisms separately in this section. A large number of studies have been performed for the characterization of its cytotoxic compounds. Some investigations suggested that the cytotoxic effect of F. communis could be related to ferulenol. For example, Bocca et al, investigated cytotoxic activity of ferulenol, and proposed that ferulenol stimulated tubulin polymerization in vitro, and inhibited the binding of radiolabeled colchicine to tubulin (61). It rearranged cellular microtubule network into short fibers, and altered nuclear morphology. Remarkably, ferulenol exerted a dose-dependent cytotoxic activity against various human tumor cell lines (61). Determination of the acute LD50s of ferulenol showed that it had a higher LD50 compared to warfarin and thus had a favorable toxicity profile. Hypoprothrombinemia with internal and external hemorrhages caused by the administration of ferulenol in mice was similar to symptoms of ferulosis and anti-vitamin K poisonings. Male mice were more sensitive to intoxication by ferulenol than females (12).
Antiproliferative effects of daucane esters (Figure 5) including certain jaesekanadiol p-hydroxy- and p-methoxybenzoates from F. communis on human colon cancer cell lines were investigated (62). Daucane esters from F. communis inhibited the growth of WiDr, COLO320-HSR and LS174 colon cancer cells in a dose-dependent manner. ID50 calculated after 72 hr of treatment, revealed that the anti-proliferative capacity of the compounds was in the following descending order: ferutinin (21) > 2α-OH-ferutidin > ferutidin (22) > siol anisate (54) > lapiferin (43) > jaeskeanadiol (68). Ferula daucane esters do not act as non-specific toxins randomly impairing the cellular metabolic machinery, but rather act by interaction with specific metabolic pathways (62).
Figure 5.
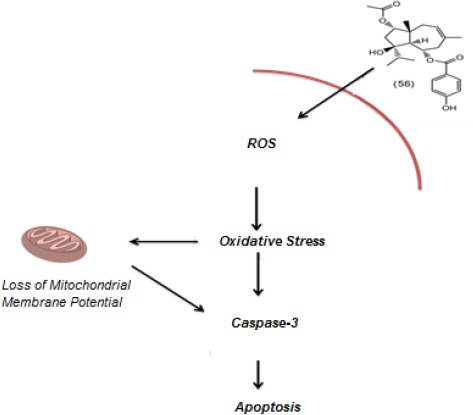
Daucane esters are able to induce apoptosis in cancer cells through interaction with mitochondrial membrane
The study on the anti-proliferative mechanism of ferutinin shows that it acts as a calcium ionophore in the T-leukemia cell line Jurkat and this event leads to a collapse of the mitochondrial membrane potential with consequent generation of reactive oxygen species (ROS) that ultimately lead to apoptosis (63).
Dall’Acqua et al investigated anti-proliferative activity of constituents from F. communis on different cell lines such as HeLa, A549, HL-60, Jurkat, K562, RS 4; 11 and SEM (64). Compounds (1-11, Figure 4) were identified and their anti-proliferative activities have been studied. Compounds (5), (7), and (9) exhibited cytotoxic effects against all the considered cell lines. Compound (7) was the most active against Jurkat while (9) showed the highest activity against all the other tested cell lines. All the most active compounds possessed ring fusion with trans geometry and the 6-(a)-ester linked group (p-methoxybenzoyl or angeloyl), while those with cis geometry (1 and 11) or 4- and 5-epoxy group (2) had poor activity. Also the double bond in the hepta-atomic ring appeared critical (64). The 10-β-hydroxy function or the 10-β-acetoxy substituent in these compounds is essential for the cytotoxic effects. Notably, for the two isomers (4) and (5) the IC50 is lower or at least the same (for HL-60) in all the cell lines for the compound bearing the 10β-hydroxy group (5). Data suggest that apoptosis may be the major cause of cell death (64).
Figure 4.
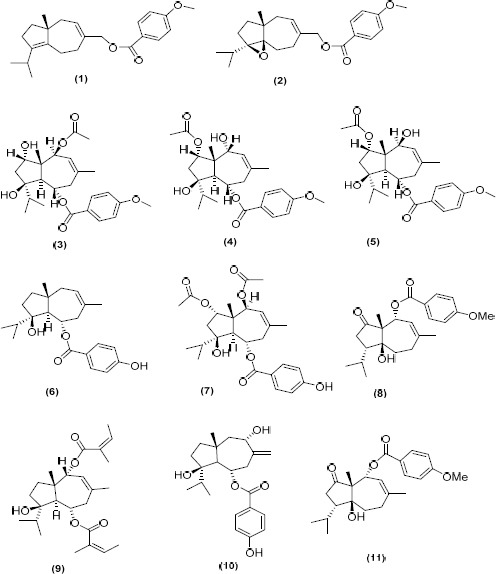
Cytotoxic sesquiterpenes isolated from Ferula communis
Dall’Acqua et al, in continuation of their research on anti-proliferative agents from plants, found natural daucane esters isolated from F. communis and Ferulago campestris induced apoptosis in G1 phase in leukaemic cells through ROS production (65). Among all isolated compounds, only one of them showed more activity against leukemic cell lines (compound 76). By using structure–activity relationship studies, they realized that there is a relation between cytotoxicity and trans geometry of the ring fusion.
Compound 76 also induces alterations of several intracellular antioxidant enzymes (65). This compound promotes oxidative stress in SEM cells via reducing antioxidant enzymes such as superoxide dismutase and other related enzymes.
Discussion
As discussed in previous sections, the most bioactive compounds were highlighted in the mind of readers; however, in this section we have a brief overlook on bioactive compounds of giant fennel. Ferulenol (1), a coumarin derivative, is the major constituent of the poisonous chemotype F. communis. Ferulenol was isolated for the first time from F. communis and is the universal precursor of farnesylated 4-hydroxycoumarins (30). As shown in Table 1, ferulenol has diverse pharmacological effects such as antimicrobial, anticoagulant, antifeedant, and antiproliferative activities. Recently, Gliszczyn´ ska et al, have reviewed sesquiterpene coumarins including ferulenol as lead compounds in drug discovery (66).
Table 1.
Biological activities reported from the bioactive compounds of Ferula communis
| Compound number | Compound name | Biological activities | Ref. |
|---|---|---|---|
| 57 and other daucane esters | Apoptosis inducer through ROS production in leukaemic cells | (56) | |
| 1 | Ferulenol and ω-hydroxyferulenol | Pro-haemorrhagic activity | (15) |
| 1, 3 | ω-oxygenated derivatives of ferulenol and ferprenin | Antithrombotic activity | (46) |
| 66 | Falcarindiol | Anti-platelet activity | (79) |
| 1, 65, 77 | Daucane ester 14-(O-hydroxycinnamoyloxy)-dauc-4,8-diene Ferulenol Ferchromone | Antibacterial activity | (30) |
| 4-hydroxycoumarins | Anticoagulant activity | (49, 80) | |
| 1 | Ferulenol | Antimycobacterial activity, inhibitory activity on succinate ubiquinone reductase, cytotoxic | (40, 52, 81, 82) |
| 21, 22 | Ferutinin, 2α-OH-Ferutidin, Ferutidin | Antiproliferative activity | (53) |
| 21 | Ferutinin | Anti-inflammatory | (82) |
| 21 | Ferutinin | Antifungal activity, Apoptosis inducer, Anti-cancer, bone formation, Calcium ionophoretic | (54, 59, 63, 65, 70, 74, 78) |
Ferutinin is a natural phytoestrogen with ionophoretic properties. The beneficial effects of non-poisonous chemotype of F. communis is attributed to phytoestrogens including ferutinin which was isolated for the first time from Ferula tenuisecta Eug. Kor. (67). It has been established that ferutinin has ionophoretic properties, i.e. it increases the permeability of thymocytes, mitochondria, sarcoplasmic reticulum, liposomes and bilayer lipid membranes (BLM) for cations, especially Ca2+ (68). It is more selective for divalent cations versus monovalent cations (69). Ferutinin is typically considered as non-toxic compound that is not able to exert anticoagulant activities like prenylated coumarins. Ferutinin, unlike the other Ca2+ ionophores which most likely increases (Ca2+] via the activation of platelet plasma membrane Ca2+ channels and the release of Ca2+ from intracellularstores, increases Ca2+ levels because of its Ca2+-ionophoretic properties, and does not induce blood platelet aggregation. Ferutinin stimulates the expression of the active form of the GPIIb-IIIa complex and whole blood platelet aggregation only weakly and has no statistically significant effect on the binding of fibrinogen. In fact, ferutinin has inconsistent effects; it raises intra-platelet Ca2+ concentration but fails to have an effect on spontaneous blood platelet aggregation. This pattern of responses may be caused by the combination of ferutinin-related Ca2+ ionophoric effect and estrogenic properties (70).
Anti-proliferative, cytotoxic and apoptotic effects of ferutinin in different cell lines have been also investigated (62, 71-73). This compound induces calcium mobilization from external and internal stores and eventually triggers apoptosis through a caspase-3 dependent pathway. It caused morphological changes, DNA damage, significant regression in tumor size in cancerous cells, while it showed less toxic effects in non-tumoral cells (74). According to structure-activity studies, p-hydroxylation of the benzoyl moiety is crucial for the activity (71, 75) while the parent polyol (jaeschkeanadiol, 2α) was inactive. Homologation, vinylation, methylation of the p-hydroxyl substituent and the introduction of oxygen functions on the adjacent carbons were essential for its activity (75).
Studying the effect of ferutinin on human red blood cells revealed that it induced in vitro apoptosis through membrane permeabilization and calcium influx (76). Death promoting activities of ferutinin in a number of cancer cells by opening the mitochondrial permeability transition pores have been documented well. The induction of apoptosis in human red blood cells is known as eryptosis or erythroptosis. Ferutinin causes eryptosis/erythroptosis in human red blood cells and simultaneously increases caspase-3 activity and the cytosolic free Ca2+ ion level (76).
Phytoestrogens as alternative drugs can be used for the treatment of osteoporosis and many studies have been investigated the anti-osteoporosis effects of phytoestrogens (77). Since ferutinin has esterogenic activity, its beneficial effect on osteoporosis has been investigated. For instance, oral administration of ferutinin in recovering severe osteoporosis has been studied in ovariectomized rats (78, 79). It could increase the recovery of bone loss caused by severe estrogen deficiency. Thus ferutinin can be listed among the potential compounds for the treatment of postmenopausal osteoporosis. Expression of the osteoblast phenotype markers osteocalcin (OCN), osteopontin (OPN), collagen I, RUNX-2 and osterix (OSX), increase in calcium deposition and osteocalcin secretion are proposed mechanisms for this activity of ferutinin (80). Anti-inflammatory (81), antibacterial (82, 83), aphrodisiac (84-86) and fungicidal (87) properties are other activities proposed for this compound.
Concluding remarks
F. communis subsp. communis, namely, giant gennel, showed versatile biological activities (Table 2) with various bioactive natural products. Two chemotypes of F. communis L. have been characterized with different chemical constituents. The toxic chemotype mainly produces prenylated coumarins such as ferulenol (1) that are responsible for a lethal haemorrhagic disorder called ferulosis. The non-toxic chemotype contains daucane type sesquiterpenoids such as ferutinin (21) and its pharmacological activities are attributed to these compounds. Antibacterial and cytotoxic activities are two areas that have been covered in previous studies. However, further studies should be focused on mechanisms behind the antibacterial and cytotoxic activities, and those biological activities that have been reported traditionally.
Table 2.
Pharmacological/biological activities reported from F. communis in detail
| Activity | Dosage form/type of extract | Concentrations/dosages | Tested living system/organ/cell | Active compound(s) | Result(s) | Ref. |
|---|---|---|---|---|---|---|
| Antibacterial | Petroleum ether extract | 1.25-5.0 mg/ml | Staphylococcus aureus, Bacillus subtilis, Streptococcus durans, Enterococcus faecalis, Mycobacterium intracellulare, Myco. xenopei, Myco. Chelonei, Myco. Smegmatis | Ester 14-(o-hydroxycinnamoyloxy)-dauc-4,8-diene, ferulenol, ferchromone | Ester 14-(O-hydroxycinnamoyloxy)-dauc-4,8-diene exhibited strong activity against Staph. aureus, Bacillus subtilis, Strep. durans and Entro. faecalis. Ferulenol showed strong antibacterial activity against S. aureus, B. subtilis, S. durans and E. faecalis and four Mycobacterium strains. Ferchromone exhibited potent activity against Bacillus subtilis, Staphylococcus aureus, Streptococcus durans, Enterococcus faecalis. | (40) |
| Antibacterial | 80% methanol crude extracts and hydro alcoholic solvent fractionates | 500 mg/ml to 1000 mg/ml | Clinical isolates of Strep. pyogenes and Strep. pneumoniae | Eighty percent ethanol solubilized fraction was found to have antibacterial effects to all assayed bacteria while aqueous solubilized fractions did not exhibit any effect | (49) | |
| Antifungal | n-hexane | ED50 almost > 400 | The colonies and conidia of Botryotinia fuckeliana, Penicillium digitatum, Pen. expansum, Monilinia laxa, Moni. fructigena and Aspergillus spp | Acetoxyferutinin, oxojaeskeanadioyl anisate, ferutidin, and ferulenol | Root extract presented a fungitoxic effect on the colony growth, but it was not able to inhibit the conidia germination. Aerial part extract showed less activity. | (51) |
| Amoebicidal | Fercoperol | |||||
| Antimycobacterial | Petroleum ether | Mycobacterium intracellulare, M. smegmatis, M. xenopei and M. chelonei | Ferulenol | Ferulenol exhibited synergism with isonicotinic acid hydrazide (INH). Its MIC decreased from 5.0 to 0.3 µg/ml. | (40, 50) | |
| Anti-coagulant | <100 nM | BabyHamster Kidney (BHK) cells | A series of prenylated 4-hydroxycoumarins including ferulenol | They did not directly affect blood coagulation but showed hepatocyte cytotoxicity and, at non-cytotoxic concentrations (<100 nM), impaired factor X biosynthesis (40% reduction) | (59) | |
| Aphid antifeedant | Hexane and ethanolic extracts | Herbivorous insects: (Spodoptera littoralis and Myzus persicae) Nematodes: (Meloidogyne javanica) Plants: (Lactuca sativa and Lolium perenne) | Ferulenol | The bioassay-guided search for aphid antifeedant compounds resulted in the isolation of ferulenol. F. communis root ethanolic exhibited a significant but moderate reduction in Spodo. littoralis larvae feeding. The hexane extracts displayed the most activity against Myzus persicae ethanolic extract showed the most phytotoxic activity. | (51) | |
| Ionophoretic and apoptotic properties | Leukemia T-cell line, Jurkat | Ferutinin | Ferutinin induces Ca2+ mobilization in Jurkat cells. It induces DCm disruption, ROS generation and apoptosis through a caspase-3 dependent pathway | (63) | ||
| The cytotoxic and apoptosis-inducing activities | 24-46 µg/ml | Human breast (MCF7) and bladder (TCC) cancer cells as well as normal fibroblasts (HFF3) | Ferutinin | Ferutinin caused DNA damage and apoptosis which was significantly (p<0.001) higher in MCF7 and TCC than that of normal cells HFF3 | (72) | |
| Antiproliferative | 0.8-60 µM | Human colon cancer cells | Daucane esters: ferutinin, 2α-hydroxyferutidin, Ferutidin, Siol anisate, Lapiferin, Jaeskeanadiol | Daucane esters inhibited the growth of WiDr, COLO320-HSR and LS174 colon cancer cells in a dose-dependent manner. the antiproliferative capacity of the compounds was in descending order: ferutinin > 2α-OH-ferutidin > ferutidin > siol anisate > lapiferin > jaeskeanadiol | (62) | |
| Aphrodisiac | Acute (2.5 mg/kg) and subchronic (0.25 mg/kg/day for 10 days) | Male rats | Ferutinin | Ferutinin is able to stimulate sexual behavior after acute ingestion in sluggish/impotent animals, but exerts a negative influence on the sexual capacity of potent male rats | (84) | |
| Aphrodisiac | Ovariectomized rats | Ferutinin | Ferutinin given alone markedly increased the intensity of the lordotic response but failed to significantly affect proceptivity. When administered in combination with estradiol, ferutinin reduced the increase in receptivity and proceptivity due to estrogen effects, acting as an antiestrogen. | (86) | ||
| Proliferation and osteoblastic differentiation | 10-8 and 10-9 M | Human amniotic fluid and dental pulp stem cells | Ferutinin | Ferutinin treatment induced greater expression of the osteoblast phenotype markers osteocalcin (OCN), osteopontin (OPN), collagen I, RUNX-2 and osterix (OSX), increased calcium deposition and osteocalcin secretion in the culture medium compared to controls | (80) |
References
- 1.Iranshahi M, Amin GR, Jalalizadeh H, Shafiee A. New germacrane derivative from Ferula persica Willd. var latisecta Chamberlain. Pharm Biol. 2003;41:431–433. [Google Scholar]
- 2.Iranshahi M, Ghiadi M, Sahebkar A, Rahimi A, Bassarello C, Piacente S, et al. Badrakemonin, a new eremophilane-type sesquiterpene from the roots of Ferula badrakema Kos.-Pol. Iran J Pharm Res. 2009;8:275–279. [Google Scholar]
- 3.Iranshahi M, Rezaee R, Sahebkar A, Bassarello C, Piacente S, Pizza C. Sesquiterpene coumarins from the fruits of Ferula badrakema. Pharm Biol. 2009;47:344–347. [Google Scholar]
- 4.Iranshahi M, Kalateghi F, Sahebkar A, Sardashti A, Schneider B. New sesquiterpene coumarins from the roots Ferula flabelliloba. Pharm Biol. 2010;48:217–220. doi: 10.3109/13880200903019226. [DOI] [PubMed] [Google Scholar]
- 5.Iranshahi M, Masullo M, Asili A, Hamedzadeh A, Jahanbin B, Festa M, et al. Sesquiterpene coumarins from Ferula gumosa. J Nat Prod. 2010;73:1958–1962. doi: 10.1021/np100487j. [DOI] [PubMed] [Google Scholar]
- 6.Kasaian J, Iranshahy M, Masullo M, Piacente S, Ebrahimi F, Iranshahi M. Sesquiterpene lactones from Ferula oopoda and their cytotoxic properties. J Asian Nat Prod Res. 2014;16:248–253. doi: 10.1080/10286020.2013.866099. [DOI] [PubMed] [Google Scholar]
- 7.Iranshahi M, Amin G, Amini M, Shafiee A. Sulfur containing derivatives from Ferula persica var. latisecta. Phytochemistry. 2003;63:965–966. doi: 10.1016/s0031-9422(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 8.Iranshahi M, Amin G, Salehi Sourmaghi MH, Shafiee A, Hadjiakhoondi A. Sulphur containing compounds in the essential oil of Ferula persica Willd. var persica. Flavour Frag J. 2006;21:260–261. [Google Scholar]
- 9.Iranshahi M, Hassanzadeh-Khayyat MH, Fazly Bazzaz BS, Sabeti Z, Enayati F. High content of polysulphides in the volatile oil of Ferula latisecta Rech. F. et Aell. fruits and antimicrobial activity of the oil. J Essent Oil Res. 2008;20:183–185. [Google Scholar]
- 10.Iranshahi M. A review of volatile sulfur-containing compounds from terrestrial plants: biosynthesis, distribution and analytical methods. J Essent Oil Res. 2012;24:393–434. [Google Scholar]
- 11.Appendino G, Tagliapietra S, Gariboldi P, Mario Nano G, Picci V. ω-Oxygenated prenylated coumarins from Ferula communis. Phytochemistry. 1988;27:3619–3624. [Google Scholar]
- 12.Fraigui O, Lamnaouer D, Faouzi MYA. Acute toxicity of ferulenol, a 4-hydroxycoumarin isolated from Ferula communis L. Vet Hum Toxicol. 2002;44:5–7. [PubMed] [Google Scholar]
- 13.Arnoldi L, Ballero M, Fuzzati N, Maxia A, Mercalli E, Pagni L. HPLC-DAD-MS identification of bioactive secondary metabolites from Ferula communis roots. Fitoterapia. 2004;75:342–354. doi: 10.1016/j.fitote.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Appendino G, Cravotto G, Sterner O, Ballero M. Oxygenated sesquiterpenoids from a nonpoisonous sardinian chemotype of giant fennel (Ferula communis) J Nat Prod. 2001;64:393–395. doi: 10.1021/np000468f. [DOI] [PubMed] [Google Scholar]
- 15.Gunther RT. The Greek Herbal of Dioscortdes. New York: Hafner; 1959. [Google Scholar]
- 16.Heywood VH. The Biology and Chemistry of the Umbellifercre. London: Academic Press; 1972. pp. 1247–1249. [Google Scholar]
- 17.Collenette S. An Illustrated Guide to the Flowers of Saudi Arabia. London: Scorpion Publishing Ltd; 1985. [Google Scholar]
- 18.Bnouham M, Mekhfi H, Legssyer A, Ziyyat A. Medicinal plants used in the treatment of diabetes in Morocco. Int J Diabetes Metabol. 2002;10:33–50. [Google Scholar]
- 19.Dioscorides. DE MATERIA MEDICA. South Africa: AIBIDIS PRESS; 2000. p. 468. [Google Scholar]
- 20.Valle MG, Appending G, Nano GM, Picci V. Prenylated coumarins and sesquiterpenoids from Ferula communis. Phytochemistry. 1986;26:253–256. [Google Scholar]
- 21.Miski M, Mabry TJ. Daucane esters from Ferula communis subsp. communis. Phytochemistry. 1985;24:1735–1741. [Google Scholar]
- 22.Miski M, Jakupovic J. Cyclic farnesyl-coumarin and farnesyl-chromone derivatives from Ferula communis subsp. communis. Phytochemistry. 1990;29:1995–1998. [Google Scholar]
- 23.Valle MG, Appendino G, Nano GM, Picci V. Prenylated coumarins and sesquiterpenoids from Ferula communis. Phytochemistry. 1986;26:253–256. [Google Scholar]
- 24.Appendino G, Tagliapietra S, Nano GM, Picci V. Ferprenin, a prenylated coumarin from Ferula communis. Phytochemistry. 1988;27:944–946. [Google Scholar]
- 25.Lamnaouer D, Bodo B, Martin MT, Molho D. Ferulenol and ω-hydroxyferulenol, toxic coumarins from Ferula communis var. genuina. Phytochemistry. 1987;26:1613–1615. [Google Scholar]
- 26.Appendino G, Tagliapietra S, Paglino L, Nano GM, Monti D, Ppicci V. Sesquiterpenoid esters from the fruits of Ferula communis. Phytochemistry. 1990;29:1481–1484. [Google Scholar]
- 27.Abd El-Razek MH, Ohta S, Hirata T. Terpenoid coumarins of the genus Ferula. Heterocycles. 2003;60:689–716. [Google Scholar]
- 28.Iranshahi M, Amanolahi F, Schneider B. New sesquiterpene coumarin from the roots of Ferula latisecta. Avicenna J Phytomed. 2012;2:133–138. [PMC free article] [PubMed] [Google Scholar]
- 29.Nazari ZE, Iranshahi M. Biologically active sesquiterpene coumarins from Ferula species. Phytother Res. 2011;25:315–323. doi: 10.1002/ptr.3311. [DOI] [PubMed] [Google Scholar]
- 30.Carboni S, Malaguzzi V, Marsili A. Ferulenol a new coumarin derivative from Ferula communis. Tetrahedron Lett. 1964;5:2783–2786. [Google Scholar]
- 31.Mamoci E, Cavoski I, Simeone V, Mondelli D, Al-Bitar L, Caboni P. Chemical composition and in vitro activity of plant extracts from Ferula communis and Dittrichia viscosa against postharvest fungi. Molecules. 2011;16:2609–2625. doi: 10.3390/molecules16032609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamnaouer D, Fraigui O, Martin MT, Gallard JF, Bodo B. Structure of isoferprenin, a 4-hydroxycoumarin derivative from Ferula communis var genuina. J Nat Prod. 1991;54:576–578. [Google Scholar]
- 33.Appendino G, Tagliapietra S, Crarotto G, Nano GM. Structure and synthesis of a prenylated acetophene from Ferula communis. Gaz Chem Ital. 1989;119:385–388. [Google Scholar]
- 34.Pinar M, Rodriguez B. A new coumarin from Ferula loscosii and the correct structure of coladonin. Phytochemistry. 1977;16:1987–1989. [Google Scholar]
- 35.Abu-Gabal NS, Edris FM, Abu Mustafa EA. Further investigation on Ferula communis grown in Saudi Arabia. Egypt J Chem. 2008;51:107–114. [Google Scholar]
- 36.Iranshahi M, Amin G, Shafiee A. A New Coumarin from Ferula persica. Pharm Biol. 2004;42:440–442. [Google Scholar]
- 37.Iranshahi M, Shahverdi AR, Mirjani R, Amin G, Shafiee A. Umbelliprenin from Ferula persica roots inhibits the red pigment production in Serratia marcescens. Z Natur forsch C. 2004;59:506–508. doi: 10.1515/znc-2004-7-809. [DOI] [PubMed] [Google Scholar]
- 38.Miski M, Mabry TJ. Fercolide, a type of sesquiterpene lactone from Ferula communis subsp. communis and the correct structure of vaginatin. Phytochemistry. 1986;25:1673–1675. [Google Scholar]
- 39.Lamnaouer D, Martin MT, Molho D, Bodo B. Isolation of daucane esters from Ferula communis var. brevifolia. Phytochemistry. 1989;28:2711–2716. [Google Scholar]
- 40.Al-Yahya MA, Muhammad I, Mirza HH, El-Feraly FS. Antibacterial constituents from the rhizomes of Ferula communis. Phytother Res. 1998;12:335–339. [Google Scholar]
- 41.Sahebkar A, Iranshahi M. Volatile constituents of the genus Ferula (Apiaceae): A review. J Essent Oil Bear Pl. 2011;14:504–531. [Google Scholar]
- 42.Sahebkar A, Iranshahi M. Biological activities of essential oils from the genus Ferula (Apiaceae) Asian Biomed. 2010;4:835–847. [Google Scholar]
- 43.Ferrari B, Tomi F, Casanova J. Composition and chemical variability of Ferula communis essential oil from Corsica. Flavour Frag J. 2005;20:180–185. [Google Scholar]
- 44.Marongiu B, Piras A, Porcedda S. Comparative analysis of the oil and supercritical CO2 extract of Ferula communis L. J Essent Oil Res. 2005;17:150–152. [Google Scholar]
- 45.Manolakou S, Tzakou O, Yannitsaros A. Volatile constituents of Ferula communis L. subsp communis growing spontaneously in Greece. Record Nat Prod. 2013;7:54–58. [Google Scholar]
- 46.Maggi F, Lucarini D, Tirillini B, Sagratini G, Papa F, Vittori S. Chemical analysis of the essential oil of Ferula glauca L. (Apiaceae) growing in Marche (central Italy) Biochem Syst Ecol. 2009;37:432–441. [Google Scholar]
- 47.Miski M, Mabry TJ, Bohlmann F. Fercoperol, an unusual cyclic-endoperoxynerolidol derivative from Ferula communis subsp communis. J Nat Prod. 1986;49:916–918. doi: 10.1021/np50047a026. [DOI] [PubMed] [Google Scholar]
- 48.De Pascual Teresa J, Villaseco MA, Hernandez JM. Complex acetylenes from the roots of Ferula communis. Planta Med. 1986;6:458–462. doi: 10.1055/s-2007-969253. [DOI] [PubMed] [Google Scholar]
- 49.Unasho A, Geyid A, Melaku A, Debela A, Mekasha A, Girma S, et al. Investigation of antibacterial activities of Albizia gummifera and Ferula communis on Streptococcus pneumoniae and Streptoccus pyogenes. Ethiop Med J. 2009;47:25–32. [PubMed] [Google Scholar]
- 50.Mossa JS, El-Feraly FS, Muhammad I. Antimycobacterial constituents from Juniperus procera Ferula communis and Plumbago zeylanica and their in vitro synergistic activity with isonicotinic acid hydrazide. Phytother Res. 2004;18:934–937. doi: 10.1002/ptr.1420. [DOI] [PubMed] [Google Scholar]
- 51.Mamoci E, Cavoski I, Andres MF, Díaz CE, Gonzalez-Coloma A. Chemical characterization of the aphid antifeedant extracts from Dittrichia viscosa and Ferula communis. Biochem Syst Ecol. 2012;43:101–107. [Google Scholar]
- 52.Bruneton J. Toxic plants dangerous to human and animals. Paris Lavoiser publishing Inc; 1999. [Google Scholar]
- 53.Rubiolo P, Matteodo M, Riccio G, Ballero M, Christen P, Fleury-Souverain S, et al. Analytical discrimination of poisonous and nonpoisonous chemotypes of giant fennel (Ferula communis L.) through their biologically active and volatile fractions. J Agr Food Chem. 2006;54:7556–7563. doi: 10.1021/jf061592t. [DOI] [PubMed] [Google Scholar]
- 54.Carta A. Ferulosis;isolation of the substance with hypoprothrombinemizing action from the galbanum of Ferula communis. La ferulosi; l’isolamento della sostanza and azione ipoprotrombinemizzante dal galbano della Ferula communis. Boll Soc Ital Biol Sper. 1951;27:690–693. [PubMed] [Google Scholar]
- 55.Lamnaouer D. Anticoagulant activity of coumarins from Ferula communis L. Therapie. 1999;54:747–751. [PubMed] [Google Scholar]
- 56.Tagliapietra S, Aragno M, Ugazio G, Nano GM. Experimental studies on the toxicity of some compounds isolated from Ferula communis in the rat. Res Commun Chem Pathol Pharmacol. 1989;66:333–336. [PubMed] [Google Scholar]
- 57.Alzweiri M, Al-Shudeifat M, Al-Khaldi K, Al-Hiari Y, Afifi FU. Acetylated ferulenol-oxy-ferulenol as a proposed marker for fresh Ferula toxicity: A metabolomic approach. J Liq Chromatogr Relat Technol. 2015;38:283–288. [Google Scholar]
- 58.Lahouel M, Zini R, Zellagui A, Rhouati S, Carrupt P-A, Morin D. Ferulenol specifically inhibits succinate ubiquinone reductase at the level of the ubiquinone cycle. Biochem Biophys Res Commun. 2007;355:252–257. doi: 10.1016/j.bbrc.2007.01.145. [DOI] [PubMed] [Google Scholar]
- 59.Monti M, Pinotti M, Appendino G, Dallocchio F, Bellini T, Antognoni F, et al. Characterization of anti-coagulant properties of prenylated coumarin ferulenol. Biochim Biophys Acta. 2007;1770:1437–1440. doi: 10.1016/j.bbagen.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 60.Tligui N, Ruth GR, Felice LJ. Plasma ferulenol concentration and activity of clotting factors in sheep with Ferula communis variety brevifolia intoxication. Am J Vet Res. 1994;55:1564–1569. [PubMed] [Google Scholar]
- 61.Bocca C, Gabriel L, Bozzo F, Miglietta A. Microtubule-interacting activity and cytotoxicity of the prenylated coumarin ferulenol. Planta Med. 2002;68:1135–1137. doi: 10.1055/s-2002-36342. [DOI] [PubMed] [Google Scholar]
- 62.Poli F, Appendino G, Sacchetti G, Ballero M, Maggiano N, Ranelletti FO. Antiproliferative effects of daucane esters from Ferula communis and F. arrigonii on human colon cancer cell lines. Phytother Res. 2005;19:152–157. doi: 10.1002/ptr.1443. [DOI] [PubMed] [Google Scholar]
- 63.Macho A, Blanco-Molina M, Spagliardi P, Appendino G, Bremner P, Heinrich M, et al. Calcium ionophoretic and apoptotic effects of ferutinin in the human Jurkat T-cell line. Biochem Pharmacol. 2004;68:875–883. doi: 10.1016/j.bcp.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 64.Dall’Acqua S, Linardi MA, Maggi F, Nicoletti M, Petitto V, Innocenti G, et al. Natural daucane sesquiterpenes with antiproliferative and proapoptotic activity against human tumor cells. Bioorg Med Chem. 2011;19:5876–5885. doi: 10.1016/j.bmc.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 65.Dall’Acqua S, Linardi MA, Bortolozzi R, Clauser M, Marzocchini S, Maggi F, et al. Natural daucane esters induces apoptosis in leukaemic cells through ROS production. Phytochemistry. 2014;108:147–156. doi: 10.1016/j.phytochem.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 66.Gliszczyńska A, Brodelius PE. Sesquiterpene coumarins. Phytochem Rev. 2012;11:77–96. [Google Scholar]
- 67.Saidkhodzhaev AI, Nikonov GK. The structure of ferutinin. Chem Nat Compd. 1975;9:25–26. [Google Scholar]
- 68.Zamaraeva MV, Hagelgans AI, Abramov AY, Ternovsky VI, Merzlyak PG, Tashmukhamedov BA, et al. Ionophoretic properties of ferutinin. Cell Calcium. 1997;22:235–241. doi: 10.1016/s0143-4160(97)90062-2. [DOI] [PubMed] [Google Scholar]
- 69.Abramov AY, Zamaraeva MV, Hagelgans AI, Azimov RR, Krasilnikov OV. Influence of plant terpenoids on the permeability of mitochondria and lipid bilayers. Biochim Biophys Acta. 2001;1512:98–110. doi: 10.1016/s0005-2736(01)00307-8. [DOI] [PubMed] [Google Scholar]
- 70.Zamaraeva M, Charishnikova O, Saidkhodjaev A, Isidorov V, Granosik M, Rózalski M, et al. Calcium mobilization by the plant estrogen ferutinin does not induce blood platelet aggregation. Pharmacol Rep. 2010;62:1117–1126. doi: 10.1016/s1734-1140(10)70374-1. [DOI] [PubMed] [Google Scholar]
- 71.Macho A, Blanco-Molina M, Spagliardi P, Appendino G, Bremner P, Heinrich M, et al. Calcium ionophoretic and apoptotic effects of ferutinin in the human Jurkat T-cell line. Biochem Pharmacol. 2004;68:875–883. doi: 10.1016/j.bcp.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 72.Matin MM, Nakhaeizadeh H, Bahrami AR, Iranshahi M, Arghiani N, Rassouli FB. Ferutinin, an apoptosis inducing terpenoid from Ferula ovina. Asian Pac J Cancer Prev. 2014;15:2123–2128. doi: 10.7314/apjcp.2014.15.5.2123. [DOI] [PubMed] [Google Scholar]
- 73.Ferretti M, Cavani F, Manni P, Carnevale G, Bertoni L, Zavatti M, et al. Ferutinin dose-dependent effects on uterus and mammary gland in ovariectomized rats. Histol Histopathol. 2014;29:1027–1037. doi: 10.14670/HH-29.1027. [DOI] [PubMed] [Google Scholar]
- 74.Arghiani N, Matin MM, Bahrami AR, Iranshahi M, Sazgarnia A, Rassouli FB. Investigating anticancer properties of the sesquiterpene ferutinin on colon carcinoma cells in vitro and in vivo. Life Sci. 2014;109:87–94. doi: 10.1016/j.lfs.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Appendino G, Spagliardi P, Cravotto G, Pocock V, Milligan S. Daucane Phytoestrogens: A Structure-Activity Study. J Nat Prod. 2002;65:1612–1615. doi: 10.1021/np0201671. [DOI] [PubMed] [Google Scholar]
- 76.Gao M, Wong SY, Lau PM, Kong SK. Ferutinin induces in vitro eryptosis/erythroptosis in human erythrocytes through membrane permeabilization and calcium influx. Chem Res Toxicol. 2013;26:1218–1228. doi: 10.1021/tx400127w. [DOI] [PubMed] [Google Scholar]
- 77.Fu SW, Zeng GF, Zong SH, Zhang ZY, Zou B, Fang Y, et al. Systematic review and meta-analysis of the bone protective effect of phytoestrogens on osteoporosis in ovariectomized rats. Nutr Res. 2014;34:467–477. doi: 10.1016/j.nutres.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 78.Ferretti M, Bertoni L, Cavani F, Zavatti M, Resca E, Carnevale G, et al. Influence of ferutinin on bone metabolism in ovariectomized rats. II: Role in recovering osteoporosis. J Anat. 2010;217:48–56. doi: 10.1111/j.1469-7580.2010.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cavani F, Ferretti M, Carnevale G, Bertoni L, Zavatti M, Palumbo C. Effects of different doses of ferutinin on bone formation/resorption in ovariectomized rats. J Bone Miner Metab. 2012;30:619–629. doi: 10.1007/s00774-012-0366-0. [DOI] [PubMed] [Google Scholar]
- 80.Zavatti M, Resca E, Bertoni L, Maraldi T, Guida M, Carnevale G, et al. Ferutinin promotes proliferation and osteoblastic differentiation in human amniotic fluid and dental pulp stem cells. Life Sci. 2013;92:993–1003. doi: 10.1016/j.lfs.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 81.Geroushi A, Auzi AA, Elhwuegi AS, Elzawam F, Elsherif A, Nahar L, et al. Antiinflammatory sesquiterpenes from the root oil of Ferula hermonis. Phytother Res. 2011;25:774–777. doi: 10.1002/ptr.3324. [DOI] [PubMed] [Google Scholar]
- 82.Abourashed EA, Galal AM, Shibl AM. Antimycobacterial activity of ferutinin alone and in combination with antitubercular drugs against a rapidly growing surrogate of Mycobacterium tuberculosis. Nat Prod Res. 2011;25:1142–1149. doi: 10.1080/14786419.2010.481623. [DOI] [PubMed] [Google Scholar]
- 83.Al-Ja’Fari AH, Vila R, Freixa B, Costa J, Cañigueral S. Antifungal compounds from the rhizome and roots of Ferula hermonis. Phytother Res. 2013;27:911–915. doi: 10.1002/ptr.4806. [DOI] [PubMed] [Google Scholar]
- 84.Zanoli P, Rivasi M, Zavatti M, Brusiani F, Vezzalini F, Baraldi M. Activity of single components of Ferula hermonis on male rat sexual behavior. Int J Impot Res. 2005;17:513–518. doi: 10.1038/sj.ijir.3901346. [DOI] [PubMed] [Google Scholar]
- 85.Zavatti M, Montanari C, Zanoli P. Role of ferutinin in the impairment of female sexual function induced by Ferula hermonis. Physiol Behav. 2006;89:656–661. doi: 10.1016/j.physbeh.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 86.Zanoli P, Zavatti M, Geminiani E, Corsi L, Baraldi M. The phytoestrogen ferutinin affects female sexual behavior modulating ERαexpression in the hypothalamus. Behav Brain Res. 2009;199:283–287. doi: 10.1016/j.bbr.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 87.Al-Mughrabi KI, Aburjai TA. Fungitoxic activity of root extracts from Ferula harmonis. Phytopathol Mediterr. 2003;42:141–148. [Google Scholar]
- 88.Appendino G, Tagliapietra S, Nano GM, Picci V. An anti-platelet acetylene from the leaves of Ferula communis. Fitoterapia. 1993;64:179. [Google Scholar]
- 89.Lamnaouer D. Anticoagulant activity of the coumarins of Ferula communis L. Activite anticoagulante des coumarines de Ferula communis L. Therapie. 1999;54:747–751. [PubMed] [Google Scholar]
- 90.Appendino G, Mercalli E, Fuzzati N, Arnoldi L, Stavri M, Gibbons S, et al. Antimycobacterial coumarins from the Sardinian giant fennel (Ferula communis) J Nat Prod. 2004;67:2108–2110. doi: 10.1021/np049706n. [DOI] [PubMed] [Google Scholar]
- 91.Lahouel M, Zini R, Zellagui A, Rhouati S, Carrupt PA, Morin D. Ferulenol specifically inhibits succinate ubiquinone reductase at the level of the ubiquinone cycle. Biochem Biophys Res Commun. 2007;355:252–257. doi: 10.1016/j.bbrc.2007.01.145. [DOI] [PubMed] [Google Scholar]


