Abstract
Objective(s):
Resveratrol (RES) is a polyphenol compound that has been shown a promising cardioprotective effect. However, some reports have yielded conflicting findings. Herein, we investigated the anti-atherosclerotic effects of RES in apolipoprotein E (apo E)-deficient mice on a high cholesterol diet.
Materials and Methods:
Firstly, atherosclerosis was induced by feeding a high cholesterol diet to apo E-deficient mice. Then, we examined its effects on weight control, and serum interleukin-6 (IL-6) levels and used histopathological methods to analyze morphology and inflammatory marker of atherosclerotic lesions in mice orally supplemented with high (25 mg/kg/day) and low (5 mg/kg/day) doses of RES for 8 weeks.
Results:
Mice with high dose of RES had reduced epididymal fat pads, and lower serum IL-6 levels compared with those of control mice. Moreover, RES in high doses also decreased the low-density lipoprotein cholesterol (LDL-C) levels and atherogenic index (LDL-C/HDL-C) in the mice. Dissection of high-dose RES-treated mice revealed a marked reduction in fat deposition, percentage of mice with atherosclerotic lesion, and intima/media ratio in the aortic areas. The expressions of macrophage-specific marker F4/80 and cardiovascular inflammatory marker NF-κB in atherosclerotic vessels were both diminished in the atherosclerotic vessels of high-dose RES-supplementated apo E-deficient mice.
Conclusion:
These results suggest that RES prevented the effects of a high cholesterol diet on the rate of accretion in atherosclerosis progression by reducing the LDL-C levels and suppressing atherosclerotic inflammation. RES can therefore be valuable in the development of new anti-atherosclerotic agents.
Keywords: Atherosclerosis, Inflammation, Low-density lipoprotein-cholesterol, Resveratrol
Introduction
Coronary artery disease (CAD) arising from atherosclerosis is a leading cause of death and morbidity worldwide (1). Atherosclerosis can be multifactorial and is generally associated with predisposing conditions including obesity, hyperlipidemia, diabetes, arterial hypertension, smoking, and inadequate exercise (2). The pathogenesis of atherosclerosis has been shown to involve an imbalanced lipid metabolism and increase in proinflammatory cytokines such as interleukin-6 (IL-6) (3), a maladaptive immune response entailing a chronic inflammatory process of the arterial wall with accumulation of cholesterol-enriched macrophages, smooth muscle cell proliferation, and extracellular matrix in the intima (4). Cholesterol-lowering therapy such as statin treatment may improve cardiovascular diseases, but it was not an independent predictor of improved outcome in patients with CAD (5). Furthermore, anti-inflammatory drugs have been demonstrated to retard the development of atherosclerosis, but the drugs examined in different animal experiments have yielded contradictory results and have side effects (6, 7). Therefore, additional treatments for inhibition or improvement of atherosclerosis with cholesterol-lowering strategies are needed, particularly naturally occurring compounds that act as protective agents (8, 9).
Resveratrol (RES), trans-3,4’,5-trihydroxy-stilbene, naturally occurs as a polyphenol found in grapes, peanuts, and red wine. It was implicated as the main active principle agent in a study that investigated its cardioprotective effects (10). In biological systems, RES has been demonstrated to exert a variety of pharmacological properties including anti-oxidant (11), anti-inflammatory, and anti-cancer (12) effects. Moreover, RES has also been shown to improve lipid profile, lipoprotein metabolism (13), and prolong survival (14). Conversely, RES significantly improves glucose control and insulin sensitivity in patients with diabetes (15) and is a leading candidate as an adjunct to the pharmacological treatment of type 2 diabetes mellitus (16). A more recent breakthrough was the discovery that RES exhibits beneficial effects in patients with cardiovascular disease (CVD) (17). These could be attributed to mechanisms involved in alterations of lipid metabolism (18).
Moreover, studies showed that treatment with RES reduced myocardial complications by anti-oxidant activity, influencing infarct size, apoptosis, and angiogenesis (19, 20). These studies also proposed that various anti-atherogenic mechanisms may be involved in the reduction of CVD risk by RES. However, various reports failed to show changes in lipid profile after treatment with RES (10, 21, 22), and others revealed that RES promoted atherosclerotic development, rather than protected against it (23, 24). Thus, the administration of RES in anti-atherogenic therapy has yielded contradictory findings and the mechanisms involved have not been fully explored. The present study was aimed to clarify how RES administration can affect the serum lipids profiles and inflammation process in apolipoprotein E (apo E)-deficient mice with atherosclerotic lesions, as an effective model for studies of human atherosclerosis. We planned to achieve this purpose by determining the effects of body weight control, lipid profile, inflammation biomarker, and serum lipid profiles in apo E-/- mice fed with a high cholesterol diet and treated orally with RES. We also evaluated the atherosclerotic lesion development, adipocyte content of aortic areas, macrophage-specific, and cardiovascular inflammatory marker using histopathological morphology and immunohistochemistry.
Materials and Methods
Animals and treatment
Male apo E-/- mice at 12 weeks of age were housed individually in standard plastic rodent cages in animal quarters maintained at 22±2 °C, 55 ± 5% relative humidity, with a light/dark cycle of 12 hrs. Food and water were given ad libitum. The animals were randomly divided into three groups: (i) control group: mice were fed with a high cholesterol diet (diet #58R5, 20.7% protein, 20.3% fat, and 45.6% carbohydrates and cholesterol at 5053 ppm of chow, PMI Nutrition International Inc, MO, USA); (ii) low-dose RES group: mice were fed with a high cholesterol diet and orally administered RES (Resveratrol®, 98%, Organic Herb INC, Changsha, Hunan Province, China) at 5 mg/kg body weight/day; (iii) high-dose RES group: mice were fed with a high cholesterol diet and oral doses of RES at 25 mg/kg body weight/day. The three groups were monitored during the experimental period of 56 days. The used protocol for the experimental mice was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the National Chung Hsing University (IACUC Approval No. 98-85). In addition, all procedures adhered to the Guidelines for the Care and Use of Laboratory Animals recommended by the Taiwanese government.
Biochemical determinations
At the end of the study period, animals were anesthetized with urethane (1.2 g/kg body weight) and sera were harvested for subsequent analysis. Clinical laboratory assessments included triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C). Measurements were taken using an automated system (Hitachi 704 automatic analyzer, Hitachi, Tokyo, Japan). Serum IL-6 concentrations were measured using a mouse ELISA kit (Invitrogen, Camarillo, CA, USA).
Histological analysis
After 8 weeks of the atherogenic or control diet, mice were sacrificed and hearts containing the aortic areas were separated and embedded in optimal cutting temperature (OCT) medium, frozen in liquid nitrogen, and stored at -80 °C for lesion analysis. Cross-sectional cryosections 5 µm thick were sequentially obtained from the base toward the apex of the heart to the appearance of the aortic roots (25). Consecutive cross-sections were stained with Oil-red-O or haematoxylin and eosin (H&E) and counterstained with haematoxylin for each tract of the 8 aorta sections, which were then morphologically analyzed for atherosclerotic lesions (26, 27). To quantify the luminal cross-sectional areas of the atherosclerotic plaques in the ascending aorta, digital images of the stained sections were obtained at the aortic valve level by using an Olympus BX51 digital microscope (Olympus Optical, Tokyo, Japan). The atherosclerotic lesions were manually traced using a computer. Images were captured and analyzed by using ImageJ 1.33 software (National Institutes of Health, USA). The size of lesions was determined using the ImageJ software. The surface area of the aortic segment was determined using autotracing feature of ImageJ 1.33. Morphometric analyses were considered to exclude normal-appearing media and to include only the intimal or subintimal atherosclerotic lesions. Data were expressed as a percentage of the total luminal surface occupied by the lesion areas of the cross-sections. On the other hand, hematoxylin and eosin-stained sections were used for morphometric analysis in the aortic intima-to-media ratio, as the method reported by von der Thusen et al (28). Cross-sections of each aortic area were assessed at 3 levels: 0.5 mm proximal to the collar, in the midsection, and 0.5 mm distal to the collar. The images were captured using an Olympus BX51 digital microscope and analyzed using ImageJ 1.33. The intimal surface area was calculated by subtracting the patent lumen area from the area circumscribed by the internal elastic lamina. The medial surface area was defined as the area between the internal and external elastic laminas. The intima-to-media ratio was determined by dividing the intimal area by the medial area. Data were expressed as the total intima-to-media area ratio of the cross-sections.
Immunohistochemistry
In each sample, 3% goat serum was added to unstained and frozen sections for 30 min at room temperature to block nonspecific activity, and then the sections were washed in phosphate-buffered salt solution (PBS) for 5 min. After three washes in PBS, sections were incubated with primary anti-mouse macrophage-specific marker F4/80 (1:200, Abcam, Cambridge, UK) and NF-kappa B (NF-κB, 1:200, Bioworld, Minnesota, USA) overnight at 4 °C. The monoclonal antibody F4/80 was used for the detection of macrophage accumulation, and NF-κB-driven inflammatory pathways in monocytes were identified, which have been implicated in the pathogenesis of atherosclerosis (29). Slides were incubated with biotinylated secondary antibody for 30 min at room temperature after washing with PBS, followed by addition of horseradish peroxidase conjugated streptavidin. Finally, sections were visualised using chromogen 3,3’-Diaminobenzidine (DAB) substrate (Immunotech, Marseille, France), and then counterstained with haematoxylin (Sigma-Aldrich, MO, USA) stain for 10 min. The immunohistochemistry staining procedure was employed according to the method developed by Chan et al (30). Image acquisitions were performed with an Olympus BX51 digital microscope and examined using ImageJ 1.33 software. The specimens were considered to be positive if staining was observed in more than 10% of samples and were then classified as exhibiting high (+++), moderate (++,), and low (+) immunoreactivity for F4/80 and NF-κB.
Statistical analysis
Results are shown as mean ± SEM. Significant differences among the groups were evaluated using one-way ANOVA, and the differences between the means were assessed using Duncan’s multiple-range test. A Mann-Whitney nonparametric test was used for comparing the scores in the histopathological analysis between the control and RES supplementation groups. A P-value of less than 0.05 was considered significant.
Results
Body weight, organ weight and serum IL-6
To test the effects of RES on physical parameters and atherosclerosis in diet-induced atherosclerotic mice, mice were preliminarily fed either a high cholesterol diet or normal diet for 8 weeks, and the prevalence of atheroselerotic lesions was then evaluated in both groups. After the induction of atheroselerotic lesions for 8 weeks, there was a higher percentage of mice in the control group fed a high cholesterol diet with aortic fatty plaques compared with that in the control mice that were fed a normal diet (23.46±0.71 in high cholesterol diet group versus 9.31±2.20 in standard diet group, P<0.01) (Supplementary Figure 1). Thus, experimental atherosclerosis was induced in mice fed a high cholesterol diet.
Supplementary Figure 1.
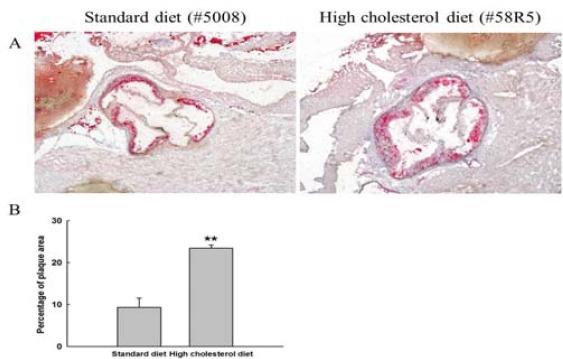
Atherosclerotic lesions in apo E-/- mice fed a standard diet or a high cholesterol diet showing (A) aortic fatty plaque and (B) percentage cross-sectional area of atherosclerotic lesion. The photographs reflect greater differences in aortic fatty plaque and percentage cross-sectional area of atherosclerotic lesion in the high cholesterol diet group compared with the standard diet group. All photomicrographs were stained with Oil-Red O and subjected to magnification for histological analysis (X 100). **, P<0.01, determined using the Mann-Whitney nonparametric test, high cholesterol diet group vs. standard diet group
1 From 12 weeks of age, male apo E-/- mice were fed with a standard diet or high cholesterol diet for 8 weeks. Results are presented as means±SEM, n = 6 for all groups
2 Standard diet (SD, diet 5008, PMI Nutrition International Inc, MO, USA): 16% of calories provided by fat and metabolizable energy, 3.3 kcal/gm
The initial body weight of mice showed no significant differences among the control, low-dose RES and high-dose RES groups. No significant difference in body weight existed between the groups (Table 1). On the other hand, the weights of liver, spleen, and kidney were also not markedly different among the three groups. However, the weight of heart and epididymal white adipose tissue (EWAT) decreased by 20% and 23% in the high-dose RES mice compared with that in the control mice (P<0.05). The weight of heart in the low-dose RES mice was also decreased by 16% compared to that of the control group. After 8 weeks of the high cholesterol diet, treatment with RES supplement resulted in a significant reduction of serum IL-6 levels by 50% in the low-dose RES group (P<0.05) and by 62% in the high-dose RES group (P<0.05), respectively, compared to that of the control group. Moreover, serum IL-6 levels were decreased in the high-dose RES-supplemented mice compared to the low-dose RES-supplemented mice. In contrast, EWAT was not affected in the mice treated with low-dose RES compared to those of the control or high-dose RES groups.
Table 1.
Effects of resveratrol (RES) supplementation on body weight and organ weight in apo E-/- mice fed a high cholesterol diet
| Control | Low-dose RES | High-dose RES | |
|---|---|---|---|
| Initial body weight (g) | 23.99 ± 1.04 | 23.98 ± 1.04 | 24.01 ± 1.04 |
| Final body weight (g) | 26.96 ± 1.59 | 26.83 ± 1.80 | 26.15 ± 1.07 |
| Heart (g) | 0.25 ± 0.01a | 0.21 ± 0.01b | 0.20 ± 0.01b |
| Liver (g) | 1.59 ± 0.06 | 1.51 ± 0.10 | 1.50 ± 0.07 |
| Spleen (g) | 0.15 ± 0.02 | 0.12 ± 0.01 | 0.13 ± 0.01 |
| Kidney (g) | 0.37 ± 0.02 | 0.35 ± 0.04 | 0.33 ± 0.02 |
| EWAT (g) | 0.48 ± 0.10a | 0.50 ± 0.08a | 0.37 ± 0.08b |
| IL-6 (pg/ml) | 208.93 ± 26.69a | 104.63 ± 18.28b | 79.25 ± 13.42c |
1 Data are presented as means ± SEM of 6 animals in each group. Means in the same row that do not share a common superscript differ significantly (P<0.05), as determined using a one-way ANOVA test
2 Control: high cholesterol diet (#58R5) without RES treatment; Low-dose RES: high cholesterol diet (#58R5) + low-dose RES supplementation; High-dose RES: high cholesterol diet (#58R5) + high-dose RES supplementation; EWAT, epididymal white adipose tissue
Serum lipid profiles
The plasma lipid concentrations are shown in Table 2. There were no significant differences among the groups in TG, TC, HDL-C, and TC:HDL-C ratio. However, the high-dose RES group had significantly lower LDL-C concentrations (565.27±16.98 in high-dose RES group versus 615.43±10.28 in control group, P<0.05) and LDL-C:HDL-C ratio (11.52±1.49 in high-dose RES group versus 14.08±2.06 in control group, P<0.05), compared to the control group with a high cholesterol diet. Accordingly, LDL-C levels and ratio of LDL-C to HDL-C, which are sensitive biomarkers of coronary heart disease risk, were significantly reduced in mice with a high cholesterol diet that received a high-dose of oral RES supplement. In contrast, mice treated with low-dose RES supplement in the low-dose RES group had unchanged serum LDL-C levels and LDL-C:HDL-C ratio compared to those of the control and high-dose RES group.
Table 2.
Effect of oral resveratrol (RES) supplementation on triglycerides, total cholesterol, LDL-C, HDL-C levels, LDL/HDL-C ratio, and TC/HDL-C ratio in high cholesterol diet-fed apo E-/- mice
| Control | Low-dose RES | High-dose RES | |
|---|---|---|---|
| TG (mg/dl) | 70.67 ± 3.29 | 78.50 ± 8.91 | 76.50 ± 6.29 |
| TC (mg/dl) | 1252.67 ± 59.93 | 1326.40 ± 59.92 | 1320.51 ± 62.37 |
| LDL-C (mg/dl) | 615.43 ± 10.28a | 608.81 ± 35.86a | 565.27 ± 16.99b |
| HDL-C (mg/dl) | 47.52 ± 7.84 | 56.61 ± 12.69 | 54.85 ± 9.34 |
| LDL/HDL-C ratio | 14.08 ± 2.06a | 12.91 ± 2.34a | 11.52 ± 1.49b |
| TC/HDL-C ratio | 28.78 ± 4.62 | 27.42 ± 4.38 | 26.66 ± 4.87 |
1 Data are presented as means±SEM of 6 animals in each group. Means in the same row that do not share a common superscript differ significantly (P<0.05), as determined using a one-way ANOVA test
2 Control: high cholesterol diet (#58R5) without RES treatment; Low-dose RES: high cholesterol diet (#58R5) + low-dose RES supplementation; High-dose RES: high cholesterol diet (#58R5) + high-dose RES supplementation; Triglyceride, TG; total cholesterol, TC; low-density lipoprotein cholesterol, LDL-C; high-density lipoprotein cholesterol, HDL-C; low-density lipoprotein cholesterol/high-density lipoprotein cholesterol ratio, LDL/HDL-C ratio and total cholesterol/high-density lipoprotein cholesterol, TC/HDL-C ratio
Histopathological morphology of atherosclerotic lesions
Morphometric analysis of arterial lesions and lipid depositions were also carried out in this study. Dissection of the aortic arch revealed that histopathological changes were prominent in the ascending aorta, as seen after staining with Oil-Red O, and H&E (Figure 1). Known as macrophage lipids, these lesions were shown to be atherosclerotic lesions and were stained by Oil-Red O, which allows for visualization of lipid accumulation in lesions. A fatty plaque could be visualized at the edge of the dissection that extended over the inside of the aortic area in the control group. Interestingly, the presence of atherosclerotic lesions in these sections showed a 25% reduction in mice treated with high-dose RES compared with that observed in the control mice fed a high cholesterol diet (19.67±2.01% for high-dose RES group versus 25.99±1.45% for control group, P<0.05, Figure 2A). However, in low-dose RES-supplemented and control mice there were no differences in the prevalence rates of atherosclerotic lesions. Relative to the control group, the ratio of aortic intima/media diameter was significantly (P<0.05) lower, by 32%, in the high-dose RES treatment group (29.63±0.30% for high-dose RES group versus 43.31±0.60% for control group, Figure 2B). However, no difference was found in intima thickness between the low-dose RES and control groups.
Figure 1.
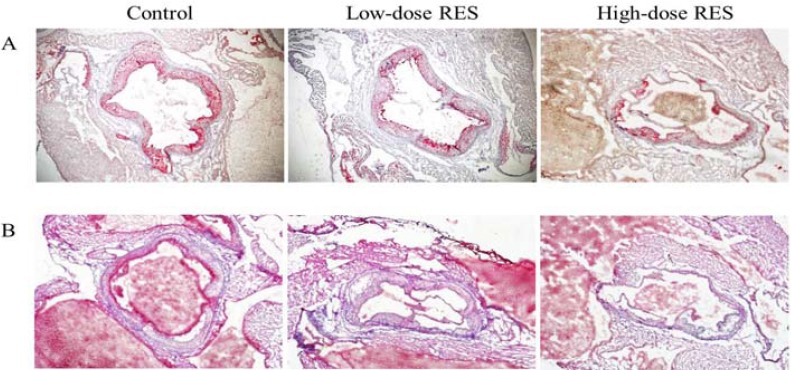
Effects of oral resveratrol supplementation on aorta morphology in apo E-/- mice fed a high cholesterol diet (A) Oil-Red O staining, (B) haematoxylin and eosin staining, illustrating greater differences for the high-dose RES treatment group than for the controls group. All photomicrographs were subjected to magnification for histological analysis (X 100). Control: high cholesterol diet (#58R5) without resveratrol (RES) treatment; Low-dose RES: high cholesterol diet (#58R5) + low-dose RES supplementation; High-dose RES: high cholesterol diet (#58R5) + high-dose RES supplementation
Figure 2.
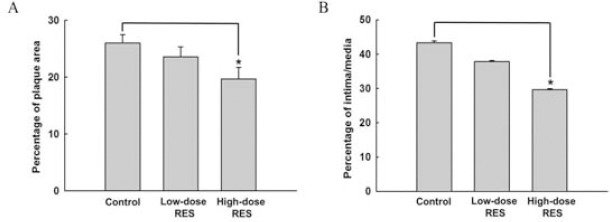
Effects of resveratrol supplementation on (A) atherosclerotic lesion area and (B) aortic intima/media area ratio in high cholesterol diet-fed apo E-/- mice. Results are expressed as mean±SEM of 6 animals in each group. *, P<0.05, determined using the Mann-Whitney nonparametric test, high-dose RES-treated animals vs. control groups. Control: high cholesterol diet (#58R5) without resveratrol (RES) treatment; Low-dose RES: high cholesterol diet (#58R5)+low-dose RES supplementation; High-dose RES: high cholesterol diet (#58R5) + high-dose RES supplementation
Immunohistochemistry
There was a significant decrease in staining for the macrophage marker, F4/80, reflecting a decrease in macrophage infiltration in the aorta of the high-dose RES-treated animals as compared with that in the control mice (Figure 3A-C). Furthermore, results indicated that the expression of F4/80 in the high-dose RES group was consistently lower than that of the control mice, with an average of 40% in the immunohistochemical immunoreactivity scoring (1.50±0.61 for high-dose RES group versus 2.50± 1.02 for control group, P<0.05, Figure 3D). Next, to evaluate the effect of RES on atherosclerotic inflammation, we studied NF-κB immunolocalization in the aortic microsections of cholesterol-fed apo E-/- mice. We found that 8 weeks of high-dose RES supplementation decreased NF-κB immunopositivity compared to the control group (Figure 4A-C). Compared to control mice, high-dose treatment with oral RES was able to significantly quench NF-κB activity, by 29%, as measured by immunohistochemical immunoreactivity scoring (1.67±0.68 for high-dose RES group versus 2.33 ±0.95 for control group, P<0.05, Figure 4D). However, compared with the low-dose group the expressions of F4/80 and NF-κB, which indicated the level of immunohistochemical immunoreactivity, were not found to be different compared to those of the control group or high-dose RES group.
Figure 3.
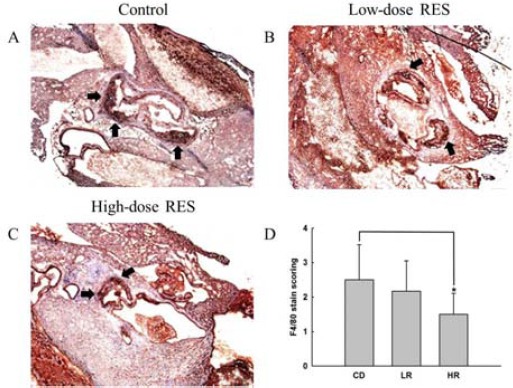
Effects of oral resveratrol (RES) supplementation on F4/80 expression in aortic arch in high cholesterol diet-fed apo E-/- mice as determined by (A), (B) and (C) immunohistochemical staining and (D) immunohistochemistry immunoreactivity scoring. All photomicrographs were subjected to magnification for histological analysis (X 100). Arrows indicate positive F4/80 immunoreactivity. Results are expressed as mean±SEM of 6 animals in each group. *, P<0.05, determined using the Mann-Whitney nonparametric test, high-dose RES-treated animals vs. controls group. Control: high cholesterol diet (#58R5) without RES treatment; Low-dose RES: high cholesterol diet (#58R5) + low-dose RES supplementation; High-dose RES: high cholesterol diet (#58R5) + high-dose RES supplementation
Figure 4.
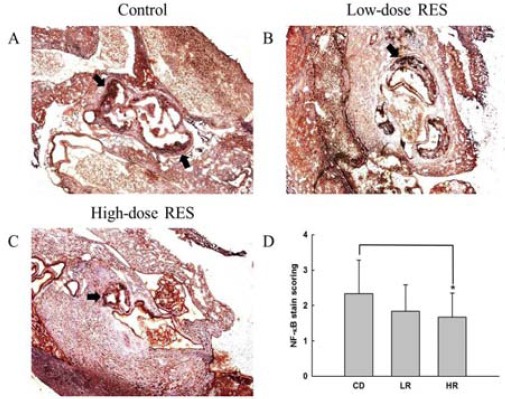
Effects of oral resveratrol (RES) supplementation on NF-κB expression in aortic arch in high cholesterol diet-fed apo E-/- mice as determined by (A), (B) and (C) immunohistochemical staining and (D) immunohistochemistry immunoreactivity scoring. All photomicrographs were subjected to magnification for histological analysis (X 100). Arrows indicate positive NF-κB immunoreactivity. Results are expressed as mean±SEM of 6 animals in each group. *, P<0.05, determined using the Mann-Whitney nonparametric test, high-dose RES-treated animals vs. controls group. Control: high cholesterol diet (#58R5) without RES treatment; Low-dose RES: high cholesterol diet (#58R5) + low-dose RES supplementation; High-dose RES: high cholesterol diet (#58R5) + high-dose RES supplementation
Discussion
The results of the present study support the notion that RES may induce beneficial anti-atherogenic effects. The effect by RES in our mouse model was highly significant and persisted over time. Typically, changes in atheroclerosis are associated with elevated plasma cholesterol levels and inflammatory factors. Despite having similar HDL-C values, by the end of the 8-weeks experimental period in which mice were fed a high cholesterol diet, mice that received high-dose oral RES supplementation exhibited significantly reduced LDL-C levels and LDL/HDL cholesterol ratio as atherogenic indicators, compared to those of the control group. This was especially surprising because the high-dose RES-treated mice showed a greater anti-inflammatory effect, compared with the control mice that were fed high cholesterol, by reducing macrophage infiltration and further inflammation development.
These broad spectrums of effects are supported by new data demonstrating the great potency of RES supplementation in relation to body fat reducing effect (31). Here, our results also demonstrated that RES may reduce diet-induced white adipose tissue gain in mice. Fat pad mass decreases as a result of reduced formation of new adipocytes from precursor cells (adipocyte hyperplasia) or by decreased adipocyte size due to fat storage (adipocyte hypertrophy) (32, 33). The lower gain of fat pad we observed in RES-supplemented mice may have been due to an inhibition in adipogenesis (34). Moreover, it is reported that RES reduced expression of most adipogenic marker genes, including peroxisome proliferators-activated receptors gamma (PPAR-γ, leptin, and fatty acid synthase, which affect lipogenesis and adipogenesis (35). On the other hand, histopathological examination revealed reduced hypertrophy of adipocytes in rat by RES treatment (36). Thus, we suggested that RES reduced fat mass via inhibition of adipocyte hyperplasia and hypertrophy in fat cells. This may have implications for a potential application of RES to reduce fat content in anti-obesity by mimicking calorie restriction.
We evaluated the concentration of serum IL-6, a pro- and inflammatory cytokine, which is synthesized primarily in monocyte and monocyte-derived macrophages, and found it to enhance fatty lesion development in atherosclerosis-prone mice (37). Increased IL-6 expression provides a chemotactic stimulus to the adherent leukocytes, directing their migration into the intima (38). We suggest that blockage of calcium ion influx into cells due to RES supplementation contributed to inhibition of IL-6 biosynthesis and inhibited IL-6 release from macrophage (39), resulting in the reduced levels of IL-6 observed in the low- and high-dose RES-treated mice in our study. It is noteworthy that, in the present study, the inhibition of IL-6 release from macrophage by RES was also evidenced by a decrease in F4/80 immunopositivity expression. Moreover, in RES-treated mice, lower IL-6 levels were involved in reduced macrophage migration, suggesting that RES decreased the development of atherosclerosis that leads to formation of fatty lesions.
Hypercholesterolemia is a well-known major risk factor for atherosclerosis, and increased concentrations of LDL-C have been shown to play a key role in the pathogenesis of atherosclerosis (40). Moreover, it is reported that treatment with RES significantly reduced TC and LDL-C levels in patients with nonalcoholic fatty liver disease (NAFLD) (41). These results contradicted those of Sahebkar (42), who found that RES supplementation did not significantly improve circulating lipid levels, including TC, LDL-C, HDL-C, and TG. The contrasting findings of these studies may be due to their unequal sample sizes, intervention durations, and participant baseline characteristics. Here, a decrease of LDL-C levels improves LDL function, which in turn either prevents aortic lesion formation or avoids the exacerbation of existing aortic lesion (43). However, treatment with RES, even with a high dose did not result in decreased TG or TC levels, or did not induce increased HDL-C levels compared to those of the control mice. This result is not consistent with the findings reported by Zern et al (44) and Do et al (45). We suggest that the discrepancy may be due to differences in the length or dose of RES treatment in these studies. The ratio of LDL-C to HDL-C, an atherogenic index which has been demonstrated to be a sensitive indicator of clinically detectable human atherosclerosis (46), showed a 12-fold decrease in the group with high-dose RES supplementation due to the decrease in LDL-C concentrations, compared to that of the control group. RES supplement increased CYP7A1 expression, which was 50% capable of degrading cholesterol to bile acids by more than 50% via classic (or neutral) and alternative (or acidic) bile acid biosynthetic pathways, resulting in decreased blood cholesterol and LDL-C concentrations (47). Consistent with former findings, our data strongly suggest that RES may have promise as a new hypolipidemic compound.
Accumulation of vascular lipids in RES-supplemented mice was significantly reduced compared with that of control mice during the 8-weeks high cholesterol diet feeding period (areas of Oil-Red O stained lipid accumulations). The previous data suggest that increased cytosolic calcium of RES modulates the PPAR-γ pathway, subsequently leading to change in expression of cholesterol transporters (48, 49). Thus, the underlying mechanism by which RES causes a decrease in atherosclerotic lesions of the aortic area in ApoE-/- mice fed a high cholesterol diet appears to be mediated directly via the reduction of lipid storage.
In a previous study, lipoprotein lipase has been proposed as a key protein involved in the retention of LDL and VLDL in the arterial intima by enhancing their adherence to the extracellular matrix (50). In addition, induction the expression of CDK inhibitors p27/KIP1 via RES treatment contributed to inhibition of intimal cell proliferation and thickening after vascular injury (51). Indeed, our results showed a decreased ratio of intima to media area in the high-dose RES group with lower LDL-C levels compared with that of the control animals. Furthermore, the highly significant decrease in the ratio of intima/media area may parallel the mitigation of the atherosclerotic process in the RES-treated mice, and therefore a lower ratio may be valuable as an indicator of reduced risk in the advanced stages of atherosclerosis.
Also, localized chronic inflammation has been shown to influence the development and progression of atherosclerosis (2). Our data showed significant differences in F4/80 and NF-κB content within the atherosclerotic lesions between the high-dose RES group and control mice, suggesting that RES supplementation exerted an anti-inflammation effect, thereby suppressing the development of atherosclerosis. It is noteworthy that, in the present study, the inhibition of cytokine release by RES contributed to a decrease in macrophage infiltration, as evidenced by the IL-6 values. Furthermore, it has been shown that inhibition of cytokine release may be due to suppression of NF-kB activation, with a consequent reduction in inflammatory cytokine expression (52). Taken together, the aforementioned findings demonstrate that RES supplementation suppresses atherogenic lesion inflammation, and further suggest that RES may have therapeutic value as an anti-atherosclerosis agent in humans.
We found that high-dose RES treatment had a markedly reduced prevalence of atherosclerotic lesion in mice compared with that in mice with low-dose RES treatment at the end of the study. In humans, RES supplementation was shown to have a cardioprotective effect by lowering plasma LDL-C levels (53), whereas in the serum lipid profile of rats, TG was not reduced (54). RES also promoted the development of atherosclerosis in a hypercholesterolemic rabbit model (23). This apparent discrepancy may be due to the different health statuses of the studied animals; the rats received a normal diet to estimate a condition of dyslipidemia from normal plasma lipid levels, and rabbits had higher cholesterol levels, whereas the human populations were myocardial infarction patients with coronary artery disease. It is also possible that differences among species receiving RES treatment may result in differing effects. The anti-atherosclerotic effects of RES may be affected by elevating the dose, duration, or frequency of RES.
Conclusion
Our study demonstrates that treatment with RES significantly reduced adiposity mass in experimental mice. Dietary supplementation with RES was also shown to significantly improve lipid metabolism, inflammatory risk factors and antigen-positive macrophages of atherosclerosis in humanized models of disease. Thus, RES appeared to suppress the formation of atherosclerotic lesion by exerting an anti-inflammatory effect. Further studies are needed to understand the complexities involved and to determine whether RES supplementation in hypercholesterolemic patients could lead to deleterious effects.
Acknowledgment
This work was supported in part by the Council of Agriculture Taiwan (102 AS-1.1.2-BQ-B3) and the Ministry of Education Taiwan under the Aim for the Top University plan.
References
- 1.Antoniadis CA, Tousoulis D. Gene therapy and coronary artery disease. Hell J Cardiol. 2002;43:226–235. [Google Scholar]
- 2.Lim S, Despres JP, Koh KK. Prevention of atherosclerosis in overweight/obese patients.- In need of novel multi-targeted approaches. Circ J. 2011;75:1019–1027. doi: 10.1253/circj.cj-10-1240. [DOI] [PubMed] [Google Scholar]
- 3.Bernberg E, Ulleryd MA, Johansson ME, Bergström GM. Social disruption stress increases IL-6 levels and accelerates atherosclerosis in ApoE-/-mice. Atherosclerosis. 2012;221:359–365. doi: 10.1016/j.atherosclerosis.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 4.Mueller MA, Beutner F, Teupser D, Ceglarek U, Thiery J. Prevention of atherosclerosis by the mTOR inhibitor everolimus in LDLR-/- mice despite severe hypercholesterolemia. Atherosclerosis. 2008;198:39–48. doi: 10.1016/j.atherosclerosis.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 6.Rott D, Zhu J, Burnett MS, Zhou YF, Zalles-Ganley A, Ogunmakinwa J, et al. Effects of MF-tricyclic, a selective cyclooxygenase-2 inhibitor, on atherosclerosis progression and susceptibility to cytomegalovirus replication in apolipoprotein-E knockout mice. J Am Coll Cardiol. 2003;41:1812–1819. doi: 10.1016/s0735-1097(03)00304-8. [DOI] [PubMed] [Google Scholar]
- 7.Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA, et al. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634–1642. doi: 10.1161/CIRCULATIONAHA.106.181424. [DOI] [PubMed] [Google Scholar]
- 8.Sahebkar A, Watts GF. New LDL-cholesterol lowering therapies: pharmacology, clinical trials, and relevance to acute coronary syndromes. Clin Ther. 2013;35:1082–1098. doi: 10.1016/j.clinthera.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Sahebkara A, Chew GT, Watts GF. Recent advances in pharmacotherapy for hypertriglyceridemia. Prog Lipid Res. 2014;56:47–66. doi: 10.1016/j.plipres.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Fremont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 11.Yao J, Wang JY, Liu L, Li YX, Xun AY, Zeng WS, et al. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch Med Res. 2010;41:288–294. doi: 10.1016/j.arcmed.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Udenigwe CC, Ramprasath VR, Aluko RE, Jones PJ. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr Rev. 2008;66:445–454. doi: 10.1111/j.1753-4887.2008.00076.x. [DOI] [PubMed] [Google Scholar]
- 13.Milde J, Elstner EF, Grabmann J. Synergistic effects of polyphenols and carotenoids on human low-density lipoprotein oxidation. Mol Nutr Food Res. 2007;51:956–961. doi: 10.1002/mnfr.200600271. [DOI] [PubMed] [Google Scholar]
- 14.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 15.Liu K, Zhou R, Wang B, Mi MT. Effect of resveratrol on glucose control and insulin sensitivity: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr. 2014;99:1510–1519. doi: 10.3945/ajcn.113.082024. [DOI] [PubMed] [Google Scholar]
- 16.Hausenblas HA, Schoulda JA, Smoliga JM. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus-systematic review and meta-analysis. Mol Nutr Food Res. 2015;59:147–159. doi: 10.1002/mnfr.201400173. [DOI] [PubMed] [Google Scholar]
- 17.Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev. 2004;22:169–188. doi: 10.1111/j.1527-3466.2004.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 18.Penumathsa SV, Thirunavukkarasu M, Koneru S, Juhasz B, Zhan L, Pant R, et al. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J Mol Cell Cardiol. 2007;42:508–516. doi: 10.1016/j.yjmcc.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan E, Zhang L, Jiang S, Bai Y. Beneficial effects of resveratrol on atherosclerosis. JMed Food. 2008;11:610–614. doi: 10.1089/jmf.2007.0091. [DOI] [PubMed] [Google Scholar]
- 20.Shigematsu S, Ishida S, Hara M, Takahashi N, Yoshimatsu H, Sakata T. Resveratrol, a red wine constituent polyphenol, prevents superoxide-dependent inflammatory responses induced by ischemia/reperfusion, platelet-activating factor, or oxidants. Free Radic Biol Med. 2003;34:810–817. doi: 10.1016/s0891-5849(02)01430-2. [DOI] [PubMed] [Google Scholar]
- 21.Turrens JF, Lariccia J, Nair MG. Resveratrol has no effect on lipoprotein profile and does not prevent peroxidation of serum lipids in normal rats. Free Radic Res. 1997;27:557–562. doi: 10.3109/10715769709097859. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Zou J, Huang Y, Cao K, Xu Y, Wu JM. Effect of resveratrol on platelet aggregation in vivo and in vitro. Chin Med J. 2002;115:378–380. [PubMed] [Google Scholar]
- 23.Wilson T, Knight TJ, Beitz DC, Lewis DS, Engen RL. Resveratrol promotes atherosclerosis in hypercholesterolemic rabbits. Life Sci. 1996;59:PL15–PL21. doi: 10.1016/0024-3205(96)00260-3. [DOI] [PubMed] [Google Scholar]
- 24.Cignarella A, Minici C, Bolego C, Pinna C, Sanvito P, Gaion RM, et al. Potential pro-inflammatory action of resveratrol in vascular smooth muscle cells from normal and diabetic rats. Nutr Metab Cardiovasc Dis. 2006;16:322–329. doi: 10.1016/j.numecd.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Saeed O, Otsuka F, Polavarapu R, Karmali V, Weiss D, Davis T, et al. Pharmacological suppression of hepcidin increases macrophage cholesterol efflux and reduces foam cell formation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:299–307. doi: 10.1161/ATVBAHA.111.240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001;103:448–454. doi: 10.1161/01.cir.103.3.448. [DOI] [PubMed] [Google Scholar]
- 27.Habets KL, van Puijvelde GH, van Duivenvoorde LM, van Wanrooij EJ, de Vos P, Tervaert JW, et al. Vaccination using oxidized low-density lipoprotein-pulsed dendritic cells reduces atherosclerosis in LDL receptor-deficient mice. Cardiovasc Res. 2010;85:622–630. doi: 10.1093/cvr/cvp338. [DOI] [PubMed] [Google Scholar]
- 28.von der Thusen JH, van Berkel TJ, Biessen EA. Induction of rapid atherogenesis by perivascular carotid collar placement in apolipoprotein E-deficient and low-density lipoprotein receptor-deficient mice. Circulation. 2001;103:1164–1170. doi: 10.1161/01.cir.103.8.1164. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt R, Bültmann A, Fischel S, Gillitzer A, Cullen P, Walch A, et al. Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor kappa B-dependent inflammation in monocytes. Circ Res. 2008;102:302–309. doi: 10.1161/CIRCRESAHA.107.157990. [DOI] [PubMed] [Google Scholar]
- 30.Chan YC, Chang SC, Liu SY, Yang HL, Hseu YC, Liao JW. Beneficial effects of yam on carbon tetrachloride-induced hepatic fibrosis in rats. J Sci Food Agric. 2010;90:161–167. doi: 10.1002/jsfa.3801. [DOI] [PubMed] [Google Scholar]
- 31.Macarulla MT, Alberdi G, Gómez S, Tueros I, Bald C, Rodríguez VM, et al. Effects of different doses of resveratrol on body fat and serum parameters in rats fed a hypercaloric diet. J Physiol Biochem. 2009;65:369–376. doi: 10.1007/BF03185932. [DOI] [PubMed] [Google Scholar]
- 32.Chang GR, Chiu YS, Wu YY, Chen WY, Liao JW, Chao TH, et al. Rapamycin protects against high fat diet-induced obesity in C57BL/6J mice. J Pharmacol Sci. 2009;109:496–503. doi: 10.1254/jphs.08215fp. [DOI] [PubMed] [Google Scholar]
- 33.Chang GR, Wu YY, Chiu YS, Chen WY, Liao JW, Hsu HM, et al. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin Pharmacol Toxicol. 2009;105:188–198. doi: 10.1111/j.1742-7843.2009.00427.x. [DOI] [PubMed] [Google Scholar]
- 34.Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res. 2008;22:1367–1371. doi: 10.1002/ptr.2503. [DOI] [PubMed] [Google Scholar]
- 35.Kim S, Jin Y, Choi Y, Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol. 2011;81:1343–1351. doi: 10.1016/j.bcp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Majumdar AS, Giri PR, Pai SA. Resveratrol- and melatonin-abated ovariectomy and fructose diet-induced obesity and metabolic alterations in female rats. Menopause. 2014;21:876–85. doi: 10.1097/GME.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 37.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 38.Libby P, Ridker PM, Attilio Maseri. Inflammation and Atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 39.Zhong M, Cheng GF, Wang WJ, Guo Y, Zhu XY, Zhang JT. Inhibitory effect of resveratrol on interleukin 6 release by stimulated peritoneal macrophages of mice. Phytomedicin. 1999;6:79–84. doi: 10.1016/S0944-7113(99)80039-7. [DOI] [PubMed] [Google Scholar]
- 40.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC, Jr, et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;192:211–217. doi: 10.1016/j.atherosclerosis.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Chen S, Zhao X, Ran L, Wan J, Wang X, Qin Y, et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Dig Liver Dis. 2015;47:226–232. doi: 10.1016/j.dld.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Sahebkar A. Effects of resveratrol supplementation on plasma lipids: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2013;71:822–835. doi: 10.1111/nure.12081. [DOI] [PubMed] [Google Scholar]
- 43.Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:473–480. doi: 10.1161/01.atv.21.4.473. [DOI] [PubMed] [Google Scholar]
- 44.Zern TL, West KL, Fernandez ML. Grape polyphenols decrease plasma triglycerides and cholesterol accumulation in the aorta of ovariectomized guinea pigs. J Nutr. 2003;133:2268–2272. doi: 10.1093/jn/133.7.2268. [DOI] [PubMed] [Google Scholar]
- 45.Do GM, Kwon EY, Kim HJ, Jeon SM, Ha TY, Park T, et al. Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem Biophys Res Commun. 2008;374:55–59. doi: 10.1016/j.bbrc.2008.06.113. [DOI] [PubMed] [Google Scholar]
- 46.Basu M, Prasad R, Jayamurthy P, Pal K, Arumughan C, Sawhney RC. Anti-atherogenic effects of seabuckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine. 2007;14:770–777. doi: 10.1016/j.phymed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 47.Chen Q, Wang E, Ma L, Zhai P. Dietary resveratrol increases the expression of hepatic 7α-hydroxylase and ameliorates hypercholesterolemia in high-fat fed C57BL/6J mice. Lipids Health Dis. 2012;11:56–63. doi: 10.1186/1476-511X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 49.Nakata R, Takahashim S, Inoue H. Recent advances in the study on resveratrol. Biol Pharm Bull. 2012;35:273–279. doi: 10.1248/bpb.35.273. [DOI] [PubMed] [Google Scholar]
- 50.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007;12:4839–4854. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- 52.Culpitt SV, Rogers DF, Fenwick PS, Shah P, De Matos C, Russell RE, et al. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax. 2003;58:942–946. doi: 10.1136/thorax.58.11.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magyar K, Halmosi R, Palfi A, Feher G, Czopf L, Fulop A, et al. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin Hemorheol Microcirc. 2012;50:179–187. doi: 10.3233/CH-2011-1424. [DOI] [PubMed] [Google Scholar]
- 54.Fukuhara K, Miyata N. Resveratrol as a new type of DNA-cleaving agent. Bioorg Med Chem Lett. 1998;8:3187–3192. doi: 10.1016/s0960-894x(98)00585-x. [DOI] [PubMed] [Google Scholar]


