Abstract
Objective(s):
Both caloric restriction (CR) and resveratrol (RSV) have been shown to improve learning and memory, but their potential effects in juvenile animals were unknown. Here, we evaluated the effects of RSV and CR on learning and memory function in juvenile mice and investigated potential molecular mechanisms.
Methods:
Six-week-old C57BL/6J mice were assigned to one of three different dietary groups: normal control (stock diet) (n=12), CR diet (30% caloric reduction diet) (n=12), and RSV diet (stock diet supplemented with 18.6 mg/kg RSV) (n=12), for 6 months. Body weight and blood glucose were measured every 4 weeks. Serum cholesterol and serum triglyceride levels were examined using biochemical methods. Serum insulin and insulin-like growth factor 1 (IGF-1) levels were evaluated using enzyme linked immunosorbant assay (ELISA), and protein expression of silent mating type information regulation 2 homology 1 (SIRT1), p53, p16, peroxisome proliferator-activated receptor γ (PPARγ), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), phosphorylated-cAMP response element-binding protein (p-CREB), and IGF-1 were examined with immunohistochemistry.
Results:
Although long-term CR diet did not alter physiological conditions in juvenile mice relative to control, RSV supplementation slightly elevated blood glucose, serum triglyceride, and serum insulin levels. Both CR and RSV improved learning and memory function, although the effect of CR was significantly greater. Both CR and RSV downregulated p53 and upregulated IGF-1 in hippocampal CA1 region of mice.
Conclusion:
We demonstrate that CR and RSV may improve learning and memory by downregulating p53 and upregulating IGF-1 in hippocampal CA1 region of juvenile mice.
Keywords: Caloric restriction, IGF-1, Learning, memory, p53, Resveratrol
Introduction
Impairment in learning and memory processes are common clinical problems in the elderly and in patients with neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease (1). Caloric restriction (CR) by decreasing dietary intake has been shown to slow aging, prevent neuronal damage, and protect against neurodegeneration (2). CR may even improve learning ability and memory function in mice (3, 4) and in early old rats (5). The insulin-PI3K/Akt signaling pathway has been implicated in these processes (3), although the precise underlying mechanisms are still poorly understood.
The benefits of resveratrol (RSV), a natural compound found in grapes, red wine, peanuts and cranberries, as a dietary supplement are well documented regarding the prevention of cardiovascular, cerebral, and metabolic diseases and cancer (6, 7). RSV, like CR, improves learning and memory in adult mice (8). However, its role in neuroprotection is controversial. Recently, it is reported that RSV treatment did not reverse scopolamine-induced deficits in cognitive function in mice (9), suggesting that RSV is likely not universally neuroprotective. To date, the effects of RSV on learning and memory in juvenile mice have not been fully clarified.
RSV, a well-known silent mating type information regulation 2 homology 1 (SIRT1) activator, induces various biological actions via activation of SIRT1 (10). SIRT1, the most extensively studied sirtuin family protein, plays an essential role in regulating brain function (11). SIRT1 regulates the expression of several downstream effectors implicated in synaptic plasticity, including p53, p16, peroxisome proliferator-activated receptor γ (PPARγ), nuclear factor kappa B, forkhead box O, and liver X receptor (11). Another signaling pathway important for neuronal function is the insulin/insulin-like growth factor (IGF-1)/phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/protein kinase B (Akt) pathway. Dysfunction of the insulin and its downstream targets have been linked to synaptic dysfunction and cognitive decline in neurodegenerative disorder such as Alzheimer’s disease (12).
Based on these studies, we hypothesized that CR and RSV would improve learning and memory function in juvenile mice, perhaps via the SIRT1 and/or insulin-IGF-1-PI3K signaling pathways. Our findings may provide valuable insight for the development of novel pharmacological targets for the treatment of deficits in learning and memory.
Materials and Methods
Animals
In total, 36 male and female C57BL/6J mice (6 week-old, weight range, 16.1-25.1 g, provided from Laboratory Animal Center of the Academy of Military Medical Sciences, China) were used in this study. Animals were housed under specific pathogen free (SPF) conditions and maintained at constant temperature (23±2 °C) and humidity (55±5%) with a 12 hr light dark cycle. All mice had free access to water and were adapted to the housing condition for 2 weeks prior to the start of experimentation. The animal studies were approved by the Animal Ethical Committee of Xuanwu Hospital, Beijing, China.
Diets and experimental assignments
Animals were randomly assigned to one of three dietary groups: control (n=12), CR group (n=12), and RSV (n=12). Mice in the control group were fed SPF-level stock diet (Laboratory Animal Center of the Academy of Military Medical Sciences). The diet of CR mice consisted of 58% stock diet, 34% dietary fiber, and 8% isolated soy protein, leading to a 30% caloric reduction (13). Mice in the RSV group were fed the stock diet supplemented with 18.6 mg/kg (equal to 30 mg/kg/day) RSV (99.9% purification; Beijing Puhuashi Technology Development Co., Ltd., Beijing, China) (14, 15). All mice were maintained on the diet for 6 months. During the experimental period, animal body weight and fasting blood-glucose level were measured every 4 weeks.
Serological test
Serum IGF-1 and insulin levels were measured using specific enzyme linked immunosorbant assay (ELISA) kits in accordance of the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). Serum cholesterol and serum triglyceride levels were also examined using biochemical methods.
Morris water maze test
The Morris water maze task was used to assess learning and memory function of animals fed different diets (16). The Morris water maze apparatus (provided by the Institute of Materia Medica, Chinese Academy of Medical Sciences, Beijing, China) consisted of a circular pool of water (24 °C + 1 °C) with a diameter of 120 cm and a height of 30 cm. The water pool was divided into four quadrants: northeast, northwest, southeast, and southwest quadrant. In the northeast quadrant, a platform was submerged 2.0 cm below of the surface of the water, which was made invisible with the addition of dry milk. During training, the mice were physically placed onto the platform, where they remained for 30 sec. On Day 1, mice were placed in the southwest quadrant and were given 60 sec to find the hidden platform. If unsuccessful within the allotted time period, they were guided to the platform. Place navigation test was conducted from Day 2 to Day 5, and the escape latency and the overall swimming distance were recorded. If a mouse was unsuccessful in finding the platform within the allotted time period, they were given a score of 60 sec.
Immunohistochemical analysis
Tissue samples from CA1 regions of hippocampus were fixed in 4% paraformaldehyde overnight, dehydrated in an ethanol series (50%, 70%, 80%, 90%, 95%, and 100%), cleared in xylene, and embedded in paraffin. Paraffin-embedded tissues were sectioned with an automatic tissue processor (Leica, Wetzlar, Germany) into 5 μm thick coronal sections. Sections were incubated in 3% hydrogen peroxide solution for 30 min to inactivate the peroxidase. After sections were washed three times with phosphate buffered saline (PBS), sections were probed with rabbit primary antibody (1: 1000 dilution) and goat anti-rabbit biotin-labeled secondary antibody (1 : 300 dilution) (Beijing Zhong Shan Golden Bridge Biological Technology Co., Ltd, Beijing, China). Primary antibodies, including anti-IGF-1, anti-PI3K, anti-phosphorylated-cAMP response element-binding protein (p-CREB), anti-SIRT1, anti-p16, anti-PPARγ and anti-p53, were synthesized in the Bioactive Peptides Laboratory of Xuanwu Hospital (Beijing, China). Immunoreactivity was visualized with 3, 3’-diaminobenzidine tetrahydrochloride (DAB) kit according to the manufacturer’s instructions (Beijing Zhong Shan Golden Bridge Biological Technology Co., Ltd). After washing, samples were further stained with hematoxylin. For a negative control, PBS was used in place of primary antibody (data not shown). The number of immunopositive neurons was analyzed using Image-Pro Plus software. For quantification, three slices were chosen at random from each animal and from those, three fields were randomly selected under 400X magnification.
Statistical analysis
All data were analyzed using SPSS 17.0 software (Chicago, IL, USA) and were expressed as mean± standard deviation (SD). Statistical significance was determined using one-way analysis of variance (ANOVA). For comparison of the body weight, blood glucose level, escape latency, speed, and swimming distances in the three groups of animal, repeated measures two-way ANOVA was used. P<0.05 or P<0.01 was considered statistically significant.
Results
Effects of resveratrol and CR on physiological indicators.
First, we examined the effect of CR and RSV diets on physiological conditions in juvenile C57BL/6J mice. Animal body weight gradually increased during the experimental period (24 weeks) in all three groups (Figure 1A). Compared to mice fed control diet, CR attenuated the increase in animal body weight significantly (P<0.05). At 8 and 12 weeks of treatment, body weight in the RSV group was significantly higher than the CR group (P<0.01). There was no significant difference in the level of blood glucose between control and CR, whereas in the RSV group, blood glucose level was elevated starting at 1 month after onset of treatment (P<0.01 compared with other groups) (Figure 1B). The serum levels of cholesterol, triglyceride, insulin, and IGF-1 were not affected by CR treatment (P>0.05) (Figure 1C). However, serum levels of triglyceride and insulin were significantly elevated in the RSV group (P<0.05) (Figure 1C). These results demonstrated that long-term CR diet did not alter physiological conditions, whereas 30 mg/kg/day RSV elevated blood glucose, serum triglyceride, and serum insulin levels in juvenile mice.
Figure 1.
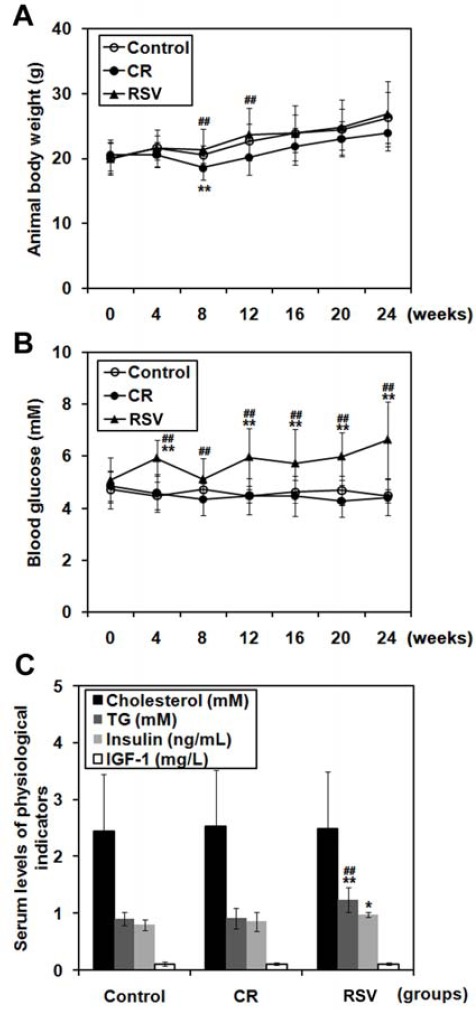
Physiological changes in mice fed caloric restriction (CR) or resveratrol (RSV) diet. Mice were fed normal, CR or RSV diet for 6 months. Animal body weight (A), blood glucose level (B), serum cholesterol, serum triglyceride (TG), serum insulin, or serum IGF-1 levels (C) are shown. *P<0.05, **P<0.01 compared with control; ##P<0.01 compared with CR. N=6 for each group
Effects of RSV and CR on learning and memory ability in mice
The Morris water maze task is a well-established behavioral test to evaluate learning and memory in rodents. Here, we examined the effect of CR and RSV on learning and memory function in mice. No significant difference was found in the speed of animals in three experimental groups (P>0.05), indicating that motor function or physical conditions was no different among the groups. Therefore, the escape latency and the overall swimming distance were used to determine cognitive function of mice. As shown in Figure 2, both escape latency and the overall swimming distance decreased over the course of the 5 day test period. CR significantly reduced escape latency and swimming distance relative to control by Day 3 (escape latency, P<0.05). With RSV treatment, escape latency and the swimming distance tended to decrease on Days 2, 3, and 5. Relative to RSV, CR was more effective for improving learning and memory function in mice.
Figure 2.
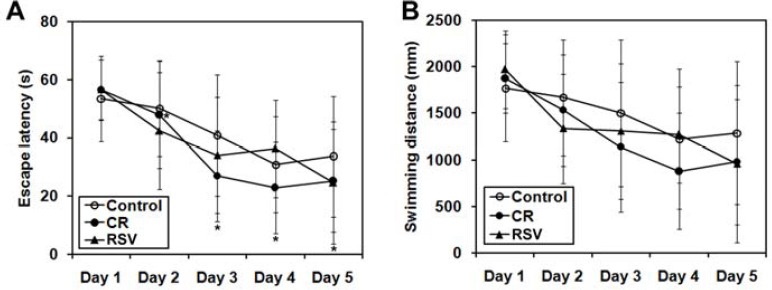
Learning and memory function of mice as assessed with the Morris water maze test. Escape latency (A) and swimming distance (B) of mice in normal control, CR, and RSV groups are shown. *P<0.05 compared with control; #P<0.05, ##P <0.01 compared with LC. N= 12 for each group
SIRT1 signaling pathway in mice fed different diets
In order to understand the molecular mechanisms underlying CR- and RSV-mediated improvement in learning and memory, we measured the expression levels of SIRT1 and its substrates p53, p16, and PPARγ in CA1 of hippocampus. We found no significant difference in the number of SIRT1-positive neurons among the three groups (P>0.05), whereas CR and RSV treatment significantly downregulated the expression of p53 in hippocampal CA1 region relative to control (P<0.01) (Figure 3). There was also no difference in the number of p16-positive neurons in CR or RSV group as compared with control (P>0.05). These findings indicated that CR and RSV may improve learning and memory via downregulation of p53 in hippocampal CA1 region.
Figure 3.
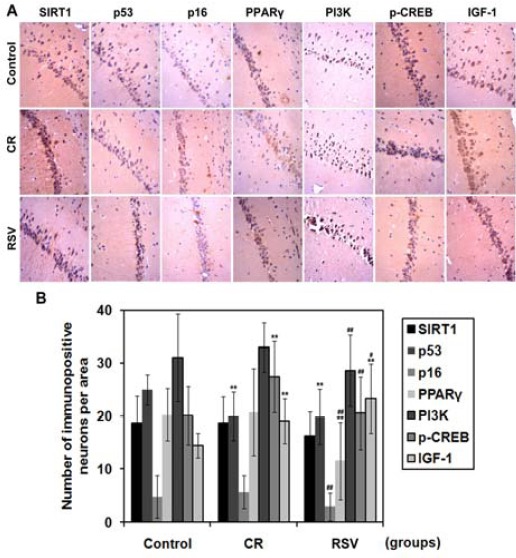
Expression of SIRT1 and insulin signaling pathway regulators. (A) The protein expressions of SIRT1, p53, p16, PPARγ, PI3K, p-CREB, and IGF-1 were assessed using immunohistochemistry. Magnification ×400. (B) The number of immunopositive neurons per area in hippocampal CA1 region is shown. **P<0.01 compared with control; #P<0.05, ##P <0.01 compared with CR. N=6 for each group
IGF-1-PI3K-p-CREB signal pathway in mice fed with different diets
The role of the IGF-1-PI3K-p-CREB signaling pathway was also investigated on CR- and RSV-mediated improvement in learning and memory. The expression of PI3K in hippocampal CA1 region was not significantly different in either the CR or RSV groups relative to control (P>0.05) (Figure 3). Regarding p-CREB, it was significantly upregulated in the CR group (P<0.01). In addition, although both CR and RSV significantly elevated the number of IGF-1-positive neurons in hippocampal CA1 region (P< 0.01 compared with control), the upregulation was greater in the RSV group (P<0.05 compared with CR). These results revealed that CR and RSV may improve learning and memory via upregulation of IGF-1 in hippocampal CA1 region.
Discussion
In this study, we examined the effects of long-term CR and RSV diet on learning and memory abilities in juvenile C57BL/6J mice. We found that a 30 mg/kg/day RSV regimen for 6 months elevated blood glucose, serum triglyceride, and serum insulin levels, although the blood glucose level was below the lower critical value for diabetes. Previously, a study demonstrated that administration of 5 mg/kg RSV for 42 days had no effect on animal blood glucose level (17), whereas another study showed that RSV reduced blood glucose levels in diabetic rats (18). This discrepancy may be due to differences in species, dose of RSV, or duration of RSV diet; and further studies are needed to determine the reason. It is important to note that CR did not alter physiological conditions, which is consistent with a previous report that demonstrated that CR did not significantly alter blood glucose or triglyceride levels in young rhesus monkeys (19).
According to the Morris water maze test results, both CR and RSV improved learning and memory in mice, where the effect of CR was greater than RSV. Previously, it was shown that CR prevented cognitive decline in old F344Xbn mice, whereas no significant difference was found in CR-fed or control middle age mice (20). Here, we showed that CR could improve learning and memory in juvenile C57BL/6J mice. However, neuroprotection by RSV is controversial. Although Zhao et al reported improved learning and memory function in normally aged mice fed with RSV (8), another study found that RSV treatment could not reverse scopolamine-induced deficit in cognitive functions in mice (9). We observed a moderate effect of RSV diet on the learning and memory improvement in juvenile mice, but the efficacy was significantly smaller than that by CR.
SIRT1 plays a critical role in longevity and regulates a key pathway regulating senescence (21). In order to understand the potential involvement of the SIRT1 signaling pathway in CR- and RSV-mediated neuroprotection, SIRT1 in addition to its downstream targets p53, p16 and PPARγ were studied. Among these regulators, the number of p53-positive neurons in hippocampal CA1 region after CR or RSV treatment was significantly reduced. Accordingly, it has been suggested that p53 is the common downstream effector of both the SIRT1 and the target of rapamycin (TOR) pathway to mediate CR-induced longevity of mammalian cells and organisms (22). p53 is a well characterized tumor suppressor gene (23). Genetically engineered mice with elevated p53 activity exhibited premature loss of neurogenic capacity and accelerated organismal aging (24). Perhaps the downregulation of p53 expression in hippocampal CA1 region by CR or RSV diet in our study was responsible for the improvement of learning and memory abilities in mice. Moreover, other regulators in addition to SIRT1 may regulate the activity of p53 in this process.
The insulin signaling pathway has been implicated in regulation of hippocampus-dependent spatial learning and memory (25, 26). Therefore, we measured the expressions of IGF-1, PI3K, and p-CREB, all of which are key regulators in the insulin signaling pathway, in hippocampal CA1 region. Only the expression of IGF-1 was significantly changed, as both CR and RSV greatly upregulated IGF-1 level in hippocampal CA1 region. Compared to CR, RSV was more effective at upregulating IGF-1 level in hippocampus. Finding of a study showed that the reduction in serum IGF-1 level was closely related to the impairment in cognitive performance in the elderly (27). Consistent with our findings, it was shown that RSV improved cognitive function by increasing expression of IGF-1 in the hippocampus (28). However, there was no significant change in the levels of PI3K or p-CREB. We did not examine the expression of phosphorylated Akt (p-Akt), so we could not rule out the possibility that the PI3K/Akt pathway was activated. Collectively, we have shown that CR and RSV may protect brain function via upregulation of IGF-1 expression. The downstream regulators involved in the insulin-IGF-1 signal remain to be elucidated.
Conclusion
In this study, we found that CR and RSV improved learning and memory function in juvenile C57BL/6J mice. Compared to RSV, CR was more effective in this process. The improvement in learning and memory induced by CR and RSV may be exerted through downregulating p53 and upregulating IGF-1 in hippocampal CA1 region. Our findings add to a growing body of evidence suggesting that CR and RSV may be beneficial clinically for treating learning and memory deficits.
Acknowledgment
This study was supported by the Beijing Natural Science Foundation (Grant No. 7132044) and Capital Health Development Research Fund (Grant No. 2011-1001-02). We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Brem AK, Ran K, Pascual-Leone A. Learning and memory. Handb Clin Neurol. 2013;116:693–737. doi: 10.1016/B978-0-444-53497-2.00055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillette-Guyonnet S, Vellas B. Caloric restriction and brain function. Curr Opin Clin Nutr Metab Care. 2008;11:686–692. doi: 10.1097/MCO.0b013e328313968f. [DOI] [PubMed] [Google Scholar]
- 3.Dong W, Wang R, Ma LN, Xu BL, Zhang JS, Zhao ZW, et al. Autophagy involving age-related cognitive behavior and hippocampus injury is modulated by different caloric intake in mice. Int J Clin Exp Med. 2015;8:11843–11853. [PMC free article] [PubMed] [Google Scholar]
- 4.Kuhla A, Lange S, Holzmann C, Maass F, Petersen J, Vollmar B, et al. Lifelong caloric restriction increases working memory in mice. PLoS One. 2013;8:e68778. doi: 10.1371/journal.pone.0068778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geng YQ, Guan JT, Xu MY, Xu XH, Fu YC. Behavioral study of calorie-restricted rats from early old age. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:2393–2395. doi: 10.1109/IEMBS.2007.4352809. [DOI] [PubMed] [Google Scholar]
- 6.Carrizzo A, Forte M, Damato A, Trimarco V, Salzano F, Bartolo M, et al. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem. 2013;61:215–226. doi: 10.1016/j.fct.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Carter LG, D’Orazio JA, Pearson KJ. Resveratrol and cancer: focus on in vivo evidence. Endocrin Relat Cancer. 2014;21:R209–25. doi: 10.1530/ERC-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao YN, Li WF, Li F, Zhang Z, Dai YD, Xu AL, et al. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem Biophys Res Commun. 2013;435:597–602. doi: 10.1016/j.bbrc.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Gupta R, Gupta LK, Mediratta PK, Bhattacharya SK. Effect of resveratrol on scopolamine-induced cognitive impairment in mice. Pharmacol Rep. 2012;64:438–444. doi: 10.1016/s1734-1140(12)70785-5. [DOI] [PubMed] [Google Scholar]
- 10.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 11.Paraiso AF, Mendes KL, Santos SH. Brain activation of SIRT1: role in neuropathology. Mol Neurobiol. 2013;48:681–689. doi: 10.1007/s12035-013-8459-x. [DOI] [PubMed] [Google Scholar]
- 12.O’Neill C, Kiely AP, Coakley MF, Manning S, Long-Smith CM. Insulin and IGF-1 signalling: longevity, protein homoeostasis and Alzheimer’s disease. Biochem SocTtrans. 2012;40:721–727. doi: 10.1042/BST20120080. [DOI] [PubMed] [Google Scholar]
- 13.Ma L, Zhao Z, Wang R, Zhang J, Dong W, Xu B, et al. Caloric restriction can improve learning ability in C57/BL mice via regulation of the insulin-PI3K/Akt signaling pathway. Neurological Sciences. 2014;35:1381–1386. doi: 10.1007/s10072-014-1717-5. [DOI] [PubMed] [Google Scholar]
- 14.Alberdi G, Rodriguez VM, Miranda J, Macarulla MT, Arias N, Andrés Lacueva C, et al. Changes in white adipose tissue metabolism induced by resveratrol in rats. Nutr Metab. 2011;8:29. doi: 10.1186/1743-7075-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tome-Carneiro J, Larrosa M, Gonzalez-Sarrias A, Tomás-Barberán FA, Almagro García-Conesa MT, Espín JC. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr Pharm Des. 2013;19:6064–6093. doi: 10.2174/13816128113199990407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 17.Silan C. The effects of chronic resveratrol treatment on vascular responsiveness of streptozotocin-induced diabetic rats. Biol Pharm Bull. 2008;31:897–902. doi: 10.1248/bpb.31.897. [DOI] [PubMed] [Google Scholar]
- 18.Palsamy P, Subramanian S. Ameliorative poten-tial of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic beta-cell dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J Cell Physiol. 2010;224:423–432. doi: 10.1002/jcp.22138. [DOI] [PubMed] [Google Scholar]
- 19.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams MM, Shi L, Linville MC, Forbes ME, Long AB, Bennett C, et al. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp Neurol. 2008;211:141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- 22.Tucci P. Caloric restriction: is mammalian life extension linked to p53? Aging. 2012;4:525–534. doi: 10.18632/aging.100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcel V, Dichtel-Danjoy ML, Sagne C, Hafsi H, Ma D, Ortiz-Cuaran S. Biological functions of p53 isoforms through evolution: lessons from animal and cellular models. Cell Death Differ. 2011;18:1815–1824. doi: 10.1038/cdd.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medrano S, Scrable H. Maintaining appearances--the role of p53 in adult neurogenesis. Biochem Biophys Res Commun. 2005;331:828–833. doi: 10.1016/j.bbrc.2005.03.194. [DOI] [PubMed] [Google Scholar]
- 25.Xu BL, Wang R, Meng XH, Zhao ZW, Wang HJ, Ma LN, et al. Effects of analog P165 of amyloid precursor protein 5-mer peptide on learning, memory and brain insulin receptors in the rat model of cognitive decline. Neurol Sci. 2014;35:1821–1826. doi: 10.1007/s10072-014-1849-7. [DOI] [PubMed] [Google Scholar]
- 26.Diegues JC, Pauli JR, Luciano E, de Almeida Leme JA, de Moura LP, Dalia RA, et al. Spatial memory in sedentary and trained diabetic rats: molecular mechanisms. Hippocampus. 2014;24:703–711. doi: 10.1002/hipo.22261. [DOI] [PubMed] [Google Scholar]
- 27.Landi F, Capoluongo E, Russo A, Onder G, Cesari M, Lulli P, et al. Free insulin-like growth factor-I and cognitive function in older persons living in community. Growth Hormone IGF Res. 2007;17:58–66. doi: 10.1016/j.ghir.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Harada N, Zhao J, Kurihara H, Nakagata N, Okajima K. Resveratrol improves cognitive function in mice by increasing production of insulin-like growth factor-I in the hippocampus. J Nutr Biochem. 2011;22:1150–1159. doi: 10.1016/j.jnutbio.2010.09.016. [DOI] [PubMed] [Google Scholar]


