Abstract
Objective(s):
Neurodegenerative Alzheimer’s disease (AD) is associated with profound deficits in synaptic transmission and synaptic plasticity. Long-term potentiation (LTP), an experimental form of synaptic plasticity, is intensively examined in hippocampus. In this study we evaluated the effect of aqueous extract of lavender (Lavandula angustifolia) on induction of LTP in the CA1 area of hippocampus. In response to stimulation of the Schaffer collaterals the baseline or tetanized field extracellular postsynaptic potentials (fEPSPs) were recorded in the CA1 area.
Materials and Methods:
The electrophysiological recordings were carried out in four groups of rats; two control groups including the vehicle (CON) and lavender (CE) treated rats and two Alzheimeric groups including the vehicle (ALZ) and lavender (AE) treated animals.
Results:
The extract inefficiently affected the baseline responses in the four testing groups. While the fEPSPs displayed a considerable LTP in the CON animals, no potentiation was evident in the tetanized responses in the ALZ rats. The herbal medicine effectively restored LTP in the AE group and further potentiated fEPSPs in the CE group.
Conclusion:
The positive effect of the lavender extract on the plasticity of synaptic transmission supports its previously reported behavioral effects on improvement of impaired spatial memory in the Alzheimeric animals.
Keywords: Hippocampus, Lavandula angustifolia, LTP, Synaptic transmission
Introduction
Alzheimer’s disease (AD) is a common form of neurodegenerative disorder associated with prog-ressive memory deficits (1, 2). Memory impairment, in particular the incapability to form and preserve new episodic memories, is characteristic of AD (3). AD is characterized by intracellular deposition of neurofibrillary tangles (NFTs) and extracellular deposition of amyloid β-protein (Aβ) (4, 5). Aβ is generated through proteolysis of amyloid precursor protein (APP) by β-secretase and subsequently by γ-secretase (6). Hyperphosphorylation of tau proteins dissociates them from the microtubules and aggregates NFTs (7).
The hippocampus is crucial in memory storage and retrieval. The aggregated form of the Aβ initiates pathological processes in neurons of the hippocampus. In humans, the CA1 area of hippocampus is one of the first brain areas to display pathology of AD (8).
It is well known that long lasting changes in synaptic efficacy is required for formation of learning and memory. Long-term potentiation (LTP), an experimental form of synaptic plasticity, has been proposed as a candidate mechanism of memory formation in hippocampal neural circuits. Description of cellular mechanisms of information storage is broadly described in the brain hippocampal LTP. Research indicates that an increase in the aggregated form of Aβ in senile plaques greatly diminishes LTP (9).
Acetylcholine is a neurotransmitter which is degraded by acetylcholinesterase (AChE), an enzyme that is considered to play a role in the pathology of AD. AChE is present in the brain and is detected in NFTs and neuritic plaques (10).
Inflammatory processes are among the candidate mechanisms involved in progression of AD. Several inflammatory mediators are increased in the brains of AD patients compared to age-matched controls, and many of these mediators have been localized to classical pathologic lesions in AD brains (11).
The medicines currently available for AD are only able to delay the progression of the disease.
Lavender (Lavandula angustifolia) is a strongly aromatic sub-shrub at the Mediterranean region. Fresh flowers of this plant are mostly used for extract preparation. Numerous pharmacological effects including anticonvulsant, sedative, antispasmodic, analgesic, antioxidant and local anesthetic are attributed to lavender (12-14). This medicinal herb is found to be effective as an anticholinesterase and inhibitor of glutamate-induced neurotoxicity as well (15). Reports indicate that lavender extract is used in treatment of inflammation, depression, stress, and headache (16). We have previously demonstrated that lavender extract effectively underlies hippocampal related cognitive function (17). The present study was undertaken to evaluate the effect of the aqueous extract of the medicinal herb on LTP of synaptic transmission in the CA1 area of hippocampus of an animal model of Alzheimeric rat.
Materials and Methods
HPLC analysis
Twenty microliters of Lavender extract that was redissolved in methanol (10 mg/ml) was analyzed using an HPLC unit and a Spherisorb ODS2 column (4.6*250 mm, 5 µm particle size). The solvent system used was a gradient of water/formic acid (19:1) (A) and acetonitrile (B). the gradient was as follows: 0 min- 17% B; 40 min – 23% B; 57 min – 49% B; 59 min – 100% B; 60 min – 100% B. Elution was performed at a solvent flow rate of 1 ml/min. Detection was achieved with a Gilson diode array detector. The data were processed on a Uniport Software system; peak purity was checked using the software contrast facilities.
Animals
Thirty two male Wistar rats weighing 250–300 g at the beginning of the experimental procedures were used. Rats were rendered Alzheimeric as described in the previous report (17). Briefly, the animals received an intracerebroventricular injection of 1 micrograms Aβ 1-42 (purchased from Sigma-Aldrich) via a Hamilton syringe. The experimental subjects were kept in a single holding cage and housed in a constant temperature of 21± 2 °C and a humidity of 55± 5% with free access to food and water ad libitum. All procedures were in accordance with the Guidelines of Ethical Committee, Deputy of Research, Kashan University of Medical Sciences, Kashan, Iran.
Preparation of lavender extract
L. angustifolia was obtained from herbarium of Shahid Beheshti University of Medical Sciences, Tehran, Iran. For extraction, 250 g dried flowers of lavender was mixed with 1000 ml boiling water. The mixture was then stirred for 4 hr, filtered, and concentrated by vaporizing. Lavender specimen was identified by Pharmaceutics Faculty of the university, where voucher specimens (1092) were kept. The extract was standardized based on 130 mg caffeic acid in each gram.
Extract administration
The concentrated aqueous extract of lavender was suspended in distilled water. The control animals were intraperitoneally (IP) injected with either distilled water (CON) or 200 mg/kg of the lavender extract (CE). Also, Alzheimeric rats received distilled water (ALZ) or 200 mg/kg of the aqueous extract (AE). The administrations were carried out 20 days after Aβ injection. The volume of injections was adjusted at 0.4 ml/kg body-weight for all groups of animals. The treatment was conducted once per day for 20 consecutive days prior to electrophysiological experiments.
Electrophysiology Animal preparation and electrode implantation
The experimental subjects were anesthetized by urethane (1.5 g/kg, IP) and placed in a stereotaxic frame. Two holes were drilled above the skull with a sterile drill bit: a hole for the recording electrode (1 mm diameter, 4.2 mm posterior to bregma, 3.8 mm lateral to the midline) and another one for the stimulating electrode (1 mm diameter, 3.4 mm posterior to bregma, 2.5 mm lateral to the midline). All coordinates were based on the Stereotaxic atlas (18). The recording and stimulating electrodes were lowered into the CA1 stratum radiatum and the Schaffer collaterals, respectively. Extracellular recordings were referenced to an indifferent site on the skull.
Hippocampal recordings
In response to test pulses applied to the Schaffer collaterals, evoked field extracellular postsynaptic potentials (fEPSPs) of the CA1 field were attained. The stimulus amplitude was determined by means of an input/output curve. The stimulation intensity was adjusted to a level that evoked a 60% of maximum fEPSPs for all phases of the experiment. The fEPSPs were recorded at 30 sec intervals for 30 min. Then, LTP was induced by high-frequency stimulation (HFS) of 100 Hz (10 bursts of 10 stimuli, 0.1 msec stimulus duration and 2 sec inter-burst interval). Following the tetanus, responses to the test pulses were collected continuously for 1 hr (Figure 1).
Figure 1.
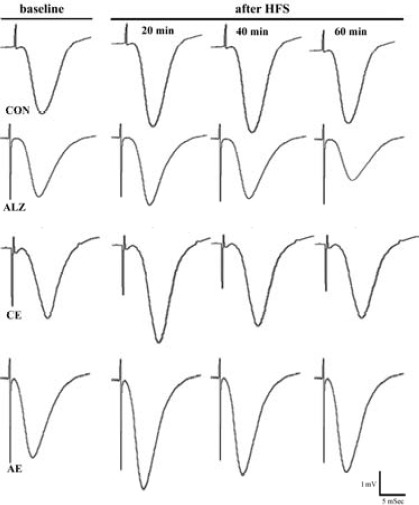
Slope of the baseline and post-tetanus field potentials recorded in the CA1 area of hippocampus evoked by stimulation of the Schaffer collaterals
Statistical analysis
The data were considered for the pre and post-tetanic response changes. The slope of fEPSPs (mili-volt/mili-sec) was considered for evaluation of recordings. The level of potentiation in the post-HFS responses was measured as:

Data are presented as mean±SEM and normalized by taking the pre-tetanus slope of fEPSPs as 100% and comparing the post-tetanus fEPSPs with it. The baseline and post-tetanus recordings of the four groups of animals were analyzed using two-way ANOVA followed by LSD posttest. The probability levels were interpreted as statistically significant if P-value was less than 0.05.
Results
By stimulating the Schaffer’s collaterals the baseline fEPSPs were recorded in the CA1 area of hippocampus. Then, the CA3-CA1 pathway was tetanized to assess the extent of post-HFS enhancement in the CA1 recordings. Analysis of variance indicated a significant general statistical difference between the data taken from the four groups of animals (F7,1342=29.079, P<0.0001).
The baseline recordings in the CA1 area of hippocampus
Basic synaptic transmission was recorded in each group prior to application of the tetanic stimulation. Our results showed that Aβ treatment at the dose of 1 µg/2 µl insignificantly influences the pre-tetanus field responses where the values in the CON and ALZ groups were 0.613±0.022 mV/ms and 0.652±0.023 mV/ms, respectively. Twenty day treatment of the animals with the lavender extract ineffectively affected the baseline fEPSPs in both CE and AE groups; values were 0.485±0.021 mV/ms and 0.649±0.021 mV/ms for the former and latter groups, respectively (Figure 2).
Figure 2.
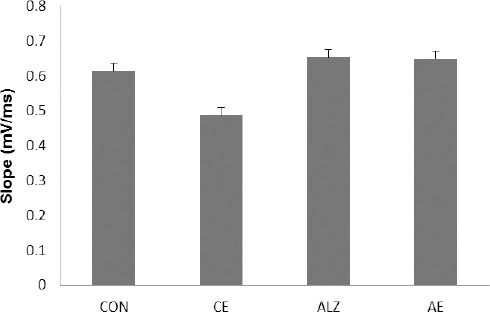
Representative traces illustrating the mean slope of the baseline responses and the changes in slope of the post-HFS fEPSPs over the first, the second and the third 20 min of recordings
Induction of LTP in the fEPSPs of the CA1 area of hippocampus Occurrence of LTP in the CON animals
Tetanization of the Schaffer’s collaterals resulted in a considerable LTP in the fEPSPs recorded in the CON group. Mean slope of post-tetanus responses increased more than 17% compared to the pre-tetanus recordings (P=0.001). The potentiation was steadily observable as long as the recording continued.
Effect of the lavender extract on the induction of LTP in the CE animals
Administration of the herbal medicine effectively influenced the post-HFS enhancement in the CE rats, where the tetanic stimulation led to a further potentiation in the fEPSPs (P=0.001). The post-tetanus mean slope in this group increased by more than 21% and the potentiation lasted throughout the recording session. ANOVA indicated a significant variation (P<0.0001) between the potentiated responses in the CON and CE groups. However, compared to the CON group, the LTP declined over recordings such that the degree of potentiation was almost similar in both CON and CE groups at the end of experiments.
Induction of LTP in the ALZ animals
Application of the tetanus failed to produce LTP in the ALZ group. The mean slope values of pre- and post-tetanus responses were 0.652±0.023 mV/ms and 0.670±0.023 mV/ms, respectively. Transient elevation of the fEPSPs slope immediately after HFS displayed a sharp decay so that the mean slope of recordings resembled that of the baseline responses about 30 min after the tetanic stimulation. The mean slope change percent was 2.7% indicating no difference between the pre- and post-tetanus responses slope (P=0.628). Additionally, the post-HFS fEPSPs exhibited a real depotentiation when the recordings continued.
Effect of the lavender extract on the induction of LTP in the AE animals
Treatment of the Alzheimeric animals with the aqueous extract of lavender restored occurrence of LTP. HFS elicited a post-tetanus enhancement by 34.1% in the AE group (P<0.0001). The statistical analysis showed a significant difference between the slope sizes of the post-HFS responses in the ALZ and AE animals (P<0.0001). Figure 3 illustrates the post-tetanus changes in the vehicle and lavender treated control and Alzheimeric rats. Table 1 summarizes the changes in slopes of the pre- and post-HFS responses in the four testing groups.
Figure 3.
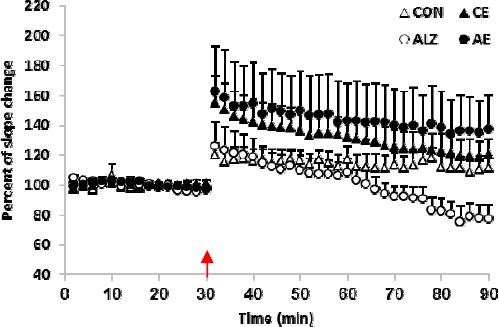
The percent of change in the slope of post-tetanus fEPSPs. While HFS considerably triggered LTP in post-tetanus responses, in the CON rats it failed to elicit a maintained potentiation in the fEPSPs in the ALZ animals. Lavender substantially lowered production of LTP in the AE group and further enhanced the synaptic plasticity in the CE group. Arrow indicates the time of application of the high frequency stimulation (HFS). Each point indicates data average obtained during 3 min
Table 1.
Values represent mean changes in the slopes of fEPSPs recorded in the four tested groups before and after tetanic stimulation
| Mean slope of fEPSP Groups |
Pre-tetanus (mV/ms) | Post-tetanus (mV/ms) | Approximate percent of potentiation |
|---|---|---|---|
| CON | 0.613±0.022 | 0.72±0.022 | 17.3% P<0.0001 |
| CE | 0.485±0.021 | 0.588±0.013 | 21% P<0.0001 |
| ALZ | 0.652±0.023 | 0.67±0.023 | 2.7% P=0.841 |
| AE | 0.649±0.021 | 0.871±0.02 | 34.1% P<0.0001 |
P-values indicate the changes in slopes of the pre- and post-tetanus fEPSPs
Discussion
The neurodegenerative AD profoundly affects the cognitive functions of the brain (19). Attempts have been dedicated to prevention of the disease or overcoming its adverse effects (20). The present study was devoted to electrophysiological assessment of synaptic transmission in the CA3-CA1 pathway, the well-known hippocampal neuronal circuit in memory consolidation. In this in vivo study the probable role of the lavender extract in the baseline as well as tetanized fEPSPs in the Aβ injected animals was evaluated. We found that the baseline responses are not sensitive to the intraventricular injection of Aβ, where the mean slope of fEPSPs was almost similar in both CON and ALZ groups. Consistently, Yamin reported that Aβ strongly inhibits LTP in the fEPSPs of the CA1 area at doses that have no acute effect on baseline excitatory transmission (21).
On the other hand, whereas the tetanic stimulation elicited a considerable LTP in the fEPSPs in the CON animals, it failed to potentiate the responses in the Aβ treated rats. These results are consistent with the report indicating that Aβ pronouncedly prevents occurrence of LTP (22).
Administration of the lavender extract restored the induction of LTP in the AE animals, and additionally, led to a further potentiation in the CON rats. What are the probable mechanisms through which the herbal medicine improves the synaptic plasticity?
Research indicates that, in addition to LTP prevention, the Aβ treatment results in impaired learning and memory. The neurotransmitter acetylcholine is crucial for memory, thinking and behavior. Involvement of cholinergic mechanisms in the biochemical and behavioral effects of soluble Aβ has been demonstrated (23). There are evidences indicating that the inhibitory effect of Aβ on LTP induction might be via inhibition of cholinergic transmission (24). Declined synthesis and/or release of acetylcholine are reported during treatment with Aβ (25, 26). Also, Magdesian et al. reported the blockade of acetylcholine receptors under Aβ administration (27). It is demonstrated that, through inhibiting AChE, lavender increases neurotransmitter activity in the brain. This may lead to increased availability of acetylcholine, which helps slow progression of AD symptoms including suppression of LTP induction (15).
It is reported that Aβ may increase synaptic concentration of glutamate by inhibiting glutamate uptake or promoting the release of this neurotrans-mitter (28). It may lead to a glutamatergic excitotoxicity, which is mediated by NMDA receptors (29). Consistently, it is shown that excessive release of glutamate plays an injurious role in ischemic injury of neurons (30). Also, evidence indicates that deficiencies in many stages of the glutamate cycle occurring in AD lead to increased synaptic concentrations of glutamate (31). It is found that aqueous extract of lavender flowers decreases glutamate induced neurotoxicity in rat pups’ cerebellar granular cell culture (32). Additionally, antioxidant characteristics and AChE inhibition is reported for linalool; one of the main components in lavender oil. This indicates that several targets relevant to treatment of AD such as the cholinergic, neuroprotective and antioxidant activities could be found in lavender (33).
Aβ induces secretion of pro-inflammatory molecules from microglia, and neuroinflammation is one of the key events in AD progress (34). In line, it is shown that intracerebroventricular injection of Aβ stimulates secretion of inflammatory agents and, thus, is one of the main reasons of inflammation in Alzheimeric brains (35). Lavender is reported to be an efficient medicinal herb in treating inflammation (16). Anti-inflammatory activities of lavender have been reported for different extracts of Laminaceae plants (36).
Altogether, the findings of the present study support the idea that the lavender extract positively underlies the NMDA receptor-mediated LTP in both the control and Aβ treated animals (37).
Conclusion
Our findings indicate that the herbal medicine extract is ineffective on basic synaptic activity in the hippocampal circuits; however, it has a positive impact on the tetanized NMDA receptor-mediated synaptic transmission in both the normal and specially the alzheimeric animals.
Acknowledgment
Deputy of Research of Kashan University of Medical Sciences, Kashan, Iran, financially supported this study (grant No. 9101 to M Salami). We greatly thank Dr GA Hamidi for his kind assistance.
References
- 1.Barry AE, Klyubin I, Mc Donald JM, Mably AJ, Farrell MA, Scott M, et al. Alzheimer’s disease brain-derived amyloid-beta-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J Neurosci. 2011;31:7259–7263. doi: 10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 3.He FQ, Qiu BY, Zhang XH, Li TK, Xie Q, Cui DJ, et al. Tetrandrine attenuates spatial memory impairment and hippocampal neuroinflammation via inhibiting NF-kappaB activation in a rat model of Alzheimer’s disease induced by amyloid-beta(1-42) Brain Res. 2011;1384:89–96. doi: 10.1016/j.brainres.2011.01.103. [DOI] [PubMed] [Google Scholar]
- 4.Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo SC, Bru-Mercier G, et al. Abeta(1-42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3beta. Nat Neurosci. 2011;14:545–547. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- 5.Jayadev S, Nochlin D, Poorkaj P, Steinbart EJ, Mastrianni JA, Montine TJ, et al. Familial prion disease with Alzheimer disease-like tau pathology and clinical phenotype. Ann Neurol. 2011;69:712–720. doi: 10.1002/ana.22264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hampel H, Shen Y, Walsh DM, Aisen P, Shaw LM, Zetterberg H, et al. Biological markers of amyloid β-related mechanisms in Alzheimer’s disease. Exp Neurol. 2010;223:334–346. doi: 10.1016/j.expneurol.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aisen PS, Cummings J, Schneider LS. Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006395. doi: 10.1101/cshperspect.a006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piermartiri TC, Figueiredo CP, Rial D, Duarte FS, Bezerra SC, Mancini G, et al. Atorvastatin prevents hippocampal cell death, neuroinflammation and oxidative stress following amyloid-beta(1-40) administration in mice: evidence for dissociation between cognitive deficits and neuronal damage. Exp Neurol. 2010;226:274–284. doi: 10.1016/j.expneurol.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Miao J, Hao J, Li Z, Xu J, Liu R, et al. Protective effect of S14G-humanin against beta-amyloid induced LTP inhibition in mouse hippocampal slices. Peptides. 2009;30:1197–1202. doi: 10.1016/j.peptides.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Ni R, Marutle A, Nordberg A. Modulation of alpha7 nicotinic acetylcholine receptor and fibrillar amyloid-beta interactions in Alzheimer’s disease brain. J Alzheimers Dis. 2013;33:841–851. doi: 10.3233/JAD-2012-121447. [DOI] [PubMed] [Google Scholar]
- 11.Fakhfouri G, Ahmadiani A, Rahimian R, Grolla AA, Moradi F, Haeri A. WIN55212-2 attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-gamma pathway. Neuropharmacology. 2012;63:653–666. doi: 10.1016/j.neuropharm.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Lehrner J, Marwinski G, Lehr S, Johren P, Deecke L. Ambient odors of orange and lavender reduce anxiety and improve mood in a dental office. Physiol Behav. 2005;86:92–95. doi: 10.1016/j.physbeh.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 13.Lin PW, Chan WC, Ng BF, Lam LC. Efficacy of aromatherapy (Lavandula angustifolia) as an intervention for agitated behaviours in Chinese older persons with dementia: a cross-over randomized trial. Int J Geriatr Psychiatry. 2007;22:405–410. doi: 10.1002/gps.1688. [DOI] [PubMed] [Google Scholar]
- 14.Kim HM, Cho SH. Lavender oil inhibits immediate-type allergic reaction in mice and rats. J Pharm Pharmacol. 1999;5:221–226. doi: 10.1211/0022357991772178. [DOI] [PubMed] [Google Scholar]
- 15.Adsersen A, Gauguin B, Gudiksen L, Jager AK. Screening of plants used in Danish folk medicine to treat memory dysfunction for acetylcholinesterase inhibitory activity. J Ethnopharmacol. 2006;104:418–422. doi: 10.1016/j.jep.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 16.Hajhashemi V, Ghannadi A, Sharif B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J Ethnopharmacol. 2003;89:67–71. doi: 10.1016/s0378-8741(03)00234-4. [DOI] [PubMed] [Google Scholar]
- 17.Kashani MS, Tavirani MR, Talaei SA, Salami M. Aqueous extract of lavender (Lavandula angustifolia) improves the spatial performance of a rat model of Alzheimer’s disease. Neurosci Bull. 2011;27:99–106. doi: 10.1007/s12264-011-1149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates - The New Coronal Set. Elsevier Science; 2004. [Google Scholar]
- 19.Taghizadeh M, Djazayery A, Salami M, Eshraghian MR, Zavareh SA. Vitamin-D-free regimen intensifies the spatial learning deficit in Alzheimer’s disease. Int J Neurosci. 2011;121:16–24. doi: 10.3109/00207454.2010.523132. [DOI] [PubMed] [Google Scholar]
- 20.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, et al. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamin G. NMDA receptor-dependent signaling pathways that underlie amyloid beta-protein disruption of LTP in the hippocampus. J Neurosci Res. 2009;87:1729–1736. doi: 10.1002/jnr.21998. [DOI] [PubMed] [Google Scholar]
- 22.Origlia N, Capsoni S, Cattaneo A, Fang F, Arancio O, Yan SD, et al. Abeta-dependent Inhibition of LTP in different intracortical circuits of the visual cortex: the role of RAGE. J Alzheimers Dis. 2009;17:59–68. doi: 10.3233/JAD-2009-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He YX, Wu MN, Zhang H, Qi JS. Amyloid beta-protein suppressed nicotinic acetylcholine receptor-mediated currents in acutely isolated rat hippocampal CA1 pyramidal neurons. Synapse. 2012;67:11–20. doi: 10.1002/syn.21611. [DOI] [PubMed] [Google Scholar]
- 24.Kar S, Issa AM, Seto D, Auld DS, Collier B, Quirion R. Amyloid beta-peptide inhibits high-affinity choline uptake and acetylcholine release in rat hippocampal slices. J Neurochem. 1998;70:2179–2187. doi: 10.1046/j.1471-4159.1998.70052179.x. [DOI] [PubMed] [Google Scholar]
- 25.Maatuk N, Samson AO. Modeling the binding mechanism of Alzheimer’s Abeta(1-42) to nicotinic acetylcholine receptors based on similarity with snake alpha-neurotoxins. Neurotoxicology. 2013;34:236–242. doi: 10.1016/j.neuro.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Wu MN, He YX, Guo F, Qi JS. Alpha4beta2 nicotinic acetylcholine receptors are required for the amyloid beta protein-induced suppression of long-term potentiation in rat hippocampal CA1 region in vivo. Brain Res Bull. 2008;77:84–90. doi: 10.1016/j.brainresbull.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Magdesian MH, Nery AA, Martins AH, Juliano MA, Juliano L, Ulrich H, et al. Peptide blockers of the inhibition of neuronal nicotinic acetylcholine receptors by amyloid beta. J Biol Chem. 2005;280:31085–31090. doi: 10.1074/jbc.M502406200. [DOI] [PubMed] [Google Scholar]
- 28.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goto Y, Niidome T, Akaike A, Kihara T, Sugimoto H. Amyloid beta-peptide preconditioning reduces glutamate-induced neurotoxicity by promoting endocytosis of NMDA receptor. Biochem Biophys Res Commun. 2006;351:259–265. doi: 10.1016/j.bbrc.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 30.Shimmyo Y, Kihara T, Akaike A, Niidome T, Sugimoto H. Three distinct neuroprotective functions of myricetin against glutamate-induced neuronal cell death: involvement of direct inhibition of caspase-3. J Neurosci Res. 2008;86:1836–1845. doi: 10.1002/jnr.21629. [DOI] [PubMed] [Google Scholar]
- 31.Alberdi E, Sanchez-Gomez MV, Cavaliere F, Perez-Samartin A, Zugaza JL, Trullas R, et al. Amyloid beta oligomers induce Ca2+dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47:264–272. doi: 10.1016/j.ceca.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Buyukokuroglu ME, Gepdiremen A, Hacimuftuoglu A, Oktay M. The effects of aqueous extract of Lavandula angustifolia flowers in glutamate-induced neurotoxicity of cerebellar granular cell culture of rat pups. J Ethnopharmacol. 2003;84:91–94. doi: 10.1016/s0378-8741(02)00286-6. [DOI] [PubMed] [Google Scholar]
- 33.Umezu T, Nagano K, Ito H, Kosakai K, Sakaniwa M, Morita M. Anticonflict effects of lavender oil and identification of its active constituents. Pharmacol Biochem Behav. 2006;85:713–721. doi: 10.1016/j.pbb.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- 35.Hoppe JB, Frozza RL, Horn AP, Comiran RA, Bernardi A, Campos MM, et al. Amyloid-beta neurotoxicity in organotypic culture is attenuated by melatonin: involvement of GSK-3 beta, tau and neuroinflammation. J Pineal Res. 2010;48:230–238. doi: 10.1111/j.1600-079X.2010.00747.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang YJ, Thomas P, Zhong JH, Bi FF, Kosaraju S, Pollard A, et al. Consumption of grape seed extract prevents amyloid-beta deposition and attenuates inflammation in brain of an Alzheimer’s disease mouse. Neurotox Res. 2009;15:3–14. doi: 10.1007/s12640-009-9000-x. [DOI] [PubMed] [Google Scholar]
- 37.Raymond CR, Ireland DR, Abraham WC. NMDA receptor regulation by amyloid-beta does not account for its inhibition of LTP in rat hippocampus. Brain Res. 2003;968:263–272. doi: 10.1016/s0006-8993(03)02269-8. [DOI] [PubMed] [Google Scholar]


