Abstract
Objective
To determine whether vitamin D levels are associated with menopause-related symptoms in older women.
Methods
A randomly selected subset of 1,407 women, among 26,104 potentially eligible participants of the Women’s Health Initiative Calcium and Vitamin D (CaD) trial of postmenopausal women aged 51-80 years, had 25-hydroxyvitamin D [25(OH)D] levels measured at the CaD trial baseline visit. Information about menopause-related symptoms at baseline was obtained by questionnaire and included overall number of symptoms and composite measures of sleep disturbance, emotional well-being, and energy/fatigue, as well as individual symptoms. After exclusions for missing data, 530 women [mean age 66.2 years (SD 6.8)] were included in these analyses.
Results
There were borderline significant associations between 25(OH)D levels and total number of menopausal symptoms (p values ranging from 0.05 to 0.06 for fully adjusted models); however, the effect was clinically insignificant and disappeared with correction for multiple testing. There were no associations between 25(OH)D levels and composite measures of sleep disturbance, emotional well-being, or energy/fatigue (p’s > 0.10 for fully adjusted models).
Conclusions
There was no evidence of a clinically important association between serum 25(OH)D levels and menopause-related symptoms in postmenopausal women.
Keywords: Vitamin D, menopause, hot flashes, mood, sleep
Introduction
Many women experience menopause-related symptoms long after the final menstrual period. For example, women often experience hot flashes (1-7) for 4 to 10 years post-menopause (8-10). Many also have mood disturbances, although the link with menopause is less clear (1; 6; 7; 11-16). These symptoms can be severe enough to negatively impact quality of life, work performance, and personal relationships (1; 17-20). Current treatments for menopause-related symptoms, such as menopausal hormone therapy, antidepressants, and anticonvulsants, may have significant side effects and serious long-term adverse consequences (21-23). After discontinuing treatment, symptoms may recur or even develop de novo (24; 25). Recognizing the possible mechanisms underlying menopause-related symptoms may lead to the development of new therapeutic strategies.
Calcium balance studies have shown that calcium absorption declines with menopause (26). Since 25(OH)D serves the purpose of calcium absorption and appears to be hormonally sensitive, we hypothesized that vitamin D also may influence vasomotor symptoms, which are clearly related to hormone levels. Also, some of the symptoms associated with vitamin D deficiency, such as mood disturbance and musculoskeletal complaints (27), are similar to symptoms women may experience during the transition through menopause (28). We therefore hypothesized that vitamin D levels may be associated with menopause-related symptoms.
We are unaware of any previous data on this association in postmenopausal women. We did find a small study conducted in middle-aged women taking aromatase inhibitors (AIs) for breast cancer (29). Although the hot flashes that occur with AI therapy are different than menopause-related vasomotor symtpoms, breast cancer patients who had higher 25-hydroxyvitamin D [25(OH)D] levels (>66 ng/ml) had fewer hot flashes and other symptoms compared to women with lower vitamin D levels.
There is some biological data suggesting that a link between vitamin D and menopause-related symptoms is plausible. Vitamin D can protect against experimental serotonin depletion in rats (30) and a menopausal decline in serotonin, a neurotransmitter with known effects on thermoregulation, may be a contributor to hot flashes (31-33). In addition, estrogen increases the activity of the enzyme responsible for activating vitamin D (34) and so declining estrogen levels during the menopausal transition could lead to symptoms of vitamin D deficiency. Indeed, vitamin D supplementation can improve mood in nonmenopausal populations (29; 35-37).
Based on the totality of evidence from limited data, we hypothesized that higher levels of vitamin D would be associated with fewer menopause-related symptoms. To test this hypothesis, we examined the cross-sectional association between baseline serum 25(OH)D levels and menopausal symptoms in a subset of women who participated in the Women’s Health Initiative Calcium and Vitamin D (CaD) trial.
METHODS
This paper presents post–hoc secondary analyses of the association between serum 25(OH)D concentrations and menopause-related symptoms in the participants of the WHI calcium and vitamin D trial (CaD) trial.
Sample selection
The WHI clinical trials (WHI-CT) enrolled postmenopausal women aged 50 to 79 into randomized trials of menopausal hormone therapy (HT) (38) and/or dietary modification(DM) (39). At the Year 1 or Year 2 WHI-CT visit, HT and DM trial participants were also invited to participate in a randomized placebo-controlled trial of calcium plus vitamin D(CaD trial) (40). A total of 36,282 WHI-CT participants enrolled in the CaD trial. For our analysis, we drew participants from the CaD trial and used data from the baseline CaD trial visit. A subset of these patients (N=4785) had vitamin D levels drawn as part of separate, unrelated case control studies; we only included controls (N=1,407) from these studies in our analysis.(41-43)
We excluded women randomized to hormone therapy as part of the WHI trial, women with possible medical conditions associated with vitamin D deficiency (following a malabsorption diet, colitis, bowel resections, pancreatic disease, current gall bladder problems, and liver disease), or women with conditions that would influence vitamin D supplementation (high blood calcium or incident cancer). Of the remaining 26,104 eligible participants, 530 had vitamin D levels and had data on important potential confounders (described below). These 530 women served as the final analytic sample; 528 of these had at least one symptom of mild severity.
The Institutional Review Boards at each participating institution approved all protocols and consent forms. All women provided signed informed consent.
Data collection
Menopause-related symptoms
At the CaD trial baseline visit, participants provided data on menopause-related symptoms via questionnaires(7)., which contained a checklist based on the Postmenopausal Estrogen/Progestin Interventions (PEPI) symptom tool (44) and other national surveys and clinical trials (45). The psychosocial forms containing these symptom items were reviewed for content validity by nationally recognized behavioral and clinical experts and were pretested extensively on age-appropriate women from diverse racial/ethnic groups (25).
For this analysis, the following symptoms were analyzed: hot flashes, night sweats, dizziness, heart racing or skipping beats, tremors, feeling restless or fidgety, feeling tired, difficulty concentrating, forgetfulness, mood swings, vaginal dryness, breast tenderness, headaches or migraines, waking up at night, waking earlier than planned, trouble falling back to sleep after waking earlier than planned, overall typical sleep quality, restless sleep, and energy level. These symptoms have been associated with menopause in previous research. For most of the symptoms, participants rated their severity on a 4-point scale, from symptom did not occur (score of 1) to did not interfere with usual activities, i.e. mild (score of 2), interfered somewhat with usual activities, i.e. moderate (score of 3), or so bothersome that usual activities could not be performed, i.e. severe (score of 4). For sleep symptoms, participants rated how often they occurred over the past 4 weeks (from none to 5 or more times per week).
Our primary outcome was total number of symptoms of any severity (mild, moderate, or severe; range from 0 to 20). Secondary outcomes included the energy/fatigue and emotional well-being subscales of the SF-36, for which higher scores indicate better health (range from 0-100) (46; 47), and a sleep disturbance construct (48). The latter was created from 5 questions about sleep (trouble falling asleep, waking up several times at night, waking earlier than planned, trouble falling back to sleep after waking earlier than planned, and overall typical sleep); the summary score ranged from 0 to 20 with a higher score indicating greater sleep disturbance. We also examined associations between 25(OH)D levels and each individual symptom (of any severity) on the checklist.
Serum 25(OH)D assay
Serum was drawn at the baseline CaD trial visit (Year 1 or 2 of the CTs) and processed and stored at −80°C, as previously described (42). Serum 25(OH)D concentrations (nmol/L) were measured by using the DiaSorin LIAISON chemiluminescence method (DiaSorin, Stillwater, MN), and the coefficient of variation (CV) determined by using blinded controls was 11.8% (49).
Potential confounders
At WHI-CT screening, participants self reported data on demographics (race, education) and current or prior medical conditions (thyroid disorders). At the CaD trial baseline, participants self reported demographics (i.e. age, years since menopause), lifestyle factors (i.e. physical activity, smoking), UV exposure (i.e. Langley’s measure of UV exposure) and underwent physical measurements (i.e., height and weight to calculate BMI) (40). A standardized, in-person, interviewer-administered form was used to collect information on the dose, frequency, and duration of current supplements (i.e. calcium, vitamin D) and medication use [i.e. menopausal hormone therapy (HT), selective serotonin reuptake inhibitors (SSRI)/ serotonin–norepinephrine reuptake inhibitors (SNRI), selective estrogen receptor modulators (SERM), hypnotics/sedatives] at CaD baseline (50; 51). If data were not available at CaD baseline (Year 1 visit), data from WHI-CT screening were used.
At WHI-CT screening, dietary vitamin D and calcium intakes over the past 3 months were estimated from a self-administered food-frequency questionnaire (FFQ) specifically designed for WHI (52). The FFQ was also administered at CaD trial baseline (Year 1 of WHI-CT), but only in the DM trial. Therefore, dietary vitamin D data collected at CaD trial baseline (concurrent with the time serum was obtained for determining 25(OH)D concentrations) were used when possible; otherwise reported screening dietary vitamin D intake was used. As previously reported, the Pearson correlation coefficient among DM trial participants for dietary vitamin D intake at baseline and Year 1 was 0.59 (P < 0.0001) (49).
Statistical Methods
We examined the cross-sectional association between 25(OH)D level and symptoms. We examined 25(OH)D cutpoints based on current clinical definitions of vitamin D deficiency, insufficiency, and sufficiency (≥ 75, 50 to < 75, 25 to < 50, < 25 nmol/L)(53) These cut-points were similar to quartile cutpoints.
We compared baseline characteristics according to categories of 25(OH)D using Chi-square tests of association for categorical variables and ANOVA F-test tests for continuous variables.
The symptom total (primary outcome) was normally distributed and therefore modeled as a continuous outcome according to 25(OH)D status using general linear models. The reference group was ≥75 nmol of 25(OH)D. These results were adjusted for age and race and then adjusted for multiple confounding variables chosen a priori based on literature review and expert opinion about factors associated with menopausal symptoms and/or vitamin D status. These included years since menopause, education, BMI category, smoking status, UV exposure, HT at screening (personal history of HT use at the screening visit), trial arm (HT or DM), and calcium and vitamin D intake (through diet and supplement).
Using logistic regression, we estimated the odds ratio of having each individual symptom according to 25(OH)D status (≥ 75, 50 to < 75, 25 to < 50, < 25 nmol/L), using the highest cut-off as the referent (≥ 75 nmol/L). We first adjusted for race and age and then adjusted the models for the potential confounders listed above. We also examined the relationship between continuous 25(OH)D levels and continuous number of symptoms and composite symptom scores using linear regression.
We did a multiple imputation analysis as a sensitivity analysis to retain the 1407 women with 25(OH)D levels regardless of whether they had complete data on confounders.
For both logistic regression and linear models, we examined p values adjusting for multiple comparisons for all analyses using a 5% false discovery rate using the Benjamini-Hochberg method (54).
This study is a post-hoc analysis of an existing dataset with fixed sample size. Assuming the total number of symptoms was the primary outcome of interest and the parameter of interest was the regression coefficient of 25(OH)D, we conducted power analyses for two-tailed linear multiple regression. With 16 predictors and N=530, we had 80% power at α=0.05 to detect an effect size of 0.014. Thus we were able to detect a difference of 0.014 in symptoms for each unit change in 25(OH)D.
RESULTS
Baseline characteristics
Women were, on average, 66 years of age and almost 16 years since menopause. Participants’ age, years since menopause, education, UV exposure, HT use pattern at screening, and randomization to diet modification trial arm did not differ by 25(OH)D status (Table 1). A higher percentage of nonwhite, obese, nonsmokers with lower activity levels and lower calcium and vitamin D intake (especially from supplements) were in the lower two 25(OH)D level categories. There were no differences in the use of relevant non-hormonal medications (i.e. serotonin–norepinephrine reuptake inhibitors, selective serotonin re-uptake inhibitors, selective estrogen receptor modulators, or hypnotics/sedatives) among 25(OH)D categories, although few women used these medications (<5% of women took at least one of these medications; data not shown). There was also no difference in distribution of thyroid disease among categories (only 1.8% and 15% had ever had overactive or underactive thyroid disease, respectively; data not shown).
Table 1.
Baseline Characteristics of Analytic Cohort by Vitamin D levels
| Characteristics (Visit where information collected) | Vitamin D Levels | ||||
|---|---|---|---|---|---|
| <25 nmol/L n=53 (10.0) |
≥25 to <50 nmol/L n=211 (39.8) |
≥50 to <75 nmol/L n=170 (32.0) |
≥75 nmol/L n=96 (18.1) |
P-value | |
| Age (Year 1) | 0.86 | ||||
| 50-59 | 11 (10.9) | 34 (33.7) | 34 (33.7) | 22 (21.8) | |
| 60-69 | 22 (9.4) | 98 (42.1) | 72 (30.9) | 41 (17.6) | |
| 70+ | 20 (10.2) | 79 (40.3) | 64 (32.7) | 33 (16.8) | |
|
| |||||
| Years since menopause (Year 1) | 0.42 | ||||
| <5 | 5 (9.4) | 19 (35.9) | 21 (39.6) | 8 (15.1) | |
| 5-10 | 10 (11.6) | 26 (30.2) | 31 (36.0) | 19 (22.1) | |
| >10 | 38 (9.7) | 166 (42.5) | 118 (30.2) | 69 (17.7) | |
|
| |||||
| Ethnicity (Screening) | <.0001 | ||||
| White | 40 (8.4) | 180 (37.9) | 163 (34.3) | 92 (19.4) | |
| Hispanic | a | 9 (69.2) | a | a | |
| Black | 8 (38.1) | 11 (52.4) | a | a | |
| Other | a | 11 (52.4) | 5 (23.8) | a | |
|
| |||||
| Education (Screening) | 0.84 | ||||
| <HS | a | 13 (50.0) | 6 (23.1) | 5 (19.2) | |
| HS | 31 (10.6) | 109 (37.3) | 97 (33.2) | 55 (18.8) | |
| College | 20 (9.4) | 89 (42.0) | 67 (31.6) | 36 (17.0) | |
|
| |||||
| BMI in kg/m2 (Year 1) | <.01 | ||||
| <25 | 11 (6.6) | 53 (31.7) | 61 (36.5) | 42 (25.2) | |
| 25-30 | 16 (8.5) | 82 (43.6) | 57 (30.3) | 33 (17.6) | |
| >30 | 26 (14.9) | 76 (43.4) | 52 (29.7) | 21 (12.0) | |
|
| |||||
| Physical activity (Year 1) | <.0001 | ||||
| Total MET hours/week | 5.8 (8.2) | 9.3 (10.3) | 14.7 (17.2) | 17.1 (13.7) | |
|
| |||||
| Smoking (Year 1) | 0.02 | ||||
| Never | 38 (12.3) | 130 (41.9) | 91 (29.4) | 51 (16.5) | |
| Past | 9 (4.9) | 71 (38.6) | 63 (34.2) | 41 (22.3) | |
| Current | 6 (16.7) | 10 (27.8) | 16 (44.4) | a | |
|
| |||||
| UV exposure (Screening) | 0.13 | ||||
| Low | 28 (10.2) | 99 (36.1) | 100 (36.5) | 47 (17.2) | |
| High | 25 (9.8) | 112 (43.8) | 70 (27.3) | 49 (19.1) | |
|
| |||||
| HT Use (Screening) | 0.03 | ||||
| Never used | 20 (8.6) | 94 (40.5) | 85 (36.6) | 33 (14.2) | |
| Past user | 14 (15.4) | 42 (46.2) | 20 (22.0) | 15 (16.5) | |
| Current user | 19 (9.2) | 75 (36.2) | 65 (31.4) | 48 (23.2) | |
|
| |||||
| DM trial arm (Screening) | 0.47 | ||||
| Not randomized to DM | 10 (8.3) | 54 (44.6) | 39 (32.2) | 18 (14.9) | |
| Intervention | 20 (12.8) | 61 (39.1) | 51 (32.7) | 24 (15.4) | |
| Control | 23 (9.1) | 96 (37.9) | 80 (31.6) | 54 (21.3) | |
|
| |||||
| Dietary Calcium (mg) (Screening) | 0.06 | ||||
| 719.6 (341.0) | 829.7 (448.7) | 904.3 (469.3) | 868.6 (476.4) | ||
|
| |||||
| Dietary Vitamin D (mg) (Screening) | 0.05 | ||||
| 3.9 (2.4) | 4.5 (2.9) | 5.1 (2.9) | 4.7 (3.1) | ||
|
| |||||
| Supplemental Calcium (mg) (Year 1) | <.01 | ||||
| 144.6 (282.9) | 347.5 (497.8) | 398.7 (446.9) | 432.8 (435.3) | ||
|
| |||||
| Supplemental Vitamin D (mcg) (Year 1) | <.0001 | ||||
| 1.9 (4.2) | 4.5 (5.4) | 5.9 (5.9) | 7.4 (6.2) | ||
|
| |||||
| Total Number of menopausal symptoms | 0.05 | ||||
| 6.8 (3.8) | 6.07 (3.3) | 5.8 (3.2) | 5.3 (3.1) | ||
Data not shown as cell size < 5
Women had from 0 to 17 symptoms of at least mild intensity with a mean of 5.9 (SD 3.3) and median of 5 (IQR 5). Only 87 women (16.3%) reported hot flashes and 109 (20.4%) reported night sweats.
Association between vitamin D status and overall number and composite measures of menopause-related symptoms
We observed no statistically significant association between total number of menopausal symptoms and 25(OH)D status in age and race adjusted (p= .11) or fully adjusted (p=0.06) models (Table 2). Similarly, we observed no significant association between 25(OH)D status and scores on composite measures of sleep disturbance, emotional well-being, and energy/fatigue in age and race adjusted (p’s>0.05) or fully adjusted models (p’s>0.10). Any associations of borderline statistical significance were no longer significant after adjustment for multiple comparisons (data not shown).
Table 2.
Adjusteda composite symptom scores according to 25(OH)D clinical cut-off categories (≥75 nmol/L as referentb)
| Menopause-related symptom | 25(OH)D level <25 nmol/Lb | 25(OH)D level ≥25 to <50 nmol/L | 25(OH)D level ≥50 to <75 nmol/L | 25(OH)D level ≥75 nmol/L | P value |
|---|---|---|---|---|---|
| Symptom Totalc | 0.84 (-1.02, 2.70) | 0.85 (-0.7, 2.39) | 0.32 (-1.27, 1.91) | Ref | 0.06 |
| Sleep disturbance constructd | 0.23 (-2.35, 2.80) | 1.04 (-1.10, 3.18) | 0.14 (-2.06, 2.34) | Ref | 0.25 |
| Emotional well beinge | -5.11 (-9.78, -0.45) | -2.87 (-6.18, 0.44) | -1.10 (-4.50, 2.31) | Ref | 0.11 |
| Energy/fatiguee | -1.29 (-11.96, 9.38) | -3.32 (-12.19, 5.54) | 1.33 (-7.78, 10.44) | Ref | 0.39 |
Results are adjusted for age, years since menopause, ethnicity, education, BMI category, smoking status, UV exposure, HT use at screening, trial arm (HT or DM), calcium (dietary and supplement), and vitamin D (dietary and supplement).
Adjusted coefficients from linear models (with 95 % CI) for difference between symptom scores in each vitamin D category relative to highest vitamin D level ≥75 nmol/L
Higher total symptom score indicates more symptoms
Higher sleep score indicates greater sleep disturbance
Higher score indicates better health
Using linear regression, we observed a statistically significant association between continuous 25(OH)D levels and total number of symptoms and composite symptom scores. Vitamin D levels were not associated with any composite symptom score. In terms of total number of symptoms, each 1 nmol/L increase in 25(OH)D level resulted in .01 fewer total number of symptoms. This degree of symptom reduction is unlikely to be clinically significant. In addition, the association was no longer statistically significant after adjustment for multiple comparisons.
Association between 25(OH)D status and individual menopause-related symptoms
No individual menopause-related symptoms were significantly associated with 25(OH)D status after adjustment for multiple comparisons (Table 3).
Table 3.
Odds Ratioa(95% Confidence Interval) for individual menopause-related symptoms according to 25(OH)D clinical cut-off categories
| Menopause-related symptomb | 25(OH)D level <25 nmol/L | 25(OH)D level ≥25 to <50 nmol/L | 25(OH)D level ≥50 to <75 nmol/L | 25(OH)D level ≥75 nmol/L | P value | P value adjusted or multiple comparisons |
|---|---|---|---|---|---|---|
| Hot flashes | 1.89 (0.64, 5.59) | 1.23 (0.55, 2.75) | 1.34 (0.61,2.93) | Ref | 0.68 | 0.85 |
| Night sweats | 1.53 (0.61, 3.83) | 0.84 (0.43, 1.66) | 1.08 (0.56, 2.08) | Ref | 0.46 | 0.63 |
| Dizziness | 2.51 (0.96, 6.56) | 1.35 (0.64, 2.84) | 1.55 (0.75, 3.23) | Ref | 0.25 | 0.63 |
| Heart racing | 1.91 (0.71, 5.12) | 1.26 (0.61, 2.58) | 1.94 (0.97, 3.90) | Ref | 0.18 | 0.41 |
| Tremors | 1.61 (0.29, 9.02) | 1.12 (0.33, 3.82) | 0.33 (0.07, 1.53) | Ref | 0.32 | 0.58 |
| Restless | 1.71 (0.76, 3.84) | 1.02 (0.58, 1.81) | 0.67 (0.37, 1.19) | Ref | 0.08 | 0.71 |
| Feeling tired | 1.73 (0.54, 5.49) | 1.07 (0.50, 2.29) | 1.21 (0.57, 2.59) | Ref | 0.78 | 0.58 |
| Difficulty concentrating | 2.14 (0.91, 5.03) | 2.40 (1.30, 4.44) | 1.60 (0.87, 2.97) | Ref | 0.04 | 0.82 |
| Forgetfulness | 1.41 (0.64, 3.10) | 1.44 (0.83, 2.48) | 1.00 (0.59, 1.70) | Ref | 0.38 | 0.24 |
| Mood swings | 1.38 (0.60, 3.20) | 1.49 (0.83, 2.67) | 1.10 (0.61, 1.97) | Ref | 0.51 | 0.24 |
| Vaginal dryness | 1.43 (0.59, 3.49) | 1.26 (0.67, 2.35) | 1.15 (0.62, 2.15) | Ref | 0.87 | 0.24 |
| Breast tenderness | 2.50 (0.98, 6.37) | 1.19 (0.59, 2.40) | 1.75 (0.89, 3.43) | Ref | 0.11 | 0.92 |
| Headache | 0.98 (0.44, 2.18) | 1.40 (0.81, 2.44) | 1.25 (0.72, 2.16) | Ref | 0.53 | 0.58 |
| Wake at night | 2.11 (0.96, 4.68) | 2.24 (1.27, 3.94) | 1.42 (0.81, 2.49) | Ref | 0.03 | 0.92 |
| Waking earlier than planned | 2.82 (0.74, 10.7) | 1.51 (0.53, 4.28) | 0.99 (0.33, 2.96) | Ref | 0.32 | 0.24 |
| Trouble going back to sleep | 2.68 (0.92, 7.77) | 1.75 (0.78, 3.92) | 1.62 (0.72, 3.67) | Ref | 0.34 | 0.41 |
| Quality of sleep | 0.74 (0.33, 1.64) | 0.70 (0.41, 1.21) | 0.99 (0.58, 1.67) | Ref | 0.43 | 0.58 |
| Restless sleep | 1.70 (0.67, 4.31) | 1.37 (0.69, 2.73) | 1.33 (0.67, 2.64 | Ref | 0.72 | 0.78 |
| Energy | 0.94 (0.40, 2.21) | 0.84 (0.45, 1.57) | 0.91 (0.49, 1.70) | Ref | 0.95 | 0.94 |
Odds ratios are adjusted for age, years since menopause, ethnicity, education, BMI category, smoking status, UV exposure, HT use at screening, trial arm (HT or DM), calcium (dietary and supplement), and vitamin D (dietary and supplement).
Higher values indicate more bothersome symptoms or more frequent occurrence
DISCUSSION
In postmenopausal women with an average age of 66 and 15.9 years since menopause. we found a borderline association between total number of menopause-related symptoms and 25(OH)D level. However, the effect size was clinically insignificant and the associations were no longer significant after correction for multiple comparisons.
We are unaware of any other studies that have examined the association between vitamin D and menopause-related symptoms. In breast cancer patients on aromatase inhibitors, low 25(OH)D levels were associated with a greater prevalence of symptoms, especially vasomotor symptoms;(29) however hot flashes in breast cancer patients are different than those in postmenopausal women. Also, the mean age of our population, 66.2 years (SD 6.8), was higher than in the study of breast cancer patients (56 years) and we excluded women with a history of cancer. In fact, only 27% of the women in our study suffered from any hot flashes or night sweats. This low symptom prevalence could have been due to women having an average age of 66 and being almost 16 years postmenopause; in addition, women with severe menopause-related symptoms were discouraged from participating because of the possibility that they might be randomized to placebo.
We had hypothesized that because estrogen increases the activity of the enzyme responsible for activating vitamin D (34), low estrogen levels in postmenopausal women could exacerbate the symptoms of subclinical vitamin D deficiency, including mood disturbances. Previous studies have found that vitamin D supplementation can improve such things as mood in nonmenopausal populations (29; 35-37). However, we did not find an association between 25(OH)D status and composite scores on sleep, emotional well-being, or energy/fatigue constructs in our population of postmenopausal women.
There were several limitations of our study. This was a posthoc analysis of women who had vitamin D testing done as part of 3 nested case control studies of fracture, breast cancer, and colorectal cancer. Participants were matched on age, race-ethnicity, blood draw date, and clinic center at CaD randomization. The breast cancer nested case-control study was also matched on HT and DM trial arm participation. This subset selection increased the age of the study cohort and reduced the number of women with symptoms related to early menopause (such as hot flashes and night sweats). Three-quarters of our participants were more than 10 years postmenopausal and women in this study who were still experiencing symptoms may not be representative of the general population of women who experience symptoms during the transition through menopause. WHI used a brief self-report instrument to collect data on menopausal symptoms. Only the presence and broad severity of the symptom could therefore be assessed. Given that few women with vasomotor symptoms were included in this sample and the lack of rigorous measurement of vasomotor symptoms, we cannot exclude a different finding in a younger population with more rigorous monitoring of hot flashes.
Because no data on a possible association between vitamin D and menopause-related symptoms were available, we examined multiple symptoms. This increased the possibility that we would find an association by chance and indeed, when we corrected for multiple comparisons, any associations we had found were no longer significant. On the other hand, the number of women who were in our final sample was relatively small and only 27% suffered from vasomotor symptoms. This low number of women with vasomotor symptoms could have limited our ability to see an association between vasomotor symptoms and 25(OH)D. We did perform sensitivity analyses using multiple imputation to examine all 1407 women with 25(OH)D levels (not just those who had data on confounders) and had similar null findings. We did not stratify by time since menopause and it is possible that 25(OH)D may be associated with menopausal symptoms in women closer to the onset of menopause. Because this was a post hoc analysis, conclusions can only be considered hypothesis generating.
CONCLUSION
Our data does not support the hypothesis that vitamin D status is associated with menopause-related symptoms in postmenopausal women with an average age of 66. We cannot exclude an association in younger women who are more symptomatic.
Figure 1.
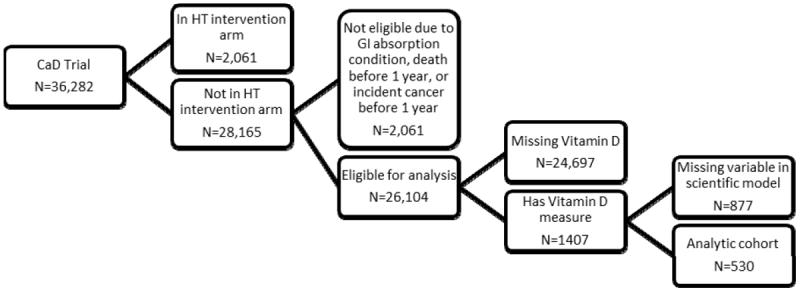
Flow of WHI participants into study cohort
Acknowledgments
Source of funding: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Appendix: SHORT LIST OF WHI INVESTIGATORS
Program Office
(National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers
(Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women’s Health Initiative Memory Study
(Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
For a list of all the investigators who have contributed to WHI science, please visit: https://cleo.whi.org/researchers/SitePages/Write%20a%20Paper.aspx
Footnotes
Conflicts of Interest: Dr. Manson and colleagues at Harvard Medical School receive NIH funding for the VITamin D and Omega-3 Trial (Vital). For the remaining authors none were declared.
Contributor Information
Erin S. LeBlanc, Kaiser Permanente Northwest, Center for Health Research, Portland, Oregon.
Manisha Desai, Quantitative Sciences Unit, Department of Medicine, Stanford University School of Medicine, Stanford, CA.
Nancy Perrin, Kaiser Permanente Northwest, Center for Health Research, Portland, Oregon.
Jean Wactawski-Wende, University of Buffalo, Buffalo, NY.
JoAnn E. Manson, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Jane A. Cauley, Department of Epidemiology, Graduate School of Public Health University of Pittsburgh, Pittsburgh, PA.
Yvonne L. Michael, Drexel University School of Public Health, Department of Epidemiology & Biostatistics, Philadelphia, PA.
Jean Tang, Department of Medicine, Stanford University School of Medicine, Stanford, CA.
Catherine Womack, University of Tennessee, Health Science Center, Memphis, TN.
Yiqing Song, Department of Epidemiology, Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, IN 46202.
Karen C. Johnson, Deaprtment of Preventive Medicine, University of Tennessee, Health Science Center, Memphis, TN.
Mary J. O’Sullivan, University of Miami, Miami, FL.
Nancy Woods, University of Washington, Biobehavioral Nursing, Seattle, WA.
Marcia L. Stefanick, Department of Medicine (Stanford Prevention Research Center), Stanford University School of Medicine, Stanford, CA.
Reference List
- 1.Simon JA, Reape KZ. Understanding the menopausal experiences of professional women. Menopause. 2009 Jan;16(1):73–6. doi: 10.1097/gme.0b013e31817b614a. [DOI] [PubMed] [Google Scholar]
- 2.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992 Jan;14(2):103–15. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 3.Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000 Sep;96(3):351–8. doi: 10.1016/s0029-7844(00)00930-3. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Wilawan K, Samsioe G, Lidfeldt J, Agardh CD, Nerbrand C. Health profile of middle-aged women: The Women’s Health in the Lund Area (WHILA) study. Hum Reprod. 2002 May;17(5):1379–85. doi: 10.1093/humrep/17.5.1379. [DOI] [PubMed] [Google Scholar]
- 5.Groeneveld FP, Bareman FP, Barentsen R, Dokter HJ, Drogendijk AC, Hoes AW. Vasomotor symptoms and well-being in the climacteric years. Maturitas. 1996 Apr;23(3):293–9. doi: 10.1016/0378-5122(95)00989-2. [DOI] [PubMed] [Google Scholar]
- 6.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004 Jan;61(1):62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 7.Barnabei VM, Cochrane BB, Aragaki AK, et al. Menopausal symptoms and treatment-related effects of estrogen and progestin in the Women’s Health Initiative. Obstet Gynecol. 2005 May;105(5 Pt 1):1063–73. doi: 10.1097/01.AOG.0000158120.47542.18. [DOI] [PubMed] [Google Scholar]
- 8.Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011 Jan 19;305(3):267–74. doi: 10.1001/jama.2010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Col NF, Guthrie JR, Politi M, Dennerstein L. Duration of vasomotor symptoms in middle-aged women: A longitudinal study. Menopause. 2009 May;16(3):453–7. doi: 10.1097/gme.0b013e31818d414e. [DOI] [PubMed] [Google Scholar]
- 10.Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: A meta-analysis. J Gen Intern Med. 2008 Sep;23(9):1507–13. doi: 10.1007/s11606-008-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt PJ, Haq N, Rubinow DR. A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. Am J Psychiatry. 2004 Dec;161(12):2238–44. doi: 10.1176/appi.ajp.161.12.2238. [DOI] [PubMed] [Google Scholar]
- 12.Freeman EW, Sammel MD, Lin H, et al. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstet Gynecol. 2007 Aug;110(2 Pt 1):230–40. doi: 10.1097/01.AOG.0000270153.59102.40. [DOI] [PubMed] [Google Scholar]
- 13.Maartens LW, Knottnerus JA, Pop VJ. Menopausal transition and increased depressive symptomatology: A community based prospective study. Maturitas. 2002 Jul 25;42(3):195–200. doi: 10.1016/s0378-5122(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 14.Bromberger JT, Meyer PM, Kravitz HM, et al. Psychologic distress and natural menopause: A multiethnic community study. Am J Public Health. 2001 Sep;91(9):1435–42. doi: 10.2105/ajph.91.9.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson HD, Haney E, Humphrey L, et al. Management of menopause-related symptoms. Evid Rep Technol Assess (Summ) 2005 Mar;(120):1–6. [PMC free article] [PubMed] [Google Scholar]
- 16.National Institutes of Health State-of-the-Science Conference statement: Management of menopause-related symptoms. Ann Intern Med. 2005 Jun 21;142(12 Pt 1):1003–13. [PubMed] [Google Scholar]
- 17.Park MK, Satoh N, Kumashiro M. Effects of Menopausal Hot Flashes on Mental Workload. Ind Health. 2011 Aug 1; doi: 10.2486/indhealth.ms1222. [DOI] [PubMed] [Google Scholar]
- 18.Geukes M, van Aalst MP, Nauta MC, Oosterhof H. The impact of menopausal symptoms on work ability. Menopause. 2012 Mar;19(3):278–82. doi: 10.1097/gme.0b013e31822ddc97. [DOI] [PubMed] [Google Scholar]
- 19.Sarrel PM. Women, work, and menopause. Menopause. 2012 Mar;19(3):250–2. doi: 10.1097/gme.0b013e3182434e0c. [DOI] [PubMed] [Google Scholar]
- 20.Conde DM, Pinto-Neto AM, Santos-Sa D, Costa-Paiva L, Martinez EZ. Factors associated with quality of life in a cohort of postmenopausal women. Gynecol Endocrinol. 2006 Aug;22(8):441–6. doi: 10.1080/09513590600890306. [DOI] [PubMed] [Google Scholar]
- 21.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002 Jul 17;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 22.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA. 2004 Apr 14;291(14):1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 23.Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: Systematic review and meta-analysis. JAMA. 2006 May 3;295(17):2057–71. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 24.Lindh-Astrand L, Brynhildsen J, Hoffman M, Hammar M. Vasomotor symptoms usually reappear after cessation of postmenopausal hormone therapy: A Swedish population-based study. Menopause. 2009 Nov;16(6):1213–7. doi: 10.1097/gme.0b013e3181a53221. [DOI] [PubMed] [Google Scholar]
- 25.Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005 Jul 13;294(2):183–93. doi: 10.1001/jama.294.2.183. [DOI] [PubMed] [Google Scholar]
- 26.Heaney RP, Recker RR, Stegman MR, Moy AJ. Calcium absorption in women: relationships to calcium intake, estrogen status, and age. J Bone Miner Res. 1989 Aug;4(4):469–75. doi: 10.1002/jbmr.5650040404. [DOI] [PubMed] [Google Scholar]
- 27.Arvold DS, Odean MJ, Dornfeld MP, et al. Correlation of symptoms with vitamin D deficiency and symptom response to cholecalciferol treatment: a randomized controlled trial. Endocr Pract. 2009 Apr;15(3):203–12. doi: 10.4158/EP.15.3.203. [DOI] [PubMed] [Google Scholar]
- 28.Brunner RL, Aragaki A, Barnabei V, et al. Menopausal symptom experience before and after stopping estrogen therapy in the Women’s Health Initiative randomized, placebo-controlled trial. Menopause. 2010 Sep;17(5):946–54. doi: 10.1097/gme.0b013e3181d76953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan QJ, Reddy PS, Kimler BF, et al. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat. 2010 Jan;119(1):111–8. doi: 10.1007/s10549-009-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cass WA, Smith MP, Peters LE. Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2006 Aug;1074:261–71. doi: 10.1196/annals.1369.023. [DOI] [PubMed] [Google Scholar]
- 31.Rossmanith WG, Ruebberdt W. What causes hot flushes? The neuroendocrine origin of vasomotor symptoms in the menopause. Gynecol Endocrinol. 2009 May;25(5):303–14. doi: 10.1080/09513590802632514. [DOI] [PubMed] [Google Scholar]
- 32.Berendsen HH. The role of serotonin in hot flushes. Maturitas. 2000 Oct 31;36(3):155–64. doi: 10.1016/s0378-5122(00)00151-1. [DOI] [PubMed] [Google Scholar]
- 33.Pinkerton JV, Zion AS. Vasomotor symptoms in menopause: Where we’ve been and where we’re going. J Womens Health (Larchmt) 2006 Mar;15(2):135–45. doi: 10.1089/jwh.2006.15.135. [DOI] [PubMed] [Google Scholar]
- 34.Buchanan JR, Santen R, Cauffman S, Cavaliere A, Greer RB, Demers LM. The effect of endogenous estrogen fluctuation on metabolism of 25-hydroxyvitamin D. Calcif Tissue Int. 1986 Sep;39(3):139–44. doi: 10.1007/BF02555109. [DOI] [PubMed] [Google Scholar]
- 35.Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: Randomized double blind trial. J Intern Med. 2008 Dec;264(6):599–609. doi: 10.1111/j.1365-2796.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- 36.Lansdowne AT, Provost SC. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology (Berl) 1998 Feb;135(4):319–23. doi: 10.1007/s002130050517. [DOI] [PubMed] [Google Scholar]
- 37.Gloth FM, III, Alam W, Hollis B. Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging. 1999;3(1):5–7. [PubMed] [Google Scholar]
- 38.Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women’s Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003 Oct;13(9 Suppl):S78–S86. doi: 10.1016/s1047-2797(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 39.Ritenbaugh C, Patterson RE, Chlebowski RT, et al. The Women’s Health Initiative Dietary Modification trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003 Oct;13(9 Suppl):S87–S97. doi: 10.1016/s1047-2797(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 40.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women’s Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003 Oct;13(9 Suppl):S98–106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 41.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006 Feb 16;354(7):669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 42.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006 Feb 16;354(7):684–96. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 43.Chlebowski RT, Johnson KC, Kooperberg C, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008 Nov 19;100(22):1581–91. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greendale GA, Reboussin BA, Hogan P, et al. Symptom relief and side effects of postmenopausal hormones: Results from the Postmenopausal Estrogen/Progestin Interventions Trial. Obstet Gynecol. 1998 Dec;92(6):982–8. doi: 10.1016/s0029-7844(98)00305-6. [DOI] [PubMed] [Google Scholar]
- 45.Ganz PA, Day R, Ware JE, Jr, Redmond C, Fisher B. Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst. 1995 Sep 20;87(18):1372–82. doi: 10.1093/jnci/87.18.1372. [DOI] [PubMed] [Google Scholar]
- 46.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–83. [PubMed] [Google Scholar]
- 47.Jenkinson C, Wright L, Coulter A. Criterion validity and reliability of the SF-36 in a population sample. Qual Life Res. 1994 Feb;3(1):7–12. doi: 10.1007/BF00647843. [DOI] [PubMed] [Google Scholar]
- 48.Levine DW, Kripke DF, Kaplan RM, et al. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003 Jun;15(2):137–48. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 49.Millen AE, Wactawski-Wende J, Pettinger M, et al. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: the Women’s Health Initiative Calcium plus Vitamin D clinical trial. Am J Clin Nutr. 2010 May;91(5):1324–35. doi: 10.3945/ajcn.2009.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr. 1999 Dec;53(12):920–6. doi: 10.1038/sj.ejcn.1600870. [DOI] [PubMed] [Google Scholar]
- 51.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of Vitamin D in obesity. Am J Clin Nutr. 2000 Sep;72(3):690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 52.Patterson RE, Levy L, Tinker LF, Kristal AR. Evaluation of a simplified vitamin supplement inventory developed for the Women’s Health Initiative. Public Health Nutr. 1999 Sep;2(3):273–6. doi: 10.1017/s1368980099000361. [DOI] [PubMed] [Google Scholar]
- 53.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011 Jul;96(7):1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 54.Benjamini B, Hochner H. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. 1995;57(1):289–300. [Google Scholar]


