Abstract
Purpose
To examine the utility of fluorescein angiography (FA) in identification of the macular center and the diagnosis of zone in patients with retinopathy of prematurity (ROP).
Design
Validity and reliability analysis of diagnostic tools
Methods
32 sets (16 color fundus photographs; 16 color fundus photographs paired with the corresponding FA) of wide-angle retinal images obtained from 16 eyes of eight infants with ROP were compiled on a secure web site. 9 ROP experts (3 pediatric ophthalmologists; 6 vitreoretinal surgeons) participated in the study. For each image set, experts identified the macular center and provided a diagnosis of zone.
Main Outcome Measures
(1) Sensitivity and specificity of zone diagnosis (2) “Computer facilitated diagnosis of zone,” based on precise measurement of the macular center, optic disc center, and peripheral ROP.
Results
Computer facilitated diagnosis of zone agreed with the expert’s diagnosis of zone in 28/45 (62%) cases using color fundus photographs and in 31/45 (69%) cases using FA. Mean (95% CI) sensitivity for detection of zone I by experts as compared to a consensus reference standard diagnosis when interpreting the color fundus images alone versus interpreting the color fundus photographs and FA was 47% (35.3% – 59.3%) and 61.1% (48.9% – 72.4%), respectively, (t(9) ≥ (2.063), p = 0.073).
Conclusions
There is a marginally significant difference in zone diagnosis when using color fundus photographs compared to using color fundus photographs and the corresponding fluorescein angiograms. There is inconsistency between traditional zone diagnosis (based on ophthalmoscopic exam and image review) compared to a computer-facilitated diagnosis of zone.
Introduction
Retinopathy of prematurity (ROP) is a vascular proliferative disease of the retina that occurs in premature infants.1 Major advances in the diagnosis and treatment of ROP have occurred as a result of the classification criteria outlined by the Cryotherapy for ROP (CRYO-ROP) and Early Treatment for ROP (ETROP) studies.2,3 Both of these clinical trials demonstrated that the classification of zone in ROP is an important metric for prognosis and treatment.
Zone I of the retina is defined as a circle, the radius of which extends from the optic disc center to twice the distance from the optic disc center to the macular center. Based on this definition, identification of the macular center is critical to diagnose zone I disease properly. Diagnosis of zone is particularly important because zone I disease has a guarded prognosis and must be treated promptly. Previous studies have shown that variability may exist in identification of the macular center, which may translate to differences in the diagnosis of zone.4
Fluorescein angiography (FA) is an imaging modality that may provide useful information regarding the retinal vasculature in the premature retina. Although a number of investigators have described FA changes in ROP, there is no consensus among vitreoretinal specialists and pediatric ophthalmologists regarding the proper utilization of FA in ROP. The current literature is limited to descriptive case series on FA findings in ROP and other pediatric vitreoretinal disorders.5–10 These initial reports suggest that FA may allow more objective assessment of disease zone.5,7 There are currently no studies, to our knowledge, on the direct comparison of expert diagnosis of ROP made from FA findings vs expert diagnosis of ROP made from color fundus photographs in patients with ROP.
The purposes of this study are to: (1) examine and compare how color fundus photographs and FA influence the identification of the macular center in ROP and (2) evaluate the influence of FA on diagnosis of zone in ROP.
METHODS
This study was approved as a prospective study by the Institutional Review Board at Weill Cornell Medical College. Informed consent was obtained from all study participants prior to participation, and waiver of consent was obtained for use of de-identified retinal images. This study was conducted in accordance with Health Insurance Portability and Accountability Act (HIPAA) guidelines.
Image Acquisition
Wide-angle images of the posterior retina and corresponding fluorescein angiograms were captured bilaterally from 8 infants with ROP (16 eyes) using a wide-angle camera (RetCam; Clarity Medical Systems, Pleasanton, CA). Images were taken of infants between 33 – 44 weeks postmenstrual age. For acquisition of FAs, 4/8 (50%) infants were imaged in the neonatal intensive care unit (NICU) without intubation or sedation, while the remaining 4/8 (50%) infants were imaged in the operating room under sedation but were not intubated.
Consensus Reference Standard Diagnosis of Zone
For each image set, a reference standard ROP diagnosis was established. This was done by combining the clinical diagnosis and the image-based diagnosis by multiple experts, as follows11: (1) The clinical diagnosis (based on complete ophthalmic exam by an experienced ROP examiner) was recorded. (2) Each set of retinal images was interpreted by 3 experienced readers using a web-based system. (3) The diagnosis that was selected by the majority of image readers (zone, stage, plus disease, overall disease category) was then compared to the clinical diagnosis. When these two diagnoses were the same, it was defined as the reference standard diagnosis. When the diagnoses were different, all of the data were reviewed by two of the investigators (RVPC, MFC) along with two study coordinators, and a consensus reference standard was determined. This consensus reference standard was then used for the purposes of this current study.
Study Experts
Study “experts” were defined as board certified practicing pediatric ophthalmologists or vitreoretinal specialists who met at least 1 of the following criteria: having been a principal investigator or certified investigator for the CRYO-ROP or ETROP study, or having published at least 2 peer-reviewed ROP articles. Furthermore, all experts in this study routinely evaluate and treat children with ROP.
Study Design
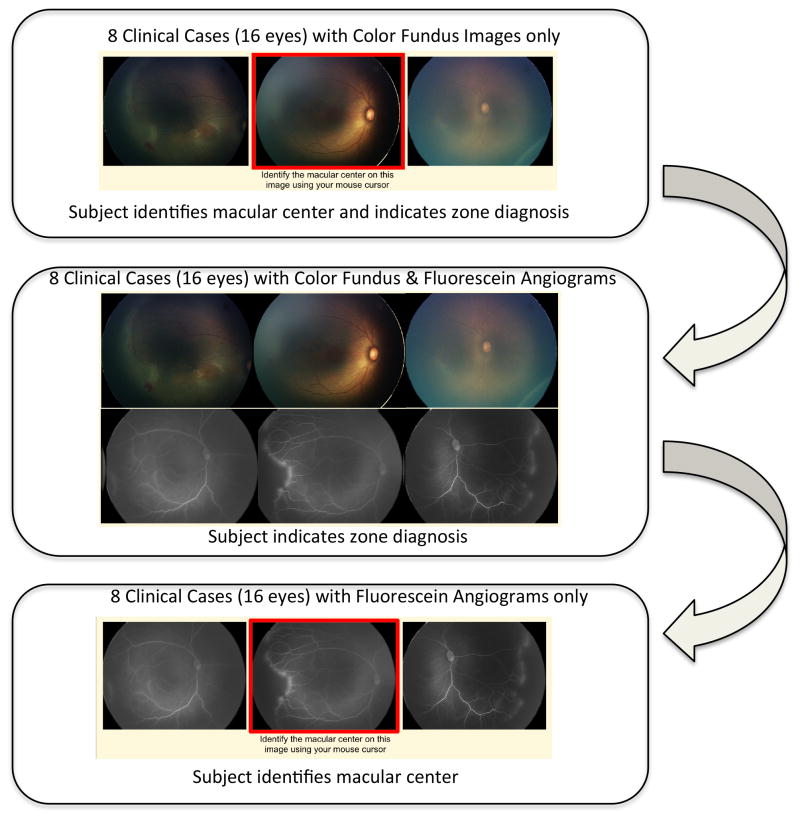
Study experts were directed to a secure website developed by the authors (SNP, MAK, MFC, RVPC) that displayed a set of three retinal images of each eye (temporal, posterior, nasal). For each image set, certain baseline demographic information from the time of imaging was provided, e.g., the birth weight, gestational age, and postmenstrual age. Experts were first directed to use their mouse cursor to identify the macular center on the posterior retinal photograph. Responses were recorded as (x,y) coordinates of the image. Color fundus photographs alone were displayed in sequential order and then fluorescein angiograms with corresponding color fundus photographs were displayed in the same order. After selecting the macular center, experts were then asked what zone (I, II, II-Posterior, III) they would classify each set of retinal images (Figure 1).
Figure 1. Sequence of Images Presented to the Experts in Identification of the Macular Center and Zone in Retinopathy of Prematurity using Color Fundus Photographs and Fluorescein Angiograms.
In the first part of this study, each expert completed 8 clinical cases in which he or she identified the macular center and provided a diagnosis of zone based only on color fundus photographs. In the second part, each expert was asked to provide the diagnosis of zone based on the same 8 clinical cases, but were now provided with the corresponding fluorescein angiograms along with the color fundus photographs. In the final part of this study, each expert was asked to identify the macular center on the fluorescein angiogram for the same 8 clinical cases.
For each color fundus photograph and fluorescein angiogram, experts were asked to rate the image quality for identification of the macular center (adequate, possibly adequate, not adequate) and their confidence in identifying the macular center (confident, somewhat confident, not confident).
Computer Facilitated Diagnosis of Zone
For every response to each image, the linear distance from the optic disc center to the marked macular center was measured. A computer facilitated diagnosis of zone was determined for images in which peripheral ROP was visible. In these images, the closest linear distance from the optic disc center to the peripheral disease was measured. The location of the optic disc center and peripheral disease was determined by the study authors (RVPC, MFC). The value, determined for each image as the distance from the macular center to the optic disc center, was the mean distance calculated by using the identification of the macular center by all study experts. To account for differences in magnification of the images, all distances were standardized by multiplying 1.05/(measured optic disc width in millimeters of each image). This standardization is based on prior published data showing the mean optic disc width in premature infants to be 1.05 mm.12 Based on these arithmetic changes in linear distance, the computer facilitated diagnosis of zone was assigned to either “Zone I” or “Zone II”.
Statistical Analysis
All data were analyzed in SPSS Version 22 (SPSS Inc., Chicago, IL). For each eye, a paired sample t-test was performed to determine whether the mean difference in linear distance between paired color fundus photograph and fluorescein angiogram was significantly different from zero. The variances of each modality in the identification of the macular center were compared using a non-parametric Wilcoxon signed-rank test.
Using the consensus reference standard diagnosis, sensitivity and specificity were computed for each expert for the diagnosis of zone I and zone II. Significant differences in sensitivity or specificity between the imaging modalities were assessed using paired sample t-tests.
The mean unweighted kappa statistic was used for analysis of agreement on zone disease diagnosis. An accepted scale was used to interpret results as follows: 0 to 0.20 indicated slight agreement; 0.21 to 0.40, fair agreement; 0.41 to 0.60, moderate agreement; 0.61 to 0.80, substantial agreement; and 0.81 to 1.00, near perfect agreement.13
RESULTS
Study Experts
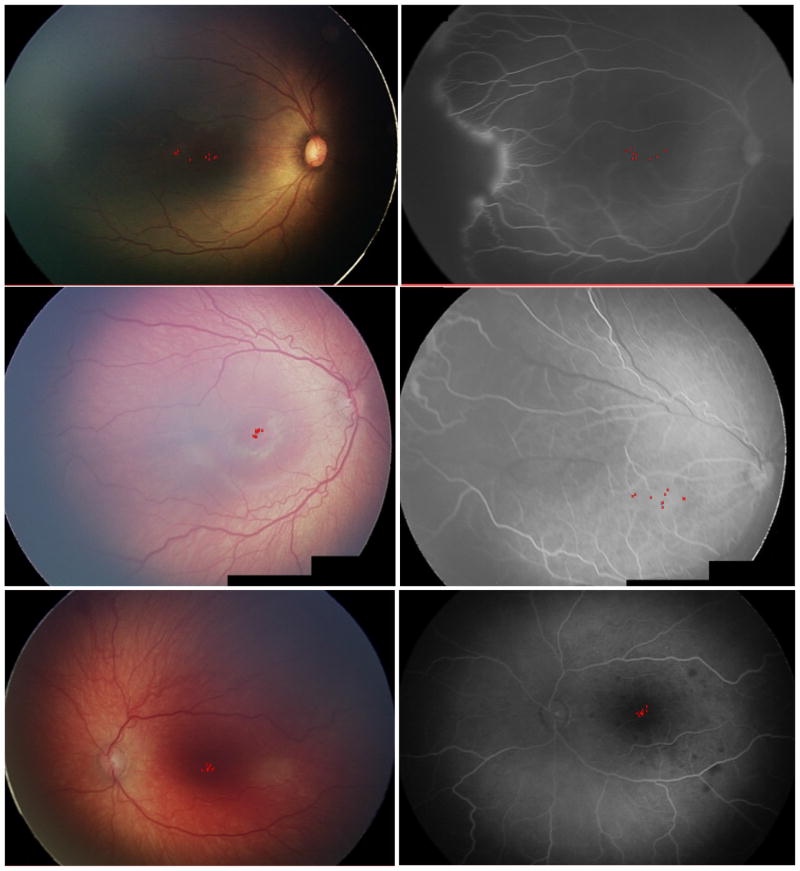
Among the 9 experts who consented to participate in the study, 6/9 (67%) were retina specialists and 3/9 (33%) were pediatric ophthalmologists. The experts have been practicing ophthalmology for a mean (SD) of 19 (8.6) years (range: 10 – 33 years). When asked if fluorescein angiography is safe in neonates and infants, all (9/9) responded “Yes”. Each expert graded 32 images (16 color fundus, 16 fluorescein angiogram) from 16 eyes, for a total of 288 readings of the macular center. Figure 2 reveals examples of concordance and discordance among experts in the identification of the macular center on color fundus photographs and fluorescein angiograms. In 3/15 (20%) eyes, there was a significant difference in intergrader agreement of macular center identification between the color fundus photograph and corresponding fluorescein angiogram, t(9) ≥ |4.807|, p < 0.001 (Table 1). There was no statistically significant differences between the variances of each modality with regard to the identification of the macular center (p = 0.75).
Figure 2. Representative Images of Concordance and Discordance Between Color Fundus Photograph Versus Fluorescein Angiogram (FA) Among Experts in Identification of the Macular Center in Retinopathy of Prematurity (ROP).
(Top Left to Top Right - Case 1 right eye): Good agreement within color fundus photograph and FA. Postmenstrual age at time of imaging: 31.5 weeks. Patient had a consensus reference standard diagnosis of zone I ROP; (Middle Left to Middle Right - Case 6 right eye): Poor agreement in identification of the macular center between color fundus photograph & FA. Postmenstrual age at time of imaging: 39.1 weeks. Patient had a consensus reference standard diagnosis of zone II ROP; (Bottom Left to Bottom Right - Case 8 left eye): Good agreement in identification of the macular center within and between color fundus photograph and FA. Postmenstrual age at time of imaging: 43 weeks. Patient had a consensus diagnosis of zone II ROP.
Table 1.
Comparison of Linear Distance from the Optic Disc Center to the Macular Center using Color Fundus Photography and Fluorescein Angiography from Patients with Retinopathy of Prematurity.
| Case–Eye | Distance to Macular Center on Color Fundus Photography (SD), mm | Distance to Macular Center on FA (SD), mm | Pa |
|---|---|---|---|
| 1-OD | 5.28 (1.20) | 4.96 (0.57) | 0.203 |
| 1-OS | 5.74 (0.91) | 6.27 (0.69) | 0.355 |
| 2-OD | 3.07 (0.23) | 3.44 (0.34) | 0.121 |
| 2-OS | 3.54 (0.46) | 3.40 (0.42) | 0.567 |
| 3-OD | 3.58 (0.46) | 4.12 (0.44) | 0.240 |
| 3-OS | 3.29 (0.38) | 3.38 (0.32) | 0.720 |
| 4-OD | 3.70 (0.58) | 2.90 (0.35) | 0.001 |
| 4-OS | 3.94 (0.87) | 2.75 (0.54) | <0.001 |
| 5-OD | 4.10 (0.28) | 3.37 (0.24) | 0.092 |
| 5-OS | 4.19 (0.48) | 3.16 (0.29) | <0.001 |
| 6-OD | 3.82 (0.12) | 3.90 (0.61) | 0.750 |
| 6-OS | 3.24 (0.33) | 3.75 (0.68) | 0.073 |
| 7-OD | 4.29 (0.07) | 4.29 (1.10) | 1.000 |
| 7-OS | NA | NA | NA |
| 8-OD | 3.54 (0.14) | 3.21 (0.35) | 0.189 |
| 8-OS | 3.58 (0.13) | 3.68 (0.13) | 0.690 |
SD = Standard Deviation; FA = Fluorescein Angiogram; OD = Oculus Dexter; OS = Oculus Sinister.
P value by paired sample t-test.
Computer Facilitated Diagnosis of Zone
10/30 (33%) digital images (5 color fundus, 5 fluorescein angiograms) had both a clearly visible optic nerve and peripheral ROP present (Table 2). The computer facilitated diagnosis of zone based on an expert’s identification of the macular center agreed with that expert’s diagnosis of zone in 28/45 (62%) cases using color fundus photographs and in 31/45 (69%) cases using FA.
Table 2.
Comparison of the Computer Facilitated Diagnosis of Zone in Retinopathy of Prematurity (ROP) and the Expert Diagnosis of Zone in ROP as Determined by Interpretation of Color Fundus Photography and Fluorescein Angiography with Visible Peripheral Disease
| Case-Eye-Image Modality | Computer Facilitated Diagnosis of Zonea | Consensus Diagnosis of Zoneb | Expert Responses for Zone Diagnosis (N = 9) | |
|---|---|---|---|---|
| Zone I | Zone II | |||
| 1-OD-Color | Zone II | Zone I | 3 | 6 |
| 1-OS-Color | Zone I | Zone I | 4 | 5 |
| 3-OD-Color | Zone I | Zone I | 5 | 4 |
| 2-OS-Color | Zone II | Zone I | 5 | 4 |
| 4-OD-Color | Zone I | Zone I | 7 | 2 |
| 1-OD-FA | Zone II | Zone I | 1 | 8 |
| 1-OS-FA | Zone I | Zone I | 5 | 4 |
| 3-OD-FA | Zone I | Zone I | 6 | 3 |
| 3-OS-FA | Zone I | Zone I | 7 | 2 |
| 6-OD-FA | Zone II | Zone II | 0 | 9 |
FA = Fluorescein Angiogram; OD = Oculus Dexter; OS = Oculus Sinister.
Computer facilitated diagnosis of Zone was calculated by comparing the distance from closest peripheral ROP to the center of the optic disc to two times the distance to the macular center from the center of the optic disc. For each case, the distance to the macular center from the center of the optic disc was determined by mean distance of all nine graders. If the location of peripheral ROP was closer than two times the mean distance to the macular center from the center of the optic disc, the computer facilitated diagnosis of Zone was Zone I. If the location of peripheral ROP was greater than two times the mean distance to the macular center from the center of the optic disc, the computer facilitated diagnosis of Zone was Zone II.
Consensus diagnosis is determined from the color fundus photographs by three independent readers in combination with the clinical diagnosis based on ophthalmoscopic examination, as the reference standard.
When the computer facilitated diagnosis indicated zone I, experts chose zone I diagnosis in 19/30 (63%) responses with the color fundus photograph versus in 20/30 (67%) responses with the color fundus photograph and corresponding fluorescein angiogram. When the computer facilitated diagnosis indicated zone II, experts chose zone II diagnosis in 10/20 (50%) responses with the color fundus photograph versus in 19/20 (95%) responses with the color fundus photograph and corresponding fluorescein angiogram.
Of the 5 eyes with color fundus photographs with visible peripheral ROP, 3/5 (60%) (Case 1OD, Case1OS, Case 3OD) had corresponding fluorescein angiograms with visible peripheral ROP (Table 2). For these cases, when the computer facilitated diagnosis indicated zone I, experts chose zone I diagnosis in 11/20 (55%) responses in the color fundus photographs and in 12/20 (60%) responses in the corresponding fluorescein angiograms.
Overall Accuracy of Zone Diagnosis with Color Fundus Photographs versus Color Fundus Photograph with corresponding Fluorescein Angiograms
There was a consensus reference standard zone I diagnosis in 8/16 (50%) eyes. Experts agreed with the consensus reference standard zone I diagnosis in 38/80 (48%) responses with the color fundus photographs and in 59/80 (74%) responses when shown the color fundus photographs and corresponding fluorescein angiograms. In cases with a consensus reference standard zone II diagnosis, experts chose zone II in 76/80 (95%) responses with the color fundus photographs, and in 74/80 (93%) responses when shown the color fundus photographs and corresponding fluorescein angiograms.
Sensitivity and Specificity of Zone Diagnosis
The sensitivity and specificity of zone I diagnosis was determined for each expert (Table 3). Mean (95% CI) sensitivity for detection of zone I by the experts as compared to the consensus reference standard diagnosis when interpreting the color fundus photographs alone versus interpreting the color fundus photographs and FA was 47.2% (35.3% – 59.3%) and 61.1% (48.9% – 72.4%), respectively (t(9) ≥ (2.063), p = 0.073). Based on the case selection, mean sensitivity for detection of Zone II was not possible to compute, however, mean (95% CI) specificity for detection of zone II by the experts as compared to the consensus diagnosis when interpreting the color fundus photographs alone versus interpreting the color fundus photographs and FA was 100% (95% – 100%) with both scenarios.
Table 3.
Sensitivity and Specificity of Zone I Diagnosis in Retinopathy of Prematurity by Experts using Color Fundus Photography and Fluorescein Angiography.
| Expert | Color Fundus Photography | Color Fundus Photography & FA | ||
|---|---|---|---|---|
| Sn (%) (95% CI) |
Sp (%) (95% CI) |
Sn (%) (95% CI) |
Sp (%) (95% CI) |
|
| Expert 1 | 25.0 | 100.0 | 75.0 | 100.0 |
| (3.2, 65.1) | (63.1, 100) | (34.9, 96.8) | (63.1, 100) | |
| Expert 2 | 87.5 | 100.0 | 100.0 | 100.0 |
| (47.3, 99.7) | (63.1, 100) | (63.1, 100) | (63.1, 100) | |
| Expert 3 | 25.0 | 100.0 | 50.0 | 100.0 |
| (3.2, 65.1) | (63.1, 100) | (15.7, 84.3) | (63.1, 100) | |
| Expert 4 | 62.5 | 100.0 | 75.0 | 100.0 |
| (24.5, 91.5) | (63.1, 100) | (34.9, 96.8) | (63.1, 100) | |
| Expert 5 | 0.0 | 100.0 | 0.0 | 100.0 |
| (0, 37.1) | (63.1, 100) | (0, 37.1) | (62.9, 100) | |
| Expert 6 | 37.5 | 100.0 | 37.5 | 100.0 |
| (8.5, 75.5) | (63.1, 100) | (8.5, 75.5) | (63.1, 100) | |
| Expert 7 | 50.0 | 100.0 | 50.0 | 100.0 |
| (15.7, 84.3) | (63.1, 100) | (15.7, 84.3) | (63.1, 100) | |
| Expert 8 | 50.0 | 100.0 | 87.5 | 100.0 |
| (15.7, 84.3) | (63.1, 100) | (47.3, 99.7) | (63.1, 100) | |
| Expert 9 | 87.5 | 100.0 | 75.0 | 100.0 |
| (47.3, 99.7) | (63.1, 100) | (34.9, 96.8) | (63.1, 100) | |
| Average | 47.2 | 100.0 | 61.1 | 100.0 |
| (35.3, 59.3) | (95, 100) | (48.9, 72.4) | (95, 100) | |
Sn = Sensitivity; Sp = Specificity; FA = Fluorescein Angiogram; CI = Confidence Interval.
Intergrader Agreement of Zone Diagnosis
Based on a 3-level categorization of zone (I, II, III), the mean (95% CI) kappa statistic for all experts was 0.291 (0.187 – 0.419) when interpreting the color fundus photographs alone. When interpreting the color fundus photographs and corresponding fluorescein angiograms, the mean (95% CI) kappa statistic was 0.428 (0.303 – 0.593).
DISCUSSION
The key findings from this study are: (1) There does not appear to be a statistically significant difference by ROP experts in identification of the macular center using color fundus photographs versus fluorescein angiograms. (2) There is a marginally significant improvement in sensitivity of zone diagnosis and no statistically significant difference in specificity of zone diagnosis when using color fundus photographs compared to using color fundus photographs with the corresponding fluorescein angiograms. (3) There is inconsistency between traditional zone diagnosis (based on ophthalmoscopic exam and image review) compared to computer-generated diagnosis of zone (based on precise measurement of optic disc and macular centers).
The first key study finding is that there is no significant difference in identification of the macular center when experts use color fundus photographs versus fluorescein angiograms. Previous studies have shown that significant variability may exist among experts in the identification of the macular center.4 In our study when taking the average of all experts, there was no significant variability in identification of the macular center in 12/15 (80%) eyes when using the color fundus photograph versus the fluorescein angiogram. In 3/15 (20%) eyes, there was significant variability in the experts’ identification of the macular center when using the color fundus photograph versus the fluorescein angiogram (Table 1). In two of these cases (Case 4OD and 4OS), more than 4/9 (>44%) experts reported ‘not adequate’ image quality on the fluorescein angiograms. In these cases, although the average of all responses for the macular center by the ROP experts was significantly different on the FA as compared to the color fundus photograph, there was agreement among experts within the imaging modality. Furthermore, there were no statistically significant differences between the variances of each modality within the cases.
The second key study finding is that there was a marginally significant improvement in sensitivity of zone diagnosis and no statistically significant difference in specificity of zone diagnosis when using color fundus photographs compared to using color fundus photographs and the corresponding FAs. We note that previous reports have suggested that FA provides a more objective assessment of zone, due in part to providing superior visualization of the peripheral retinal vasculature.5,7 Our current study did find that intergrader agreement of the zone diagnosis changed from fair to moderate agreement, and that there was a trend for the mean sensitivity of a zone I diagnosis to improve with the addition of the fluorescein angiogram. From a clinical perspective, however, disease in zone I and posterior zone II may behave similarly, but because FA may provide high contrast images and improve identification of avascular retina, it warrants consideration as a method for improving the identification of vascular features of ROP that could aid in determining zone diagnosis.
The third key study finding is that there was discordance between an ROP expert’s traditional diagnosis of zone and a computer facilitated diagnosis of zone based on the average of the experts’ identification of the macular center. Zone I is defined as the area within a circle that extends from the optic disc center to twice the distance from the optic disc center to the macular center.4 Therefore, an examiner’s identification of the macular center should correlate with their diagnosis of zone. On average, in this study, an expert’s identification of the macular center (for those images where there was both a clearly visible optic nerve and peripheral ROP present) agreed with that same expert’s diagnosis of zone in only 28/45 (62%) cases using color fundus photographs and in only 31/45 (69%) cases using FA. This disagreement may be due to experts relying on additional information for the diagnosis of zone besides the location of the macular center. This discordance may demonstrate that a computer facilitated diagnosis of zone based on the expert’s identification of the macular center may not correlate with that expert’s diagnostic interpretation of what zone disease is present based on image review. This may also partly explain why experts agreed with the consensus diagnosis of zone I at a much lower rate than in cases with a consensus diagnosis of zone II. With the development of new automated ROP management systems, further investigation is needed to ascertain if the design of a computer facilitated diagnosis of zone is a viable option. In addition, in the era of telemedicine for ROP, the ability to have the system identify zone based on the examiner’s identification of the macular center may be a useful tool.
Currently, ROP classification using color fundus photographs is standardized according to the criteria outlined by ICROP.14 Because no formal guidelines exist with regard to the classification of fluorescein angiographic findings in ROP, it is unclear what metrics were employed when experts interpreted FA images. However, FA has been shown to be valuable in the treatment of other vitreoretinal diseases like neovascular age-related macular degeneration (AMD) after the development of standardized classification schema for FA interpretation.15–18 Potential future roles for FA in the standard classification of ROP may warrant additional study.
The risk-benefit ratio of FA in ROP diagnosis and management currently remains unclear. It is important to consider the potential risks associated with FA in the neonatal population. However, FA does appear to be safe in children, including neonates with ROP, as no adverse effects have been reported in several series.5–8 In addition, all experts in this current study agreed that FA is safe in neonates and infants. Given our findings, elucidating the risk-benefit ratio of FA imaging may warrant additional study.
Several limitations should be noted: (1) Image quality of the fluorescein angiograms: Fluorescein angiography is an imaging modality that is very operator dependent to retrieve high quality images. As detected in Case 4 OD and OS, when the image quality is not adequate, there may not be accurate identification of the macular center. Overall, experts rated the quality of the images to be adequate in 85/144 (59%) of the color fundus image sets and 72/144 (50%) of the FA image sets, which may reflect inherent limitations that remain with digital imaging for ROP diagnosis. However, among all the cases, statistical analysis found no difference in confidence in identification of the macular center using color fundus photographs or FA imaging. (2) Timing of the fluorescein angiograms: The fluorescein angiograms that readers were provided did not contain the specific transit time. Time-stamped images were not obtainable in all cases. In order to limit stress on the baby, only a finite number of FA images were obtained (3) Images were taken from infants ranging from 33 to 44 weeks PMA. During this time period, morphological characteristics of the macula are still developing and may not reach full maturity until nearly 45 months of age.19 To account for such discrepancies, the study design included relevant demographical information about the case including birth weight, gestational age, and postmenstrual age at time of imaging.
Overall, this study contributes to the body of ROP knowledge by showing that there is a marginally significant improvement in sensitivity of zone diagnosis and no statistically significant difference in specificity of zone diagnosis when color fundus photographs are supplemented with fluorescein angiograms, and by showing that there is inconsistency between the traditional zone diagnosis (based on ophthalmoscopic exam and image review) and a computer-facilitated diagnosis of zone (based on precise measurement of the optic disc and macular centers). The utility of FA in the management algorithms of ROP is currently unclear, and future studies aimed at establishing consensus guidelines for fluorescein findings in ROP are warranted. This will provide opportunities to improve clinical diagnosis, and to provide added value to clinicians through automated programs for ROP zone diagnosis using retinal images.
Acknowledgments
Funding/Support: This investigation was supported by National Center for Advancing Translational Sciences (NCATS) grant # UL1TR00457 of the Clinical and Translational Science Center at Weill Cornell Medical College (New York, New York), grant EY19474 from the National Institutes of Health (Bethesda, Maryland), unrestricted departmental funding from Research to Prevent Blindness (New York, New York), The St. Giles Foundation (New York, New York) and The iNsight Foundation (New York, New York). All authors attest that the sponsors and funding organizations had no role in the reporting of the study data and interpretation of the data.
Footnotes
Financial Disclosures: MFC is an unpaid member of the Scientific Advisory Board for Clarity Medical Systems (Pleasanton, California). AMB – Allergan (Irvine, California): Consultant, Honoraria; Alcon (Forth Worth, Texas): Consultant, Honoraria; Clarity Medical Systems (Pleasanton, California): Speakers bureau. SNP, MAK, MCR, KEJ, SO, MAM, AMB, RVPC: None
Author Contributions: design and conduct of the study (SNP, MAK, KEJ, SO, MAM, AMB, MFC, RVPC); collection, management, analysis, and interpretation of the data (SNP, MAK, MCR, KEJ, MAM, MFC, RVPC); preparation, review, or approval of the manuscript (SNP, MAK, MCR, MFC, RVPC); and responsibility for the integrity of the entire study and manuscript (SNP, MAK, MCR, KEJ, SO, MAM, AMB, MFC, RVPC). All authors meet ICMJE requirements for authorship.
References
- 1.Gilbert C. Retinopathy of prematurity: A global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84(2):77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results. Pediatrics. 1988;81(5):697–706. [PubMed] [Google Scholar]
- 3.Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 4.Chiang MF, Thyparampil PJ, Rabinowitz D. Interexpert agreement in the identification of macular location in infants at risk for retinopathy of prematurity. Arch Ophthalmol. 2010;128(9):1153–1159. doi: 10.1001/archophthalmol.2010.199. [DOI] [PubMed] [Google Scholar]
- 5.Ng EY, Lanigan B, O’Keefe M. Fundus fluorescein angiography in the screening for and management of retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2006;43(2):85–90. doi: 10.3928/0191-3913-20060301-07. [DOI] [PubMed] [Google Scholar]
- 6.Yokoi T, Hiraoka M, Miyamoto M, et al. Vascular abnormalities in aggressive posterior retinopathy of prematurity detected by fluorescein angiography. Ophthalmology. 2009;116(7):1377–1382. doi: 10.1016/j.ophtha.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 7.Purcaro V, Baldascino A, Papacci P, et al. Fluorescein angiography and retinal vascular development in premature infants. J Matern Fetal Neonatal Med. 2012;25(S3):53–56. doi: 10.3109/14767058.2012.712313. [DOI] [PubMed] [Google Scholar]
- 8.Zepeda-Romero LC, Oregon-Miranda AA, Lizarraga-Barron DS, Gutierrez-Camarena O, Meza-Anguiano A, Gutierrez-Padilla JA. Early retinopathy of prematurity findings identified with fluorescein angiography. Graefes Arch Clin Exp Ophthalmol. 2013;251(9):2093–2097. doi: 10.1007/s00417-013-2321-8. [DOI] [PubMed] [Google Scholar]
- 9.Henaine-Berra A, Garcia-Aguirre G, Quiroz-Mercado H, Martinez-Castellanos MA. Retinal fluorescein angiographic changes following intravitreal anti-VEGF therapy. J AAPOS. 2014;18(2):120–123. doi: 10.1016/j.jaapos.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Tahija SG, Hersetyati R, Lam GC, Kusaka S, McMenamin PG. Fluorescein angiographic observations of peripheral retinal vessel growth in infants after intravitreal injection of bevacizumab as sole therapy for zone I and posterior zone II retinopathy of prematurity. Br J Ophthalmol. 2014;98(4):507–512. doi: 10.1136/bjophthalmol-2013-304109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan MC, Ostmo S, Jonas K, et al. Development and evaluation of reference standards for image-based telemedicine diagnosis and clinical research studies in ophthalmology. AMIA Annu Sym Proc. 2014:1902–1910. [PMC free article] [PubMed] [Google Scholar]
- 12.De Silva DJ, Cocker KD, Lau G, Clay ST, Fielder AR, Moseley MJ. Optic disk size and optic disk-to-fovea distance in preterm and full-term infants. Invest Ophthalmol Vis Sci. 2006;47(11):4683–4686. doi: 10.1167/iovs.06-0152. [DOI] [PubMed] [Google Scholar]
- 13.Chiang MF, Jiang L, Gelman R, Du YE, Flynn JT. Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmol. 2007;125(7):875–880. doi: 10.1001/archopht.125.7.875. [DOI] [PubMed] [Google Scholar]
- 14.International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123(7):991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 15.Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the macular photocoagulation study. Arch Ophthalmol. 1991;109(9):1242–1257. [PubMed] [Google Scholar]
- 16.Bressler NM Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Archives of Ophthalmology. 2001;119(2):198–207. [PubMed] [Google Scholar]
- 17.Verteporfin Roundtable Participants, Treatment of Age-Related Macular Degeneration with Photodynamic Therapy Study Group Principal Investigators, Verteporfin in Photodynamic Therapy Study Group Principal Investigators. Guidelines for using verteporfin (visudyne) in photodynamic therapy to treat choroidal neovascularization due to age-related macular degeneration and other causes. Retina. 2002;22(1):6–18. doi: 10.1097/00006982-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Chamberlin JA, Bressler NM, Bressler SB, et al. The use of fundus photographs and fluorescein angiograms in the identification and treatment of choroidal neovascularization in the macular photocoagulation study. Ophthalmology. 1989;96(10):1526–1534. doi: 10.1016/s0161-6420(89)32707-2. [DOI] [PubMed] [Google Scholar]
- 19.Hendrickson AE, Yuodelis C. The morphological development of the human fovea. Ophthalmology. 1984;91(6):603–612. doi: 10.1016/s0161-6420(84)34247-6. [DOI] [PubMed] [Google Scholar]