Abstract
Black raspberries (BRBs) demonstrate potent inhibition of aerodigestive tract carcinogenesis in animal models. However, translational clinical trials evaluating the ability of BRB phytochemicals to impact molecular biomarkers in the oral mucosa remain limited. The present phase 0 study addresses a fundamental question for oral cancer food-based prevention: Do BRB phytochemicals successfully reach the targeted oral tissues and reduce pro-inflammatory and anti-apoptotic gene expression profiles? Patients with biopsy-confirmed oral squamous cell carcinomas (OSCCs) administered oral troches containing freeze-dried BRB powder from the time of enrollment to the date of curative intent surgery (13.9 ± 1.27 days). Transcriptional biomarkers were evaluated in patient-matched OSCCs and non-involved high at-risk mucosa (HARM) for BRB-associated changes. Significant expression differences between baseline OSCC and HARM tissues were confirmed using a panel of genes commonly deregulated during oral carcinogenesis. Following BRB troche administration, the expression of pro-survival genes (AURKA, BIRC5, EGFR) and pro-inflammatory genes (NFKB1, PTGS2) were significantly reduced. There were no BRB-associated Grade 3–4 toxicities or adverse events and 79.2% (N = 30) of patients successfully completed the study with high levels of compliance (97.2%). The BRB phytochemicals cyanidin-3-rutinoside and cyanidin-3-xylosylrutinoside were detected in all OSCC tissues analyzed, demonstrating that bioactive components were successfully reaching targeted OSCC tissues. We confirmed that hallmark anti-apoptotic and pro-inflammatory molecular biomarkers were over-expressed in OSCCs and that their gene expression was significantly reduced following BRB troche administration. Since these molecular biomarkers are fundamental to oral carcinogenesis and are modifiable, they may represent emerging biomarkers of molecular efficacy for BRB-mediated oral cancer chemoprevention.
Keywords: black raspberries, phase 0, phytochemicals, biomarkers
Introduction
Oral cancer is a growing global health problem due to the continued expansion of tobacco product use in the developing world contributing to significant morbidity, mortality, and economic burden (1,2). Oral cancers, including oral cavity and oropharyngeal cancers, are some of the most common cancers worldwide with an expected 300,000 new cases and 145,000 deaths. Men are twice as likely as women to be diagnosed with oral cancers, with worldwide incidence and death rates highest in southern Asia, the Indian sub-continent, and many developing countries (3–5). Oral squamous cell carcinomas (OSCCs) account for 90% of oral malignancies and are strongly associated with the use of tobacco products and alcohol, as well as poor nutrition, periodontal disease, exposure to high-risk human papillomaviruses, and genetic susceptibility (2,6–13). More than 45,700 Americans will be newly diagnosed with oral cancer in 2015 and while the death rate has been dropping for the last several decades, an estimated 8,650 Americans will die from the disease this year (14).
Oral carcinogenesis is a multistep process marked by characteristic molecular changes in key regulators of cell growth (13,15). The accumulation of molecular deficiencies results in unrepaired DNA damage, inappropriate transcriptional expression, epigenetic modifications, and the loss of orchestrated growth control (16). In OSCCs as well as other cancers, the role of the immune system and the inflammatory response appears to be biphasic. The acute and early responses directed by immune surveillance against the developing tumor are considered beneficial. However, the chronic immune milieu created by an unresolved immune response in the tumor microenvironment promotes carcinogenic progression (17–21).
Cancer chemoprevention strategies are used to inhibit or delay the multistep carcinogenic process, and reduce the risk for recurrence or development of future cancer (22–27). Food-based models of cancer prevention leverage the complex interactions between bioactive phytochemicals to facilitate the reduction in procarcinogenic markers and negative outcomes concomitant with cancer risk. Black raspberries (BRBs) feature many bioactive phytochemicals, including a rich complement of anthocyanins, flavonoids, and fruit phenolic acids (28). The antioxidant and anticancer activities of many of these individual agents are well documented; however, it is the potential for further synergistic contributions by multiple bioactive food components on the cancer microenvironment that makes a food-based intervention strategy most attractive (28–31).
The current phase 0 study design was chosen to answer two fundamental questions. First, can newly diagnosed oral cancer patients complete a short-term BRB protocol using oral lozenges (troches) with high compliance? Second, could regional targeting using BRB troches significantly inhibit the transcriptional profile of key pro-carcinogenic genes in oral squamous cell carcinoma (OSCC) tissues? We hypothesized that oral cancer patients would self-administer BRB troches with strong compliance, and that this exposure to BRB phytochemicals would reduce pro-carcinogenic gene expression in contacted OSCC tissues. We employed a pre-surgical model that focused on obtaining oral tissues during the interval between OSCC diagnosis and scheduled surgical resection as part of curative therapeutics. This model provides the opportunity to assess compliance, safety, and toxicity while providing oral biopsy tissues to characterize transcriptional biomarkers without impeding normal standard-of-care clinical practices.
Fundamental to the success of this early biomarker study was the presence of BRB phytochemicals (i.e. anthocyanins) in the targeted tumor tissues and a testable/predictable biological outcome in response to those phytochemicals. Detection of BRB components in OSCC tissues acts as an analytic assessment of BRB exposure, confirms the utility of the oral troches as a delivery vehicle, and corroborates patient self-reported compliance. In conjunction with BRB bioactive components present in the oral tumor tissues, the reduced expression of genes fundamental to oral cancer progression following BRB troche administration further supports the role of these genes as reversible biomarkers of molecular efficacy for this BRB troche intervention.
Materials and Methods
BRB source, troche compounding, and topical delivery
Black raspberries (Rubus occidentalis Jewel variety) were obtained from Dale Stokes Raspberry Farm, LLC in Wilmington, OH. A dedicated single lot of BRBs was mechanically harvested, washed, and frozen at −20°C. BRBs were shipped frozen to Van Drunen Farms (Momence, IL), lyophilized in a VirTis Sublimator Freeze Dryer (SP Scientific), and ground into a freezedried powder. Dissolvable BRB troches were compounded to prolong oral mucosa contact time and facilitate bioactive component delivery using established industry standards (Central Ohio Compounding Pharmacy; Columbus, OH). BRB troches were packaged in protective plastic containers, containing 30 pre-scored troches (Fig. 1A). Each 25 mm × 25 mm square troche contained 360 mg BRB freeze-dried powder plus inert ingredients and binders, including mannitol USP, citric acid monohydrate USP, polyethylene glycol 1450 NF, and sodium benzoate. The daily administered BRB dosage for the current study was markedly less than the BRB amounts previously administered to either healthy participants (45 g/day)(32) or Barrett’s Esophagus patients (32–45 g/day)(33) without toxicity. Importantly, in the current study the effective BRB dose was based on accessible oral cavity surface area rather than total body weight, and focused on a localized delivery instead of systemic dissemination of BRB bioactive components. Oral cancer patients were instructed to actively “tumble” the BRB troches during administration to facilitate oral cavity coverage and dissolution.
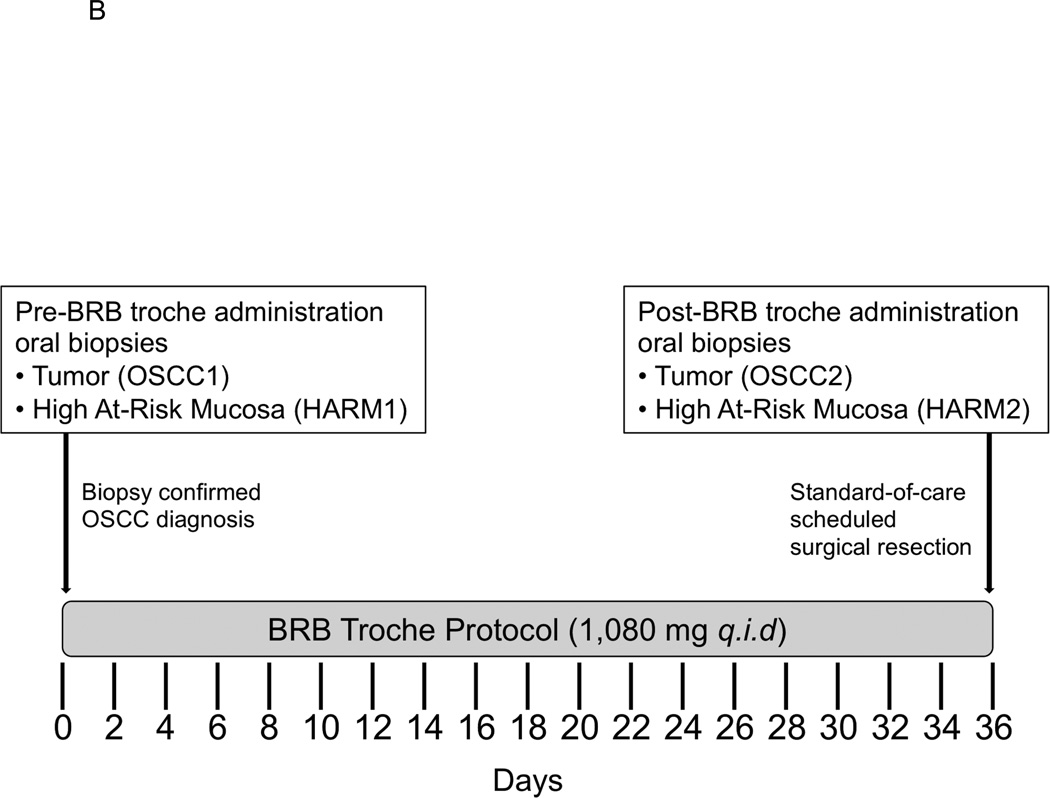
Figure 1. Compounded BRB troches and Phase 0 clinical trial design.
A, Slow release dissolvable BRB troches packaged in protective plastic container containing 30 pre-scored troches. B, Cancer patients with biopsy-confirmed OSCC were consented and enrolled into the study protocol. Participants consumed three dissolvable slow-release BRB troches q.i.d. for a cumulative daily dose of 4.3g freeze-dried BRB powder. Each patient provided oral tissue biopsies from oral tumor before and following BRB administration (OSCC1, OSCC2, respectively) and histologically “normal,” high at-risk mucosa before and following oral BRB administration (HARM1, HARM2, respectively).
BRB dosing rationale and physico-chemical properties
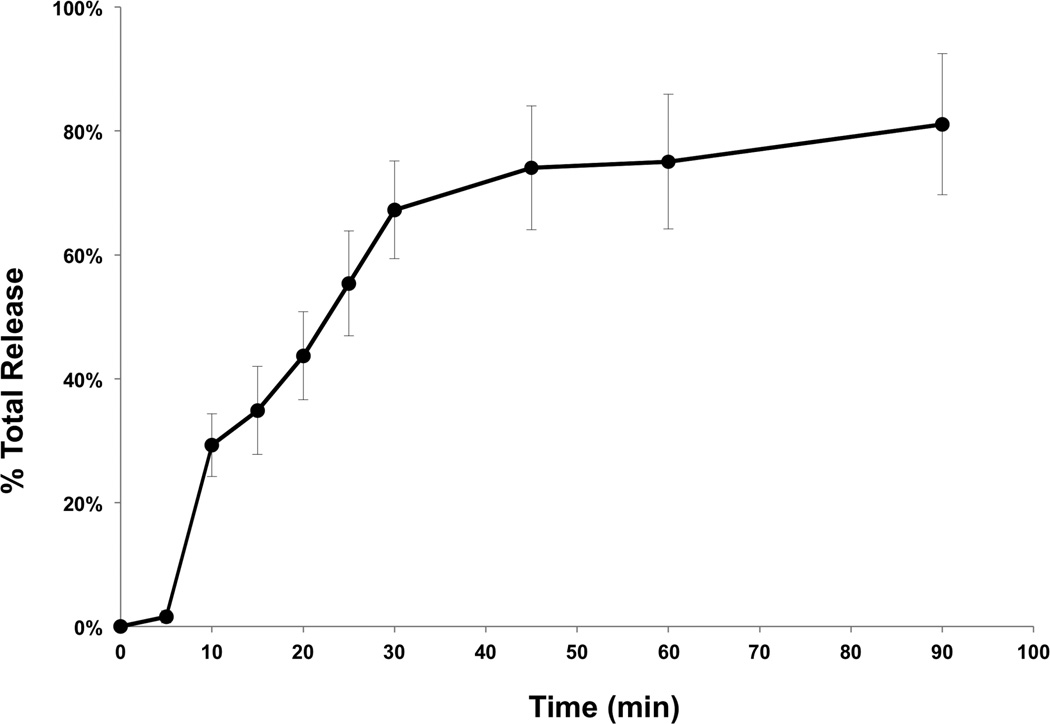
BRB dose was extrapolated from accessible oral cavity surface area data from previous pre-clinical animal studies that demonstrated a significant reduction (44%, P < 0.05) in the number of oral cavity tumors (34). Consequently, the equivalent topical daily dose of orally administered BRBs for humans was estimated at 4.3 g of freeze-dried BRB powder. BRB troches were characterized for phytochemical release using dissolution kinetic analysis. Total phenolic release measurements (λmax 765 nm, N = 9) in a pH6.5 phosphate buffer system were obtained for BRB troches over 90 minutes.
Phase 0 human clinical trial (Fig. 1B)
Eligibility and Inclusion: Male and female OSCC cancer patients (N = 38) ≥ 21 years of age of any race or ethnicity with newly diagnosed, untreated, biopsy confirmed OSCC of any stage were consented and enrolled onto the study protocol in accordance with Internal Review Board directives for The Ohio State University Wexner Medical Center/The Arthur G. James and Richard J. Solove Research Institute. Participants were instructed to follow a low-phenolic diet, document (self-report) alcohol and tobacco use, and record adherence/compliance with daily BRB troche administration using provided log books. Patients were excluded for any of the following criteria: (i) Inability to provide informed consent, (ii) requirement of chemotherapy and/or radiation therapy prior to scheduled standard-of-care surgery, (iii) pregnancy, (iv) use of cyclooxygenase inhibitors that could not be discontinued, (v) inability to take nutrition orally, (vi), intolerance or hypersensitivity to BRB products, (vii) exclusive vegetarian or vegan diet In this short-term pre-surgical protocol, participants consumed three dissolvable slow-release BRB troches q.i.d. for a cumulative daily dose of 4.3 g freeze-dried BRBs. Each patient provided oral tissue biopsies from oral tumor (OSCC) and distant, histologically “normal,” high at-risk mucosa (HARM) before (OSCC1, HARM1) and following (OSCC2, HARM2) BRB troche administration. The duration of BRB administration corresponded to existing standard-of-care scheduled surgical resection for curative therapeutic appointments. (Fig. 1B). Compliance: Adherence to the study protocol was assessed by comparing the number of BRB troches assigned for pre-surgical administration, the number of unused BRB troches returned at the date of surgery, and the number of BRB doses documented in their daily log books.
Analytic measurements of BRB components in OSCC tissues
LC-MS assays were performed based on previously described methodologies (35,36)(Supplemental Data). Briefly, representative oral tumor tissues obtained during surgical resection (OSCC2) were suspended in 5% (v/v) formic acid, sonicated, precipitated, dried under nitrogen gas, and resuspended in 5% (v/v) formic acid/methanol. Extracted samples were applied to an ACQUITY UPLC System (Waters Corporation) operated with BEH C18 column and eluent introduced to a Micromass Quattro Ultima triple quadrupole mass spectrometer with an electrospray probe in positive ion mode. Anthocyanins were detected by selected reaction monitoring (SRM) with the following m/z transitions: cyanidin-3-rutinoside 595>287, cyanidin-3-xylosylrutinoside 727>287, cyanidin-3-glucoside 449>287, cyanidin-3-sambubioside 581>287 using collision energies between 15–25 eV.
Total RNA isolation from HARM and OSCC tissues
Oral tissue incisional biopsies were collected into Ambion RNAlater reagent and batch processed for RNA isolation using the Qiagen RNeasy Fibrous Tissue Kit. Total RNA was treated with DNase I to remove contaminating co-isolated genomic DNA. The DNA-free RNA was assessed for yield using a NanoDrop ND-1000 microvolume spectrophotometer and integrity using an Agilent Technologies Bioanalyzer 2100. RNA samples with RNA Integrity Number (RIN) values between 6–9 were used as templates for cDNA syntheses and RT-qPCR analysis.
Prognostic biomarkers of OSCC and biomarkers of BRB exposure/molecular efficacy
Prognostic cancer biomarkers define the likely course of carcinogenic progression in the absence of treatment. Pathways associated with “hallmarks of cancer,” (16) including apoptosis and inflammation, were used to focus on known prognostic biomarkers that could further represent relevant biomarkers of BRB molecular efficacy in some OSCC patients. Potential gene targets were identified that demonstrated deregulated patterns of expression in tumor tissues with an emphasis on OSCC and HNSCC cancers. Clinical tissue samples from current oral cancer patients were obtained from tumor (OSCC) and distant, non-involved, phenotypically “normal” tissues (HARM)(37,38) at the time of surgical resection. HARM tissues, while distant from the tumor, also represent potential regions high at-risk for oral cancer development as a consequence of field cancerization defects. First, genes were characterized for differential expression between baseline tumor and high at-risk mucosal tissues (OSCC1−HARM1), thereby supporting a biological role in oral carcinogenesis. Second, genes demonstrating a differential expression between baseline tumor tissue and post-BRB administered tumor tissue (OSCC2−OSCC1) were identified as biomarkers of BRB associated exposure and molecular efficacy in OSCC tissues.
Reverse transcription quantitative PCR (RT-qPCR)
An 11-gene panel of oral cancer prognostic biomarkers (AURKA, BAX, BCL2, BIRC5, CASP3, CASP14, EGFR, NFKB1, PTGS1, PTGS2, TBXA2R) was validated using RT-qPCR and 384-well microfluidic cards in triplicate. Pre-validated Applied Biosystems human TaqMan Assays and qPCR chemistry were used to interrogate molecular biomarker expression patterns in OSCC tumor tissues prior to and following short-term, low-dose administration of BRB troches.
Statistical analysis of gene expression profiles
Gene expression values were normalized to an evidence-based active reference gene (DUSP1) and examined for significant changes in OSCC1–HARM1 (oral carcinogenesis prognostic biomarkers) and OSCC2–OSCC1 (OSCC BRB predictive biomarkers) gene expression. Differences in ΔCq values were examined for significance using a linear model adjusting for the effects of clinical stage of disease, Body Mass Index (BMI), smoking status, and age.
Results
BRB composition and dosing regimen
Polyphenolic profiles were obtained for the BRB powder (BRB-P) used for pharmaceutical compounding of the BRB troches (BRB-T) as well as the BRB-T (Table 1). Total anthocyanin, ellagitannins (ETs), methyl ellagic acid, and quercetin glycoside levels were determined, as well as measurements for free ellagic acid and individual phytochemical components. Anthocyanins were the dominant polyphenol class with the disaccharide and trisaccharide substitutions most abundant. Due to the complexity of the ellagitannins and difficulty of their analysis, it is common to hydrolyze extracts to liberate ellagic acid and report total ellagic acid. The various species were quantified individually since ellagic acid and ellagitannins could demonstrate distinct bioactive activities in vivo. Sanguiin H-6 and lambertiannin C were the two most prominent ETs and tentatively identified according to their parent masses and liberation of ellagic acid in the MSe experiment. The m/z 783 ET species are likely pedunculagin or structural isomers as found in pomegranate (39). The total ellagitannins represent a very large class of polyphenols in BRBs at 24% of the total polyphenols. Although essentially non-bioavailable as ETs, once ingested ellagitannins can hydrolyze in the gut to give free ellagic acid, which in turn can be absorbed or metabolized to urolithins by gut bacteria. ETs may also impact gut physiology locally without being absorbed. Quercetin glycosides are another bioactive class of polyphenols and they are substituted with sugars similar to the anthocyanins with rutinoside and xylosylrutinosides predominating. They are not absorbed intact but rather must be deglycosylated, absorbed as aglycone quercetin and reconjugated to glucuronide and sulphate species in the enterocytes and in circulation. Total anthocyanin and free ellagic acid levels are consistent (<10% difference) with previous estimates reported by Stoner et al. across multiple BRB harvest years and studies (40).
Table 1.
BRB phytochemical components and potential chemopreventive agents present in freeze-dried BRB powder and BRB troches.
| Component | BRB-P mg/kg |
mg/troche | BRB-T mg/dose |
mg/day |
|---|---|---|---|---|
| Total Anthocyanins | 33,360.50 | 18.26 | 54.78 | 219.13 |
| Cyanidin-3-O-glucoside | 12,924.40 | 7.54 | 22.62 | 90.48 |
| Cyanidin-3-O-sambubioside | ||||
| Cyanidin-3-O-rutinoside | 20,436.10 | 10.72 | 32.16 | 128.64 |
| Cyanidin-3-O-xylosylrutinoside | ||||
| Ellagitannins | 6,026.38 | 1.65 | 4.95 | 19.79 |
| ET 933-1 | 1,279.73 | 0.07 | 0.20 | 0.81 |
| ET 783-1 | 459.90 | 0.00 | 0.00 | 0.00 |
| ET 933-2 | 762.06 | 0.07 | 0.22 | 0.88 |
| ET 783-2 | 400.07 | 0.05 | 0.15 | 0.61 |
| ET(s) | 468.88 | 0.14 | 0.43 | 1.73 |
| Sanguiin H6 | 2,246.80 | 1.13 | 3.38 | 13.53 |
| MeEA Pent | 212.61 | 0.07 | 0.22 | 0.89 |
| MeEA MalPent | 196.32 | 0.11 | 0.33 | 1.34 |
| Ellagic Acid | 238.77 | 0.02 | 0.05 | 0.19 |
| Quercetin Glycosides | 1,244.40 | 0.83 | 2.48 | 9.91 |
| QxRut | 334.90 | 0.18 | 0.54 | 2.17 |
| Rutin | 602.80 | 0.31 | 0.92 | 3.68 |
| QGluA, MeEAGluA | 306.70 | 0.34 | 1.02 | 4.06 |
| Myericetin Glycosides | 40.60 | – | – | – |
BRB-P, freeze-dried black raspberry powder source material
BRB-T, compounded freeze-dried black raspberry troches
QGluA/GalA, quercetin glucuronide/galacturonide
MeEAGluA/GalA, methyl ellagic acid glucuronide/galacturonide
ET, ellagitannin; numbers following ET refer to m/z isobaric peak number
Pent, pentoside
Mal, malonyl
Participation and adherence of OSCC patients
Sixty-two eligible newly diagnosed OSCC patients were successfully identified by systematic medical records review for the Head and Neck Oncology Clinic at The Ohio State University, Arthur G. James Cancer Hospital and Richard J. Solove Research Institute (Table 2). After obtaining an informed consent in accordance with Institutional guidelines, 38 OSCC patients were enrolled into this phase 0 study with intent to complete the NCT01465776 protocol for short-term administration of BRB troches. Following consent, two patients declined to provide pre-surgical biopsy tissues. An additional three patients provided pre-surgical tissues, but declined to consume the BRB troches. Consequently, 33 patients fully initiated the NCT01465776 protocol by provided tissues and administering BRB troches. Since the study was contoured to the patient’s existing standard-of-care clinic visits, BRB troche administration ranged from 0.25–34 days, with a mean duration of 12.9 days and 153 troches, in those patients administering at least one BRB dose (N = 33).
Table 2.
Phase 0 trial participant demographics, clinico-pathologic features, and adherence measurements for the NCT01465776 protocol.
| Patients, N | (%) | ||
|---|---|---|---|
| Sex, Race/Ethnicity | |||
| Male | 26 | 68% | |
| Female | 12 | 32% | |
| NHW | 37 | 97% | |
| NHB | 1 | 3% | |
| Age (years) | |||
| < 45 | 6 | 16% | |
| 45–60 | 16 | 42% | |
| > 60 | 16 | 42% | |
| Range | 37 – 79 | ||
| Average | 57.5 | ||
| Tumor Location | |||
| Gingival | 1 | 3% | |
| Floor of Mouth | 6 | 16% | |
| Mandibular | 2 | 5% | |
| Maxillary | 1 | 3% | |
| Retromolar | 3 | 8% | |
| Tongue | 24 | 65% | |
| Tumor Stage | |||
| 1 | 10 | 27% | |
| 2 | 9 | 24% | |
| 3 | 6 | 16% | |
| 4 | 12 | 32% | |
| HPV Status | |||
| − | 24 | 83% | |
| + | 5 | 17% | |
| Smoking Status | |||
| Never | 12 | 32% | |
| Former | 9 | 24% | |
| Current | 17 | 45% | |
| Alcohol Use | |||
| Never | 10 | 28% | |
| Former | 8 | 22% | |
| Current | 18 | 50% | |
| BMI | |||
| < 18.50 | underweight | 1 | 3% |
| 18.50 – 24.99 | normal | 8 | 21% |
| ≥ 25.00 | overweight | 29 | 76% |
| ≥ 30.00 | obese | 19 | 50% |
| Range | 16 – 48 | ||
| Average | 29.5 | ||
| Duration (Days) | |||
| < 7 | 4 | 13% | |
| 7–21 | 23 | 77% | |
| > 21 | 3 | 10% | |
| Range | 5 – 34 | ||
| Average | 13.9 | ||
| Median | 12.5 | ||
| Dose (Troches) | |||
| < 166 | 18 | 60% | |
| 166 – 250 | 8 | 27% | |
| > 250 | 4 | 13% | |
| Range | 60 – 408 | ||
| Average | 165.5 | ||
| Median | 141.0 |
Adherence to the study protocol was assessed with respect to self-reported BRB troche administration compliance documented in a daily food consumption and tobacco usage diary, as well as analytic measurements of BRB components in the OSCC tissues following short-term BRB troche administration. OSCC patients demonstrated a willingness to participate in the study (61.3% enrollment of eligible candidates), with 78.9% (30/38) successfully completing the study protocol as measured by documented, self-reported BRB administration. Adherence to BRB troche administration and documentation was 97.2% in the 30 OSCC patients completing the protocol during an average two-week (13.9 ± 1.3 days, 165.6 ± 15.3 troches) period of administration.
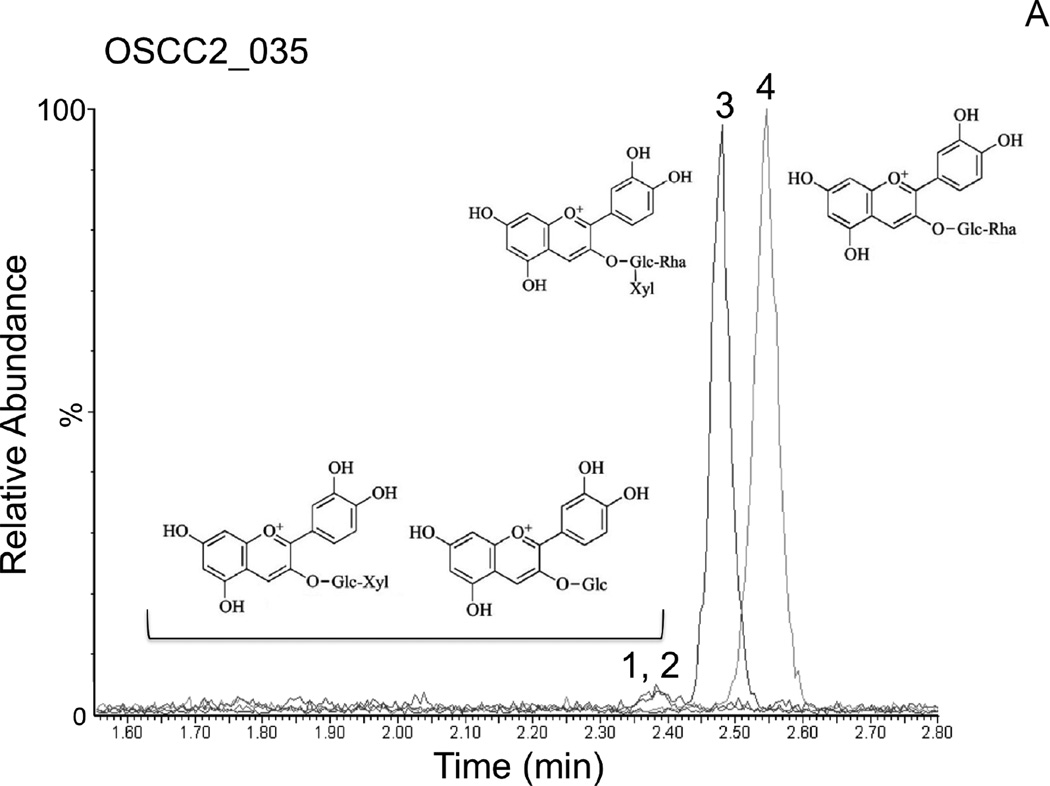
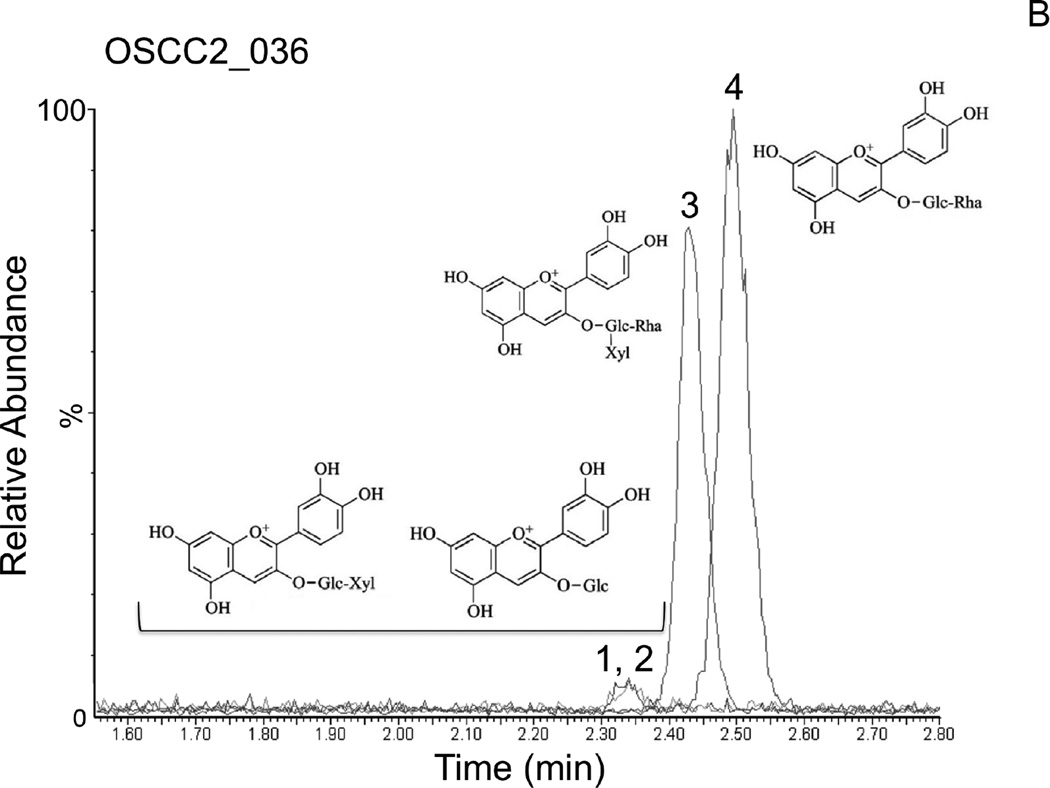
In order to support the self-reported compliance, analytic measurements of BRB components were performed in OSCC tissues following BRB troche administration. In vitro dissolution kinetic studies demonstrated that 55.4% of the total phenolics present in the BRB troches were released within 25 minutes (Fig. 2), a duration that mimicked the patient-driven “tumbling” times for the current protocol. Furthermore, analytic measurements using HPLC Electrospray Ionization/Tandem Mass Spectrometry (HPLC ESI-MS/MS) demonstrated the presence of established BRB components in the OSCC tissues (N = 15) following completion of the study protocol (Fig. 3). The dominant anthocyanins, cyanidin-3-rutinoside and cyanidin-3-xylosylrutinoside (Fig. 3A, 3B), were readily detectable in all tested OSCC tissue biopsies obtained at the time of surgical resection for cure. Furthermore, the minor content anthocyanins cyanidin-3-glucoside and cyanidin-3-sambubioside (Fig. 3A, 3B) were detected in 100% and 93%, respectively, of the tissue samples following short-term BRB troche administration.
Figure 2. Dissolution kinetics for total phenolics of BRB troches in vitro.
Total polyphenolic release from BRB oral troches was estimated from λmax 765 nm measurements using a pH6.5 phosphate buffer system over 90 minutes.
Figure 3. Analytic phytochemistry for malignant oral tissues following short-term BRB troche administration.
Detection of the anthocyanins cyanidin-3-sambubioside and cyanidin-3-glucoside (peaks 1,2), cyanidin-3-xylosylrutinoside (peak 3), and cyanidin-3-rutinoside (peak 4) by LC MS/MS in representative OSCC biopsy tissues following short-term BRB troche administration. A, Patient PT035, 45y, stage 4 lateral tongue OSCC, never-smoker, 8-day BRB troche exposure. B, Patient PT036, 75y, stage 2 retromolar mucosa OSCC, current smoker, 7-day BRB exposure.
Adverse experiences and toxicity of BRB troches
In accordance with NCI Data and Safety Monitoring Guidelines, events were monitored and reported consistent with “the anticipated level of risk involved” in the study at the Investigator’s discretion. Adverse events were monitored and reported to ensure participant safety and to facilitate identification of emerging differences within the study population. There were no Grade 3–4 adverse experiences reported in association with the short-term administration of BRB troches. OSCC patients most commonly reported some mild irritation or soreness in the oral cavity (Grade 1, “related”: 6/38, 15.8%), typically proximal to the surgical biopsy site and not necessitating clinical intervention, while self-administering BRB troches. One patient reported pain or swelling (Grade 2, “unrelated”: 1/38, 2.6%) near the surgical biopsy site and was similarly determined by the attending physician to be independent of BRB troche administration and “unlikely” related to the BRB intervention. Three study participants reporting an adverse experience of Grade 1 or Grade 2 (3/38, 7.9%) eventually discontinued BRB troche use, while five additional study participants (5/38, 13.2%) voluntarily discontinued BRB troche administration without any indication of an adverse experience.
Down-regulation of anti-apoptotic transcriptional biomarkers following short-term delivery of BRB troches
Predictive cancer biomarkers are surrogates for measuring potential responsiveness to a treatment or intervention in patients already presenting with disease. A prognostic 7-gene panel of apoptosis-associated molecular biomarkers (AURKA, BAX, BCL2, BIRC5, CASP3, CASP14, EGFR) was used to explore relevant gene expression changes in OSCC tissues after short-term delivery of BRB troches. Five apoptosis-associated genes (AURKA, BAX, BIRC5, EGFR, and NFKB1; Table 3) demonstrated significant (P < 0.05) over-expression in OSCC tissues with respect to patient-matched HARM tissues. Importantly, five genes (AURKA, BCL2, BIRC5, CASP3, EGFR) further demonstrated a significantly reduced (P < 0.05) level of expression in oral tumor tissues following short-term administration of BRB troches (Table 3), with three of these genes (AURKA, BIRC5, EGFR) remaining significant following multiple-comparison correction.
Table 3.
Fold differences in expression for prognostic molecular biomarkers of oral carcinogenesis and BRB predicative molecular biomarkers in OSCC patients.
| Prognostic Molecular Biomarkers |
Predictive Molecular Biomarkers |
|||||||
|---|---|---|---|---|---|---|---|---|
| OSCC1 – HARM1 | OSCC2 – OSCC1 | |||||||
| Fold-Change | CI | Fold-Change | CI | |||||
| AURKA | +4.47 | +2.62 | +7.64 | a,b | −3.12 | −5.45 | −1.78 | a,b |
| BAX | +2.48 | +1.66 | +3.70 | a,b | −0.94 | −2.02 | +2.28 | a |
| BCL2 | +1.01 | −1.45 | +1.49 | −1.84 | −2.90 | −1.17 | ||
| BIRC5 | +3.92 | +2.48 | +6.18 | a | −2.60 | −3.71 | −1.83 | a,b |
| CASP3 | +1.91 | −1.26 | +4.58 | −6.15 | −18.79 | −2.01 | a | |
| CASP14 | +1.73 | −1.63 | +4.88 | −1.96 | −3.93 | +1.02 | ||
| EGFR | +2.33 | +1.33 | +4.07 | a | −2.60 | −3.79 | −1.67 | a,b |
| NFKB1 | +2.68 | +1.68 | +4.27 | a,b | −3.76 | −6.14 | −2.30 | a,b |
| PTGS1 | +3.41 | +2.24 | +5.20 | a,b | −4.14 | −7.41 | −2.32 | a,b |
| PTGS2 | +20.97 | +13.44 | +32.71 | a,b | −2.87 | −6.52 | −1.26 | a |
| TBXA2R | +5.74 | +3.32 | +9.91 | a,b | −1.43 | −3.11 | +1.51 | |
BRB effect is >2 s.e.m.
BRB effect is > Bonferroni bound CI, upper and lower limits for 90% confidence interval
Inhibition of pro-inflammation transcriptional biomarkers following short-term administration of BRB troches
A prognostic 4-gene panel of inflammation-associated molecular biomarkers (NFKB1, PTGS1, PTGS2, and TBXA2R) was used to investigate gene expression changes in oral tumors following BRB troche administration. All four genes (Table 3) demonstrated significant (P < 0.05) over-expression in OSCC tissues with respect to patient-matched HARM tissues, and three genes (NFKB1, PTGS1, and PTGS2) demonstrated a significantly reduced (P < 0.05) level of expression in tumor tissues following short-term BRB administration (Table 3). BRB effects for NFKB1 and PTGS1 remained significant following Bonferroni correction.
While eight candidate genes for BRB driven molecular efficacy demonstrated significant expression changes (AURKA, BAX, BIRC5, EGFR, NFKB1, PTGS1, PTGS2, and TBXA2R) following short-term BRB troche administration, only five retained significance subsequent to multiple comparison adjustments. These five genes (AURKA, BIRC5, EGFR, NFKB1, PTGS1) represent a signature for BRB exposure and potential molecular efficacy in oral malignant tissues.
Patient stratification by BRB molecular efficacy
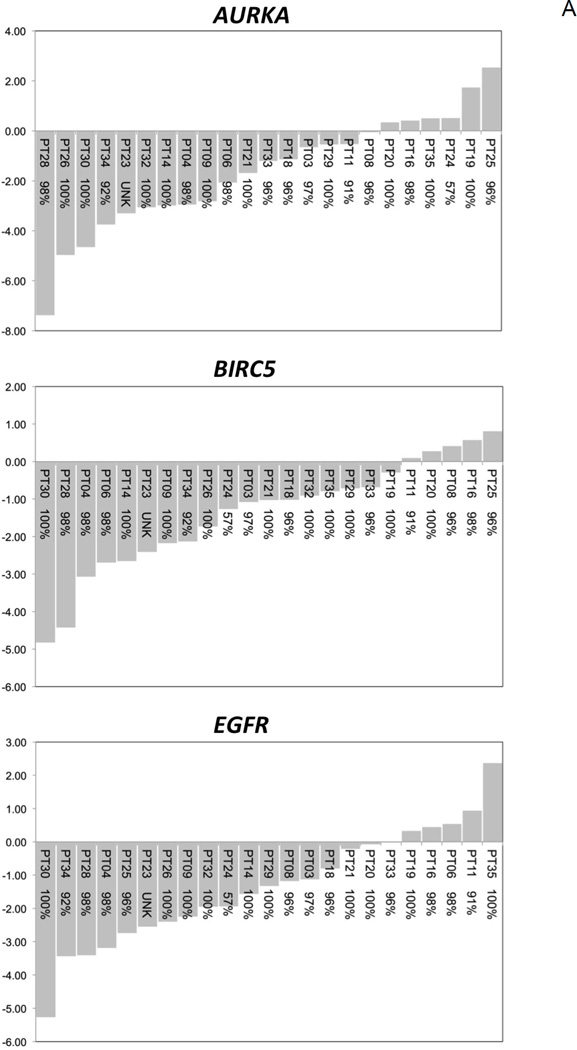
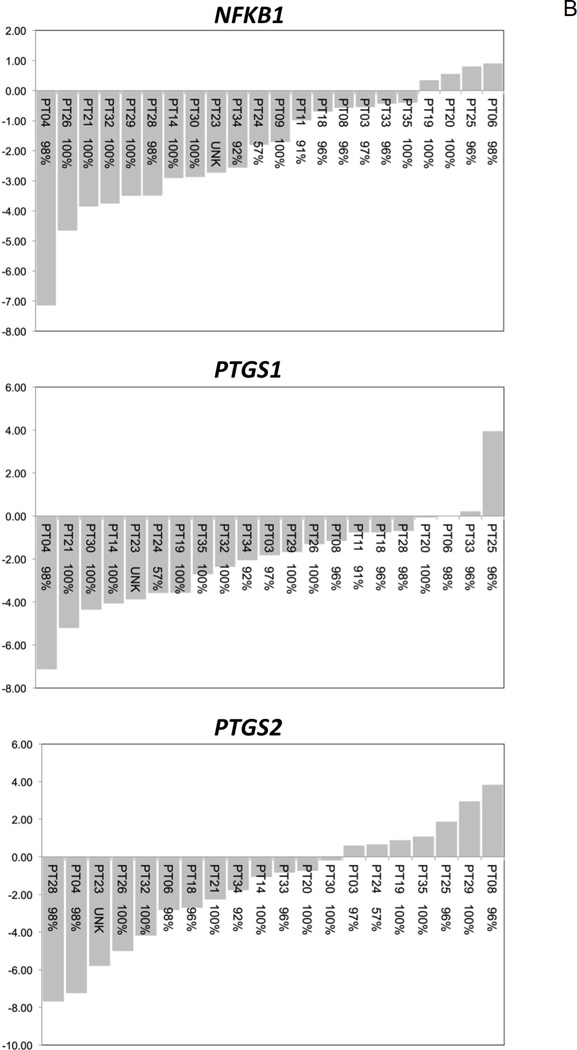
Normalized gene expression profiles revealed that prognostic pro-inflammatory and pro-survival biomarkers were over-expressed in OSCC tissues compared to patient-matched non-involved HARM tissues. Notably, 11/12 (91.7%) biomarkers demonstrated a decrease in expression in OSCC tissues following short-term delivery of BRB troches (Table 3). Patient-level response profiles for each molecular biomarker demonstrate a significant (Fig. 4A) or general trend (Fig. 4B) in decreased expression in OSCC patients following administration of BRB troches. On average each molecular biomarker demonstrated a BRB response (transcriptional inhibition) that would favor cancer prevention in 79% of the OSCC patients. Furthermore, patient-level response profiles reveal that there is a minority of cases that demonstrate an increase in transcriptional expression in individual molecular biomarkers, suggesting potential for the predictive value of these biomarkers.
Figure 4. Patient-level transcriptional responses in OSCC biopsy tissues following short-term BRB troche administration.
A, Significant reduction in gene expression levels was evident for AURKA, BIRC5, and EGFR (P < 0.05). B, The molecular biomarkers NFKB1, PTGS1, and PTGS2, which demonstrated similar transcriptional inhibition but did not reach statistical significance following Bonferroni correction, are included for comparison. x-axis, OSCC patient ID and self-reported compliance to the BRB protocol; y-axis, fold-change in gene expression.
Molecular-clinical correlations in OSCC patients after short-term administration of BRB troches
Molecular biomarkers were interrogated against common clinical features following BRB troche delivery in order to reveal potential associations between transcriptional regulation and clinicopathologic modifiers (Table 4). The biomarkers BAX, BCL2, BIRC5, PTGS1, and TBXA2R demonstrated a negative correlation with molecular efficacy; in other words, the greatest molecular efficacy (inhibition of gene expression) following BRB administration was demonstrated in younger patients. Similarly, PTGS2 molecular efficacy was more pronounced in OSCC patients with lower tumor staging, while higher BMI indices dampened the BRB effects in AURKA and EGFR.
Table 4.
Clinico-pathologic features demonstrating a dampened BRB effect following short-term topical administration in OSCC patients.
| OSCC2 – OSCC1 | |||
|---|---|---|---|
| Age | Stage | BMI | |
| AURKA | ↓ | ↓ | |
| BAX | ↓ | ↓ | |
| BCL2 | ↓ | ||
| BIRC5 | ↓ | ↓ | |
| CASP3 | |||
| CASP14 | ↓ | ↓ | |
| EGFR | ↓ | ||
| NFKB1 | |||
| PTGS1 | ↓ | ||
| PTGS2 | ↓ | ||
| TBXA2R | ↓ | ||
Discussion
Chemoprevention and nutritional prevention strategies seek to reduce cancer risk and/or inhibit carcinogenesis through the administration of agents and modification of life style behaviors. The ideal paradigm strategy would be effective, safe, easy, cost-effective, and nominally alter existing life style behaviors. Early phase trials of novel delivery systems of food-based products, such as the current study protocol, are challenged by conducting the intervention in partnership with the highest at-risk or actively manifest cancer patient populations in a manner that does not compromise existing standard-of-care practices. As recently discussed by Brenner and Hawk (41), the necessary clinical design of early phase cancer risk reduction interventions imposes many restrictions on the ability to obtain biomarker data efficiently. Combining an appropriately targetable patient population and cancer with a pertinent cancer prevention strategy remains an ongoing clinical challenge. In contrast to definitive therapeutic strategies for malignant disease, cancer prevention actions require relatively long-term administration in advance of overt disease in recognized at-risk populations to reduce the risk of future carcinogenesis.
Importantly, it has been estimated that a modifiable factor, diet, contributes to nearly 35% of all cancers (22). It is hypothesized that food-based cancer prevention strategies can leverage the complex interactions and complementarity of multiple bioactive phytochemicals to reduce the risk for cancer development. Physiological targets of these strategies include curbing deregulated cell growth/death and alleviating chronic pro-inflammatory responses within the solid tumor microenvironment. The current phase 0 trial protocol explored the potential for repeated low-dose, short-term BRB troches to successfully deliver bioactive components to OSCC tissues and to effect relevant transcriptional changes in those tissues. By design, the short-term duration of the intervention, the single low daily dose of BRBs, and the selected cohort of current OSCC patients awaiting curative surgical therapy, acknowledges that there is no reasonable expectation of disease preventive efficacy. However, this study does demonstrate that hallmark features of oral carcinogenesis are modifiable targets of BRB phytochemicals, and that the transcriptional changes that follow the BRB intervention support a role for these phytochemical in a food-based oral cancer prevention strategy. These long-term goals include incorporation of BRBs or their components and metabolites into primary chemopreventive practices, as well as to define the potential utility of BRB phytochemicals towards secondary/tertiary prevention and cancer control measures in oral cancer patients. Importantly, our analysis of molecular efficacy and clinical correlates provides the opportunity for identifying specific BRB-responsive surrogate endpoints for primary prevention trials in populations, such as cigarette smokers, who are high at-risk for subsequently developing oral cancer.
The aerodigestive tract, especially the oral cavity, is compromised by contact with several significant risk factors for cancer development, predominantly exposure to alcohol and tobacco smoke. These insults confer a persistent field of damage (15,42,43) in the epithelial cells and subsequently emerge as malignant lesions (OSCCs) and non-tumorigenic but high at-risk mucosa (HARM)(37,38). These OSCC and HARM tissues further afford the unique opportunity for minimally invasive surveillance as well as active targeting of an intervention, such as demonstrated with our BRB troches.
Several research groups prior studies exploring the tolerability and feasibility of BRB bioactive components on reducing the malignant phenotype in the aerodigestive tract. Stoner et al. (32) conducted an early phase pharmacokinetic study in 11 healthy participants who received 45 g of BRB powder once daily for 7 days and were assessed for safety, tolerability, and presence of systemic BRB phytochemicals. Urine and plasma samples demonstrated the presence of ellagic acid, and the anthocyanin metabolites cyanidin-3-glucoside, cyanidin-3-sambubioside, cyanidin-3-rutinoside, and cyanidin-3-xylosylrutinoside. Importantly, this study demonstrated that there were nominal safety/tolerability concerns following daily administration of 45 g of BRB powder, more than 10-times the amount delivered in our current protocol. Kresty et al. (33) similarly administered 32–45 g of BRB powder daily for 26 weeks to 10 Barrett’s Esophagus patients. Patient compliance to the BRB protocol was reported overall as extremely good with no significant adverse events, and demonstrated that high levels of compliance could be achieved during a 6-month BRB powder intervention. Systemic metabolic biomarkers of DNA damage (8-epi-prostaglandin F2α, 8-hydroxy-2-deoxyguanosine) were decreased in the urine of patients following the 6-month BRB intervention.
Work by Mallery et al. (36) extended the potential use of BRBs into the oral cavity and established the utility of a “mucoadhesive gel” formulation for focused delivery within the oral cavity of healthy volunteers. The differences in strategy between these prior studies and the current clinical trial are significant. The mucoadhesive patch is used as a very focal mechanism to deliver BRB bioactives to a known lesion. The surrounding high “at-risk” mucosa is not targeted or treated by design. In contrast, our troches will ultimately be used in a different scenario where manifest lesions are not present, but rather we are treating the at-risk mucosal field by accessing the entire oral mucosa. For example, after successful treatment of a tobacco-related oral cancer, an incidence rate for a second tobacco related cancer can be 15–20% per year in high risk populations. This would be an ideal target population for an intervention that accesses the entire HARM field of cancerization. In the work by Ugalde et al. (44) and Mallery et al. (45) 0.5g of a 10% freeze-dried BRB mucoadhesive gel was applied to premalignant lesions for 12 weeks while undergoing clinical observations for progression. This corresponds to an estimated 50mg of freeze-dried BRB powder delivery to the defined premalignant tissues of interest. In the current biomarkers study, each troche contained 360mg of freeze-dried BRB powder (7× the mucoadhesive gel dosage) delivered by oral tumbling to the entire mucosal tissues of the oral cavity for an average of two weeks. While studies by the Mallery group targeted discrete premalignant lesions at a lower relative dose, the current study attempted to deliver a larger total phytochemical dose to the complete oral cancerization field (HARM and OSCC tissues). Consequently, these studies are distinct but complementary in approach and intent.
Local and systemic detection of anthocyanins strongly supported the functional effectiveness of a low-dose targeted topical delivery of BRB phytochemicals in the oral cavity. Shumway et al. (46) showed the potential molecular efficacy of topical BRBs within the oral cavity following application of a 10% BRB powder bioadhesive gel q.i.d to established premalignant lesions. Despite appreciable inter-patient variation, topical application of BRBs significantly reduced the prevalence of LOH in a subset of IEN patients, with 41% demonstrating a favorable response (decreased lesion grade) following BRB gel application. While complicated by the dynamic progression/regression profile of IEN, these studies reveal the potential of BRBs to favorably mediate molecular profiles in at-risk oral tissues and demonstrated a BRB delivery system that allowed rapid local transmucosal and systemic delivery of BRB phytochemicals. The current study established that BRB phytochemicals were: (i) readily dispersed from the troche delivery system with kinetics that allowed 55.4% of total phenolics to be released within 25 minutes (Fig. 2) and (ii) incorporated into the target tumor tissues in the oral cavity (Fig. 3). Consequently, measurements of protocol adherence were assessed measurements of protocol adherence using both self-reported compliance documentation and analytical profiling for BRB phytochemicals. Importantly, the presence of BRB phytochemicals in the oral tumor tissues is essential to support our hypothesis that BRB bioactives are capable of modulating transcriptional profiles in these tissues in a manner that supports oral cancer chemopreventive strategies.
Hanahan and Weinberg describe a conceptual model (16) that forms the framework for multistep carcinogenesis (47,48). Among the complementary processes proposed are the “hallmarks” of sustained cell proliferation and cell death avoidance, and the “enabling characteristics” of a pro-inflammatory microenvironment and the loss of genomic integrity (16,19,49–51). Short-term administration of BRB troches to current OSCC patients demonstrated a molecular efficacy that reduced transcriptional biomarkers associated with these current and emerging fundamental characteristics. Chronic non-resolving inflammation is an intrinsic factor supporting diverse disease etiologies and is vital to the creation of high at-risk microenvironments that promote poor health and disease development. In the oral cavity, inflammatory responses are driven by innate immunity effectors and mediated by NFκB signaling. Accordingly, individuals exposed to tobacco smoke are an archetypal population to assess environmental inflammation-associated risk biomarkers and further define proinflammatory risk components within a multistep process leading to a poor oral health outcome. Extensive preclinical in vitro and animal studies have demonstrated the remarkable anti-inflammatory risk-reduction activities of BRBs (28,40). While the inhibition of cancer-specific biomarkers and endpoints (tumor reduction) is striking in preclinical models, studies demonstrating the potential of BRB phytochemicals to reduce inflammatory risk factors in high at-risk, but non-cancerous, human populations remain limited.
The acquisition of deregulated cell growth by cancer cells is a defining trait early in tumorigenesis. Cancer cells readily lose responsiveness to inhibitory proliferative signals, while gaining the capacity to enhance the contribution of growth promoting factors. In OSCCs, the over-expression and deregulated expression of the Epidermal Growth Factor Receptor (EGFR) is an early and common feature of neoplastic progression (16,52,53). The vast majority (85–100%) of head and neck squamous cell carcinomas (HNSCCs) over-express EGFR (54,55), and it has represented an important preventive and therapeutic target for several years (17). However, despite promising indicators of therapeutic effectiveness and increased survival times in some studies, mitigating the over-expressed status of EGFR alone using tyrosine kinase (e.g. erlotinib, gefitinib) or monoclonal antibody (cetuximab, panitumumab) inhibitors has demonstrated important but limited efficacy (17). While it is noteworthy that short-term administration of BRB troches significantly decreased EGFR expression in OSCC tissues, it is also important to acknowledge that the true value of this inhibition may be in conjunction with concurrent modifications in parallel signalling pathways (56).
Oral cancer progression is also marked by the ability of localized cells to survive in a chronic pro-inflammatory microenvironment, as well as evade immune surveillance and counter immune response mechanisms (16,19,21,51). Recently, we described how a BRB extract could modulate the immune suppressive activity of myeloid-derived suppressor cells often found upregulated in cancer patients, as well as inhibit regulatory T cell survival/proliferation and subsequent immune suppressive activities (57). Furthermore, NFκB is at the crossroads of inflammatory, immune response, proliferative, and apoptotic signalling events, and its over-expression fosters a tissue microenvironment promoting carcinogenesis. Consequently, it is fundamentally important to be able to arbitrate its expression in a manner that favors cancer prevention strategies. NFκB induces key components within the arachidonic acid metabolism pathway, including cyclooxygenase enzymes as well as cell survival factors such as B-cell CLL/lymphoma 2 (BCL2). The presence and over-expression of proinflammatory mediators, such as PTGS1 (COX-1), PTGS2 (COX-2), and NOS2 (iNOS) in the tumor microenvironment are consistent events within a deregulated chronic immune response. Abnormal NFκB signaling contributes to avoidance of immune surveillance, diminished apoptotic cell removal, and increased chronic oxidative stress as a consequence of elevated COX-1 and COX-2 activities. Short-term administration of BRB troches to OSCC patients was able to significantly decrease tumor expression levels of PTGS1, PTGS2, and NFKB1, and consequently provide a window of opportunity for an intervention strategy that reduces the chronic pro-inflammatory signalling environment intrinsic to the OSCC tumor microenvironment.
Apoptotic programmed cell death and control of the cell cycle Restriction Point are essential for maintaining cellular homeostasis and genomic integrity. Since progressively advanced tumorigenic cells are increasingly effective at avoiding these mechanisms, interventions that arrest cell cycle progression and allow DNA damage discrimination and removal of field-defective cells continue to represent promising preventive or therapeutic strategies. The Aurora kinase family of serine/threonine kinases play instrumental roles during mitotic assembly and progression. Deregulation of AURKA results in aberrant cyclin B/CDK1 control, defective G2/M checkpoint transition, chromosomal segregation instabilities, and malignant transformation, and over-expression is common in many solid tumors, including HNSCCs (48,50,58). Inappropriate over-expression of AURKA can direct p53 phosphorylation, obstruct its transactivation activities, and supersede cell proliferation and apoptotic control measures (59), while suppressing over-expression can reintroduce apoptotic selection and growth control (48). AURKA can also positively regulate NFκB activities by phosphorylating IκBα and Ras signalling via RalA phosphorylation. Evaluation of small molecule inhibitors MK8745 (60), MLN8237 (61,62), TC-28 (63), MLN8054 (47), VX680 (47) have demonstrated some efficacy in inhibiting AURKA-associated mitotic transition defects, but several have been subsequently discontinued following clinical evaluation (VX680, Phase II; MLN8054, Phase I) (64). In partnership with deregulated cell cycle checks, the loss of apoptotic control during carcinogenesis results in the inability to remove (and persistence of) genetically compromised cells. In OSCCs, deregulation of the apoptotic machinery is commonly associated with disease progression and tumor formation. Over-expression of pro-survival biomarkers BCL2 and BIRC5 (Survivin) are hallmark features of HNSCCs. BCL2 is the canonical member of the BCL2 family of intrinsic mitochondrial pro-survival proteins, while Survivin (BIRC5) is an inhibitor of apoptosis protein (IAP) that orchestrates cytoprotective activities by inhibiting caspase signaling events. Short-term administration of BRB troches was able to successfully down-regulate the expression of these pro-survival factors in a manner consistent with suppressing the “resisting cell death” hallmark of cancer (16). Therapeutic interventions have explored modulation of BCL2, mostly in hematopoietic malignancies, via BH3 antagonistic mimetics, small molecules, anti-mitotic agents, and natural compound derivatives [reviewed (65)], and Survivin using the small molecule inhibitor YM155. However, BH3 mimetic peptides demonstrate sub-optimal pharmacologic properties, anti-mitotic agents show a broad specificity, promote resistance selection, and demonstrate significant toxicities, while the targeted efficacy of YM155 remains under active investigation (66).
The ability of BRBs to modulate signaling events associated with neoplastic progression in pre-clinical and clinical models is well documented (28,40,67); however, the translation of these findings to human oral carcinogenesis is only beginning to be addressed. The current study demonstrates for the first time that short-term administration of BRB troches to current OSCC patients results in a significant reduction of keystone molecular features of oral cancer progression in a manner that favors chemoprevention. In addition, this predictive biomarker signature illustrates that there are patient-level variations that can assist in identifying potential “responder” and “non-responder” populations for molecular efficacy. Previously, Mallery et al. described potential responder categories following a BRB topical intervention in patients with premalignant lesions, in part by individual patient gene expression profile changes (68). Although it may seem that there should be an a priori argument for the benefit and safety of food-based chemopreventive strategies, the decision to participate in such interventions must be evaluated for patient-level responses in concert with epidemiologic population level data. Recently, Tsimberidou et al. (69) described the clinical utility of a personalized medicine approach using a non-randomized Phase I trial. Despite the latitudes allowed in patient tumor type and prior therapeutic interventions, the study was able to demonstrate the translational value in identifying molecular alterations and structuring subsequent patient care practices using molecular efficacy profiles. An overall favorable risk-to-benefit ratio can be greatly enhanced if appropriately (and inappropriately) targeted patient categories can be identified. It has been proposed previously that inter-patient variation in response to BRB-based interventions may correspond to differential profiles for absorption, processing, and localized tissue uptake of BRB phytochemicals (44). The complex microenvironmental parameters of the oral cavity include interaction networks between the pharmacologic agents contained in BRBs, BRB-derived metabolites, the host oral epithelium, resident bacterial biofilms, and insults such as alcohol and tobacco smoke exposure. Recently, emerging criteria have identified targetable populations demonstrating differential responses based upon oral microbiome-associated anthocyanin degradation capacities (70,71). Despite these variabilities and the intrinsic heterogeneity of cancerous lesions, 64.3% (18/28) OSCC tissues demonstrated a favorable response (reduced pro-carcinogenic gene expression) in all five molecular biomarkers in the BRB effects signature, while only 6/28 tumors showed a favorable response in ≤ 2 molecular biomarkers in the transcriptional signature. These “poor” response profiles were not associated with low self-reported compliance measurements or lack of BRB components when assessable.
Food-based aerodigestive cancer prevention strategies using BRBs have demonstrated both clinical and molecular efficacy in a series of early phase clinical trials, and have established favorable responses in both precursor and malignant epithelial lesions. Seminal translational studies by Stoner and his collaborators have demonstrated the consistent efficacy of BRBs and their bioactive components in esophagus and colon cancers (28,40). We demonstrate for the first time that short-term, low dose administration of BRB troches to current oral cancer patients is well tolerated, without significant adverse experiences, and that patients exhibit high levels of protocol compliance (97.2% self-reported, 100% analytic measurements) and study completion (79.2%). Furthermore, bioactive components of BRBs, namely the anthocyanins cyanidin-3-rutinoside, cyanidin-3-xylosylrutinoside, cyanidin-3-glucoside, and cyanidin-3-sambubioside, were retained and readily detected in the target tumor tissues despite a pre-surgical fasting period in excess of 8 hours. BRB troche administration resulted in a significant reduction of canonical biomarkers of oral carcinogenesis in malignant tissues and defined a BRB signature of molecular exposure and efficacy incorporating the AURKA, BIRC5, EGFR, NFKB1, and PTGS1 genes. These transcriptional biomarkers represent established key mediators in epithelial carcinogenesis and rational targets for chemoprevention strategies. BRBs represent a food-based approach to cancer prevention that leverages favorable bioactivities of well-known compounds such as anthocyanins, as well as potential interactive effects of other phenolic components, including ellagic acid and quercetin, to provide a strategy to reduce molecular risk factors for oral cancer.
Supplementary Material
Acknowledgments
Financial Support: NIH NIDCR R21 DE016361 (T.J. Knobloch, B.C. Casto, C.M. Weghorst), NIH NIDCR T32 DE014320 (J.M. Ferguson, B.M. Warner, C.M. Weghorst), NIH NCI P30 CA016058 (T.J. Knobloch, L.K. Uhrig, D.K. Pearl, B.C. Casto, S.K. Clinton, C.L. Sardo-Molmenti, K. Riedl, S. J. Schwartz, Y. Vodovotz, D.E. Schuller, E. Ozer, A. Agrawal, C.M. Weghorst).
Footnotes
Conflict of Interest Statement: “The authors state that there are no conflicts of interests to disclose.”
References
- 1.Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ. 2005;83:661–669. [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Petersen PE. Oral cancer prevention and control--the approach of the World Health Organization. Oral Oncology. 2009;45:454–460. doi: 10.1016/j.oraloncology.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Coelho KR. Challenges of the oral cancer burden in India. J Cancer Epidemiol. 2012;2012:1–17. doi: 10.1155/2012/701932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray F, Ren J-S, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2012;132:1133–1145. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 6.Hashibe M, Brennan P, Chuang S-C, Boccia S, Castellsague X, Chen C, et al. Interaction between Tobacco and Alcohol Use and the Risk of Head and Neck Cancer: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:541–550. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein BY, Chang S-C, Hashibe M, La Vecchia C, Zhang Z-F. Alcohol consumption and cancers of the oral cavity and pharynx from 1988 to 2009: an update. Eur J Cancer Prev. 2010;19:431–465. doi: 10.1097/CEJ.0b013e32833d936d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillison ML, Broutian T, Pickard RKL, Tong Z-Y, Xiao W, Kahle L, et al. Prevalence of Oral HPV Infection in the United States, 2009–2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, et al. Alcohol Drinking in Never Users of Tobacco, Cigarette Smoking in Never Drinkers, and the Risk of Head and Neck Cancer: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 10.Hillbertz NS, Hirsch J-M, Jalouli J, Jalouli MM, Sand L. Viral and molecular aspects of oral cancer. Anticancer Res. 2012;32:4201–4212. [PubMed] [Google Scholar]
- 11.Chuang S-C, Jenab M, Heck JE, Bosetti C, Talamini R, Matsuo K, et al. Diet and the risk of head and neck cancer: a pooled analysis in the INHANCE consortium. Cancer Causes Control. 2012;23:69–88. doi: 10.1007/s10552-011-9857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tezal M, Sullivan MA, Hyland A, Marshall JR, Stoler D, Reid ME, et al. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:2406–2412. doi: 10.1158/1055-9965.EPI-09-0334. [DOI] [PubMed] [Google Scholar]
- 13.Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends in Genetics. 1993;9:138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- 14.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 15.Califano JA, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Can Res. 1996;56:2488–2492. [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Tsien CI, Nyati MK, Ahsan A, Ramanand SG, Chepeha DB, Worden FP, et al. Effect of erlotinib on epidermal growth factor receptor and downstream signaling in oral cavity squamous cell carcinoma. Head Neck. 2012;35:1–8. doi: 10.1002/hed.23128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini P-L. 2011: the immune hallmarks of cancer. Cancer Immunol, Immunother. 2011;60:319–326. doi: 10.1007/s00262-010-0968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Visser KE, Coussens LM. The inflammatory tumor microenvironment and its impact on cancer development. Contrib Microbiol. 2006;13:118–137. doi: 10.1159/000092969. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 22.Anand P, Kunnumakkara AB, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sporn MB. Approaches to prevention of epithelial cancer during the preneoplastic period. Can Res. 1976;36:2699–2702. [PubMed] [Google Scholar]
- 24.Wattenberg LW. Chemoprevention of Cancer. Can Res. 1985;45:1–8. [PubMed] [Google Scholar]
- 25.Greenwald P, Kelloff G, Burch-Whitman C, Kramer BS. Chemoprevention. CA Cancer J Clin. 1995;45:31–49. doi: 10.3322/canjclin.45.1.31. [DOI] [PubMed] [Google Scholar]
- 26.Alberts DS, Colvin OM, Conney AH, Ernster VL, Garber JE, Greenwald P, et al. Prevention of cancer in the next millennium: Report of the Chemoprevention Working Group to the American Association for Cancer Research. Can Res. 1999;59:4743–4758. [PubMed] [Google Scholar]
- 27.Mukhtar H. Chemoprevention: making it a success story for controlling human cancer. Cancer Lett. 2012;326:123–127. doi: 10.1016/j.canlet.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Stoner GD, Wang L-S, Zikri N, Chen T, Hecht SS, Huang C, et al. Cancer prevention with freeze-dried berries and berry components. Semin Cancer Biol. 2007;17:403–410. doi: 10.1016/j.semcancer.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YS, Young MR, Bobe G, Colburn NH, Milner JA. Bioactive Food Components, Inflammatory Targets, and Cancer Prevention. Cancer Prev Res. 2009;2:200–208. doi: 10.1158/1940-6207.CAPR-08-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis CD. Nutritional Interactions: Credentialing of Molecular Targets for Cancer Prevention. Exp Biol Med (Maywood) 2007;232:176–183. [PubMed] [Google Scholar]
- 31.Surh Y-J. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 32.Stoner GD, Sardo C, Apseloff G, Mullet D, Wargo W, Pound V, et al. Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. J Clin Pharmacol. 2005;45:1153–1164. doi: 10.1177/0091270005279636. [DOI] [PubMed] [Google Scholar]
- 33.Kresty LA, Frankel WL, Hammond CD, Baird ME, Mele JM, Stoner GD, et al. Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: interim results show berries modulate markers of oxidative stress in Barrett’s esophagus patients. Nutr Cancer. 2006;54:148–156. doi: 10.1207/s15327914nc5401_15. [DOI] [PubMed] [Google Scholar]
- 34.Casto BC, Kresty LA, Kraly CL, Pearl DK, Knobloch TJ, Schut HA, et al. Chemoprevention of oral cancer by black raspberries. Anticancer Res. 2002;22:4005–4015. [PubMed] [Google Scholar]
- 35.Tian Q, Giusti MM, Stoner GD, Schwartz SJ. Urinary excretion of black raspberry (Rubus occidentalis) anthocyanins and their metabolites. J Agric Food Chem. 2006;54:1467–1472. doi: 10.1021/jf052367z. [DOI] [PubMed] [Google Scholar]
- 36.Mallery SR, Stoner GD, Larsen PE, Fields HW, Rodrigo KA, Schwartz SJ, et al. Formulation and in-vitro and in-vivo evaluation of a mucoadhesive gel containing freeze dried black raspberries: implications for oral cancer chemoprevention. Pharm Res. 2007;24:728–737. doi: 10.1007/s11095-006-9192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poi MJ, Knobloch TJ, Sears MT, Warner BM, Uhrig LK, Weghorst CM, et al. Alterations in RD INK4/ARF-mediated en bloc regulation of the INK4-ARF locus in human squamous cell carcinoma of the head and neck. Mol Carcinog. 2015;54:532–542. doi: 10.1002/mc.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poi MJ, Knobloch TJ, Sears MT, Uhrig LK, Warner BM, Weghorst CM, et al. Coordinated expression of cyclin-dependent kinase-4 and its regulators in human oral tumors. Anticancer Res. 2014;34:3285–3292. [PMC free article] [PubMed] [Google Scholar]
- 39.Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 40.Stoner GD. Foodstuffs for Preventing Cancer: The Preclinical and Clinical Development of Berries. Cancer Prev Res. 2009;2:187–194. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brenner DE, Hawk E. Trials and Tribulations of Interrogating Biomarkers to Define Efficacy of Cancer Risk Reductive Interventions. Cancer Prev Res. 2013;6:71–73. doi: 10.1158/1940-6207.CAPR-12-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 43.Braakhuis BJM, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Can Res. 2003;63:1727–1730. [PubMed] [Google Scholar]
- 44.Ugalde CM, Liu Z, Ren C, Chan KK, Rodrigo KA, Ling Y, et al. Distribution of anthocyanins delivered from a bioadhesive black raspberry gel following topical intraoral application in normal healthy volunteers. Pharm Res. 2009;26:977–986. doi: 10.1007/s11095-008-9806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mallery SR, Tong M, Shumway BS, Curran AE, Larsen PE, Ness GM, et al. Topical application of a mucoadhesive freeze-dried black raspberry gel induces clinical and histologic regression and reduces loss of heterozygosity events in premalignant oral intraepithelial lesions: results from a multicentered, placebo-controlled clinical trial. Clin Cancer Res. 2014;20:1910–1924. doi: 10.1158/1078-0432.CCR-13-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shumway BS, Kresty LA, Larsen PE, Zwick JC, Lu B, Fields HW, et al. Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clin Cancer Res. 2008;14:2421–2430. doi: 10.1158/1078-0432.CCR-07-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manfredi MG, Ecsedy JA, Meetze KA, Balani SK, Burenkova O, Chen W, et al. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc Natl Acad Sci USA. 2007;104:4106–4111. doi: 10.1073/pnas.0608798104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazumdar A, Henderson YC, El-Naggar AK, Sen S, Clayman GL. Aurora kinase A inhibition and paclitaxel as targeted combination therapy for head and neck squamous cell carcinoma. Head Neck. 2009;31:625–634. doi: 10.1002/hed.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gizatullin F, Yao Y, Kung V, Harding MW, Loda M, Shapiro GI. The Aurora kinase inhibitor VX-680 induces endoreduplication and apoptosis preferentially in cells with compromised p53-dependent postmitotic checkpoint function. Can Res. 2006;66:7668–7677. doi: 10.1158/0008-5472.CAN-05-3353. [DOI] [PubMed] [Google Scholar]
- 50.Kao S-Y, Chen Y-P, Tu H-F, Liu C-J, Yu A-H, Wu C-H, et al. Nuclear STK15 expression is associated with aggressive behaviour of oral carcinoma cells in vivo and in vitro. J Pathol. 2010;222:99–109. doi: 10.1002/path.2737. [DOI] [PubMed] [Google Scholar]
- 51.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 52.Taoudi Benchekroun M, Saintigny P, Thomas SM, El-Naggar AK, Papadimitrakopoulou V, Ren H, et al. Epidermal Growth Factor Receptor Expression and Gene Copy Number in the Risk of Oral Cancer. Cancer Prev Res. 2010;3:800–809. doi: 10.1158/1940-6207.CAPR-09-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shin DM, Ro JY, Hong WK, Hittelman WN. Dysregulation of epidermal growth factor receptor expression in premalignant lesions during head and neck tumorigenesis. Can Res. 1994;54:3153–3159. [PubMed] [Google Scholar]
- 54.Ang KK, Berkey BA, Tu X, Zhang H-Z, Katz R, Hammond EH, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Can Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 55.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 56.Baba Y, Fujii M, Tokumaru Y, Kato Y. Present and Future of EGFR Inhibitors for Head and Neck Squamous Cell Cancer. J Oncol. 2012;2012:986725. doi: 10.1155/2012/986725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mace TA, King SA, Ameen Z, Elnaggar O, Young G, Riedl KM, et al. Bioactive compounds or metabolites from black raspberries modulate T lymphocyte proliferation, myeloid cell differentiation and Jak/STAT signaling. Cancer Immunoll Immunother. 2014;63:889–900. doi: 10.1007/s00262-014-1564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reiter R, Gais P, Jütting U, Steuer-Vogt MK, Pickhard A, Bink K, et al. Aurora kinase A messenger RNA overexpression is correlated with tumor progression and shortened survival in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:5136–5141. doi: 10.1158/1078-0432.CCR-05-1650. [DOI] [PubMed] [Google Scholar]
- 59.Iacono Lo M, Monica V, Saviozzi S, Ceppi P, Bracco E, Papotti M, et al. Aurora Kinase A expression is associated with lung cancer histological-subtypes and with tumor de-differentiation. J Transl Med. 2011;9:100. doi: 10.1186/1479-5876-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nair JSJ, Ho ALA, Schwartz GKG. The induction of polyploidy or apoptosis by the Aurora A kinase inhibitor MK8745 is p53-dependent. Cell Cycle. 2012;11:807–817. doi: 10.4161/cc.11.4.19323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maris JM, Morton CL, Gorlick R, Kolb EA, Lock R, Carol H, et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP) Pediatr Blood Cancer. 2010;55:26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carol H, Boehm I, Reynolds CP, Kang MH, Maris JM, Morton CL, et al. Efficacy and pharmacokinetic/pharmacodynamic evaluation of the Aurora kinase A inhibitor MLN8237 against preclinical models of pediatric cancer. Cancer Chemother Pharmacol. 2011;68:1291–1304. doi: 10.1007/s00280-011-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sardon T, Cottin T, Xu J, Giannis A, Vernos I. Development and biological evaluation of a novel aurora A kinase inhibitor. Chembiochem. 2009;10:464–478. doi: 10.1002/cbic.200800600. [DOI] [PubMed] [Google Scholar]
- 64.Lens SMA, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10:825–841. doi: 10.1038/nrc2964. [DOI] [PubMed] [Google Scholar]
- 65.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- 66.Holmes D. Cancer drug’s survivin suppression called into question. Nat Med. 2012;18:842–843. doi: 10.1038/nm0612-842b. [DOI] [PubMed] [Google Scholar]
- 67.Seeram NP. Berry fruits for cancer prevention: current status and future prospects. J Agric Food Chem. 2008;56:630–635. doi: 10.1021/jf072504n. [DOI] [PubMed] [Google Scholar]
- 68.Mallery SR, Zwick JC, Pei P, Tong M, Larsen PE, Shumway BS, et al. Topical application of a bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein in human premalignant oral lesions. Can Res. 2008;68:4945–4957. doi: 10.1158/0008-5472.CAN-08-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsimberidou AM, Iskander NG, Hong DS, Wheler JJ, Falchook GS, Fu S, et al. Personalized Medicine in a Phase I Clinical Trials Program: The MD Anderson Cancer Center Initiative. Clin Can Res. 2012;18:6373–6383. doi: 10.1158/1078-0432.CCR-12-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamonpatana K, Giusti MM, Chitchumroonchokchai C, MorenoCruz M, Riedl KM, Kumar P, et al. Susceptibility of anthocyanins to ex vivo degradation in human saliva. Food Chem. 2012;135:738–747. doi: 10.1016/j.foodchem.2012.04.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamonpatana K, Failla ML, Kumar PS, Giusti MM. Anthocyanin structure determines susceptibility to microbial degradation and bioavailability to the buccal mucosa. J Agric Food Chem. 2014;62:6903–6910. doi: 10.1021/jf405180k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.