Abstract
The innate immune cell sensor leucine-rich–containing family, pyrin domain containing 3 (NLRP3) inflammasome controls the activation of caspase-1, and the release of proinflammatory cytokines interleukin (IL)-1β and IL-18. The NLRP3 inflammasome is implicated in adipose tissue inflammation and the pathogenesis of insulin resistance. Herein, we tested the hypothesis that adipose tissue inflammation and NLRP3 inflammasome are linked to the downregulation of subcutaneous adipose tissue (SAT) adipogenesis/lipogenesis in obese adolescents with altered abdominal fat partitioning. We performed abdominal SAT biopsies on 58 obese adolescents and grouped them by MRI-derived visceral fat to visceral adipose tissue (VAT) plus SAT (VAT/VAT+SAT) ratio (cutoff 0.11). Adolescents with a high VAT/VAT+SAT ratio showed higher SAT macrophage infiltration and higher expression of the NLRP3 inflammasome–related genes (i.e., TLR4, NLRP3, IL1B, and CASP1). The increase in inflammation markers was paralleled by a decrease in genes related to insulin sensitivity (ADIPOQ, GLUT4, PPARG2, and SIRT1) and lipogenesis (SREBP1c, ACC, LPL, and FASN). Furthermore, SAT ceramide concentrations correlated with the expression of CASP1 and IL1B. Infiltration of macrophages and upregulation of the NLRP3 inflammasome together with the associated high ceramide content in the plasma and SAT of obese adolescents with a high VAT/VAT+SAT may contribute to the limited expansion of the subcutaneous abdominal adipose depot and the development of insulin resistance.
Introduction
Increasing evidence supports a crucial role of the adipose tissue in initiating the chronic inflammatory disease known as obesity-related insulin resistance (1–4). Important metabolic effects have been recently attributed to the protein complex known as the “inflammasome” (5). There are several different types of inflammasomes, as follows: activation of a particular type is dependent on the specific agonist involved, but the end result of every inflammasome assembly and activation is the maturation of pro–interleukin (IL)-1β and pro–IL-18 into their active forms via the enzyme caspase-1 (5–10). Both animal and human studies (7–9) implicate the inflammasome in the development of obesity itself, as well as its associated chronic inflammatory state and manifestations of the metabolic syndrome. The inflammasome containing leucine-rich–containing family, pyrin domain containing 3 (NLRP3) proteins have been reported to sense a vast array of nonmicrobial molecules, for example, ceramides (8,10), fatty acids, or glucose (9,10). Genetic deletion or pharmacological inhibition of the NLRP3 inflammasome results in reduced weight gain and adipocyte size, protection from obesity-induced insulin resistance and pancreatic damage, lower hepatic triglyceride levels, and reduced macrophage infiltration and overall adipose tissue inflammation in murine models of diet-induced obesity (9,10). In obese individuals with type 2 diabetes, weight loss leads to reduced activation of the inflammasome in adipose tissue (8).
Recently, we described a phenotype of obese adolescents with a high visceral and low subcutaneous abdominal fat distribution (i.e., insulin resistant) who have increased ectopic fat accumulation and show a low adipogenic/lipogenic capacity in the subcutaneous adipose tissue (SAT) (11). To understand whether the NLRP3 inflammasome machinery is involved in the inability of the SAT to store excess fat, we combined lipidomics to study the effect of ceramides as danger signals and transcriptomics to understand downstream effects of inflammasome activation in SAT biopsy samples from obese adolescents with an unhealthy abdominal fat distribution versus equally obese adolescents with a more healthy abdominal fat distribution.
Research Design and Methods
Subjects
The Yale Pathophysiology of Type 2 Diabetes in Obese Youth Study is a long-term project aimed at examining early alterations in glucose metabolism in relation to fat patterning in obese adolescents. As part of this study, all subjects undergo a detailed assessment of abdominal fat distribution by MRI. As previously described (12), we found that the metabolic profile worsens with the increasing visceral adipose tissue (VAT) to VAT plus SAT (VAT/VAT+SAT) ratio. Given the distribution of the VAT/VAT+SAT ratio obtained in our entire multiethnic cohort of ∼500 adolescents, we used the 50th percentile (0.11) as a cutoff value to recruit and enroll subjects in the current biopsy study. From this cohort, 58 obese adolescents agreed to have a subcutaneous periumbilical adipose tissue biopsy and were divided into the following two groups: low (<0.11) and high (>0.11) VAT/VAT+SAT ratio. Their clinical characteristics are described in Table 1. Thirty-eight of these subjects were reported in a previous study (11). None of the subjects were receiving treatment with any medications nor had any known disease. The nature and potential risks of the study were explained to all subjects before obtaining their written informed consent. The study was approved by the ethics committees of the Yale University Hospital.
Table 1.
Metabolic characteristics of the obese adolescents (n = 58)
| Low VAT/VAT+SAT ratio (n = 29) (0.08 ± 0.02) | High VAT/VAT+SAT ratio (n = 29) (0.15 ± 0.04) | P value | Adjusted P value (age, race, sex, BMI) | |
|---|---|---|---|---|
| Anthropometric | ||||
| Age (years) | 15.3 ± 3.1 | 15.1 ± 3.0 | 0.795 | |
| Sex (female/male) | 22/7 | 16/13 | 0.097# | |
| Race (C/AA/H) | 6/16/7 | 15/8/6 | 0.014# | |
| BMI (kg/m2) | 37.3 ± 8.6 | 37.3 ± 6.6 | 0.991 | |
| BMI z-score | 2.3 ± 0.5 | 2.3 ± 0.3 | 0.499 | 0.236 |
| % Fat | 42.0 ± 7.0 | 39.6 ± 6.4 | 0.260 | 0.143 |
| Abdominal fat distribution | ||||
| Visceral fat (cm2) | 54.0 ± 27.6 | 90.4 ± 29.4 | 0.000 | 0.000 |
| Subcutaneous fat (cm2) | 610.2 ± 246.8 | 546.7 ± 197.6 | 0.284 | 0.016 |
| VAT/VAT+SAT ratio | 0.08 ± 0.02 | 0.15 ± 0.04 | 0.000 | 0.000 |
| HFF (%) | 2.5 ± 6.6 | 10.5 ± 12.0 | 0.003 | 0.057 |
| Metabolic | ||||
| Insulin sensitivity | ||||
| Matsuda index (WBISI) | 2.4 ± 1.7 (n = 28) | 1.6 ± 1.0 (n = 27) | 0.050 | 0.008* |
| Glucose disposal rate (mg/kg LBM · min) | 10.7 ± 5.4 (n = 23) | 7.6 ± 3.4 (n = 21) | 0.025 | 0.037* |
| Adipokines and lipids | ||||
| Adiponectin (μg/mL) | 9.4 ± 5.1 | 6.2 ± 3.4 | 0.010 | 0.018 |
| Leptin (ng/mL) | 35.8 ± 16.3 | 34.6 ± 17.5 | 0.804 | 0.600 |
| HDL (mg/dL) | 47.6 ± 9.7 | 39.3 ± 8.0 | 0.001 | 0.010 |
| TG (mg/dL) | 82.8 ± 59.9 | 132.9 ± 88.8 | 0.020 | 0.426 |
| FFA (μmol/L/L) | 561.7 ± 166.2 | 616.5 ± 177.9 | 0.267 | 0.312 |
Metabolic characteristics of the obese adolescents undergoing fat biopsy (n = 58), with values reported as the mean ± SD. AA, African Americans; C, Caucasians; FFA, free fatty acid; H, Hispanics; HFF, hepatic fat fraction; LBM, lean body mass; TG, triglycerides; WBISI, whole-body insulin sensitivity index.
#χ2 test, ANCOVA adjusted for age, sex, race, and BMI.
*Values are log transformed. Values in bold are P < 0.05.
Metabolic Studies
Oral Glucose Tolerance Test
All subjects were invited to the Yale Center for Clinical Investigation for an oral glucose tolerance test at 8:00 a.m. after an overnight fast. Baseline fasting blood samples were obtained with the use of an indwelling venous line for the measurement of glucose, insulin, lipid profile, free fatty acids, adiponectin, and leptin levels, as previously described (12). The composite whole-body insulin sensitivity index was calculated using the formula described by Matsuda and DeFronzo (13).
The Hyperinsulinemic-Euglycemic Clamp
In the morning at 8:00 a.m., after an overnight fast of 10–12 h, insulin sensitivity was measured with a hyperinsulinemic-euglycemic clamp, during which time insulin was administered as a prime continuous infusion of 80 mU/m2/min for 120 min (11).
Total Body Composition and Abdominal Fat Distribution
Whole-body composition was measured by DEXA with a Hologic scanner (Boston, MA).
Abdominal MRI studies were performed on a GE or Siemens Sonata 1.5-Tesla system (12). Five slices, obtained at the level of the L4/L5 disc space, were analyzed for each subject, as previously reported (12).
Liver Fat Assessment: Fast-Gradient MRI
Hepatic fat fraction, an estimate of the percentage of fat in the liver, was measured by fast-gradient MRI (14), and we validated this method against proton MRS (1H-MRS) and found a strong correlation (r = 0.93, P < 0.001) (15).
Adipocyte Size Distribution
The 1- to 2-g samples of SAT were obtained via surgical excision inferior to the umbilicus after administration of 0.25 lidocaine with adrenaline (epinephrine) for local anesthesia, from which two 20- to 30-mg samples were used immediately for adipose cell size distribution analysis by osmium fixation (Multisizer 3; Beckman Coulter, Miami, FL), using a curve-fitting analysis technique that has been described previously (11).
Quantitative Real-Time PCR
Total RNA was isolated using TRIzol reagent and was further purified using an RNeasy kit (Qiagen, Valencia, CA). The quantification of several differentially expressed genes by real-time RT-PCR was performed using an ABI 7000 Sequence Detection System (Applied Biosystems, Foster City, CA), as described previously (11).
Immunohistochemistry
Subcutaneous biopsy samples from 20 subjects were used for immunohistochemical staining, and CD68 and CD115 were used as a marker for total macrophages. Staining was performed using a standard protocol on sections from formalin-fixed, paraffin-embedded tissue blocks. Serial sections were deparaffinized, rehydrated, and treated with 10 mmol/L citrate buffer (pH 6.0) in a steamer, and then endogenous peroxidase was blocked with 3% H2O2. The sections were then incubated for 1 h at room temperature with primary antibodies, mouse monoclonal anti-CD68 (Ab-3 clone KP1; Thermo Fisher Scientific, Fremont, CA). After rinsing in Tris-buffered saline solution containing 0.25% Triton X-100 (pH 7.2), sections were incubated with ENVISION+ (K4007 or K4011; DAKO, Carpinteria, CA), followed by visualization with 3.3′-diaminobenzidine tetrachloride (DAKO). All sections were counterstained with GILL III hematoxylin, dehydrated, and coverslipped with a resinous mounting media. For each subject, the number of macrophages (identified as CD68+ cells) and the number of crown-like structures (CLSs) within 10 regions of interest were counted by two independent observers using a light microscope and normalized to the number of counted adipocytes.
Lipidomics
Lipidomic analysis from SAT samples from 44 subjects were measured via liquid chromatography–tandem mass spectrometry (16). Briefly, flash-frozen adipose tissue samples (40 mg) were homogenized in 2.0 mL of organic extraction solvent (isopropanol/water/ethyl acetate, 25:10:65 [v/v/v]). Immediately afterward, 20 µL of internal standard solution was added (AL Ceramide/Sphingoid Internal Standard Mixture II diluted 1:4 in ethanol; Avanti Polar Lipids). The mixture was vortexed and sonicated in an ultrasonic bath for 40 min at 40°C. Then samples were allowed to reach room temperature and 1.5 mL of aqueous sucrose buffer (25 mmol/L, pH 7.0). Two-phase liquid extraction was performed, the supernatant was transferred to a new tube, and the pellet was re-extracted. Supernatants were combined and evaporated under nitrogen. The dried residue was reconstituted in 200 µL of high-performance liquid chromatography solvent B (methanol/formic acid, 99:1, v/v, containing 5 mmol/L ammonium formate) for liquid chromatography–tandem mass spectrometry analysis. Lipid separation was achieved on a 2.1 (internal diameter) × 150 mm Kinetex C8, 2.6-μm core-shell particle (Phenomenex, Torrance, CA) column. Plasma sphingolipids were quantified using a similar methodology requiring 50 µL of plasma.
Analytical Methods
Plasma glucose levels were measured using the YSI 2700 STAT Analyzer (Yellow Springs Instruments) and lipid levels were measured using an autoanalyzer (model 747–200; Roche-Hitachi). Plasma insulin, adiponectin, and leptin levels were measured with a radioimmunoassay (Linco, St. Charles, MO).
Statistical Analysis
Student unpaired t tests or the χ2 tests (for categorical variables) were used to compare the clinical and laboratory characteristics between the two groups. Potential confounders (sex, age, race, and BMI) were entered in an ANOVA model in which the metabolic and cell size parameters were dependent variables and the VAT/VAT+SAT ratio group was the primary grouping variable, nonnormally distributed parameters were log transformed before entry into the model. Comparisons of relative gene expression as quantitated by RT-PCR were compared using the Mann-Whitney test. These analyses as well as Pearson correlation and multiple linear regression analyses were performed using SPSS version 19 (SPSS, Chicago, IL). For all analyses, a P value <0.05 was considered statistically significant.
Results
Anthropometric, Clinical, and Metabolic Characteristics of the Study Population
As shown in Table 1, sex, age and BMI were similar in the two groups, but race differed; therefore, when comparing the two groups, we adjusted for all four parameters (see adjusted P value). As expected, the high VAT/VAT+SAT ratio group not only had a higher amount of VAT and a lower amount of abdominal SAT but also a higher hepatic fat content and was significantly more insulin resistant than the low VAT/VAT+SAT group (Table 1). Additionally, levels for adiponectin, HDL, and plasma triglycerides were significantly different between the groups (Table 1).
Inflammation Is Increased in the SAT of Obese Adolescents With a High VAT/VAT+SAT Ratio
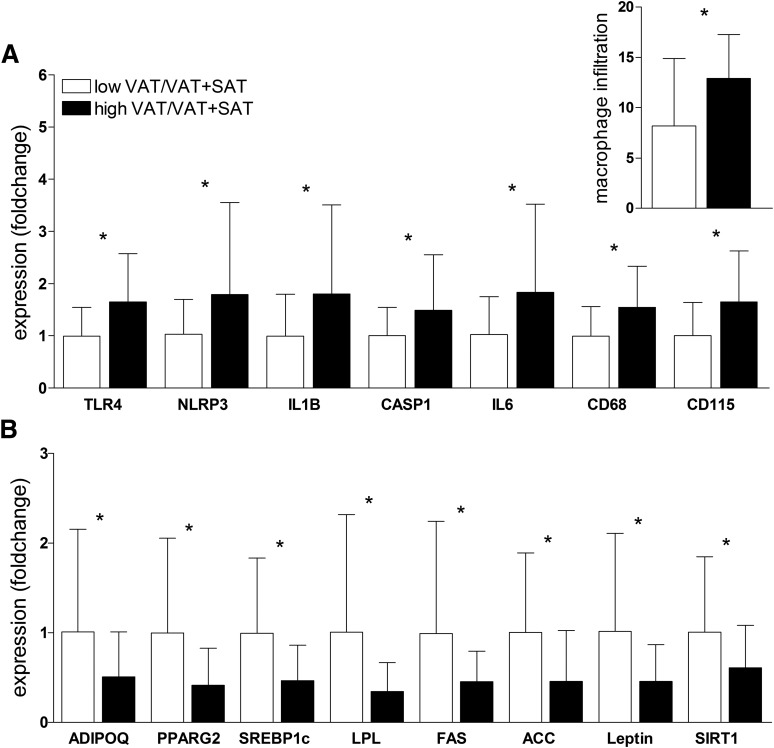
On the basis of the results from GeneChip analyses (11), which revealed significantly higher expression of NLRP3 in obese adolescents with a high ratio (n = 18) versus a low ratio (n = 16) (fold change 1.2, P = 0.02), we measured mRNA levels of NLRP3, as well as other components of the NLRP3 inflammasome machinery (IL1B, CASP1, and TLR4), in our cohort of 58 subjects by quantitative RT-PCR. The expression of TLR4 (P = 0.04), NLRP3 (P = 0.03), IL1B (P = 0.03), and CASP1 (P = 0.03) was significantly increased in the high ratio group (Fig. 1A). Notably, immunohistochemistry of the abdominal SAT biopsy samples showed significantly higher macrophage infiltration in the high ratio group (P = 0.01) (Fig. 1, inset), which was confirmed by measuring the expression of the macrophage markers CD68 (P = 0.01) and CD115 (P = 0.02) (Fig. 1A). Macrophage infiltration inversely correlated with insulin sensitivity measured by the clamp (immunohistochemistry data: r = −0.451, P = 0.01; quantitative RT-PCR data: r = −0.389, P = 0.03). An example of the difference in macrophage infiltration between obese insulin-resistant and obese insulin-sensitive subjects is shown in Supplementary Figs. 1 and 2. Not only the total macrophage count but also the number of CLSs was significantly different between the groups (P = 0.045). Although the number of CLSs in the low ratio group was only 1.3, the number was significantly increased in the high ratio group to an average of 5.2.
Figure 1.
A: Increased inflammatory gene expression and macrophage infiltration in abdominal SAT of the high VAT/VAT+SAT group. B: Decreased lipogenic gene expression in abdominal SAT of the high VAT/VAT+SAT group. Subcutaneous expression of specific genes was normalized to the expression of 18S ribosomal RNA and based on the expression of a human control adipose tissue (2ΔΔCt). Expression values of the low VAT/VAT+SAT group (white bars) were set to 1, and values for the high VAT/VAT+SAT group (black bars) are expressed as fold changes compared with 1 (mean ± SD, n = 58). Macrophage infiltration (inset in A) was assessed by immunohistochemistry. *Indicates that the t test between the two groups was significant at the <0.05 level.
Furthermore, SIRT1 expression was also significantly lower in the high ratio group and, as reported earlier (17), significantly correlated with macrophage infiltration in the SAT (r = −0.404, P = 0.00).
While the expression of IL6 in the SAT was significantly different between the groups (P = 0.01), no difference was observed for TNFα and MCP1 (data not shown). Of note, serum levels for inflammation markers IL-6, tumor necrosis factor-α (TNF-α), MCP1, and C-reactive protein were not significantly elevated in the high ratio group.
In contrast with the upregulation of the inflammasome genes, the high ratio group showed a lower expression of adipogenic and lipogenic genes in the SAT (Fig. 1B), as reported previously in a smaller cohort (11).
Inflammation in the SAT Is Predictive of the High VAT/VAT+SAT Ratio
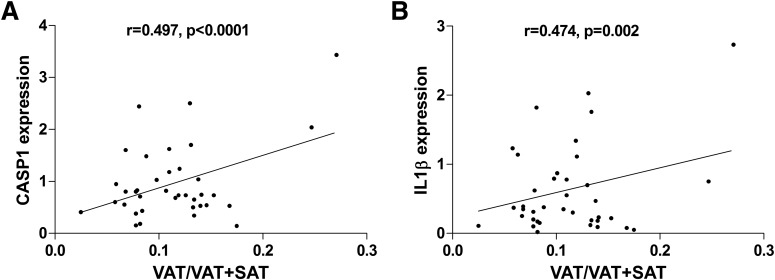
Correlation analyses between SAT inflammation gene markers and VAT/VAT+SAT ratio were significant for CASP1 (r = 0.497, P = 0.00) and IL1B (r = 0.474, P = 0.002) and for the total macrophage marker CD68 (r = 0.348, P = 0.01) (Fig. 2). Because the expression of these inflammation markers correlated significantly with the abdominal fat distribution, we ran stepwise regression models to identify the best predictor of VAT/VAT+SAT ratio among the inflammatory markers measured in SAT. The model that predicted VAT/VAT+SAT ratio in the best possible way (0.562; P = 0.00) included CD68 (β = 0.363, P = 0.00), CASP1 (β = 0.388, P = 0.00), and race (β = −0.420, P = 0.00) as predictors, whereas the other variables (age; sex; BMI; and expression of TLR4, IL6, NLRP3, and IL1B) were excluded. The best predictor of an unhealthy abdominal fat distribution, excluding all other variables, was CASP1, with an r2 of 0.287 (P = 0.00).
Figure 2.
Positive correlation of VAT/VAT+SAT ratio to subcutaneous adipose expression of caspase-1 (A) and IL-1β (B).
Ceramide Content Is Higher in the Plasma and SAT of Obese Adolescents With High VAT/VAT+SAT Ratios
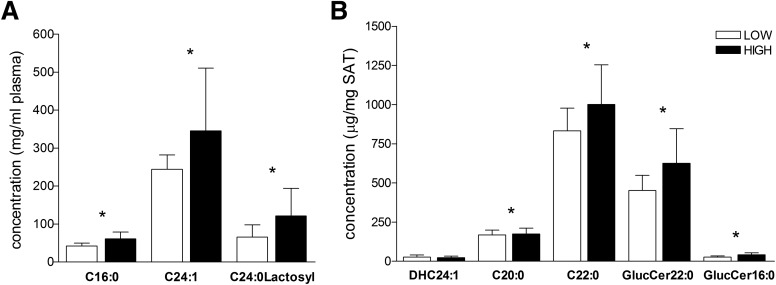
Because ceramides have recently been identified as activators of the NLRP3 inflammasome (8), we measured a number of different ceramide species in SAT in a subset of 44 obese adolescents (27 low ratio group vs. 17 high ratio group). In addition, in 21 of these subjects we analyzed plasma ceramide levels from samples obtained on the same day of the biopsy. Multivariate analysis of total plasma ceramide levels showed a significant difference between the high and the low ratio groups (MANOVA, P = 0.03). Specifically, levels of ceramide subtypes C24:0-lactosyl (P = 0.04), C24:1 (P = 0.03), and C16:0 (P = 0.00) were higher in the high ratio group (Fig. 3A).
Figure 3.
Ceramide level in abdominal SAT of obese adolescents. A: Level of plasma ceramides is significantly different between low ratio (white bars, n = 4) and high ratio (black bars, n = 17) group (mean ± SD). *Indicates that the t test between the two groups was significant at the <0.05 level. B: Level of tissue ceramides is significantly different between low ratio (white bars, n = 27) and high ratio (black bars, n = 17) groups (mean ± SD). *Indicates that the t test between the two groups was significant at the <0.05 level.
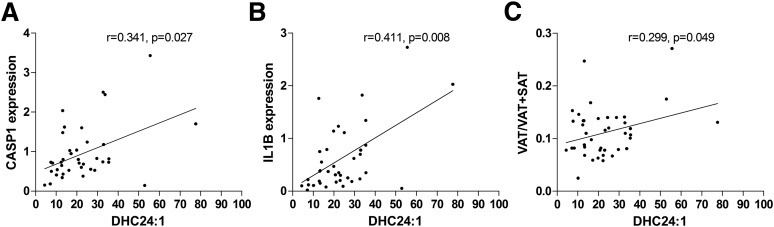
Furthermore, we also found a higher level of SAT ceramides in the high VAT/VAT+SAT ratio group, with C20:0 (P = 0.02), C22:0 (P = 0.03), GlucCer-22:0 (P = 0.047), and GlucCer-16:0 (P = 0.006) reaching significance (Fig. 3B). In addition, levels of sphingosine (P = 0.01), sphinganine (P = 0.008), hexadecanoyl sphingomyelin (SM16:0) (P = 0.006), and N-nervonoylsphingomyelin (SM24:1) (P = 0.03) were also significantly higher in SAT from subjects in the high ratio group. The ratio of plasma ceramides to tissue ceramides was significantly different between the two groups (Supplementary Fig. 3). Levels of ceramide DHC24:1 (r = 0.496, P = 0.02) in SAT correlated with VAT/VAT+SAT ratio, whereas C20:0 and C22:0 fell short of significance. Significantly positive correlations were found between the high concentrations of pathogenic ceramides in the SAT of obese adolescents with different patterns of VAT/VAT+SAT ratio and the gene expression of CASP1 mRNA and IL1B mRNA (Fig. 4)(SAT DHC24:1 level with CASP1 mRNA [r = 0.341, P = 0.027] and IL1B mRNA [r = 0.411, P = 0.008]), suggesting a potential link between ceramide levels and the activation of components of the inflammasome cascade in the abdominal SAT of obese adolescents.
Figure 4.
Positive correlation of SAT DHC24:1 level to subcutaneous adipose expression of caspase-1 (A) and IL-1β (B) and VAT/VAT+SAT ratio (C).
Discussion
In the current study, we report for the first time the coexistence of the two parallel phenomena occurring independently of overall obesity: altered abdominal fat distribution and upregulation of markers of inflammation in the superficial abdominal depot (i.e., SAT) of obese adolescents. Notably, the expression of NLRP3 inflammasome-related genes and the macrophage content in abdominal SAT were significantly higher in subjects with a high visceral/subcutaneous fat depot compared with BMI-matched obese adolescents with a low visceral/subcutaneous fat depot. Also, we demonstrated that increased expression of the inflammasome components CASP1 and macrophage marker CD68 were strongly correlated with visceral/subcutaneous fat distribution, which inversely correlated with insulin sensitivity. Given our previous report (11) indicating that a high ratio of visceral to subcutaneous fat is associated with impaired adipogenesis/lipogenesis, the current study uncovered the underlying excessive inflammatory state of the SAT as a potential contributor to its low storage capacity, causing lipid spillover into the visceral depot and the liver and insulin resistance in these obese adolescents.
The Role of Caspase-1
Recent studies have shown that the NLRP3 inflammasome may contribute to obesity-related metabolic complications in adults (6,8). In the current study, we demonstrated that abdominal SAT gene expression of CASP1 was elevated in obese adolescents with a high visceral/subcutaneous fat depot. It must be emphasized that caspase-1 mRNA still needs to be translated, and the resulting pro–caspase-1 needs further processing by the NLRP3 inflammasome before activating IL-1β, but because of the combined increase in CASP1, NLRP3, and IL1B expression, we speculate that SAT of obese adolescents with high VAT/SAT ratio may be more easily primed to release the major proinflammatory proteins IL-1β and IL-18.
Linear regression model revealed that CD68 and CASP1 expression in SAT is the best predictor for the ratio of impaired SAT to increased VAT. The role of caspase-1 has been implicated in the control of body weight and glucose homeostasis (6,8,18), with caspase-1–dependent cytokines IL-1β and IL-18 affecting adipogenesis, adiposity, and lipid metabolism (18). The direct effect of IL-1β on adipogenesis has been shown in primary human adipocyte culture where long-term treatment with IL-1β decreased the expression of adipogenic markers (PPARγ2, SREBP1c, FASN, ACAC, and LPL) and decreased the cellular lipid content (19). Moreover, caspase-1 activation seems to direct adipocytes toward a more insulin-resistant phenotype (6), and weight loss in obese individuals with type 2 diabetes leads to reduced activation of the inflammasome in adipose tissue (8).
We were able to show for the first time in a group of obese adolescents with an increased visceral to subcutaneous fat distribution that higher expression of the NLRP3 inflammasome–related genes (i.e., NLRP3, CASP1, and IL1B) was associated with a higher expression of inflammatory markers and a lower expression of adipogenic/lipogenic genes in the SAT. In line with these observations, Nlrp3−/− mice were protected from obesity-associated activation of proinflammatory macrophages and adipose tissue effector T-cell–mediated inflammation (9). Furthermore, Esser et al. (20) found an increased expression of the NLRP3 inflammasome, as well as an increased number of macrophages in the visceral depot of metabolically unhealthy compared with metabolically healthy individuals. This is in line with a recent study (21), showing that gene expression of NLRP3 and CASP1 in the SAT of obese men compared with lean control subjects was positively correlated with adipose tissue inflammation and CASP1 expression was strongly associated with insulin resistance and impairments in glucose homeostasis. Because the NLRP3 is predominantly expressed in macrophages and not in the adipocytes of human SAT (8), the NLRP3 measured in the SAT reflects the contribution of macrophages to adipose tissue inflammation.
The upregulation of the inflammasome complex in the high ratio group of more insulin-resistant obese adolescents was associated with a downregulation of SIRT1. This supports data published by Chalkiadaki and Guarente (22), showing that high-fat diet–induced inflammation activates caspase-1, which in turn cleaves SIRT-1 (sirtuin-1).
Ceramides as Activator of the NLRP3 Inflammasome in SAT
Type 2 diabetes is a metabolic disease that is characterized by insulin resistance, primarily within adipose, liver, and muscle tissues. Initial evidence suggesting that sphingolipids might play a role in insulin signaling came from experiments demonstrating the accumulation of ceramides in insulin-resistant tissues in obese animals as well as humans (23–25). Importantly, ceramide and its complex derivatives were shown to mediate the effects of agents, such as TNF-α and saturated fatty acids that promote insulin resistance. Recently, TNF-α–induced elevation of the ganglioside GM3 was proposed as a novel mechanism for insulin resistance (26). Ceramides are tightly linked with inflammation, because ceramide synthesis is essential for Toll-like receptor-4–dependent insulin resistance (27). Only two other studies (28,29) have so far evaluated the role of plasma ceramides in adolescents. First, Majumdar and Mastrandrea (29) showed a correlation of plasma ceramides with insulin resistance in overweight adolescents. Second, Lopez et al. (28) showed elevated plasma ceramide levels in female obese adolescents with type 2 diabetes compared with lean control subjects. Multivariate analysis of all measured plasma ceramides in our study showed a significant difference between the high ratio and the low ratio groups (MANOVA, P = 0.03), suggesting that high levels of plasma ceramides might be one cause for the inflammasome activation in the SAT. Furthermore, not only the plasma but also the SAT ceramide level were increased in the high ratio group and correlated with the VAT/VAT+SAT ratio, as well as inflammasome components CASP1 and IL1B. Greater total ceramide content in plasma and adipose tissue of obese mice has been reported (30), although the predominant species that were increased were the long-chain ceramides. Similarly, in obese adults the greatest elevation in C14-Cer, C16-Cer, and C24-Cer in adipocytes, and C14-Cer, C16-Cer, C18:1-Cer, C18-Cer and in C24:1-Cer in plasma was observed (31). Furthermore, the high ratio group has not only an impaired SAT capacity, which might be caused by inflammation, but also an increased amount of liver fat accumulation. Kolak et al. (32) showed that adipose tissue from obese subjects with increased liver fat is infiltrated with macrophages, and its content of long-chain triacylglycerols and ceramides is increased compared with equally obese subjects with normal liver fat content.
Even though a causal relationship between ceramides and insulin resistance in rodents is well established, the clinical observations reported here are strictly correlational. In addition, although some ceramide species have been more directly implicated in cellular insulin resistance, very little is known for the vast majority of the other sphingolipid subspecies, including the ones we have measured for this study.
In this study we only measured ceramides and have not measured diacylglycerol (DAG) in plasma or SAT and can therefore not make any assumption about an additional effect of DAG on the activation of the inflammasome. It is known that one cause of increased DAG content is overnutrition, which increases the rate of fatty acid delivery to muscle and liver, exceeding the rates of intracellular fat oxidation and triglyceride synthesis and storage (33). Although our study is mainly cross-sectional and does not prove causality, the direct correlation among VAT/VAT+SAT ratio, inflammation markers, and ceramide levels in the SAT, as well as the indirect correlation with insulin sensitivity, suggests that excessive adipose inflammation may be involved in regulating insulin sensitivity by impairing SAT function/expansion early in the course of obesity in adolescents. The phenotype of impaired subcutaneous fat storage and ectopic fat accumulation coexist with a phenotype of hepatic steatosis and insulin resistance.
Limitations and Strengths
Limitations of the study are caused by the fact that we measured only expression and not protein content, due to the limited amount of tissue obtained from these adolescents. Furthermore, we sampled only SAT and cannot conclude whether the results would be different in VAT. It should be noted that racial differences between the groups of high and low VAT/VAT+SAT ratio exist, but, as discussed in our previous report (11), they do not account for the differences seen in gene expression or adipose size. In addition, differences in the distribution of females and males in both groups were significant in this study. Even though we adjusted for this difference in sex when analyzing the differences between the groups, we cannot exclude the possibility that the differences seen in SAT inflammation are sex related. Moreover, the lack of measurement of IL-6, TNF-α, and MCP1 protein levels is a limitation of the current study and quantifying the proteins may reveal differences in TNF-α and MCP1 between the groups that were not detected by measuring mRNA levels. We also acknowledge that these findings are correlative and therefore do not imply causation.
Several strengths of this study need to be highlighted, including the following: the detailed metabolic characterization of two equally obese groups of adolescents who differed in their abdominal fat distribution, the use of state-of-the-art measurements of insulin sensitivity, imaging techniques for the assessment of lipid content in liver and abdominal fat distribution, and measurements of ceramides in SAT and plasma.
Summary
Our data suggest that macrophage-adipocyte cross talk mediated by NLRP3 inflammasome–dependent inflammation may have important functional consequences for adipose tissue biology and insulin resistance. Although the NLRP3 inflammasome is critical for defense against pathogens, the aberrant chronic activation of inflammasome by metabolic “danger signals” seems to be important in obesity-associated chronic diseases. This evidence suggests that the inhibition of the NLRP3 inflammasome through dietary or pharmacological approaches may have the potential to prevent the progression of dyslipidemia and its pathologic consequences in humans.
Supplementary Material
Article Information
Acknowledgments. The authors thank all of the volunteers and Karen Allen, Kim Nguyen, and Yun-Hee Youm, Yale University, for their skillful help in the study.
Funding. This study was supported by National Institutes of Health (NIH) National Institute of Child Health and Human Development grants R01-HD-40787, R01-HD-28016, and K24-HD-01464 to S.C.; Clinical and Translational Science Award grant UL1-RR-0249139 from the National Center for Research Resources, a component of the NIH; grant R01-EB006494 (Bioimage Suite); and Distinguished Clinical Scientist Award from the American Diabetes Association (S.C.), as well as grants DK-49230 and DK-085638 (G.I.S.), grants DK-090556 and AG045712 (V.D.D.), the Diabetes Research Center grant P30-DK-045735, and the European Society for Paediatric Endocrinology Long-term Research Fellowship (C.G.). N.S. is funded by the American Heart Association (grant 13SDG14640038) and the Allen Foundation.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.K. initiated the concept of the study, performed the transcriptomics and statistical analyses, and wrote the article. V.D.D. provided expertise in the measurements and interpretation of data relevant to inflammasome cascade. P.E.S., R.G., and X.L. measured and analyzed the plasma and subcutaneous adipose tissue ceramides. N.S. performed the hyperinsulinemic-euglycemic clamps and was responsible for recruiting the subjects and performing the MRI analysis. D.N. was the surgeon for the biopsies. C.G. performed the hyperinsulinemic-euglycemic clamps. B.P. and J.N. were responsible for recruiting the subjects and performing the MRI analysis. G.I.S. provided laboratory space and help with the setup of the used methods. S.C. initiated the concept of the study and wrote the article. All authors contributed to the interpretation of the data. S.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1478/-/DC1.
This publication and its contents are solely the responsibility of the authors and do not necessarily represent the official view of National Center for Research Resources or the National Institutes of Health.
References
- 1.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010;72:219–246 [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 3.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2006;116:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001;7:941–946 [DOI] [PubMed] [Google Scholar]
- 5.Stienstra R, van Diepen JA, Tack CJ, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci USA 2011;108:15324–15329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stienstra R, Joosten LA, Koenen T, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab 2010;12:593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Youm YH, Adijiang A, Vandanmagsar B, Burk D, Ravussin A, Dixit VD. Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology 2011;152:4039–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 2011;17:179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen H, Gris D, Lei Y, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 2011;12:408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science 2010;327:296–300 [DOI] [PubMed] [Google Scholar]
- 11.Kursawe R, Eszlinger M, Narayan D, et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: association with insulin resistance and hepatic steatosis. Diabetes 2010;59:2288–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taksali SE, Caprio S, Dziura J, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes 2008;57:367–371 [DOI] [PubMed] [Google Scholar]
- 13.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing. Diabetes Care. 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 14.Cali AM, De Oliveira AM, Kim H, et al. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology 2009;49:1896–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H, Taksali SE, Dufour S, et al. Comparative MR study of hepatic fat quantification using single-voxel proton spectroscopy, two-point dixon and three-point IDEAL. Magn Reson Med 2008;59:521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland WL, Miyauchi Y, Gordillo R, Scherer PE. Sphingolipid profiling of adipose tissue: specific overexpression of ceramidase improves whole body glucose and lipid metabolism. Late-breaking poster presented at the ASMS 61st Conference on Mass Spectrometry and Allied Topics, 9–13 June 2013, Minneapolis, Minnesota [Google Scholar]
- 17.Gillum MP, Kotas ME, Erion DM, et al. SirT1 regulates adipose tissue inflammation. Diabetes 2011;60:3235–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant RW, Dixit VD. Mechanisms of disease: inflammasome activation and the development of type 2 diabetes. Front Immunol 2013;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagathu C, Yvan-Charvet L, Bastard JP, et al. Long-term treatment with interleukin-1beta induces insulin resistance in murine and human adipocytes. Diabetologia 2006;49:2162–2173 [DOI] [PubMed] [Google Scholar]
- 20.Esser N, L’homme L, De Roover A, et al. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia 2013;56:2487–2497 [DOI] [PubMed] [Google Scholar]
- 21.Goossens GH, Blaak EE, Theunissen R, et al. Expression of NLRP3 inflammasome and T cell population markers in adipose tissue are associated with insulin resistance and impaired glucose metabolism in humans. Mol Immunol 2012;50:142–149 [DOI] [PubMed] [Google Scholar]
- 22.Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab 2012;16:180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams JM 2nd, Pratipanawatr T, Berria R, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 2004;53:25–31 [DOI] [PubMed] [Google Scholar]
- 24.Samad F, Hester KD, Yang G, Hannun YA, Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes 2006;55:2579–2587 [DOI] [PubMed] [Google Scholar]
- 25.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA 1998;95:2498–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabayama K, Sato T, Kitamura F, et al. TNFalpha-induced insulin resistance in adipocytes as a membrane microdomain disorder: involvement of ganglioside GM3. Glycobiology 2005;15:21–29 [DOI] [PubMed] [Google Scholar]
- 27.Holland WL, Bikman BT, Wang LP, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 2011;121:1858–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez X, Goldfine AB, Holland WL, Gordillo R, Scherer PE. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J Pediatr Endocrinol Metab 2013;26:995–998 [DOI] [PubMed] [Google Scholar]
- 29.Majumdar I, Mastrandrea LD. Serum sphingolipids and inflammatory mediators in adolescents at risk for metabolic syndrome. Endocrine 2012;41:442–449 [DOI] [PubMed] [Google Scholar]
- 30.Shah C, Yang G, Lee I, Bielawski J, Hannun YA, Samad F. Protection from high fat diet-induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. J Biol Chem 2008;283:13538–13548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blachnio-Zabielska AU, Koutsari C, Tchkonia T, Jensen MD. Sphingolipid content of human adipose tissue: relationship to adiponectin and insulin resistance. Obesity (Silver Spring) 2012;20:2341–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolak M, Westerbacka J, Velagapudi VR, et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes 2007;56:1960–1968 [DOI] [PubMed] [Google Scholar]
- 33.Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med 2010;16:400–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.