Abstract
Descriptions of insulitis in human islets throughout the natural history of type 1 diabetes are limited. We determined insulitis frequency (the percent of islets displaying insulitis to total islets), infiltrating leukocyte subtypes, and β-cell and α-cell mass in pancreata recovered from organ donors with type 1 diabetes (n = 80), as well as from donors without diabetes, both with islet autoantibodies (AAb+, n = 18) and without islet autoantibodies (AAb−, n = 61). Insulitis was observed in four of four donors (100%) with type 1 diabetes duration of ≤1 year and two AAb+ donors (2 of 18 donors, 11%). Insulitis frequency showed a significant but limited inverse correlation with diabetes duration (r = −0.58, P = 0.01) but not with age at disease onset. Residual β-cells were observed in all type 1 diabetes donors with insulitis, while β-cell area and mass were significantly higher in type 1 diabetes donors with insulitis compared with those without insulitis. Insulitis affected 33% of insulin+ islets compared with 2% of insulin− islets in donors with type 1 diabetes. A significant correlation was observed between insulitis frequency and CD45+, CD3+, CD4+, CD8+, and CD20+ cell numbers within the insulitis (r = 0.53–0.73, P = 0.004–0.04), but not CD68+ or CD11c+ cells. The presence of β-cells as well as insulitis several years after diagnosis in children and young adults suggests that the chronicity of islet autoimmunity extends well into the postdiagnosis period. This information should aid considerations of therapeutic strategies seeking type 1 diabetes prevention and reversal.
Introduction
Type 1 diabetes (T1D) is a chronic autoimmune disorder resulting from poorly understood combinations of immunologic, genetic, and environmental factors that drive immune responses against multiple β-cell antigens, resulting in the irreversible loss of functional pancreatic β-cells (1). These destructive processes are thought to begin months to years before the clinical symptoms of T1D occur. Ongoing autoimmunity and β-cell destruction are asymptomatic during this prediabetic period, but can be identified serologically by the presence of autoantibodies against one or more of several β-cell autoantigens, including GAD antibody (GADA), islet antigen 2 antibody (IA-2A), insulin autoantibody (IAA), and zinc transporter 8 (ZnT8A) (2). The number, rather than the titer, of these so called “islet autoantibodies” can be used to determine risk for T1D development (reviewed in Brorsson et al. [3]).
Whereas the initial description for inflammation of pancreatic islet cells (i.e., insulitis) in individuals with T1D occurred more than a century ago, a limited number of studies have characterized this lesion in patients with the disease or during the preclinical phase (4). Certain exceptions exist, yet a meta-analysis of the literature would suggest that insulitis is present in young donors (<14 years of age) within 1 year of T1D diagnosis as well as in donors with multiple islet autoantibodies who did not have diabetes (5–9). Difficulties in studying human islets/β-cells in vivo can be ascribed to several factors, including their relative scarcity within the pancreas (1–2%), anatomical inaccessibility, declining patient autopsy rates, and inherent risks associated with pancreatic biopsy (reviewed in Krogvold et al. [10]). This inability to perform pathological evaluations is unfortunate as such evaluations hold the potential to help explain, in part, multiple facets of disease heterogeneity, including age variation at diagnosis and disease progression including rate of C-peptide decline after onset or with experimental therapy (11,12). In an attempt to overcome these limitations, organized efforts have been developed to obtain high-quality pancreas biospecimens from organ donors to study mechanisms of β-cell loss in T1D (e.g., PanFinn, Belgian Diabetes Registry, JDRF Network for Pancreatic Organ Donors with Diabetes [nPOD]) (7,13,14).
In the current study, our major objective was to screen pancreata from nPOD donors with and without T1D, as well as from donors with and without islet autoantibodies but no diabetes, for islets with insulitis followed by leukocyte subtyping of infiltrating cells. Insulitis frequency and leukocyte subtypes in islets still expressing insulin as well as insulin− islets were correlated with donor clinical attributes (age at onset or demise, diabetes duration, autoantibody numbers, HLA, and diabetic ketoacidosis [DKA]). The β-cell and α-cell area and mass were also determined for each donor group and were tested for correlations to insulitis frequency and diabetes duration.
Research Design and Methods
Study Design
Pancreata were recovered from organ donors during a 7-year period through the JDRF nPOD program according to procedures previously described (14–16). This report provides results from donors with the following: 1) no history of diabetes or other pancreatic disease and negative for islet cell autoantibodies (AAb−, n = 61); 2) no history of diabetes or other pancreatic disease but positive for islet autoantibodies (AAb+, n = 18); or 3) T1D (n = 80). The lower age limit in this study was 4 years because the youngest donor with T1D was 4.4 years of age and estimates of β-cell proliferation were reported to be near adult levels by this age (17,18). Clinical and demographic data are summarized (Supplementary Table 1) and include the proportions of donors by numbers of islet autoantibodies and those with DKA in relation to cause of death. Diabetes durations were known for 79 of 80 donors with T1D; 1 donor with unknown diabetes duration was a 34-year-old Caucasian male with a clinical history of insulin-dependent diabetes, undetectable C-peptide levels, and no insulin+ islets, as determined by histopathology (nPOD case 6032). Ethical permission was obtained from the Institutional Review Board at the University of Florida, and informed consent was obtained from a legal representative of each donor.
Laboratory Assessments
Islet autoantibody assays, serum C-peptide levels, and high-resolution HLA genotyping were performed as previously described (14–16). Islet autoantibodies were measured by radioimmunoassay against all four major T1D-associated autoantigens (GADA, IA-2A, ZnT8A, and IAA) in all but one donor, in whom T1D was diagnosed at 10.7 years of age and who had diabetes for a duration of 6 years (nPOD 6062). Of note, after institution of insulin therapy for T1D, IAAs could not be distinguished from antibodies induced by insulin injections, so IAAs were excluded from autoantibody counts for the donors in the T1D subgroup in Table 1 and Supplementary Tables 1 and 3 (19).
Table 1.
Insulitis frequency in donors
| Case ID | Sections (n) | Ins+ CD3− | Ins+ CD3+ | Ins− CD3+ | Ins− CD3− | Total islets* | Insulitis frequency (%)† | Age at onset (years)‡ | Diabetes duration (years) | Age at demise (years) | Autoantibody results§‖ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1D | 6264 | 6 | 7 | 3 | 0 | 1,017 | 1,027 | 0.3 | 3.00 | 9.00 | 12.00 | Negative |
| 6212 | 12 | 51 | 13 | 10 | 1,601 | 1,675 | 1.4 | 15.00 | 5.00 | 20.00 | ||
| 6268 | 5 | 0 | 1 | 13 | 808 | 822 | 1.7 | 9.00 | 3.00 | 12.00 | ||
| 6265 | 6 | 8 | 6 | 5 | 508 | 527 | 2.1 | 3.00 | 8.00 | 11.00 | GADA+ | |
| 6039 | 4 | 0 | 2 | 5 | 324 | 331 | 2.1 | 16.70 | 12.00 | 28.70 | GADA+ IA-2A+ ZnT8A+ | |
| 6046 | 5 | 92 | 2 | 6 | 268 | 368 | 2.2 | 10.80 | 8.00 | 18.80 | IA-2A+ ZnT8A+ | |
| 6113 | 14 | 21 | 28 | 9 | 1,640 | 1,698 | 2.2 | 11.52 | 1.58 | 13.10 | ||
| 6224 | 8 | 30 | 24 | 12 | 1,486 | 1,552 | 2.3 | 19.50 | 1.50 | 21.00 | Negative | |
| 6088 | 7 | 73 | 7 | 6 | 433 | 519 | 2.5 | 26.20 | 5.00 | 31.20 | GADA+ IA-2A+ ZnT8A+ | |
| 6245 | 6 | 131 | 7 | 28 | 1,021 | 1,187 | 2.9 | 15.00 | 7.00 | 22.00 | GADA+ IA-2A+ | |
| 6247 | 6 | 77 | 20 | 25 | 1,068 | 1,190 | 3.8 | 23.40 | 0.60 | 24.00 | ||
| 6195 | 16 | 0 | 2 | 53 | 1,385 | 1,440 | 3.8 | 14.20 | 5.00 | 19.20 | GADA+ IA-2A+ ZnT8A+ | |
| 6070 | 4 | 56 | 15 | 8 | 333 | 412 | 5.6 | 15.60 | 7.00 | 22.60 | IA-2A+ | |
| 6198 | 2 | 16 | 9 | 5 | 181 | 211 | 6.6 | 19.00 | 3.00 | 22.00 | GADA+ IA-2A+ ZnT8A+ | |
| 6052 | 9 | 10 | 27 | 41 | 921 | 999 | 6.8 | 11.00 | 1.00 | 12.00 | IA-2A+ | |
| 6209 | 11 | 32 | 42 | 54 | 1,247 | 1,375 | 7.0 | 4.75 | 0.25 | 5.00 | IA-2A+ ZnT8A+ | |
| 6243 | 8 | 162 | 55 | 32 | 859 | 1,108 | 7.9 | 8.00 | 5.00 | 13.00 | ||
| 6228 | 15 | 250 | 246 | 74 | 2,447 | 3,017 | 10.6 | 13.00 | 0.00 | 13.00 | GADA+ IA-2A+ ZnT8A+ | |
| Total | 144 | 1,016 | 509 | 386 | 17,547 | 19,458 | ||||||
| AAb+¶ | 6197 | 5 | 1,981 | 28 | 0 | 4 | 2,013 | 1.4 | 22.00 | GADA+ IA-2A+ | ||
| 6267 | 13 | 2,149 | 139 | 8 | 18 | 2,314 | 6.4 | 23.00 | GADA+ IA-2A+ | |||
| Total | 18 | 4,130 | 167 | 8 | 22 | 4,327 |
Multiple slide sections were reviewed from each donor pancreas, and islets were counted by insulin immunopositivity (Ins+, Ins−) and insulitis status (CD3−, CD3+). The total number of insulitic islets divided by the total number of islet was used to calculate insulitis frequency (%) for each donor. Donors were listed in order of increasing insulitis frequency (%). Demographic information for ages at demise and diabetes onset, diabetes duration, and autoantibody types were also shown.
*Total islets calculated as the sum of four islet subtypes.
†Insulitis frequency (%) calculated as sum insulitic islets: (Ins+ CD3+ and Ins− CD3+)/total islets.
‡Calculated as age at demise minus diabetes duration.
§All donors with insulitis had autoantibody testing by radioimmunoassay. Radioimmunoassay data values were converted to National Institute of Diabetes and Digestive and Kidney Diseases units and defined as positive if one or more of the following applied: GADA if ≥20, IA-2A if ≥5, or ZnT8A if ≥0.020.
‖IAA were not listed for donors with T1D as they could not be distinguished as autoantibodies versus antibodies to parenteral insulin as described in research design and methods. An empty field indicates that only insulin autoantibodies were detected by radioimmunoassay.
¶AAb+ donors without diabetes.
Insulitis Screening and Insulitic Islet Subtyping for Insulitis Frequencies
Pancreata were processed to formalin-fixed paraffin blocks for each pancreas region (head, body, and tail) as previously described (20). For each donor, serial sections (average two blocks per region) were stained by hematoxylin-eosin and two double-immunohistochemistry (IHC) stains (Ki67 and insulin, CD3 and glucagon) (Supplementary Table 2) (21). When insulitic islets were found in a given donor, additional blocks were screened (as detailed below). Stained sections were scanned at ×20 magnification using an Aperio CS scanner (Leica/Aperio, Vista, CA), and all images were stored in an online pathology database (eSLIDE; Leica/Aperio).
Screening for insulitic islets was performed on CD3+ glucagon–stained sections. An islet was defined as ≥10 α-cells. Insulitis was defined as an islet with six or more CD3+ cells immediately adjacent to or within the islet with three or more islets per pancreas section, according to recent criteria (4). Islets with insulitis were marked in an image layer using ImageScope software (Leica/Aperio). The two IHC serial images were aligned using the synchronization tool, and insulin+ islets were also marked on the image layer. All islets/sections from donors with insulitis were subsequently subtyped as follows: 1) insulin+ CD3−, 2) insulin+ CD3+, 3) insulin− CD3+, and 4) insulin− CD3− (see Table 1 for numbers of islets analyzed). Then, all islets were counted by subtype. The process was reversed for AAb+ donors (i.e., islet subtypes were counted using the Ki67-insulin image after markup for CD3+ insulitic islets and insulin− islets using the CD3-glucagon image). The number of pancreas sections subtyped for insulitis ranged from 2 to 16 sections/donor (8.1 ± 4.1 sections/donor, n = 162 sections) (Table 1). The lowest number of available sections was due to partial pancreas recovery (tail only in nPOD 6198).
Insulitis frequency (percent) was calculated as the total number of insulitic islets (sum of insulin+ CD3+ and insulin− CD3+ islets) divided by the total number of islets (sum of four subtypes). The frequency of insulin+ insulitic islets in relation to the total number of insulin+ islets was determined by the ratio of (insulin+ CD3+ islets)/(sum of insulin+ CD3− and insulin+ CD3+ islets) with similar calculations for the frequency of insulin− insulitic islets (insulin− CD3+)/(sum of insulin− CD3+ and insulin− CD3−).
Insulitis Leukocyte Phenotyping
Paraffin sections from blocks having the maximum insulitis frequency for each donor were stained, and positive leukocytes/insulitic islets (six or more CD3+ cells) were counted using multi-immunofluorescence. Serial sections (4 μm) were dewaxed and rehydrated with Tris buffer. Heat-induced antigen retrieval was performed using Trilogy (Cell Marque, Rocklin, CA) at 95° for 20 min followed by rinsing in water for 20 min. The staining series was designed to phenotype leukocytes (total leukocytes [CD45], T [CD3] and B [CD20] lymphocytes, T-lymphocyte subsets [CD4 and CD8], and monocytes [dendritic cells (CD11c) and macrophages (CD68)]) (21) in conjunction with subtyping islets for insulin immunopositivity. The staining series was as follows: 1) CD45+glucagon+insulin, 2) CD20+CD3+glucagon, 3) CD8+CD4+glucagon, and 4) CD11c+CD68+insulin. Chromogranin A staining was also used to delineate endocrine cells. Antigens are listed in order of primary antibody incubation, and the corresponding secondary antibody and conjugated fluorochrome (AF488-AF555-AF647) (Supplementary Table 2). After blocking, sections were sequentially incubated with the primary antibody followed by the appropriate secondary antibody. For anti-CD4, a Cy3 Tyramide Signal Amplification Kit (PerkinElmer, Waltham, MA) was used according to the manufacturer's instructions. All sections were mounted with ProLong Gold Antifade mounting media containing DAPI (Life Technologies, Grand Island, NY). Positive controls included human spleen, tonsil, and donor intrapancreatic lymph nodes, and negative controls included omission of the primary antibody.
The numbers of leukocytes/insulitic islets were determined using multichannel image acquisition software on a Zeiss Axiophot microscope (AxioVision; Carl Zeiss Inc., Thornwood, NY). Fluorescent channels were viewed in combination with DAPI to count the number of positive leukocytes/islet.
β-Cell and α-Cell Area and Mass
Insulin- and glucagon-immunopositive areas were determined using the IHC sections to estimate β-cell and α-cell areas, respectively, in relation to total tissue area using a single Aperio colocalization algorithm (22). An average of six sections was used per donor (two sections/head, body, and tail regions). The β-cell and α-cell areas were expressed as a ratio (percent) to the total sectional area, including acinar and interstitial regions, to permit the use of pancreata weights. The average β-cell and α-cell area per pancreas was calculated from regional area averages. The β-cell or α-cell mass (in milligrams) was calculated by multiplication of the respective average area and pancreas weight (in grams).
Statistical Analysis
Numbers and percents were reported for all categorical factors, the mean (±SD, n = number of donors unless noted in figure legend) was reported for normally distributed continuous variables, and the median (minimum, maximum) was reported for continuous variables with a skewed distribution. Donor characteristics were analyzed using a Satterthwaite-corrected t test if continuous and parametric, a Kruskal-Wallis test if continuous and nonparametric, a Pearson χ2 test if categorical values in all cells exceeded 5, or a Fisher exact test if categorical values in one or more cells were <5. P values were not calculated if values from two or more cells were equal to 0. Donor characteristics were correlated to insulitis frequency, and leukocyte subtype numbers/islets were correlated to other subtypes by Spearman correlation and plotted with linear regression lines. Data were analyzed with the exclusion of the AAb+ donors with insulitis due to the small sample size (n = 2).
To obtain a subset of T1D donors and to compare clinical characteristics in the presence or absence of insulitis, a selection of controls for all 18 T1D insulitis cases was attempted using a 1:1 ratio to identify the single best match. A matching method was used to minimize bias in lesion effect estimates from key covariates (i.e., age at diabetes onset, sex, ethnicity, and BMI). A propensity score, given the selected covariates, was calculated for all T1D donors (23). The logit of the propensity score was used to match donors without insulitis to those with insulitis. A randomly sorted nearest-available-neighbor matching method without replacement was used with an optimal caliper width equal to 0.20 SD of the logit value (0.15), as has been shown to be optimal (24), along with an SAS Software (SAS Institute, Cary, NC) macro (25,26). All reported P values are two-tailed and significant if <0.05. All analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA) or SAS Software version 9.3.
Results
Insulitis Screening in Donors With T1D and AAb+ Donors With No Diabetes
A total of 159 pancreata were reviewed for insulitis (no diabetes [n = 61 AAb−; n = 18 AAb+]; n = 80 T1D) (Supplementary Table 1). Insulitis was observed in 18 T1D donors (18 of 80 donors, 23%) and 2 AAb+ donors (2 of 18 donors, 11%). Variability in the lobular distribution of insulin+ islets and insulitic islets was high in donors with T1D (Fig. 1A–D). In AAb+ donors with insulitis, insulitis was primarily observed in insulin+ islets (Supplementary Fig. 1). The number of CD3+ cells/islet varied in a given donor, irrespective of islet insulin immunopositivity or size, and CD3+ cells were observed diffusely infiltrating islets, in variously sized aggregates, or both (Fig. 2 and Supplementary Fig. 2).
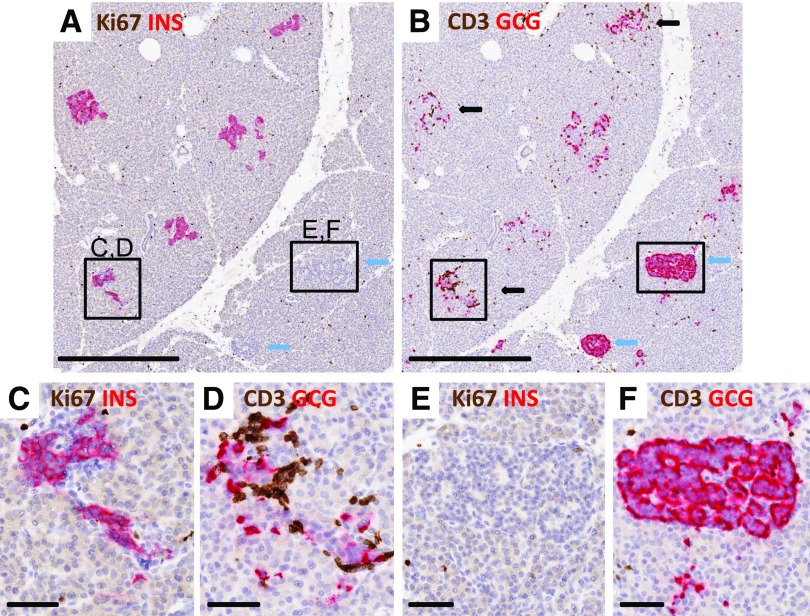
Figure 1.
Lobular variability in insulin+ islets and insulitis. Islets were imaged from a 13-year-old patient with T1D for 5 years (nPOD 6243). Serial paraffin sections were stained for Ki67 plus insulin (INS) (A, C, and E) and CD3 plus glucagon (GCG) (B, D, and F), and islets were subtyped as described in research design and methods. Five insulin+ islets (A) are seen in a lobule adjacent to a lobule with two insulin− islets in the patient with T1D (A and B, blue arrows). Three insulin+ islets had insulitis (B, black arrows), and both insulin− islets did not have insulitis. One of the insulin+ islets with insulitis is shown at higher magnification (C and D) as well as an insulin− insulitis− islet (E and F). Few islet cells were Ki67+ (A and C), indicating no effect of insulitis on proliferating cell numbers. Scale bars: A and B, 500 μm; C–F, 50 μm.
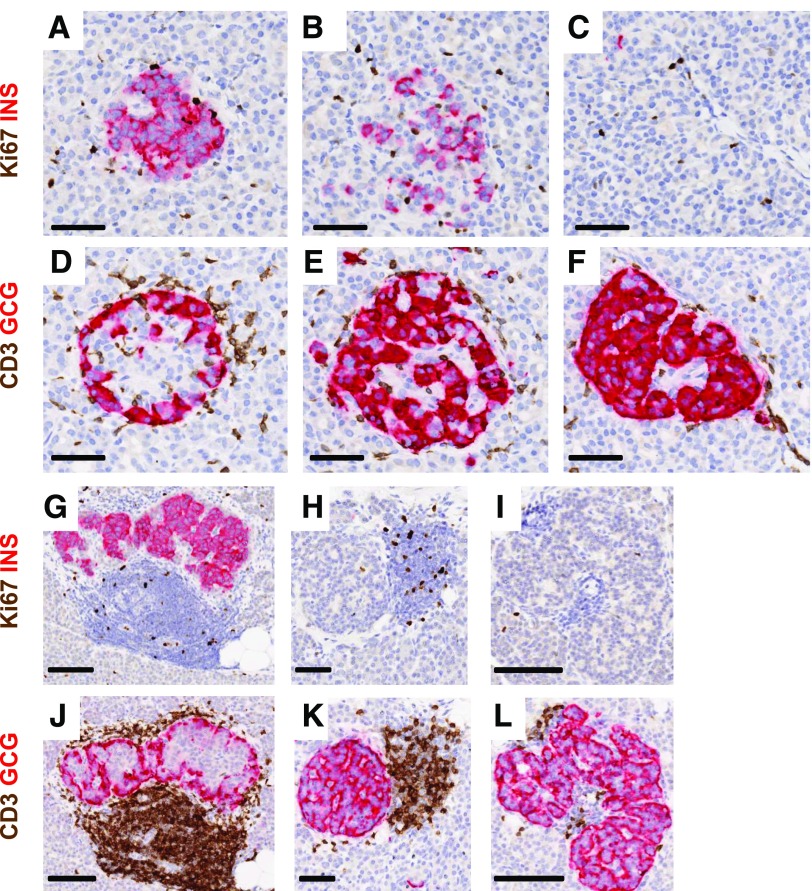
Figure 2.
Heterogeneity of islet β-cells and the numbers of CD3+ cells in insulitic islets in young donors at diabetes onset and after 5 years of disease duration. Representative islets were imaged for a 13-year-old at diabetes onset (nPOD 6228; A–F) and for another 13-year-old with T1D for 5 years (6243; G–L). Serial paraffin sections were stained using dual-IHC (Ki67 plus insulin [INS], CD3 plus glucagon [GCG]), as described in research design and methods. Decreasing proportions of β-cells are shown in different islets from both donors (A–C and G–I) as well as heterogeneity in numbers of CD3+ cells per islet (D–F and J–L). Heterogeneity in CD3+ counts between two insulin− islets of similar sizes is also shown for one donor (K and L). Scale bars: A–F, H, and K, 50 μm; G, I, J, and L, 100 μm.
All 18 donors with T1D and insulitis had insulin+ islets detected in at least one section, while only 5 of 62 donors (8%) with T1D and no insulitis had insulin+ islets. Insulitis was found in 509 of 1,525 insulin+ islets (33%) and in 379 of 17,718 insulin− islets (2%) in donors with T1D (Table 1). In AAb+ donors with insulitis, the opposite was found; insulitis affected 167 of 4,297 insulin+ islets (4%) and 8 of 30 insulin− islets (27%). Insulitis was found in T1D donors ranging in age from 3 to 26.2 years (mean age 13.3 ± 6.5 years) at disease onset and with disease durations of 0–12 years (mean duration 4.6 ± 3.4 years) (Table 1). The highest insulitis frequency (10.6%) was observed in a 13-year-old who presented with DKA at disease onset (nPOD 6228). The lowest insulitis frequency (0.3%) was observed in a 12-year-old who had T1D for 9 years (nPOD 6264). The two AAb+ donors with insulitis had multiple autoantibodies and were 22 and 23 years old (Table 1). The AAb+ donors had insulitis frequencies (1.4%, 6.4%) within the range observed for donors with T1D; few insulin− islets, with or without insulitis, were observed in these donors.
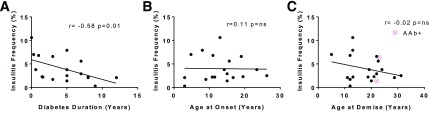
The percent of islets with insulitis (insulitis frequency) was tested for a correlation with diabetes duration. Insulitis frequency showed a low, yet significant, inverse relationship to diabetes duration, whereas age at onset or demise did not (Fig. 3).
Figure 3.
Insulitis frequency in relation to diabetes duration and donor ages at demise or disease onset. The insulitis frequency (%, total number of insulitic islets/total number of islets) is shown on the y-axis in comparison with years for diabetes duration (A), age at T1D onset (B), and age at demise (C). Data were displayed (pink) for the two AAb+ donors with insulitis for age at demise but were excluded from statistical analyses because of the small sample size. Insulitis frequency (%) had a low but significant correlation to diabetes duration, but not to donor age at demise or disease onset. The linear regression line with Spearman r and P values are shown.
Correlation of Insulitis Prevalence With Diabetes Duration
Because past studies reporting insulitis primarily examined young donors with T1D within a year of onset, the prevalence of donors with insulitis was analyzed in this study using similar criteria (6). All four donors with recent-onset T1D (duration ≤1 year) had insulitis; three of four donors were <15 years of age at disease onset (Table 2). In donors with disease durations >1 year, insulitis was found in 7 of 51 donors (14%) who were <15 years of age at disease onset and in 7 of 24 donors (29%) who were ≥15 years of age at disease onset. Using 12 years of diabetes duration as the cutoff, the prevalence of insulitis was 45% in donors (18 of 40 donors) with T1D.
Table 2.
Insulitis prevalence in nPOD donors by age of onset and diabetes duration
| Diabetes duration ≤1 year | Diabetes duration >1 year | Totals* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number donors insulitis+ | Total number donors | Proportion with insulitis (%) | Number donors insulitis+ | Total number donors | Proportion with insulitis (%) | Number donors insulitis+ | Total number donors | Proportion with insulitis (%) | |
| Age at onset (years) | |||||||||
| 1.4–14.2 | 3 | 3 | 100 | 7 | 51 | 14 | 10 | 54 | 19 |
| 15–36 | 1 | 1 | 100 | 7 | 24 | 29 | 8 | 25 | 32 |
| Total | 4 | 4 | 100 | 14 | 75 | 19 | 18 | 79 | 23 |
Age-groups and diabetes durations adapted from In’t Veld (6).
*One donor with T1D had an unknown duration of disease (see research design and methods).
To further define other potential clinical variables as they relate to insulitis, 15 T1D donors with insulitis were matched for age at onset, ethnicity, sex, and BMI with 15 T1D donors without insulitis (Supplementary Table 3; in three cases, no matches were found and thus were excluded). Diabetes duration was significantly shorter in T1D donors with insulitis versus those without insulitis (5 vs. 14.5 years, respectively). Correspondingly, donors with insulitis were significantly younger at demise compared with matched donors without insulitis (20 vs. 27.6 years, respectively). Interestingly, islet autoantibody numbers were not different between the matched T1D donors. Hospitalization durations were not significantly different in this matched subset and ranged from a mean of 3.2 to 3.7 days across all donor groups (Supplementary Tables 1 and 3). Correlation of DKA during the hospitalization showed a significantly higher proportion of donors with insulitis (7 of 15 donors, 47%) versus those without insulitis (1 of 15 donors, 7%, P = 0.04).
Infiltrating Leukocytes Have Similar Proportions of B and T Cells and T-Cell Subsets, and Numbers Were Proportional to Insulitis Frequency
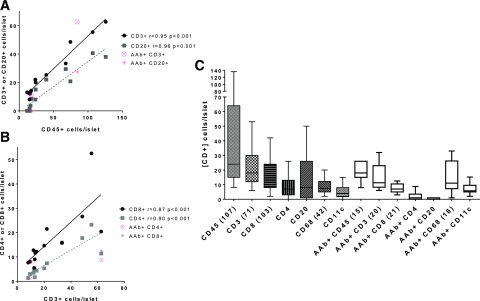
Infiltrating leukocytes in insulitic islets were examined by multi-immunofluorescence on serial sections (Fig. 4). Mean numbers of CD45+ cells/islet in each donor were significantly correlated to mean numbers of both T cells (CD3+) and B cells (CD20+) per islet (Fig. 5). Absolute numbers of CD3+ and CD20+ cells varied between islets in a given donor, depending on the nature of the infiltrate (e.g., diffusely associated with islet, aggregate, and mixture). The numbers of both CD3+ and CD20+ cells appeared independent of islet insulin immunopositivity and diabetes duration (Supplementary Figs. 2 and 3). The numbers of CD3+ cells were significantly correlated with the numbers of CD8+ and CD4+ cells/islet (Fig. 5B). Leukocyte numbers per islet were also plotted for both donor groups with insulitis, and high variability was observed, particularly for CD45+, CD3+ and CD20+ (Fig. 5C). This variability was also seen in the AAb+ donors without diabetes (Fig. 5C). Five donors had insulitis aggregates with >50 CD3+ cells/islet; four were donors with T1D who were all <12 years of age but had widely varying diabetes durations (nPOD 6209, 6243, 6052, and 6070), and one was an AAb+ donor without diabetes (nPOD 6267).
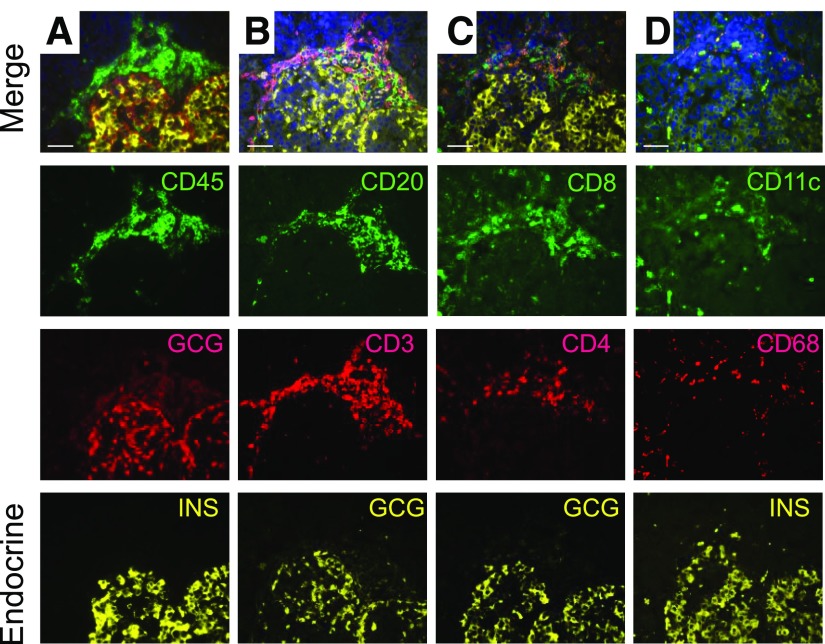
Figure 4.
Insulitis leukocyte subtyping by multiple immunofluorescence. Serial sections of an islet with insulitic aggregate from a 5-year-old donor with T1D for 4 months (nPOD 6209) were imaged. The numbers of leukocytes/islet were counted for total leukocytes (CD45, A), B and T lymphocytes (CD20 and CD3, B), T-lymphocyte subsets (CD8 and CD4, C), and dendritic cells and macrophages (CD11c and CD68, D) as described in research design and methods. Islet endocrine cells were identified using glucagon (GCG) and insulin (INS) stains. The merged images (A–D) include DAPI-stained DNA, and the individual fluorescence channels are shown below the merged images. Scale bars: 50 µm.
Figure 5.
Leukocyte numbers increase in parallel with CD45+ and CD3+ numbers but are heterogeneous between islets. Numbers of CD45+, CD3+, CD20+, CD8+, and CD4+ cells were counted for each insulitic islet and averaged per donor (n = 13 donors with T1D, 2–10 islets/donor). Data were also displayed (pink) for each leukocyte marker for the two AAb+ donors with insulitis for comparison purposes but were excluded from statistical analyses because of the small sample size. Correlations between the numbers of leukocytes per islet were performed by Spearman analysis with linear regression lines shown. The numbers of CD45+ cells per islet were significantly correlated with the numbers of CD3+ and CD20+cells per islet (A). The numbers of CD3+ cells per islet were also significantly correlated with the numbers of CD8+ and CD4+ cells per islet (B). The numbers of leukocyte subtypes per islet were also analyzed to examine the variability in cell numbers among all islets for donors with T1D and two AAb+ donors with insulitis, with means depicted by box-and-whisker plots with Tukey test error bars (nonparametric) (C). Numbers in parentheses following each marker indicate the total number of individual islets analyzed for each leukocyte marker (CD45) or set of markers. Variability in the overall numbers of leukocytes per islet was observed. The numbers of leukocytes per islet had similar trends in AAb+ donors without diabetes.
We also tested whether the numbers of leukocytes per islet were correlated with insulitis frequency, and found significant correlations with the numbers of CD45+, CD3+, CD20+, CD8+, and CD4+ cells per islet, but not for the numbers of CD68+ and CD11c+ cells per islet (Supplementary Fig. 4 and Supplementary Table 4). Because of the variability between islets in the numbers of leukocytes per insulitic islet, we also tested whether a ratio of markers (CD3+ cells/sum of CD3+ and CD20+ cells) would normalize interislet variability but found no significant correlation to insulitis frequency (Supplementary Fig. 4).
β-Cell and α-Cell Mass and Insulitis
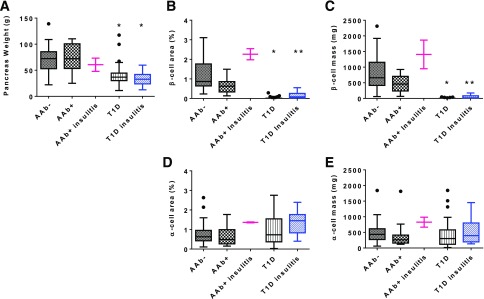
Pancreata weights were variable in donors without diabetes: 71.1 ± 24.2 g (n = 49) in AAb− donors, 70.7 ± 28.0g (n = 15) in AAb+ donors without insulitis, and 60.7 ± 17.9 in two AAb+ donors with insulitis (Fig. 6A). As anticipated, both donor groups without diabetes (AAb− and AAb+) showed increasing pancreas weight after age 4 years up to ∼30 years (Supplementary Fig. 5A and B). Donors with T1D had a significantly lower mean pancreas weight (39.51 ± 17.9 g no insulitis, n = 60; 33.8 ± 12.9 g with insulitis, n = 16) compared with donors without diabetes (Fig. 6A). Pancreata weights were decreased at all ages in donors with T1D compared with donors without diabetes (Supplementary Fig. 5C).
Figure 6.
Pancreas weight and β-cell area and mass are decreased in donors with T1D. Group means were depicted by box-and-whisker plots with Tukey test error bars (nonparametric, outliers shown as black circles). Data were displayed (pink) for the two AAb+ donors with insulitis and were excluded from statistical analyses because of the small sample size (n = 2). Pancreata weights were significantly lower in donors with T1D compared with AAb− and AAb+ donors without diabetes, and no difference was detected between donors with T1D based on insulitis status (A). The β-cell area was significantly lower in both donor groups with T1D compared with AAb− and AAb+ donors without diabetes (B). T1D donors with insulitis had a significantly higher β-cell area compared with T1D donors without insulitis (B). The β-cell mass was similar between AAb− and AAb+ donors and was significantly different from that in groups of donors with T1D (C). T1D donors with insulitis had significantly higher insulin+ β-cell mass compared with T1D donors without insulitis (C). The α-cell area (D) and mass (E) were not significantly different between groups. *Significantly different from AAb− and AAb+ donors (P < 0.001). **Significantly different from AAb− and AAb+ donors (P < 0.001) and T1D donors without insulitis (P < 0.05).
β-Cell area was also variable in donors without diabetes: 1.18 ± 0.71% for AAb− donors (n = 57) and 0.67 ± 0.38% for AAb+ donors without insulitis (n = 16) (Fig. 6B). The two AAb+ donors with insulitis had a mean β-cell area of 2.26 ± 0.4%. As expected, significant reductions in β-cell area were observed for donors with T1D compared with those for donors without diabetes (Fig. 6B). β-Cell area was also significantly different between the two donor groups with T1D (0.02 ± 0.05% no insulitis, n = 54; 0.15 ± 0.17% with insulitis, n = 18) (Fig. 6B).
The mean β-cell mass was 797 ± 523 mg for AAb− donors (n = 45) and 448 ± 268 mg for AAb+ donors without insulitis (n = 15) (Fig. 6C). β-Cell mass was 1,407 ± 649 mg in the two AAb+ donors with insulitis. β-Cell mass was decreased in donors with T1D, regardless of insulitis status, compared with those without T1D; however, β-cell mass was significantly higher in T1D donors with insulitis (47.9 ± 53.4 mg, n = 17, P < 0.05) compared with those without insulitis (5.5 ± 10.1 mg, n = 53) (Fig. 6C). The mass of β-cells from five donors without diabetes (three AAb−, two AAb+) overlapped with the highest values for β-cell mass in donors with T1D and insulitis due to low pancreas weight (3) or low β-cell area (2). Estimated β-cell mass was further tested for correlation with insulitis frequency, but no association was found (Supplementary Fig. 6A, P > 0.05). Similarly, we compared β-cell mass to T1D duration and age at onset to determine whether insulin mass was greater in donors with shorter diabetes durations or younger donors; again, no significant associations were found (Supplementary Fig. 6B and C, P > 0.05).
In marked contrast to differences in β-cell area and mass, α-cell area and mass were not significantly different between groups (Fig. 6D and E). The mean α-cell area was 0.73 ± 0.45% in AAb− donors (n = 57), 0.62 ± 0.44% in AAb+ donors without insulitis (n = 16), 1.37 ± 0.04% in AAb+ donors with insulitis (n = 2), 1.04 ± 0.84% in donors with T1D and no insulitis (n = 57), and 1.37 ± 0.62% in donors with T1D and insulitis (n = 17). The mean α-cell mass was 495 ± 320 mg in AAb− donors, 402 ± 428 mg in AAb+ donors without insulitis, 827 ± 224 mg in AAb+ donors with insulitis, 396 ± 377 mg in donors with T1D and no insulitis, and 513 ± 375 mg in donors with T1D and insulitis.
Influence of HLA on Insulitis
Donors with T1D had a higher frequency of DRB1*03:01-DQB1*02:01 or DRB1*04-DQB1*03:02 genotypes (DR3/DR4) compared with AAb− or AAb+ donors (35.9% vs. 0%, P < 0.0001 and P = 0.0012, respectively). HLA DR3/DR4 frequencies were not different when comparing the matched donors within the T1D group, with and without insulitis (Supplementary Table 3). The DRB1*1501-DQB1*0602 haplotype was very rare and considered protective for T1D. The overall frequency of this haplotype in AAb− donors (25%) was similar to the general U.S. Caucasian population (22%) (27). This haplotype was found in four AAb+ donors with single autoantibodies and in none of those with multiple autoantibodies. In the T1D donors, only one had the DRB1*1501-DQB1*0602 haplotype, and, quite interestingly, this donor also had insulitis (nPOD 6195).
Discussion
The concept of insulitis has been the subject of previous reports; however, this study is the first to report insulitis frequency, leukocyte subtypes, pancreatic weights, and β-cell and α-cell mass in organ donors encompassing the entire natural history of T1D (i.e., preclinical phase [no diabetes, islet autoantibody positive] as well as acute and chronic phases]. Beyond this, the current study is large, used standard operating procedure–driven protocols, and used only high-quality tissues. As a result, we believe the results noted herein provide much in the way of new information as well as validating, or refuting, past observations.
Insulitis was defined by the presence of six or more CD3+ cells/islet and three insulitic islets/section (4). Overall, insulitis was identified in 18 of 80 donors with T1D (23%) and 2 of 18 AAb+ donors without diabetes (11%). Notably, the numbers of donors with insulitis (insulitis prevalence) was higher for older donors and for longer disease durations in both young and older patients than reported using a meta-analysis of existing studies (6). Historically, insulitis prevalence has been reported in 51% of patients (41 of 81 patients) with disease duration of ≤1 year, compared with only 3% of patients (4 of 132 patients) with durations >1 year (6). As shown in Table 2, we observed an insulitis prevalence of 100% (4 of 4 donors) in donors with T1D durations of ≤1 year and 19% (14 of 75 of donors) in donors with durations of >1 year. We also observed a high proportion of donors with insulitis for diabetes durations up to 12 years (18 of 40 donors, 45%). At the same time, our overall insulitis prevalence of 23% (18 of 79 donors) in donors with T1D compares well with the overall insulitis prevalence rate of 21% (45 of 213 donors) from former studies (6). Heterogeneity in the distribution of insulitic islets could partly explain the lower detection rates from former studies using autopsy collections or biopsy specimens because fewer blocks and pancreas regions could be tested per patient (5,7).
Studies by Oram et al. (11) and several others (28–30) support the persistence of β-cells in patients with long-standing T1D. Our data also show the persistence of β-cells in donors with T1D, with and without insulitis, up to 12 years after disease onset. Although other investigators have also reported insulin+ islets without insulitis in patients with T1D, few have emphasized that insulin− islets are also involved in the insulitic process (reviewed in Gepts [31]). The proportions of insulin+ and insulin− insulitic islets in our study (33% insulin+, 2% insulin−) were similar to those reported by Foulis et al. (5) and others (32) (28–35% insulin+, 1–5% insulin−). Persistent insulitis after the loss of β-cells could imply the presence of degranulated and/or dedifferentiated β-cells, undetected by these methods, or, alternatively, the spread of autoimmunity or “innocent bystander” effects on other endocrine cells. Prospective studies demonstrated that the appearance of multiple islet autoantibodies is usually sequential rather than simultaneous and that their number (two or more) is a strong predictor of progression to T1D (1). Herein, insulitis was observed in two of five donors (40%) with multiple autoantibodies, a finding that is similar to that in the study by In’t Veld et al. (7), in which insulitis was detected in two of seven donors (29%) with multiple autoantibodies. Of note, the AAb+ donors with insulitis in our study were younger (21 and 22 years of age compared with 46 and 52.5 years of age), and the entire pancreas was available for study compared with the former study of biopsy material. Interestingly, all four donors showed similar insulitis frequencies (1.4–6.4%, 3–9%) and normal β-cell mass, and all four donors had the autoantibody combination of GADA+ and IA-2A+.
Another novel aspect of our study is in showing similarities between the preclinical and clinical phases of T1D in the leukocyte subtypes in insulitic islets and independent of the presence of residual β-cells. Both phases were characterized by diffuse and/or focal aggregates and varying numbers of leukocytes/islet within and between donors. Several studies (7,30,32–38) have shown a predominance of CD8+ T lymphocytes and macrophages in insulitic islets. The defining study was considered to be that of Willcox et al. (32), using 29 patients from the Foulis collection (5), and reexamination of 21 patients from the Foulis collection with two nPOD donors (6052 and 6113) was recently reported by Arif et al. (39). In the former study, insulitic islets were ranked by both insulin percentage and immune cell numbers (n = 279 insulitic islets), whereas only insulin+ islets were evaluated in the latter study. All leukocytes were significantly reduced in insulin− islets (32). In marked contrast, our study found that CD45+, CD3+, CD20+, and T-cell subsets increased linearly with the numbers of insulitic islets (insulitis frequency), irrespective of islet β-cells. Potential differences between studies may result from differences in age or disease duration or in technical differences in methods.
An intriguing hypothesis by Skog et al. (40) proposed that ascending infection through the pancreatic ducts could result in insulitis due to the greater susceptibility of β-cells compared with other endocrine cells. Though insulitic islets were frequently observed near pancreatic ducts in this study, no consistent pattern was observed for the presence of periductal infiltrates and insulitic islets. The heterogeneous leukocyte composition of insulitis might support this hypothesis if the offending agent (virus or bacterium) or its toxic by-products had minimal proclivity for ductular epithelial cells.
In the current study, donors with T1D had small pancreata regardless of age or disease duration. Pancreata weights were not different between AAb− or AAb+ donor groups without diabetes and are similar to the age-related changes reported in normal pancreas volume by Saisho et al. (41). Our current findings contrast with those of our previous report (42) using a subset (n = 8) of the 18 AAb+ donors in this study. In our first study, all donors were >18 years of age and excluded if pancreatitis was noted in the medical chart or was discovered by histopathology. Age or pancreatitis exclusion criteria were not used in the current study. Because it is difficult to predict when organ donors with multiple AAb+ could progress to T1D, pancreas size loss in the natural history of T1D might be best studied in longitudinal clinical trials of AAb+ subjects by noninvasive radiography (43,44). Beyond this, the notion of pancreatitis in individuals with T1D is of evolving interest, given the histopathological findings of multifocal CD3+ infiltrates in acinar regions of donors with T1D. Indeed, Rodriguez-Calvo et al. (45) reported an increased number of exocrine CD8+ T cells in nPOD donors with T1D and type 2 diabetes.
Reports indicate marked variability in β-cell area or mass in normal adults, with adult levels reached by 5 years of age (17,18,46,47). Indeed, some donors without diabetes (both AAb+ and AAb−) exhibit β-cell mass within the range observed for T1D donors. Reasons for this heterogeneity are unknown but could result from host genetic and environmental factors, including in utero and neonatal periods when β-cell mass is increasing. Rising rates of obesity could also account for some variability. Our β-cell area and mass data are in agreement with past literature that also reported high variability in both measures of islet β-cell content. For instance, our mean control β-cell area (1.2%) was similar to that reported in children and young adults by Meier et al. (18) (1.7% [age range 5.7–15 years of age], 1.3% [18.5–21.5 years of age]), as well as that reported by In’t Veld et al. (7) in AAb+ donors without diabetes (mean relative β-cell area of 1.2% [0.51–2.61 years of age]). In the report by In’t Veld et al. (7), comparison of β-cell areas and mass in AAb+ and AAb− donors did not show a significant difference. Two other groups (48,49) have also reported normal β-cell mass in patients with autoantibodies. Interestingly, β-cell mass in five donors without diabetes was in the upper range found for donors with T1D and insulitis and was related to young donor age, low pancreas weight, and/or low β-cell area.
In terms of potential limitations, it must be emphasized that these data were obtained from pancreata of multiorgan donors with brain death who were subjected to intensive care procedures. Interpretations of data must be considered, as posed by In’t Veld et al. (7), in light of the medications received during critical care and the duration of intensive care (reviewed in the study by Atkinson [50]). The majority of donors in this study had short hospitalization durations, yet further studies are needed to both validate and expand these findings. The computer-assisted image analysis algorithm used in this study has inherent limitations, with single minimum and maximum intensity thresholds applied to all sections. Alternatively, its unbiased approach using cross-sectional areas of the entire section could add greater consistency compared with manual point-counting methods. When studying organ donors, there is the possibility that the referring/initial diagnosis provided to nPOD may be of a questionable nature. To address this, full medical histories are carefully reviewed by board-certified endocrinologists and pathologists. Even when histories are limited, we carefully consider age at diagnosis, BMI, time to insulin dependence, history of insulin− dependence, treatment with oral agents, C-peptide level, HLA level, the presence of DKA, ketosis, and more.
In sum, the proportion of insulitic islets (insulitis frequency) in donors with T1D was inversely associated with disease duration, and not by age at onset, number of autoantibodies, or HLA genotype. The number of donors with insulitis (insulitis prevalence) was higher in older patients and for longer disease durations in both young and older patients than previously reported. In donors with T1D, the proportions of insulin+ islets with insulitis was higher than in insulin− islets, yet insulin− islets were also affected by insulitis, particularly in donors with multiple AAb+. Insulitic lesions were composed of a mixture of B and T lymphocytes, the numbers of which increased with insulitis frequency. The β-cell mass was significantly higher in donors with T1D and insulitis compared with donors without insulitis, whereas β-cell mass was similar between AAb+ donors and AAb− donors. These data underscore the chronic and heterogeneous nature of insulitis in individuals with T1D. The presence of insulitis as well as insulin+ islets several years after diagnosis, and the limited correlation between insulitis frequency and disease duration suggest that the chronicity of islet autoimmunity extends well into the postdiagnosis period. Together with growing evidence for the chronicity of autoimmune responses and for the persistence of C-peptide secretion in patients, even decades after onset, our findings support the existence of a potentially long window of therapeutic opportunity (51,52).
Supplementary Material
Article Information
Acknowledgments. The authors thank Dr. Alvin Powers (Vanderbilt University, Memphis, TN) and Dr. Jake Kushner (Baylor College of Medicine, Houston, TX) for nPOD donor referrals and Robert Pietras for image analysis software design. The authors also thank Dr. Amanda Posgai (University of Florida, Gainesville, FL) for editorial assistance. In addition, the authors thank nPOD staff members for donor recovery, data collection, and helpful discussions. Organ procurement organizations partnering with nPOD are listed at http://www.jdrfnpod.org/for-partners/npod-partners. Immunohistochemistry slides used in this study are available for viewing through the nPOD Online Pathology database (21). This study used data from the Organ Procurement and Transplantation Network (OPTN) supplied by the United Network for Organ Sharing as the contractor for the OPTN.
Funding. This work was supported by the JDRF (grants 25-2013-268, 17-2012-3, and 25-2012-516 to M.C.-T., J.S.K., A.P., and M.A.A.) and National Institute of Diabetes and Digestive and Kidney Diseases (UC4-DK-104155 01 and 1DP3-DK-101120-01 to M.C.-T., D.A.S., and M.A.A.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.C.-T. designed the experiments; performed pathology reviews, image analyses, and statistical calculations; and wrote the article. A.F. performed experiments and edited the article. J.S.K. and A.P. researched the data, performed the statistical calculations, and contributed to discussion and the editing of the article. C.W. designed the autoantibody studies, performed experiments, and edited the article. D.A.S. and M.A.A. contributed to the study design and discussion and edited the article. M.C.-T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Preliminary immunotyping results of this study were presented at the Immunology of Diabetes Society 12th International Congress, Vancouver Island, BC, Canada, 15–19 June 2012, and 13th International Congress, Lorne, Victoria, Australia, 7–11 December 2013. Pancreata weights were reported in a subset of these donors (53). Of the 159 nPOD cases presented herein, 43 were previously examined by Coppieters et al. (30) (Supplementary Table 5).
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-0779/-/DC1.
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the Organ Procurement and Transplantation Network or the U.S. Government.
See accompanying article, p. 545.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winter WE, Schatz DA. Autoimmune markers in diabetes. Clin Chem 2011;57:168–175 [DOI] [PubMed] [Google Scholar]
- 3.Brorsson CA, Onengut S, Chen WM, et al.; Type 1 Diabetes Genetics Consortium . Novel association between immune-mediated susceptibility loci and persistent autoantibody positivity in type 1 diabetes. Diabetes 2015;64:3017–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell-Thompson ML, Atkinson MA, Butler AE, et al. The diagnosis of insulitis in human type 1 diabetes. Diabetologia 2013;56:2541–2543 [DOI] [PubMed] [Google Scholar]
- 5.Foulis AK, Liddle CN, Farquharson MA, Richmond JA, Weir RS. The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia 1986;29:267–274 [DOI] [PubMed] [Google Scholar]
- 6.In’t Veld P. Insulitis in human type 1 diabetes: the quest for an elusive lesion. Islets 2011;3:131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.In’t Veld P, Lievens D, De Grijse J, et al. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes 2007;56:2400–2404 [DOI] [PubMed] [Google Scholar]
- 8.Gianani R, Putnam A, Still T, et al. Initial results of screening of nondiabetic organ donors for expression of islet autoantibodies. J Clin Endocrinol Metab 2006;91:1855–1861 [DOI] [PubMed] [Google Scholar]
- 9.Wiberg A, Granstam A, Ingvast S, et al. . Characterization of human organ donors testing positive for type 1 diabetes-associated autoantibodies. Clin Exp Immunol 2015;182:278–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krogvold L, Edwin B, Buanes T, et al. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia 2014;57:841–843 [DOI] [PubMed] [Google Scholar]
- 11.Oram RA, Jones AG, Besser RE, et al. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 2014;57:187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenbaum CJ, Beam CA, Boulware D, et al.; Type 1 Diabetes TrialNet Study Group . Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes 2012;61:2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tauriainen S, Salmela K, Rantala I, Knip M, Hyöty H. Collecting high-quality pancreatic tissue for experimental study from organ donors with signs of β-cell autoimmunity. Diabetes Metab Res Rev 2010;26:585–592 [DOI] [PubMed] [Google Scholar]
- 14.Campbell-Thompson M, Wasserfall C, Kaddis J, et al. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev 2012;28:608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pugliese A, Vendrame F, Reijonen H, Atkinson MA, Campbell-Thompson M, Burke GW. New insight on human type 1 diabetes biology: nPOD and nPOD-transplantation. Curr Diab Rep 2014;14:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pugliese A, Yang M, Kusmarteva I, et al. The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) Program: goals, operational model and emerging findings. Pediatr Diabetes 2014;15:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregg BE, Moore PC, Demozay D, et al. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab 2012;97:3197–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fineberg SE, Kawabata TT, Finco-Kent D, Fountaine RJ, Finch GL, Krasner AS. Immunological responses to exogenous insulin. Endocr Rev 2007;28:625–652 [DOI] [PubMed] [Google Scholar]
- 20.Campbell-Thompson ML, Montgomery EL, Foss RM, et al. Collection protocol for human pancreas. J Vis Exp 2012;e4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell-Thompson ML, Heiple T, Montgomery E, Zhang L, Schneider L. Staining protocols for human pancreatic islets. J Vis Exp 2012;e4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013;62:2595–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Agostino RB, Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–2281 [DOI] [PubMed] [Google Scholar]
- 24.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coca-Perraillon M. Local and global optimal propensity score matching. Late-breaking abstract presented at SAS Global Forum, 16–19 April 2007, Orlando, Florida [Google Scholar]
- 26.Coca-Perraillon M. Matching with propensity scores to reduce bias in observational studies. Late-breaking abstract presented at Northeast SAS Users Group Conference: NESUG 2006, 17–20 September 2006, Philadelphia, PA [Google Scholar]
- 27.Gonzalez-Galarza FF, Christmas S, Middleton D, Jones AR. Allele frequency net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res 2011;39:D913–D919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic ß-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010;59:2846–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu EH, Digon BJ 3rd, Hirshberg B, et al. Pancreatic beta cell function persists in many patients with chronic type 1 diabetes, but is not dramatically improved by prolonged immunosuppression and euglycaemia from a beta cell allograft. Diabetologia 2009;52:1369–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med 2012;209:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 1965;14:619–633 [DOI] [PubMed] [Google Scholar]
- 32.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 2009;155:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanafusa T, Imagawa A. Insulitis in human type 1 diabetes. Ann N Y Acad Sci 2008;1150:297–299 [DOI] [PubMed] [Google Scholar]
- 34.Itoh N, Hanafusa T, Miyazaki A, et al. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest 1993;92:2313–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dotta F, Censini S, van Halteren AG, et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci USA 2007;104:5115–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med 1985;313:353–360 [DOI] [PubMed] [Google Scholar]
- 37.Hänninen A, Jalkanen S, Salmi M, Toikkanen S, Nikolakaros G, Simell O. Macrophages, T cell receptor usage, and endothelial cell activation in the pancreas at the onset of insulin-dependent diabetes mellitus. J Clin Invest 1992;90:1901–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Somoza N, Vargas F, Roura-Mir C, et al. Pancreas in recent onset insulin-dependent diabetes mellitus. Changes in HLA, adhesion molecules and autoantigens, restricted T cell receptor V beta usage, and cytokine profile. J Immunol 1994;153:1360–1377 [PubMed] [Google Scholar]
- 39.Arif S, Leete P, Nguyen V, et al. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes 2014;63:3835–3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skog O, Korsgren S, Melhus A, Korsgren O. Revisiting the notion of type 1 diabetes being a T-cell-mediated autoimmune disease. Curr Opin Endocrinol Diabetes Obes 2013;20:118–123 [DOI] [PubMed] [Google Scholar]
- 41.Saisho Y, Butler AE, Meier JJ, et al. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat 2007;20:933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell-Thompson M, Wasserfall C, Montgomery EL, Atkinson MA, Kaddis JS. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA 2012;308:2337–2339 [DOI] [PubMed] [Google Scholar]
- 43.Gaglia JL, Guimaraes AR, Harisinghani M, et al. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest 2011;121:442–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams AJ, Thrower SL, Sequeiros IM, et al. Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab 2012;97:E2109–E2113 [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes 2014;63:3880–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. β-Cell mass and turnover in humans: effects of obesity and aging. Diabetes Care 2013;36:111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008;10(Suppl. 4):32–42 [DOI] [PubMed] [Google Scholar]
- 48.Wagner R, McNally JM, Bonifacio E, et al. Lack of immunohistological changes in the islets of nondiabetic, autoimmune, polyendocrine patients with beta-selective GAD-specific islet cell antibodies. Diabetes 1994;43:851–856 [DOI] [PubMed] [Google Scholar]
- 49.Oikarinen M, Tauriainen S, Honkanen T, et al. Analysis of pancreas tissue in a child positive for islet cell antibodies. Diabetologia 2008;51:1796–1802 [DOI] [PubMed] [Google Scholar]
- 50.Atkinson MA. Losing a grip on the notion of β-cell specificity for immune responses in type 1 diabetes: can we handle the truth? Diabetes 2014;63:3572–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilmot-Roussel H, Lévy DJ, Carette C, et al. Factors associated with the presence of glutamic acid decarboxylase and islet antigen-2 autoantibodies in patients with long-standing type 1 diabetes. Diabetes Metab 2013;39:244–249 [DOI] [PubMed] [Google Scholar]
- 52.Oram RA, McDonald TJ, Shields BM, et al.; UNITED Team . Most people with long-duration type 1 diabetes in a large population-based study are insulin microsecretors. Diabetes Care 2015;38:323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell-Thompson ML, Kaddis JS, Wasserfall C, et al. The influence of type 1 diabetes on pancreatic weight. Diabetologia 2016;59:217–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.