Abstract
The Müllerian duct (MD) is the anlage of the oviduct, uterus and upper part of the vagina, the main parts of the female reproductive tract. Several wingless-type mouse mammary tumor virus (MMTV) integration site family member (Wnt) genes, including Wnt4, Wnt5a and Wnt7a, are involved in the development of MD and its derivatives, with Wnt4 particularly critical, since the MD fails to develop in its absence. We use, here, Wnt4EGFPCre-based fate mapping to demonstrate that the MD tip cells and the subsequent MD cells are derived from Wnt4+ lineage cells. Moreover, Wnt4 is required for the initiation of MD-forming cell migration. Application of anti-Wnt4 function-blocking antibodies after the initiation of MD elongation indicated that Wnt4 is necessary for the elongation as well, and consistent with this, cell culture wound-healing assays with NIH3T3 cells overexpressing Wnt4 promoted cell migration by comparison with controls. In contrast to the Wnt4 null embryos, some Wnt4monomeric cherry/monomeric cherry (Wnt4mCh/mCh) hypomorphic mice survived to adulthood and formed MD in ∼45% of cases. Nevertheless, the MD of the Wnt4mCh/mCh females had altered cell polarization and basement membrane deposition relative to the controls. Examination of the reproductive tract of the Wnt4mCh/mCh females indicated a poorly coiled oviduct, absence of the endometrial glands and an undifferentiated myometrium, and these mice were prone to develop a hydro-uterus. In conclusion, the results suggest that the Wnt4 gene encodes signals that are important for various aspects of female reproductive tract development.
Introduction
The mammalian sex ducts are formed from the female Müllerian duct (MD) and the male Wolffian duct (WD) during embryogenesis. The paired MD, also called the paramesonephros, represents the primordium of the oviduct, the uterus and the upper part of the vagina, and is an integral part of the embryonic urogenital system. The MD initially forms in both the male and the female, but degenerates later in the male under the influence of the anti-Müllerian hormone (1,2).
The MD was described more than 200 years ago, but there are still lively discussions going on even today about its origin. It is now well-established that the MD cells do not originate from the WD cell population (2–4), even though the WD does provide important mechanical guidance cues and secretes certain signals that promote MD development (5). It is known that the coelomic epithelium (CoE) contributes to MD development, but it is unclear (in Amniota) whether it is invagination or a local thickening of CoE that establishes the primordium of the MD (3,4). What controls and guides the posterior elongation of the MD is also an open question.
Gene targeting experiments have indicated that several factors such as Gata3, paired-box gene 2 (Pax2), paired-box gene 8 (Pax8), LIM homeobox protein 1 (Lhx1), empty spiracles homeobox 2 (Emx2), homeobox A13 (Hoxa13) and wingless-type mouse mammary tumor virus (MMTV) integration site family member 4 (Wnt4), Wnt7a, Wnt9b regulate MD development (5–14). Of these, the Wnt4 gene encodes one of the key signals, since the MD fails to develop in its absence and only the extreme anterior Wnt7a-positive primordium is formed (13). Otherwise, the role of the Wnt4 gene during MD development remains unclear.
The MD-derived oviduct, uterus and upper part of the vagina reach sexual maturity around puberty. The mature uterus is composed of the endometrial stromal cells and the myometrium, which has inner and outer layers. During postnatal development the endometrial glands, which provide nutrients, growth factors and cytokines to prepare the uterus for possible pregnancy are derived from the luminal epithelium. Failure of these sequential steps is connected with infertility. Mutations in the human WNT4 gene are associated with Mayer–Rokitansky–Kuster–Hauser–Biason–Lauber (MRKHBL) and female SEx Reversal and dysgenesis of Kidneys, Adrenals, and Lungs (SERKAL) syndromes, which involve severe defects in the female reproductive tract but the underlying molecular mechanisms that distinguish between a normal and a pathological uterus are still for the most part poorly understood (15–20).
We have been able to show by means of time-lapse organ culture that the Wnt4+ progenitor cells contribute to the MD primordium and represent an important cell population that is required for MD assembly. Our findings also demonstrate that Wnt4 is needed not only for initiation of MD-forming cell migration and tip cell differentiation, but also for MD elongation. They suggest that the MD initiates its growth from the coelomic epithelial cells that invade the space beneath it and establish a funnel-shaped MD progenitor cellular unit. Moreover, using a novel hypomorphic Wnt4 monomeric cherry (Wnt4mCh) mouse model we have shown that Wnt4 is needed for cell polarization and proper basement membrane (BM) deposition in the developing MD and that it is also required later in uterine ontogenesis for endometrial gland and myometrium organization. Thus, the hypomorphic Wnt4mCh mice serve as a useful model for studying the mechanisms lying behind hyperplastic MD malformations and agenesis. The results suggest that Wnt4 may be involved in the development of endometrial disease and female infertility, thereby extending the role of this female sex determinant as a signal for the ontogenesis of the female reproductive tract.
Results
Initiation of MD growth requires Wnt4 signalling
The MD fails to form in Wnt4 knock-out embryos and only the anterior MD precursor cells differentiate, as depicted by the Wnt7a MD marker in in situ hybridization (Supplementary Material, Fig. S1, compare B with A, arrow) (13). To understand better how Wnt4 coordinates MD development, we examined the location of lineages derived from cells that have expressed Wnt4 by making the use of Wnt4EGFPCre mice crossed with floxed RosaR26R LacZ (R26R LacZ) so that the LacZ gene was flanked by LoxP sites and became activated by the Cre reaction.
Although no LacZ staining was seen in the controls at any of the stages analysed (Supplementary Material, Fig. S1C, E, G and I), the Wnt4EGFPCre; R26RLacZ embryos had positive cells scattered within the urogenital ridge at E12.5-E15.5 (Supplementary Material, Fig. S1D, F, H and J). These were more numerous in the anterior side of the urogenital ridge at E12.5 (Supplementary Material, Fig. S1D, arrow), whereas later, at E14.5, they tended to accumulate in the posterior portion (Supplementary Material, Fig. S1H, arrow).
Such observations led us to hypothesize that Wnt4 may be a factor that controls MD development by promoting cell migration. To study this, we performed lineage tracing of the cells expressing Wnt4 by time-lapse imaging of ex vivo urogenital ridge explant cultures from Wnt4EGFPCre; RosaR26R YFP (R26R YFP) embryos (Supplementary Material, Movies S1 and S2).
The movies revealed YFP expression in the region of the mesonephric tubules of the urogenital ridge, but not in the WD (Supplementary Material, Fig. S2A–F and Movie S1). Moreover, the YFP+ cells started to migrate from the anterior region towards the posterior region laterally with respect to the WD and parallel to it (arrows in Supplementary Material, Fig. S2A–F and Movie S1). Urogenital ridges of Wnt4EGFPCre embryos served as control material (Supplementary Material, Fig. S2G–L and Movie S2). This migration of the YFP+ cells correlated with the formation of the MD and occurred in both male and female embryonic explants.
The YFP+ cells migrated along the WD and CoE and appeared to shape the MD in the urogenital ridges (Supplementary Material, Movies S1). They then continued their migration along the resulting MD primordium even after it had reached the urogenital sinus (Supplementary Material, Movies S1). We conclude that cell lineages defined by Wnt4 expression participate in growth of the MD.
To characterize the spatial and temporal organization of the MD with respect to the WD and the CoE, we next performed anti-Pax2 immunostaining of the urogenital ridges at the age of 23–26 tail somites. The staining depicted both MD and WD structures (Supplementary Material, Fig. S2M–O). Further optical projection tomography (OPT) then provided 3D evidence of the funnel-shaped appearance of the anterior part of the MD and its close proximity to the WD at E12.5 (Supplementary Material, Fig. S2P, arrows).
The MD tip cells and some coelomic epithelial cells are derived from cells expressing Wnt4
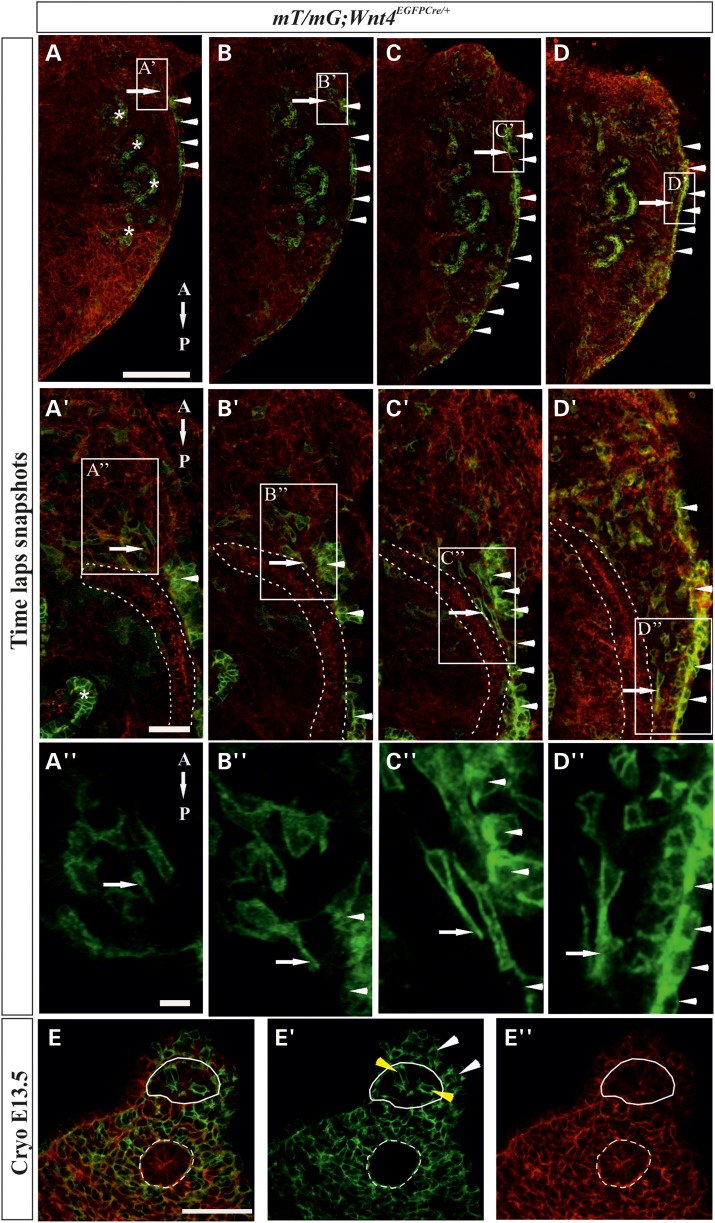
For a better insight into the dynamics of how Wnt4+ lineage cells participate in MD generation, we set out to obtain confocal time-lapse images of the Wnt4EGFPCre; mT/mG-positive embryonic urogenital ridge explants. In the mT/mG reporter, a membrane-targeted tomato (mT, red) is normally expressed in all the urogenital ridge cells. Once recombined with the Wnt4EGFPCre the cells start to express cell membrane-targeted green fluorescence protein (mG) and are considered to be the Wnt4+ lineage cells.
The time-lapse imaging revealed a specific group of GFP+ cells that formed the MD primordium (Fig. 1A–A″, arrows and Supplementary Material, Movie S3). It is significant that GFP was also expressed in the CoE (Fig. 1A′–D′, arrowheads) and the mesonephric tubules (Fig. 1A′–D″star), but not in the WD (Fig. 1A′–D″ and E, dashed line).
Figure 1.
The MD is constructed of cells of Wnt4EGFPCre lineage via cell migration, as depicted in time-lapse imaging. (A–D″) Selected snapshots from the time-lapse movie presented in Supplementary Material, Movie S3 depict an organ culture of a Wnt4EGFPCre; mT/mG embryonic urogenital ridge. (A) The MD primordium forms in the anterior part of the urogenital ridge in close proximity to the WD. Note the GFP+ Wnt4 lineage cells (A′ and A″ arrows). The CoE serves as a landmark for the migrating MD-forming cells (A′–D″, arrowhead). (B–B″) Micrographs depict a cell located in the MD primordium that has generated extensions (arrow) between the WD and the CoE (arrowhead). (C–C″) After differentiation of the tip cells (arrow) the MD-forming cells start to migrate in a posterior direction. (D–D″) The cellular processes protrude though the extracellular matrix between the WD and the CoE, which is positive for GFP+ (arrowheads). (E–E′″) Cryo-sections of the genital ridges of Wnt4EGFPCre; mT/mG embryos. (E) Merged images of the GFP+ (green) and mTomato (red) fluorescent cells marking Wnt4 lineage. (E′) The GFP+ cells of Wnt4+ lineage are seen (in green). The yellow arrowheads point to the Wnt4+ lineage cells contributing to MD assembly. Note that, there are also GFP− cells among the MD cells. Cells of Wnt4+ lineage are also present in the CoE, while the WD is negative for GFP. (E′″) Hoechst staining depicts the nuclei of the cells. The dotted line underlines the WD and the solid line the MD. Scale bars (A–D) 250 µm, (A′–D′) 50 µm, (A″–D″) 10 µm and (E–E′″) 50 µm.
We observed both GFP+ and GFP− cells that moved beneath the CoE and on the anterior side of it, and laterally with respect to the WD. Based on these data we define this heterogenous cell population as representing the MD node in the urogenital ridge (Supplementary Material, Movie S3). After several hours of culture, the GFP-positive cells derived from the MD node were seen to develop cytoplasmic extensions (Fig. 1A″–D″, arrows) and to protrude between the WD (Fig. 1A″–D″, dashed line) and the CoE (Fig. 1A″–D″, arrowheads). The migratory stream of GFP+ and GFP− cells behind the leading GFP+ cells also took part in shaping the MD primordium (Fig. 1A′–D′ and Supplementary Material, Movie S3). The CoE cells turned the GFP expression on via Wnt4EGFPCre in correlation with the process of MD elongation in developing urogenital ridge (Fig. 1A–D and Supplementary Material, Movie S3).
After the MD primordium had extended and reached the developing urogenital sinus, other cells continued to migrate towards the urogenital sinus via the maturing MD (Supplementary Material, Movie S3). Interestingly, some cells forming the MD were capable of changing their direction of migration and reverted from posterior movement to migrate anteriorly, against the dominant stream (Supplementary Material, Movie S3).
To depict the localization of the GFP+ cells during MD development, we made cryostat cross-sections of the Wnt4EGFPCre; mT/mG (Fig. 1E–E″′) and control (Supplementary Material, Fig. S2Q–Q′″) urogenital ridges at E13.5. The CoE and mesenchyme surrounding the developing MD and WD contained GFP+ cells (Fig. 1E′ white arrows), and some cells in the epithelial MD were GFP+ (Fig. 1E′, yellow arrow heads), whereas no GFP expression was noted in the WD (Fig. 1E–E″).
The presence of Wnt4+ lineage cells in the CoE alongside the elongating MD primordium suggested that the coelomic epithelium might play a role in MD growth. We made a mechanical incision in the CoE and studied the outcome by time-lapse imaging. Analysis of the movies showed that growth of the MD terminated at the level of the mechanical incision in the CoE and that the tip cells also made several attempts to migrate beyond the incision site, but failed (Supplementary Material, Movie S4 and Fig. S2R).
In order to reliably assign the failure in cell migration to the CoE, we analysed the integrity of the WD via confocal sections visible in the individual frames of the time-lapse movies. The results revealed that the mechanical insult was present only in the CoE and suggested an important role for the CoE in MD development. The study also indicated that the Wnt4 signal is critical for MD tip cell specification and for the initiation of migration of the MD-forming cells.
The Wnt4 signal is important for elongation of the MD
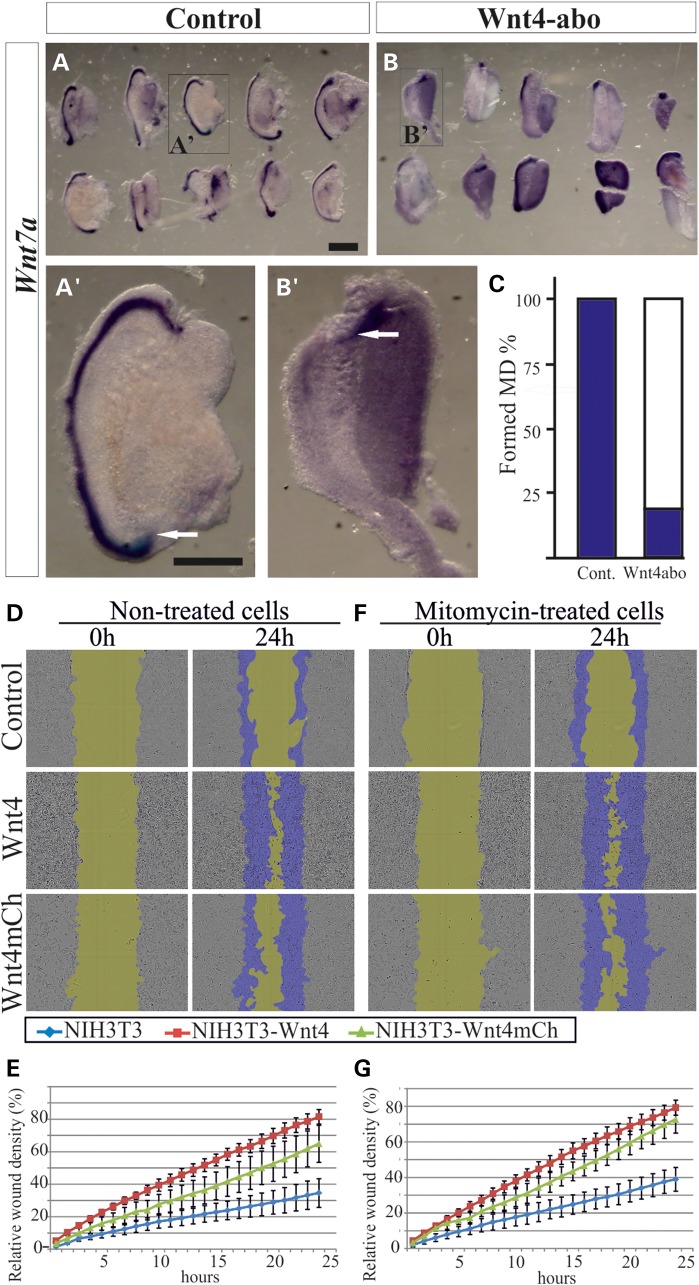
The data raised the question of whether Wnt4 would have a role in the subsequent growth of the MD. To study this possibility, we used anti-Wnt4 antibodies to inhibit Wnt4 activity in urogenital ridge explants from E11.5 embryos in an organ culture. The control specimens were treated with goat anti-human IgG. After 48 h of culture the explants were fixed, stained with Wnt7a RNA probe and analysed.
In the presence of the control IgGs the MD grew and reached the posterior end of the WD in all the samples studied (24/24) (Fig. 2A, enlarged in A′), whereas MD growth was inhibited in 79% (19/24) of cases in the presence of the anti-Wnt4 antibody (Fig. 2B, enlarged in B′), but the MD did develop in the remaining 21% (5/24) (Fig. 2C). We conclude that Wnt4 signalling is required not only for initiation of the cell migration that leads to MD formation, but also for MD extension.
Figure 2.
The Wnt4 function is required for the extension of the MD and scratch-wound recovery in vitro. Urogenital ridges were dissected at E11.5 and grown for 48 h in the presence of goat IgG immunoglobulins as controls (A). The MD was detected with the Wnt7a marker (A, and enlarged in A′, arrow). The MD failed to develop in ∼79% of the cases upon supplementation with anti-Wnt4 antibody (B, and enlarged in B′, arrow) (C). (D) Micrographs from the in vitro wounding assay with control NIH3T3, NIH3T3Wnt4+ and NIH3T3Wnt4mCh/mCh+ cells reveal a positive influence of Wnt4 on wound closing, as quantified in (E). (F) Treatment of the cells with mitomycin did not alter the behaviour of these cells significantly, as quantified in (G). Scale bars (A, B and A′–B″) 500 µm.
Wnt4 promotes cell migration in vitro, as judged by cell scratch-wound assay
Since the time-lapse studies of the Wnt4EGFPCre+ cells in the urogenital ridge explants suggested a role for Wnt4 in the control of cell migration, we analysed this further by means of in vitro scratch-wound assays of NIH3T3 cells that expressed Wnt4 and Wnt4mCh, the latter being a fusion between Wnt4 and the mCherry protein, representing a hypomorphic allele (21). The parental NIH3T3 cells, which did not express Wnt4 protein (22), were used as a control.
In order to determine whether scratch-wound closure was driven by cell proliferation or cell migration properties, we treated one set of cells with mitomycin, an inhibitor of cell proliferation. After wounding, the cells were cultured and imaged once per hour for 24 h. We then analysed the resulting time-lapse images and calculated the relative scratch-wound density after 24 h of culture (Fig. 2D).
The relative wound density was 42% in the control cells, 74% in the NIH3T3Wnt4 cells and 67% in the NIH3T3Wnt4mCh cells (Fig. 2E). Similar results were obtained in the presence of mitomycin (Fig. 2F). The relative scratch-wound densities were close to those for untreated cells after 24 h of culture, which were 54% in the control cells, 72% in the NIH3T3Wnt4 cells and 59% in the NIH3T3Wnt4mCh cells (Fig. 2G). Taken together, the data suggest that Wnt4 may control MD development by inducing cell migration.
The canonical Wnt signalling pathway inhibiting small molecule XAV939 influences MD formation
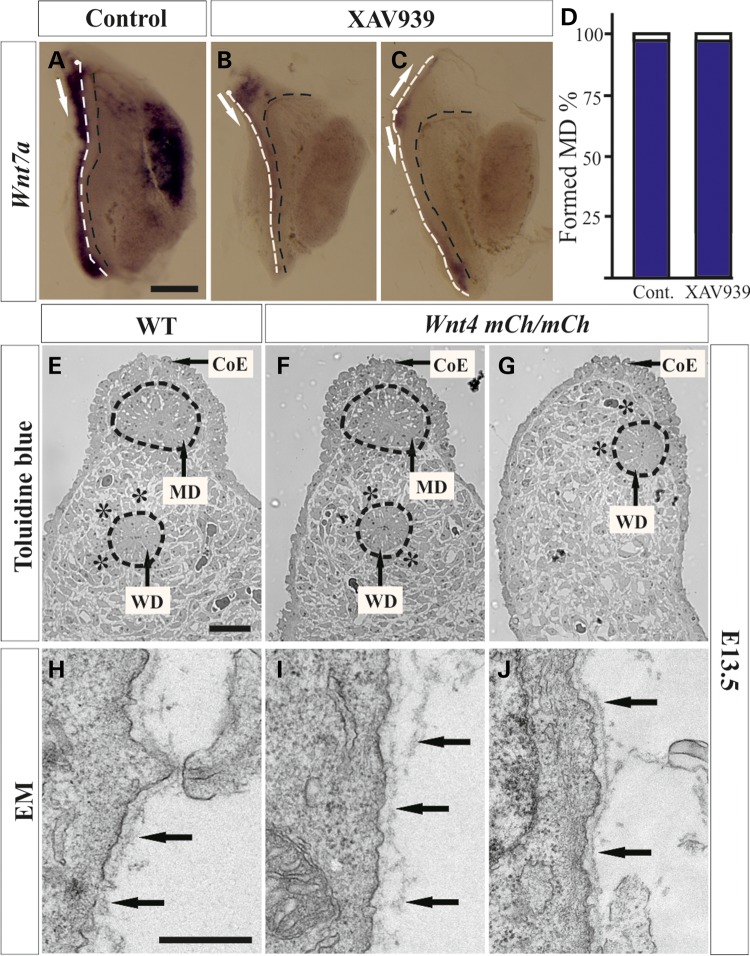
As Wnt4 may signal via both the canonical/β-catenin pathway and non-canonical pathways (23–25), we used the tankyrase inhibitor XAV939 to ascertain whether β-catenin-mediated signalling is involved in MD development (26). The XAV939 was diluted in dimethyl sulphoxide (DMSO) and control specimens were supplemented with media containing a corresponding amount of DMSO alone. After 48 h of culture, the degree of MD development was evaluated by in situ hybridization with the Wnt7a probe.
MD growth was initiated and the duct had extended posteriorly in both the control urogenital ridge specimens (96%) and those supplemented with XAV939 (95.8%) although the latter had an ambiguous MD and only weak Wnt7a RNA probe staining (Fig. 3A and B). In some cases, the MD extended not only posteriorly, as in the non-treated specimens, but also more apically (Fig. 3C). We conclude that the β-catenin signalling pathway inhibitor XAV939 does not perturb MD growth, but it does influence the overall MD morphology in vitro.
Figure 3.
Changes in MD growth caused by the tankyrase inhibitor XAV939 and the hypomorphic Wnt4mCh/mCh allele. The MD develops normally in the presence of DMSO, serving as a control (A, white line, arrow). The Wnt7a MD marker is weak in the presence of XAV939, so that ∼41% of the MDs have branched on the apical side (B, arrows) and some have extended not only posteriorly, but also anteriorly (C, arrows). XAV939 does not block MD growth, but it severely affects the properties of the resulting MD (D). (E) Toluidine blue staining detects the wild-type urogenital ridge at E13.5. The MD was formed in 45% of the Wnt4mCh/mCh mice (F) and failed to form in 55%. The mesenchymal cells were not organized in a concentric manner around the WD at E13.5 in the Wnt4mCh/mCh mice, in contrast to the controls (stars in E and G). The failure of MD formation influences the localization of the WD and CoE (arrows in E and G). The transmission electron micrographs depict a well-condensed BM around the control MD (H, arrows) but a loose, malformed one around its Wnt4mCh/mCh counterpart (I, arrows). The BM surrounding the WD remains intact (J, arrows). MD, white dashed line, and WD, black dashed line in (A–C). CoE, coelomic epithelium. Scale bars (A–G) 500 µm and (H–J) 250 nm.
The hypomorphic allele depicts roles for Wnt4 in reproductive tract development during embryogenesis
To address the putative later functions of Wnt4 in the development of the urogenital ridge, we took advantage of the recently developed novel Wnt4mCh/mCh mouse model, which represents a fusion protein and provides a hypomorphic Wnt4 allele (21).
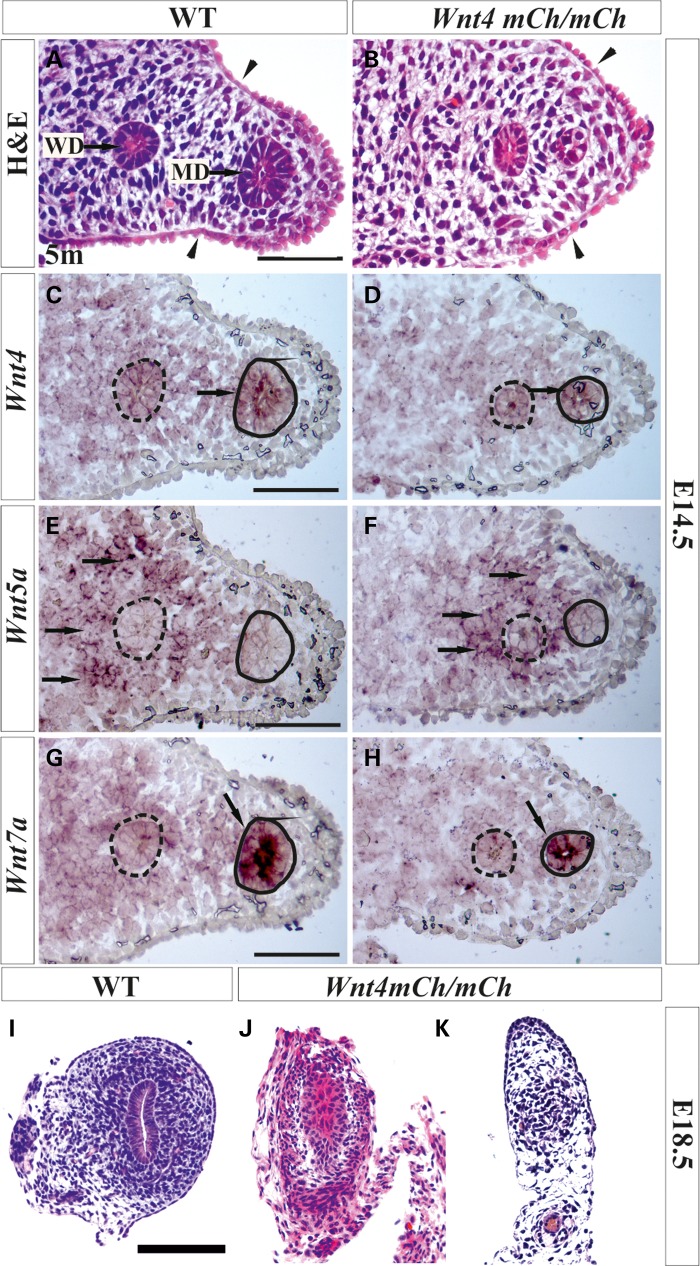
Interestingly, the MD was found to be formed in ∼45% of the Wnt4mCh/mCh embryos, but in the remaining 55% it had failed to develop (Fig. 3E–G). This made it possible to study the role of Wnt4 later on in sex-duct development in more detail. We analysed the degree of MD development in the urogenital ridge at three time points: E13.5, when the MD has just formed, E14.5 when it is still undifferentiated, and before birth, at E18.5, when it has become differentiated into the oviduct, uterus and upper part of the vagina.
The Wnt4mCh/mCh MD phenotype varied from being close to the wild-type to complete ductal agenesis, as depicted at E13.5 (Fig. 3E–G). The lack of any Wnt4 signal also affected the CoE (Fig. 3E, arrows). In the cases where the duct did not form, the CoE cells became cuboidal and were displaced together with the WD (Fig. 3G, compare with E). The mesenchymal cells normally establish a concentric organization around the WD in wild-type urogenital ridges, whereas the Wnt4mCh/mCh mesenchymal cells failed to do so. The defect was more prominent in those urogenital ridges where the MD did not form at all (Fig. 3G, compare with E and F stars).
Examination of the ultrastructure of the Wnt4mCh/mCh MD cells revealed that the BM was slack in its deposition at E13.5 and formed loops, whereas the wild-type BM was more solid (Fig. 3H compare with I, arrows). In contrast, the WD had a properly developed BM in both the wild-type (data not shown) and the Wnt4mCh/mCh specimens (Fig. 3J, arrows). The grooves of the MD ridge, as seen in the wild-type, were often missing in the Wnt4mCh/mCh embryos, as shown at the age of E14.5 (Fig. 4A and B, arrowheads). The MD differed notably in diameter between the Wnt4mCh/mCh and control specimens (Fig. 4A and B).
Figure 4.
The Wnt4mCh/mCh hypomorph influences prenatal development of the MD and uterus. (A) The wild-type mice have a well-developed MD at E14.5, whereas MD formation is impaired in the Wnt4mCh/mCh mice (B). The arrowheads in (A and B) depict the urogenital ridge groove that typically fails to form in the Wnt4mCh/mCh embryos (compare B with A). The Wnt4 gene is expressed in the MD (C and D, arrow) and in the mesenchyme around the MD and the WD in both the control (C) and Wnt4mCh/mCh embryos (D). Wnt5a is expressed in the mesenchyme (E and F, arrows), while Wnt7a mRNA is located in the epithelial cells of the MD (E and G, respectively, arrows) in the wild-type and Wnt4mCh/mCh embryos (F and H, arrows). (I) The wild-type uterus has an open lumen at E18.5, whereas the Wnt4mCh/mCh embryos have a rudimentary uterus (J) or none at all (K). The continuous line depicts the MD and the dashed line the WD (C–H). Scale bars (A–H) 50 µm, (E–G) 100 µm.
In situ hybridization analysis of Wnt4, Wnt5a and Wnt7a, the Wnt genes involved in MD development, indicated that the MD and its surrounding stromal cells in both the wild-type and the Wnt4mCh/mCh embryos were positive for the Wnt4 RNA probe at E14.5 (Fig. 4C and D). There was extensive Wnt5a expression in the stroma of the urogenital ridge around the WD in the controls, whereas the expression was more compact in the Wnt4mCh/mCh mice (Fig. 4E and F). Wnt7a was expressed in both the control and Wnt4mCh/mCh epithelial cells, (Fig. 4G and H). We conclude that the reduction in Wnt4 signalling does not alter the expression of the Wnt5a or Wnt7 genes in the urogenital ridge to any great extent.
The wild-type uterus was robust and oviduct had formed folds at E18.5 (Supplementary Material, Fig. S3A and B), whereas the uterus was ambiguous and the oviducts lacked folds in the Wnt4mCh/mCh embryos (Supplementary Material, Fig. S3C and D). Histological sections of the wild-type embryo uterus exhibited a well-defined, open uterine lumen (Fig. 4I), while the uterus in the Wnt4mCh/mCh embryos was either rudimentary (Fig. 4J) or had completely failed to form (Fig. 4K).
Wnt4 signalling is critical for formation of the endometrial glands of the uterus
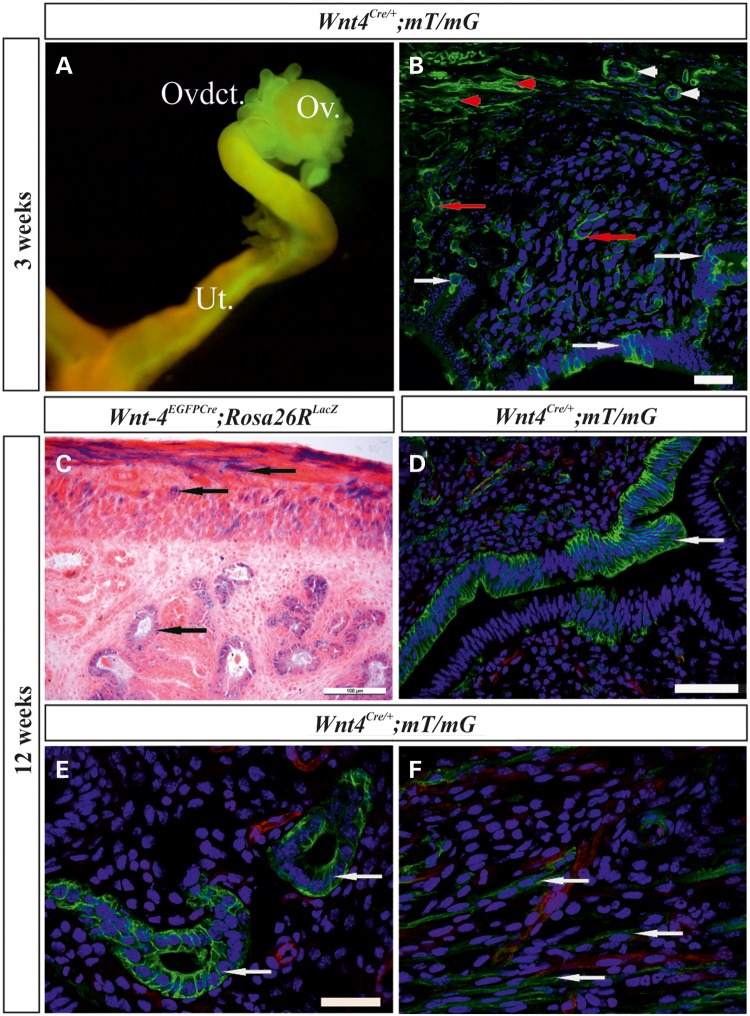
Analysis of the distribution of Wnt4+ lineage cells in the uterus of postnatal Wnt4EGFPCre; mT/mG mice at 3 weeks of age (postnatal day P21, Fig. 5A) showed these to be present in the uterine epithelium (Fig. 5B, white arrows), luminal glands (Fig. 5B, red arrows), myometrium (Fig. 5B, red arrowheads) and endothelial cells surrounding the blood vessels (Fig. 5B, white arrowheads). A corresponding analysis performed on Wnt4EGFPCre; RosaR26RLacZ mice showed that cells of Wnt4+ lineage were located among the myometrium cells of the uterus (Fig. 5C, black arrows) and in the endometrial gland epithelium (Fig. 5C, white arrows).
Figure 5.
Fate mapping of the established Wnt4EGFPCre progenitor cell lineage reveals daughter cells in the female reproductive tract. (A) Marked GFP+ cells are detected in the ovary (Ov), oviduct (Ovdct) and uterus (Ut) of the Wnt4EGFPCre;mT/mG mice at 3 weeks of age (P21). (B) GFP+ cells are situated in the uterine luminal epithelium, the luminal glands (white arrows) and the interstitial (red arrows), myometrium (red arrowheads) and endothelial cells around the blood vessels (white arrowheads). (C) β-galactosidase staining of the uterus of the Wnt4EGFPCre; RosaR26RLacZ females reveals positive cells in the myometrium and endometrial glands (arrows). (D) Marked GFP+ cells are present in the epithelium of the uterus (arrow), the endometrial glands (E, arrows) and the myometrium (F, arrows) of the Wnt4EGFPCre;mT/mG mice at 12 weeks of age (C–F). Scale bar (B–F) 100 µm.
The 12-week-old Wnt4EGFPCre;mT/mG mice showed GFP-positive cells in the luminal epithelium (Fig. 5D, arrow), endometrial glands (Fig. 5E, arrow) and myometrium of the uterus (Fig. 5F, arrows). In conclusion, cells of Wnt4+ lineage participate in MD growth and contribute later to the formation of the uterine myometrium and endometrium and to its vasculature development.
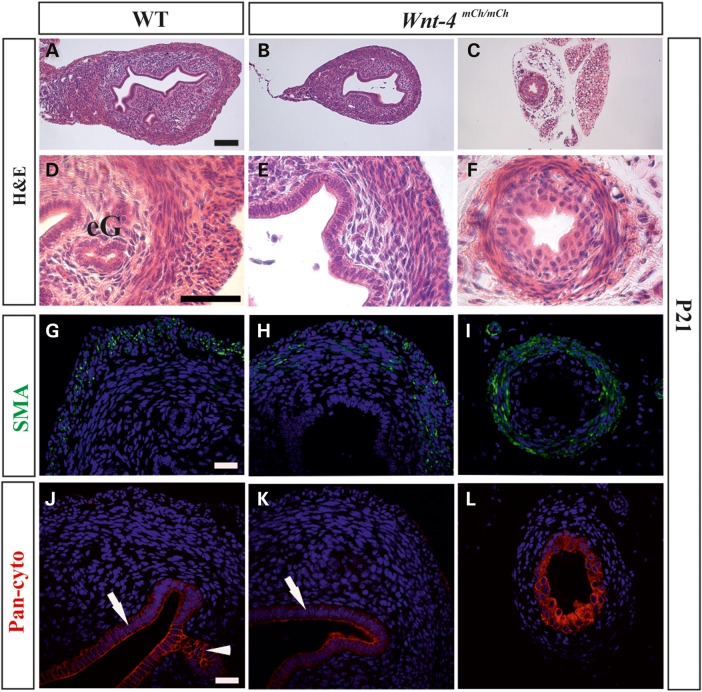
Further analysis of the uterus before sexual maturity, on Day P21, demonstrated that the Wnt4mCh/mCh mice had a thinner uterus than the wild-type mice (Fig. 6B and E compare with A and D). Notably, in line with Wnt4 knock-out females that become masculinized (13), the Wnt4mCh/mCh female mice had WD derivatives, an epididymis (21) and a vas deferens type of structure embedded in fat-like tissue (Fig 6C and F). Staining with alpha-smooth muscle actin (α-SMA) antibody showed smooth muscle cell differentiation in both the wild-type and the Wnt4mCh/mCh uterus (Fig. 6G and H), and a smooth muscle layer had also formed in the vas deferens-like tube (Fig. 6I).
Figure 6.
The uterus is severely compromised in adult hypomorphic Wnt4mCh/mCh females. The uterus is reduced in size in Wnt4mCh/mCh adult mice (P21) and no luminal or endometrial glands are formed, whereas these develop in the wild-type mice (compare B and E with A and D). (C and F) In some cases the WD derivatives remained in the Wnt4mCh/mCh females and formed a vas deferens-like structure (C and F). SMA (green) staining depicts two distinct muscle layers in the wild-type uterus at P21 (G), whereas the Wnt4mCh/mCh uterus has a thinner myometrium layer (H). The vas deferens-like structure in the Wnt4mCh/mCh females has a smooth muscle distribution typical of a wild-type male (I). (J) Pan-cytokeratin staining highlights the luminal epithelium and forming luminal glands (arrow) in the wild-type, whereas no luminal glands have formed in the Wnt4mCh/mCh mice at 5 months of age (K, arrow). (L) The epithelium of the vas deferens-like structure in the Wnt4mCh/mCh mice had a distorted cell distribution, as depicted by pan-cytokeratin staining (P21). Scale bars (A–C) 100 µm, (E and F) 50 µm and (G–L) 100 µm.
The pan-cytokeratin antibody had stained the luminal epithelium and the emerging endometrial glands in the wild-type, but importantly, no endometrial gland formation was detected in the Wnt4mCh/mCh uterus (Fig. 6J and K). The vas deferens-type structure had epithelial cells that were positive for pan-cytokeratin staining, but they were cuboidal in shape rather than pseudo-stratified and columnar as is typical of the male vas deferens (Fig. 6L).
Some of the Wnt4mCh/mCh mice reached sexual maturity and survived to 5 months of age, but most of them had unilateral or bilateral hydro-uterus, probably caused by the congenital vaginal abnormalities (Supplementary Material, Fig. S3E compare with F and G). In the cases where the uterus had failed to form, only a primitive mesometrium was present (Supplementary Material, Fig. S3H, arrow heads).
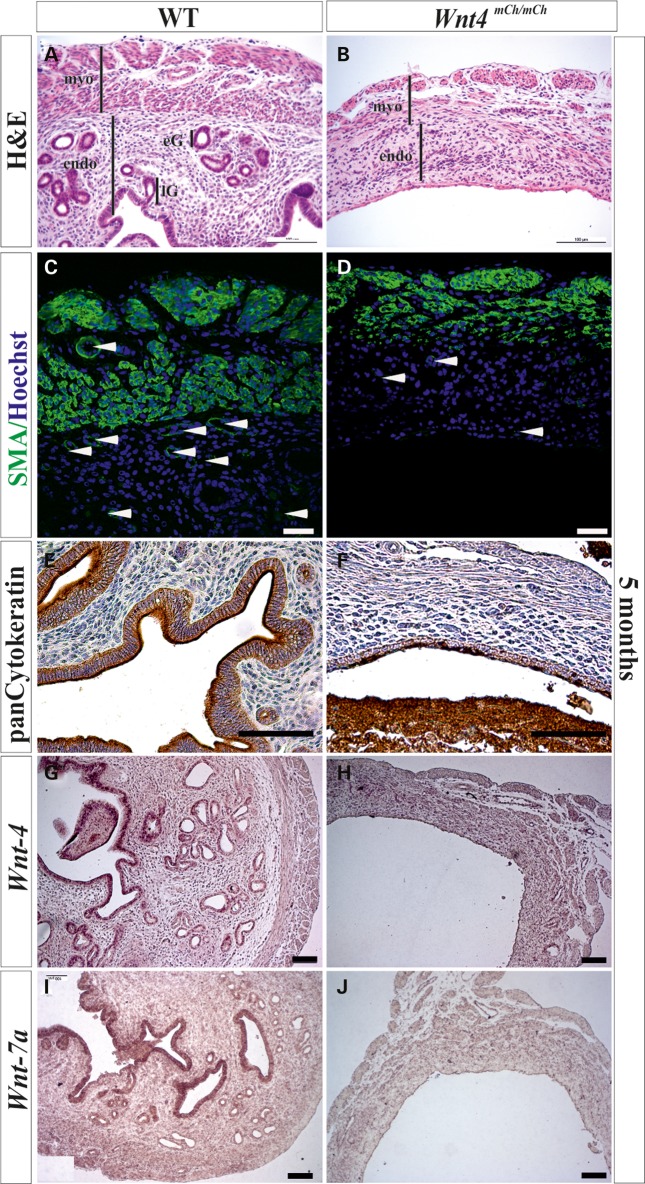
Histological examination revealed that while the wild-type uterus had a clearly distinguishable myometrium, endometrium and lumen (Fig. 7A), the uterus in the severe cases among the Wnt4mCh/mCh mice was dilated and largely lacking in uterine components (Fig. 7B). The smooth muscle layer of the uterine myometrium is normally composed of two parts, an inner circular one and an outer longitudinal one (Fig. 7C), but in the Wnt4mCh/mCh case these two parts were indistinguishable, as revealed by immunostaining for α-SMA (Fig. 7D). The endometrium of the Wnt4mCh/mCh uterus did not have the well-developed vasculature system as seen in the wild-type mice (Fig. 7D compare with C, arrowheads). The pan-cytokeratin antibody depicted the luminal epithelium and endometrial glands in the wild-type mice (Fig. 7E), whereas these were missing in the severely malformed uterus of the Wnt4mCh/mCh females (Fig. 7F).
Figure 7.
The myometrium and endometrium of the uterus are compromised in Wnt4mCh/mCh females. (A) The wild-type uterus has a well-differentiated myometrium and endometrium with endometrial glands. (B) The Wnt4mCh/mCh uterus has a thin layer of myometrium and endometrium without any luminal epithelium or endometrial glands. (D)The uterus normally has two layers of smooth muscle cells and a well-vascularized endometrium (C, arrowheads), whereas the Wnt4mCh/mCh uterus fails to form two layers within the myometrium and the number of blood vessels is reduced (compare D with C, arrowheads). Pan-cytokeratin antibody staining shows the formation of endometrial glands in the wild-type mice (E) and failure of endometrial gland formation in the rudimentary epithelium of the Wnt4mCh/mCh mice (F). (G) The Wnt4 gene is expressed normally in the luminal epithelium and endometrial glands of the wild-type uterus, but is lost in the case of Wnt4mCh/mCh hypomorphism (compare H with G). (I) Wnt7a is expressed in the luminal epithelium and luminal glands of the wild-type uterus, but no mRNA is detected in the Wnt4mCh/mCh uterus (J) at 5 months of age. Myo, myometrium; endo, endometrium; eG, endometrial gland; lG, luminal glands. Scale bar (A–J) 100 µm.
When we analysed the expression pattern of Wnt genes in the 5-month-old mouse uterus by in situ hybridization Wnt4 expression was depicted in the luminal epithelium, endometrial glands and stromal cells of the wild-type uterus (Fig. 7G), but none was present in the Wnt4mCh/mCh mice, since development of the luminal epithelium and endometrial glands had been impaired (Fig. 7H). Wnt7a expression is normally restricted to the uterine luminal epithelium (Fig. 7I), but this expression was lost in the Wnt4mCh/mCh mice (Fig. 7J). Taken together, these observations suggest that ablated Wnt4 signalling causes defects at very early stages in MD development which lead to further flaws in uterus development and endometrial gland formation.
Discussion
The mechanism of formation of the MD anlage is still being actively discussed. Although one model has suggested that WD-derived cells contribute to MD growth, lineage tracing experiments have shown that this is not the case (2,4). The WD is needed as a physical guide in the elongation of the MD, however, and it also provides important signals, as demonstrated in Wnt9b knock-out mice. These mice have a WD that is the source of Wnt9b in the wild-type, but MD elongation is perturbed once Wnt9b signalling has been ablated (5). The model that is currently favoured suggests that the MD originates from the CoE, which extends caudally (1,3,4,6,27).
Our time-lapse movies provided evidence that the cell population of the MD primordium indeed originates from the coelomic epithelial cells by migration rather than invagination, in line with earlier studies (3). Our findings indicate that Wnt4 expression is needed for differentiation of the tip cells and initiation of migration in the MD precursor cells. These cells are guided not only by the WD, but also by signals coming from the CoE. In line with this, we observed that an intact CoE is vital for MD growth, as damage to it prevented MD elongation even when the WD remained intact. Interestingly, the cells of the CoE initiated Wnt4 expression concurrently with MD elongation, which suggests that certain signals such as Wnt4 are expressed in a specific spatiotemporal manner and serve to guide MD growth. We also found that the CoE of the Wnt4mCh/mCh embryos which failed to form any MD had shifted in position and altered in its cell morphology. These observations suggest that the CoE may have an important, but as yet uncharacterized role in controlling MD growth.
Cells of multiple lineages may be needed to construct the MD. Our Wnt4+ cell-lineage tracing experiments with a dual reporter mouse strain (mT/mG) showed that the MD had not only Wnt4EGFPCre-activated GFP+ cells, but also GFP− cells, i.e. that multiple cell populations could be involved in forming the MD. The possibility also remains, however, that some cells (in the Wnt4EGFPCre;mT/mG mice) might have been Wnt4EGFPCre GFP-negative due to epigenetic silencing of the modified Wnt4 locus.
The Wnt4-positive cells at the tip had cytoplasmic extensions and represented the cells that were leading the migration involved in MD development, while those that migrated behind the leading edge consisted of both Wnt4+ and Wnt4− lineage cells and served to form the duct-like structure. The movies indicated that cells did indeed continue to migrate caudally after the MD primordium had reached the urogenital sinus (1).
The cells that assembled the duct demonstrated epithelial characteristics in that they were located close to each other, they had a limited extracellular matrix, their apical surface was exposed towards the lumen and they had deposited a basement membrane. Nevertheless, despite these numerous morphological epithelial cell features, the embryonic MD at E13.5 in the urogenital ridge did not express typical epithelial cell polarity markers such as cytokeratin-8 or E-cadherin, and it should therefore be referred to as a mesoepithelial duct (2). The tip cells that were derived from the Wnt4-positive founder cells became integrated as part of the MD primordium and underwent a mesenchymal-epithelial transition, thereby confirming previous observations that MD tip cells have mesenchymal cell characteristics (4). Our current results demonstrate that the MD tip cells were derived from the Wnt4+ founder cell population.
We were also interested in studying whether Wnt4 is functional after the initiation of the migration of MD-forming cells. An antibody-blocking assay, a useful tool for studying protein functions (28), showed that anti-Wnt4 antibodies did indeed inhibit elongation of the MD. This suggests that the Wnt4 signal is required not only for the initiation of MD growth, but also for subsequent elongation of the duct. Further evidence of the role of Wnt4 as a promoter of cell migration was obtained in our in vitro scratch-wound-healing assays with NIH3T3 cells that stably expressed Wnt4 or Wnt4mCh. The results indicated that cells expressing Wnt4 closed the scratch-wound faster than did the controls. The Wnt4mCh cells, which represent a hypomorphic Wnt4 allele, migrated with an intermediate speed relative to NIH3T3Wnt4 and the controls. Thus, the data support the conclusion that Wnt4 regulates cell migration and in that way controls MD assembly. Similarly Wnt4 is also involved in controlling the migration of endothelial and steroidogenic cells (29,30). Moreover, the anti-androgen flutamide that inhibits androgen action did not rescue formation of the MD in Wnt4−/− embryos and adult mice (29), a circumstance that is in line with the role of Wnt4 in controlling MD growth.
Wnt4 transduces its signalling via both canonical and non-canonical pathways. We used the tankyrase inhibitor XAV939, a molecule that has been successfully used before in an organ culture set-up (31), to inhibit the β-catenin signalling pathway in urogenital ridge explant cultures (26), and found it to have a negative effect on MD formation in vitro, causing defects resembling the ones seen in mice with ablated β-catenin signalling (27,32,33). The compound did not inhibit MD growth to any great extent, however. It was also shown that lymphoid enhancer factor 1 (the downstream target of β-catenin signalling) and Wnt4 signals overlap only in the mesenchyme cells that surround the MD, although Wnt4 expression appears to be broader, to include the CoE as well (27). All told, this suggests that Wnt4 could signal via alternative pathways during MD development, a matter that warrants further investigation.
An MD formed in ∼45% of the samples from Wnt4mCh/mCh hypomorphic mice, but the reduction in the Wnt4 function caused notable defects in differentiation, including alterations in MD cell polarization, MD tube organization and BM deposition. These findings are in line with our earlier observations that Wnt4 regulates the organization of granulose cells in ovaries and influences BM deposition in growing ovarian follicles (21).
Our analysis of the uterus in the Wnt4mCh/mCh females at P21 revealed that the uterine glands had failed to form the myometrium and that the uterus was smaller and dilated due to the formation of a hydro-uterus. Moreover, our previous work had shown a prolonged oestrous cycle in the Wnt4flox/flox; Amhr2Cre conditional knock-out mouse model (21). Fewer uterine glands and a defective decidualization process have been reported in Wnt4flox/flox; PRCre conditional knock-out mice (34). Our current findings indicate that Wnt4 is required for the epithelization process both during embryogenesis, when the MD forms, and after birth, when the endometrial glands develop. Similarly, Wnt4 controls conversion of the mesenchymal pre-tubular aggregates to epithelial vesicles during kidney development (35). The actual detailed molecular mechanism of the mesenchymal-to-epithelial transition in the MD nevertheless remains to be elucidated.
In summary, Wnt4 is a crucial factor for the initiation and maintenance of the cell migration that leads to MD formation during female reproductive tract development. In the adult mouse, it is needed for proper myometrium layering and for luminal and endometrial gland formation (Fig. 8). We show that balanced Wnt4 signalling is constantly needed during embryogenesis, adolescence and adult life to ensure the proper formation and functioning of the female reproductive tract. A deeper understanding of the function of Wnt4 in female sexual-duct development would help us to explain the pathophysiological mechanisms causing MRKHBL and SERKAL syndromes, endometriosis and infertility.
Figure 8.
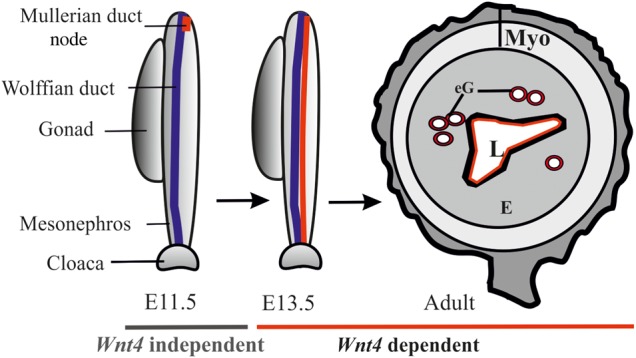
Schematic illustration of the processes regulated by Wnt4 during female reproductive tract development. Wnt4 is required during prenatal and postnatal development of female reproductive tract. The initial MD primordium occurs independently of Wnt4 function (E11.5, the MD primordium in red). After initiation of the process, differentiation of the MD tip cells, prenatal elongation of the MD and postnatal formation of the endometrial gland (eG) all depend on Wnt4 signalling. The daughter cells that are initially Wnt4+ contribute to MD and eG formation. Myo, myometrium; E, endometrium; eG, endometrial glands; L, lumen.
Materials and Methods
Maintenance and genotyping of the transgenic mouse lines
The Wnt4 knock-out (−/−) (35), Wnt4EGFPCre (36), RosaR26RLacZ (Soriano 1999), RosaR26RYFP (37) and mT/mG (38) mice were maintained and genotyped as reported. The generation of the Wnt4mCh/+ knock-in transgenic mice is described below. The transgenic mice were maintained in the C57Bl/6J OlaHsd background and littermates were used as controls. All the studies were conducted in accordance with the Finnish national legislation, the European Convention (ETS 123) and EU Directive 86/609/EEC.
Generation of Wnt4mCh knock-in transgenic mice
The Wnt4mCh cDNA was produced by polymerase chain reaction (PCR) and targeted to the first exon of the Wnt4 locus (Supplementary Material, Fig. S4A). Southern blot analysis was used to screen the targeting event and a HindIII-KpnI fragment HK1.3 that corresponds to the nucleotides 2054 and 21773. The 5′ of the homologous arms of the targeting construct was used as a probe for the DNA screening of embryonic stem cells (Supplementary Material, Fig. S4B).
The activity of the fusion protein was analysed in the NIH3T3 cells, and mCherry-derived florescence served as an indication of expression (Supplementary Material, Fig. S4C). The Wnt4mCh mice were generated in the transgenic core facility at the Biocenter Oulu and genotyped by PCR analysis using DNA isolated from ear clip DNA. The wild-type Wnt4 allele was identified with the primers 5′-ACTCCTCGTCTTCGCCGTGT and 5′-CAGACGCACTGCCAGCCC, while the mCherry allele was depicted with the primers 5′-CGGGAGGCGGCCTTTGTAT and 5′-GGCTTTAGATGTCTTGTTGC. The PCR programme was 94°C for 10 min, 34 cycles at 94°C for 30 s and 58°C for 30 s, followed by an extension at 72°C for 1 min and a final extension step at 72°C for 7 min. Five-percent DMSO was added to the wild-type PCR. The size of the amplicon of the wild-type PCR is 257 bp, while that of the targeted PCR is 593 bp (Supplementary Material, Fig. S4D).
Histology, immunohistochemistry and β-galactosidase assay
The tissues were dissected in Dulbecco’s phosphate buffered saline (PBS), fixed overnight at +4°C in 4% paraformaldehyde (PFA), washed briefly, dehydrated and embedded in paraffin and sectioned (6 µm). The tissue sections were stained with haematoxylin and eosin or used for immunohistochemistry. Antigen retrieval was enhanced by boiling the slides in 0.02 m citric acid buffer, pH 6, for 20 min or by incubating them in 0.04% pepsin, pH 2, for 30 min. followed by blocking with the appropriate serum for 1 h.
The primary antibodies used were Pax2 (Covance, PRB-276P) (1:100), α-SMA (Abcam ab5694) (1:100) and pan-cytokeratin (1:100) (Santa Cruz, sc-15367). These were incubated overnight at +4°C, washed with PBS, followed by 1 h of staining with the respective Alexa Fluor 488/546 or HRP-conjugated secondary antibody at a dilution of 1:1000. Hoechst 33258 (Polyscience, Inc.) was used to stain the nucleus. The specimens were mounted with Immu-Mount (Fisher Scientific), inspected using a confocal microscope and photographed (Olympus Fluoview FV10-ASW).
The activity of the floxed RosaR26R-derived β-galactosidase was identified as published (36), counterstained with eosin for 2 min and mounted with Immu-Mount (Fisher Scientific).
In situ hybridization
The non-radioactive in situ hybridization with digoxigenin-labelled RNA probes was performed with the aid of the Intavis AG Bio analytical instrument for the whole mounts, and the BioLane HTI for the slides, as described in (29). The Wnt4, Wnt5a and Wnt7a plasmids required for generating the RNA probes were gifts from A. P. McMahon (Eli & Edythe Broad Center for Regenerative Medicine and Stem Cell Research at USC, USA).
Organ culture and time-lapse video microscope analysis of the urogenital ridges
The urogenital ridges were micro-dissected from the Wnt4EGFPCre; R26RYFPflox/+ and Wnt4EGFPCre; mT/mG embryos at E11.5 in ice-cold Dulbecco's PBS and placed on Transwell plates (Costar 3450, Corning, Inc.). The tissues were cultured in Dulbecco's modified Eagle's medium (DMEM) 21063 (Gibco) for the time-lapse, whereas DMEM, GlutaMAX (GibcoBRL, Gaithersburg) medium supplemented with 10% foetal bovine serum (FBS), 0.5 mm 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid, N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (Sigma, H0887), and 1% penicillin/streptomycin was employed in the antibody-blocking assay. The temperature was maintained at 37°C by means of the TControl Basic 2.3. Program (OkoLab) and a carbon dioxide level of 5% was maintained via the microscope stage incubator (OkoLab). Time-lapse images of the Wnt4EGFPCre; R26RYFPflox/+ explants were taken at 20 min intervals with an Olympus XM10 digital camera using the Olympus Cell^P program.
The time-lapse images of the urogenital ridges prepared from the Wnt4EGFPCre; mT/mG embryos were taken at 5 min intervals with a Zeiss LSM 780 confocal microscope. The novel organ culture set-up used will be described in detail elsewhere. Briefly, the samples were placed on glass cover slips glued to the bottom of a six-well plate and gently compressed by Transwell inserts. In this setting, the images were taken directly through the glass cover slips to obtain the best optical resolution.
We examined time-lapse movies of eight Wnt4EGFPCre; mT/mG, two mTmG (control), five Wnt4EGFPCre; R26RYFPflox/+ and two R26RYFPflox/+ (control) urogenital ridges.
The antibody-blocking assay was performed with polyclonal anti-Wnt4 goat IgG (R&D Systems) in a concentration of 20 ng/µl, the control specimens being supplemented with 20 ng/µl of goat anti-human IgG antibodies (Jackson Immuno Research, Inc.). The tankyrase inhibitor XAV939 (Sigma-Aldrich) was diluted to 10 µm in DMSO (Sigma-Aldrich), and the controls were supplemented with DMSO alone. Urogenital ridges dissected from E11.5 embryos were placed on Transwell inserts in a medium with the antibodies or XAV939 and cultured for 48 h, washed briefly in PBS, fixed in 4% PFA and processed for whole mount in situ hybridization.
Optical projection tomography
Whole mount urogenital ridges from E12.5 embryos were fixed in 4% PFA and stained with Pax2 (Covance, PRB-276P) primary antibody (1:100) followed by washes and staining with the Alexa Fluor 546 secondary antibody (1:800). The specimens were embedded in low-melting point agar, cleared in a mixture of benzyl benzoate and benzyl alcohol (2:1) for 24 h and imaged with an OPT Scanner 3001 m (Bioptonics Microscopy, UK). The OPT data were analysed with the Imaris software (Bitplane, Zurich Switzerland).
Transmission electron microscopy
The toluidine blue staining and processing of the samples for transmission electron microscopy (TEM) was carried out using the Tecnai GS Spirit Bio Twin microscope (FEI Europe, Edinhoven, Netherlands) as described (39). The images were acquired with a Quemesa CCD camera controlled by the iTEM software (Olympus Soft Imaging Solutions GmbH, Munster Germany).
Scratch-wound cell migration assay
Mouse embryonic fibroblast NIH3T3 cells, NIH3T3 Wnt4 and NIH3T3 Wnt4mCh cells were used in the in vitro scratch-wound assay. 2 × 104 cells/well were seeded onto 96-well plates (Essence Bioscience Image Lock 4379) and grown to confluence. The DMEM 21063 (Gibko) medium was supplemented with 1% penicillin/streptomycin and 10% FBS. A set of cells was incubated in medium containing 0.5 mg/ml of mitomycin (Sigma) for 3 h prior to making the scratch-wound to inhibit cell proliferation. After 24 h of cell culture one central scratch-wound per well was made using the 96-pin WoundMaker (Essence BioScience). After this the cells were grown for a further 24 h and the recovery of the scratch-wound was analysed by taking images at 1 h intervals with an IncuCyte™ automated microscope (Essence BioScience).
The images were analysed with the IncuCyte HD software (Essence BioScience) and the results presented in the form of relative wound densities and standard deviations for each time point. Relative wound density (%) represents the cell density in the wound area expressed relative to that outside the wound area as a function of time. Experiments were performed a minimum of three times in six replicates per experiment the averaged data of experiments is presented (Fig. 2D–G). The percent changes densities were as follows non-treated cells: control 1876%, Wnt4-3T3 1717% and Wnt4mCh-3T3 2068%; mitomycin-treated cells: control 2133%, Wnt4-3T3 1488% and Wnt4mCh-3T3 1764%.
Supplementary Material
Funding
This work was supported by grants from the Academy of Finland (206038 and 121647), the Centre of Excellence Grant 2012 – 2017 of the Academy of Finland (251314), the Sigrid Jusélius Foundation and the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement FP7-HEALTH-F5-2012-INNOVATION-1 EURenOmics (305608). Funding to pay the Open Access publication charges for this article was provided by Centre of Excellence Grant 2012 – 2017 of the Academy of Finland (284605).
Supplementary Material
Acknowledgements
We thank Härkman H., Kekolahti-Liias J., Haipus P., Eklund L. (PhD) and Viklund A. for their excellent technical assistance, Raija Sormunen, PhD, for expert assistance and advice concerning the transmission electron microscopy and Timo Pikkarainen, PhD, for his critical reading of the manuscript.
Conflict of Interest statement. None declared.
References
- 1.Fujino A., Arango N.A., Zhan Y., Manganaro T.F., Li X., MacLaughlin D.T., Donahoe P.K. (2009) Cell migration and activated PI3K/AKT-directed elongation in the developing rat Müllerian duct. Dev. Biol., 325, 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orvis G.D., Behringer R.R. (2007) Cellular mechanisms of Müllerian duct formation in the mouse. Dev. Biol., 306, 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacob M., Konrad K., Jacob H.J. (1999) Early development of the Müllerian duct in avian embryos with reference to the human. An ultrastructural and immunohistochemical study. Cells Tissues Organs, 164, 63–81. [DOI] [PubMed] [Google Scholar]
- 4.Guioli S., Sekido R., Lovell-Badge R. (2007) The origin of the Müllerian duct in chick and mouse. Dev. Biol., 302, 389–398. [DOI] [PubMed] [Google Scholar]
- 5.Carroll T.J., Park J.S., Hayashi S., Majumdar A., McMahon A.P. (2005) Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell., 9, 283–292. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi A., Behringer R.R. (2003) Developmental genetics of the female reproductive tract in mammals. Nat. Rev. Genet., 4, 969–980. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi A., Shawlot W., Kania A., Behringer R.R. (2004) Requirement of Lim1 for female reproductive tract development. Development, 131, 539–549. [DOI] [PubMed] [Google Scholar]
- 8.Grote D., Boualia S.K., Souabni A., Merkel C., Chi X., Costantini F., Carroll T., Bouchard M. (2008) Gata3 acts downstream of beta-catenin signaling to prevent ectopic metanephric kidney induction. PLoS Genet., 4, e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller C., Pavlova A., Sassoon D.A. (1998) Differential expression patterns of Wnt genes in the murine female reproductive tract during development and the estrous cycle. Mech. Dev., 76, 91–99. [DOI] [PubMed] [Google Scholar]
- 10.Simpson J.L. (1999) Genetics of the female reproductive ducts. Am. J. Med. Genet., 89, 224–239. [DOI] [PubMed] [Google Scholar]
- 11.Taylor H.S., Fei X. (2005) Emx2 regulates mammalian reproduction by altering endometrial cell proliferation. Mol. Endocrinol., 19, 2839–2846. [DOI] [PubMed] [Google Scholar]
- 12.Torres M., Gomez-Pardo E., Dressler G.R., Gruss P. (1995) Pax-2 controls multiple steps of urogenital development. Development, 121, 4057–4065. [DOI] [PubMed] [Google Scholar]
- 13.Vainio S., Heikkila M., Kispert A., Chin N., McMahon A.P. (1999) Female development in mammals is regulated by Wnt-4 signalling. Nature, 397, 405–409. [DOI] [PubMed] [Google Scholar]
- 14.Huang C.C., Orvis G.D., Kwan K.M., Behringer R.R. (2014) Lhx1 is required in Müllerian duct epithelium for uterine development. Dev. Biol., 389, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hempstock J., Cindrova-Davies T., Jauniaux E., Burton G.J. (2004) Endometrial glands as a source of nutrients, growth factors and cytokines during the first trimester of human pregnancy: a morphological and immunohistochemical study. Reprod. Biol. Endocrinol., 2, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi K., Yoshioka S., Reardon S.N., Rucker E.B. III, Spencer T.E., DeMayo F.J., Lydon J.P., MacLean J.A. II (2011) WNTs in the neonatal mouse uterus: potential regulation of endometrial gland development. Biol. Reprod., 84, 308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philibert P., Biason-Lauber A., Gueorguieva I., Stuckens C., Pienkowski C., Lebon-Labich B., Paris F., Sultan C. (2011) Molecular analysis of WNT4 gene in four adolescent girls with Müllerian duct abnormality and hyperandrogenism (atypical Mayer-Rokitansky-Kuster-Hauser syndrome). Fertil. Steril., 95, 2683–2686. [DOI] [PubMed] [Google Scholar]
- 18.Philibert P., Biason-Lauber A., Rouzier R., Pienkowski C., Paris F., Konrad D., Schoenle E., Sultan C. (2008) Identification and functional analysis of a new WNT4 gene mutation among 28 adolescent girls with primary amenorrhea and Müllerian duct abnormalities: a French Collaborative Study. J. Clin. Endocrinol. Metab., 93, 895–900. [DOI] [PubMed] [Google Scholar]
- 19.Biason-Lauber A., Konrad D., Navratil F., Schoenle E.J. (2004) A WNT4 mutation associated with Müllerian-duct regression and virilization in a 46,XX woman. N. Engl. J. Med., 351, 792–798. [DOI] [PubMed] [Google Scholar]
- 20.Mandel H., Shemer R., Borochowitz Z.U., Okopnik M., Knopf C., Indelman M., Drugan A., Tiosano D., Gershoni-Baruch R., Choder M. et al. (2008) SERKAL syndrome: an autosomal-recessive disorder caused by a loss-of-function mutation in WNT4. Am. J. Hum. Genet., 82, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prunskaite-Hyyrylainen R., Shan J., Railo A., Heinonen K.M., Miinalainen I., Yan W., Shen B., Perreault C., Vainio S.J. (2014) Wnt4, a pleiotropic signal for controlling cell polarity, basement membrane integrity, and anti-Müllerian hormone expression during oocyte maturation in the female follicle. FASEB J., 28, 1568–1581. [DOI] [PubMed] [Google Scholar]
- 22.Kispert A., Vainio S., McMahon A.P. (1998) Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development, 125, 4225–4234. [DOI] [PubMed] [Google Scholar]
- 23.Tanigawa S., Wang H., Yang Y., Sharma N., Tarasova N., Ajima R., Yamaguchi T.P., Rodriguez L.G., Perantoni A.O. (2011) Wnt4 induces nephronic tubules in metanephric mesenchyme by a non-canonical mechanism. Dev. Biol., 352, 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chassot A.A., Gillot I., Chaboissier M.C. (2014) R-spondin1, WNT4, and the CTNNB1 signaling pathway: strict control over ovarian differentiation. Reproduction, 148, R97–110. [DOI] [PubMed] [Google Scholar]
- 25.Chang J., Sonoyama W., Wang Z., Jin Q., Zhang C., Krebsbach P.H., Giannobile W., Shi S., Wang C.Y. (2007) Noncanonical WNT-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J. Biol. Chem., 282, 30938–30948. [DOI] [PubMed] [Google Scholar]
- 26.Huang S.M., Mishina Y.M., Liu S., Cheung A., Stegmeier F., Michaud G.A., Charlat O., Wiellette E., Zhang Y., Wiessner S. et al. (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature, 461, 614–620. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi A., Stewart C.A., Wang Y., Fujioka K., Thomas N.C., Jamin S.P., Behringer R.R. (2011) Beta-catenin is essential for Müllerian duct regression during male sexual differentiation. Development, 138, 1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oberlender S.A., Tuan R.S. (1994) Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development, 120, 177–187. [DOI] [PubMed] [Google Scholar]
- 29.Heikkila M., Prunskaite R., Naillat F., Itaranta P., Vuoristo J., Leppaluoto J., Peltoketo H., Vainio S. (2005) The partial female to male sex reversal in Wnt-4-deficient females involves induced expression of testosterone biosynthetic genes and testosterone production, and depends on androgen action. Endocrinology, 146, 4016–4023. [DOI] [PubMed] [Google Scholar]
- 30.Jeays-Ward K., Hoyle C., Brennan J., Dandonneau M., Alldus G., Capel B., Swain A. (2003) Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development, 130, 3663–3670. [DOI] [PubMed] [Google Scholar]
- 31.Ahtiainen L., Lefebvre S., Lindfors P.H., Renvoise E., Shirokova V., Vartiainen M.K., Thesleff I., Mikkola M.L. (2014) Directional cell migration, but not proliferation, drives hair placode morphogenesis. Dev. Cell., 28, 588–602. [DOI] [PubMed] [Google Scholar]
- 32.Deutscher E., Hung-Chang Yao H. (2007) Essential roles of mesenchyme-derived beta-catenin in mouse Müllerian duct morphogenesis. Dev. Biol., 307, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart C.A., Wang Y., Bonilla-Claudio M., Martin J.F., Gonzalez G., Taketo M.M., Behringer R.R. (2013) CTNNB1 in mesenchyme regulates epithelial cell differentiation during Müllerian duct and postnatal uterine development. Mol. Endocrinol., 27, 1442–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franco H.L., Dai D., Lee K.Y., Rubel C.A., Roop D., Boerboom D., Jeong J.W., Lydon J.P., Bagchi I.C., Bagchi M.K. et al. (2011) WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J., 25, 1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stark K., Vainio S., Vassileva G., McMahon A.P. (1994) Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by WNT-4. Nature, 372, 679–683. [DOI] [PubMed] [Google Scholar]
- 36.Shan J., Jokela T., Skovorodkin I., Vainio S. (2010) Mapping of the fate of cell lineages generated from cells that express the Wnt4 gene by time-lapse during kidney development. Differentiation, 79, 57–64. [DOI] [PubMed] [Google Scholar]
- 37.Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. (2001) Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol., 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muzumdar M.D., Tasic B., Miyamichi K., Li L., Luo L. (2007) A global double-fluorescent Cre reporter mouse. Genesis, 45, 593–605. [DOI] [PubMed] [Google Scholar]
- 39.Naillat F., Prunskaite-Hyyrylainen R., Pietila I., Sormunen R., Jokela T., Shan J., Vainio S.J. (2010) Wnt4/5a signalling coordinates cell adhesion and entry into meiosis during presumptive ovarian follicle development. Hum. Mol. Genet., 19, 1539–1550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.