Abstract
The blood glucose meter (BGM) is the most successful and widely used portable device for point-of-care (POC) tests. However, its usage is limited to self-monitoring of blood glucose level only. To expand the targets that BGM can monitor while taking advantage of more than 50 years of technology development, we report herein the use of BGM to detect and quantify insulin and glycated hemoglobin (HbA1c), which are useful hormone for diabetes treatment and biomarker for diabetes monitoring, respectively. The method is based on invertase enzyme-linked immunosorbent assay (iELISA) and phosphatase enzyme-linked immunosorbent assay (pELISA) that convert BGM-inert sucrose or glucose-1-phosphate into glucose in the presence of insulin and glycated hemoglobin, respectively. In both assays, monoclonal antibodies specific to the targets (insulin or HbA1c) are immobilized onto magnetic beads to capture the targets in samples, followed by the formation of sandwich complex with the polyclonal antibodies conjugated to either invertase or phosphatase. The quantification of the targets is then realized by the production of glucose from the biochemical reactions catalyzed by the polyclonal antibody-enzyme conjugates bound on the surface of the magnetic beads. Such a method can be generally applied to a wide range of other biomarkers using the corresponding antibodies.
Keywords: blood glucose meter, enzyme-linked immunosorbent assay, glycated hemoglobin, insulin, point-of-care test
As a widely used portable device for glucose monitoring, blood glucose meter (BGM) is currently the most successfully commercialized point-of-care (POC) self-monitoring device.1,2 With over 50 years of engineering and development since its first report in 1962,3 BGMs possess all the features for a POC device: simple, portable, low-cost, fast, and quantitative.1,2 As a result, BGMs are available worldwide and dominate the current POC device market. In addition, many BGMs are compatible with smartphones,4,5 which enrich user experience through different apps and enable wireless data transmission for mobile health care (mHealth) applications.6 However, the BGMs are dedicated device, used only for monitoring blood glucose in diabetics. If the BGMs can be expanded to detect other blood biomarkers, especially those related to diabetes, the well-established device platform will then be applicable to monitor a much broader range of biomarkers besides blood glucose to help monitor and manage diabetes and other related diseases. In this way, the costly development process of other devices can be bypassed to take advantage of the BGM device that is already familiar to millions of diabetes. To realize this goal, we have previously reported invertase-based approaches to repurpose BGMs to detect a variety of analytes, including metal ions, organic molecules and biomolecules.7-10 In these approaches, the selective binding of functional DNA (eg, DNAzymes and DNA aptamers) and antibodies to their respective targets is quantitatively converted to glucose production through invertase-catalyzed hydrolysis of sucrose into glucose for BGM measurement. Recently, Yan et al. have also reported target-responsive hydrogel with glucose meter readout for detection of nonglucose targets.11 While the general principle of detection was shown, its specific applications in detecting biomarkers related to diabetes have not been demonstrated.
In this report, we describe proof-of-concept methods that use BGMs to detect and quantify insulin12 and HbA1c,13 as an example of a protein target and a biomarker for diabetes, respectively. The methods are based on the principle of enzyme-linked immunosorbent assay (ELISA), using invertase and phosphatase as the signal producing enzymes, thus establishing invertase enzyme-linked immunosorbent assay (iELISA) and phosphatase enzyme-linked immunosorbent assay (pELISA) approaches for insulin and Hb1Ac detections, respectively.
Methods
The BGM used in this study was an ACCU-CHEK Aviva glucose meter from Roche (Indianapolis, IN) with coded strips. Other BGMs should also be applicable for the same purpose. Invertase from yeast (Grade VII), bovine serum albumin (BSA), human hemoglobin (Hb) and reagents for buffers were purchased from Sigma-Aldrich (St. Louis, MO). The biotinylation reagents, Biotin-PEG4-NHS and streptavidin, were purchased from Pierce (Rockford, IL). A commercial insulin detection kit (containing insulin antibody-coated 96 well plates, binding buffer, washing buffer, biotinylated insulin antibody and insulin standard solution) from Millipore (Billerica, MA) and an antibody conjugation kit for epoxy magnetic beads (MBs) from Invitrogen (Carlsbad, CA) were also used. Human Hb1Ac protein, mouse monoclonal antibody (mAb) to HbA1c, rabbit polyclonal antibody (pAb) to HbA1c, and alkaline phosphatase (ALP) conjugated mouse secondary mAb to rabbit IgG were obtained from Abcam (Cambridge, MA).
Invertase-biotin conjugates were prepared similarly to a published procedure,9 where 20 mg/mL invertase in a 100 mM sodium phosphate buffer (pH 7.2) containing 100 mM NaCl was mixed with around 2 mg/mL Biotin-PEG4-NHS for 2 hours under ambient condition and subsequently the conjugate product was purified using Amicon-10K centrifuge filters from Millipore for 6 times using the sodium phosphate buffer.
Antibody-conjugated magnetic beads were also prepared through a reported procedure9 with minor changes following the protocol in the commercial conjugation kit (Dynabeads® Antibody Coupling Kit). Solutions C1, C2, HB, LB, SB and Dynabeads M-270 were provided in the kit (the supplier does not reveal the components of each solution) and were used directly without any treatment in this report. A portion of 8 mg Dynabeads M-270 MBs was washed with 1 mL C1 solution and the supernatant was removed by a magnet. Then, the residue was dispersed in a 0.5 mL mixed solution of C1 and C2 (v/v = 1:1) and added with 50 μg mouse mAb to HbA1c. The solution was then kept on a roller at room temperature overnight. Later, the supernatant was removed by a magnet, and the solid residue was washed by 0.8 mL solution HB. This washing step was then repeated twice using 0.8 mL LB and SB instead of HB, respectively. Then, the solid residue was redispersed in 0.8 mL SB and kept on a roller for 15 minutes before the removal of supernatant by a magnet. Finally, the solid residue was dispersed in 0.5 mL SB and stored at 4°C.
In the BGM detection of insulin by iELISA, 100 μL insulin standard solutions of different concentrations in the binding buffer were added to each well of the insulin antibody-coated 96 well plate, respectively. After 1 hour, the solutions were discarded from the plate, and each well was washed for 3 times using the washing buffer. Then, 100 μL biotinylated insulin antibody was added to each well, followed by the addition of 1 μM streptavidin in the binding buffer containing 1 g/L BSA, and the plate was incubated for 0.5 hours. After 3 times washing by the washing buffer, each well of the plate was treated with 100 μL 1 μM invertase-biotin conjugate in the binding buffer for 0.5 hours and then washed by the washing buffer for 3 times again. Subsequently, 100 μL 0.5 M sucrose in the sodium phosphate buffer was added to each well and the plate was sealed to avoid water evaporation. After kept on a gentle roller for 72 hours, the solution in each well was measured by the BGM.
In the BGM detection of HbA1c by pELISA, 100 μL 4 mg/mL (0.4 mg) antibody conjugated MBs was used for each test. The supernatant was removed by a magnet, and then 100 μL HbA1c standard solutions of different concentrations of HbA1c in the assay buffer (100 mM sodium phosphate at pH 7.0 containing 100 M NaCl and 1 g/L BSA) were added to the MB residue and mixed on a roller. After 30 minutes, the supernatant was removed by a magnet and the MB residue was washed by the assay buffer for 3 times. Then, 100 μL 25 mg/L rabbit polyclonal antibody to hemoglobin A1c (ab31152) in the assay buffer was added to the MB residue and mixed at room temperature for 30 minutes. After removal of supernatant and twice wash using the assay buffer, 100 μL 1/100 diluted of mouse monoclonal secondary antibody to rabbit IgG conjugated with ALP (ab99701) was added to the MB residue and reacted at room temperature for 30 minutes, followed by 3 times wash using the assay buffer and then dispersed in 100 μL 0.1 M sodium carbonate buffer (pH 9.9) containing 3 g/L glucose-1-phosphate for 2.5 hours. Finally, 6 μL 1 M NaH2PO4 was added to the solution to neutralize the pH and the solution was measured by a BGM.
Results
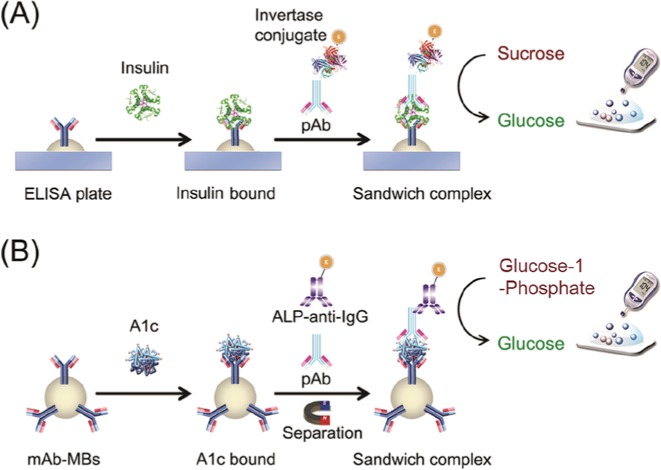
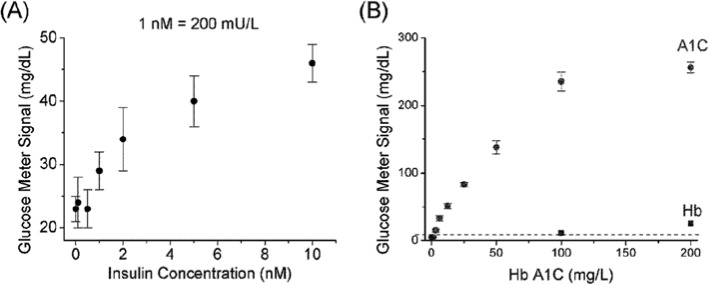
The methods in this report are based on the iELISA and pELISA approaches for insulin and Hb1Ac detections, respectively. The principle under both approaches is similar to a typical ELISA, but with invertase or phosphatase as the enzyme to generate glucose for BGM quantification of the targets (Figure 1). Insulin or Hb1Ac as analyte was captured by antibodies coated on a surface, and then another type of antibodies were added to the surface to form the antibody-target-antibody sandwich. Subsequently, secondary antibodies conjugated with either invertase or phosphatase were added to produce glucose for BGM quantification, through invertase-catalyzed or phosphatase-catalyzed hydrolysis of sucrose or glucose-1-phosphate, respectively. Typical results using iELISA and pELISA for the detection of insulin and Hb1Ac are shown in Figure 2, demonstrating the successful quantification of the targets by a BGM. Detection limits of 1 nM (200 mU/mL) insulin and 3 mg/L HbA1c were achieved, and high concentrations of BSA in washing buffer did not interfere the detection. Interestingly, the HbA1c detection by pELISA was not affected by normal Hb, except for the trace amount of HbA1c that might be present in the Hb samples.
Figure 1.
Scheme of insulin and HbA1c detections using BGM based on iELISA (A) and pELISA (B), respectively.
Figure 2.
Typical results of insulin (A), and HbA1c (B) detection using BGM. The interference of Hb in (B) may be caused by the trace amount of HbA1c in the Hb sample.
Discussions
Based on the typical ELISA principle with invertase and phosphatase as the enzyme, insulin and HbA1c have been successfully quantified by a BGM in this report by the iELISA and pELISA approaches, respectively. All the reagents involved in the method are either commercially available or can be prepared in a simple step, making it easier for the establishment of the standard protocols. Sensitivity has been reached to 1 nM and 3 mg/L for insulin and HbA1c, respectively, and competing proteins such as BSA and Hb do not interfere the detection. The current proof-of-concept method uses basic ELISA protocols, which still involves several liquid transfer and washing steps. To make the HbA1c detection more suitable for POC applications for patients and doctors, integrating all the reagents and sample pretreatment units into lateral-flow devices, as well as making the method applicable for real blood sample tests, are also demanded.
Conclusions
Based on ELISA principle and utilizing invertase or phosphatase as the enzyme to produce BGM detectable glucose from inert precursors, the proof-of-concept method in this report (eg, iELISA and pELISA) enables the current widely available BGMs to quantitatively monitor insulin and HbA1c besides blood glucose. While insulin serves as an example of protein targets for detection, the POC detection of HbA1c along with glucose monitoring are very useful for patients and doctors during the control and treatment of diabetes. Although the method in this report at its current stage still needs further optimizations to realize blood sample tests and integration into test kits to automate and shorten the detection, it provides a promising proof-of-concept technology to enable BGM and BGM-integrated wireless devices to detect biomarkers besides blood glucose for the monitoring of diabetes status. The concept in this method can also be expanded to the POC monitoring of other diseases biomarkers as long as suitable antibodies and enzymes are used.
Footnotes
Abbreviations: ALP, alkaline phosphatase; BGM, blood glucose meter; BSA, bovine serum albumin; C1, solution C1 provided with the Dynabeads Antibody Coupling Kit; C2, solution C2 provided with the Dynabeads Antibody Coupling Kit; ELISA, enzyme-linked immunosorbent assay; HB, solution HB provided with the Dynabeads Antibody Coupling Kit; Hb, hemoglobin; HbA1c, glycated hemoglobin; iELISA, invertase enzyme-linked immunosorbent assay; LB, solution LB provided with the Dynabeads Antibody Coupling Kit; mAb, monoclonal antibody; MB, magnetic bead; mHealth, mobile health care; pAb, polyclonal antibody; pELISA, phosphatase enzyme-linked immunosorbent assay; POC, point-of-care; SB, solution SB provided with the Dynabeads Antibody Coupling Kit .
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study in this report is supported by the U.S. National Institutes of Health (ES16865) and GlucoSentient, Inc.
References
- 1. Heller A, Feldman B. Electrochemical glucose sensors and their applications in diabetes management. Chem Rev. 2008;108(7):2482-2505. [DOI] [PubMed] [Google Scholar]
- 2. Montagnana M, Caputo M, Giavarina D, Lippi G. Overview on self-monitoring of blood glucose. Clin Chim Acta. 2009;402(1-2):7-13. [DOI] [PubMed] [Google Scholar]
- 3. Clark LC, Lyons C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann N Y Acad Sci. 1962;102(1):29-45. [DOI] [PubMed] [Google Scholar]
- 4. iBGStar® Blood Glucose Meter. Available at: http://www.bgstar.com/web/ibgstar.
- 5. Carroll AE, Marrero DG, Downs SM. The HealthPia GlucoPack (TM) diabetes phone: a usability study. Diabetes Technol Ther. 2007;9(2):158-164. [DOI] [PubMed] [Google Scholar]
- 6. GSMA mHealth. Available at: http://www.gsma.com/connectedliving/wp-content/uploads/2012/04/22-November12_MobileHealth_Device_Listing.pdf.
- 7. Xiang Y, Lu Y. Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nature Chem. 2011;3(9):697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiang Y, Lu Y. Using Commercially available personal glucose meters for portable quantification of DNA. Anal Chem. 2012;84(4):1975-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiang Y, Lu Y. Portable and quantitative detection of protein biomarkers and small molecular toxins using antibodies and ubiquitous personal glucose meters. Anal Chem. 2012;84(9):4174-4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xiang Y, Lu Y. An invasive DNA approach toward a general method for portable quantification of metal ions using a personal glucose meter. Chem Commun. 2013;49(6):585-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan L, Zhu Z, Zou Y, et al. Target-responsive “sweet” hydrogel with glucometer readout for portable and quantitative detection of non-glucose targets. J Am Chem Soc. 2013;135(10):3748-3751. [DOI] [PubMed] [Google Scholar]
- 12. Yalow RS, Berson SA. Assay of plasma insulin in human subjects by immunological methods. Nature. 1959;184(4699):1648-1649. [DOI] [PubMed] [Google Scholar]
- 13. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]