Abstract
Background:
There is a perception that patients with diabetes struggle to produce sufficient blood to fill glucose test strips, including strips with 1-µL fill requirements. The purpose of this study was to determine the volume of blood expressed when these patients perform routine fingersticks using their own lancing device and sampling technique and to evaluate the relationship between blood volume and pain.
Methods:
Sixty-four patients (type 1 or type 2 diabetes) performed 8 fingersticks using their own lancing device and preferred depth setting and lancing technique. Eight different commercially available lancing systems were used (8 patients/system). Blood volume and perceived pain were recorded after each fingerstick.
Results:
The mean blood volume across all patients was 3.1 µL (512 fingersticks), with 97% of patients expressing a mean of ≥1.0 µL of blood. There was no correlation between pain response and the volume of blood expressed. Nearly all patients agreed that they could easily and comfortably obtain a 1-µL blood sample, and most patients actually preferred a larger drop size to ease sampling and avoid wasting strips.
Conclusion:
These results provide evidence across 8 lancing systems that challenge the current perceptions that patients with diabetes struggle to produce sufficient blood samples to fill most test strips, including those with 1-µL fill requirements, and that obtaining larger volumes of blood is more painful. These results are consistent with the previous literature suggesting that patients derive no real benefits from very low strip volumes and generally prefer a blood drop size that enables them to confidently fill their test strip.
Keywords: blood volume, pain, diabetes, lancing, self-monitoring of blood glucose
Self-monitoring of blood glucose (SMBG) using a finger or alternate blood sampling sites to obtain blood provides important information regarding glycemic control for patients with diabetes, and current guidelines recommend appropriate testing for such patients.1 Furthermore, a recent publication2 described a strong association between higher SMBG frequency and lower hemoglobin A1c (HbA1c) levels, highlighting the need for patients to perform fingersticks and reinforcing the need for more data on blood sampling given its critical role in SMBG. A recent review by Heinemann and Boecker3 summarized lancing technologies and provided insight into fingerstick blood sampling. The authors posed the question “What size blood drop do we need?” and suggested that handling blood volumes of less than 1 to 2 µL on a routine basis is impractical for most users because of impaired vision and dexterity. Furthermore, the authors suggested that because of the small diameter of a blood drop that is less than 1 to 2 µL, there is a potential for misalignment when trying to fill a strip, resulting in smearing of the sample and perhaps the wasting of strips. The authors concluded that patients do not really benefit from the advertised “low volume strip” claims of manufacturers. Despite the practical concerns raised by this review article on handling smaller blood drops, there remains a widespread perception that many patients struggle to produce blood drops ≥1 µL in volume. This perception may be especially prevalent with respect to patients who are accustomed to using test strips that specify low blood volume requirements (0.3 or 0.6 µL). It is postulated that such patients might routinely express smaller blood volumes because they are aware that their strip requires less than 1 µL of blood.
To explore these perceptions and characterize the actual blood volume expressed, we specifically recruited patients (56 of 64) currently using glucose test strips that require only 0.3 or 0.6 µL of blood. In addition, to explore the impact of the lancing device, we recruited patients using a wide range of current lancing devices. Another common perception is that generating larger blood volumes (≥1 µL) during fingersticks will cause more pain than generating smaller blood volumes. Therefore, the current study also evaluated if there is a relationship between the blood volume expressed after routine fingersticks and pain perceived by the patient.
Methods
This single-center, open, nonrandomized clinical study was intended to mimic the routine blood sampling practices of patients with diabetes. The study was approved by the relevant investigational review board, and all patients provided written informed consent before initiation of the study. Patients were 18 to 75 years old, had type 1 or type 2 diabetes, and performed SMBG at least twice per day for at least 6 months before the study. All patients used a currently marketed lancing system. Twenty-nine patients were currently using 1 of the 8 lancing systems included in the study. The remaining 35 patients were assigned a system to use at home for at least 1 week before returning for the second visit to ensure that they were proficient in using the lancing device and were deemed current users of the device.
The study was conducted over 2 visits at 1 center by FACET Technologies (Atlanta, GA, USA). During the first visit, patients consented to participate and were evaluated with the inclusion/exclusion criteria, and demographic information was collected. The second visit consisted of a maximum of 12 lancing events (per institutional review board approval) and completion of a survey. Patients brought their lancing device to visit 2 and used the same depth setting typically used at home to lance. In addition, they were instructed to express capillary blood from their fingertips using the same technique that they typically use at home and consistent with their specific lancing device instructions. This minimized any bias that may have been introduced by dictating a lancing depth or prescribing a specific blood sampling technique. Eight different lancing devices (Table 1) were used with 8 different patients per device. Patients were asked to perform 8 separate lancing events using both sides of 2 fingers on each hand. The order and side of finger used were randomized across patients.
Table 1.
Lancing Devices and Lancet Gauges.
| Lancing group | No. of patients | Manufacturer | Lancing device | Lancet gauge | Depth setting |
|---|---|---|---|---|---|
| 1 | 8 | Menarini | Glucoject Dual S | 30 | 0-6 |
| 2 | 8 | Roche | Accu-Chek FastClix | 30 | 0.5-5.5 |
| 3 | 8 | Roche | Accu-Chek Multiclix | 30 | 0.5-5.5 |
| 4 | 8 | Bayer | Microlet 2 | 28 | 1-5 |
| 5 | 8 | Abbott | EasyTouch | 28 | 1-8 |
| 6 | 8 | Abbott | FreeStyle Flash | 28 | 1-4 |
| 7 | 8 | LifeScan | OneTouch Delica | 30 | 1-7 |
| 8 | 8 | ReliOn | ReliOn Lancer | 30 | 1-5 |
Once the blood droplet was formed on the patient’s finger, the study nurse drew up the blood in either one or two 5-µL calibrated glass capillary tubes. The capillary tube was then placed on a 5-µL calibrated linear scale (with 0.1-µL increments), and the value was read by the study nurse and recorded. Up to a maximum of 10 µL of blood generated on the skin surface was collected for each patient by the study nurse. Any additional blood was not used to ensure that extreme blood collection values did not skew the data. Based on previous measurement system analyses, the standard deviation for this collection method is 0.15 µL, with an 8.28% blood collection variance. Lancing events that produced <0.3 µL were considered unsuccessful because patients in a home setting would need to relance to obtain sufficient blood to fill their test strip, and patients were instructed to relance up to 4 times (on each side of 2 different fingers) to achieve blood volumes ≥0.3 µL. Following each lancing event, patients scored their perceived pain using the well-established Gracely pain scale from 0 to 20 points (Table 2).4-10
Table 2.
Gracely Pain Scale.
| Score | Description of pain |
|---|---|
| 20 | |
| 19 | |
| 18 | Extremely intense |
| 17 | Very intense |
| 16 | Intense |
| 15 | Strong |
| 14 | |
| 13 | Slightly intense |
| 12 | Barely strong |
| 11 | Moderate |
| 10 | |
| 9 | |
| 8 | Mild |
| 7 | Very mild |
| 6 | |
| 5 | Weak |
| 4 | Very weak |
| 3 | |
| 2 | |
| 1 | Faint |
| 0 | No pain sensation |
Statistical Analyses
Statistics are mainly descriptive for blood volume means ± standard deviations and medians. Mean blood volumes were calculated for each patient, which were then used to determine the mean blood volume across all 64 patients. Blood volume proportions were examined using a success criterion of ≥1.0-µL mean blood volume across patients. To estimate the blood volume across all lancing systems and patients within lancing systems, an ANOVA test was used with a significance level of .05. Pain responses were correlated to the blood volume and depth setting using the Pearson correlation coefficient. Survey responses were described using proportions.
Results
Patients
All 64 patients completed the study. Baseline characteristics are shown in Table 3. There were 37.5% of patients who had type 1 diabetes, and 62.5% had type 2 diabetes. The SMBG test frequency was at least twice per day in all patients, with 46.9% testing ≥3 times per day.
Table 3.
Patient Demographics (N = 64).
| n (%) | |
|---|---|
| Sex | |
| Female | 33 (51.5) |
| Male | 31 (48.4) |
| Age, y | |
| 21-30 | 9 (14.1) |
| 31-40 | 6 (9.4) |
| 41-50 | 16 (25.0) |
| 51-60 | 19 (29.7) |
| 61-70 | 10 (15.6) |
| ≥71 | 4 (6.3) |
| Diabetes type | |
| Type 1 | 24 (37.5) |
| Type 2 | 40 (62.5) |
| Medication regimen | |
| Both insulin and oral | 11 (17.2) |
| Insulin only | 28 (43.8) |
| Oral only | 19 (29.7) |
| Neither | 6 (9.4) |
| Frequency of testing | |
| 2 times daily | 34 (53.1) |
| 3 times daily | 7 (10.9) |
| 4 times daily | 9 (14.1) |
| 5 times daily | 8 (12.5) |
| ≥6 times daily | 6 (9.4) |
| Callused fingers | |
| Yes | 9 (14.1) |
| No | 55 (85.9) |
Characterization of Blood Volume
Sixty-four patients performed 512 fingersticks always using their own preferred depth setting. Descriptive statistics of patient blood volume are shown in Table 4. The mean blood volume across all 64 patients was 3.1 ± 2.1 µL. Repeat or second lancing events were required (per protocol) in 11 patients (88 lancing events) when the initial volume collected was less than 0.3 µL. The mean blood volume from these lancing events was 2.9 ± 2.4 µL. The mean blood volume collected from 53 patients who had first-time lancing events only (424 events) was 3.1 ± 2.0 µL. The median blood volume was 2.7 µL across all 64 patients . The median blood volume in patients who required a repeat lancing event was 2.3 µL (Table 4).
Table 4.
Summary of Patient Blood Volume Collection.
| All lancing eventsa | One lancing eventb | Repeat lancing eventc | |
|---|---|---|---|
| No. of patients | 64 | 53 | 11 |
| No. of fingersticks | 512 | 424 | 88 |
| Blood volume, µL | |||
| Mean ± standard deviation | 3.1 ± 2.1 | 3.1 ± 2.0 | 2.9 ± 2.4 |
| Median | 2.7 | 2.7 | 2.3 |
Each patient performed a minimum of 8 fingersticks (lancing events). Eleven patients required second lancing attempts to express ≥0.3 µL. Volume data were recorded only for the second fingerstick attempt in these patients.
The mean blood volume per patient was used to calculate the mean and median blood volume across all 64 patients.
Mean and median blood volume calculated using all 424 fingersticks.
Mean and median blood volume calculated using all 88 fingersticks.
Patients performed at least 8 fingersticks randomized across 3 fingers on each hand and used either side of each finger, as required. There were 97% of patients (62/64) who expressed a mean of ≥1.0 µL of blood based on performing at least 8 individual fingersticks (Figure 1). The ANOVA test indicated that there was no significant contribution due to the lancing system on the individual (Figure 1) or mean blood volume (F = 1.623, P = .15) (Table 5). In addition to the mean blood volumes for each patient, individual blood volume data are shown for each of the 512 individual fingersticks across the 64 patients (Figure 2). Patients used their preferred lancing depth setting, which varied across devices and patients (data not shown). Although there is no direct match across the depth settings on the 8 different devices, and although we did not measure the actual penetration depth across the 8 devices used in the study, there was no statistically significant correlation between the depth setting used and blood volume expressed (r = 0.135, P = .29) (Figure 3).
Figure 1.
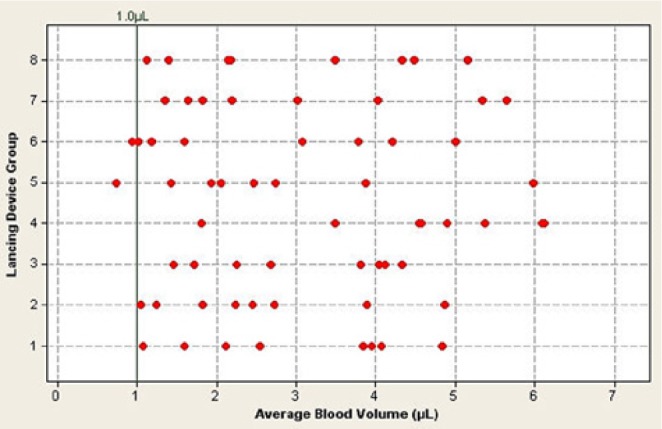
Mean fingerstick blood volume across all 64 patients versus lancing group (see Table 1 for lancing device groups). Each data point represents the mean of 8 fingersticks per patient for that lancing device. Some data points may be obscured by multiple, similar mean blood volumes for a particular lancing device.
Table 5.
Blood Volumes Collected Across 8 Different Lancing Devices.
| Lancing device group | n | Blood volume, mean ± standard deviation, µL |
|---|---|---|
| Glucoject Dual S | 8 | 3.0 ± 1.4 |
| Accu-Chek FastClix | 8 | 2.5 ± 1.3 |
| Accu-Chek Multiclix | 8 | 3.0 ± 1.2 |
| Microlet 2 | 8 | 4.6 ± 1.4 |
| EasyTouch | 8 | 2.7 ± 1.6 |
| FreeStyle Flash | 8 | 2.6 ± 1.6 |
| OneTouch Delica | 8 | 3.1 ± 1.7 |
| ReliOn Lancer | 8 | 3.0 ± 1.5 |
Figure 2.
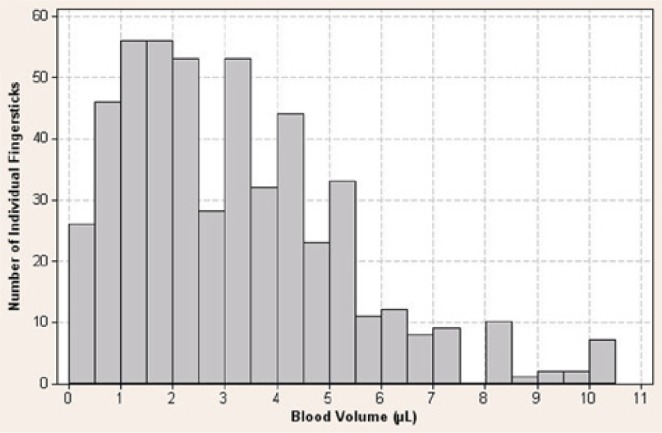
Individual fingerstick blood volume collected from 512 individual fingersticks (8 fingersticks per patient across 64 patients).
Figure 3.
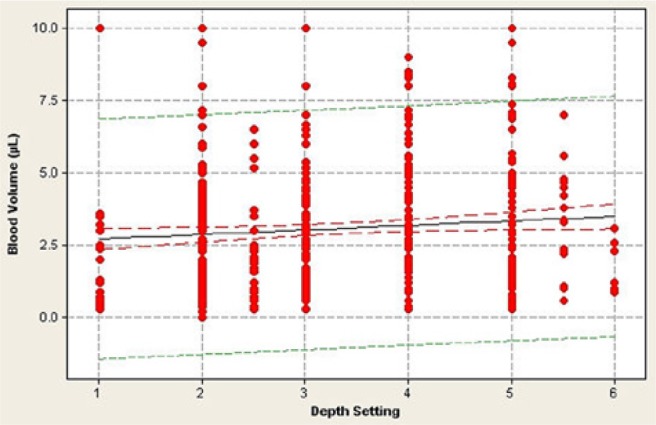
Relationship between expressed blood volume and lancing device’s depth setting. Each data point represents 1 of 512 fingersticks performed in 64 patients using 8 different lancing systems. Some data points may be obscured by multiple similar blood volumes at a particular depth setting. There is no significant relationship between blood volume and depth setting (r = 0.135, P = .29).
Association of Blood Volume With Depth Setting and Pain Experienced
The mean pain score across all 512 lancing events was 3.96 on the Gracely pain scale (corresponding to “very weak pain”) and ranged between 0 and 17 (Figure 4). There was no relationship between blood volume collected and the pain response (r = −0.054, P = .67), with less than 0.01% of the data variance attributed to this relationship (Figure 5). Similarly, there was no statistically significant correlation (r = 0.164, P = .19) between the depth setting and pain reported by the patient (Figure 6).
Figure 4.
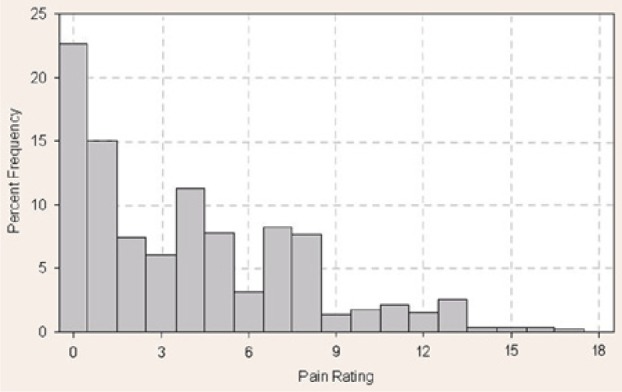
Distribution of pain scores. Frequency (%) of pain score responses after 512 fingersticks by 64 patients (8 fingersticks per patient) using the Gracely pain scale. Median pain rating = 3; mean pain rating = 3.96.
Figure 5.
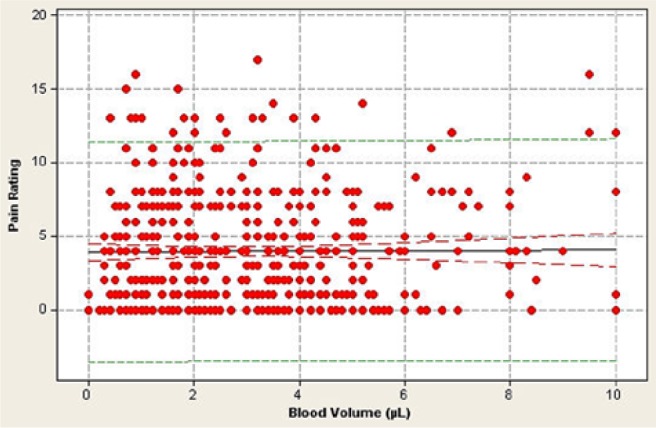
Relationship between pain, as measured by the Gracely pain scale, and blood volume expressed during 512 fingersticks by 64 patients. Each data point represents a single fingerstick. Some data points may be obscured by multiple similar pain ratings at a particular blood volume. There is no significant relationship between pain rating and blood volume (r = −0.054, P = .67).
Figure 6.
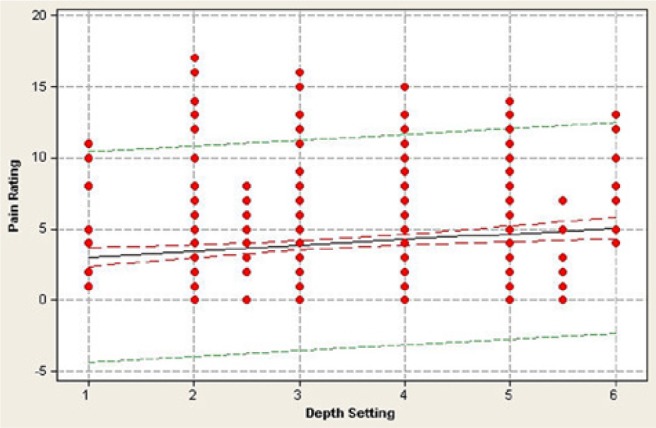
Relationship between pain, as measured by the Gracely pain scale, and lancing device’s depth setting during 512 fingersticks in 64 patients. Each data point represents a single fingerstick. Some data points may be obscured by multiple similar pain ratings at a particular depth setting. There is no significant relationship between pain rating and depth setting (r = 0.164, P = .19).
Patient Survey Feedback on Blood Sampling and Pain
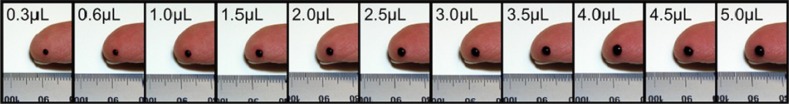
To facilitate survey responses from patients, a visual chart was provided comparing blood volume in microliters to blood drop size before patients completed the survey (Figure 7). Table 6 shows that 97% of patients agreed that they could easily and comfortably obtain more than 1 µL of blood to fill a strip, and 97% also agreed that getting enough blood was both quick and easy to do. Moreover, 89% of patients agreed that getting enough blood to fill a 1-µL test strip was not painful. Also, 80% and 79% of patients, respectively, agreed that filling their strips using larger blood drops (>1 µL) helped them to avoid wasting strips or lancing twice. In addition, 77% responded that they actually preferred a larger blood drop to ensure that they did not waste a strip.
Figure 7.
Comparison chart of blood volume (µL) compared to a visual chart of the same blood drop size shown to patients before responding to the survey of blood sampling practices.
Table 6.
Survey of Blood Sampling Practices.
| Survey statement | Agree or strongly agree, % |
|---|---|
| I can easily get more than 1 µL of blood to fill a strip. | 97 |
| I can comfortably get more than 1 µL of blood to fill a strip. | 97 |
| Getting enough blood to fill a 1-µL strip is quick and easy to do. | 97 |
| Getting more than 1 µL of blood from my skin is quick and easy to do. | 94 |
| I rarely or never have trouble getting more than 1 µL of blood from my skin. | 92 |
| Getting enough blood to fill a 1-µL strip is not painful. | 89 |
| Filling my strip using larger blood drops (more than 1 µL) helps me avoid lancing twice. | 80 |
| Filling my strip using larger blood drops (more than 1 µL) helps me avoid wasting strips. | 79 |
| I prefer a larger blood drop (more than 1 µL) to ensure I don’t waste a strip. | 77 |
Sixty-four patients responded to a 5-point scale for each survey statement: strongly agree, agree, neither agree nor disagree, disagree, or strongly disagree.
Safety
No unanticipated device-related adverse effects were reported.
Discussion
The primary objective of the study was to explore preconceived perceptions regarding the expressed blood volume in patients performing fingersticks during SMBG. The study demonstrated that the mean blood volume collected across 64 patients was 3.1 µL. This blood volume is 3 times the volume requirement for a 1.0-µL test strip and highlights a large discrepancy between the stated blood volume requirements of test strips and the actual sampling behavior of patients currently using the latest “low blood volume” (0.3 or 0.6 µL) glucose monitoring systems. It is worth noting that by having patients use multiple sampling sites (rather than simply preferred fingers on a particular hand), we may have actually underestimated the volumes expressed in that some patients may have struggled to obtain a blood sample from less familiar sites or sites that they would have ordinarily avoided because of handling or skin issues. Even with these limitations, the mean blood volume collected across patients was significantly higher than what might have been predicted in this population. In addition, 97% of the patients were able to produce on average at least 1.0 µL of blood. The reason for asking patients to perform multiple fingersticks was to achieve greater consistency in the data by offsetting slight changes in technique across each hand and different fingers, including site-to-site differences in skin conditions that in practice could impact blood sampling. The wide range of blood volumes observed within individual patients reflects the fact that blood sampling across finger sites is inherently variable. It also supports the view that patients may develop preferences for which fingers to lance based on how successful that site is for providing a sample or simply for device-handling reasons. In practice, patients are encouraged to rotate which fingers are lanced to ensure that skin condition, pain, or even cosmetic impacts of lancing are minimized, although to our knowledge, there is no evidence on how frequently finger sites are actually rotated.
It is important to note that it was not the intent of this study to compare differences between individual lancing devices with respect to blood volume collection because it is assumed that all current lancing devices perform well in the hands of practiced users. Furthermore, there are numerous factors that affect sampling that are not associated with the initial lancing event per se or the device itself, such as the postlancing technique (eg, pressure applied) used to express the blood sample from the skin.3,11-13 This study did not seek to control these factors.
Numerous studies have shown that within a patient, steadily increasing the depth settings results in higher expressed blood volumes.14-19 However, the current study did not attempt to confirm or refute these findings because such studies often prescribe a specific technique to express blood after lancing, which tends to mask variation in blood expression, regardless of the depth setting. The design of the current study simply allowed patients to use their accustomed depth setting and technique while evaluating the resulting expressed blood volume. We found that there was no correlation between the chosen depth setting and expressed blood volume or pain, with the caveat that the depth setting scales were not identical across lancing systems utilized. Although fingerstick blood volume is influenced by the depth setting, patients generally use other techniques (eg, mild pressure) to produce a blood drop size that they feel comfortable handling when filling strips, regardless of the stated blood volume requirement of the strip. Given the elasticity of the skin, the minor and transient nature of the wound, and the natural tendency of skin to immediately close after lancing, it is challenging to achieve a blood drop on the skin (regardless of depth) without some postlancing technique to encourage blood to the surface.19-22
Following each lancing event, each patient scored his or her perceived pain using the Gracely pain scale. The results indicated that there was no direct relationship between the blood volume collected and pain, with less than 0.01% of the data variance attributed to this relationship. Fruhstorfer et al16 also noted this lack of relationship between blood volume and pain and concluded that although there is a clear dependency of both puncture pain and blood volume on penetration depth, there is no direct interrelationship between pain and blood volume. Despite this evidence, there remains a perception that expressing a larger blood sample creates more pain for the user, an assumption based perhaps on a belief that the depth setting must be increased to achieve more blood. However, this argument fails to recognize that lancing itself and sample generation, although linked, are in fact unique events, each contributing to the overall blood drop size. In fact, the wide range of blood volumes obtained within patients (at the same depth settings) demonstrates how much site-to-site differences and personal postlancing techniques (eg, pressure application) strongly influence the blood volume expressed. Furthermore, although manufacturers focus on strip volume requirements in microliters, patients tend to use a blood drop size that they can actually see, handle, and manipulate to fill their strip. Although it has been widely reported that there is a correlation between increasing depth setting and pain experienced within individual patients,14-19 we did not observe a correlation between these 2 variables when depth setting and pain were analyzed across patients. This points strongly to the influence of other factors in pain perception across patients other than the depth setting, such as individual sensitivity to pain, skin thickness variations, specific lancing site variations, lancing mechanism specifications (speed, trajectory, and vibration), noise of the lancing mechanism, and lancet gauge and geometry.3,23-25 It is likely that multiple factors combine to influence an individual’s overall experience and response to pain. When the contribution of a single factor (such as depth) is analyzed across patients, the correlation is not as strong as it might have been had the study design required individual patients to perform multiple fingersticks at steadily increasing depth settings. In studies using systematic designs of this nature, a stronger correlation between an individual’s lancing depth and pain experienced has been reported.14
Finally, another key influence on pain perception (in a home setting) is the common practice of patients using lancets more than once perhaps because of a lack of time, inconvenience of changing lancets, or simply an attempt to economize. This practice results in progressive dulling of the lancing blade over time and is thought to have a major influence on the pain experienced. Although a systematic clinical study to evaluate pain associated with reusing lancets cannot be performed because of ethical considerations, a survey of patients indicated that over 65% use the same lancet at least 5 times, with only 10% changing lancets after every test.3,26
In addition to the quantitative measurements in this study, qualitative data were obtained to determine the personal views of patients regarding blood sampling. The vast majority (97%) of patients in this study agreed that they could easily and comfortably obtain more than 1 µL of blood to fill a strip and that getting enough blood was both quick and easy to do. This response is not entirely surprising given that the mean blood volume collected across all 64 patients was 3.1 µL and that 97% of the patients achieved a mean of ≥1 µL of blood. In terms of pain experienced, 89% of patients agreed that getting enough blood to fill a 1-µL test strip was not painful. Again, this is not surprising given that the mean pain score across all 512 fingersticks was only 3.96 (on a 0- to 20-point scale), which equated to a very weak pain sensation. Furthermore, our data indicate that there is no correlation between pain and the amount of blood collected. This result runs counter to the perceptions of some patients and perhaps certain health care professionals.
In a recent lancing review article, Heinemann and Boecker3 questioned the value of low volume strips (<1 µL) in terms of how impractical it is for patients to reliably fill strips using such a small blood drop. The patients in our study tended to agree with this concern, as 80% and 79%, respectively, agreed that filling their strips using larger blood drops (>1 µL) helped them to avoid wasting strips or lancing twice. Moreover, 77% stated that they actually preferred a larger blood drop to ensure that they did not waste a strip.
Conclusion
These results provide evidence across 8 lancing systems that challenge the current perceptions that patients with diabetes struggle to produce sufficient blood samples to fill most test strips, including those with 1-µL fill requirements, and that obtaining larger volumes of blood is more painful. These results are consistent with the previous literature suggesting that patients derive no real benefits from very low strip volumes and generally prefer a blood drop size that enables them to confidently fill their test strip.
Acknowledgments
The authors thank Mary Ann Kinard for her review and input to the article and Stephen Popielarski for review of the protocol and support of the study.
Footnotes
Abbreviations: HbA1c, hemoglobin A1c; SMBG, self-monitoring of blood glucose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.G. is an employee of LifeScan Scotland Ltd. L.B.K. is an employee of LifeScan Inc. B.G. is a consultant to FACET Technologies Inc. M.P. and M.K.P. are each employees of FACET Technologies Inc.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by LifeScan Inc.
References
- 1. American Diabetes Association. Position statement: standards of medical care in diabetes. Diabetes Care. 2013;36,S16-S18 (Suppl 1). [Google Scholar]
- 2. Miller KM, Beck RW, Bergenstal RM, et al. ; T1D Exchange Clinic Network. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 2013;36(7):2009-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heinemann L, Boecker D. Lancing: quo vadis. J Diabetes Sci Technol. 2011;5(4):966-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harkins W. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;65:217-226. [DOI] [PubMed] [Google Scholar]
- 5. Gracely RH. Psychophysical assessment of human pain. In: Bonica JJ, Liebeskind JC, Albe-Fessard D, eds. Advances in Pain Research and Therapy. Vol 3 New York: Raven Press; 1979:805-824. [Google Scholar]
- 6. Gracely RH, Kwilosz DM. The descriptor differential scale: applying psychological principles to clinical pain assessment. Pain. 1988;35:279-288. [DOI] [PubMed] [Google Scholar]
- 7. Gracely RH, Naliboff BD. Measurement of pain sensation. In: Kruger L, ed. Pain and Touch. New York: Academic Press; 1996:243-313. [Google Scholar]
- 8. Gracely RH, McGrath P, Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978;5:5-18. [DOI] [PubMed] [Google Scholar]
- 9. Gracely RH. Measuring pain in the clinic. Anesth Prog. 1990;34:88-92. [PMC free article] [PubMed] [Google Scholar]
- 10. Oliveria A, Teixeira N. Multidimensional quantitative semantics of pain: a nomothetic-idiographic approach through functional measurement. Teorie & Modelli, n.s., XII, 1-2, 2007. (155-166). Bologna, Pitagora Press. [Google Scholar]
- 11. Heinemann L. Finger pricking and pain: a never ending story. J Diabetes Sci Technol. 2008;2(5):919-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le Floch JP, Bauduceau B, Levy M, Mosnier-Pudar H, Sachon C, Kakou B. Self-monitoring of blood glucose, cutaneous finger injury, and sensory loss in diabetic patients. Diabetes Care. 2008;31(10):e73. [DOI] [PubMed] [Google Scholar]
- 13. Klein SL, Heisler M, Piette JD, Makki F, Kerr EA. The effect of chronic pain on diabetes patients’ self-management. Diabetes Care. 2005;28:65-70. [DOI] [PubMed] [Google Scholar]
- 14. Fruhstorfer H, Schmelzeisen-Redeker G, Weiss T. Capillary blood volume and pain intensity depend on lancet penetration. Diabetes Care. 2000;23(4):562-563. [DOI] [PubMed] [Google Scholar]
- 15. Fruhstorfer H. Capillary blood sampling: the pain of single-use lancing devices. Eur J Pain. 2000;4(3):301-305. [DOI] [PubMed] [Google Scholar]
- 16. Fruhstorfer H, Müller T, Scheer E. Capillary blood sampling: how much pain is necessary? Part 2: relation between penetration depth and puncture pain. Pract Diabetes Int. 1995;12(4):184-185. [Google Scholar]
- 17. Fruhstorfer H, Lange H. Capillary blood sampling: how much pain is necessary? Part 3: pricking the finger can be less painful. Pract Diabetes Int. 1995;12(6):253-254. [Google Scholar]
- 18. Fruhstorfer H, Selzer K, Selbman O. Capillary blood sampling: how much pain is necessary? Part 4: comparison of lancets for automatic lancing devices. Pract Diabetes Int. 1996;13(2):58-60. [Google Scholar]
- 19. Fruhstorfer H, Schmelzeisen-Redeker G, Weiss T. Capillary blood sampling: relation between lancet diameter, lancing pain and blood volume. Eur J Pain. 1999;3(3):283-286. [DOI] [PubMed] [Google Scholar]
- 20. Kocher S, Tshiananga JK, Koubek R. Comparison of lancing devices for self-monitoring of blood glucose regarding lancing pain. J Diabetes Sci Technol. 2009;3(5):1136-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lekarcyk J, Ghiloni S. Analysis of the comparison of lancing devices for self-monitoring of blood glucose regarding lancing pain. J Diabetes Sci Technol. 2009;3(5):1144-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pacaud D, Lemay J, Buithieu M, Yale J. Blood volumes and pain following capillary punctures in children and adolescents with diabetes. Diabetes Care. 1999;22(9):1592-1594. [DOI] [PubMed] [Google Scholar]
- 23. Fruhstorfer H, Abel U, Garthe CD, Knüttel A. Thickness of the stratum corneum of the volar fingertips. Clin Anat. 2000;13(6):429-433. [DOI] [PubMed] [Google Scholar]
- 24. Warunek D, Stankovic AK. Evaluation of lancets for pain perception and capillary blood volume for glucose monitoring. Clin Lab Sci. 2008;21(4):215-218. [PubMed] [Google Scholar]
- 25. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koschinsky T. Blood glucose self-monitoring report 2006 reveals deficits in knowledge and action. Diabetes Stoffwechsel Herz. 2007;16:185-192. [Google Scholar]