Abstract
Background:
Insulin pump therapy may be offered to patients with type 2 diabetes that is not controlled by multiple daily injections. Patients with type 2 diabetes may suffer from unrecognized cognitive disabilities, which may compromise the use of a pump device.
Methods:
To predict patient autonomy, we evaluated 39 patients with type 2 diabetes from our database (n = 143) after continuous subcutaneous insulin infusion (CSII) initiation using (1) an autonomy questionnaire evaluating the patient’s cognitive and operative capacities for CSII utilization, (2) the Montreal Cognitive Assessment (MOCA) for the detection of mild cognitive disabilities, (3) the Hospital Anxiety and Depression Scale (HADS) for the detection of anxiety and depression, and (4) the Diabetes Treatment Satisfaction Questionnaire (DTSQ). Patients were selected to constitute 3 groups matched for age, with different degrees of autonomy at discharge after the initial training program: complete (n = 13), partial (n = 13), or no autonomy (n = 13).
Results:
The satisfaction level with the pump device was high. At the last follow-up visit, only 23% of patients did not reach complete autonomy. The autonomy score correlated fairly with the MOCA score (R = 0.771, P < .001). A receiver operating characteristic (ROC) analysis showed that at a cut-off score of 24, the MOCA identified autonomous versus dependent patients at long-term follow-up (area under the ROC curve [AUC], 0.893; sensitivity, 81%; specificity, 81%). The HADS correlated negatively with the autonomy score, and the sociocultural level also influenced autonomy with pump utilization.
Conclusion:
Patients with type 2 diabetes with partial autonomy at discharge may progress to complete autonomy. The MOCA and HADS may help predict a patient’s ability to manage with a pump device.
Keywords: cognitive disability, DTSQ, HADS, insulin infusion system, MOCA, type 2 diabetes
Progressive β-cell dysfunction and chronic insulin resistance are the pivotal mechanisms for type 2 diabetes,1,2 which worsen over time, resulting in the need for exogenous insulin administration in patients who no longer respond to antihyperglycemic agents.3 Although the use of continuous subcutaneous insulin infusion (CSII) in patients with type 1 diabetes is widespread, pump utilization for type 2 diabetes is eligible for reimbursement in few countries including France.4
The effectiveness of CSII versus multiple daily injections (MDI) for lowering glucose was questioned in 4 randomized controlled studies.5-8 In 2 parallel-group studies, both treatments demonstrated similar efficacy.5,6 However, a higher efficacy of CSII over traditional MDI regimens was observed in 2 crossover studies.7,8 Recent open-label observational studies have shown the potential benefits of CSII on glycemic control in patients with type 2 diabetes. One 24-week study performed in 46 patients previously on a basal/bolus regimen demonstrated a −0.5% glycated hemoglobin (HbA1c) drop under CSII.9 One retrospective longitudinal study performed in 102 patients with poorly controlled diabetes reported a −1.4% HbA1c decrease, which was maintained throughout the study’s 5-year follow-up period.10 These studies provide meaningful information on CSII and its efficacy in the treatment of type 2 diabetes. A large international randomized study now in progress will determine the efficacy of CSII in comparison with the gold standard of a basal/bolus insulin regimen in patients with type 2 diabetes.11
A patient’s ability to manage a CSII device was previously evaluated in a single-center French retrospective study, showing that 65% of patients were not fully autonomous at discharge from the initial training session and that at long-term follow-up, 25% of patients remained dependent on aid from a nurse.10 The identification of a patient’s capacity for using a pump is based on medical staff experience, but objective criteria are actually lacking. Unrecognized cognitive or operative disabilities, but also mood alterations such as depression or anxiety, may impair a patient’s ability to use a CSII device. Assessment tools such as the Montreal Cognitive Assessment (MOCA) have been validated for the detection of cognitive impairment in clinical situations such as predementia and cerebral ischemic attacks.12-15 It was the aim of this study to evaluate tools that may help determine the ability of patients with type 2 diabetes to autonomously manage with a pump device.
Patients and Methods
The present study retrospectively selected 39 patients with type 2 diabetes treated by CSII, from the Caen University Hospital type 2 CSII database (n = 143), to identify predictive criteria for determining their ability to manage diabetes using a CSII device. All patients gave their informed consent for the study. Patients were selected if their age was less than 80 years; if they were not prone to recurrent hypoglycemia; and if they had no known cognitive impairment, cerebrovascular disease, or Alzheimer disease. The patients were selected for the study to constitute 3 subgroups based on their autonomy for the management of a CSII device at the time of discharge from a 5-day diabetes center training program. Technical autonomy was defined as the ability to select a basal or bolus program, to perform a bolus, to select a temporary basal, to switch off the pump, and to change the catheter and reservoir. Cognitive autonomy was defined as the patient’s ability to appropriately change a basal or bolus rate according to the glycemic level, to correct hypoglycemic or hyperglycemic events, to anticipate the occurrence of hypoglycemia, and to deal with a session of physical exercise.
From the 39 selected patients, 13 displayed complete autonomy (ie, technical and cognitive), 13 displayed partial autonomy (ie, technical but not cognitive), and 13 were dependent on a nurse or family member for the management of the CSII device. The 3 groups were matched for age. The 39 patients of the present study did not differ from the 104 other patients of the type 2 CSII database according to age, baseline HbA1c value, diabetes duration, duration of pump therapy, total daily insulin dose, and comorbidities. Moreover, we also checked that the 3 groups of 13 patients identified by the criteria of autonomy did not differ from each other according to age, baseline HbA1c value, diabetes duration, duration of pump therapy, total daily insulin dose, and comorbidities.
Before pump initiation, insulin therapy regimens included a basal-bolus regimen (52%), premixed insulin twice daily (34%), or basal therapy (14%). Fifty-two percent of patients used metformin, 3% used glitazone, and none used other antidiabetic agents.
Patients were admitted for pump deployment and pump training. All patients were evaluated during a 45- to 60-minute visit performed between December 2010 and May 2011 using the following: (1) an autonomy questionnaire developed by the medical staff of the Caen University Hospital’s Endocrinology Department for assessing a patient’s cognitive and operative abilities to use the CSII device: a total score of 1 to 10 of 31 characterized a low degree of autonomy, 11 to 20 characterized a medium autonomy score, and 21 to 31 characterized a high autonomy score. (2) The MOCA for the detection of cognitive dysfunction and operative disabilities: the MOCA was designed as a rapid screening instrument for mild cognitive dysfunction. It assesses different cognitive domains such as attention and concentration, executive functions, memory, language, visuoconstructional skills, conceptual thinking, calculation, and orientation. The maximal score is 30; a score of ≥26 is considered normal.15 (3) The Hospital Anxiety and Depression Scale (HADS) for the detection of anxiety and/or depression,16 and (4) the Diabetes Treatment Satisfaction Questionnaire (DTSQ) for evaluation on a visual scale of the satisfaction level for the pump device, its convenience and flexibility, the perception of diabetes, the level of glycemic control, and willingness to remain on pump therapy.17 (5) In addition, the patient’s social and cultural level was evaluated by a French scale from Poitrenaud18 using a 4-level graded scale (level 1: no diploma; level 2: 4 years before baccalaureate; level 3: 1-3 years before baccalaureate; level 4: baccalaureate or postbaccalaureate).
Statistical Analysis
Quantitative variables were described using means ± standard deviations. Qualitative variables were described using frequencies and percentages. An ANOVA was used to compare the means of quantitative variables in ≥2 independent groups. The relationship between 2 quantitative variables was assessed using the Pearson correlation coefficient. The receiver operating characteristic (ROC) method was used to define the threshold of the MOCA score to discriminate cognitive dysfunction. The area under the ROC curve (AUC) was calculated to quantify the discriminative ability of the MOCA. Factor analysis of the scale was performed using the principal component analysis as the method of extraction. The retained factors had eigenvalues >1. Independent factors were obtained using the varimax rotation method. All the tests were 2-tailed, and their level of significance was defined as P < .05. SPSS 20.0 for Windows (IBM, Armonk, NY, USA) was the statistical software used.
Results
Thirty-nine patients with type 2 diabetes were included in the study. Analysis of baseline patient characteristics showed a mean age at the time of the study of 61.0 ± 8.5 years, a mean body mass index of 33.7 ± 6.2 kg/m2, a mean HbA1c value before pump initiation of 8.9% (74 mmol/mol) ± 1.7%, and a mean insulin dose of 1.18 ± 0.57 U/kg/d. Patients had a mean duration of diabetes of 14.0 ± 7.1 years, with a mean duration of pump utilization of 6.9 ± 5.6 years (60% of patients were pump users for 1-4 years, 30% for 5-7 years, and 10% for 8-9 years). Comorbid conditions included nephropathy (33%), neuropathy (38%), retinopathy (28%), coronary artery disease (23%), and peripheral artery disease (8%).
Clinical and Metabolic Evolution Under CSII
After CSII initiation, a −1.4% HbA1c decrease was observed at 1 year (7.5%, 58 mmol/mol), which was maintained at last follow-up (7.7%, 61 mmol/mol). The total insulin daily dose decreased by 25% at 1 year (0.88 ± 0.42 U/kg/d; P < .001) and by 18% at last follow-up (0.97 ± 0.47 U/kg/d; P < .02) in comparison with the total daily dose under MDI. No change was made in oral antidiabetic agents after insulin pump initiation. No significant weight change was observed at 1 year and at last follow-up.
Satisfaction With the Pump Device
The mean DTSQ score at last follow-up showed a high level of satisfaction with the CSII device related to its convenience and flexibility. Most patients were willing to remain on long-term pump therapy. No important change in perception by the patient of the quality of glycemic control was observed on CSII (Figure 1).
Figure 1.
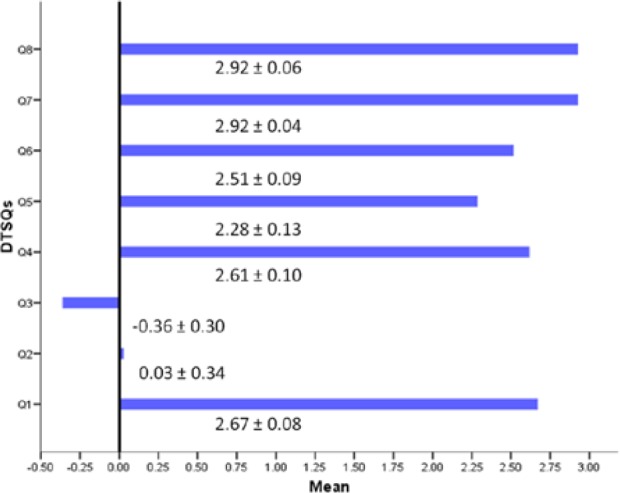
Patient satisfaction with the device at pump initiation according to the Diabetes Treatment Satisfaction Questionnaire (DTSQ) (mean ± standard deviation).
The 3 subgroups of patients respectively exhibited complete autonomy, partial (technical) autonomy, or no autonomy at discharge from the initial 5-day training program in our diabetes center. At the 1-year visit and at last follow-up, most patients improved their autonomy level, with 77% of the patients displaying complete autonomy and 23% remaining partially or completely dependent (Table 1). The cognitive and operative autonomy questionnaire completed at last follow-up visit showed that 25% of patients had a low degree of autonomy, 59% had medium autonomy, and 15% had high autonomy.
Table 1.
Patient Autonomy at Pump Initiation, 1 Year, and Last Evaluation.
| Autonomy level | After pump initiation, n | At 1 year, n | At last evaluation, n |
|---|---|---|---|
| No autonomy | 13 | 2 | 2 |
| Partial autonomy | 13 | 7 | 7 |
| Complete autonomy | 13 | 30 | 30 |
Evaluation of Mood Disorders by the HADS
The HADS completed at last follow-up identified overt anxiety items in 33% of patients and overt depressive items in 18% of patients.
Sociocultural Assessment
Only 13% of patients exhibited a high sociocultural level (level 4), while most patients had a low sociocultural level (69% level 2 and 18% level 1).
Cognitive Evaluation by the MOCA and Correlations With the Autonomy Score
The MOCA completed at last follow-up visit showed that 43.6% of patients exhibited a score below the normal level of 26 (mean level, 22.3 ± 1.8), while 56.4% exhibited a score above 26 (mean level, 28.0 ± 1.2). A relationship was observed between the MOCA score at the last follow-up visit and the degree of autonomy at discharge from the 5-day training program: 61.5% of patients with no autonomy at discharge had a MOCA score <26, while 84.6% of patients with complete autonomy at discharge had a MOCA score >26. The autonomy score calculated from the cognitive and operative ability questionnaire fairly correlated with the MOCA score (R = 0.771, P ≤ .001 ; R2 = 59.4%) (Figure 2). At last follow-up visit, 100% of patients with a high autonomy score exhibited a MOCA score >26, and 100% of patients with a low autonomy score exhibited a MOCA score <26. A ROC analysis showed that the MOCA score predicted complete autonomy with the CSII device (vs partial or absence of autonomy) at discharge from the 5-day initial training session with a cut-off score of 27 (AUC, 81.7%; sensitivity, 81.0%; specificity, 71.0%) and predicted complete autonomy at long-term follow-up with a cut-off score of 24 (AUC, 89.3%; sensitivity, 81.0%; specificity, 81.0% ) (Figure 3).
Figure 2.
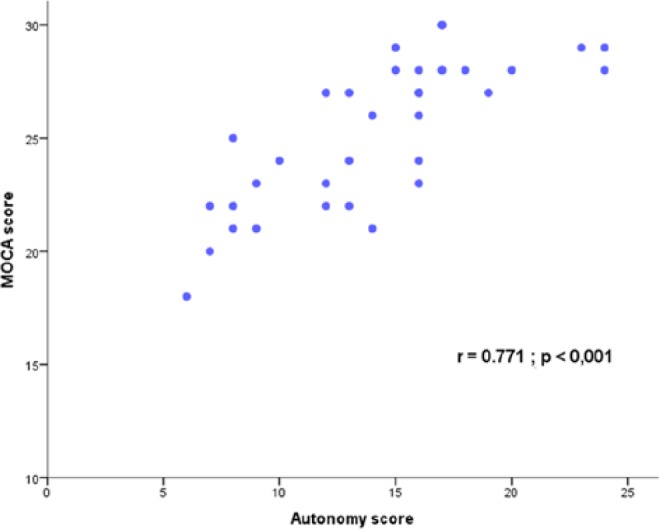
Correlation between the continuous subcutaneous insulin infusion (CSII) autonomy score and the Montreal Cognitive Assessment (MOCA) score.
Figure 3.
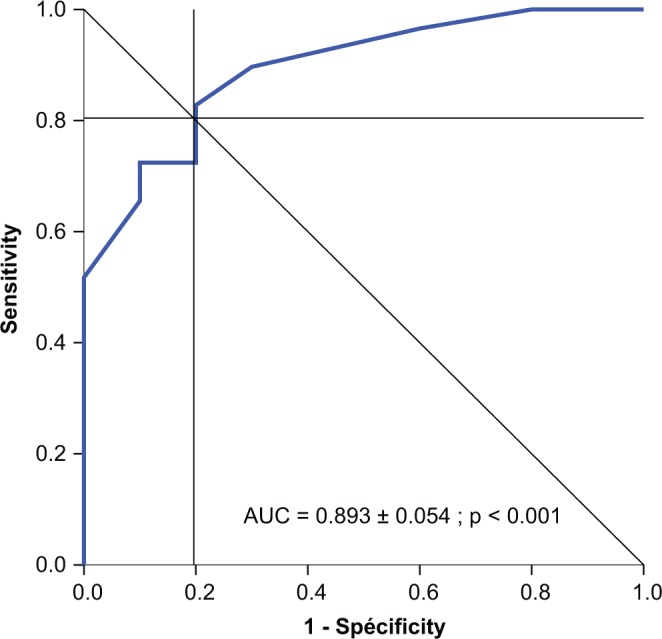
Receiver operating characteristic (ROC) analysis: prediction of patient autonomy with the pump device at 1 year by the Montreal Cognitive Assessment (MOCA) score <24 (area under the ROC curve [AUC], 0.893; sensitivity, 0.810; specificity, 0.810).
Influence of Other Variables on Autonomy With the Pump Device
Autonomy with the pump device at long-term follow-up was influenced by mood disorders, as shown by the negative correlation existing between the autonomy score on one hand and the global HADS score (R = −0.345, P < .05) and the HADS depression score (R = −0.387, P < .05) on the other hand. The autonomy score was significantly lower in patients exhibiting overt depressive items in comparison with patients without depressive items (mean HADS depression score: 11.1 ± 4.3 vs 15.7 ± 4.3, respectively; P = .02). The HADS anxiety score did not significantly influence the autonomy score. A low sociocultural level possibly influenced the degree of autonomy because patients with a low autonomy score had a trend toward having lower university degrees in comparison with those with a high autonomy score (1.6 ± 0.7 vs 3.0 ± 0.9, respectively; P = .068). The degree of autonomy did not correlate with the level of glycemic control, age, duration of prior insulin therapy, and duration of pump therapy. The influence of the different variables on the autonomy score is illustrated by the factorial analysis showing that the autonomy score positively correlates with the MOCA score and the sociocultural level, negatively correlates with the HADS depression score, but is independent of patient age at pump initiation and the duration of diabetes (Figure 4).
Figure 4.
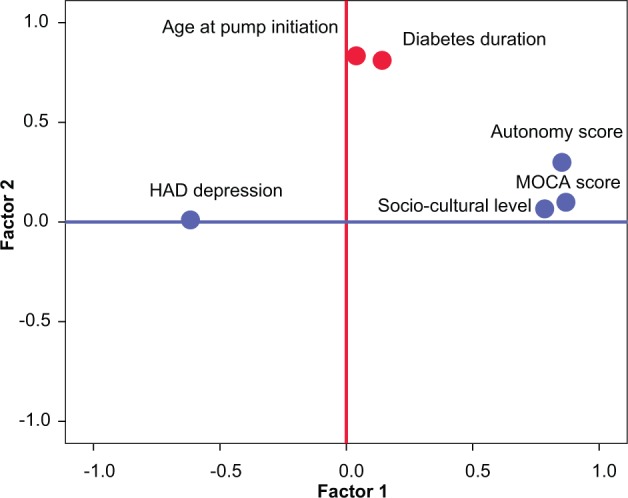
Factorial analysis: interaction between the autonomy score and other variables.
Discussion
The present study was designed to evaluate the autonomy of patients with type 2 diabetes with their pump device when CSII was preferred to the standard MDI option for insulin intensification. Pump therapy may be offered to patients with type 2 diabetes who fail to respond to an intensified MDI course, mostly in situations of severe chronic hyperglycemia despite high insulin requirements.1 A regimen of 4 to 5 daily injections combining a slow-acting analog plus a rapid-acting analog, and alternatively a combination of 2 to 3 daily doses of premixed neutral protamine Hagedorn (NPH)/rapid analog, are both considered as valuable tools for the intensification of insulin therapy after failure of a basal insulin regimen.19 Nevertheless, several observational studies have highlighted the durable benefit of CSII on glycemic control.10,20 Pump therapy may therefore be an option for maintaining fair glycemic control and for limiting weight gain in obese patients with type 2 diabetes. The ability to manage a pump device is a question raised in patients with type 2 diabetes who are older than patients with type 1 diabetes and are not always familiar with the utilization of electronic devices such as insulin pumps. Furthermore, executive functioning and speed may be impaired in older patients with type 2 diabetes in comparison with their healthy counterparts.21
In the present study, we observed in a group of patients who were initially trained to pump utilization during a standardized 5-day session that half of patients were not completely autonomous at discharge but that 77% reached complete autonomy after 1 year of pump utilization and 23% remained partially or completely dependent on a nurse’s assistance. In a recent retrospective cohort study, 25% of patients on long-term pump therapy remained dependent on a nurse’s or family member’s assistance.10 The selection of candidates for pump therapy may therefore include an analysis of the patient’s cognitive and operative capacities and the detection of any mental disability or mood disorder that would compromise the success of the switch from MDI to CSII. The absence of a disability ought to be evaluated by a team with good experience in the management of pump therapy. Mild cognitive dysfunction and anxious or depressive mood can be detected with specialized questionnaires validated in this setting to reinforce the educational knowledge in these patients with proper training sessions and a simplified approach to pump therapy. Apart from specialized diabetes questionnaires that investigate a patient’s cognitive and operative capacities for pump utilization, no data were available concerning the influence of mild cognitive disabilities or mood disorders on patient autonomy with the CSII device.
The present study concludes that the MOCA may be administered to candidates for insulin pump therapy and may help to distinguish those patients who will need intensified training and/or assistance for the management of their CSII device. The MOCA is a rapid screening instrument that assesses different cognitive domains including attention and concentration, executive functions, and calculation,15 which are important capacities for the correct and safe utilization of CSII devices. The MOCA was proven to detect mild cognitive impairment in patients with Parkinson disease or dementia when standard tests such as the Mini-Mental State Examination (MMSE) failed to detect such disabilities.12,13 The MOCA was also found to be more effective than the MMSE for the cognitive evaluation of patients with transient ischemic attacks and stroke.14 In the present study, the MOCA was able to predict short-term but also long-term autonomy with fair sensitivity and specificity. Although a significant proportion of the patients participating in the study had an abnormal MOCA score, they were not identified as having significant cognitive impairment before selection, which would have been an exclusion criterion for pump therapy. In a recent large international study, as much as 36% of enrolled patients had an abnormal MOCA score and were randomized thereafter.22 The HADS also helped to detect patients suffering from depressive traits, which may impair their capacity to manage their own CSII devices. We suggest that such tools may be administered to any patient with type 2 diabetes who is a candidate for pump therapy to help evaluate his or her ability to deal with the pump device and to reinforce the educational training of patients with cognitive or mood disabilities.
Another finding of the present study is the influence of social and cultural characteristics of our patients on the ability to deal with the pump device. The detection of cognitive or mood disabilities is to be taken into account for personalizing the educational approach of patients with type 2 diabetes but is not a determinant for the indication of pump therapy because in our retrospective cohort study, pump therapy provided good efficacy even in the noncompletely autonomous subgroup of patients.10 Patients selected for the present study were fairly representative of a previously described cohort, as suggested by the same demographic profile and the same rate of efficacy of pump therapy in the former compared to the latter.10 The satisfaction level for the pump device and its convenience in real life were high, confirming data from previous studies.5-7
Conclusion
Pump therapy may be a valuable tool in the management of type 2 diabetes that is uncontrolled by traditional MDI regimens. Successful CSII utilization depends on a patient’s ability to engage the device effectively and consistently, and this process can be limited by unrecognized cognitive and/or operative disabilities. Patients with type 2 diabetes who exhibit limited autonomy at discharge may be able to progress to complete autonomy during the first year of CSII. Cognitive and mood evaluation tools such as the MOCA and HADS may be helpful for detecting cognitive and operative disabilities and therefore identify patients who may benefit from personalized training programs or alternatively a nurse’s assistance for the safe utilization of CSII devices.
Footnotes
Abbreviations: AUC, area under the ROC curve; CSII, continuous subcutaneous insulin infusion; DTSQ, Diabetes Treatment Satisfaction Questionnaire; HADS, Hospital Anxiety and Depression Scale; HbA1c, glycated hemoglobin; MDI, multiple daily injections; MOCA, Montreal Cognitive Assessment; ROC, receiver operating characteristic.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Yves Reznik and Michael Joubert carried out clinical trials as co-investigators for Medtronic, Eli Lilly, and Novo Nordisk. Yves Reznik provided advisory services to Medtronic and attended conferences organized by Eli Lilly and Medtronic as a contributor. Michael Joubert served as a consultant and attended conferences organized by Medtronic. Rémy Morello, Amel Zenia, Julia Morera, and Anne Rod have no conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Temple RC, Carrington CA, Luzio SD, et al. Insulin deficiency in non-insulin-dependent diabetes. Lancet. 1989;1(8633):293-295. [DOI] [PubMed] [Google Scholar]
- 2. Gerich J. The genetic basis of type 2 diabetes mellitus: impaired insulin secretion versus impaired insulin sensitivity. Endocr Rev. 1998;19(4):491-503. [DOI] [PubMed] [Google Scholar]
- 3. Group UKPDS. Overview of 6 years’ therapy of type II diabetes: a progressive disease (UKPDS 16). Diabetes. 1995;44(11):1249-1258. [PubMed] [Google Scholar]
- 4. Lassmann-Vague V, Clavel S, Guerci B, et al. ; Société Francophone du Diabète (ex ALFEDIAM). Position statement: when to treat a diabetic patient using an external insulin pump. Expert consensus: Société Francophone du Diabète (ex ALFEDIAM) 2009. Diabetes Metab. 2010;36(1):79-85. [DOI] [PubMed] [Google Scholar]
- 5. Raskin P, Bode BW, Marks JB, et al. Continuous subcutaneous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes. Diabetes Care. 2003;26(9):2598-2603. [DOI] [PubMed] [Google Scholar]
- 6. Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care. 2005;28(7):1568-1573. [DOI] [PubMed] [Google Scholar]
- 7. Berthe E, Lireux B, Coffin C, et al. Effectiveness of intensive insulin therapy by multiple daily injections and continuous subcutaneous insulin infusion: a comparison study in type 2 diabetes with conventional insulin regimen failure. Horm Metab Res. 2007;39(3):224-229. [DOI] [PubMed] [Google Scholar]
- 8. Wainstein J, Metzger M, Boaz M, et al. Insulin pump therapy vs multiple daily injections in obese type 2 diabetic patients. Diabetes Med. 2005;22(8):1037-1046. [DOI] [PubMed] [Google Scholar]
- 9. Kesavadev J, Balakrishnan S, Ahammed S, Jothydev S. Reduction of glycosylated haemoglobin following 6 months of continuous subcutaneous insulin infusion in an Indian population with type 2 diabetes. Diabetes Technol Ther. 2009;11(8):517-521. [DOI] [PubMed] [Google Scholar]
- 10. Reznik Y, Morera J, Rod A, et al. Efficacy of continuous subcutaneous insulin infusion (CSII) in type 2 diabetes mellitus: a survey on a cohort of 102 patients with prolonged follow-up. Diabetes Technol Ther. 2010;12(12):931-936. [DOI] [PubMed] [Google Scholar]
- 11. Opt2mise glucose control in type 2 diabetes mellitus (DM) with insulin pump therapy. Available at: http://ClinicalTrials.gov/show/NCT01182493. Sponsor Medtronic. First received August 11, 2010.
- 12. Nazem S, Siderowf AD, Duda JE, et al. Montreal Cognitive Assessment performance in patients with Parkinson’s disease with “normal” global cognition according to Mini-Mental State Examination score. J Am Geriatr Soc. 2009;57(2):304-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Costa AS, Fimm B, Friesen P, et al. Alternate-form reliability of the Montreal Cognitive Assessment screening test in a clinical setting. Dement Geriatr Cogn Disord. 2012;33(6):379-384. [DOI] [PubMed] [Google Scholar]
- 14. Pendlebury ST, Cuthbertson FC, Welch SJV, Mehta Z, Rothwell PM. Underestimation of cognitive impairment by Mini-Mental State Examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke. Stroke. 2010;41(6):1290-1293. [DOI] [PubMed] [Google Scholar]
- 15. Nassreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. [DOI] [PubMed] [Google Scholar]
- 16. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361-370. [DOI] [PubMed] [Google Scholar]
- 17. Bradley C. Diabetes Treatment Satisfaction Questionnaire. In: Bradley C, ed. Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Chur, Switzerland: Harwood Academic; 1994:111-132. [Google Scholar]
- 18. Poitrenaud J. Niveau socio-culturel. In: Hugonot-Diener L, ed. Guide Pratique de la Consultation de Gériatrie: Evaluation du Niveau Socio-culturel. Paris: Masson; 2001:5. [Google Scholar]
- 19. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55(6):1577-1596. [DOI] [PubMed] [Google Scholar]
- 20. Reznik Y, Cohen O. Insulin pump for type 2 diabetes : use and misuse of continuous subcutaneous insulin infusion in type 2 diabetes. Diabetes Care. 2013; 36 (Suppl2):S219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yeung SE, Fischer AL, Dixon RA. Exploring effects of type 2 diabetes on cognitive functioning in older adults. Neuropsychology. 2009;23(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aronson R, Cohen O, Conget I, et al. OPT2MISE: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes. Research design and methods. Diabetes Technol Therap 2014. April 15 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]


